Abstract
The brain-derived neurotrophic factor gene (BDNF) is one of many genes thought to influence synaptic plasticity in the adult brain and shows a common single nucleotide polymorphism (BDNF Val66Met) in the normal population that is associated with differences in hippocampal volume and episodic memory. It is also thought to influence possible synaptic changes in motor cortex following a simple motor learning task. Here we extend these studies by using new non-invasive transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (TDCS) techniques that directly test the excitability and plasticity of neuronal circuits in human motor cortex in subjects at rest. We investigated whether the susceptibility to TMS probes of plasticity is significantly influenced by the BDNF polymorphism. Val66Met carriers were matched with Val66Val individuals and tested on the following protocols: continuous and intermittent theta burst TMS; median nerve paired associative stimulation; and homeostatic plasticity in the TDCS/1 Hz rTMS model. The response of Met allele carriers differed significantly in all protocols compared with the response of Val66Val individuals. We suggest that this is due to the effect of BNDF on the susceptibility of synapses to undergo LTP/LTD. The circuits tested here are implicated in the pathophysiology of movement disorders such as dystonia and are being assessed as potential new targets in the treatment of stroke. Thus the polymorphism may be one factor that influences the natural response of the brain to injury and disease.
A number of TMS protocols lead to after-effects on the excitability of the human motor cortex that persist for 30 min to several hours (see review by Ziemann et al. 2008). Evidence from pharmacological studies suggests that at least three of these may involve NMDA-mediated changes at synaptic connections. These are: transcranial direct current stimulation (TDCS), paired associative stimulation (PAS) and theta burst stimulation (TBS). Given the postulated role of synaptic plasticity in recovery of function after brain damage, there is currently great interest in applying these methods therapeutically to enhance function after stroke as well as chronic neurodegenerative disease. However, even in healthy subjects, the response to these protocols is highly variable between different individuals. A number of factors have already been described that contribute to this variation such as the prior history of brain activation (Gentner et al. 2008; Huang et al. 2008), the subject's age (Müller-Dahlhaus et al. 2008), the time of day (Sale et al. 2008), and the menstrual cycle (Inghilleri et al. 2004). Here we ask whether genetic factors might also influence these measures.
Brain-derived neurotrophic factor (BDNF) has a variety of roles in development and in the adult has been shown in animal models (Aicardi et al. 2004) to modulate NMDAR-dependent LTP (Figurov et al. 1996) and LTD (Woo et al. 2005a). Like many neurotrophins BDNF is initially produced as a longer precursor molecule, ProBDNF, which is then cleaved into mature BDNF. Both forms predominantly undergo activity-dependent release (rather than predominant constitutive release like other neurotrophins) at central synapses (Lu, 2003), possibly by virtue of a ‘sorting motif’ in the ‘pro’ region of ProBDNF. The emerging molecular biology of BDNF suggests that the intrasynaptic ratio of BDNF to ProBDNF may influence the relative ease of producing increases or decreases in synaptic efficacy (Woo et al. 2005b).
In humans, the ‘pro’ region of BDNF shows a single nucleotide polymorphism (SNP) –BDNF Val66Met – that has known functional consequences in healthy human subjects including reduced hippocampal volume (Pezawas et al. 2004) and episodic memory (Egan et al. 2003; Hariri et al. 2003; Pezawas et al. 2004). The polymorphism is relatively common (65% Val66Val to 35% Val66Met in the Caucasian population), making any functional consequence potentially significant and enabling studies with a smaller target recruitment. While several genes have been implicated in synaptic plasticity, the critical role of BDNF in LTP/LTD, the advances in molecular biology, the presence of a common polymorphism and increasing evidence of a functional role for this polymorphism makes it an attractive target for further investigation in humans.
Single pulse TMS has been used previously to demonstrate that BDNF genotype is associated with changes in excitability of primary motor cortex that occur after practising a motor task (Kleim et al. 2006). This study made use of the fact that there is an increase in excitability of the hand area of motor cortex (measured both as an expansion of the somatotopic motor map as well as an increase in the slope of the stimulus intensity/Motor Evoked Potential (MEP) amplitude curve) after subjects perform a repetitive key press/pinch grip task designed to activate the first dorsal interosseous (FDI) muscle. The authors found that individuals with the Val/Val polymorphism showed the expected changes whereas those carrying the Met allele did not. At the present time it is unclear to what extent the excitability changes are caused by changes in the efficacy of synaptic connections in the cortex or to changes in neuronal excitability. Nevertheless, the result would be consistent with the idea that polymorphisms of BDNF can directly influence synaptic plasticity in the adult human brain.
