Abstract
We tested the hypothesis that increasing blood amino acid (AA) availability would counter the physical inactivity-induced reduction in muscle protein synthesis. We determined how 14 days of unilateral knee immobilization affected quadriceps myofibrillar protein synthesis (MPS) in young healthy subjects (10 men, 2 women, 21 ± 1 years; 80.2 ± 4.0 kg, mean ±s.e.m.) in the post-absorptive state and after infusing AA (10% Primene) at low or high doses (43 and 261 mg kg−1 h−1). Muscle cross-sectional area (MRI) and peak isometric torque declined in the immobilized leg (−5.0 ± 1.2% and −25 ± 3%, respectively, both P < 0.005), but were unchanged (all P > 0.6) in the non-immobilized leg. Immobilization induced a 27% decline in the rate of post-absorptive MPS (immobilized, 0.027 ± 0.003: non-immobilized, 0.037 ± 0.003% h−1; P < 0.001). Regardless of dose, AA infusion stimulated a greater rise in MPS in the non-immobilized legs; at 4 h MPS was greater by +54 ± 12% with low dose and +68 ± 17% with high dose AA infusion (both P < 0.001). There was some evidence of delayed responsiveness of phosphorylation of Akt to high doses of AA and p70S6k at both doses but no marked differences in that of mTOR, GSK3β or eEF2. Phosphorylation of focal adhesion kinase (Tyr576/577) was reduced (P < 0.05) with immobilization. We observed no change in polyubiquitinated protein content after immobilization. We confirm that 14 days of immobilization reduces MPS in the post-absorptive state and this diminution is reduced but not abolished by increased provision of AA, even at high rates. The immobilization-induced decline in post-absorptive MPS with the ‘anabolic resistance’ to amino acids can account for much of immobilization-induced muscle atrophy.
Disuse atrophy is characterized by a reduction in muscle fibre cross sectional area. The consequences of inactivity-induced muscle wasting are reductions in strength and muscle quality (for review see Adams et al. 2003), with deleterious effects on quality of life and independence. Furthermore, this reduction in metabolically active lean tissue results in decreases in the capacities of whole-body glucose storage and metabolism (Stein & Wade, 2005; Wolfe, 2006), which contribute to insulin resistance, and a lower whole-body metabolic rate (Johnstone et al. 2005). Disuse of human muscle has been studied after a variety of interventions including bed rest, casting, limb suspension and spaceflight (Adams et al. 2003). Whenever human muscle protein synthesis has been measured after inactivity, a marked decline has been observed in fasted-state muscle protein synthesis (MPS) (Gibson et al. 1987; Ferrando et al. 1996; Paddon-Jones et al. 2006; de Boer et al. 2007). The reduced fasted-state MPS occurs relatively early in immobilization (10 days) and does not decline further (de Boer et al. 2007). However, the possible effect of immobilization on the stimulation of MPS to essential amino acids, the primary drivers of anabolism (Bohe et al. 2001, 2003; Fujita et al. 2007), remains unstudied. The expected increase in whole-body protein synthesis in response to amino acid feeding is impaired after bed rest (Biolo et al. 2004), which was interpreted as being due to a reduction in muscle protein synthesis, but this hypothesis was not tested by those authors.
Regulation of MPS on an hour-to-hour basis is predominantly at the translational level with changes in phosphorylation of the Akt–mTOR–p70S6 pathway proteins playing a critical role (for reviews see Kimball et al. 2002; Wang & Proud, 2006; Proud, 2007). Such signalling proteins in human muscle are normally responsive to feeding (Fujita et al. 2007), high-intensity exercise (Dreyer et al. 2006; Eliasson et al. 2006) and combinations of the two (Karlsson et al. 2004; Cuthbertson et al. 2006; Dreyer et al. 2008). However, 10 or 21 days of immobilization of human muscle are reported to have no effects on the states of phosphorylation of components of this pathway (Akt/PKB, TSC-2, p70S6 and 4EBP1) in the post-absorptive state in humans (de Boer et al. 2007) or rats (Vargas & Lang, 2008).
