Abstract
We have documented gestation- and labour- (preterm and term) dependent changes in expression of genes encoding contraction associated proteins in the rat uterus and correlated these changes with various parameters of uterine contractility. The data demonstrate increased expression of contractile agonist systems concurrent with decreased expression of relaxant systems after gestational day 20. Significant increases in expression of oxytocin (OT), its receptor (OTR), prostaglandin (PG) H synthase isoform 1 (PGHS-1) and PGF2α receptor (FP) occurred first, followed by increases in PGHS-2, connexin-43, endothelin-1 (ET-1) and the ET-1 receptor isoform ETA. Expression of OTR and FP was significantly reduced during mid-gestation compared to non-pregnant animals. Expression of inducible nitric oxide synthase (iNOS) increased significantly during pregnancy then decreased concurrently with the increase in OTR and FP. Functional changes in uterine contractility accompany changes in gene expression. OT was the most potent contractile stimulant. Sensitivity of uterine strips to OT was reduced in early and mid-pregnancy then increased at uterine activation. Progesterone antagonist-induced preterm labour caused changes similar to those at normal term. Comparison of mRNA transcripts in separated endometrium and myometrium suggested that the endometrium is an important regulator of myometrial contractility, analogous to the relationship between endothelium and vascular smooth muscle. This novel combination of functional and genetic expression analyses provides new insight into the physiology of parturition.
For most of gestation, the uterus remains in a state of relative quiescence. Despite the stretching effect of the rapidly growing intrauterine contents, the uterine smooth muscle (myometrium) maintains a low level of spontaneous contractility and responsiveness to contractile stimulants. In contrast, at the time of parturition, the myometrium is a responsive muscle capable of powerful, coordinated contractions with sufficient force to expel the products of conception. The transformation between these states is called uterine activation. The factors that regulate uterine quiescence or activation remain incompletely resolved. Knowledge of these processes is essential to understand the timing of parturition and, in particular, the mechanisms involved in spontaneous preterm birth. Preterm birth is associated with > 75% of death and long-term disability resulting from pregnancy.
Most of our knowledge concerning the regulation of parturition has been derived from animal species, particularly the sheep and rat. While it is clear that there are several important differences in mechanisms regulating parturition among the species, there also are many similarities at the level of uterine activation and uterine smooth muscle contraction. In both the sheep and rat, a reduction in maternal progesterone concentrations is essential to enable the mechanisms that culminate in parturition (Challis et al. 2000). In pregnant women, there is no such decline in maternal progesterone concentrations. However, there is evidence that other mechanisms may result in a functional progesterone withdrawal. This includes changes in production or metabolism of progesterone within the pregnant uterus (Mitchell & Wong, 1993) or a change in the expression of isoforms of the progesterone receptor that may minimize biological activity of progesterone in the uterus (Mesiano et al. 2002).
Uterine activation in the rat is accompanied by increased expression of a set of genes encoding contraction-associated proteins (CAPs) (Challis et al. 2000). The CAPs include receptors to contractile stimulants such as the oxytocin (OT) receptor (OTR) and the receptor for prostaglandin F2α (FP). Other CAPs include the enzyme regulating prostaglandin (PG) synthesis, PGH synthase isoform 2 (PGHS-2) and the major gap junction protein, connexin-43 (Cx-43). In addition, during pregnancy in both the rat and human, the endometrium expresses the enzyme inducible nitric oxide synthase (iNOS), which produces nitric oxide, a smooth muscle relaxant (Buhimschi et al. 1995, 1996; Bansal et al. 1997; Okawa et al. 1998). In contrast to the CAPs, rat uterine concentrations of mRNA encoding iNOS decrease prior to parturition (Okawa et al. 2004; Mitchell et al. 2005).
The identity of the contractile stimulants that result in increased contractions of the activated uterus remains unclear. OT and PGF2α are well-described uterine agonists. Endothelin-1 (ET) has also been noted to be a potent uterine contractile stimulant (Kozuka et al. 1989; Word, 1992). Prostaglandins are abundantly produced within the late gestation uterus in several species. Our own and other laboratories have demonstrated synthesis of OT, predominantly in the endometrial lining layer in late human and rat gestation (Lefebvre et al. 1992; Chibbar et al. 1993).
The current understanding of the mechanisms of uterine activation and stimulation has resulted from many studies that largely have been narrowly focused on one particular CAP independently from the others. However, the precise temporal sequence of appearance of the CAPs as gestation progresses and labour approaches has not been addressed systematically. Neither has there been an attempt to correlate the changes in gene expression with the functional contractility of the uterus. We have undertaken such a study to describe in detail the sequence and timing of the biochemical and physiological changes in several uterine contractile systems through late gestation. We also have determined the effects of the progesterone antagonist RU486 on these processes. We chose to perform these studies in the rat for several reasons: we are familiar with this model and have demonstrated its relevance to the human uterus (Fang et al. 1996; Mitchell et al. 2005); the model can be easily manipulated; and there is adequate tissue to complete the combined biochemical and physiological studies. Changes in expression of several CAP genes are similar in rat and human. Though there remain questions regarding the relevance of progesterone withdrawal in human parturition, a functional withdrawal may occur at the molecular level (Zakar & Hertelendy, 2007), which makes the rat model even more relevant. The data from our current experiments reveal novel findings regarding the sequence of expression of several recognized CAP genes, particularly concerning the expression of ET-1 and its relevant receptor as a potentially important agonist signalling system in the uterus. These changes are correlated with remarkably subtle changes in uterine contractility. Our findings show that the changes induced by a progesterone antagonist administered preterm are similar to those of spontaneous parturition at term. Our data also support the presence of a paracrine system within the late pregnant uterus wherein contractile factors are produced in the endometrium to have their effect on the adjacent myometrium, not unlike the relationship between the endothelium and vascular smooth muscle. This information provides a clearer and more integrated understanding of the series of events that culminate in parturition and suggests new strategies to further explore these important physiological events.
Methods
Animals
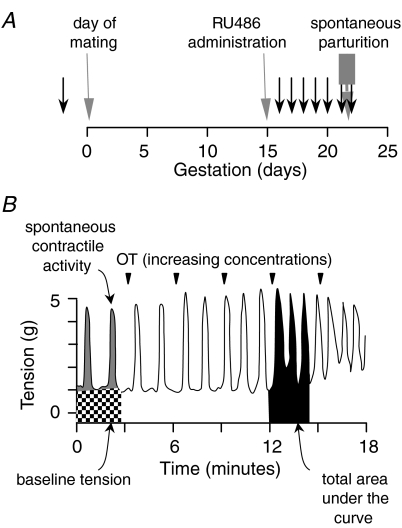
The University of Alberta Health Sciences Animal Policy and Welfare Committee approved all animal protocols and the experiments were conducted in accordance with the Guidelines and Policies of the Canadian Council on Animal Care. A total of 77 female Sprague–Dawley rats weighing approximately 250 g were used in these studies. For the protocols requiring pregnant animals, virgin rats were time-mated in the facilities of the University of Alberta Health Sciences Laboratory Animal Services. The morning of discovery of the semen plug is considered day 0. The pregnant animals in this colony deliver on the afternoon of day 21 or on day 22. To obtain uterine tissues for the in vitro studies, the rats were given a lethal dose of isofluorane (Bimeda-MTC, Cambridge, ON, Canada) by inhalation in a large glass beaker. Tissues were obtained at several points through late gestation as illustrated in Fig. 1A. For the ‘active labour’ group, animals were killed following delivery of the first pup. For all other groups, the animals were killed in the early morning. In several animals, the progesterone receptor antagonist RU486 was administered by a single injection of 2.5 mg in oil (controls received a corresponding amount of corn oil) subcutaneously on the morning of day 15. These animals were killed for tissue collection after 24 h on gestational day 16. In all RU486-treated animals, there was evidence of active labour on day 16 (vaginal bleeding delivery of a pup) whereas the placebo-treated animals showed no such signs. The uterus was removed immediately and, after removal of the remaining pups, placentas and fetal membranes from the pregnant animals, one horn of the uterus was frozen and stored at –80°C until subsequent analysis for mRNA. From the other horn, full thickness strips of uterine wall were collected for the myographic studies.
Figure 1. Experimental protocol for collection of uterine tissues and example of a myographic tracing in response to increasing concentrations of oxytocin.
A, experimental protocol for collection of uterine tissues. The day of mating, day of RU486 administration and time of spontaneous parturition are noted. The black arrows indicate the times that animals were killed for tissue collection. B, typical example of a myographic tracing in response to increasing concentrations of oxytocin (OT; noted by the inverted triangles). Based on analysis of length–tension experiments, the baseline tension was set to approximately 1 g. The AcqKnowledge software provides a measure of the total integrated area under the response curve (black shading). As a measure of spontaneous contractile activity (grey shading), we subtracted the baseline tension (stippled shading) from the total area under the curve prior to addition of the stimulant. To assess the net effect of the uterine stimulant, we subtracted the total area under the curve in the prestimulant tracing from the total area under the curve after addition of each concentration of stimulant.
