Abstract
The membrane-associated guanylate kinases (MAGUKs) PSD-95, PSD-93 and SAP102 are thought to have crucial roles in both AMPA receptor trafficking and formation of NMDA receptor-associated signalling complexes involved in synaptic plasticity. While PSD-95, PSD-93, and SAP102 appear to have similar roles in AMPA receptor trafficking, it is not known whether these MAGUKs also have functionally similar roles in synaptic plasticity. To explore this issue we examined several properties of basal synaptic transmission in the hippocampal CA1 region of PSD-93 and PSD-95 mutant mice and compared the ability of a number of different synaptic stimulation protocols to induce long-term potentiation (LTP) and long-term depression (LTD) in these mutants. We find that while both AMPA and NMDA receptor-mediated synaptic transmission are normal in PSD-93 mutants, PSD-95 mutant mice exhibit clear deficits in AMPA receptor-mediated transmission. Moreover, in contrast to the facilitation of LTP induction and disruption of LTD observed in PSD-95 mutant mice, PSD-93 mutant mice exhibit deficits in LTP and normal LTD. Our results suggest that PSD-95 has a unique role in AMPA receptor trafficking at excitatory synapses in the hippocampus of adult mice and indicate that PSD-93 and PSD-95 have essentially opposite roles in LTP, perhaps because these MAGUKs form distinct NMDA receptor signalling complexes that differentially regulate the induction of LTP by different patterns of synaptic activity.
Excitatory synapses in the brain are characterized by a dense network of proteins called the postsynaptic density (PSD), which contains transmembrane receptors, scaffold proteins, and signalling molecules. Four members of a family of scaffold proteins called membrane-associated guanylate kinases (MAGUKs) are abundantly expressed in the PSD: PSD-95/SAP-90/dLg4, PSD-93/chapsyn-110/dLg2, SAP-97/dLg1 and SAP102/dLg3. Each contains three PDZ domains, an SH3 domain, and a catalytically inactive guanylate kinase domain which together mediate protein–protein interactions important for channel clustering and recruitment of signalling complexes (Kim & Sheng, 2004). Importantly, MAGUKs associate with a host of signalling proteins which coalesce into large complexes of approximately 2 MDa called MAGUK-associated signalling complexes (MASCs) (Husi et al. 2000; Collins et al. 2005; Dosemeci et al. 2007). Moreover, through their PDZ domains, PSD-93, PSD-95 and SAP102 bind to the C-terminus of N-methyl-d-aspartate receptor (NMDAR) type-2 subunits (Kornau et al. 1995) and thus couple NMDARs to MASCs. This suggests that MAGUKs couple synaptic NMDARs to signalling complexes that regulate activity-dependent changes in synaptic strength. Indeed, genetic and pharmacological disruption of numerous MASC proteins results in defects in synaptic plasticity and learning as well as human brain diseases (Pocklington et al. 2006; Grant et al. 2005; http://www.genes2cognition.org). Moreover, mutations in genes encoding SAP102 and PSD-93 have recently been identified in X-linked mental retardation (Tarpey et al. 2004) and schizophrenia (Walsh et al. 2008), respectively. Decreased hippocampal levels of PSD-95 are also found in schizophrenia (Toro & Deakin, 2005).
MAGUKs also interact with α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR) binding proteins (TARPs), which play a pivotal role in AMPAR trafficking at excitatory synapses (Nicoll et al. 2006). Manipulation of MAGUK levels in cultured neurons shows that they strongly influence AMPAR content in synapses, probably through TARP binding (Elias & Nicoll, 2007). For example, overexpression of PSD-95, PSD-93 or SAP102 increases AMPAR incorporation into excitatory synapses (El-Husseini et al. 2000; Schnell et al. 2002; Béïque & Andrade, 2003; Stein et al. 2003; Ehrlich & Malinow, 2004; Nakagawa et al. 2004; Elias et al. 2006). Conversely, acute shRNA-mediated knockdown of either PSD-95 or PSD-93 decreases AMPAR-mediated synaptic transmission in cultured hippocampal pyramidal cells (Elias et al. 2006). Thus, these MAGUKs appear to have similar roles in recruiting AMPARs to synapses (Elias & Nicoll, 2007).
There is now abundant evidence that MAGUKs also participate in NMDAR-dependent forms of synaptic plasticity such as long-term potentiation (LTP) and long-term depression (Migaud et al. 1998; Béïque & Andrade, 2003; Colledge et al. 2003; Stein et al. 2003; Yao et al. 2004; Béïque et al. 2006; Cuthbert et al. 2007). It is unclear, however, whether different MAGUKs have unique roles in LTP despite their apparently overlapping roles in AMPAR trafficking. To address this issue we examined properties of basal synaptic transmission in PSD-95 and PSD-93 knockout mice as well as the ability of different synaptic stimulation protocols to induce LTP in the hippocampal CA1 region in these mutants. We find that AMPAR-mediated basal synaptic transmission is impaired in PSD-95 mutants but normal in PSD-93 mutant mice. Thus, PSD-95 has a unique role in AMPAR trafficking at excitatory synapses in the adult hippocampus which is not apparent in cultured neurons. Moreover, we find that PSD-93 knockout mice exhibit deficits in LTP and normal LTD, in contrast to the facilitated LTP and impaired LTD observed in the absence of PSD-95. Thus, PSD-95 and PSD-93 appear to have distinct roles in synaptic plasticity, perhaps through differential recruitment of signalling molecules to the NMDAR.
Methods
Slice preparation and extracellular recording
Hippocampi were obtained from mice that were deeply anaesthetized with halothane and then killed by cervical dislocation. Hippocampal slices (400 μm thick) were then prepared using standard techniques and maintained in an interface-slice type recording chamber (at 30°C) perfused with oxygenated (95% O2–5% CO2) artificial cerebrospinal fluid (ACSF) containing 124 mm NaCl, 4.4 mm KCl, 25 mm NaHCO3, 1 mm NaH2PO4, 2 mm CaCl2, 1.2 mm MgSO4 and 10 mm glucose. All techniques were approved by the UCLA Institutional Animal Care and Use Committee and done in accordance with UK Home Office Guidelines. After allowing slices to recover for at least 1 h, a bipolar, nichrome wire stimulating electrode was placed in stratum radiatum of the hippocampal CA1 region to activate Schaffer collateral–commissural fibre synapses and an extracellular glass microelectrode filled with ACSF (resistance = 5–10 MΩ) was used to record evoked field excitatory postsynaptic potentials (fEPSPs). All extracellular recordings were done under interface conditions. The intensity of presynaptic fibre stimulation was adjusted to evoke fEPSPs with amplitude approximately 50% of the maximal fEPSP amplitude that could be elicited in each slice. fEPSPs were then elicited at 0.02 Hz throughout the experiment. The theta pulse stimulation (TPS) trains used in the majority of LTP experiments consisted of single pulses of presynaptic fibre stimulation delivered at 5 Hz. The average fEPSP slope measured between 40 and 45 min post-TPS was used for statistical comparisons (two tailed t tests, n = number of animals in each group). A conventional low-frequency stimulation (LFS) protocol (1 Hz for 15 min) was used to induced long-term depression (LTD). In these experiments slices were submerged in a modified ACSF containing 4.0 mm CaCl2 to facilitate the induction of LTD in adult slices (Norris et al. 1996; Delgado et al. 2007) and the average slope of fEPSPs measured between 55 and 60 min post-LFS was used for statistical comparisons (two tailed t tests). The PSD-95 (Migaud et al. 1998) and PSD-93 mutant mice (McGee et al. 2001; Elias et al. 2006) used in our experiments were between 4 and 12 months of age. In some experiments (Figs 5 and 6) we also used slices obtained from male C57Bl/6 mice (6–12 weeks of age).
Figure 5. Pairing EPSPs with a single postsynaptic action potential (AP) does not induce LTP in pyramidal cells in hippocampal CA1 region of adult wild-type mice.