In the present paper we have followed up this observation in more detail by employing a number of non-invasive TMS techniques that directly test the excitability and plasticity of neuronal circuits in human motor cortex. Using these protocols instead of a motor task allows us to study increases, decreases and homeostatic changes in cortical excitability, enabling greater resolution into the component processes of synaptic plasticity. The data suggest that genetic factors influence the response to TMS plasticity protocols. If correct this would potentially have implications for diagnostic and therapeutic trials that harness the ability of rTMS protocols to probe and modulate neuroplasticity (e.g. in stroke rehabilitation or depression).
Methods
Ethical approval
The UCL/UCLH Regional Ethics Committee approved all experimental procedures. Sixty one volunteers were recruited after informed consent was obtained. Subjects recruited did not have any chronic illnesses requiring treatment. Epilepsy and chronic or recent use of prescription medication (like antidepressants, analgesics, etc.) other than the oral contraceptive pill were specifically excluded.
Recruitment of subjects
Subjects were genotyped after informed consent was obtained, using a previously described method and primers (see below).
All subjects carrying a ‘Met’ allele were invited for all experiments and recruited into the non-Val/Val group. Only two Met allele homozygotes were identified and only one volunteered for experiments 1a, 2 and 3. Subjects homozygous for the ‘Val’ allele, matched for age, sex and ethnicity (see Table 1), were then recruited into the ‘Val/Val’ group. Female volunteers were not matched for phase of menstrual cycle, but since the timing of the experiments was random, this would be unlikely to bias the results in any significant way. In total, 18 subjects (9 in each group) took part in experiments 1a, 1b and 3. Sixteen subjects (8 in each group) took part in experiment 2. Investigators blinded to the subject's genotype collected electrophysiological measures. Experiments were conducted at least 1 week apart.
Table 1.
Demographics of volunteers included in experiments
| Exp 1a: cTBS | Exp 1b: iTBS | Exp 2: TDCS preconditioning | Exp 3: PAS | |||||
|---|---|---|---|---|---|---|---|---|
| Genotype | Val/Val | Non-Val/Val | Val/Val | Non-Val/Val | Val/Val | Non-Val/Val | Val/Val | Non-Val/Val |
| (n = 9) | (n = 9) | (n = 9) | (n = 9) | (n = 8) | (n = 8) | (n = 9) | (n = 9) | |
| Mean age (±s.d.) (years) | 26.45 (±5) | 26.45 (±5) | 29.3 (±3) | 28.7 (±3) | 25.8 (±5) | 26.5 (±5) | 27.1 (±4) | 28 (±5) |
| Female | 5 | 5 | 3 | 3 | 3 | 3 | 4 | 4 |
| Caucasian | 5 | 6 | 6 | 7 | 5 | 5 | 6 | 6 |
| Asian | 4 | 3 | 3 | 2 | 3 | 3 | 3 | 3 |
BDNF genotyping technique
Genotyping was carried out twice with known positive controls. In GenBank sequences and the public SNP database (http://www.ncbi.nlm.nih.gov/), we identified a common coding variant in the BDNF gene, a G→A polymorphism responsible for a Val66Met change. Whole blood was taken into EDTA tubes and DNA was extracted using a standard phenol–chloroform method and checked for quality and concentration using a spectrophotometer. Part of exon 2 of the BDNF gene was amplified using the polymerase chain reaction (PCR) and primers (SBDNF1-AAA GAA GCA AAC ATC CGA GGA CAA G; SBDNF2-ATT CCT CCA GCA GAA AGA GAA GAG G) resulting in a 274 base pair (bp) PCR product. A Perkin Elmer 9700 thermal cycler was used for DNA amplification. Amplification reactions were performed in a total volume of 25 μl, containing approximately 50 ng of genomic template, 1 μm of each primer, 200 μm deoxyribonucleotide triphosphate (dNTP), 10× buffer inclusive of 2.5 mm magnesium chloride and 1 U of Taq polymerase. The PCR cycling conditions consisted of an initial denaturation for 10 min at 94°C, followed by touchdown program of 25 cycles of 94°C for 30 s, 60°C for 30 s and 72°C for 45 s. After each cycle the annealing temperature was reduced by 0.4°C down to 50°C. There were then 12 cycles of 94°C for 30 s, 50°C for 30 s and 72°C for 45 s and a final extension at 72°C for 10 min. The PCR was checked for success on a 2% agarose gel. The PCR product was then digested with the restriction enzyme Hsp92II. The reaction consisted of 10 μl of PCR product, 2 μl buffer, 1 μl Hsp92II, 0.2 μl bovine serum albumin and 6.8 μl of water. In the presence of the G allele, Hsp92II digestion produced two products, 57 and 217 bp, whereas the A allele produced three products, 57, 77 and 140 bp. The presence of a second Hsp92II site served as a restriction digest control, identifying incomplete digests for repeat analysis. Polymerase chain reaction products were electrophoresed on a 2% agarose gel and visualized using a transilluminator and ethidium bromide staining. All participants were successfully genotyped.