Quantitative reverse transcription-polymerase chain reaction (RT-PCR)
RNA was extracted using the GenElute Mammalian Total RNA Miniprep kit (Sigma-Aldrich, St Louis, MO, USA). Samples were treated with Amplification grade DNase I (Sigma-Aldrich). The purity of the extracted RNA was assessed by spectrophotometry and the quality assessed by electrophoresis on 1% agarose gel. The sample was diluted to 50 ng mRNA per μl. Reverse transcription was performed using the Taqman Reverse Transcription Reagents kit (Applied Biosystems, Foster City, CA, USA). The resultant cDNA was stored at −20°C until ready for PCR. As a negative control the reaction was also performed in randomly chosen samples in the absence of the reverse transcriptase to ensure the absence of genomic or other DNA contamination in the samples.
Real time PCR was performed in triplicate using the SYBR Green PCR Core Reagents Kit (Applied Biosystems) and the iCycler apparatus (Bio-Rad, Montreal, QC, Canada). The forward and reverse primer sequences, gene accession numbers and amplicon locations within the gene are presented in Table 1. The protocol for each PCR reaction was individually validated and optimized. Efficiency curves were assessed for each set of primers and the PCR products were assessed using 1% agarose gels to ensure the presence of one amplicon at the expected size and the absence of primer-dimer contamination. The blank control was one tube in which autoclaved H2O replaces the cDNA template. The fluorescence was measured at the end of each PCR cycle and plotted against the cycle number to determine the threshold cycle. After each PCR reaction, the purity of the amplified cDNA was confirmed by the presence of a single melt curve for all samples.
Table 1.
Sequences used for forward (f) and reverse (r) primers for PCR
| mRNA | gene ID | Primers | bp | Location in gene |
|---|---|---|---|---|
| OTR | AF257237 | f. AATGAAGAGCACCGACAGC | 161 | exon 1 |
| r. CCGCCGCTGCCGTCTTGA | ||||
| FP | NM013115 | f. CTGGCCATAATGTGCGTCTC | 105 | exon 2–exon 3 |
| r. TGTCGTTTCACAGGTCACTGG | ||||
| PGHS-1 | U18060 | f. GCCGAGGATGTCATCAAGGA | 168 | exon 10–exon 11 |
| r. GAACTCTAAAGCATCGATGTCACCA | ||||
| PGHS-2 | NM017232 | f. CCTTGAACACGGACTTGCTCAC | 131 | exon 8–exon 9 |
| r. TCTCTCTGCTCTGGTCAATGGA | ||||
| Cx-43 | NM012567 | f. TGACTTCAGCCTCCAAGG | 171 | 5′UTR exon 1–exon 3 |
| r. AATGAAGAGCACCGACAGC | ||||
| iNOS | AJ234082 | f. TTGGTGAAAGCGGTGTT | 81 | exon 13–exon 14 |
| r. AGCAAAGAGGACTGTGGC | ||||
| OT | K01494 | f. CTTGGCCTACTGGCTCTGAC | 216 | exon 1–exon 2 |
| r. GGGCAGGTAGTTCTCCTCCT | ||||
| ET-1 | AF122903 | f. CTCCTCCTTGATGG ACAAGG | 370 | exon 2–exon 5 |
| r. CTTGATGCTGTTGCTGATGG | ||||
| ETA | M60787 | f. TTCCCTCTTCACTTAAGCCGAA | 202 | exon 5–exon 7 |
| r. GCAACAGAGGCATGACTGAAAA | ||||
| Cyclophylin | NM017101 | f. CACCGTGTTCTTCGACATCAC | 114 | exon 1–exon 3 |
| r. CCAGTGCTCAGAGCTCGAAAG |
For quantification, we used the ‘Δ–Δ’ method to determine the relative expression between groups (Pfaffl, 2001). This calculation provides the relative expression of each sample, normalized to the expression of a housekeeping gene (HKG). We chose cyclophylin as our HKG since it is expressed in approximately the same quantity as our genes of interest (GOI) and it remains constant in the rat uterus through late gestation (Fang et al. 1996). The threshold cycle (CT) was determined for the GOI and the HKG. The data were normalized by subtracting the CT for the GOI from the CT for cyclophylin to determine the ΔCT. After this ‘loading control’ correction the relative expression levels of the GOI in an experimental sample was compared to that of a control or calibrator sample by subtracting their ΔCT values. By using the same, randomly chosen calibrator sample for every experimental sample, the relative expression for every sample was calculated using the formula: relative fold change = 2−x, where x is the difference between the ΔCT of the experimental sample and the calibrator samples.
Uterine myography
The muscle bath preparation was established based on published methodology (Molnar et al. 1993). Uterine horns were removed immediately and incised longitudinally. No attempt was made to separate circular from longitudinal muscle or to remove attached endometrium. Longitudinal muscle strips approximately 8 mm in length and 3 mm in width were excised, attempting to avoid implantation sites.
Muscle strips were mounted vertically onto four separate transducers in separated jacketed 10 ml organ baths filled with physiological saline solution (PSS) of the following composition (mm): 140 NaCl2, 4 KCl, 1.0 KH2PO4, 2.0 MgSO4.7H2O, 1.0 Hepes, 5.0 CaCl2.2H2O, 5.5 glucose at pH 7.5 at 37°C with 95% O2–5% CO2. One end of the tissue was tied to a fixed hook using a silk suture and the other end was attached to a Bridge 8 force-displacement transducer connected to a Biopac Systems MP100 unit with AcqKnowledge software, version 3.7.2 (World Precision Instruments, Sarasota, FL, USA).
Preliminary length–tension studies (Knudsen et al. 1998) were performed to determine the optimal basal tension. The uterine strips were attached to the strain gauge with just sufficient tension to be recorded by the transducer. The initial length (L0) was taken as this point. The tissue length was increased by increments of 0.25 mm using the micromanipulator attached to the transducer. The resting or ‘passive’ tension was recorded after the strip had stabilized at the new length (4–5 min). The ‘total’ tension at this stretch was measured in response to a high potassium ion (K+) solution (mm: 2 MgSO4.7H2O, 5 CaCl2.2H2O, 1 Hepes, 24 NaCl2, 124 KCl, 1.2 KH2PO4, 5.5 glucose). The total tension was determined at the peak of the initial transient contraction observed within 60 s of high K+ exposure. The high K+ solution was thoroughly washed from the bath and replaced with PSS. The active tension at each time point was calculated as follows: active tension = total tension – passive tension. This procedure was repeated at several tissue lengths until the tissue became overstretched in order to construct a length–tension curve. The passive tension at which the maximum active tension was generated was used in subsequent experiments. The general patterns of the length–tension curves for uterine muscle strips were similar in tissues obtained from non-pregnant or pregnant rats. The major difference between the uterine strips from the two groups was in their compliance. The amount of stretch required to generate a given increase in passive tension was much greater in the tissues from the pregnant animals. Importantly, the passive tension at which the maximal active response was obtained occurred at between 0.5 and 1 g tension for both non-pregnant and pregnant animals. Interestingly, the active tension generated by OT was fairly constant for passive tensions between 0.5 and 2 g. Based on these data, we selected 1.0 g resting tension as the baseline from which to evaluate the parameters of contractility and to assess the sensitivities and responses to uterine agonists for strips from either non-pregnant or pregnant animals.
The muscle strips were incubated for 60–90 min to establish a stable baseline. Cumulative concentration–response curves were established for OT, ET-1 (0.1–320 nmol l−1 for each) or PGF2α (1 nmol l−1 to 10 μmol l−1) added at 3 min intervals. The EC50 concentration (concentration at which half-maximal tension occurred) was calculated as a measure of sensitivity for each of the contractile stimulants using nonlinear regression for a sigmoid curve (Prism version 4.0 software, GraphPad Software, San Diego, CA, USA). Only those strips where the correlation coefficient (r2) for the curve fit was > 0.80 were used for the data reported. The responsiveness of the strips was assessed using three approaches (Fig. 1B). First, we calculated the spontaneous baseline contractility by subtracting the resting tension from the integrated area over the 3 min of recording prior to addition of agonist. Next, the maximal response to OT was calculated by measuring the area (integrated over the 3 min time course) under the concentration of agonist that resulted in the maximal contraction minus the area under the curve of the 3 min immediately prior to addition of the stimulant. For calculation of the EC50 values, this was considered as 100% and all other values were normalized to this. Finally, we assessed the total area under the concentration–response curve determined by summation of the integrated responses at each concentration after subtraction of the area under the curve of the 3 min immediately prior to addition of the stimulant. For purposes of the discussion, we have defined the maximal OT-inducible contractile activity as the sum of the spontaneous contractile activity plus the maximal response to OT.