A, examples of responses during the single spike (left) and burst pairing (right) used in these experiments. B, 100 pulses of presynaptic fibre stimulation (delivered at time = 0) were paired with single postsynaptic action potentials (○, n = 12 cells) or a burst of postsynaptic action potentials (•, n = 13 cells). Postsynaptic spikes were elicited 10 ms after EPSP onset and pairing was done at 5 Hz. The inset shows EPSPs recorded during baseline and 30 min post-pairing in an experiment where EPSPs were paired with single postsynaptic action potentials (left) or a burst of postsynaptic action potentials (right). Calibration bars are 20 ms and 5 mV. B, summary of several experiments like that shown in A where EPSP-spike pairing was performed at different stimulation frequencies. While EPSPs paired with bursts of postsynaptic action potentials induce robust LTP, EPSPs paired with single postsynaptic action potentials failed to induce LTP at all frequencies tested (n = 4–12 cells per group). **P < 0.01 compared to baseline, *P < 0.05 compared to baseline. Postsynaptic infusion of the Na+ channel blocker QX314 blocked the induction of LTP by pairing EPSPs with bursts of postsynaptic action potentials (shaded bar, n = 4).
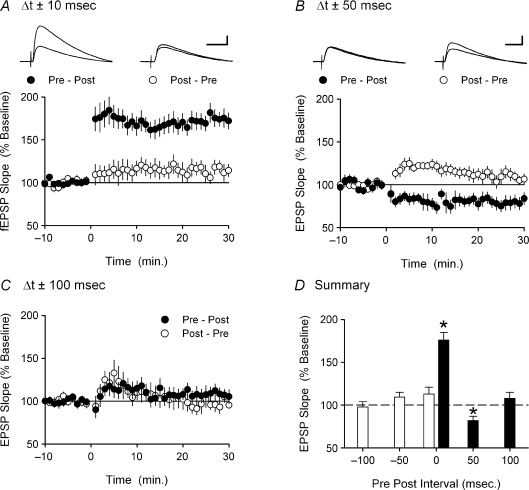
Figure 6. Spike timing-dependent changes in synaptic strength induced by EPSP–burst pairing.
A, presynaptic fibre stimulation followed 10 ms later by a burst of postsynaptic action potentials induces LTP (•, n = 12) while postsynaptic bursting elicited 10 ms prior to presynaptic fibre stimulation has no lasting effect on synaptic strength (○, n = 7). B, increasing the time interval between EPSPs and postsynaptic bursting to 50 ms elicits LTD when postsynaptic bursts follow presynaptic fibre stimulation (•, n = 7) but has no lasting effect on synaptic strength when postsynaptic bursting precedes presynaptic fibre stimulation (○, n = 10). C, neither pre then post (•, n = 6) nor post then pre (○, n = 5) pairing has any lasting effect on synaptic transmission when presynaptic fibre stimulation and postsynaptic bursting are separated by 100 ms. D, summary of the effects of spike timing and order on changes in synaptic strength induced by EPSP–burst pairing. Values correspond to the mean ±s.e.m. change in synaptic transmission measured 30 min post-pairing (*P < 0.01).
Whole-cell recordings
Low resistance (2–6 MΩ) patch electrodes filled with a solution containing 127.5 mm potassium gluconate, 17.4 KCl, 1.0 MgCl2, 0.2 mm EGTA, 2 mm Mg-ATP, 0.3 mm GTP and 10 mm Hepes (pH 7.2) were used to record excitatory postsynaptic potentials (EPSPs). In some experiments we also used an electrode-filling solution containing 120 mm potassium gluconate, 20 mm KCl, 2 mm MgCl2, 4 mm Na2-ATP, 0.3 mm Tris-GTP, 14 mm phosphocreatine and 10 mm Hepes (pH 7.3). Results from experiments using these two electrode solutions were the same and were therefore combined. In all experiments slices were maintained in a submerged-slice recording chamber and picrotoxin (100 μm) was added to the ACSF to block inhibitory synaptic transmission. To prevent bursting in the absence of inhibition, the CA3 region of the slices was removed and slices were bathed in a modified ACSF containing 4.0 mm CaCl2, 4.0 mm MgSO4 and 2.4 mm KCl. EPSPs were elicited once every 20 s using a stimulation intensity that evoked approximately 5–8 mV EPSPs. Constant injections of hyperpolarizing current were used to maintain membrane potentials between −70 and −80 mV and both input and series resistance were monitored throughout the experiment by a 50 ms-long pulse of hyperpolarizing current delivered 150–170 ms after each evoked EPSP. To study the effects of EPSPs paired with different patterns of postsynaptic action potentials we paired 100 EPSPs (evoked at 2–20 Hz) with either single action potentials elicited by a short (5–7 ms) pulse of depolarizing current (0.5–1.0 nA) or a burst of three to four postsynaptic action potentials elicited by a 50 ms pulse of depolarizing current. In both cases the timing of presynaptic fibre stimulation and postsynaptic current injection was adjusted so that postsynaptic action potentials (first spike in the case of bursts) followed EPSP onset by approximately 10 ms (range 8–12 ms). Cells that failed to show consistent bursting (2 or more action potentials) throughout the pairing protocol were discarded. In other experiments we varied the timing and order of pre- and postsynaptic stimulation to study the properties of spike timing-dependent plasticity (STDP) induced by pairing EPSPs with bursts of postsynaptic action potentials. Pairing was done at resting membrane potential (−56 to −64 mV) and performed within 20 min of whole-cell recording. There were no obvious differences in depolarization-induced action potential firing during pairing in wild-type, PSD-93−/−, and PSD-95 mutant cells. Statistical comparisons (Student's two-tailed t test) were done using the average EPSP slope measured 25–30 min post-pairing. To examine how the intensity of presynaptic fibre stimulation used in our whole cell recordings compares to that used in our extracellular recordings we used dual, extra- and intracellular recordings to simultaneously record fEPSPs and intracellular EPSPs in the same slice. In seven separate experiments we found that presynaptic fibre stimulation intensities that evoked half-maximal fEPSPs elicited ∼12 mV EPSPs in individual pyramidal cells (EPSPs were 12 ± 0.6 mV in amplitude, mean ±s.e.m.). Thus the presynaptic fibre stimulation intensity used in our whole-cell experiments was considerably less than that used in our extracellular recording experiments.
Whole-cell voltage-clamp techniques were used to record AMPAR and NMDAR-mediated excitatory postsynaptic currents (EPSCs). In general the techniques used in these experiments were similar to those described above except that the patch electrodes were filled with a solution containing 102 mm caesium gluconate, 17.5 CsCl, 10 mm TEA-Cl, 5 mm QX314, 4.0 mm Mg-ATP, 0.3 mm Tris-GTP, and 20 mm Hepes (pH 7.2). EPSCs evoked by presynaptic fibre stimulation (0.2 Hz) were recorded at membrane potentials of −80 or +40 mV and the AMPAR and NMDAR-mediated components of the synaptic currents were estimated by measuring EPSC amplitude 5 and 50 ms after EPSC onset, respectively. In these experiments the intensity of presynaptic fibre stimulation was adjusted to elicit EPSCs (at −80 mV) with peak amplitudes of approximately 200 pA. In addition, we also measured the relative contributions of AMPARs and NMDARs to the postsynaptic currents by recording EPSCs at +40 mV before and after bath application of the NMDAR antagonist d-APV (100 μm). The pharmacologically isolated AMPAR-mediated current was then subtracted from the compound EPSC recorded in the absence of APV to give the NMDAR-mediated component of the EPSC. Statistical comparisons in these experiments were performed using ANOVAs followed by Student–Newman–Keuls tests for multiple pairwise comparisons. Miniature EPSCs (mEPSCs) were recorded at −80 mV in cells bathed in a modified ACSF containing 4.0 mm CaCl2, 2.4 mm MgSO4, 2.4 mm KCl, 100 μm picrotoxin, and 0.5–0.75 μm tetrodotoxin. mEPSCs were analysed using a template-based event detection routine in pCLAMP 10 (Molecular Devices, Union City, CA, USA) and a threshold of 6 pA. Statistical comparisons of mEPSC amplitude and interevent interval distributions were performed using the Kolmogorov–Smirnov test.
Breeding and genotyping of mutant mice
Homozygous knockout mice were generated from heterozygous intercrosses of PSD-95 mutant mice (Migaud et al. 1998) and PSD-93 mutant mice (McGee et al. 2001) that were backcrossed > 10 generations on a C57BL/6 background. Genotyping using PCR protocols were performed as published (Migaud et al. 1998; McGee et al. 2001). The PSD-95 mutants used in our experiments were generated by introducing a stop codon into the third PDZ domain and thus express a truncated version of PSD-95 containing the N-terminus and first two PDZ domains (Migaud et al. 1998). The truncated protein is absent from excitatory synapses (Migaud et al. 1998), most likely because of the absence of C-terminal portions of the protein required for synaptic localization of PSD-95 (Xu et al. 2008).