Transcranial magnetic stimulation methods
The primary motor cortex of the dominant hemisphere was stimulated in all experiments. One subject in each group in experiments 1a, 2 and 3 was left handed and therefore the right hemisphere was stimulated in these two subjects in these experiments. Two figure-of-eight coils with outer diameter of 70 mm (Magstim Co., Whitland, Dyfeld, UK) were used for the experiments. A monophasic Magstim 200 was used to define the motor hot-spot and to assess MEP size. The motor hot-spot was defined as the location where TMS consistently produced the largest MEP size at 120% RMT in the target muscle. A second coil was connected to a biphasic stimulator, a Super Rapid Magstim package (Magstim Co., UK), and was used to deliver rTMS. The coils were held at an angle of 45 deg away from the mid-sagittal line with the handle pointing backwards. According to the guidelines of the International Federation of Clinical Neurophysiology, we defined the resting motor threshold (RMT) as the minimum stimulation intensity over the motor hot-spot, which can elicit a MEP of no less than 50 μV in 5 out of 10 trials. Active threshold (AMT) was defined as the intensity necessary to evoke a 200 μV MEP while subjects maintained approximately 10% contraction of the target muscle.
The change in corticospinal excitability produced by each intervention was assessed by measuring the amplitude of the MEP response to a standard test pulse that remained constant throughout the experiment. In each subject the intensity of this pulse was individually adjusted at the start of the experiment to produce a stable MEP (of 0.5–1 mV) with the subject at rest (see Table 2B for mean baseline MEP amplitudes for each experiment).
Table 2.
Stimulation intensity (expressed as percentage of maximum stimulator output) and baseline MEP amplitudes (mV)
| A | |||
|---|---|---|---|
| % Stimulation intensity | Val/Val | Non-Val/Val | t test |
| cTBS (AMT) | 37 | 40 | 0.47 |
| iTBS (AMT) | 37 | 39 | 0.48 |
| TDCS (RMT) | 40 | 42 | 0.37 |
| B | |||
| MEP (Baseline) | Val/Val | Non-Val/Val | t test |
| cTBS | 0.97 | 0.86 | 0.44 |
| iTBS | 0.87 | 0.81 | 0.66 |
| TDCS | 1.1 | 1 | 0.39 |
| PAS (ADM) | 0.55 | 0.57 | 0.88 |
| PAS (APB) | 0.82 | 0.97 | 0.38 |
No significant differences were noted between groups.
Experimental set-up
During the experiments, subjects were sitting comfortably in an armchair with their eyes open. For experiments 1 and 2, EMGs were recorded via Ag–AgCl electrodes placed over the first dorsal interosseous (FDI) of the dominant hand using a belly tendon montage. For experiment 3, EMGs were recorded via Ag–AgCl electrodes placed over the abductor pollicis brevis (APB) and the abductor digiti minimi (ADM) of the dominant hand using a belly tendon montage. Signals were filtered (30 Hz to 2 kHz) and amplified (Digitimer 360, Digitimer Ltd, Welwyn Garden City, Herts, UK) and then stored on computer via a Power 1401 data acquisition interface (Cambridge Electronic Design Ltd, Cambridge, UK). Analysis was carried out using Signal Software (Cambridge Electronic Design).
Theta burst stimulation (TBS)
TBS was applied over the motor cortex hot-spot as described by Huang et al. (2005). Each burst consisted of three stimuli (80% AMT) given at 50 Hz. Continuous TBS (cTBS), which usually suppresses corticospinal excitability, was delivered as a sequence of 100 bursts (300 stimuli) given at a rate of 5 Hz (total duration of 20 s); intermittent TBS (iTBS) involved giving a 2 s train repeated every 10 s for 20 repetitions (600 stimuli).