Previous work on disuse atrophy of human muscle (de Boer et al. 2007) showed that focal adhesion kinase (FAK) phosphorylation was reduced with immobilization. As a mechanically sensitive transduction protein (Fluck et al. 1999; Gordon et al. 2001), we wished to measure FAK phosphorylation to possibly demonstrate a functional link between reduced FAK phosphorylation and dampened responsiveness of the Akt–mTOR–p70S6 pathway to feeding.
We aimed to test the hypothesis that during immobilization, amino acid-induced stimulation of human myofibrillar protein synthesis (MPS) and anabolic signalling would be preserved, tending to counter the immobilization-induced deficit in the rate of post-absorptive MPS, but that the effects would not restore the rate to that observed in the non-immobilized leg after amino acid feeding.
Methods
Subjects
Twelve recreationally active (i.e. exercise ≤ 2 days week−1) men (n = 10, 21 years, 81 kg, 24.7 kg m−2) and women (n = 2, 21 years, 82 kg, 27.3 kg m−2) participated in the study. Subjects were screened to exclude smokers, females taking oral contraceptives, any person with lower-limb injury within 1 year prior to the start of the study, or family history of thrombosis. All subjects underwent 14 days of unilateral knee-brace-mediated immobilization; see Yasuda et al. (2005) for details. Subjects were divided into two groups of six (5 men and 1 woman in each) to receive either a low or a high dose (43 or 261 mg kg−1 h−1) amino acid infusion after 14 days of immobilization, so the groups were equivalent until they were infused with amino acids. The groups were similar in terms of mean lean mass (low dose: 57.9 ± 2.9 kg; high dose: 58.0 ± 4.5 kg), total body mass (low dose: 79.2 ± 5.3 kg; high dose: 80.3 ± 6.5 kg) and BMI (low dose: 24.3 ± 1.3 kg m−2; high dose: 24.8 ± 1.2 kg m−2) (all P > 0.78). The study was approved by the McMaster University and the Hamilton Health Sciences Research Ethics Boards according to the Declaration of Helsinki and informed written consent was obtained from each participant before each study.
Muscle size and function tests
All subjects were familiarized with the muscle strength testing procedure at least 1 week before beginning. Each subject's voluntary single-repetition maximum (1-RM) for isometric knee extension was determined on the morning of immobilization (day 1) and after the infusion trial on day 15 using the Biodex System 3 (Shirley, NY, USA). Prior to and on day 14 of immobilization, measurements were made using dual-energy X-ray absorptiometry (DXA; Hologic 4500A, Bedford, MA, USA) for leg lean mass, and magnetic resonance imaging (MRI) for mid-thigh cross sectional area (CSA). MRI was performed in a 3 Tesla HD scanner (Signa MRI system, GE Medical, Milwaukee, WI, USA) at the Brain–Body Institute, Imaging Research Centre, St Joseph's Healthcare (Hamilton, ON, Canada). Image acquisition was carried out using a T1 flair in the axial plane with the following parameters: repetition time/echo time, 2574 ms/6.7 ms; field of view, 25–30 cm; matrix size, range from 320/320 to 512/512 phase/frequency; inversion time, 958 ms; slice thickness, 5 mm. Thigh image acquisition utilized an 8-channel torso coil with two excitations (EX). During the pre-immobilization scan, the distance from a bony landmark to the first axial scan was recorded. This distance was used in the post-immobilization scan to ensure identical positioning. The MRI image analysis was performed using Medical Image Processing, Analysis and Visualization (MIPAV) software (downloaded with permission from the National Institutes of Health; http://mipav.cit.nih.gov/).