Results
Basal synaptic function is impaired in PSD-95 mutants but normal in PSD-93 KO mice
To explore the roles of PSD-95 and PSD-93 in basal synaptic function we first compared the input/output function for basal synaptic transmission in slices from these MAGUK mutants and their wild-type littermates. In these experiments the size of presynaptic fibre volleys (input) was compared to the slopes of fEPSPs (output) measured from responses generated by different intensities of presynaptic fibre stimulation. Consistent with previous findings (Elias et al. 2006), the input/output curves from PSD-93−/− and wild-type mice were not significantly different indicating that PSD-93 is not required for normal levels of basal synaptic transmission in the hippocampal CA1 region (Fig. 1A and B). Paired-pulse facilitation was, however, significantly enhanced in slices from PSD-93−/− mutant mice (Fig. 1C). This is similar to the increase in paired-pulse facilitation seen in PSD-95 mutants (Fig. 1C) and suggests that there may be changes in presynaptic neurotransmitter release probability in these mutants.
Figure 1. Properties of basal synaptic transmission in PSD-93−/− and PSD-95 mutant mice.
A, left, input/output curves were generated by comparing fibre volley amplitude (mV) to field EPSP slope (in mV ms−1) across four different intensities of presynaptic fibre stimulation that elicited field EPSPs corresponding to 25, 50, 75 and 100% of the maximum field EPSP amplitude. The responses recorded in slices from PSD-93−/− mice (▲, n = 15 slices from 6 mice) are similar to those seen in slices from wild-type mice (○, n = 15 slices from 9 mice). In contrast, the input/output curve is right-shifted in slices from PSD-95 mutants (•, n = 10 slices from 4 mice). Right, example fEPSPs recorded in slices from wild-type, PSD-93−/− and PSD-95 mutant mice. Calibration bars are 2 mV and 2 ms. B, fibre volley/fEPSP slope ratios for each of the stimulation intensities shown in A. A repeated-measure ANOVA revealed a significant main effect of genotype and follow up Dunnett t test comparisons showed that ratios from PSD-95 mutants were significantly different (*P < 0.01) from wild-type values at all stimulation strengths. C, paired-pulse facilitation is enhanced in the hippocampal CA1 region of PSD-93−/− mutant mice. Pairs of presynaptic fibre stimulation pulses were delivered with interpulse intervals of 25, 50, 100, or 200 ms. Significantly greater paired-pulse facilitation is seen at interpulse intervals between 25 and 100 ms in slices from PSD-93−/− mutant mice (▲, n = 14 slices from 7 mice) and PSD-95 mutant mice (•, n = 13 slices from 4 mice, *P < 0.02, #P < 0.01 compared to wild-type littermates). The open circles show paired-pulse facilitation in slices from all wild-type mice (n = 27 slices from 11 mice).
Although some studies of basal synaptic transmission have failed to find deficits in AMPAR-mediated responses in PSD-95 mutants (Migaud et al. 1998; Elias et al. 2006), a more recent study has reported that AMPAR-mediated synaptic currents are significantly reduced in PSD-95 mutant mice (Béïque et al. 2006). Consistent with this later finding, input/output curves in slices from PSD-95 mutant mice were significantly shifted to the right compared to wild-type slices indicating that AMPAR-mediated basal synaptic transmission is altered in PSD-95 mutant mice (Fig. 1A and B). We reported previously that peak fEPSP amplitudes are the same in PSD-95 mutants compared to wild-type littermates and concluded that basal, AMPAR-mediated synaptic transmission was normal (Migaud et al. 1998). Although there are a number of variables (genetic background, animal age) that could explain the different results from our current and previous studies, we reanalysed our previously published data to determine whether we may have missed an alteration in basal synaptic strength in these mutants. Using data from previous experiments where fEPSPs were evoked using presynaptic fibre stimulation intensities that elicited responses with amplitudes equal to 50% of the maximal fEPSP amplitude, we found that fEPSP slopes were not significantly different between wild-type and PSD-95 mutant mice (slopes were 2.5 ± 0.06 mV ms−1 in wild-type slices, n = 69 slices from 17 mice, and were 2.5 ± 0.08 mV ms−1 in slices from PSD-95 mutants, n = 70 slices from 16 mice, P = 0.98). However, although equivalent postsynaptic responses were elicited, presynaptic fibre volley amplitudes were significantly larger in slices from PSD-95 mutant mice (fibre volley amplitudes were 0.48 ± 0.04 mV in wild-type slices compared to 1.1 ± 0.06 mV in slices from PSD-95 mutant mice, P < 0.001). Thus, reanalysis of our previous data from these PSD-95 mutant mice is consistent with our current observations that these mutants exhibit deficits in basal synaptic transmission.
To examine the deficit in basal synaptic transmission in PSD-95 mutants in more detail we next used whole-cell voltage-clamp techniques to compare the relative contribution of AMPA and NMDA receptors to EPSCs in CA1 pyramidal cells from wild-type and MAGUK mutant mice. As shown in Fig. 2A, the ratio of NMDAR- to AMPAR-mediated EPSCs estimated by the magnitude of the synaptic current measured 50 and 5 ms post-EPSC onset, respectively, was the same in wild-type (n = 28 cells from 6 mice) and PSD-93−/− cells (n = 25 cells from 4 mice). However, the NMDAR/AMPAR ratios at +40 mV were significantly larger in cells from PSD-95 mutant mice (n = 21 cells from 4 mice, P < 0.01 compared to wild-type) (Fig. 2A). Although the increase in the NMDAR/AMPAR ratios could be due to either a decrease in AMPAR-mediated currents or an increase in NMDAR-mediated currents, the significant rightward shift in the input/output observed in slices from PSD-95 mutant mice suggests that the increase in NMDAR/AMPAR ratios for the synaptic currents measured in PSD-95 mutants is most likely to be due to deficits in AMPAR-mediated synaptic transmission. To confirm this we also compared pharmacologically isolated AMPAR-mediated EPSCs in wild-type and MAGUK mutant mice. As shown in Fig. 2B, the ratio of AMPAR- to NMDAR-mediated synaptic currents was significantly decreased in pyramidal cells from PSD-95 mutant mice but normal in PSD-93−/− mice. Because AMPARs lacking edited GluR2 subunits exhibit pronounced inward rectification (Jonas & Burnashev, 1995), it is possible that a change in the subunit composition of synaptic AMPARs resulting in an increase in GluR2-lacking AMPARs could account for the decrease in AMPAR-mediated EPSCs measured at +40 mV in PSD-95 mutants. We found, however, that the GluR2-lacking AMPAR blocker IEM 1460, which strongly inhibits synaptic transmission in GluR2 null mutant mice (Gray et al. 2007), had no effect on synaptic transmission in PSD-95 mutant mice (after a 20 min bath application of 100 μm IEM 1460 fEPSPs were 99 ± 3% of baseline in slices from PSD-95 mutant mice, n = 5 slices from 5 mice, compared to 98 ± 2% of baseline in slices from wild-type controls, n = 4 slices from 3 mice).
Figure 2. AMPAR-mediated synaptic transmission is normal in PSD-93−/− mice but disrupted in PSD-95 mutant mice.
A, the ratio of NMDAR- to AMPAR-mediated synaptic currents is altered in PSD-95 mutant mice. Excitatory postsynaptic currents (EPSCs) were recorded at membrane potentials of −80 and +40 mV and AMPAR- and NMDAR-mediated components of the EPSCs were estimated from the magnitude of the currents at 5 and 50 ms post EPSC onset. Although NMDAR/AMPAR ratios for wild-type (n = 28 cells from 6 mice) and PSD-93−/− cells (n = 25 cells from 4 mice) are the same at both membrane potentials, the NMDAR/AMPAR ratio at +40 mV was significantly larger in cells from PSD-95 mutant mice (n = 21 cells from 4 mice, *P < 0.01 compared to wild-type). The inset at right shows example EPSCs recorded at −80 and +40 mV in cells from a wild-type (left), PSD-93−/− (middle) and PSD-95 mutant mice (right). Calibration bars are 20 ms and 100 pA. B, NMDAR- and AMPAR-mediated components of EPSCs were examined by using the NMDAR antagonist APV to pharmacologically isolate AMPAR-mediated currents. Cells were voltage-clamped at +40 mV and EPSCs were recorded before and after bath application of 100 μm d-APV. The NMDAR-mediated component of the EPCS was determined by subtracting the AMPAR-component recorded in the presence of APV from the compound EPSC recorded in the absence of APV. Although the ratio of AMPAR- to NMDAR-mediated currents is similar in wild-type (open bars, n = 15 cells from 5) and PSD-93−/− cells (n = 13 cells from 3 mice), it is significantly reduced in PSD-95 mutant cells (n = 15 cells from 4 mice, P < 0.01 compared to wild-type). The traces show example EPSCs recorded in a wild-type (top), PSD-93−/− (middle), and PSD-95 mutant cells (bottom). Calibration bars are 10 ms and 50 pA. C, cumulative probability distributions for amplitudes (left) and interevent intervals of mEPSCs recorded in wild-type (○, n = 19 cells from 4 mice) and PSD-95 mutant cells (•, n = 19 cells from 3 mice). Although the mEPSC amplitude distribution is similar in wild-type and PSD-95 mutant cells, mEPSC frequency is strongly reduced in PSD-95 mutant cells (P-values from Kolmogorov–Smirnov test). Traces show example mEPSCs recorded in a wild-type cell (left) and a PSD-95 mutant cell (right). Calibration bars are 10 pA and 100 ms.