In preliminary experiments, we calculated the cross-sectional area of the individual muscle strips and normalized the contractile responses on this basis. We also assessed normalizing the data to the spontaneous contractile activity. Neither of these normalization methods changed the interpretation of the responsiveness data so, for the data presented, such normalizations were not performed.
Statistics
Comparisons between groups of rats were performed using one-way analysis of variance (ANOVA) with post hoc Tukey–Kramer test only when the ANOVA revealed a significant (P < 0.05) difference. When data were not normally distributed (tested using the method of Kolmogorov and Smirnov) or the standard deviations were non-homogeneous (as assessed by Bartlett's test), a log transformation was performed. Comparisons among the three uterine agonists over pregnancy were performed using two-way ANOVA. If the two-way ANOVA demonstrated a significant effect with one of the variables (e.g. the effect of gestation on EC50), a two-tail Student's unpaired t test was used to make comparisons between the non-pregnant and late pregnant groups. In the figures, data are presented as the mean ± standard error of the mean.
Results
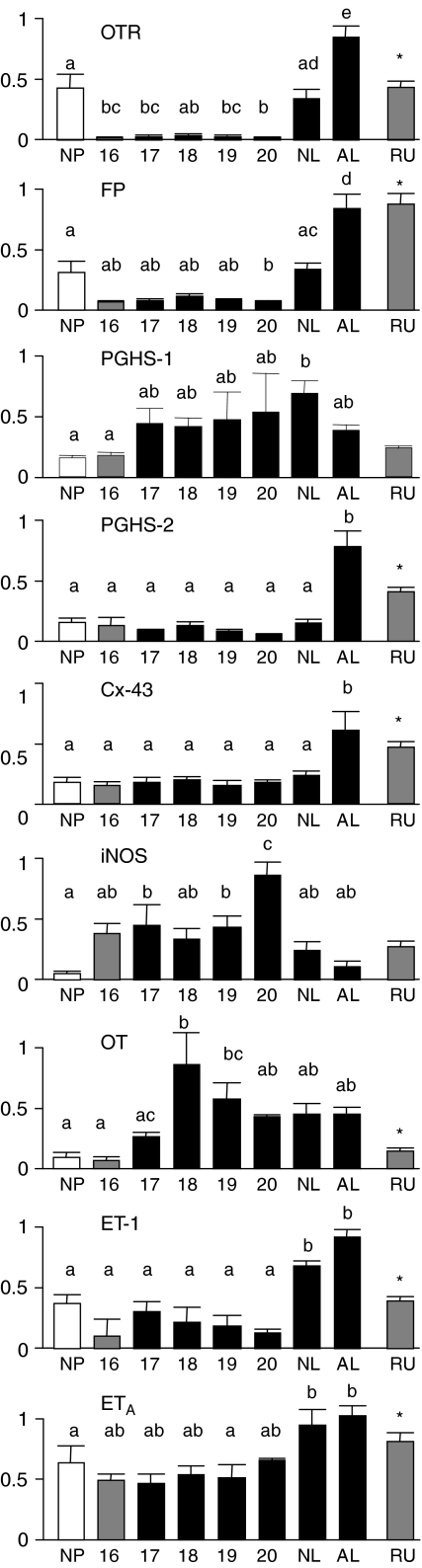
The data regarding relative expression of key uterine genes are presented in Fig. 2. Our primary objective was to assess the changes that occurred at the time of uterine activation. The mRNA expressions for PGHS-1 and OT were the first to increase significantly in mid–late gestation. The relative concentrations of mRNA for OTR and FP were significantly suppressed in the tissues from the day 16–20 animals compared to the non-pregnant group (Fig. 2; P < 0.001). However, these were significantly increased by gestational day 21 (P < 0.001) to levels similar to the non-pregnant animals and increased further during labour (P < 0.001). Notably, the mRNA for PGHS-2 and Cx-43 did not decrease significantly during pregnancy and increased only when the rats were in active labour. In contrast to OTR and FP, mRNA for iNOS increased markedly in pregnancy (P < 0.001) then decreased significantly (P < 0.01) at the same time as the increase for OTR and FP.
Figure 2. Relative expression of mRNA for key uterine genes.
Relative expression of mRNA for oxytocin receptor (OTR), prostaglandin F2α receptor (FP), prostaglandin H synthase-1 (PGHS-1), PGHS-2, connexin 43 (Cx-43), inducible nitric oxide synthase (iNOS), oxytocin (OT), endothelin-1 (ET-1) and the endothelin-1 receptor isoform A (ETA) in rat uterine tissues obtained from non-pregnant animals (NP; n = 10–14) and from pregnant animals at gestational day 16 (n = 5–6), day 17 (n = 6–7), day 18 (n = 4–5), day 19 (n = 6–10), day 20 (n = 8–22) or from gestational day 21 or 22, not in labour (NL; n = 10–14) or animals in active labour (AL; n = 9–10). Some animals were treated with a single subcutaneous injection of the antiprogestin RU486 on gestational day 15 and tissues collected 24 h later on day 16 (RU; n = 18). Within each mRNA group, excluding the RU486-treated animals group, data were assessed using one-way ANOVA and the bars with unique superscripts are significantly different (P < 0.05) from bars without overlapping superscripts. To determine the effects of the antiprogestin treatment, the treated animals were compared to the animals on gestational day 16 (both groups illustrated using grey bars). An asterisk denotes a statistically significant difference between these two groups using the two-tailed t test.
In addition to the genes regulating the CAPs, the rat uterus also contained mRNA for OT, ET-1 and the endothelin receptor A isoform (Fig. 2). For these genes, there was no change in mRNA between the tissues from the non-pregnant animals and the tissues from gestation day 16. However, expression of all three genes increased significantly in late pregnancy. There were interesting differences in the timing of the increases. Like OTR and FP, ET-1 increased prior to labour onset. ETA was similar to PGHS-2 and Cx-43 with the significant increase during labour. The patterns of mRNA for PGHS-1 and OT were unique with peak mRNA concentrations occurring at late mid-gestation prior to marked increase in expression of the CAP genes.
In response to treatment with the progesterone antagonist RU486, most amplicons were altered significantly (Fig. 2). The mRNA for OTR, FP, PGHS-2, Cx-43, OT, ET-1 and ETA were significantly increased by RU486 compared to the placebo-treated d16 controls. The mRNAs for PGHS-1 and iNOS did not change significantly.
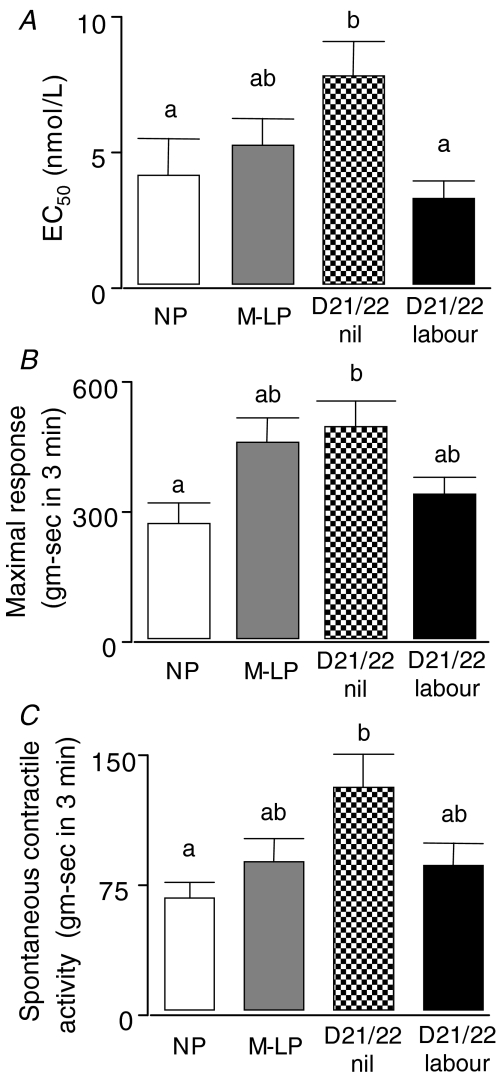
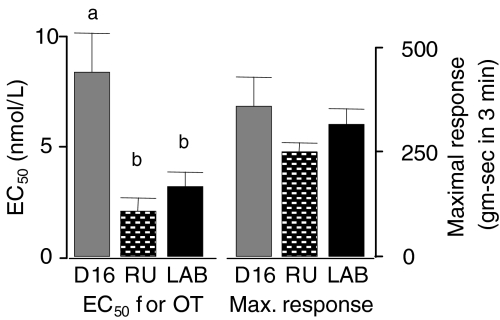
The amount of OT required to produce a response 50% of maximal (EC50) increased significantly from the non-pregnant animals (4.2 ± 1.3 nm) to the group at term but not in labour (7.6 ± 1.3 nm), reflecting a decreased sensitivity to OT in mid–late pregnancy (Fig. 3A). The sensitivity to OT then increased significantly in the tissues from the animals in active labour, returning to a level (3.1 ± 0.6 nm) that was not different from the non-pregnant group. Similarly, the uterine contractility parameters showed a significant increase in both maximal response (Fig. 3B) and spontaneous contractile activity (Fig. 3C) between the strips from the non-pregnant animals and the term, not in labour group. Interestingly, treatment with the antiprogesterone caused a significant decline in the EC50 for OT (Fig. 4) without any effect on the maximal response to the agonist.
