Abstract
Vagal pulmonary myelinated afferents are normally not activated by capsaicin, a selective agonist of transient receptor potential vanilloid type 1 (TRPV1) receptors. This study was carried out to investigate whether the expression of TRPV1 in these afferents is altered when chronic airway inflammation is induced by ovalbumin (Ova) sensitization. Two groups of Brown–Norway rats (sensitized and control) were exposed to aerosolized Ova and vehicle, respectively, 3 days per week for 3 weeks. After the C-fibre conduction in both vagus nerves was blocked, right-atrial injection of capsaicin elicited augmented breaths in sensitized rats breathing spontaneously, but not in control rats, indicating a stimulation of rapidly adapting receptors (RARs) by capsaicin. Single-unit fibre activities of RARs and slow adapting receptors (SARs), identified by their firing behaviour and adaptation indexes in response to lung inflation, were recorded in anaesthetized, vagotomized and artificially ventilated rats. Capsaicin injection evoked either negligible or no response in both RARs and SARs of control rats. However, in striking contrast, the same dose of capsaicin evoked an immediate stimulatory effect on these myelinated afferents in sensitized rats. Furthermore, the immunohistochemistry experiments showed that there was a significant increase in the proportion of TRPV1-expressing pulmonary neurones in nodose ganglia of sensitized rats; this increase in TRPV1 expression was found mainly in neurofilament-positive (myelinated) neurones. In conclusion, allergen-induced airway inflammation clearly elevated capsaicin sensitivity in myelinated pulmonary afferents, which probably resulted from an increased expression of TRPV1 in these sensory nerves.
Vagus nerves carry sensory information from the lung and airways to the brainstem. Among these vagal bronchopulmonary afferents, approximately 75% conduct action potentials in axons of non-myelinated (C-) fibres (Jammes et al. 1982). The remaining myelinated afferents can be further divided into two broadly defined receptor types, based upon their firing behaviour and rates of adaptation in response to maintained lung inflation: rapidly adapting receptors (RARs), and slowly adapting receptors (SARs) (Widdicombe, 1981; Coleridge & Coleridge, 1986; Sant'Ambrogio, 1987). Each of these three major types of lung receptors has its own characteristic afferent properties, and upon stimulation, elicits distinctly different reflex responses (Widdicombe, 1981; Coleridge & Coleridge, 1986; Sant'Ambrogio, 1987). In general, RARs and SARs are more sensitive to mechanical stimulation, and less sensitive to chemical stimuli. In contrast, C-fibre afferents exhibit more pronounced chemosensitive properties, but have a much lower sensitivity to lung inflation than their myelinated counterparts (Ho et al. 2001). Indeed, the sensitivity to capsaicin is one of the most prominent characteristics of bronchopulmonary C-fibre afferents (Ho et al. 2001); capsaicin, the pungent ingredient in hot peppers, is a specific activator of the transient receptor potential vanilloid type 1 (TRPV1) channel, a polymodal nociceptive transducer expressed predominantly in non-myelinated sensory afferents. Electrophysiological studies indicated that TRPV1 is either not or only sparsely expressed in myelinated afferents in rat lung (Ho et al. 2001).
Brown–Norway (BN) rats sensitized by chronic inhalation of ovalbumin (Ova) aerosol have been used extensively as an animal model of allergic asthma because of its well-documented pathophysiological features such as inflammatory cell infiltration, expression of TH2 cytokines interleukin-4 (IL), IL-5 and IL-13, and airway hyper-responsiveness (Elwood et al. 1992,1993; Nagase et al. 1994; Tschernig et al. 2008). A recent study in our lab demonstrated that chronic Ova exposure induced airway inflammation, eosinophilic inflitration and non-specific airway hyper-reactivity in BN rats, which closely resembled those observed in human allergic asthma (Zhang et al. 2008). Furthermore, our study showed a significant increase in the sensitivity of bronchopulmonary non-myelinated (C-) fibres to capsaicin in Ova-sensitized rats (Zhang et al. 2008). A similar observation was also recently reported by Kuo & Lai (2008). We postulated that a contributing factor might be an increase in the expression of TRPV1 in pulmonary C neurones resulting from the chronic inflammatory reaction (Zhang et al. 2008). More interestingly, results obtained in our pilot experiments carried out in anaesthetized and spontaneously breathing rats further revealed another possibility that chronic airway inflammation induced by allergen sensitization might elevate the sensitivity of RARs to capsaicin (e.g. Fig. 1). To test this hypothesis, the present study was carried out to investigate whether the sensitivity of vagal myelinated afferents to capsaicin is altered in Ova-sensitized rats, and if so, whether the expression of the TRPV1 channel is up-regulated in pulmonary sensory neurones of the nodose and jugular ganglia.
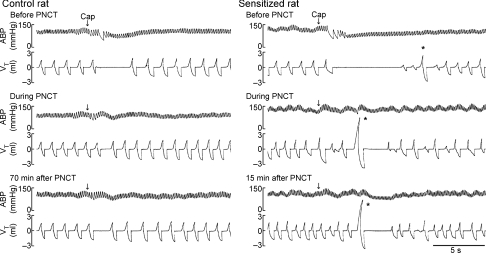
Figure 1. Experimental records illustrating the effect of perineural capsaicin treatment (PNCT) of both cervical vagi on cardiorespiratory responses to capsaicin injection.
Upper, middle and lower panels: responses to right-atrial injection of capsaicin before, during and after PNCT. Right-atrial injection of capsaicin (1 μg kg−1 in 0.15 ml volume) was slowly injected into the catheter and then flushed (at arrow) into the right atrium as a bolus with saline (0.3 ml). Asterisks were added to depict the augmented breaths. Note that the augmented breath was consistently elicited by capsaicin in sensitized rat (243 g; right) and its volume was amplified when C-fibre conduction in the vagus nerve was selectively blocked either during or after PNCT. Vagal-mediated reflex apnoea elicited by lung inflation (Pt= 10 cmH2O) was not blocked by PNCT in either control or sensitized rat (records not shown), indicating that neural conduction was intact in myelinated afferents. Partial recovery of the response was shown at 70 min after the termination of PNCT in the control rat (257 g; left). Note the augmented breath was always followed immediately by a short apnoea (e.g. right lower panel), which was probably caused by the hypocapnia generated by the augmented breath.
Methods
Animals
All protocols were performed in accordance with Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health and were also approved by the University of Kentucky Institutional Animal Care and Use Committee.
Allergen sensitization and animal exposure
Adult male BN rats were separated into two groups (control, n = 19; sensitized, n = 21). Sensitized rats received an initial intraperitoneal (i.p.) injection of a suspension containing 2 mg Ova in 1 ml ImjectAlum as adjuvant. Three days later, these rats were exposed to an aerosol of 5% Ova diluted in saline for 15 min each time, 3 times per week for 3 weeks. During exposure, the unanaesthetized rat was placed in a Plexiglas restrainer (University of Kentucky, Center for Manufacturing), and breathed spontaneously and continuously through a nose cone connected to a free stream of air–aerosol mixture under a negative-pressure exhaust hood. Ova solution (wt/vol concentration: 5% in saline) was nebulized and delivered by an ultrasonic nebulizer (Model 099HD; Devilbiss) at a droplet size ranging from 0.5 to 5 μm. Control rats received the i.p. injection and aerosol inhalation of the vehicle (isotonic saline) following identical procedures.
Animal preparation
Rats were initially anaesthetized with an i.p. injection of α-chloralose (100 mg kg−1) and urethane (500 mg kg−1) dissolved in a 2% borax solution; supplemental doses of the same anaesthetics (one-tenth of the initial dose) were injected intravenously (i.v.) to maintain abolition of pain reflexes. One femoral artery was cannulated for recording the arterial blood pressure (ABP). For administration of pharmacological agents, the left jugular vein was cannulated and a catheter was advanced until its tip was positioned just above the right atrium. A short tracheal cannula was inserted just below the larynx via a tracheotomy. Tracheal pressure (Pt) was measured (Validyne MP45-28, Validyne) via a side-port of the tracheal cannula. Body temperature was maintained at ∼36°C by means of a heating pad placed under the animal lying in a supine position.
Measurements of pulmonary chemoreflex responses
Rats breathed spontaneously via the tracheal cannula. Respiratory flow was measured with a heated pneumotachograph connected to the tracheal cannula and a differential pressure transducer (Validyne MP45-14), and integrated to give tidal volume (VT). Respiratory frequency, expiratory duration (TE), ABP and heart rate were analysed (Biocybernetics TS-100; Biocybernetics) on a breath-by-breath basis by an on-line computer.