Finally, we also examined the deficit in AMPAR-mediated synaptic transmission in PSD-95 mutant mice by comparing the amplitude and frequency of mEPSCs in CA1 pyramidal cells from mutant and wild-type mice. As shown in Fig. 2C, although the mean amplitude of mEPSCs in PSD-95 mutant cells tended to be somewhat smaller than that seen in wild-type cells (mean amplitudes were 10.5 ± 0.2 pA in PSD-95 mutant cells, n = 19 cells from 3 mice, compared to 11.1 ± 0.2 pA in wild-type cells, n = 19 cells from 4 mice), the distribution of mEPSC amplitudes in PSD-95 mutant cells was not significantly different from wild-type cells (Fig. 2C). There was, however, a pronounced reduction in mEPSC frequency in PSD-95 mutant cells (mean mEPSC frequency in wild-type cells was 5.9 ± 0.2 Hz compared to 1.5 ± 0.2 Hz in PSD mutant cells, P < 0.01). The reduction of mEPSC frequency in pyramidal cells from PSD-95 mutant cells is consistent with the results of a recent study showing that the number of silent synapses is increased in PSD-95 mutant mice (Béïque et al. 2006). However, because paired-pulse facilitation is also enhanced in PSD-95 mutants (Fig. 1C), it seems likely that a decrease in presynaptic neurotransmitter release probability also contributes to the reduction in mEPSC frequency.
TPS-induced LTP is facilitated in PSD-95 mutant and impaired in PSD-93 KO mice
Theta frequency (5 Hz) stimulation of presynaptic fibres in the hippocampal CA1 region can induce a long-lasting potentiation of synaptic transmission that, like high-frequency stimulation (HFS)-induced LTP, is NMDAR-dependent (Thomas et al. 1999). A unique feature of TPS-induced LTP, however, is that the magnitude of LTP induced by 5 Hz stimulation exhibits an ‘inverted U’ shaped dependence on the duration of stimulation (Thomas et al. 1996, 1999). As shown in Fig. 3A, a 5 s-long train of TPS has little lasting effect on synaptic strength in wild-type slices, presumably because the levels of pre- and postsynaptic activity produced by this brief train of TPS are below threshold for LTP induction. In contrast, increasing the duration of TPS to 15 s induces significant LTP (fEPSPs were potentiated to 157 ± 9% of baseline, n = 6, P < 0.001 compared to baseline) (Fig. 3B). Although significant LTP can also be induced by a 30 s-long train of TPS (fEPSPs were potentiated to 152 ± 8% of baseline, n = 5, P < 0.002), no LTP is induced when the duration of TPS is further increased to 60 s (Fig. 3C, fEPSPs were 117 ± 13% of baseline, n = 5). Previously we found that inhibitors of protein phosphatases 1 and 2A strongly facilitate the induction of LTP by long trains of TPS (Thomas et al. 1996), indicating that protein phosphatase activation is responsible for the inhibition of LTP induction during prolonged bouts of TPS.
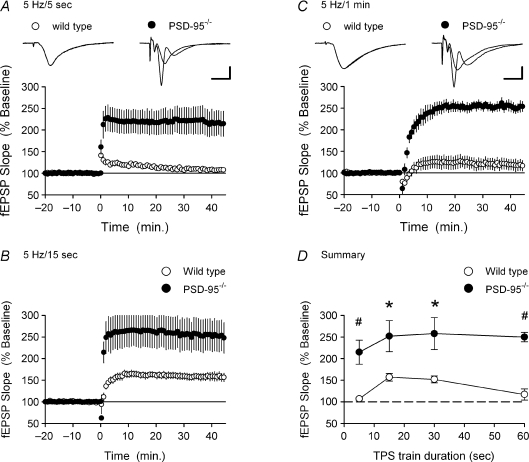
Figure 3. TPS-induced LTP is enhanced in the hippocampal CA1 region of PSD-95 mutant mice.
A, a brief train of TPS (5 s) delivered at time = 0 had little lasting effect on synaptic transmission in slices from wild-type mice (○, n = 5 slices from 5 mice) but induced robust LTP in slices from PSD-95 mutant mice (•, n = 5 slices from 5 mice). The inset shows superimposed fEPSPs recorded during baseline and 45 min post-TPS in a wild-type slices (left) and PSD-95 mutant slices (right). Calibration bars are 5 ms and 2 mV. B, the induction of LTP by a longer train of TPS (15 s, delivered at time = 0) is enhanced in slices from PSD-95 mutant mice (•, n = 7 slices from 5 mice) compared to wild-type littermates (○, n = 7 slices from 6 mice). C, while prolonged TPS (1 min, delivered at time = 0) did not induce significant LTP in wild-type slices (○, n = 6 slices from 5 mice), it induced more than 2-fold LTP in slices from PSD-95 mutant mice (•, n = 7 slices from 5 mice). The inset shows superimposed fEPSPs recorded during baseline and 45 min post-TPS in a wild-type (left) and PSD-95 mutant slice (right). Calibration bars are 5 ms and 2 mV. D, summary of the effects of different duration trains of TPS on synaptic strength in slices from wild-type (○) and PSD-95 mutant mice (•). Values correspond to the mean (±s.e.m.) potentiation recorded 40–45 min post-TPS (#P < 0.01 compared to wild-type, *P < 0.025 compared to wild-type).
Strikingly, the induction of LTP by all four TPS protocols was strongly enhanced in slices obtained from PSD-95 mutant mice. As shown in Fig. 3A and C, both the 5 and 60 s-long trains of TPS that failed to induce significant LTP in slices from wild-type mice induced more than 2-fold LTP in slices from PSD-95 mutant mice (fEPSPs were potentiated to 215 ± 28% and 250 ± 11% of baseline, respectively, P < 0.02 compared to baseline, n = 5 for both). Moreover, the induction of LTP by 15 s-long trains of TPS was also significantly enhanced in slices from PSD-95 mutants (fEPSPs were potentiated to 252 ± 36% of baseline, n = 5, P < 0.025 compared to wild-type, Fig. 3B) as was the induction of LTP by a 30 s-long train of TPS (Fig. 3D). Thus, as summarized in Fig. 3D, TPS stimulation-induced LTP is enhanced in PSD-95 mutants and the ability of synapses to discriminate TPS trains of different durations and selectively undergo LTP in response to trains lasting approximately 15–30 s is completely disrupted in the absence of PSD-95.
To determine whether PSD-93 and PSD-95 have similar roles in LTP induction we next examined both HFS- and TPS-induced LTP in slices from PSD-93−/− mutant mice (McGee et al. 2001). Although HFS stimulation-induced LTP is enhanced in PSD-95 mutants (Migaud et al. 1998; Béïque et al. 2006), the magnitude of LTP induced by HFS in PSD-93−/− mutants was not significantly different from that seen in slices from wild-type littermates (Fig. 4A). Surprisingly, although induction of LTP by a 30 s long train of TPS is strongly enhanced in slices from PSD-95 mutant mice, the induction of LTP by this pattern of TPS was significantly reduced compared to wild-type littermates in slices from PSD-93−/− mice (40–45 min post-TPS fEPSPs were potentiated to 166 ± 6% of baseline in wild-type slices, n = 5, but were only 122 ± 4% of baseline in slices from PSD-93−/− mice, n = 5, P < 0.001 compared to wild-type) (Fig. 4B). Moreover, unlike PSD-95 mutants, a short train of TPS (5 s) had no lasting effect on synaptic transmission in slices from PSD-93−/− mice (Fig. 4C). Together with our findings shown in Fig. 3, these results indicate that mutations in PSD-95 and PSD-93 produce essentially opposite effects on TPS-induced LTP.