The effect of TBS on corticospinal excitability was quantified by measuring the amplitude of MEPs evoked in the FDI by a constant-intensity TMS pulse given over the contralateral motor cortex. At the start of the experiment the intensity of this pulse was adjusted so that it evoked an MEP of about 1 mV peak-to-peak amplitude in each individual. Twenty such MEPs were collected and averaged at baseline. Then, after cTBS (experiment 1a) and iTBS (experiment 1b) over the same hot-spot, 20 MEPs were recorded at 1–5, 6–8, 9–11, 12–15 and 16–24 min after TBS and averaged.
Transcranial direct current stimulation (TDCS) preconditioned 1 Hz stimulation
In a second set of experiments, we examined the role of the polymorphism in the control of synaptic plasticity. To study homeostatic plasticity, 10 min of cathodal transcranial direct current stimulation (TDCS) was given initially to reduce motor cortical excitability; it was then followed by a short period of sub-threshold 1 Hz rTMS.
TCDS is also a non-invasive means of stimulating the cortex. Stimulation is delivered using a battery-driven DC stimulator (Schneider Electronic, Germany) via two conductive rubber electrodes, placed in saline-soaked sponges (5 cm × 7 cm), positioned over the primary motor cortex (the TMS hot-spot for FDI was used) and above the contralateral eyebrow. A constant current flow of 1 mA was applied for 10 min. The current was always ramped up/down slowly in the first and last 10 s of stimulation to reduce local skin stimulation. We used cathodal stimulation (cathode over the FDI TMS hot-spot), which produces an inhibitory effect if applied for 15 min.
rTMS of 1 Hz delivered at intensities at or above RMT suppresses corticospinal excitability. The duration of the after-effects depends on the total number of pulses given. In this study, 1 Hz rTMS was delivered for 15 min (900 pulses) at sub-threshold intensity (85% RMT) 10 min after the end of TDCS. RMT was assessed with the biphasic stimulation using the same criteria as above.
For the TDCS experiment (experiment 2), 20 MEPs were collected and averaged at baseline as for experiment 1 (time-point T0). Subjects then received 10 min of priming with cathodal TDCS, followed by 15 min of sub-threshold 1 Hz rTMS (both to the hand area of the motor cortex). MEPs were recorded immediately after TDCS (time-point T1), immediately after rTMS (time-point T2) and at 10 min after rTMS (time-point T3), and then averaged. Sub-threshold 1 Hz rTMS alone is insufficient to induce any after-effects, but when pre-conditioned by cathodal TDCS it generates facilitation of the motor cortex, producing a homeostatic-like effect (Siebner et al. 2004) that has been shown to be impaired in patients with focal dystonia (Quartarone et al. 2005).
Paired associative stimulation (PAS)
PAS (Stefan et al. 2000; Quartarone et al. 2006) was delivered using pairs of median nerve electrical and single pulse TMS over the abductor pollicis brevis (APB) hot-spot at an inter-stimulus interval of 25 ms. The intensity of the TMS was set to evoke an MEP of 0.5–1 mV in APB while the intensity of the median nerve stimulus (0.2 ms duration) was set at 3 times perceptual threshold. Two hundred pairs were given at a rate of 0.25 Hz.
Several studies have demonstrated that MEP facilitation induced by PAS is greater when subjects were tested at 8 o'clock in the evening than at 8 o'clock in the morning, possibly due to diurnal variations in the levels of neuromodulators like cortisol (Sale et al. 2008). To minimize daytime-dependent changes, in the present experiments PAS was always delivered between 11:00 and 15:00 h.
MEPs were recorded from the median-innervated APB and the ulnar-innervated abductor digiti minimi (ADM) muscles at baseline (T0) and at 1 min (T1), 15 min (T2), 30 min (T3), 45 min (T4) and 60 min (T5) after PAS, and then averaged. Note that the test stimulus was optimized for APB.
Data analysis
Data were analysed using SPSS for Windows version 11.0 on log transformed peak–peak amplitudes of the mean MEPs of each subject. Note that graphs show untransformed data. Repeated measures ANOVA with within subject factor of TIME (before/after intervention) and between subjects factor of GENOTYPE (Val/Val/non-Val/Val) was used to compare variables before and after each experimental intervention. Dose effect analysis (i.e. Val/Val versus Val/Met versus Met/Met) was not done as only a single Met allele homozygote participated in the study (and in 3 of 4 experiments only). Post hoc paired t tests were applied when necessary. In all figures, error bars refer to the standard error.