Measurement of single fibre activity
Pulmonary single-unit fibre activities were recorded in anaesthetized, vagotomized, open-chest and artificially ventilated rats, as described in detail previously (Ho et al. 2001). Briefly, after a midline thoracotomy was performed, the lung was artificially ventilated with a respirator (Model 7025; UGO Basile); the expiratory outlet of the respirator was placed under 3 cmH2O pressure to maintain a near-normal functional residual capacity. Volume and frequency were set at 8–10 ml kg−1 and 50 breaths min−1, respectively. To prevent a possible indirect stimulatory effect caused by reflex bronchoconstriction on these afferents, bilateral vagotomy was performed on these animals. The caudal end of the cut right vagus nerve was placed on a small dissecting platform and immersed in a pool of mineral oil. A thin filament was teased away from the desheathed nerve trunk and further split until the afferent activity arising from a single unit was electrically isolated. The nerve trunk was ligated just above the diaphragm to eliminate afferent signals arising from lower visceral organs.
SARs and RARs were searched initially by their responses to lung inflation and deflation, and then further identified by their adaptation indexes (AIs) in response to lung inflation; AI was calculated in each fibre by dividing the difference in fibre activity (FA) between the first 2 s during a constant-pressure (Pt= 30 cmH2O; e.g. Figs 2 and 3) lung inflation by the FA of the first second, and expressed as a percentage (Widdicombe, 1954). Fibres with AIs of < 80% and > 80% were classified as SARs and RARs, respectively. The signals of the afferent activity, Pt and ABP were recorded on a thermal writer (Gould TW11; Gould Instrument Inc.), and analysed by a computer and a data acquisition system (Biocybernetics TS-100) in 0.1 s intervals.
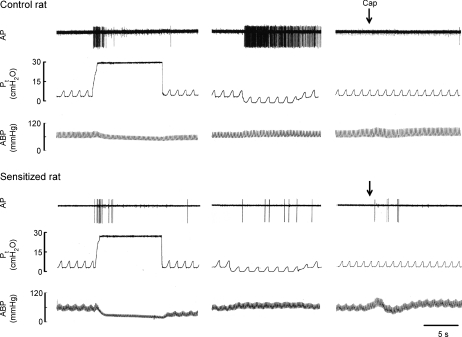
Figure 2. Experimental records illustrating the responses of silent RARs to lung inflation, deflation and capsaicin injection in anaesthetized, vagotomized, open-chest and artificially ventilated rats.
Left, responses to lung inflation (Pt= 30 cmH2O for 10 s); middle, responses to lung deflation (Pt= 0 cmH2O at the end of expiration); right, responses to right-atrial injection of capsaicin (1 μg kg−1; arrow). Note the lack of bradycardia and hypotensive reflex responses to capsaicin injection because of the bilateral vagotomy performed in these rats. AP, action potential; Pt, tracheal pressure; ABP, arterial blood pressure. Control rat (299 g): receptor location, right middle lobe; conduction velocity, 15.3 m s−1. Sensitized rat (258 g): receptor location, right lower lobe; conduction velocity, 16.7 m s−1.
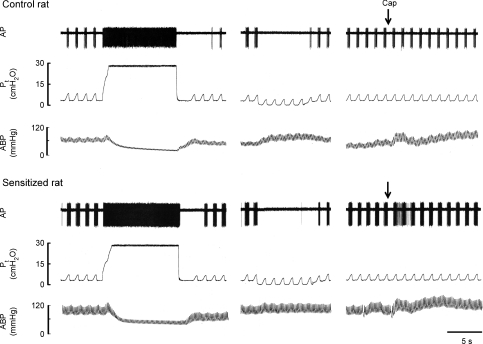
Figure 3. Experimental records illustrating the responses of SARs to lung inflation, deflation and capsaicin injection in anaesthetized, vagotomized, open-chest and artificially ventilated rats.
Left, responses to lung inflation (Pt= 30 cmH2O for 10 s); middle, responses to lung deflation (Pt= 0 cmH2O at the end of expiration); right, responses to right-atrial injection of capsaicin (1 μg kg−1). Control rat (291 g): receptor location, right middle lobe; conduction velocity, 12.9 m s−1. Sensitized rat (272 g): receptor location, right middle lobe; conduction velocity, 16.3 m s−1. For detailed descriptions, see legend to Fig. 2.
Measurement of conduction velocity
As described in detail previously (Ho et al. 2001), we isolated the intrathoracic segment of the right vagus nerve in each animal, and placed a pair of stimulating electrodes under the vagus nerve above the exit of its pulmonary branches. A rectangular constant-current pulse (duration: 0.1–0.3 ms; intensity: 0.2–1.0 mA) was generated by a pulse generator (WPI A310) and a stimulus isolation unit (WPI A360R-C), and delivered to the stimulatory electrodes. The exact distance between stimulating and recording electrodes was measured to calculate the conduction velocity. The general locations of all fibres were identified by their responses to gently pressing the lungs with a saline-wetted cotton Q-tip. Only receptors with their locations identified in the lung or airways were included for data analysis in this study. Finally, animals were euthanized after the experiment by an i.v. injection of KCl.
Retrograde neuronal tracing
The cell bodies of sensory neurones innervating the lungs and airways were identified by retrograde labelling from the lungs using the fluorescent tracer 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI; Sigma). A polyethylene catheter (PE-150) was inserted into the trachea of the anaesthetized (pentobarbital sodium, i.p., 40 mg kg−1) rat via the larynx, and its tip was positioned above the thoracic inlet. DiI (1 mg ml−1) was sonicated and dissolved in ethanol, diluted in saline (1% ethanol vol/vol), and instilled into the lungs (0.2 ml × 2) with the animal's head tilted up at ∼30 deg. Five days later, the rats were subjected to chronic exposure to saline or Ova aerosol.
Tissue preparation
On day 24 (3 days after the last exposure to saline or Ova aerosol), rats were anaesthetized with halothane inhalation, euthanized and transcardially perfused with 0.01 m phosphate-buffered saline (PBS), followed by freshly prepared 0.01 m phosphate buffer (pH 7.4) containing 4% paraformaldehyde. The nodose and jugular ganglia were dissected and fixed in 4% paraformaldehyde for 4 h. The ganglia were incubated in 20% sucrose in PBS overnight at 4°C, embedded in Tissue-Tek OCT compound (VWR Scientific), and frozen in dry ice for serial cryosectioning at 12 μm on a cryostat (Model550; Microm International GmbH). The sections were mounted on SuperFrost Plus slides (Fisher Scientific), and air dried.
Immunohistochemistry
Tissue sections were first pre-incubated for 1 h at room temperature with 0.01 m PBS containing 5% normal donkey serum to block non-specific protein binding sites. Then, the sections were incubated in a mixture of 1 : 500 TRPV1 antibody (rabbit polyclonal antibody), or in combination with 1 : 1000 neurofilament (NF) 160/200 antibody (mouse monoclonal antibody) overnight at 4°C, which were each diluted in PBS 5% normal donkey serum and 0.1% Triton X-100. After three 10 min washes in PBS, the sections were incubated with a mixture of 1 : 200 FITC conjugated donkey anti-rabbit IgG and 1 : 200 AMCA conjugated donkey anti-mouse IgG for 1 h at room temperature. Finally, the sections were washed again in PBS and mounted in Vectorshield (Vector Laboratories).
The immunoreactivity of each neurone was analysed using epifluorescence microscopy (Zeiss Axiovert 100AV) with appropriate filter combinations. DiI was detected with the filter module (wavelengths of 552 nm for excitation and 565 nm for emission); FITC (495 nm excitation and 528 nm emission); and AMCA (353 nm excitation and 440 nm emission). The sections were visualized with an AxioCam MRc colour CCD camera (Zeiss, Inc.). All images were captured using the same brightness and contrast setting.
For negative controls, primary antibodies were omitted. At least two negative control slides were done in each animal. The specificity of the TRPV1 antibody was tested using two procedures: (1) neutralizing the primary antibody with a blocking peptide (Neuromics) and (2) applying TRPV1 antibody to the nodose–jugular ganglia of TRPV1-null mice (B6.129 × 1-Trpv1tm1Jul/J; the Jackson Laboratory). Neither of the two procedures showed TRPV1 immunoreactivity.