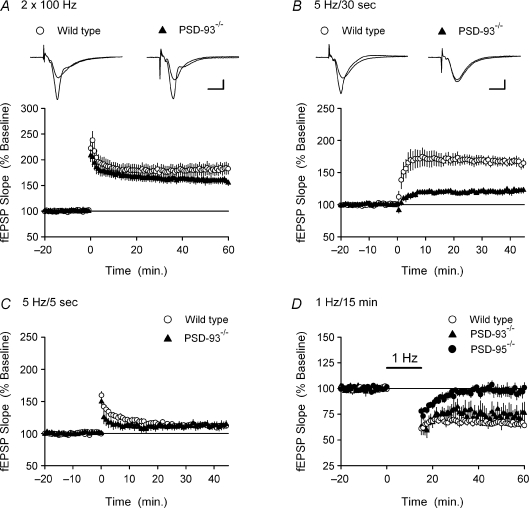
Figure 4. TPS-induced LTP is impaired in PSD-93−/− mice.
A, HFS (two, 1 s-long trains of 100 Hz stimulation, intertrain interval = 10 s) was delivered at time = 0. Although the magnitude of LTP in PSD-93−/− slices (▲, fEPSPs were potentiated to 158 ± 6% of baseline, n = 10 slices from 7 mice) was less that that seen in slices from wild-type littermates (○, fEPSPs were 184 ± 12% of baseline, n = 10 slices from 7 mice) this difference was not statistically significant (P = 0.09). The inset shows fEPSPs recorded during baseline and 60 min post-HFS in a wild (left) and PSD-93 mutant slices (right). Calibration bars are 5 ms and 2 mV. B, the induction of LTP by a 30 s-long train of TPS (delivered at time = 0) is impaired in slices from PSD-93−/− mice. fEPSPs were potentiated to 166 ± 6% of baseline in wild-type slices (○, n = 11 slices from 5 mice) but were only 122 ± 4% of baseline in slices from PSD-93−/− mice (▲, n = 11 slices from 5 mice, P < 0.001 compared to wild-type). The inset shows fEPSPs recorded during baseline and 45 min post-TPS in a wild-type (left) and PSD-93 mutant slice (right). Calibration bars are 5 ms and 2 mV. C, a short train of TPS (5 s, delivered at time = 0) has no lasting effect on synaptic transmission in slices from PSD-93−/− (▲, fEPSPs were 112 ± 6% of baseline, n = 9 slices from 5 mice) and wild-type mice (○, fEPSPs were 112 ± 3% of baseline, n = 9 slices from 5 mice). D, LTD is normal in PSD-93−/− mice (▲, n = 7 slices from 4 mice) but impaired in PSD-95 mutant mice (•, n = 7 slices from 4 mice). LTD induction in wild-type littermates for these mutants were the same and the results were combined (○, n = 14 slices from 7 mice).
Long-term depression in normal in PSD-93−/− mutant mice
Importantly, MAGUKs are not only thought to be involved in LTP but are also likely to have an important role in LTD. For example, LTD is impaired in PSD-95 mutant mice (Migaud et al. 1998) and by shRNA-mediated knockdown of PSD-95 in organotypic slices (Xu et al. 2008). Moreover, over-expression of PSD-95 enhances LTD in both cortical (Béïque & Andrade, 2003) and hippocampal neurons (Stein et al. 2003). Thus, to determine whether PSD-93 has a similar role in LTD we next examined LFS-induced LTD in wild-type, PSD-93−/− and PSD-95 mutant mice. As shown in Fig. 4D, LFS (1 Hz for 15 min) induced similar levels of LTD in both PSD-93−/− mutants and wild-type littermates (45 min after LFS fEPSPs were reduced to 73 ± 8% of baseline in slices from PSD-93−/− mice, n = 4, compared to 65 ± 4% of baseline in wild-type slices, n = 4, P = 0.41). In contrast, the induction of LTD was impaired in slices from PSD-95 mutant mice (45 min post-LFS fEPSPs were 98 ± 5% of baseline in slices from PSD-95 mutants, n = 4, compared to 66 ± 2% of baseline in wild-type slices, n = 3, P < 0.01). Thus, in contrast to PSD-95, our results suggest that PSD-93 is not required for normal levels of LTD in the hippocampal CA1 region of adult animals.
Spike timing-dependent LTP is enhanced in PSD-95 mutants and disrupted in PSD-93 mutant mice
Studies of spike timing-dependent plasticity (STDP) have shown that LTP can be induced by back-propagating action potentials that occur within several milliseconds after presynaptic action potentials and provide what are perhaps the most physiologically realistic patterns of synaptic stimulation for inducing LTP in vitro (Paulsen & Sejnowski, 2000; Dan & Poo, 2004). We thus examined whether the ability of STDP-like stimulation protocols to induce LTP is altered in the hippocampal CA1 region of PSD-95 and PSD-93 mutant mice.
In these experiments we first examined how synaptic strength in slices from adult, C57BL/6 mice was modified by pairing EPSPs with different patterns of postsynaptic action potential firing (Fig. 5A). Although pairing EPSPs with single postsynaptic action potentials induces LTP in developmentally immature preparations such as dissociated cell culture (Bi & Poo, 1998; Wang et al. 2005), organotypic slices (Debanne et al. 1998), or acute slices from very young animals (Meredith et al. 2003), we found that pairing a 5 Hz train of 100 EPSPs with single postsynaptic action potentials had no significant effect on synaptic strength in acute slices from adult mice (30 min post-pairing were 97 ± 6% of baseline, n = 12 cells) (Fig. 5B). In contrast, pairing a 5 Hz train of 100 EPSPs with a burst of three to four postsynaptic action potentials reliably induced significant LTP (EPSPs were potentiated to 170 ± 13% of baseline 30 min post-EPSP–burst pairing, n = 13 cells, P < 0.001 compared to baseline). Thus, in agreement with previous studies (Thomas et al. 1999; Pike et al. 1999; Meredith et al. 2003; Wittenberg & Wang, 2006; but see Nishiyama et al. 2000), these results indicate that single postsynaptic action potentials are not sufficient for the induction in CA1 pyramidal cells in the adult hippocampus.
Because the induction of spike timing-dependent LTP appears to be sensitive to the frequency of EPSP–action potential pairing (Sjöström et al. 2001), we repeated the STDP experiments using a range of presynaptic fibre stimulation frequencies during the pairing protocol (from 2 to 20 Hz) but again found that pairing EPSPs with single postsynaptic action potentials failed to induce significant LTP (Fig. 5C). LTP was reliably induced, however, by all frequencies of presynaptic fibre stimulation when EPSPs were paired with bursts of postsynaptic action potentials (Fig. 5C). Blocking postsynaptic action potentials by including the Na+ channel blocker QX314 (5 mm) in the recording electrode solution completely blocked the induction of LTP by EPSP–burst pairing (Fig. 5C). This indicates that postsynaptic action potentials, rather than the longer pulse of depolarizing current injection itself, are required for LTP induction. These results are consistent with the notion that bursts of postsynaptic action potentials, rather than single spikes, are the essential postsynaptic associative signal for LTP induction in the adult brain (Paulsen & Sejnowski, 2000) and suggest that the ability of synapses to discriminate single versus bursts of postsynaptic action potentials is a fundamental property of LTP induction at excitatory synapses onto CA1 pyramidal cells in the adult hippocampus.
To determine whether the EPSP–burst pairing protocols used in these experiments induces a spike timing-dependent form of LTP, we next examined the effects of systematically varying the time interval between presynaptic fibre stimulation and postsynaptic bursting on pairing-induced changes in synaptic strength in wild-type cells. As shown in Fig. 6A–C, pairing EPSPs evoked at 2 Hz with bursts of postsynaptic action potentials evoked 100, 50, or 10 ms before presynaptic fibre stimulation had no lasting effect on synaptic strength. Thus, unlike conventional STDP protocols, EPSP–burst pairing does not induce LTD when postsynaptic action potentials precede presynaptic fibre stimulation. However, the effect of presynaptic fibre stimulation followed by bursts of postsynaptic action potentials on synaptic strength was highly sensitive to the time interval between pre- and postsynaptic stimulation. As shown in Fig. 6B, pairing presynaptic fibre stimulation at 2 Hz with bursts of postsynaptic action potentials elicited 50 ms after EPSP onset did not induce LTP but instead induced significant LTD (30 min post-pairing EPSPs were reduced to 81 ± 5% of baseline, n = 7, P < 0.01 compared to baseline). In contrast, 2 Hz EPSP–burst pairing had no lasting effect on synaptic strength when the interval between EPSP onset and postsynaptic bursting was increased to 100 ms (30 min post-pairing EPSPs were 108 ± 7% of baseline, n = 7) (Fig. 6C).