Figure 3. Myographic parameters of uterine contractility.
A, the concentrations required to achieve half-maximal response (EC50) for oxytocin-induced contractions in non-pregnant animals (open bars; n = 10), animals at mid-late pregnancy (grey bars; n = 14), day 21/22 not in labour (stippled bars; n = 21) or in active labour (black bars; n = 9). B, the maximal responses (area under the curve for the concentration of oxytocin giving the maximal response) for the same animals as in panel A. C, the spontaneous contractile activity for the same animals as in panels A and B. For each panel, bars with unique superscripts are significantly different (ANOVA; P < 0.05) from bars without overlapping superscripts.
Figure 4. The effects of treatment with the antiprogestin RU486 on EC50 and maximal response parameters for OT.
The data were obtained using uterine strips from rats at gestational day 16 following 24 h treatment with placebo (grey bars; n = 5) or RU486 (stippled bars; n = 18). For comparison, data are also presented for rats at day 21 or 22 in active labour (black bars; n = 9 or 10). Bars with unique superscripts are significantly different from bars without overlapping superscripts.
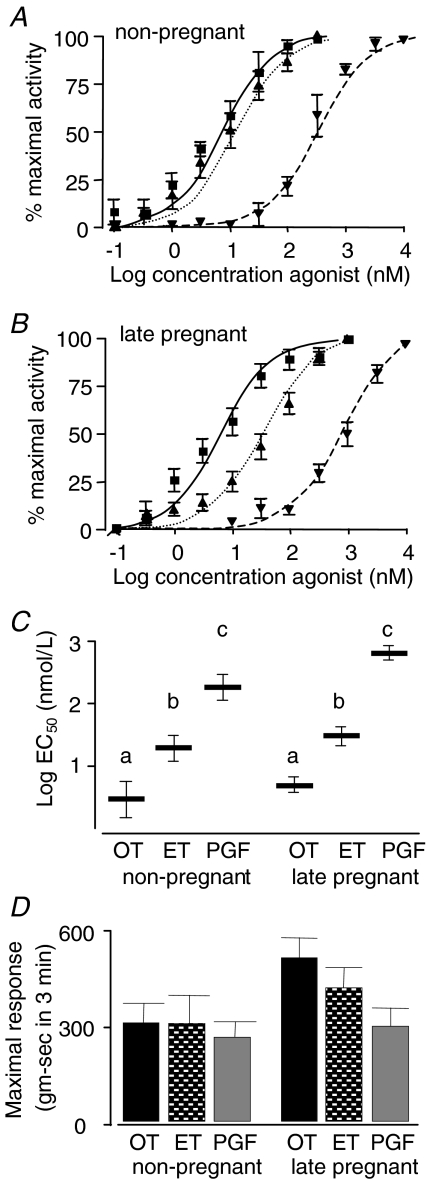
Summary concentration–response curves are illustrated for the three uterine agonists in the non-pregnant and pregnant animals (Fig. 5A and B). There were no differences between the D18 and the D21 animals so they have been grouped for purposes of the figures and referred to as late pregnant animals. For the EC50 data calculated from the concentration–response curves (Fig. 5C), two-factor ANOVA revealed a significant effect of uterine agonist (P < 0.0001) and also of pregnancy (P < 0.0001) with a significant interaction between these two variables (P < 0.001). The sensitivity of the uterus to OT was significantly greater than to ET or PGF2α in both the non-pregnant (5.2, 27.3 and 285.8 nmol l−1, respectively) and pregnant animals (6.8, 49.4 and 933.9 nmol l−1, respectively). For both pregnant and non-pregnant groups, each agonist was different from the other by one-way ANOVA and subsequent post hoc Tukey–Kramer multiple comparisons test, P < 0.001). Although there was an overall effect of gestation on the EC50 values, direct comparison of the EC50 values (two-tailed, unpaired Student's t test) for OT and ET showed no significant change whereas the EC50 for PGF2α increased significantly, indicating a decreased sensitivity to PGF2α in the tissues from late pregnancy.
Figure 5. Myographic parameters of uterine contractility comparing the three uterine agonists oxytocin (OT), endothelin-1 (ET-1) and prostaglandin (PG) F2α.
A and B, the summary concentration–response curves in non-pregnant (n = 6, 5 and 6 for OT, ET-1 and PGF2α, respectively) and late pregnant (gestation day 18–21) animals (n = 19, 19 and 14 for OT, ET-1 and PGF2α, respectively) for OT (▪), ET-1 (▴) and PGF2α (▾). C, the log concentrations required to achieve half-maximal response (EC50) for agonist-induced contractions for rat uterine strips calculated from individual concentration–response curves for the same animals as in panels A and B. Because of the wide variation in EC50 among the different agonists, the data were log-transformed for statistical analyses and the transformed data are illustrated. Bars with unique superscripts are significantly different from bars without overlapping superscripts. D, the maximal responses (area under the curve for the concentration of agonist giving the maximal response) for the same animals. There were no significant differences among groups, though for the late pregnant animals the differences approached statistical significance (ANOVA, P = 0.0511).
In contrast to the sensitivity data, the responsiveness data (Fig. 5D) for the three uterine agonists showed little change with differing state (pregnant versus non-pregnant) or differing agonist. Using two-factor ANOVA, the changes in maximal response for the strips from non-pregnant rats compared to the late pregnancy group approached statistical significance (P = 0.0511). Similarly, within the late pregnancy group, the differences in responses to the different agonists approached statistical significance (P = 0.0515). There was a significant (P = 0.0487) interaction between the two factors. There were no significant changes for spontaneous contractile activity or the total area under the concentration–response curves (data not shown).
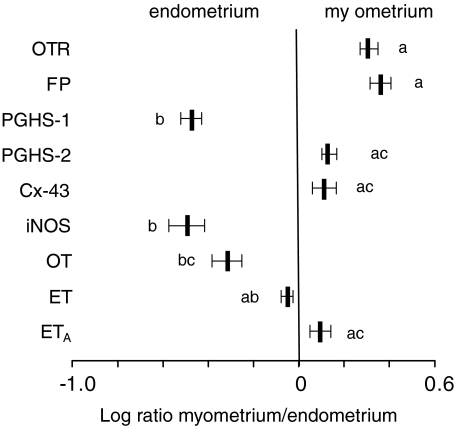
In 11 animals (4 between day 18–20, 3 in active labour, 4 treated with RU486 on day 15 and samples obtained 24 h later) we attempted to derive qualitative information regarding the tissues of origin for the mRNA being measured. The endometrial lining was bluntly dissected from the underlying myometrium and the tissues analysed separately. For each of the mRNAs, the ratio was calculated for the relative expression in the myometrium divided by the relative expression in the endometrium. The higher this ratio, the greater is the proportion of the mRNA that originated in the myometrium. As is evident in Fig. 6, OTR and FP (and perhaps PGHS-2, Cx-43 and ETA) were predominantly in the myometrium whereas PGHS-1, iNOS and OT (and perhaps ET) appeared to be mostly from the endometrium. From these limited data, there was no apparent effect of RU486 treatment on the tissue distributions in concentrations of mRNA.
Figure 6. Ratios of relative expression of mRNA for uterine genes in myometrial and endometrial tissues from rats during late gestation.