Quantitative analysis
One nodose and one jugular ganglia were studied in each animal. A total of ∼100 sections were obtained from the entire length of each ganglion (∼1.5 mm). To avoid the double counting of the same neurones, every eighth section (≥ 96 μm apart) was selected from serial sections for each ganglion. About 12 sections were analysed per ganglion in each trial. Two regions of interest were captured and analysed per section at 20× magnification.
Computer-assisted image analysis was used to analyse quantitatively the expression of TRPV1 and NF immunoreactivity. Digital images were captured using an Axiovert 100AV microscope with a CCD camera (AxioCam MRc colour CCD; Zeiss, Inc.) interfaced through a Dell Dimensions 4600 computer using Axiovision 4.6 software (Carl Zeiss Imaging Systems). The TRPV1, NF and DiI thresholds were set based on their densities on the control slides. DiI-labelled, TRPV1-positive and NF-positive neurones were counted separately, and co-localizations were determined for each region. The proportion of a neurone phenotype in a given population of neurones is expressed as a percentage and then averaged for group analysis; for example, the percentage of TRPV1-positive neurones in pulmonary (DiI-labelled) neurones was determined in each section, and averaged for the entire ganglion in each animal. Data were then pooled in each group of animals for statistical analysis.
Experimental design and protocol
Three series of experiments were carried out: in Series 1, we investigated the effect of Ova sensitization on pulmonary chemoreflex sensitivity in anaesthetized, spontaneously breathing rats. The cardiorespiratory reflex responses to right-atrial injection of the same dose of capsaicin (0.5–1.0 μg kg−1) were compared between control and sensitized rats. To determine the responses mediated through vagal myelinated afferents to capsaicin, perineural capsaicin treatment (PNCT) of both cervical vagus nerves was applied to selectively block the neural conduction in vagal C-fibre afferents as previously described (Lin & Lee, 2002). Briefly, cotton strips soaked in capsaicin solution (250 μg ml−1) were wrapped around a 2–3 mm segment of the isolated cervical vagus nerves for 30 min. The apnoeic response to right-atrial injection of capsaicin was measured as the ratio between apnoeic duration and baseline TE; apnoeic duration is the longest TE occurring within 5 s after the capsaicin injection.
In Series 2, the effects of Ova sensitization on the baseline FA and the responses of RARs and SARs to lung inflation and capsaicin were determined. Baseline FA was averaged over 20 consecutive breaths in each fibre. Constant-pressure lung inflation was applied by inflating the lung with a constant air flow (∼12 ml s−1) until Pt reached 30 cmH2O, and was maintained at that pressure for 10 s after turning off the respirator. Responses to lung inflation were calculated as the FA in the first second during lung inflation. Capsaicin (0.5 and 1.0 μg kg−1) was first injected as a bolus (0.15 ml volume) into the catheter (dead space ∼0.2 ml) and then flushed into the right atrium by an injection of 0.3 ml saline. The ΔFA in response to capsaicin was calculated as the difference between the peak FA (averaged over two consecutive breaths) within 5 s after capsaicin injection and the baseline FA (averaged over 20 consecutive breaths) in each fibre. To avoid any accumulated effect, at least 10 min elapsed between two injections.
Series 3 was carried out to investigate whether the ratios of TRPV1-positive neurones in pulmonary myelinated and non-myelinated neurones have been altered by Ova sensitization. NF has been used as the specific marker for identifying myelinated neurones (Lawson & Waddell, 1991; Hunter et al. 2000). Thus, DiI-labelled NF-positive neurones were considered as pulmonary myelinated neurones. The number of pulmonary non-myelinated neurones (DiI-labelled NF-negative) was calculated as the number of pulmonary neurones (DiI-labelled) minus the number of pulmonary myelinated neurones (DiI-labelled NF-positive).
Materials
A solution of Ova was prepared daily at the concentration described earlier. Solutions of chemical agents at the desired concentrations for injection or aerosolization were prepared daily by dilution with isotonic saline based on the animal's body weight. All chemical agents were purchased from Sigma Chemical, except Imject Alum (Pierce Biotechnology), rabbit polyclonal TRPV1 antibody (Chemicon Inc), FITC conjugated donkey anti-rabbit IgG, AMCA conjugated donkey anti-mouse IgG and normal donkey serum (Jackson ImmunoResearch Laboratories).
Statistical analysis
Statistical analysis of the difference in the responses between control and sensitized groups was performed using one-way or two-way ANOVA. When the two-way ANOVA showed a significant interaction, pair-wise comparisons were made with a post hoc analysis (Fisher's least significant difference). A P value < 0.05 was considered significant. Data were reported as mean ±s.e.m.
Results
A total of 40 adult male BN rats were used in this study. Average body weight of sensitized rats was significantly lower (266.7 ± 5.5 g; n = 21) than that of control rats (300.4 ± 5.6 g; n = 19, P < 0.01).
Series 1
In anaesthetized, spontaneously breathing rats, right-atrial injection of capsaicin (0.5–1.0 μg kg−1) immediately elicited apnoea, bradycardia and hypotension in control rats; these ‘pulmonary chemoreflex’ responses are well documented in the literature and known to be mediated through stimulation of pulmonary C-fibre afferents (Coleridge & Coleridge, 1984; Lee & Pisarri, 2001). In comparison, the same dose of capsaicin challenge elicited clearly more pronounced pulmonary chemoreflex responses in Ova-sensitized rats (e.g. Fig. 1). The apnoeic ratio (apnoeic duration/average baseline TE) was 265 ± 88% in control rats (n = 7) and 605 ± 193% in sensitized rats (n = 7; P < 0.05) after injection of a low dose of capsaicin (0.5 μg kg−1). More importantly, capsaicin injection frequently elicited an augmented breath (inspiratory VT exceeding the baseline VT by twofold), which usually occurred shortly following the initial apnoea in sensitized rats, but not in control rats (e.g. Fig. 1). Augmented breath is a vagal-mediated reflex response known to be triggered by activation of RARs (Glogowska et al. 1972; Lee et al. 1992) and can be completely eliminated by vagotomy (data not shown). Furthermore, after the C-fibre conduction was selectively blocked by PNCT of both cervical vagus nerves, the same capsaicin injection no longer elicited the apnoea, whereas the occurrence of augmented breath became more consistent and its volume was further amplified in sensitized rats (Fig. 1), probably resulting from the removal of C-fibre-mediated respiratory inhibition by PNCT (Lee et al. 1992). Results of this series imply that sensitivity to capsaicin of RARs was elevated in Ova-sensitized rats.
Series 2
A total of 75 vagal bronchopulmonary afferents were studied in 18 anaesthetized, open-chest rats (18 RARs and 13 SARs in 8 control rats and 26 RARs and 18 SARs in 10 sensitized rats): in control rats, 9.6, 41.9, 38.6 and 12.9% receptors were in upper, middle, lower and accessory lobes, respectively; in sensitized rats, 17.8, 46.7, 28.9 and 6.6% receptors were in upper, middle, lower and accessory lobes, respectively. The average conduction velocities were 12.6 ± 1.5 m s−1 for RARs and 14.8 ± 2.0 m s−1 for SARs in the control group, and were 13.2 ± 1.5 m s−1 for RARs and 15.2 ± 1.7 m s−1 for SARs in the sensitized group; no significant difference in the conduction velocity was found between control and sensitized rats in either RARs or SARs.