To determine whether MAGUKs might have a role in coupling NMDA receptors to signalling pathways that enable synapses to discriminate single action potentials versus bursts of postsynaptic spikes in LTP induction, we next examined the ability of EPSPs paired with either bursts or single postsynaptic action potentials to induce LTP in pyramidal cells from PSD-93 and PSD-95 mutant mice. As shown in Fig. 7A, pairing EPSPs elicited during a 10 Hz train of synaptic stimulation with single postsynaptic action potentials had no lasting effect on synaptic transmission in wild-type littermates (30 min post-pairing EPSPs were 106 ± 6% of baseline, n = 13 cells from 9 mice). This same pairing protocol, however, reliably induced LTP at synapses onto CA1 pyramidal cells from PSD-95 mutant mice (EPSPs were potentiated to 216 ± 16% of baseline, n = 7 cells from 6 mice) (Fig. 7A). In contrast to PSD-95 mutants, pairing EPSPs with single postsynaptic action potentials failed to induce significant LTP in CA1 pyramidal cells from PSD-93−/− mice (EPSPs were 100 ± 8% of baseline, n = 7 cells from 4 mice). Thus, the normal ability of synapses to selectively undergo LTP when EPSPs coincide with bursts of postsynaptic action potentials rather than single spikes is profoundly altered in the CA1 region of PSD-95, but not PSD-93 mutant mice. Importantly, a 10 Hz train of presynaptic fibre stimulation alone had no lasting effect on synaptic strength in PSD-95 mutant pyramidal cells (30 min post-10 Hz stimulation EPSPs were 96 ± 8% of baseline, n = 5 cells from 2 mice), indicating that presynaptic activity alone is not sufficient for LTP induction in PSD-95 mutant cells.
Figure 7. PSD-95 and PSD-93 have unique roles in pairing-induced LTP.
A, pairing EPSPs elicited by a 10-Hz train of presynaptic fibre stimulation with single postsynaptic action potentials failed to induce LTP in wild-type cells (○, n = 13 cells from 9 mice) and PSD-93−/− cells (▲, n = 8 cells from 4 mice) but induced robust LTP in PSD-95 mutant cells (•, n = 7 cells from 6 mice). Results were the same in cells from PSD-93 and PSD-95 wild-type littermates and were therefore combined (n = 7 cells from 4 mice and 6 cells from 5 mice, respectively). The inset shows superimposed EPSPs recorded during baseline and 30 min post-pairing in a wild-type (left), PSD-95 (middle) and PSD-93 mutant cell (right). Calibration bars are 15 ms and 5 mV. B, cumulative probability distribution plot showing the amount of potentiation present 30 min post-pairing in wild-type (○) as well as PSD-93 (▲) and PSD-95 (•) mutant cells in experiments where EPSPs were paired with single postsynaptic action potentials. C, pairing EPSPs elicited during a 10 Hz train of presynaptic fibre stimulation with a burst of postsynaptic action potentials induces similar amounts of LTP in wild-type (○, n = 14 cells from 9 mice) and PSD-95 mutant cells (•, n = 7 cells from 5 mice). In contrast, the induction of LTP by EPSPs paired with bursts of postsynaptic action potentials was disrupted in PSD-93−/− cells (▲, n = 9 cells from 4 mice). Results from wild-type littermates were the same and were therefore combined (n = 8 cells from 4 PSD-93 wild-type littermates and 5 cells from 4 PSD-95 wild-type littermates). The inset shows superimposed EPSPs recorded during baseline and 30 min post-pairing in a wild-type (left), PSD-95 (middle) and PSD-93 mutant cell (right). Calibration bars are 15 ms and 5 mV. D, cumulative probability distribution plot showing the amount of potentiation present 30 min post-pairing in wild-type (○), PSD-93 mutant (▲) and PSD-95 mutant cells (•) in experiments where EPSPs were paired with a burst of postsynaptic action potentials.
We also observed a striking difference between PSD-93 and PSD-95 mutant mice when we examined how synaptic strength was altered by pairing EPSPs with bursts of postsynaptic action potentials. As shown in Fig. 7C and D, pairing EPSPs with bursts of postsynaptic action potentials induced similar levels of LTP in wild-type and PSD-95 mutant cells (30 min post-pairing EPSPs were potentiated to 195 ± 13% of baseline in wild-type cells, n = 13 cells from 9 mice, and were potentiated to 200 ± 9% of baseline in PSD-95 mutant cells, n = 7 cells from 4 mice). The induction of LTP by this pattern of pre- and postsynaptic activity was, however, strongly disrupted in PSD-93−/− mutants (30 min post-pairing EPSPs were 110 ± 5% of baseline, n = 9 cells from 4 mice). Together, these findings indicate that PSD-95 and PSD-93 have unique roles in regulating the induction of LTP by EPSP–burst pairing.
Discussion
Despite the high sequence homology between PSD-95 and PSD-93 and previous studies suggesting that these MAGUKs have similar roles in AMPAR trafficking in cultured hippocampal neurons (Elias & Nicoll, 2007), our experiments show that these MAGUKs have remarkably distinct roles in both basal AMPAR function and NMDAR-dependent LTP in the hippocampus of adult mice. While high-frequency stimulation-induced LTP is normal in PSD-93 null mutant mice, both TPS- and pairing-induced LTP are reduced compared to wild-type control mice. In contrast, both TPS-induced and pairing-induced LTP are significantly enhanced in PSD-95 mutant mice. Thus, these MAGUKs appear to have essentially opposite roles in hippocampal LTP. Moreover, the fact that only PSD-95 mutants exhibit LTD deficits suggests that PSD-95 and PSD-93 have distinct roles in LTD. Importantly, our results indicate that NMDAR-mediated synaptic transmission is normal in the hippocampus of PSD-93 mutants and suggest that the same is true in PSD-95 mutant mice. Similarly, shRNA-mediated knockdown of PSD-93 or PSD-95 has no effect on NMDAR-mediated synaptic currents in cultured hippocampal slices (Elias et al. 2006; also see Xu et al. 2008). This suggests that the alterations in LTP observed in our experiments are not due to changes in NMDAR function. Instead, our findings suggest that PSD-95 and PSD-93 act to couple NMDARs to distinct MASCs that have essentially opposite roles in LTP. Although the underlying molecular alterations responsible for the changes in LTP induction in PSD-95 and PSD-93 mutant mice remain to be identified, the mutant LTP phenotypes suggest that PSD-95 normally couples NMDARs to signalling pathways that act as inhibitors or suppressors of LTP induction while PSD-93 is responsible for coupling NMDARs to signalling molecules that facilitate LTP induction.