Ratios of relative expression of mRNA for oxytocin receptor (OTR), prostaglandin F2α receptor (FP), prostaglandin H synthase-1 (PGHS-1), PGHS-2, connexin 43 (Cx-43), inducible nitric oxide synthase (iNOS), oxytocin (OT), endothelin-1 (ET) and the endothelin receptor isoform A (ETA) in myometrial and endometrial tissues from rats during late gestation. The endometrium was bluntly dissected from the uterine wall (n = 11) and the horizontal bars represent the quotients of the concentration of each mRNA in the myometrium divided by the concentration found in the endometrium from the same samples. Thus, transcripts with log ratios less than 0 are predominantly endometrial in origin and those with log ratios greater than 0 are predominantly from myometrium. The bars with unique lower case designations are significantly different from those without overlapping designations.
Discussion
This combination of biochemical and physiological data has provided more insight into the events that occur in the late gestation rat uterus around the time of the initiation of parturition. The process of uterine activation appears to occur in the pregnant rat over a 24–36 h period around gestation day 21. The variability around this timing is likely to be a result of biological variability but may also be influenced by the fact that our mating period for the virgin females occurred over a 20–24 h period. A shorter mating episode (such as 4 h) may provide less variable data in future experiments. Several studies in rat and human tissues have demonstrated increases in expression of genes encoding the CAPs in late pregnancy but this is the first description of the precise chronological appearance of these mRNAs. The data suggest an ordered process. As expected, expression of PGHS-1 appeared in late mid-pregnancy and peaked by day 20. This enzyme is responsible for generation of the PGF2α that will initiate luteolysis resulting in the progesterone withdrawal (Gross et al. 2000). The data regarding OTR confirm our earlier assessment of OT binding in the rat (Fang et al. 1996) and are in agreement with the original OT-binding studies of Alexandrova & Soloff (1980a) and Fuchs et al. (1982) in the rat and human uterus, respectively. This suggests that regulation of OTR expression in vivo is primarily at the level of transcription. It has been demonstrated in vitro that progesterone or its metabolites can directly and non-genomically inhibit OT binding to OTR (Grazzini et al. 1998) though the physiological relevance of this finding is unclear.
In agreement with our findings for FP receptor, previous studies using semiquantitative methodologies in the rat (Brodt-Eppley & Myatt, 1999; Dong & Yallampalli, 2000; Ou et al. 2000) and human (Brodt-Eppley & Myatt, 1999; Al-Matubsi et al. 2001) showed a significant increase in mRNA and protein prior to labour. Interestingly, our data show that this increased expression of FP occurs when uterine contractile sensitivity to PGF2α is decreasing, in agreement with earlier findings from Word et al. (1992).
Our study provides strong evidence for a logical mechanism to suppress contractility when uterine quiescence is essential. The expression of agonist receptor systems and uterine relaxant systems vary inversely and concurrently. Our demonstration of a significant decrease in mRNA for OTR and FP in early pregnancy is in agreement with previous studies in the rat (Alexandrova & Soloff, 1980b; Liu et al. 1996) and human myometrium (Matsumoto et al. 1997). This occurs coordinately with increased expression of a relaxatory pathway involving production of nitric oxide by the enzyme iNOS. Our data show a 10-fold increase in mRNA for iNOS during pregnancy followed by a marked decline at the time of uterine activation, concurrent with the increase in expression of mRNA for OTR. This confirms previous findings for iNOS in the uterus of both rat and human (Ali et al. 1997; Bansal et al. 1997; Okawa et al. 2004; Mitchell et al. 2005). These findings are in agreement with the concept (Lopez Bernal et al. 1995) that the process of uterine activation includes not only increased expression of uterine contractile systems but also a repression of uterine relaxant systems. This is the complete reversal of the changes that are responsible for uterine quiescence.
The mRNA for PGHS-1 began to increase in late mid-gestation, peaking on day 20, then declining. This pattern is in keeping with its role in providing the PGF2α that causes luteolysis and initiates the progesterone withdrawal (Gross et al. 1998). By comparison, mRNA concentrations for PGHS-2 and Cx-43 were increased subsequently to changes in OTR, FP and iNOS and the increase was apparent only in the tissues from the animals in active labour. The relatively late appearance of PGHS-2 suggests that it may be a result of the labour process. A potential stimulus for the increase in PGHS-2 could be the increase in pro-inflammatory cytokines that accompanies labour. The physiological function of this is not clear. Our finding of maximal uterine concentrations of mRNA for Cx-43 during labour is in agreement with previous studies using semiquantitative methodology that also show this relatively late appearance of mRNA and protein for Cx-43 (Petrocelli & Lye, 1993; Chow & Lye, 1994; Sparey et al. 1999) in both rat and human. This suggests that the enhancement of Cx-43-mediated gap junctional communication between uterine myocytes is a relatively late event in parturition. This would allow for other regulatory mechanisms to be activated prior to further facilitation of electrical transmission of action potentials through the myometrium. It is possible that this development is an important factor in the transformation of weak and uncoordinated contractions present throughout pregnancy into the powerful coordinated contractions of active labour.
Our findings also strongly support the concept that uterine agonists that play important roles in parturition are produced within the decidua. Our data demonstrate an interesting pattern of expression of the OT gene, confirming our earlier studies utilizing ribonuclease protection assays to assess mRNA for OT (Fang et al. 1996). Peak expression of OT mRNA occurs by gestational day 18. However, in our earlier study, the significamt increase in OT protein did not occur until immediately prior to the increase in mRNA for OTR, which occurred concomitantly with the increase in OTR protein as assessed by radiolabelled-OT binding (Fang et al. 1996). This temporal sequence strongly supports a role for the OT system in the initiation of parturition in this species. In the rat and mouse, low concentrations of OT actually delay parturition, perhaps through a luteotropic effect (Antonijevic et al. 1995; Imamura et al. 2000). It is interesting that the increase in mRNA for OT was concurrent with the increase in PGHS-1, which regulates the initiation of luteolysis. Together, these findings suggest that OT may be an important factor in the precise timing mechanism of parturition.
Our data also suggest a potentially important role for ET-1 in the uterine activation process and the initiation of labour. Though previous studies have demonstrated the presence of ET-1 and ETA in the uterus (O’Reilly et al. 1992), we believe that these are the first data to systematically examine their expression around the time of uterine activation. ET-1 is a contractile stimulant for uterine smooth muscle (Kozuka et al. 1989; Word et al. 1992; Osada et al. 1997) and these effects are mediated through the ETA receptor isoform (Tsunoda et al. 1993; Osada et al. 1997). There is a significant increase in mRNA for ET-1 early in the activation process, concomitantly with OTR and FP. Expression of ETA peaks the following day.
We chose to use OT as our primary uterine agonist since it has a very high binding affinity in the myometrium (Soloff, 1975) and is the most potent physiological, contractile stimulant for the uterus. During late human pregnancy, there is an increased sensitivity of the human uterus in vivo to exogenous OT (Caldeyro-Barcia & Poseiro, 1959; Takahashi et al. 1980). This pattern is compatible with our myographic findings in the rat. Using OT as a uterine agonist, the EC50 increased significantly through gestation, peaking on the day preceding labour. Then, in concert with the increase in mRNA for OTR, and at the time of the increased tissue concentration of OT protein (Fang et al. 1996) and the decrease in mRNA for iNOS, there is a marked increase in sensitivity to OT. Though the OTR concentrations increased more than 50-fold, uterine sensitivity to OT increased only 2-fold. Our previous studies using a radioligand-binding assay estimated an increase in OTR protein of approximately 5-fold (Fang et al. 1996). This suggests that uterine sensitivity may be limited by translation of OTR, postreceptor signalling mechanisms or the ability of the contractile apparatus to respond.
Previous studies have reported variable effects of pregnancy on myometrial contractility. Myometrial strips from pregnant Wistar rats have an increased response to a 100 nm OT challenge compared to tissues from non-pregnant animals (Kim et al. 1998; Li et al. 2003). This was due largely to an increase in contractions amplitude. We also found an increase in the maximal response to OT in strips at term compared to non-pregnant animals and this was accompanied by a similar increase in spontaneous contractile activity. In both cases, this was due predominantly to increases in contraction amplitude rather than frequency. Interestingly, using permeabilized muscle strips, the absolute force-generation capability of the myometrium was similar in tissues from non-pregnant and late pregnant animals (Kim et al. 1998).
The data presented in Fig. 5 clearly demonstrate that, of the three uterine agonists tested, OT was by far the most potent with respect to uterine sensitivity. Though two-way ANOVA revealed a significant overall effect of pregnancy on the EC50 values, only for PGF2α was there a significant change in EC50 (P = 0.0014), which indicated a 4-fold reduction in uterine sensitivity to this agonist in late pregnancy. This is similar to previous data using uterine strips from non-pregnant and pregnant women at term or in labour (Word et al. 1992). We cannot explain the discrepancy between this finding and the increase in mRNA for FP. It is possible that the mRNA is not translated or that the receptor signal transduction pathway is disrupted. Overall, the myography data suggest that PGF2α is unlikely to be an important direct uterine contractile stimulant for parturition. For OT and ET-1, the changes in sensitivity between the tissues from non-pregnant and late pregnant rats were not significant when analysed individually (Fig. 5A–C). The apparent discrepancy between these data and those for OT in Fig. 3A may be explained by the fact that the former are from a slightly earlier gestational age. Previous studies have shown a synergy between OT and ET-1 wherein the latter enhances the responsiveness to the former in uterine strips from pregnant women at term (Valenzuela et al. 1995).