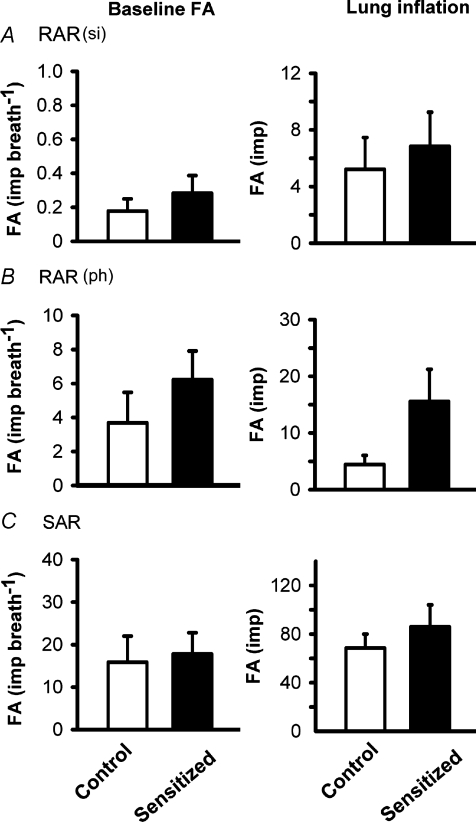
Approximately a half of the RARs exhibited distinct phasic baseline activity that was synchronous with either expiratory or inspiratory phase of respiratory cycles. The remaining RARs had no detectable or very little respiratory-related baseline activity (Fig. 2). These two types of RARs were named phasic RARs and silent RARs in this study; in order to balance the distribution, the same number of each of these two types of RARs was selected from both control and sensitized groups for this study. In phasic RAR groups, there was no significant difference in the intensity and pattern of the baseline activity between control (3.7 ± 1.8 impulses breath−1 (imp breath−1), n = 9) and sensitized rats (6.2 ± 1.7 imp breath−1, n = 13) (Fig. 4). Similarly, in silent RAR groups, there was also no significant difference in the baseline activity between control (0.2 ± 0.1 imp breath−1, n = 9) and sensitized rats (0.3 ± 0.1 imp breath−1, n = 13). When constant-pressure lung inflation (Pt= 30 cmH2O) was applied for 10 s, the discharge in RARs increased abruptly in response to inflation and then rapidly declined despite the maintained Pt (Fig. 2). When lung deflation was applied by exposing the expiratory outlet of the respirator to atmospheric pressure (Pt= 0 cmH2O), the activity of RARs increased and did not show any sign of rapid adaptation during the prolonged deflation (e.g. Fig. 2). In phasic RARs, the FA in response to lung inflation in the first second were 4.4 ± 1.6 and 15.5 ± 5.7 impulses in control (n = 9) and sensitized rats (n = 13) (Fig. 4), respectively, but there was no significant difference between them (P = 0.13). In view of the threefold difference in the responses and the small sample size of these data, a non-parametric statistical analysis (Wilcon rank sum test) was also applied, but a significant difference was not found (P = 0.18). Similarly, in silent RARs, there was also no significant difference in the first–second response to the lung inflation between control and sensitized rats (Figs 2 and 4).
Figure 4. Average baseline fibre activities (FA) and the responses of silent (si) RARs, phasic (ph) RARs and SARs to lung inflation in anaesthetized, vagotomized, open-chest and artificially ventilated rats.
Baseline FA was averaged over 20 consecutive breaths in each fibre. Response to lung inflation was measured as the FA during the first second of lung inflation (Pt= 30 cmH2O). Values are means ±s.e.m. Open bars, control group (n = 9 fibres in A, n = 9 in B, n = 13 in C); filled bars, sensitized group (n = 13 fibres in A, n = 13 in B, n = 18 in C). No statistical difference was found in any of the corresponding data between control and sensitized groups.
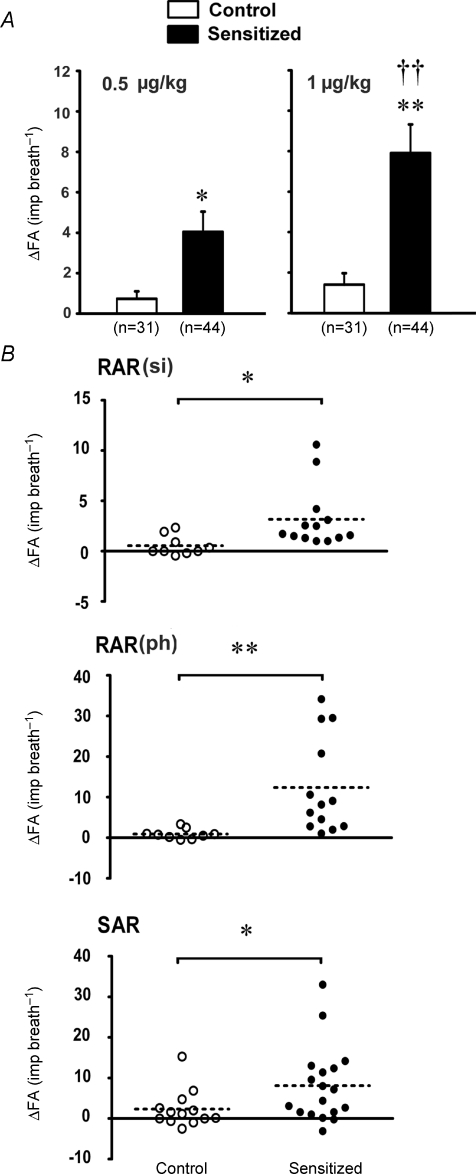
In sharp contrast, the responses of these afferents to capsaicin were markedly elevated in sensitized rats. In silent RARs, the high dose (1 μg kg−1) of capsaicin triggered a significantly higher discharge in sensitized rats (ΔFA = 3.2 ± 0.9 imp breath−1, n = 13) than those in control rats (ΔFA = 0.5 ± 0.3 imp breath−1, n = 9, P < 0.05; Figs 2 and 5B). The response of phasic RARs to the same dose of capsaicin showed a similar pattern as that in silent RARs (Fig. 5B; in control rats, ΔFA = 0.9 ± 0.4 imp breath−1, n = 9; in sensitized rats, ΔFA = 12.4 ± 3.3 imp breath−1, n = 13; P < 0.01).
Figure 5. Effect of Ova sensitization on responses of myeliated afferents to right-atrial injection of capsaicin in anaesthetized, vagotomized, open-chest and artificially ventilated rats.
A, average responses of all the myelinated afferents to right-atrial injection of capsaicin at 0.5 μg kg−1 and 1 μg kg−1. Open bars, control group (n = 31 fibres); filled bars, sensitized group (n = 44 fibres). Values are means ±s.e.m.ΔFA, difference between the peak FA (averaged over 2 consecutive breaths) within 5 s after capsaicin capsaicin injection and the baseline FA (averaged over 20 consecutive breaths) in each fibre. B, scatterplot diagrams of the responses of silent RARs, phasic RARs and SARs to right-atrial injection of capsaicin (1 μg kg−1). RAR(si), silent RAR; RAR(ph), phasic RAR. ^, data obtained in control group (n = 9, 9 and 13 in silent RARs, phasic RARs and SARs, respectively); •, sensitized group (n = 13, 13 and 18 in silent RARs, phasic RARs and SARs, respectively). Dash line: mean of the ΔFA. *P < 0.05 and **P < 0.01, significant difference in the responses between control and sensitized rats. ††P < 0.01, significant difference in the responses between low and high doses of capsaicin.
All of the SARs (13 in control and 18 in sensitized rats) exhibited distinct phasic baseline activity that peaked during the inspiratory phase of the respiratory cycles. The discharge of SARs showed very little adaptation during the constant-pressure lung inflation (AI < 80%), but ceased during deflation (e.g. Fig. 3). No significant difference in either the baseline activity (FA = 15.8 ± 6.1 and 17.8 ± 5.0 imp breath−1 in control and sensitized rats, respectively) or the responses to lung inflation in the first second (FA = 68.5 ± 11.5 and 86.1 ± 18.0 impulses, in control and sensitized rats, respectively) was found between the two groups (Fig. 4). In contrast, the response to capsaicin (1 μg kg−1) was significantly elevated in sensitized rats (ΔFA = 8.1 ± 2.2 imp breath−1, n = 18) compared to controls (ΔFA = 2.3 ± 1.3 imp breath−1, n = 13, P < 0.05) (Figs 3 and 5B).
When all the pulmonary myelinated afferents, including both RARs and SARs, were grouped together, right-atrial injection of either a low or high dose of capsaicin evoked significantly higher responses in sensitized rats than in controls (e.g. at the capsaicin dose of 1.0 μg kg−1, ΔFA = 7.9 ± 1.4 imp breath−1, n = 44 in sensitized rats, ΔFA = 1.4 ± 0.7 imp breath−1, n = 31 in control rats; P < 0.01; Fig. 5A).