Results
Induction of LTP/LTD-like change
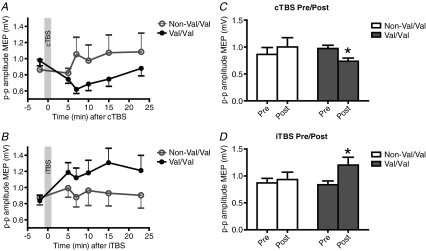
In the first set of experiments we tested whether the Val/Met polymorphism in the BDNF gene would affect the response to human theta burst stimulation. The data from all individual time points are plotted in Fig. 1A and B for the inhibitory cTBS and excitatory iTBS interventions, respectively. Since there was no difference in the post-TBS values at any time point in either group, these were averaged and the mean pre/post data are shown in the corresponding Fig. 1C and D. Two-way ANOVA of the log transformed data revealed a significant TIME*GENOTYPE interaction for both cTBS (F1,16= 16.08; P = 0.001) and iTBS (F1,16= 8.59; P = 0.01). This was due to the fact that there was a significant decrease in MEPs after cTBS in the Val/Val individuals (P = 0.0002; paired t test) but not in the non-Val/Val group. Similarly, there was a significant increase in MEPs after iTBS in the Val/Val individuals (P = 0.003; paired t test) but not in the non-Val/Val group.
Figure 1. Effect of BDNF Val66Met polymorphism on cortical excitability in response to cTBS (top row) and iTBS (bottom row).
Data are mean (+s.e.m.) peak-to-peak amplitudes of MEP. A and B plot data at all time points; C and D, the data from the 4 post-TBS sessions have been averaged to allow direct comparison of overall pre- vs post-TBS MEP amplitudes in the two groups of subjects (*P < 0.05).
Control of homeostatic plasticity
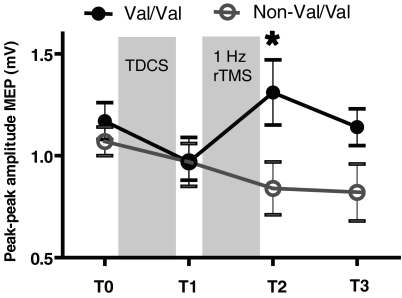
In a second set of experiments, we examined the role of the polymorphism in the control of synaptic plasticity. Analysis was performed using a mixed ANOVA design on the log transformed data with GENOTYPE as between-subjects factor (levels Val/Val and non-Val/Val) and TIME as within-subject factor; levels for factor TIME were baseline (T0), T1, T2 and T3. Figure 2 shows that subjects in the Val/Val group showed the expected pattern of effects: cathodal TDCS initially suppressed corticospinal excitability and this was followed by facilitation after 1 Hz rTMS. Subjects in the non-Val/Val group showed the same suppression after TDCS but no further effect after 1 Hz rTMS. ANOVA showed a significant GENOTYPE×TIME interaction (F3,42= 4.44, P = 0.009). Pair-wise comparisons revealed significantly higher MEP amplitudes after 1 Hz (T3) (t test: P = 0.025) in the Val/Val group compared to the non-Val/Val group.
Figure 2. Effect of BDNF Val66Met polymorphism on cortical excitability in response to cathodal TDCS preconditioning followed by sub-threshold 1 Hz rTMS.
Data are mean (+s.e.m.) peak-to-peak MEP amplitude (*P < 0.05).
Paired associative stimulation
In the final experiment, we explored the spread of LTP-like excitability using the paired associative stimulation protocol (experiment 3). Figure 3A and B plots the mean data at each individual time point for the APB and ADM muscles, respectively. Since there was no difference in any of the post-PAS values these were averaged together to form the summary pre/post comparisons in Fig. 3C and D. Three-way ANOVA on the log transformed data with within subject factors of MUSCLE (APB, ADM) and TIME (Pre, Post), and between subject factor of GENOTYPE revealed a significant TIME*GENOTYPE interaction (F1,16= 4.41; P = 0.05) as well as a significant main effect of MUSCLE (F1,16= 8.39; P = 0.011). Post hoc paired t tests showed that interaction was due to the fact that in Val/Val subjects, PAS produced a significant increase of the MEPs in ADM (P = 0.01) and a borderline significant increase in APB (P = 0.07). There were no significant effects in non-Val/Val individuals. The effect of MUSCLE was due to the fact that the MEPs were larger overall in the APB than the ADM.
Figure 3. Effect of BDNF Val66Met polymorphism on cortical excitability in response to paired associative stimulation in the target (homotypic) abductor pollicis brevis (top row) and (heterotopic) ulnar-innervated abductor digiti minimi (bottom row).