In the rat, maintenance of gestation is clearly dependent on maternal progesterone. The corpora lutea are the source of the elevated maternal progesterone concentrations throughout pregnancy and the process of luteolysis initiates parturition (Thorburn & Challis, 1979). Every mRNA transcript measured, except for iNOS and PGHS-1, increased with RU486 treatment. Previous studies have used progesterone or RU486 to demonstrate the suppressive effects of progesterone on the expression of genes encoding OTR, FP and Cx-43 (Soloff et al. 1983; Schumacher et al. 1990; Petrocelli & Lye, 1993; Wathes et al. 1996; Fang et al. 1997; Dong & Yallampalli, 2000). Our previous studies indicate that the effects of RU486 are measurable at the mRNA level by 6 h and the increase in protein by 12 h (Fang et al. 1997). On the basis of these studies, we chose the single time point of 24 h to capture the breadth of changes in gene expression. Little is known regarding steroid regulation of ETA in rat uterus. However, treatment of human uterine myocytes with medroxyprogesterone acetate decreased ETA receptor binding (Fomin et al. 1999) and progesterone treatment diminished the contractile response to ET-1 in uterine strips from postmenopausal women (Domali et al. 2005).
The uterine agonists were affected by RU486 in a fashion similar to their receptors. As expected, there was no change in expression of PGHS-1 by the antiprogestin. There was a significant increase in mRNA for OT following in vivo treatment with the antiprogestin RU486. This differs from our previous findings in the same strain of rats (Fang et al. 1997) where we found that such treatment actually prevented the increase that was observed in the placebo-treated control rats. The difference between the two studies may be due to the time of RU486 administration. In the former study, this was given on day 16 whereas in the present study it was administered on day 15. The increase following RU486 treatment was quite modest compared to the increase that took place in the current controls between gestational day 16 and 17. Unfortunately, we did not study a group of untreated rats at day 15 so we do not know what the increase would have been from day 15–16 without treatment. Treatment of non-pregnant rats with progesterone alone has no effect but synergizes with oestrogen treatment to markedly enhance uterine synthesis of mRNA for OT (Lefebvre et al. 1994). Our data strongly suggest that progesterone also suppresses synthesis of ET-1 in the rat uterus. In agreement with this, other studies have noted that progesterone withdrawal increases release of ET-1 from uterine-derived endothelial cells (Edlund et al. 2004) and synthesis of mRNA for ET-1 is much higher in the oestrogen-dominated follicular phase of the human menstrual cycle compared to the progesterone-dominated luteal phase (Kubota et al. 1995).
Little is known regarding the role of iNOS in regulating uterine contractility. However, as our data demonstrate, it is almost absent in uterine tissue from non-pregnant animals but increases rapidly through pregnancy before declining significantly prior to parturition (Ali et al. 1997; Bansal et al. 1997). For iNOS, there was a decrease with RU486 treatment but this did not reach statistical significance. Previous studies show conflicting evidence regarding the role of progesterone on expression of iNOS. In agreement with our studies, progesterone decreased and a progesterone antagonist increased mRNA for iNOS in rat cervical tissues (Marx et al. 2006). However, progesterone inhibited transcription of iNOS in murine macrophages (Miller et al. 1996) and intestinal epithelium (Salzman et al. 2000). Thus, regulation of iNOS is likely to be tissue specific. Its pattern of expression suggests that it may be an important factor in the maintenance of uterine quiescence through pregnancy.
The myographic changes in uterine function reflected the changes in mRNA. Following RU486 treatment, there was a significant increase in uterine sensitivity to OT to a level similar to active labour. However, there was no accompanying change in the maximal responses to OT following the antiprogesterone treatment.
Our concept of regulatory substances being produced in the decidua and having effects on adjacent smooth muscle is further supported by the present findings. All our experiments were intentionally performed with intact uterine tissues including both myometrium and endometrium with accompanying connective tissue and immunological cells. The placenta and fetal membrane tissues were scraped from the uterine tissue prior to preparation for the physiological or biochemical measurements. In selected samples, we attempted to gain some insight into the tissue of origin of the various mRNA transcripts by bluntly dissecting the endometrium from the myometrium and comparing the concentration of mRNA in each of the pairs of tissues. The data suggest that OTR and FP are predominantly of myometrial origin and OT and iNOS mostly from the endometrium. This is in agreement with previous studies that have demonstrated OTR in both endometrium and myometrium but predominantly in the latter for both rat and human (Alexandrova & Soloff, 1980a; Fuchs et al. 1982). We and others have measured OT protein and mRNA to determine its presence mainly in the endometrium in both rat and human (Lefebvre et al. 1992; Chibbar et al. 1993). Using western analyses, iNOS also has been localized mainly to the endometrial layer in rats and human (Buhimschi et al. 1996; Bansal et al. 1997). In many respects, the paracrine system in the uterus appears analogous to the well-documented relationship between the endothelium and vascular smooth muscle. The mRNA for PGHS-1 appeared to be predominantly in the decidua.
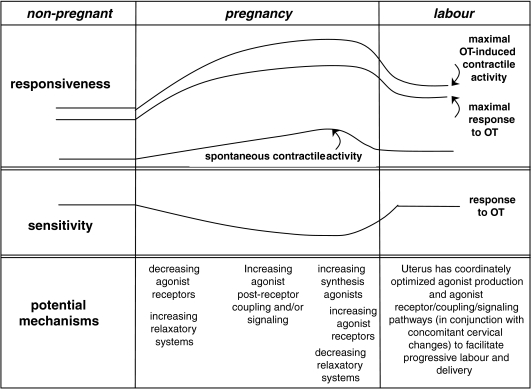
In conclusion, our study of sequential changes in uterine gene expression with the accompanying physiological assessments have provided a more integrated view of uterine contractility through pregnancy (Fig. 7). The sensitivity and maximal responsiveness to OT diminish significantly during the period of uterine quiescence. This is likely to be due to diminished expression of OTR and perhaps an increase in iNOS-mediated relaxation. Spontaneous contractility increases gradually through gestation, which is likely to be due to enhanced coupling between agonist receptors and their effector mechanisms. At the time of uterine activation, there is increased expression of uterine agonist genes and decreased relaxatory genes in the endometrium. This is accompanied by increased myometrial expression of agonist receptors and connexin-43 with a resultant increased sensitivity to OT. There is considerable support in the literature for a similar system in the pregnant human uterus, but further studies will be necessary to establish this with more certainty. In the rat model, these changes were progesterone dependent but it will be of interest to determine whether the parallel changes in human uterine myocytes are also influenced by progesterone. Clearer understanding of these paracrine and molecular mechanisms may foster development of improved methods for prevention, earlier diagnosis or more effective treatment for preterm labour.
Figure 7. Conceptual diagram of changes occurring in the uterus during pregnancy and labour.
Early in pregnancy the uterus becomes quiescent with a decrease in responsiveness to contractile agonists. This is accompanied by a decrease in agonist receptor expression and increased expression of relaxatory mechanisms. During pregnancy, there is gradually increasing postreceptor coupling of agonist receptors to the mechanisms that increase contractility leading to increasing spontaneous contractile activity and greater maximal response to OT. At the time of uterine activation, there is increased production of uterine agonists (such as OT and ET-1) from the endometrium, accompanied by increased expression of agonist receptors in the myometrium and decreased expression of relaxatory systems. At this time, there also is increased expression of other genes (e.g. Cx-43) that facilitate the coordination and efficiency of uterine contractions that are characteristic of active labour.
Acknowledgments
We gratefully acknowledge the support of the Canadian Institutes for Health Research (MOP 160799 to BFM). P.A. was supported by a Fellowship from the Perinatal Research Centre, University of Alberta and the CIHR Strategic Training Grant in Maternal, Fetal and Neonatal Health. We also are grateful to the staff of the Health Sciences Animal Facility for their care of the animals.