Series 3
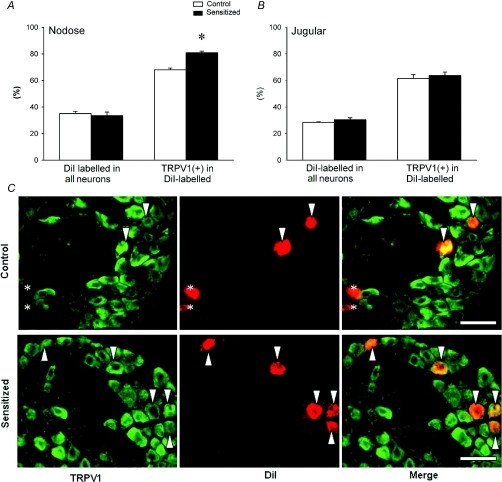
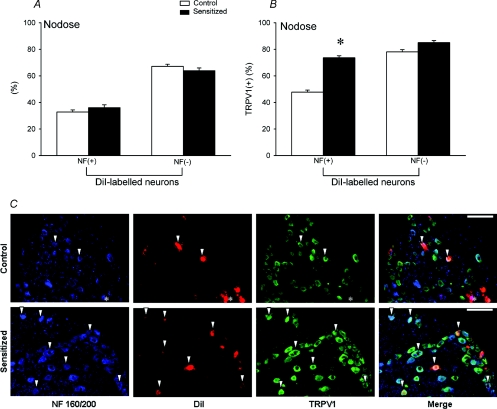
To investigate whether TRPV1 expression in pulmonary neurones in nodose and jugular ganglia is altered by Ova sensitization, bronchopulmonary neurones were identified using DiI labelling. In nodose ganglia obtained from control rats, 35.1% of the neurones were retrogradely labelled with DiI, which was similar to that of sensitized rats (33.6%). Among these DiI-labelled neurones, 68.1 ± 1.3% exhibited TRPV1 immunoreactivity in control rats. In comparison, a significantly higher percentage of the DiI-labelled neurones (80.9 ± 1.2%; n = 4, P < 0.05) expressed TRPV1 in sensitized rats (Fig. 6A). To further investigate the relative increase of TRPV1 expression in myelinated and non-myelinated neurones, we used NF immunoreactivity as the criterion to identify myelinated neurones. Previous studies showed that all NF-positive neurones were large, myelinated neurones and essentially all NF-negative neurones were small, non-myelinated C neurones (Lawson & Waddell, 1991). Among these DiI-labelled neurones, 32.8 ± 1.6% and 36.1 ± 2.1% were NF-positive neurones in control and sensitized rats, respectively (Fig. 7A). The percentage of TRPV1-positive pulmonary myelinated (DiI-labelled and NF-positive) neurones was significantly higher in sensitized rats (73.7 ± 1.5%) than in control rats (47.8 ± 1.6%; n = 4, P < 0.05) (Fig. 7B and C). Surprisingly, there was no significant difference in the percentage of TRPV1-immunoreactive pulmonary non-myelinated (C-) (NF-negative and DiI-labelled) neurones between control (78.1 ± 1.7%) and sensitized rats (85.1 ± 1.5%; n = 4, P > 0.05) (Fig. 7B). Thus, the staining for TRPV1 in nodose ganglia revealed a substantial increase of TRPV1 expression in DiI-labelled neurones induced by sensitization. The increase was mainly found in NF-positive DiI-labelled neurones (Table 1; Fig. 7B and C).
Figure 6. Effect of Ova sensitization on TRPV1 expression in pulmonary neurones in nodose and jugular ganglia.
Percentages of DiI-labelled (pulmonary) neurones in all neurones, and TRPV1-positive neurones in pulmonary neurones in nodose (A) and jugular (B) ganglia. Open bars, average data obtained from control rats (n = 4); filled bars, sensitized rats (n = 4). Values are means +s.e.m.*P < 0.05, significant difference between control and sensitized rats. C, representative photographs of double-labelling immunohistochemistry of TRPV1 and DiI in nodose ganglia of both control and sensitized rats. Left, TRPV1 staining in green; middle, DiI labelling in red; right, the merged image. Arrowheads are added to depict co-localization of TRPV1 staining and DiI labelling in the same neurones. Asterisks depict the DiI labelled neurones without TRPV1 staining. Scale bar, 100 μm.
Figure 7. Effect of Ova sensitization on TRPV1 expression in NF-positive and NF-negative neurones with DiI labelling in nodose ganglion.
A, percentages of NF-positive and NF-negative neurones in DiI-labelled pulmonary neurones. B, percentages of TRPV1-positive neurones in NF-positive and NF-negative neurones labelled with DiI. Open bars, average data obtained from control rats (n = 4); filled bars, sensitized rats (n = 4). Values are means +s.e.m.*P < 0.05, significant difference between control and sensitized rats. C, representative photographs of triple-labelling immunohistochemistry of NF, DiI and TRPV1 in nodose ganglia of both control and sensitized rats. The first column from left, NF staining in blue; second, DiI labelling in red; third, TRPV1 staining in green; fourth, the merged image. Arrowheads are added to depict co-localization of NF staining, DiI labelling and TRPV1 staining in the same neurones. Asterisks depict the neurone with co-localization of NF staining and DiI labelling, but without TRPV1 staining. Scale bar, 100 μm.
Table 1.
Expression of TRPV1-immunoreactivity in myelinated and non-myelinated pulmonary neurones in nodose and jugular ganglia
| Nodose (%) | DiI/All | NF(+)/DiI | NF(–)/DiI | TRPV1(+)/DiI | TRPV1(+) | TRPV1(+) |
|---|---|---|---|---|---|---|
| /DiI NF(+) | /DiI NF(–) | |||||
| Control | 35.1 ± 1.6 | 32.8 ± 1.6 | 66.2 ± 1.6 | 68.1 ± 1.3 | 47.8 ± 1.6 | 78.1 ± 1.7 |
| Sensitized | 33.6 ± 2.6 | 36.1 ± 2.1 | 63.9 ± 2.1 | 80.9 ± 1.2* | 73.7 ± 1.5* | 85.1 ± 1.5 |
| Jugular (%) | DiI/All | NF(+)/DiI | NF(–)/DiI | TRPV1(+)/DiI | TRPV1(+)/DiI NF(+) | TRPV1(+)/DiI NF(–) |
| Control | 28.5 ± 0.4 | 31.8 ± 1.8 | 67.2 ± 1.9 | 61.5 ± 3.0 | 51.5 ± 1.1 | 69.9 ± 4.1 |
| Sensitized | 30.5 ± 1.5 | 28.5 ± 1.8 | 70.5 ± 1.9 | 63.8 ± 2.5 | 47.9 ± 0.6 | 70.0 ± 3.2 |
The number of DiI-labelled, NF-negative (NF(–)) neurones was calculated by subtracting the number of DiI-labelled NF-positive neurones from the total number of DiI-labelled neurones; the TRPV1 expression in pulmonary non-myelinated neurones as the number of TRPV1-positive DiI-labelled neurones minus the number of TRPV1-postive NF-positive neurones with DiI. Control rats, n = 4; sensitized rats, n = 4. Values are means ±s.e.m.*P < 0.05, significant difference between the corresponding data of control and sensitized rats.
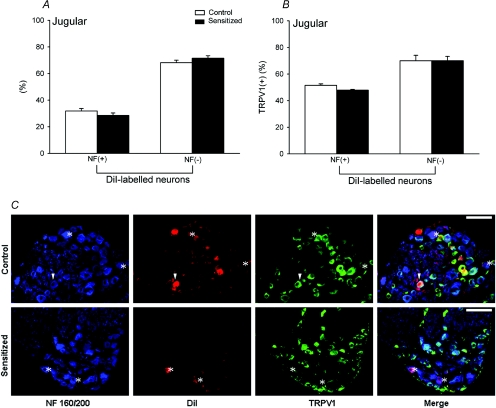
Among jugular ganglion neurones obtained from the same groups of animals, approximately 28.5 ± 0.4% were labelled with DiI in control rats, which was not significantly different from those of the jugular neurones in sensitized rats (30.5 ± 1.5%). There was no difference in TRPV1 expression in DiI-labelled neurones between control and sensitized rats (61.5 ± 3.0% in control rats; 63.8 ± 2.5% in sensitized rats; Fig. 6B). Furthermore, 31.8 ± 1.8% and 28.5 ± 1.8% of the DiI-labelled neurones in control and sensitized rats, respectively, were NF positive (Fig. 8A). In contrast to what we found in nodose ganglia, the expression of TRPV1 in DiI-labelled NF-positive neurones was not different between control and sensitized rats. The percentages of TRPV1-positive neurones in bronchopulmonary myelinated neurones were 51.5 ± 1.1% and 47.9 ± 0.6% (P > 0.05) in control and sensitized rats, respectively (Fig. 8B). Therefore, TRPV1 expression in either myelinated or non-myelinated neurones was not altered by Ova sensitization in jugular ganglia (Table 1).
Figure 8. Effect of Ova sensitization on TRPV1 expression in NF-positive and NF-negative neurones with DiI labelling in jugular ganglion.