Experimental protocol
We chose to use 14 days of unilateral knee immobilization. The knee chosen to be immobilized was identified randomly, counter-balanced for dominance based on strength in each leg such that six subjects each had their stronger and thus six had their weaker legs immobilized. Testing was performed after immobilization using the non-immobilized leg as a control.
On the first day of immobilization, subjects arrived in the laboratory by 08.00 h and after strength testing, were fitted with a knee immobilization brace (Donjoy IROM; Vista, CA, USA) and instructed in the use of the provided set of crutches. The Velcro straps of the brace were bound with plastic adhesive tape over which the investigators’ signatures were inscribed. Breaking the tape seal, so the brace could be adjusted or removed, would thus render the tape irreplaceable without damaging the signature, ensuring compliance with the immobilization procedures. Subjects returned to the lab daily throughout the immobilization period, when they were permitted to remove the brace, under supervision, for approximately 15 min to allow visual inspection of the immobilized leg and knee brace. Any signs of chaffing or swelling were noted, the brace was adjusted as necessary, re-applied and secured as described above. All subjects found the brace tolerable, and none reported any adverse events such as tightness or swelling.
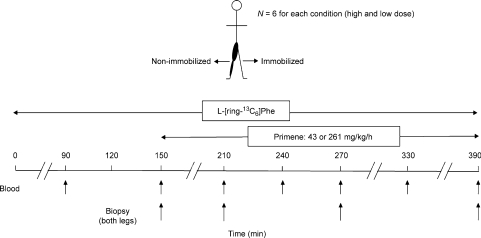
Infusion and sampling protocol (Fig. 1)
Figure 1. Schematic representation of the infusion protocol.
Participants reported to the laboratory at 06.30 h after an overnight fast. The immobilizing knee brace was removed and subjects remained supine for the remainder of the infusion protocol. One catheter was inserted in the medial vein of one arm for tracer infusion and the other was inserted in a dorsal hand vein for arterialized blood sampling. Arterialized blood samples were obtained by wrapping the hand and forearm in a heating blanket warmed to 45°C for the duration of the infusion. Baseline blood samples were drawn and then participants received priming doses of l-[ring-13C6]phenylalanine (2 μmol kg−1, 99 atom %; Cambridge Isotopes) prior to beginning a constant infusion of l-[ring-13C6]phenylalanine (0.05 μmol kg−1, 99 atom %; Fig. 1). l-[ring-13C6]phenylalanine was added to the amino acid mixture (Primene) to maintain plasma enrichments. Arterialized blood samples were collected every 0.5–1 h into evacuated heparinized tubes (Fig. 1) and chilled on ice. Blood was processed to yield perchloric acid extracts and plasma as previously described (Tang et al. 2007).
Muscle biopsies were taken from the vastus lateralis using a 5 mm Bergström needle (custom modified for manual suction) under 2% xylocaine local anaesthesia. Muscle biopsies were freed from any visible blood, fat and connective tissue and rapidly frozen in liquid nitrogen for further analysis. Four muscle biopsies were taken from each leg, but at a different incision site at least 4–5 cm proximal to the previous incision, at 150, 210, 270 and 390 min after start of the l-[ring-13C6] phenylalanine infusion for a given trial.
Blood sample analysis
The PCA blood extract was analysed for amino acid concentrations as described (Wilkinson et al. 2007). Blood l-[ring-13C6] phenylalanine enrichments were determined by heptaflurorobutyric acid (HFBA) derivatization of cation exchange resin-purified PCA plasma extracts and subsequent quantification using capillary gas chromatography–electron impact ionization–quadrupole mass spectrometry (GCMS; GC Hewlett Packard 6890, Palo Alto, CA, USA; MS Agilent 5973, Palo Alto) by monitoring ions at m/z (mass/charge) 316 and 322. Insulin levels were determined using a commercially available radioimmunoassay kit from Diagnostic Products Corporation (Los Angeles, CA, USA). Coefficients of variation (CV) for this assay did not exceed 7% for between-sample duplicates. Blood glucose was measured using a standard assay as previously described (Moore et al. 2005; Wilkinson et al. 2007), with duplicate sample CV of less than 4%.