Previous studies showing that acute down-regulation of either PSD-95 or PSD-93 expression strongly disrupts basal synaptic function in organotypic slices from young animals, suggesting that PSD-95 and PSD-93 have similar roles in AMPAR trafficking at excitatory synapses in the hippocampus (Elias et al. 2006). Although our results are consistent with the notion that MAGUKs have an important role in AMPAR trafficking, we find that only PSD-95 mutant mice exhibit significant deficits in basal synaptic transmission while PSD-93 and SAP102 mutants (Cuthbert et al. 2007) do not. This suggests that PSD-95 is especially important for normal AMPAR function in the hippocampus of adult animals. Although the input/output function for basal synaptic transmission is normal in PSD-93 mutants, these mutants exhibit enhanced paired-pulse facilitation similar to that seen in PSD-95 mutant mice (Fig. 1C). Because increases in paired-pulse facilitation are typically thought to reflect a decrease in presynaptic neurotransmitter release probability, we cannot exclude the possibility that PSD-93 mutants may exhibit a subtle deficit in basal synaptic transmission. In any event, it seems clear that while PSD-95 and SAP102 can largely compensate for the loss of PSD-93 in adult hippocampal neurons, PSD-93 and SAP102 cannot compensate for the absence of PSD-95. It is interesting, however, that both PSD-93−/− and PSD-95 mutant mice exhibit enhanced paired-pulse facilitation, which suggests a reduction in neurotransmitter release probability from the presynaptic terminal. Thus, while PSD-93 and PSD-95 may have very different roles in postsynaptic AMPAR trafficking, they appear to have similar roles in modulating presynaptic function. How might loss of these scaffolding proteins within the PSD regulate presynaptic release probability? MAGUKs can bind to neuroligins (Irie et al. 1997), which through their extracellular domains can interact with β-neurexin in presynaptic terminals (Ichtchenko et al. 1995). Through a network of protein–protein interactions β-neurexin, in turn, is coupled to regulators of the vesicle release machinery (for review see Dean & Dresbach, 2006). Thus, MAGUKs may be a crucial link in a trans-synaptic network of proteins that connects the PSD to presynaptic function. Consistent with this notion, Futai et al. (2007) recently found that acute knockdown of PSD-95 enhances transmitter release in a neuroligin-dependent manner. Importantly, our results indicate that neither PSD-95 nor PSD-93 is required for the insertion of AMPARs into excitatory synapses following the induction of high-frequency stimulation-induced LTP. Likewise, LTP is not disrupted, but instead enhanced, in SAP102 null mutant mice (Cuthbert et al. 2007). Thus, although PSD-95 may be especially important for the trafficking of AMPARs at basal synapses, none of the NMDAR-associated MAGUKs appear to be uniquely required for the insertion of AMPARs into the postsynaptic membrane that occurs following LTP induction. All of the MAGUK mutants we have studied to date do, however, exhibit unique changes in NMDAR-dependent LTP. As we show here, LTP is strongly enhanced in PSD-95 mutants and significantly disrupted in PSD-93−/− mice. Previously we found that SAP102 mutant mice, like PSD-95 mutants, exhibit enhanced TPS and pairing-induced LTP (Cuthbert et al. 2007). However, PSD-95 mutants exhibit enhanced LTP across a wide rage of stimulation protocols while SAP102 mutant mice exhibit increases in LTP with some but not all patterns of synaptic stimulation (Cuthbert et al. 2007). Furthermore, inhibitors of extracellular signal regulated kinase (ERK) activation restore LTP to wild-type levels in SAP102 mutants (Cuthbert et al. 2007) but have no effect on the enhancement of LTP in PSD-95 mutants (Opazo et al. 2003). Together, these results indicate that distinct molecular alterations are responsible for enhancement of LTP in SAP102 and PSD-95 mutant mice. Thus, all three MAGUKs that associate with NMDARs appear to have unique, subtype-specific roles in LTP, most likely because they couple NMDARs to distinct downstream signalling pathways that regulate LTP induction.
Although the results of a recent study suggest that enhancement of LTP in PSD-95 mutant mice is due to a larger number of synapses that lack AMPARs or ‘silent synapses’ in these mutants (Béïque et al. 2006), it seems unlikely that this alone can account for the enhancement of LTP in the absence of PSD-95. For example, inhibitors of both protein kinase A (PKA) and ERK signalling strongly inhibit TPS-induced LTP in hippocampal slices from wild-type mice but have no effect on TPS-induced LTP in PSD-95 mutant mice (Opazo et al. 2003; Makhinson et al. 2006). This indicates that the biochemical signalling pathways underlying LTP in PSD-95 mutant mice are strikingly different from that seen in wild-type mice and suggests that PSD-95 plays a pivotal role in organizing the postsynaptic signalling pathways responsible for LTP. Moreover, the magnitude of LTP in the hippocampal CA1 region of PSD-95 mutant mice is not only larger but also induced by patterns of synaptic stimulation that normally have no lasting effect on synaptic transmission in wild-type mice. Unlike wild-type mice, Schaffer collateral fibre synapses onto CA1 pyramidal cells in PSD-95 mutants can discriminate neither TPS trains of different duration nor presynaptic activity that is coincident with bursts of postsynaptic action potentials versus single postsynaptic spikes. This suggests that PSD-95 has a crucial role in linking NMDARs to MASCs that enables synapses to discriminate different patterns of pre- and postsynaptic activity. Disrupting the fundamental ‘rules’ that dictate which patterns of activity elicit LTP would be likely to cause degradation of information coding necessary for performing memory tasks. We hypothesize that this might account for the strong impairment in hippocampal-dependent forms of learning observed in PSD-95 mutant mice (Migaud et al. 1998).
Studies using knockout and shRNA approaches to reduce levels of PSD-93 and PSD-95 expression have shown that these MAGUKs are likely to regulate synaptic AMPAR numbers at distinct, non-overlapping populations of synapses (Béïque et al. 2006; Elias et al. 2006). This indicates that excitatory synapses onto CA1 pyramidal cells may exhibit a considerable degree of heterogeneity with respect to levels of PSD-93 and PSD-95. Indeed, only about 30% of the synapses onto CA1 pyramidal cells express both of these MAGUKs in vivo (Sans et al. 2000). Together with these findings, our results suggest that fundamental properties of LTP induction may also vary in a synapse-specific manner depending on synaptic levels of PSD-95 and PSD-93. In other words, synapses expressing mainly PSD-93 may contain NMDAR signalling complexes that strongly facilitate the induction of LTP whereas LTP will be down-regulated at synapses where NMDA receptor signalling complexes are predominantly formed via association with PSD-95. Formation of unique NMDAR signalling complexes in response to the levels of PSD-93 and PSD-95 at individual synapses may thus provide a mechanism for generating synapse-specific alterations in the rules regulating LTP induction.
Acknowledgments
This work was supported by the National Science Foundation under grant number 0543651 and National Institute of Mental Health grant number MH609197 to T.J.O. Additional support was provided by the Wellcome Trust Genes to Cognition programme (http://www.genes2cognition.org) and the Wellcome Trust Sanger Institute to S.G.N.G. We are grateful to Dr David Bredt for PSD-93 mutant mice and Dr Maksym Kopanitsa for helpful comments on the manuscript. We thank Jane Robinson and Georgina Berry for genotyping and mouse colony management.