References
- Alexandrova M, Soloff MS. Oxytocin receptors and parturition. I. Control of oxytocin receptor concentration in the rat myometrium at term. Endocrinology. 1980a;106:730–735. doi: 10.1210/endo-106-3-730. [DOI] [PubMed] [Google Scholar]
- Alexandrova M, Soloff MS. Oxytocin receptors and parturition. II. Concentrations of receptors for oxytocin and estrogen in the gravid and nongravid uterus at term. Endocrinology. 1980b;106:736–738. doi: 10.1210/endo-106-3-736. [DOI] [PubMed] [Google Scholar]
- Ali M, Buhimschi I, Chwalisz K, Garfield RE. Changes in expression of the nitric oxide synthase isoforms in rat uterus and cervix during pregnancy and parturition. Mol Hum Reprod. 1997;3:995–1003. doi: 10.1093/molehr/3.11.995. [DOI] [PubMed] [Google Scholar]
- Al-Matubsi HY, Eis AL, Brodt-Eppley J, MacPhee DJ, Lye S, Myatt L. Expression and localization of the contractile prostaglandin F receptor in pregnant rat myometrium in late gestation, labor, and postpartum. Biol Reprod. 2001;65:1029–1037. doi: 10.1095/biolreprod65.4.1029. [DOI] [PubMed] [Google Scholar]
- Antonijevic IA, Douglas AJ, Dye S, Bicknell RJ, Leng G, Russell JA. Oxytocin antagonists delay the initiaion of parturition and prolong its active phase in rats. J Endocrinol. 1995;145:97–103. doi: 10.1677/joe.0.1450097. [DOI] [PubMed] [Google Scholar]
- Bansal RK, Goldsmith PC, He Y, Zaloudek CJ, Ecker JL, Riemer RK. A decline in myometrial nitric oxide synthase expression is associated with labor and delivery. J Clin Invest. 1997;99:2502–2508. doi: 10.1172/JCI119434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodt-Eppley J, Myatt L. Prostaglandin receptors in lower segment myometrium during gestation and labor. Obstet Gynecol. 1999;93:89–93. doi: 10.1016/s0029-7844(98)00378-0. [DOI] [PubMed] [Google Scholar]
- Buhimschi I, Ali M, Jain V, Chwalisz K, Garfield RE. Differential regulation of nitric oxide in the rat uterus and cervix during pregnancy and labour. Hum Reprod. 1996;11:1755–1766. doi: 10.1093/oxfordjournals.humrep.a019481. [DOI] [PubMed] [Google Scholar]
- Buhimschi I, Yallampalli C, Chwalisz K, Garfield RE. Pre-eclampsia-like conditions produced by nitric oxide inhibition: effects of L-arginine, D-arginine and steroid hormones. Hum Reprod. 1995;10:2723–2730. doi: 10.1093/oxfordjournals.humrep.a135775. [DOI] [PubMed] [Google Scholar]
- Caldeyro-Barcia R, Poseiro JJ. Oxytocin and contractility of the pregnant human uterus. Ann N Y Acad Sci. 1959;75:813–830. doi: 10.1111/j.1749-6632.1959.tb44593.x. [DOI] [PubMed] [Google Scholar]
- Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21:514–550. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- Chibbar R, Miller FD, Mitchell BF. Synthesis of oxytocin in amnion, chorion, and decidua may influence the timing of human parturition. J Clin Invest. 1993;91:185–192. doi: 10.1172/JCI116169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L, Lye SJ. Expression of the gap junction protein connexin-43 is increased in the human myometrium toward term and with the onset of labor. Am J Obstet Gynecol. 1994;170:788–795. doi: 10.1016/s0002-9378(94)70284-5. [DOI] [PubMed] [Google Scholar]
- Domali E, Molyvdas PA, Messinis IE. In vitro responsiveness of human post-menopausal myometrium to endothelin-1 and ovarian steroids. J Endocrinol Invest. 2005;28:485–493. doi: 10.1007/BF03347235. [DOI] [PubMed] [Google Scholar]
- Dong YL, Yallampalli C. Pregnancy and exogenous steroid treatments modulate the expression of relaxant EP2 and contractile FP receptors in the rat uterus. Biol Reprod. 2000;62:533–539. doi: 10.1095/biolreprod62.3.533. [DOI] [PubMed] [Google Scholar]
- Edlund M, Andersson E, Fried G. Progesterone withdrawal causes endothelin release from cultured human uterine microvascular endothelial cells. Human Reprod. 2004;19:1272–1280. doi: 10.1093/humrep/deh256. [DOI] [PubMed] [Google Scholar]
- Fang X, Wong S, Mitchell BF. Relationships among sex steroids, oxytocin, and their receptors in the rat uterus during late gestation and at parturition. Endocrinology. 1996;137:3213–3219. doi: 10.1210/endo.137.8.8754742. [DOI] [PubMed] [Google Scholar]
- Fang X, Wong S, Mitchell BF. Effects of RU486 on estrogen, progesterone, oxytocin, and their receptors in the rat uterus during late gestation. Endocrinology. 1997;138:2763–2768. doi: 10.1210/endo.138.7.5247. [DOI] [PubMed] [Google Scholar]
- Fomin VP, Cox BE, Word RA. Effect of progesterone on intracellular Ca2+ homeostasis in human myometrial smooth muscle cells. Am J Physiol Cell Physiol. 1999;276:C379–C385. doi: 10.1152/ajpcell.1999.276.2.C379. [DOI] [PubMed] [Google Scholar]
- Fuchs AR, Fuchs F, Husslein P, Soloff MS, Fernstrom MJ. Oxytocin receptors and human parturition: a dual role for oxytocin in the initiation of labor. Science. 1982;215:1396–1398. doi: 10.1126/science.6278592. [DOI] [PubMed] [Google Scholar]
- Grazzini E, Guillon G, Monillac B, Zingg HH. Inhibition of oxytocin receptor function by direct binding of progesterone. Nature. 1998;392:509–512. doi: 10.1038/33176. [DOI] [PubMed] [Google Scholar]
- Gross GA, Imamura T, Luedke C, Vogt SK, Olson LM, Nelson DM, Sadovsky Y, Muglia LJ. Opposing actions of prostaglandins and oxytocin determine the onset of murine labor. Proc Natl Acad Sci U S A. 1998;95:11875–11879. doi: 10.1073/pnas.95.20.11875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross G, Imamura T, Muglia LJ. Gene knockout mice in the study of parturition. J Soc Gynecol Invest. 2000;7:88–95. [PubMed] [Google Scholar]
- Imamura T, Luedke CE, Vogt SK, Muglia LJ. Oxytocin modulates the onset of murine parturition by competing ovarian and uterine effects. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1061–R1067. doi: 10.1152/ajpregu.2000.279.3.R1061. [DOI] [PubMed] [Google Scholar]
- Kim BK, Ozaki H, Hori M, Takahashi K, Karaki H. Increased contractility of rat uterine smooth muscle at the end of pregnancy. Comp Biochem Physiol A Mol Integr Physiol. 1998;121:165–173. doi: 10.1016/s1095-6433(98)10118-6. [DOI] [PubMed] [Google Scholar]
- Knudsen UB, Svane D, Forman A. Length tension relationships in the nonpregnant and pregnant rat uterus and the effect of antiprogestin. J Reprod Fertil. 1998;113:75–81. doi: 10.1530/jrf.0.1130075. [DOI] [PubMed] [Google Scholar]
- Kozuka M, Ito T, Hirose S, Takahashi K, Hagiwara H. Endothelin induces two types of contractions of rat uterus: phasic contractions by way of voltage-dependent calcium channels and developing contractions through a second type of calcium channels. Biochem Biophys Res Commun. 1989;159:317–323. doi: 10.1016/0006-291x(89)92440-6. [DOI] [PubMed] [Google Scholar]
- Kubota T, Taguchi M, Kamada S, Imai T, Hirata Y, Marumo F, Aso T. Endothelin synthesis and receptors in human endometrium throughout the normal menstrual cycle. Hum Reprod. 1995;10:2204–2208. doi: 10.1093/oxfordjournals.humrep.a136269. [DOI] [PubMed] [Google Scholar]
- Lefebvre DL, Farookhi R, Giaid A, Neculcea J, Zingg HH. Uterine oxytocin gene expression. II. Induction by exogenous steroid administration. Endocrinology. 1994;134:2562–2566. doi: 10.1210/endo.134.6.8194483. [DOI] [PubMed] [Google Scholar]
- Lefebvre DL, Giaid A, Bennett H, Lariviere R, Zingg HH. Oxytocin gene expression in rat uterus. Science. 1992;256:1553–1555. doi: 10.1126/science.1598587. [DOI] [PubMed] [Google Scholar]