A, percentages of NF-positive and NF-negative neurones in DiI-labelled (pulmonary) neurones. B, percentages of TRPV1-positive neurones in NF-positive and NF-negative neurones labelled with DiI. Open bars, average data obtained from control rats (n = 4); filled bars, sensitized rats (n = 4). Values are means +s.e.m.C, representative photographs of triple-labelling immunohistochemistry of NF, DiI and TRPV1 in jugular ganglia of both control and sensitized rats. For detailed descriptions, see the legend of Fig. 7. Scale bars, 100 μm.
To avoid possible bias and to verify data accuracy, the quantitative immunohistochemistry analyses were re-performed in 21% of the images that were randomly selected from both groups and blindly coded by a person unfamiliar with the experiment. The difference between the two analyses of the same slides was relatively small; for example, the difference for NF, DiI and TRPV1 stainings were 2.1 ± 0.4%, 4.1 ± 0.8% and 2.6 ± 0.4%, respectively.
Discussion
Results obtained in the present study clearly show for the first time that sensitivity to capsaicin of vagal bronchopulmonary myelinated afferents, including both RARs and SARs, was significantly elevated when chronic inflammation was induced by allergen sensitization in rats. Consistent with electrophysiological recording experiments, our immunohistochemistry data also show a significant increase in the proportion of TRPV1-expressing bronchopulmonary neurones in nodose ganglia of sensitized rats, compared to that of control rats. This increase of TRPV1 expression was found mainly in neurofilament-positive neurones (myelinated neurones). Rather surprisingly, we did not find any significant difference in the proportion of TRPV1-expressing cells in either myelinated or non-myelinated neurones of the jugular ganglia between control and sensitized rats.
RARs are generally recognized as mechanoreceptors in the lung. These receptors can be activated by both inflation and deflation of the lung, a decrease in lung compliance and an increase in the interstitial fluid volume in the lung (Widdicombe, 1954; Kappagoda et al. 1987; Lee & Undem, 2005). A small percentage of RARs are also sensitive to various inhaled irritants including cigarette smoke, sulphur dioxide and acid (Widdicombe, 1954; Ho et al. 2001). Stimulation of RARs can elicit reflex responses such as augmented breath, bronchoconstriction and hypersecretion of mucus. In addition, a subgroup of RARs is believed to act as cough receptors in response to inhaled irritants (Widdicombe, 1998). On the other hand, SARs can be activated by volume expansion of the lung or an increase in transluminal pressure in the airways (Bartlett et al. 1976). SARs are generally considered to be insensitive to capsaicin or any other chemical irritants (Ho et al. 2001). The activity arising from SARs is known to play an important role in the regulation of breathing patterns (Widdicombe, 1981; Coleridge & Coleridge, 1986). Both RARs and SARs are myelinated afferents, as verified by the measurement of conduction velocity (> 5 m s−1) in each fibre. RARs and SARs were classified based upon their adaptation indexes in response to maintained lung inflation at 30 cmH2O pressure in this study. Similar to that reported in our previous studies (Ho et al. 2001), the heterogeneity of the discharge pattern of both SARs and RARs, and the variability of adaptation index in their responses to different inflation pressures are both quite evident. A small percentage (∼20%) of these receptors may fall into the category of ‘intermediate type’ (Bergren & Peterson, 1993). However, this variation in the classification criteria between RARs and SARs should not be viewed as a confounding factor in our conclusion because the overall response to capsaicin is still markedly greater in sensitized rats even when the data from all myelinated afferents (including both RARs and SARs) were pooled for comparison (Fig. 5A).
Capsaicin, a selective and potent agonist of TRPV1, has been used extensively as a tool for identifying non-myelinated (C-) nociceptive afferents in various organ systems (Holzer, 1991). A recent study in our lab showed that right-atrial injection of capsaicin stimulated only 6.3% of the RARs and no SARs in the lungs of Sprague–Dawley rats (Ho et al. 2001). Similar results were also found in the control rats in the present study. However, in stark contrast, right-atrial injection of capsaicin exerted a distinct stimulatory effect on RARs and SARs in Ova-sensitized rats. Thus, these results demonstrated that chronic airway inflammation induced by Ova sensitization triggered a phenotypic switch in myelinated bronchopulmonary afferents and clearly up-regulated their sensitivity to capsaicin.
TRPV1 is a polymodal nociceptive transducer containing six transmembrane domains that form non-selective, Ca2+–prefering cation channels. TRPV1 is expressed predominantly in non-myelinated neurones, and can be also activated by low pH (< 6.0) and high temperature (>39°C) (Caterina et al. 1997). It has been shown that several endogenous mediators, such as hydrogen ions, anandamide and certain lipoxygenase metabolites of arachidonic acid, can also activate TRPV1 expressed in airway sensory nerve (Geppetti et al. 2006; Jia & Lee, 2007). The involvement of TRPV1 receptors in the development of airway hypersensitivity in asthma has been suggested. For example, the sensitivity of capsaicin-induced cough is increased in asthmatics (O'Connell et al. 1996; Doherty et al. 2000). In addition, TRPV1 antagonists can inhibit cough elicited by inhalation of Ova aerosol in sensitized guinea pigs (McLeod et al. 2006). The results obtained in this study further indicate that a high percentage of pulmonary myelinated neurones expressed TRPV1 when chronic airway inflammation was induced by allergen sensitization. Therefore, it seems reasonable to hypothesize that the over-expression of TRPV1 in vagal pulmonary afferents may contribute, at least in part, to the development of hypersensitivity in allergic airways.
The mechanisms possibly responsible for the phenotypic change in TRPV1 expression in pulmonary myelinated neurones are not known. However, neurotrophins such as brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) should be considered because their synthesis and release have been shown to increase in allergic airways (Bonini et al. 1996; Lommatzsch et al. 2005). Furthermore, they are known to up-regulate the expression and function of TRPV1 in sensory neurones (Winter et al. 1988; Winter, 1998; Shu & Mendell, 2001). For example, it has been suggested that BNDF may contribute to the development of hypersensitive airways since mice treated with anti-BNDF antibodies showed reduced neuronal hypersensitivity to capsaicin (Braun et al. 2004). Indeed, BDNF can up-regulate the capsaicin sensitivity of nodose ganglion cells in culture (Winter, 1998). More importantly, the BDNF serum levels are elevated in asthmatic patients (Lommatzsch et al. 2005). NGF is another important neurotrophic factor to be considered in the pathogenesis of asthma. It has been reported that serum levels of NGF in patients with asthma were positively correlated with the severity of the disease (Bonini et al. 1996). Meanwhile, recent studies have clearly demonstrated that NGF can increase capsaicin sensitivity of sensory neurones both in vivo and in vitro (Winter et al. 1988; Winter, 1998; Shu & Mendell, 2001; Galoyan et al. 2003), which may be mediated through several possible mechanisms, such as increasing the expression of the TRPV1 channel protein (Winston et al. 2001), promoting translocation of the TRPV1 to cell membrane (Chuang et al. 2001), and modulating the TRPV1 function by releasing the receptor inhibition from phosphotidylinasitol-4,5-bisphosphate (Chuang et al. 2001).
The relationship between those neurotrophins and the expression of TRPV1 may also help to explain another interesting observation in the present study. Increased expression of TRPV1 occurred only in nodose pulmonary myelinated neurones, but not in their jugular counterparts. Different embryonic origins of these two types of ganglia may play a part in causing this difference. Nodose ganglia have a placodal origin, where as jugular ganglia arise from the neural crest (Undem et al. 2004). It has been suggested that the different phenotypes of neurones arising from these two ganglia may be related to different requirements in neurotrophic factors. In the chick embryo, placode-derived neurones, such as ventrolateral trigeminal neurones (Davies et al. 1986) and nodose ganglion neurones (Buj-Bello et al. 1994), mainly survived and grew in the presence of BDNF, whereas neural crest-derived neurones, such as dorsomedial trigeminal neurones and jugular neurones were supported preferentially by NGF (Buj-Bello et al. 1994). Meanwhile, more abundant BDNF are found in the nodose ganglion than in the jugular ganglion (Zhou et al. 1998; Ichikawa et al. 2007). It has been reported that the majority of nodose ganglion neurones expressed tyrosine receptor kinase B (TrkB), the preferred receptors for BDNF (Wetmore & Olson, 1995; Michael & Priestley, 1999; Kashiba et al. 2003). Considering the observation that the level of BDNF, but not NGF, is probably involved in determining the level of capsaicin sensitivity of vagal sensory neurones (Winter, 1998), we suggest that BDNF may be responsible for the inflammation-induced TRPV1 up-regulation in nodose ganglia. This postulation is further supported by the fact that the majority of small neurones of the nodose ganglion express TRPV1 mRNA in conjunction with BDNF receptor TrkB, but not NGF receptor TrkA (Michael & Priestley, 1999). It has been shown that after nerve injury, small neurones (usually C neurones) in DRG switched off their normal synthesis of BDNF, whereas large ones (myelinated neurones) switched to a BDNF phenotype (Zhou et al. 1999), which seems also in general agreement with our postulation. Taken together, the observations described above offer compelling support to our hypothesis that these neurotrophic factors may be involved, at least in part, in the over-expression of TRPV1 in myelinated neurones induced by allergic airway inflammation. Obviously, further investigation is required to test this hypothesis. Furthermore, possible contributions to the TRPV1 up-regulation by other chemical mediators synthesized and released in the airways during the chronic allergic inflammation can not be overlooked.