References
- Béïque J-C, Andrade R. PSD-95 regulates synaptic transmission and plasticity in rat cerebral cortex. J Physiol. 2003;546:859–867. doi: 10.1113/jphysiol.2002.031369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béïque J-C, Lin D-T, Kang M-G, Aizawa H, Takamiya K, Huganir RL. Synapse-specific regulation of AMPA receptor function by PSD-95. Proc Natl Acad Sci U S A. 2006;103:19535–19540. doi: 10.1073/pnas.0608492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Q-G, Poo M-M. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18:10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colledge M, Snyder EM, Crozier RA, Soderling JA, Jin Y, Langeberg LK, Lu H, Bear MF, Scott JD. Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron. 2003;40:595–607. doi: 10.1016/s0896-6273(03)00687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MO, Husi H, Yu L, Brandon JM, Anderson CN, Blackstock WP, Choudhary JS, Grant SG. Molecular characterization and comparison of the components of mulitprotein complexes in the postsynaptic proteome. J Neurochem. 2005;97(Suppl. 1):16–23. doi: 10.1111/j.1471-4159.2005.03507.x. [DOI] [PubMed] [Google Scholar]
- Cuthbert PC, Stanford LE, Coba MP, Ainge JA, Fink AE, Opazo P, Delgado JY, Komiyama NH, O'Dell TJ, Grant SG. Synapse-associated protein 102/dlgh3 couples the NMDA receptor to specific plasticity pathways and learning strategies. J Neurosci. 2007;27:2673–2682. doi: 10.1523/JNEUROSCI.4457-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan Y, Poo M-M. Spike timing-dependent plasticity of neural circuits. Neuron. 2004;44:23–30. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Dean C, Dresbach T. Neuroligins and neurexins: linking cell adhesion, synapse formation and cognitive function. Trends Neurosci. 2006;29:21–29. doi: 10.1016/j.tins.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Debanne D, Gahwiler BH, Thompson SM. Long-term synaptic plasticity between pairs of individual CA3 pyramidal cells in rat hippocampal slice cultures. J Physiol. 1998;507:237–247. doi: 10.1111/j.1469-7793.1998.237bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado JY, Coba M, Anderson CN, Thompson KR, Gray EE, Heusner CL, Martin KC, Grant SG, O’Dell TJ. NMDA receptor activation dephosphorylates AMPA receptor glutamate receptor 1 subunits at threonine 840. J Neurosci. 2007;48:13210–13221. doi: 10.1523/JNEUROSCI.3056-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosemeci A, Makusky AJ, Jankowska-Stephens E, Yang X, Slotta DJ, Markey SP. Composition of the synaptic PSD-95 complex. Mol Cell Proteomics. 2007;6:1749–1760. doi: 10.1074/mcp.M700040-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Malinow R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J Neurosci. 2004;24:916–927. doi: 10.1523/JNEUROSCI.4733-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- Elias GM, Funke L, Stein V, Grant SG, Bredt DS, Nicoll RA. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron. 2006;52:307–320. doi: 10.1016/j.neuron.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Elias GM, Nicoll RA. Synaptic trafficking of glutamate receptors by MAGUK scaffolding proteins. Trends Cell Biol. 2007;17:343–352. doi: 10.1016/j.tcb.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Futai K, Kim MJ, Hashikawa T, Scheiffele P, Sheng M, Hayashi Y. Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-neuroligin. Nat Neurosci. 2007;10:186–195. doi: 10.1038/nn1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant SGN, Marshal MC, Page K-L, Cumiskey MA, Armstrong JD. Synapse proteomics of multiprotein complexes: en route from genes to nervous system diseases. Human Mol Genet. 2005;14:R225–R234. doi: 10.1093/hmg/ddi330. [DOI] [PubMed] [Google Scholar]
- Gray EE, Fink AE, Sarinan J, Vissel B, O’Dell TJ. Long-term potentiation in the hippocampal CA1 region does not require insertion and activation of GluR2-lacking AMPA receptor. J Neurophysiol. 2007;98:2488–2492. doi: 10.1152/jn.00473.2007. [DOI] [PubMed] [Google Scholar]
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SGN. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci. 2000;3:661–669. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, Südhof TC. Neuroligin 1: a splice site-specific ligand for b-neurexins. Cell. 1995;81:435–443. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl TW, Südhof TC. Binding of neuroligins to PSD-95. Science. 1997;277:1511–1515. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
- Jonas P, Burnashev N. Molecular mechanisms controlling calcium entry through AMPA-type glutamate receptor channels. Neuron. 1995;15:987–990. doi: 10.1016/0896-6273(95)90087-x. [DOI] [PubMed] [Google Scholar]
- Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Makhinson M, Opazo P, Carlisle JH, Godsil B, Grant SG, O’Dell TJ. A novel role of cyclic quanosine 3′,5′ monophosphate signaling in synaptic plasticity: a selective suppressor of protein kinase A-dependent forms of long-term potentiation. Neurosci. 2006;140:415–431. doi: 10.1016/j.neuroscience.2006.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee AW, Topinka JR, Hashimoto K, Petralia RS, Kakizawa S, Kauer FW, Aguilera-Moreno A, Wenthold RJ, Kano M, Bredt DS. PSD-93 knock-out mice reveal that neuronal MAGUKs are not required for development or function of parallel fiber synapses in cerebellum. J Neurosci. 2001;21:3085–3091. doi: 10.1523/JNEUROSCI.21-09-03085.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith RM, Floyer-Lea AM, Paulsen O. Maturation of long-term potentiation induction rules in rodent hippocampus. role of GABAergic inhibition. J Neurosci. 2003;23:11142–11146. doi: 10.1523/JNEUROSCI.23-35-11142.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RG, Morrison JH, O’Dell TJ, Grant SG. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396:433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Futai K, Lashuel HA, Lo I, Okamoto K, Walz T, Hayashi Y, Sheng M. Quaternary structure, protein dynamics, and synaptic function of SAP97 controlled by L27 domain interactions. Neuron. 2004;44:453–467. doi: 10.1016/j.neuron.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Tomita S, Bredt DS. Auxiliary subunits assist AMPA-type glutamate receptors. Science. 2006;311:1253–1256. doi: 10.1126/science.1123339. [DOI] [PubMed] [Google Scholar]
- Nishiyama Hong K, Mikoshiba K, Poo M-M, Kato K. Calcium stores regulate the polarity and input specificity of synaptic modification. Nature. 2000;408:584–588. doi: 10.1038/35046067. [DOI] [PubMed] [Google Scholar]
- Norris CM, Korol DL, Foster TC. Increased susceptibility to induction of long-term depression and long-term potentiation reversal during aging. J Neurosci. 1996;17:5382–5392. doi: 10.1523/JNEUROSCI.16-17-05382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo P, Watabe AM, Grant SGN, O’Dell TJ. Phosphatidylinositol 3-kinase regulates the induction of long-term potentiation through extracellular signalrelated kinase-independent mechanisms. J Neurosci. 2003;23:3679–3688. doi: 10.1523/JNEUROSCI.23-09-03679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen O, Sejnowski TJ. Natural patterns of activity and long-term synaptic plasticity. Cur Opin Neurobiol. 2000;10:172–179. doi: 10.1016/s0959-4388(00)00076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike FG, Meredith RM, Olding AW, Paulsen O. Postsynaptic bursting is essential for ‘Hebbian’ induction of associative long-term potentiation at excitatory synapses in rat hippocampus. J Physiol. 1999;518:571–576. doi: 10.1111/j.1469-7793.1999.0571p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocklington AJ, Cumiskey M, Armstrong JD, Grant SGN. The proteomes of neurotransmitter receptor complexes form modular networks with distributed functionality underlying plasticity and behavior. Mol Sys Biol 2006. 2006;0023:1–14. doi: 10.1038/msb4100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans N, Petralia RS, Wang Y-X, Blahos J, Hell JW, Wenthold R. A developmental changes in NMDA receptor-associated proteins at hippocampal synapses. J Neurosci. 2000;20:1260–1271. doi: 10.1523/JNEUROSCI.20-03-01260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell E, Sizemore M, Karimxadegan S, Chen L, Bredt DS, Nicoll RA. Direct interactions between PSD-95 and stargazing control synaptic AMPA receptor number. Proc Natl Acad Sci U S A. 2002;99:13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström PJ, Turrigiano GG, Nelson SB. Rate, timing and cooperativity jointly determine cortical synaptic plasticity. Neuron. 2001;32:1149–1164. doi: 10.1016/s0896-6273(01)00542-6. [DOI] [PubMed] [Google Scholar]
- Stein V, House DRC, Bredt DS, Nicoll RA. Postsynaptic density-95 mimics and occludes hippocampal long-term potentiation and enhances long-term depression. J Neurosci. 2003;23:5503–5506. doi: 10.1523/JNEUROSCI.23-13-05503.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarpey P, Parnau J, Blow M, Woffendin H, Bignell G, Cox C, et al. Mutations in the DLG3 gene cause nonsyndromic X-linked mental retardation. Am J Hum Genet. 2004;75:318–324. doi: 10.1086/422703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Moody TD, Makhinson M, O’Dell TJ. Activity-dependent β-adrenergic modulation of low frequency stimulation induced LTP in the hippocampal CA1 region. Neuron. 1996;17:475–482. doi: 10.1016/s0896-6273(00)80179-8. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Watabe AM, Moody TD, Makhinson M, O’Dell TJ. Postsynaptic complex spike bursting enables the induction of LTP by theta frequency synaptic stimulation. J Neurosci. 1999;18:7118–7126. doi: 10.1523/JNEUROSCI.18-18-07118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro C, Deakin JFW. NMDA receptor subunit NR1 and postsynaptic protein PSD-95 in hippocampus and orbitofrontal cortex in schizophrenia and mood disorder. Schizophr Res. 2005;80:323–330. doi: 10.1016/j.schres.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Wang HX, Gerkin RC, Nauen DW, Bi GQ. Coactivation and timing-dependent integration of synaptic potentiation and depression. Nat Neurosci. 2005;8:187–193. doi: 10.1038/nn1387. [DOI] [PubMed] [Google Scholar]
- Wittenberg GM, Wang SSH. Malleability of spiketiming-dependent plasticity at the CA3-CA1 synapse. J Neurosci. 2006;26:6610–6617. doi: 10.1523/JNEUROSCI.5388-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Schlüter OM, Steiner P, Czervlonke BL, Sabatini B, Malenka RC. Molecular dissociation of the role of PSD-95 in regulating synaptic strength and LTD. Neuron. 2008;57:248–262. doi: 10.1016/j.neuron.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao WD, Gainetdinov RR, Arbuckle MI, Sotnikova TD, Cyr M, Beaulieu JM, Torres GE, Grant SG, Caron MG. Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron. 2004;41:625–638. doi: 10.1016/s0896-6273(04)00048-0. [DOI] [PubMed] [Google Scholar]