Muscle tissue analysis
Sarcoplasmic and myofibrillar muscle proteins were isolated from biopsy specimens (∼40–50 mg wet weight) by homogenizing in 7.5 μl mg−1 homogenization buffer (50 mm Tris-HCl pH 7.5, 1 mm EGTA, 1 mm EDTA, 1% Triton-X 100, 0.1% 2-mercaptoethanol, protease inhibitor tablet per 10 ml (Roche, Indianapolis, IN, USA), 10 mmβ-glycerophosphate, 0.5 mm Na3VO4) on ice. The samples were vortexed and centrifuged (950 g, 10 min, 4°C). The supernatant was removed and the pellet re-spun (6550 g, 3 min, 4°C) to remove remaining supernatant. Supernatant concentrations were determined by Bradford assay (Sigma). Aliquots were diluted to 3 μg μl−1 with homogenization buffer and 5× Laemmli buffer, boiled at 100°C for 5 min, then stored at −80°C until Western blot analysis (see below). The remaining pellet was washed with homogenization buffer and centrifuged (6550 g, 3 min, 4°C). The supernatant was discarded and the pellet incubated with 0.3 m NaOH (30 min, 37°C) and centrifuged (950 g, 10 min, 4°C). This was repeated and the supernatants were pooled. PCA (1 m) was added to precipitate protein, samples vortexed and centrifuged as above, then the pellet was washed twice with 70% ethanol and allowed to dry. Myofibrillar pellets were hydrolysed overnight in a slurry of 0.1 m HCl and Dowex 50WX8-200 resin (110°C; Sigma, St Louis, MO, USA). Free amino acids were purified using cation exchange chromatography (Dowex 50WX8–200 resin) and converted to their N-acetyl-n-propyl ester derivatives for analysis by gas chromatography combustion–isotope ratio mass spectrometry (Hewlett Packard 6890; IRMS model Delta Plus XP, Thermo Finnigan). To estimate 13C background enrichment in body phenylalanine, plasma protein from the baseline blood sample was isolated by precipitation with ice-cold ethanol, centrifuged, and washed with 70% ethanol. The pellet was then processed as described for the myofibrillar pellet.
Intracellular amino acids were extracted from a separate ∼20 mg piece of muscle by incubation in ice-cold (12.5 μl mg−1) 0.6 m PCA and 62.5 ng mg−1 norleucine with agitation for 10 min at 4°C. Samples were centrifuged (15000 g, 2 min, 4°C) and extraction was repeated with (7.5 μl mg−1) 0.6 m PCA. The pooled extract was neutralized with 1.25 m KHCO3, centrifuged and transferred to fresh tubes. Aliquots (30 μl) were set aside for determination of intracellular amino acid concentrations as previously described (Wilkinson et al. 2006). The remainder of the extract was purified by cation exchange chromatography and derivatized with HFBA as above to obtain intracellular enrichments.