An allergic inflammation-induced phenotypic change of neuropeptide (tachykinins and calcitonin gene-related peptide) synthesis in sensory nerves has been previously reported in guinea pig airways (Myers et al. 2002). However, the expression of TRPV1 and neuronal sensitivity to capsaicin were not found in the nodose Aδ neurones innervating the trachea and major bronchi of Ova-sensitized guinea pigs in that study (Myers et al. 2002), which is different from our observation in this study (Figs 5 and 6). Possible causes of this discrepancy are not determined, but may be related to several factors such as the severity of the allergic inflammation, innervations of different regions of the lung structures, species difference, or other unknown factors.
The expression of TRPV1 immunoreactivity was only determined in neuronal cell bodies in this study. Therefore, it did not offer any direct evidence of the TRPV1 over-expression in the sensory terminals of myelinated afferents in sensitized rats. Coincidentally, a recent short study reported the number of TRPV1-positive axons in the trachea increases in Ova-sensitized guinea pigs (Watanabe et al. 2008), which is in general agreement with our finding. Furthermore, our data in the in vivo study indicating that the sensitivities of both RARs and SARs to capsaicin were markedly increased in sensitized rats (Figs 2, Figs 3 and 5) lend strong support to our conclusion.
In the present study, we did not find a difference between control and sensitized rats in the percentage of pulmonary non-myelinated (NF-negative) neurones that express TRPV1 channels. However, we want to emphasize that the contribution of pulmonary C-fibre afferents in the airway hypersensitivity in this allergic asthma model should not be underestimated. Indeed, we have recently demonstrated that there was an increase in the excitability of pulmonary C-fibres to mechanical and chemical stimuli, especially the increased sensitivity to capsaicin, in Ova-sensitized rats (Zhang et al. 2008). Furthermore, we also need to point out the limitation in the data analysis of our immunohistochemistry experiments; the computer analysis measured only the percentage of bronchopulmonary neurones that expressed the TRPV1 receptor, rather than the density or the total number of the TRPV1 channels expressed in each neurone. Thus, it is very possible that the density and/or the sensitivity of TRPV1 channels was elevated in pulmonary non-myelinated neurones of sensitized rats, despite a lack of increase in the percentage of TRPV1-expressing neurones. In fact, our recent finding in the in vivo study (Zhang et al. 2008) has offered intriguing evidence for such a possibility. Similarly, we cannot rule out the possibility that the sensitivity and/or density of TRPV1 were also up-regulated in the jugular pulmonary neurones in sensitized rats.
In conclusion, the present study demonstrates that allergen-induced airway inflammation caused a distinct increase in the sensitivity of vagal myelinated afferents to capsaicin. The increased capsaicin sensitivity of these afferents was further supported by a pronounced increase of TRPV1 expression in pulmonary myelinated neurones in nodose ganglia. In distinct contrast, these vagal afferents did not exhibit any significant response to the same dose of capsaicin injection in control rats. We believe that the effects of endogenous chemical mediators including neurotrophic factors released from airway structure cells and inflammatory cells are probably involved in the up-regulation of TRPV1 expression in these myelinated afferent fibres during chronic airway allergic inflammation in sensitized rats.
Acknowledgments
The authors thank Robert F. Morton and Michael Dodd for their technical assistances in this study, Dr Qihai Gu for critical reading and comments on the manuscript, and Dr Richard Kryscio for statistical consultation. This study was supported in part by grants from National Institutes of Health (HL58686 to L.-Y.L.; NS53470 to D.M.S.).
References
- Bartlett D, Jr, Sant'ambrogio G, Wise JC. Transduction properties of tracheal stretch receptors. J Physiol. 1976;258:421–432. doi: 10.1113/jphysiol.1976.sp011428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergren DR, Peterson DF. Identification of vagal sensory receptors in the rat lung: are there subtypes of slowly adapting receptors? J Physiol. 1993;464:681–698. doi: 10.1113/jphysiol.1993.sp019657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini S, Lambiase A, Bonini S, Angelucci F, Magrini L, Manni L, Aloe L. Circulating nerve growth factor levels are increased in humans with allergic diseases and asthma. Proc Natl Acad Sci U S A. 1996;93:10955–10960. doi: 10.1073/pnas.93.20.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A, Lommatzsch M, Neuhaus-Steinmetz U, Quarcoo D, Glaab T, McGregor GP, Fischer A, Renz H. Brain-derived neurotrophic factor (BDNF) contributes to neuronal dysfunction in a model of allergic airway inflammation. Br J Pharmacol. 2004;141:431–440. doi: 10.1038/sj.bjp.0705638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buj-Bello A, Pinon LG, Davies AM. The survival of NGF-dependent but not BDNF-dependent cranial sensory neurons is promoted by several different neurotrophins early in their development. Development. 1994;120:1573–1580. doi: 10.1242/dev.120.6.1573. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5),P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol. 1984;99:1–110. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JCG. Reflexes evoked from tracheobronchial tree and lungs. In: Cherniak NS, Widdicombe JG, editors. Handbook of Physiology, section 3, The Respiratory System, vol II, Control of Breathing, part I. Washington, DC: American Physiological Society; 1986. pp. 395–429. [Google Scholar]
- Davies AM, Thoenen H, Barde YA. The response of chick sensory neurons to brain-derived neurotrophic factor. J Neurosci. 1986;6:1897–1904. doi: 10.1523/JNEUROSCI.06-07-01897.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty MJ, Mister R, Pearson MG, Calverley PM. Capsaicin responsiveness and cough in asthma and chronic obstructive pulmonary disease. Thorax. 2000;55:643–649. doi: 10.1136/thorax.55.8.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwood W, Barnes PJ, Chung KF. Airway hyperresponsiveness is associated with inflammatory cell infiltration in allergic brown-Norway rats. Int Arch Allergy Immunol. 1992;99:91–97. doi: 10.1159/000236340. [DOI] [PubMed] [Google Scholar]
- Elwood W, Sakamoto T, Barnes PJ, Chung KF. Allergen-induced airway hyperresponsiveness in Brown-Norway rat: role of parasympathetic mechanisms. J Appl Physiol. 1993;75:279–284. doi: 10.1152/jappl.1993.75.1.279. [DOI] [PubMed] [Google Scholar]
- Galoyan SM, Petruska JC, Mendell LM. Mechanisms of sensitization of the response of single dorsal root ganglion cells from adult rat to noxious heat. Eur J Neurosci. 2003;18:535–541. doi: 10.1046/j.1460-9568.2003.02775.x. [DOI] [PubMed] [Google Scholar]
- Geppetti P, Materazzi S, Nicoletti P. The transient receptor potential vanilloid 1: role in airway inflammation and disease. Eur J Pharmacol. 2006;533:207–214. doi: 10.1016/j.ejphar.2005.12.063. [DOI] [PubMed] [Google Scholar]
- Glogowska M, Richardson PS, Widdicombe JG, Winning AJ. The role of the vagus nerves, peripheral chemoreceptors and other afferent pathways in the genesis of augmented breaths in cats and rabbits. Respir Physiol. 1972;16:179–196. doi: 10.1016/0034-5687(72)90050-3. [DOI] [PubMed] [Google Scholar]