Data are mean (+s.e.m.) peak-to-peak amplitudes of MEP. A and B plot data at all time points; C and D, the data from the 5 post-PAS sessions have been averaged to allow direct comparison of overall pre- vs post-PAS MEP amplitudes in the two groups of subjects (*P < 0.05).
Discussion
The present data show that the response of healthy subjects to three different plasticity-inducing protocols in motor cortex is associated with the polymorphism of the BDNF gene that they carry. The implication is that genetic variation in the normal population can produce significant differences in the after-effects of rTMS protocols. If this conclusion is valid in more physiological conditions, then the same variations may influence behavioural learning as well as recovery from brain damage.
Experiment 1 examined the after-effect of an inhibitory (cTBS) and an excitatory (iTBS) rTMS protocol on corticospinal excitability. In non-genotyped healthy controls, cTBS suppresses MEPs for 30 min or so whereas iTBS facilitates them. The present results show that the after-effects of both iTBS and cTBS are reduced or absent in subjects carrying the ‘Met’ allele of the BDNF gene. This was not related to any initial differences in thresholds or MEP size in the two groups, and presumably indicates that ‘Met’ carriers are less susceptible to the effects of TBS than the Val66Val individuals.
There are several possible reasons for the difference in response between the groups. The most likely is that it is more difficult to induce plasticity of neural circuits in the non-Val/Val individuals. However, the present experiments do not address the question of whether this difference arises because non-Val/Val individuals lack any response to TBS, or because they have a different input–output relationship between the intensity of stimulation and the duration/depth of the after-effects. Further experiments with a range of TBS intensities would be needed to address this. A rather different possibility is raised by the recent report of Gentner et al. (2008) in which they pointed out that the after-effects of cTBS are extremely sensitive to the past history of motor cortex activation (‘rapid metaplasticity’). Although there was no difference in the amount of voluntary movement prior to stimulation in the two groups, it is possible that subjects differ in their sensitivity to prior activation and this could account for the apparently different response to TBS protocols. Despite the exact mechanism, we conclude that the after-effects of TBS protocols are affected by genetic variation in the normal population.
In experiment 2 we selected a ‘metaplastic’ conditioning protocol that employed 10 min cathodal TDCS to prime the response to presentation of 900 sub-threshold TMS pulses at 1 Hz. In a non-genotyped population of healthy subjects, MEPs are suppressed by the TDCS. This then transforms a subsequent period of 1 Hz rTMS, which on its own has no effect on corticospinal excitability, into facilitation. In the present experiments, cathodal TDCS produced the same amount of LTD-like suppression of corticospinal activity in all subjects, although there was a tendency for a smaller effect in the ‘Met’ carriers. More impressive, however, was a lack of the expected homeostatic effect of this stimulation on subsequent 1 Hz rTMS in the same subjects: the Val/Val subjects showed the expected reversal of corticospinal excitability towards facilitation, whereas MEPs remained suppressed in the non-Val/Val individuals.
Given the rather small number of subjects studied we cannot say with certainty that subsequent work will never reveal a difference in the response to TDCS. Nevertheless if the conclusion holds it would be consistent with the idea that TDCS and rTMS act on different neural circuits which are differentially responsive to the BDNF polymorphism (Liebetanz et al. 2002; Lang et al. 2005). The lack of any pre-conditioning effect on the response to a subsequent period of 1 Hz rTMS in the non-Val/Val group could be due to a number of reasons. For example, the duration of the ‘metaplastic window’ following TDCS could be shorter in ‘Met’ carriers, so that if we could have applied TMS more quickly after stopping TDCS, or if we had prolonged the duration of TDCS to increase the duration of the ‘metaplastic window’, we may have seen a smaller difference between the groups. Another possibility is that ‘Met’ carriers have an increased sensitivity to 1 Hz rTMS compared with Val/Val subjects. This could make it more difficult to reverse into facilitation than in Val/Val group. However, this seems unlikely in view of the generally reduced level of plastic changes we observe in the non-Val/Val individuals.
If the group differences reflect a true reduction in metaplastic interactions, then the results may relate to those in experiment 1. As noted above, one possible explanation of the lack of response to TBS protocols in non-Val/Val subjects is a lack of ‘rapid metaplasticity’ in motor cortex, where the prior level of activation preceding the TBS protocols determines the duration and direction of the after-effects on MEP amplitude.