Samples of protein homogenate (30–60 μg) were loaded on to 7.5% (mTOR, FAK, eEF, p70s6 k) or 10% (Akt, GSK3β) SDS–polyacrylamide gels prior to being transferred to a PVDF membrane for blotting. Membranes were blocked with 5% BSA (w/v) in Tris-buffered saline with 0.1% Tween (v/v) (TBST) and then incubated overnight in primary antibody at 4°C: Akt Ser473 (Cell Signalling Technology, Danvers, MA, USA, no. 4058, 1 : 4000); total Akt (Cell Signalling, no. 9272; 1 : 4000); mTOR Ser2448 (Cell Signalling, no. 2971; 1 : 1000); total mTOR (Cell Signalling, no. 2972; 1 : 1000); p70S6K1 Thr389 (Santa Cruz Biotechnology, no. 11759; 1 : 5000); total p70S6K1 (Santa Cruz, no. 230, 1 : 4000); GSK-3β Ser9 (Cell Signalling, no. 9336; 1 : 2000); total GSK-3β (Cell Signalling, no. 9332; 1 : 6000); eukaryotic elongation factor-2 (eEF2) Thr56 (Cell Signalling, no. 2331; 1 : 3000); total eEF2 (Cell Signalling, no. 2332; 1 : 3000); FAK Tyr576/577 (Santa Cruz, no. 21831, 1 : 16000), total FAK (Santa Cruz, no. 558; 1 : 8000). After washing in TBST, membranes were incubated in HRP-linked anti-rabbit IgG secondary antibody (Amersham Biosciences, Piscataway, NJ, USA, product NA934V; 1 : 10000), washed with TBST, and developed using SuperSignal West Dura substrate (Pierce) and wrapped in Saran wrap. Signals were digitally captured on a Fluorochem SP imaging system and quantified using AlphaEase Fluorchem SP software (Alpha Innotech, San Leandro, CA, USA). Membranes were also probed for actin (BD Biosciences, Mississauga, ON, Canada; no. 612656; 1 : 10000, anti-mouse secondary (Amersham Biosciences, Piscataway, NJ, USA; NA931, 1 : 10000). Phosphorylated proteins are expressed relative to actin, except for FAK, which is expressed relative to total protein. Actin concentrations of the vastus lateralis have not been found to change with unilateral lower limb suspension (Haus et al. 2007), nor did we find a change in actin levels with Western blotting (data not shown). Ubiquitin–protein conjugates were quantified by probing blots (15 μg protein load) with a rabbit polyclonal antibody against ubiquitin protein conjugates (Biomol International, Plymouth Meeting, PA, USA; no. UG-9510, 1 : 4000) and corrected for loading with Ponceau S (Sigma) staining of sample lanes.
Calculations
The rates of myofibrillar protein synthesis were calculated using the standard precursor–product method:
where ΔEb is the change in bound protein enrichment between two times, Eic is the mean enrichment over time of the muscle intracellular pool, and t is the time between biopsies. Eic was a weighted average calculated as the area under the intracellular enrichment by time curve divided by time.
Statistics
As the present study used a within subject design, changes in MPS and the phosphorylation status of signalling proteins were analysed using a two-factor ANOVA with conditions for leg (immobilized and non-immobilized) and time (during the infusion protocol) where appropriate. Differences in ANOVA means were determined using only relevant pre-planned pair-wise comparisons and a Holm–Sidak post hoc test. For all analyses, statistical significance was set at P≤ 0.05. Values are expressed as means ±s.e.m.
Results
Muscle cross sectional area (CSA), mass and leg extension strength
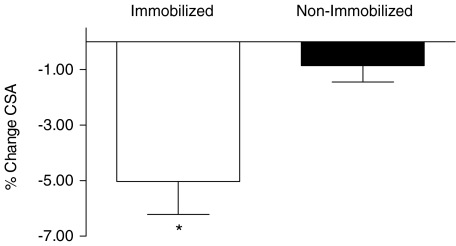
Muscle CSA measured by MRI at mid-thigh was reduced as a result of immobilization (−5.0 ± 1.2%, P < 0.005), whereas CSA of the non-immobilized leg remained unchanged (−0.9 ± 0.5, P = 0.51) (Fig. 2). Muscle mass assessed using dual-energy X-ray absorptiometry yielded revealed similar, albeit more variable, results to those of the MRI-based CSA analysis: mean leg muscle mass in the immobilized leg declined from 10251 ± 420 g to 10003 ± 432 g (−2.5 ± 1.1%, P < 0.005), whereas it remained unchanged in the non-immobilized leg (10117 ± 362 g and 10106 ± 383 g (−0.2 ± 0.9%, P = 0.77). There were no significant declines in total lean or body mass for either group, suggesting that subjects maintained adequate energy and protein intakes over the immobilization period. Isometric strength declined by −25 ± 3% (range −9 to −42%, P < 0.001) in the immobilized leg but was unchanged in the non-immobilized leg (3 ± 3%; range −3 to +17%, P = 0.67).