- Ho CY, Gu Q, Lin YS, Lee LY. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol. 2001;127:113–124. doi: 10.1016/s0034-5687(01)00241-9. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- Hunter DD, Myers AC, Undem BJ. Nerve growth factor-induced phenotypic switch in guinea pig airway sensory neurons. Am J Respir Crit Care Med. 2000;161:1985–1990. doi: 10.1164/ajrccm.161.6.9908051. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Terayama R, Yamaai T, Yan Z, Sugimoto T. Brain-derived neurotrophic factor-immunoreactive neurons in the rat vagal and glossopharyngeal sensory ganglia; co-expression with other neurochemical substances. Brain Res. 2007;1155:93–99. doi: 10.1016/j.brainres.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Jammes Y, Fornaris E, Mei N, Barrat E. Afferent and efferent components of the bronchial vagal branches in cats. J Auton Nerv Syst. 1982;5:165–176. doi: 10.1016/0165-1838(82)90037-6. [DOI] [PubMed] [Google Scholar]
- Jia Y, Lee LY. Role of TRPV receptors in respiratory diseases. Biochim Biophys Acta. 2007;1772:915–927. doi: 10.1016/j.bbadis.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Kappagoda CT, Man GC, Teo KK. Behaviour of canine pulmonary vagal afferent receptors during sustained acute pulmonary venous pressure elevation. J Physiol. 1987;394:249–265. doi: 10.1113/jphysiol.1987.sp016869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiba H, Uchida Y, Senba E. Distribution and colocalization of NGF and GDNF family ligand receptor mRNAs in dorsal root and nodose ganglion neurons of adult rats. Brain Res Mol Brain Res. 2003;110:52–62. doi: 10.1016/s0169-328x(02)00584-3. [DOI] [PubMed] [Google Scholar]
- Kuo YL, Lai CJ. Ovalbumin sensitizes vagal pulmonary C-fiber afferents in Brown Norway rats. J Appl Physiol. 2008;105:611–620. doi: 10.1152/japplphysiol.01099.2007. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Waddell PJ. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J Physiol. 1991;435:41–63. doi: 10.1113/jphysiol.1991.sp018497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BP, Morton RF, Lee LY. Acute effects of acrolein on breathing: role of vagal bronchopulmonary afferents. J Appl Physiol. 1992;72:1050–1056. doi: 10.1152/jappl.1992.72.3.1050. [DOI] [PubMed] [Google Scholar]
- Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol. 2001;125:47–65. doi: 10.1016/s0034-5687(00)00204-8. [DOI] [PubMed] [Google Scholar]
- Lee LY, Undem BJ. Bronchopulmonary vagal sensory nerves. In: Undem BJ, Weinreich D, editors. Advances in Vagal Afferent Neurobiology. Frontiers in Neuroscience. Boca Raton, FL: Taylor & Frances; 2005. pp. 279–313. [Google Scholar]
- Lin YS, Lee LY. Stimulation of pulmonary vagal C-fibres by anandamide in anaesthetized rats: role of vanilloid type 1 receptors. J Physiol. 2002;539:947–955. doi: 10.1113/jphysiol.2001.013290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommatzsch M, Schloetcke K, Klotz J, Schuhbaeck K, Zingler D, Zingler C, Schulte-Herbruggen O, Gill H, Schuff-Werner P, Virchow JC. Brain-derived neurotrophic factor in platelets and airflow limitation in asthma. Am J Respir Crit Care Medical. 2005;171:115–120. doi: 10.1164/rccm.200406-758OC. [DOI] [PubMed] [Google Scholar]
- McLeod RL, Fernandez X, Correll CC, Phelps TP, Jia Y, Wang X, Hey JA. TRPV1 antagonists attenuate antigen-provoked cough in ovalbumin sensitized guinea pigs. Cough. 2006;2:10. doi: 10.1186/1745-9974-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael GJ, Priestley JV. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci. 1999;19:1844–1854. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers AC, Kajekar R, Undem BJ. Allergic inflammation-induced neuropeptide production in rapidly adapting afferent nerves in guinea pig airways. Am J Physiol Lung Cell Mol Physiol. 2002;282:L775–L781. doi: 10.1152/ajplung.00353.2001. [DOI] [PubMed] [Google Scholar]
- Nagase T, Moretto A, Dallaire MJ, Eidelman DH, Martin JG, Ludwig MS. Airway and tissue responses to antigen challenge in sensitized brown Norway rats. Am J Respir Crit Care Med. 1994;150:218–226. doi: 10.1164/ajrccm.150.1.8025752. [DOI] [PubMed] [Google Scholar]
- O'Connell F, Thomas VE, Studham JM, Pride NB, Fuller RW. Capsaicin cough sensitivity increases during upper respiratory infection. Respir Med. 1996;90:279–286. doi: 10.1016/s0954-6111(96)90099-2. [DOI] [PubMed] [Google Scholar]
- Sant'Ambrogio G. Nervous receptors of the tracheobronchial tree. Annu Rev Physiol. 1987;49:611–627. doi: 10.1146/annurev.ph.49.030187.003143. [DOI] [PubMed] [Google Scholar]
- Shu X, Mendell LM. Acute sensitization by NGF of the response of small-diameter sensory neurons to capsaicin. J Neurophysiol. 2001;86:2931–2938. doi: 10.1152/jn.2001.86.6.2931. [DOI] [PubMed] [Google Scholar]
- Tschernig T, Neumann D, Pich A, Dorsch M, Pabst R. Experimental bronchial asthma – the strength of the species rat. Curr Drug Targets. 2008;9:466–469. doi: 10.2174/138945008784533543. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Chuaychoo B, Lee MG, Weinreich D, Myers AC, Kollarik M. Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol. 2004;556:905–917. doi: 10.1113/jphysiol.2003.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Horie S, Spina D, Michael GJ, Page CP, Priestley JV. Immunohistochemical localization of transient receptor potential vanilloid subtype 1 in the trachea of ovalbumin-sensitized Guinea pigs. Int Arch Allergy Immunol. 2008;146(Suppl. 1):28–32. doi: 10.1159/000126057. [DOI] [PubMed] [Google Scholar]
- Wetmore C, Olson L. Neuronal and nonneuronal expression of neurotrophins and their receptors in sensory and sympathetic ganglia suggest new intercellular trophic interactions. J Comp Neurol. 1995;353:143–159. doi: 10.1002/cne.903530113. [DOI] [PubMed] [Google Scholar]
- Widdicombe JG. Receptors in the trachea and bronchi of the cat. J Physiol. 1954;123:71–104. doi: 10.1113/jphysiol.1954.sp005034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe JG. Nervous receptors in the respiratory tree and lungs. In: Hornbein T, editor. Lung Biology in Health and Disease, Vol. 17, Regulation of Breathing. New York: Marcel Dekker; 1981. pp. 429–472. [Google Scholar]
- Widdicombe JG. Afferent receptors in the airways and cough. Respir Physiol. 1998;114:5–15. doi: 10.1016/s0034-5687(98)00076-0. [DOI] [PubMed] [Google Scholar]
- Winston J, Toma H, Shenoy M, Pasricha PJ. Nerve growth factor regulates VR-1 mRNA levels in cultures of adult dorsal root ganglion neurons. Pain. 2001;89:181–186. doi: 10.1016/s0304-3959(00)00370-5. [DOI] [PubMed] [Google Scholar]
- Winter J. Brain derived neurotrophic factor, but not nerve growth factor, regulates capsaicin sensitivity of rat vagal ganglion neurones. Neurosci Lett. 1998;241:21–24. doi: 10.1016/s0304-3940(97)00978-6. [DOI] [PubMed] [Google Scholar]
- Winter J, Forbes CA, Sternberg J, Lindsay RM. Nerve growth factor (NGF) regulates adult rat cultured dorsal root ganglion neuron responses to the excitotoxin capsaicin. Neuron. 1988;1:973–981. doi: 10.1016/0896-6273(88)90154-7. [DOI] [PubMed] [Google Scholar]
- Zhang G, Lin RL, Wiggers ME, Lee LY. Sensitizing effects of chronic exposure and acute inhalation of ovalbumin aerosol on pulmonary C-fibers in rats. J Appl Physiol. 2008;105:128–138. doi: 10.1152/japplphysiol.01367.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XF, Chie ET, Deng YS, Zhong JH, Xue Q, Rush RA, Xian CJ. Injured primary sensory neurons switch phenotype for brain-derived neurotrophic factor in the rat. Neuroscience. 1999;92:841–853. doi: 10.1016/s0306-4522(99)00027-5. [DOI] [PubMed] [Google Scholar]
- Zhou XF, Chie ET, Rush RA. Distribution of brain-derived neurotrophic factor in cranial and spinal ganglia. Exp Neurol. 1998;149:237–242. doi: 10.1006/exnr.1997.6716. [DOI] [PubMed] [Google Scholar]