Experiment 3 probed the effects of paired associative stimulation of median nerve and motor cortex on MEPs on the median nerve innervated APB muscle and the ulnar innervated ADM muscle. Using an interstimulus interval of 25 ms in non-genotyped healthy controls, this leads to a variable 0 to <100% facilitation of MEPs in the APB lasting 30–60 min after the end of PAS. Effects in the ADM are also variable: Stefan et al. (2000) originally reported that there was no significant difference in the facilitation of APB and ADM, but others have suggested that effects in ADM are generally smaller than in APB, consistent with a topographic specificity of PAS. In the present experiments, non-Val/Val subjects had no significant response to PAS in either muscle, whereas Val/Val individuals responded with an increase that was significant in ADM, and borderline in APB. Again, this suggests that individuals carrying the Met allele have a reduced response to LTP-like plasticity induction by rTMS protocols.
At first sight it may seem odd that the amounts of PAS-induced facilitation in APB were less than those in ADM. However, if we had mixed the data from all subjects in the present experiments, as would have been the case in previous reports, we would have found a 20–30% mean increase in both muscles, which is within the range of values reported by others. It should also be noted that we carried out all the PAS examinations between 11:00 and 15:00 h in order to avoid daytime-related changes in levels of PAS that have been reported in early evening vs early morning comparisons (Sale et al. 2008).
Since we examined only one homozygous Met/Met carrier, we were not able to make any analyses of ‘dose’ effect. However, in some other studies (like Egan et al. 2003) Met/Met carriers had more pronounced differences compared with Val/Val or even Val/Met individuals, and we presume that the same may well be true of the measures we examined here. Kleim et al. (2006), however, did not demonstrate such an allele dose effect on motor map expansion after FDI exercise tasks.
Relation to previous findings on Val66Met BDNF polymorphism
Egan et al. (2003) reported that compared to Val/Val subjects, ‘Met’ carriers have smaller hippocampal volumes and reduced grey matter in several areas of frontal cortex. Since there was no relation between age and the anatomical findings, the authors speculated that they were related to a role of BDNF in neural development. In behavioural studies, Met/Met homozygotes have been reported to have impaired episodic memory, whereas Val/Met heterozygotes have impaired recognition accuracy of visual scenes in a declarative memory task. The latter was associated with changes in functional activation of hippocampus during both encoding and retrieval of the images. Thus BDNF polymorphisms have both anatomical and behavioural consequences in healthy human populations.
In these previous studies it has not been clear to what extent the changes in anatomy are responsible for the impairments in memory, or whether there are additional effects on synaptic plasticity that interfere with encoding and retrieval during task performance. However, a study by Kleim et al. (2006) suggests that BDNF polymorphisms may indeed influence short-term synaptic plasticity. They asked subjects to learn a simple finger movement task and then tested whether this produced an increase in corticospinal excitability to the exercised muscles using TMS pulses to evoke MEPs. Although there was no difference in the degree of skill acquisition, Val/Val individuals had increased excitability after learning whereas Val/Met individuals did not. Since these practice-induced changes in excitability are believed, at least in part (from parallel studies in animals), to involve changes in synaptic plasticity, the implication was that the BDNF genotype had a direct influence on short-term plasticity in human cortex. The present study extends this conclusion through three physiological probes of known NMDA-dependent LTP and LTD-like effects in motor cortex.
Implications of this work
Many previous studies have pointed out the variability of individual responses to the newly developed TMS and TDCS protocols that probe synaptic plasticity in motor cortex. The present data suggest that genotype is one factor that can influence these effects, and it may therefore be useful to include this as a potential co-variate in analysis of the data, particularly in studies utilizing these protocols as a therapeutic intervention (for example in stroke rehabilitation or depression). In smaller studies utilizing rTMS as an experimental intervention, our results highlight the importance of ethnicity matching, as the prevalence of SNPs like BDNF Val66Met varies widely among different populations.
Several neurological conditions such as dystonia (Edwards et al. 2006; Quartarone et al. 2003) and phantom limb pain (Karl et al. 2001) have been proposed to involve abnormal plasticity at central synapses. Similarly, disorders of metaplasticity have been postulated to underlie susceptibility to l-DOPA-induced dyskinesia in Parkinson's disease (Picconi et al. 2003; Linazasoro, 2005). The recovery of function after brain injury (e.g. stroke), is also thought to be modulated by the ability of synapses to undergo plastic change. The fact that this common polymorphism of the BDNF gene influences experimental protocols that are thought to induce synaptic plasticity in the adult human brain suggests that this polymorphism could be a factor in the development of or recovery from certain neurological disorders. We conclude that, like imaging genomics (Hariri & Weinberger, 2003), rTMS plasticity probes can offer a unique insight into the physiological consequences of functional human polymorphisms.