Figure 2. Quadriceps femoris cross-sectional area.
Values are means +s.e.m.*Significantly different from pre-immobilization in immobilized (P < 0.005) and non-immobilized (P = 0.47).
Blood amino acids and insulin
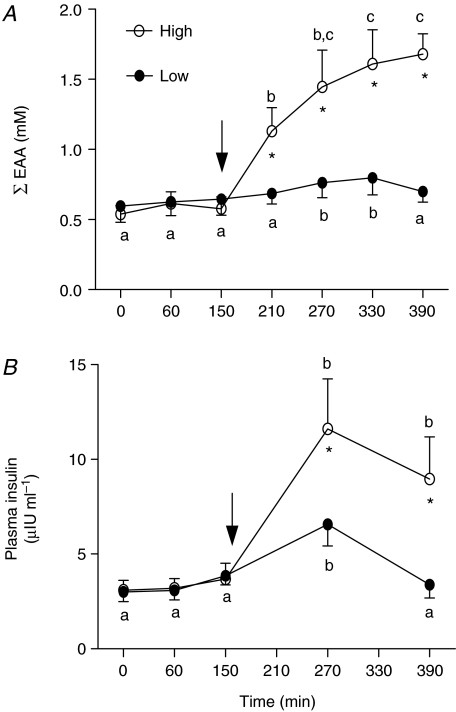
Patterns of aminoacidaemia were broadly similar for total, essential (EAA) and branched chain amino acids; we have presented data only for the EAA (Fig. 3A), which are more likely to be important for driving MPS. At the low dose (43 mg kg−1 h−1), EAA concentration was significantly elevated above the post-absorptive basal level (by about 50%) only at 270 and 330 min of infusion (both P < 0.05 versus basal; Fig. 3A). During the high dose (261 mg kg−1 h−1), EAA concentrations were rapidly elevated, reaching a plateau of ∼3 times post-absorptive values between 330 and 390 min (Fig. 3A, P < 0.01 versus 210 min). High dose EAA concentrations were significantly greater than those achieved during the low dose infusion at all times (P < 0.001, Fig. 3A).
Figure 3. Summed concentration of essential amino acids (EAA; A) and plasma insulin (B).
Values are means +s.e.m. Values associated with different letters are significantly different from each other (P < 0.05). *Significantly different from low dose condition (P < 0.01).
Plasma insulin increased moderately from 3 to 10 μIU ml−1 during the low dose amino acid infusion (Fig. 3B, P < 0.05), but then subsequently declined to baseline levels. At the high dose, insulin was increased by 270 min versus basal but thereafter remained elevated (Fig. 3B). Blood glucose remained stable throughout both low and high dose amino acid infusions at 4.5 ± 0.4 mm (data not shown).
Muscle intracellular essential amino acid content
In general immobilization resulted in an elevation in some muscle intracellular EAA and their summed content (Table 1). A rise in intracellular EAA content was seen in the high dose AA infusion in both the immobilized and non-immobilized leg (Table 1).
Table 1.