- Li Y, Je HD, Malek S, Morgan KG. ERK1/2-mediated phosphorylation of myometrial caldesmon during pregnancy and labor. Am J Physiol Regul Integr Comp Physiol. 2003;284:R192–R199. doi: 10.1152/ajpregu.00290.2002. [DOI] [PubMed] [Google Scholar]
- Liu P, Ying Y, Ko YG, Anderson RG. Localization of platelet-derived growth factor-stimulated phosphorylation cascade to caveolae. J Biol Chem. 1996;271:10299–10303. doi: 10.1074/jbc.271.17.10299. [DOI] [PubMed] [Google Scholar]
- Lopez Bernal A, Rivera J, Europe-Finner GN, Phaneuf S, Asboth G. Parturition: activation of stimulatory pathways or loss of uterine quiescence? Adv Exp Med Biol. 1995;395:435–451. [PubMed] [Google Scholar]
- Marx SG, Wentz MJ, Mackay LB, Schlembach D, Maul H, Fittkow C, Given R, Vedernikov Y, Saade GR, Garfield RE. Effects of progesterone on iNOS, COX-2, and collagen expression in the cervix. J Histochem Cytochem. 2006;54:623–639. doi: 10.1369/jhc.5A6759.2006. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Sagawa N, Yoshida M, Mori T, Tanaka I, Mukoyama M, Kotani M, Nakao K. The prostaglandin E2 and F2 alpha receptor genes are expressed in human myometrium and are down-regulated during pregnancy. Biochem Biophys Res Commun. 1997;238:838–841. doi: 10.1006/bbrc.1997.7397. [DOI] [PubMed] [Google Scholar]
- Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab. 2002;87:2924–2930. doi: 10.1210/jcem.87.6.8609. [DOI] [PubMed] [Google Scholar]
- Miller L, Alley EW, Murphy WJ, Russell SW, Hunt JS. Progesterone inhibits inducible nitric oxide synthase gene expression and nitric oxide production in murine macrophages. J Leukoc Biol. 1996;59:442–450. doi: 10.1002/jlb.59.3.442. [DOI] [PubMed] [Google Scholar]
- Mitchell BF, Wong S. Changes in 17 beta,20 alpha-hydroxysteroid dehydrogenase activity supporting an increase in the estrogen/progesterone ratio of human fetal membranes at parturition. Am J Obstet Gynecol. 1993;168:1377–1385. doi: 10.1016/s0002-9378(11)90768-6. [DOI] [PubMed] [Google Scholar]
- Mitchell BF, Zielnik B, Wong S, Roberts CD, Mitchell JM. Intraperitoneal infusion of pro-inflammatory cytokines does not cause activation of the rat uterus during late gestation. Am J Physiol Endocrinol Metab. 2005;289:E658–664. doi: 10.1152/ajpendo.00058.2005. [DOI] [PubMed] [Google Scholar]
- Molnar M, Romero R, Hertelendy F. Interleukin-1 and tumor necrosis factor stimulate arachidonic acid release and phospholipid metabolism in human myometrial cells. Am J Obstet Gynecol. 1993;169:825–829. doi: 10.1016/0002-9378(93)90011-7. [DOI] [PubMed] [Google Scholar]
- O’Reilly G, Charnock-Jones DS, Davenport AP, Cameron IT, Smith SK. Presence of messenger ribonucleic acid for endothelin-1, endothelin-2, and endothelin-3 in human endometrium and a change in the ratio of ETA and ETB receptor subtype across the menstrual cycle. J Clin Endocrinol Metab. 1992;75:1545–1549. doi: 10.1210/jcem.75.6.1464662. [DOI] [PubMed] [Google Scholar]
- Okawa T, Asano K, Takahashi H, Hashimoto S, Anbe H, Sato A, Vedernikov YP, Saade GR, Garfield RE. Expression of inducible nitric oxide synthase messenger RNA, but not guanylate cyclase messenger RNA, depends on gestational age in rat myometrium. Gynecol Endocrinol. 2004;19:146–151. doi: 10.1080/09153590400007317. [DOI] [PubMed] [Google Scholar]
- Okawa T, Syal AS, Vedernikov YP, Saade GR, Chwalisz K, Garfield RE. The effects of nitric oxide on the contractility of isolated uterine and aortic rings from pregnant rats. Am J Obstet Gynecol. 1998;179:721–726. doi: 10.1016/s0002-9378(98)70071-7. [DOI] [PubMed] [Google Scholar]
- Osada K, Tsunoda H, Miyauchi T, Sugishita Y, Kubo T, Goto K. Pregnancy increases ET-1-induced contraction and changes receptor subtypes in uterine smooth muscle in humans. Am J Physiol Regul Integr Comp Physiol. 1997;272:R541–R548. doi: 10.1152/ajpregu.1997.272.2.R541. [DOI] [PubMed] [Google Scholar]
- Ou CW, Chen ZQ, Qi S, Lye SJ. Expression and regulation of the messenger ribonucleic acid encoding the prostaglandin F2α receptor in the rat myometrium during pregnancy and labor. Am J Obstet Gynecol. 2000;182:919–925. doi: 10.1016/s0002-9378(00)70347-4. [DOI] [PubMed] [Google Scholar]
- Petrocelli T, Lye SJ. Regulation of transcripts encoding the myometrial gap junction protein, connexin-43, by estrogen and progesterone. Endocrinology. 1993;133:284–290. doi: 10.1210/endo.133.1.8391423. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman AL, Linn SC, Szabo C. Progesterone inhibits inducible nitric oxide synthase mRNA expression in human intestinal epithelial cells. Int J Mol Med. 2000;6:209–216. doi: 10.3892/ijmm.6.2.209. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Coirini H, Pfaff DW, McEwen BS. Behavioural effects of progesterone associated with rapid modulation of oxytocin receptors. Science. 1990;250:691–694. doi: 10.1126/science.2173139. [DOI] [PubMed] [Google Scholar]
- Soloff MS. Uterine receptor for oxytocin: effects of estrogen. Biochem Biophys Res Commun. 1975;65:205–212. doi: 10.1016/s0006-291x(75)80080-5. [DOI] [PubMed] [Google Scholar]
- Soloff MS, Fernstrom MA, Periyasamy S, Soloff S, Baldwin S, Wieder M. Regulation of oxytocin receptor concentration in rat uterine explants by estrogen and progesterone. Can J Biochem Cell Biol. 1983;61:625–630. doi: 10.1139/o83-078. [DOI] [PubMed] [Google Scholar]
- Sparey C, Robson SC, Bailey J, Lyall F, Europe-Finner GN. The differential expression of myometrial connexin-43, cyclooxygenase-1 and -2, and Gs α proteins in the upper and lower segments of the human uterus during pregnancy and labor. J Clin Endocrinol Metab. 1999;84:1705–1710. doi: 10.1210/jcem.84.5.5644. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Diamond F, Bieniarz J, Yen H, Burd L. Uterine contractility and oxytocin sensitivity in preterm, term, and postterm pregnancy. Am J Obstet Gynecol. 1980;136:774–779. doi: 10.1016/0002-9378(80)90455-x. [DOI] [PubMed] [Google Scholar]
- Thorburn GD, Challis JR. Endocrine control of parturition. Physiol Rev. 1979;59:863–918. doi: 10.1152/physrev.1979.59.4.863. [DOI] [PubMed] [Google Scholar]
- Tsunoda H, Miyauchi T, Fujita K, Kubo T, Goto K. Mechanism of rat uterine smooth muscle contraction induced by endothelin-1. Br J Pharmacol. 1993;110:1437–1446. doi: 10.1111/j.1476-5381.1993.tb13981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela GJ, Hewitt CW, Ducsay CA. Endothelin-1 potentiates the in vitro contractile response of pregnant human myometrium to oxytocin. Am J Obstet Gynecol. 1995;172:1573–1576. doi: 10.1016/0002-9378(95)90499-9. [DOI] [PubMed] [Google Scholar]
- Wathes DC, Mann GE, Payne JH, Riley PR, Stevenson KR, Lamming GE. Regulation of oxytocin, oestradiol and progesterone receptor concentrations in different uterine regions by oestradiol, progesterone and oxytocin in ovariectomized ewes. J Endocrinol. 1996;151:375–393. doi: 10.1677/joe.0.1510375. [DOI] [PubMed] [Google Scholar]
- Word RA, Kamm KE, Casey ML. Contractile effects of prostaglandins, oxytocin, and endothelin-1 in human myometrium in vitro: refractoriness of myometrial tissue of pregnant women to prostaglandins E2 and F2α. J Clin Endocrinol Metab. 1992;75:1027–1032. doi: 10.1210/jcem.75.4.1400867. [DOI] [PubMed] [Google Scholar]
- Zakar T, Hertelendy F. Progesterone withdrawal: key to parturition. Am J Obstet Gynecol. 2007;196:289–296. doi: 10.1016/j.ajog.2006.09.005. [DOI] [PubMed] [Google Scholar]