Acknowledgments
This work was funded by the Medical Research Council, UK.
References
- Aicardi G, Argilli E, Cappello S, Santi S, Riccio M, Thoenen H, et al. Induction of long-term potentiation and depression is reflected by corresponding changes in secretion of endogenous brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 2004;101:15788–15792. doi: 10.1073/pnas.0406960101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MJ, Huang YZ, Mir P, Rothwell JC, Bhatia KP. Abnormalities in motor cortical plasticity differentiate manifesting and nonmanifesting DYT1 carriers. Mov Disord. 2006;21:2181–2186. doi: 10.1002/mds.21160. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Gentner R, Wankerl K, Reinsberger C, Zeller D, Classen J. Depression of human corticospinal excitability induced by magnetic theta-burst stimulation: Evidence of rapid polarity-reversing metaplasticity. Cereb Cortex. 2008;18:2046–2053. doi: 10.1093/cercor/bhm239. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Weinberger DR. Imaging genomics. Br Med Bull. 2003;65:259–270. doi: 10.1093/bmb/65.1.259. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC, Edwards MJ, Chen RS. Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cereb Cortex. 2008;18:563–570. doi: 10.1093/cercor/bhm087. [DOI] [PubMed] [Google Scholar]
- Inghilleri M, Conte A, Currà A, Frasca V, Lorenzano C, Berardelli A. Ovarian hormones and cortical excitability. An rTMS study in humans. Clin Neurophysiol. 2004;115:1063–1068. doi: 10.1016/j.clinph.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Karl A, Birbaumer N, Lutzenberger W, Cohen LG, Flor H. Reorganization of motor and somatosensory cortex in upper extremity amputees with phantom limb pain. J Neurosci. 2001;21:3609. doi: 10.1523/JNEUROSCI.21-10-03609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, et al. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci. 2006;9:735–737. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, et al. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci. 2005;22:495–504. doi: 10.1111/j.1460-9568.2005.04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche M, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002;125:2238–2247. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- Linazasoro G. New ideas on the origin of 1-dopa-induced dyskinesias: Age, genes and neural plasticity. Trends Pharmacol Sci. 2005;26:391–397. doi: 10.1016/j.tips.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Dahlhaus J, Orekhov Y, Liu Y, Ziemann U. Interindividual variability and age-dependency of motor cortical plasticity induced by paired associative stimulation. Exp Brain Res. 2008;187:467–475. doi: 10.1007/s00221-008-1319-7. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picconi B, Centonze D, Hakansson K, Bernardi G, Greengard P, Fisone G, et al. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat Neurosci. 2003;6:501–506. doi: 10.1038/nn1040. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Bagnato S, Rizzo V, Siebner HR, Dattola V, Scalfari A, et al. Abnormal associative plasticity of the human motor cortex in writer's cramp. Brain. 2003;126:2586–2596. doi: 10.1093/brain/awg273. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Rizzo V, Bagnato S, Morgante F, Sant'Angelo A, Girlanda P, et al. Rapid-rate paired associative stimulation of the median nerve and motor cortex can produce long-lasting changes in motor cortical excitability in humans. J Physiol. 2006;575:657–670. doi: 10.1113/jphysiol.2006.114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartarone A, Rizzo V, Bagnato S, Morgante F, Sant'Angelo A, Romano M, et al. Homeostatic-like plasticity of the primary motor hand area is impaired in focal hand dystonia. Brain. 2005;128:1943–1950. doi: 10.1093/brain/awh527. [DOI] [PubMed] [Google Scholar]
- Sale MV, Ridding MC, Nordstrom MA. Cortisol inhibits neuroplasticity induction in human motor cortex. J Neurosci. 2008;28:8285–8293. doi: 10.1523/JNEUROSCI.1963-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, et al. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: Evidence for homeostatic plasticity in the human motor cortex. J Neurosci. 2004;24:3379–3385. doi: 10.1523/JNEUROSCI.5316-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, et al. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005a;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, et al. The yin and yang of neurotrophin action. Nat Neurosci. 2005b;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Paulus W, Nitsche MA, Pascual-Leone A, Byblow W, Berardelli A, et al. Consensus: motor cortex plasticity. Brain Stimulation. 2008;1:164–182. doi: 10.1016/j.brs.2008.06.006. [DOI] [PubMed] [Google Scholar]