Muscle intracellular essential amino acid concentrations (pmol mg wet muscle−1)
| Low dose | ||||||||
|---|---|---|---|---|---|---|---|---|
| Immobilized | Non-immobilized | |||||||
| Fasted | 1 h | 2 h | 4 h | Fasted | 1 h | 2 h | 4 h | |
| Ile | 93 ± 11* | 96 ± 9* | 84 ± 11 | 83 ± 11 | 61 ± 3 | 61 ± 5 | 63 ± 8 | 64 ± 8 |
| Leu | 169 ± 23* | 159 ± 14* | 135 ± 20 | 145 ± 18 | 120 ± 7 | 121 ± 5 | 106 ± 9 | 110 ± 6 |
| Val | 206 ± 22 | 203 ± 13* | 183 ± 17 | 174 ± 18 | 172 ± 5 | 168 ± 7 | 152 ± 8 | 148 ± 6 |
| ∑BCAA | 469 ± 54* | 457 ± 35* | 403 ± 45 | 402 ± 44 | 353 ± 12 | 350 ± 14 | 321 ± 23 | 322 ± 17 |
| Lys | 152 ± 19+ | 132 ± 15+ | 154 ± 16 | 163 ± 32 | 256 ± 25 | 242 ± 28 | 230 ± 32 | 248 ± 36 |
| Met | 29 ± 7 | 31 ± 6 | 21 ± 4 | 23 ± 2 | 18 ± 4 | 18 ± 3 | 18 ± 4 | 19 ± 2 |
| Phe | 81 ± 11 | 80 ± 4+ | 83 ± 5 | 73 ± 6 | 58 ± 4 | 60 ± 4 | 71 ± 8 | 60 ± 5 |
| Thr | 605 ± 80 | 422 ± 43 | 409 ± 55 | 524 ± 50 | 545 ± 53 | 522 ± 90 | 543 ± 35 | 428 ± 33 |
| ∑EAA | 1336 ± 145 | 1122 ± 47 | 1070 ± 66 | 1185 ± 38* | 1329 ± 23 | 1193 ± 114 | 1184 ± 68 | 1076 ± 29 |
| High dose | ||||||||
| Immobilized | Non-immobilized | |||||||
| Fasted | 1 h | 2 h | 4 h | Fasted | 1 h | 2 h | 4 h | |
| Ile | 74 ± 7+a | 120 ± 9b | 136 ± 8+bc | 148 ± 13c | 49 ± 3a | 95 ± 6b | 102 ± 6b | 120 ± 7c |
| Leu | 147 ± 16#a | 230 ± 17+b | 246 ± 13#bc | 288 ± 29c | 107 ± 14a | 174 ± 17b | 198 ± 14b | 256 ± 15c |
| Val | 176 ± 9*a | 257 ± 16b | 296 ± 13#bc | 346 ± 30c | 148 ± 11a | 226 ± 16b | 248 ± 16b | 338 ± 22c |
| ∑BCAA | 397 ± 30#a | 607 ± 41*b | 678 ± 32#bc | 782 ± 70c | 304 ± 26a | 495 ± 26b | 548 ± 35b | 704 ± 42c |
| Lys | 120 ± 17*a | 186 ± 30ab | 220 ± 42*bc | 282 ± 50c | 211 ± 23a | 271 ± 40ab | 284 ± 39ab | 399 ± 57b |
| Met | 25 ± 5 | 21 ± 5 | 19 ± 5 | 28 ± 4 | 20 ± 2 | 22 ± 3 | 17 ± 4 | 23 ± 5 |
| Phe | 70 ± 5*a | 89 ± 6*b | 102 ± 3#b | 95 ± 5b | 56 ± 4a | 66 ± 7ab | 78 ± 3b | 78 ± 7b |
| Thr | 530 ± 58 | 609 ± 76 | 617 ± 51 | 642 ± 92 | 486 ± 64 | 602 ± 58 | 606 ± 104 | 568 ± 54 |
| ∑EAA | 1141 ± 55a | 1511 ± 76b | 1636 ± 58bc | 1830 ± 140c | 1077 ± 69a | 1456 ± 83b | 1543 ± 151bc | 1792 ± 72c |
Significant differences: *P < 0.05, +P < 0.01, #P < 0.005 between legs (immobilized versus non-immobilized) at the same time point. Means with different letters are significantly different (P < 0.05) across time, within the same leg.
Myofibrillar protein synthesis
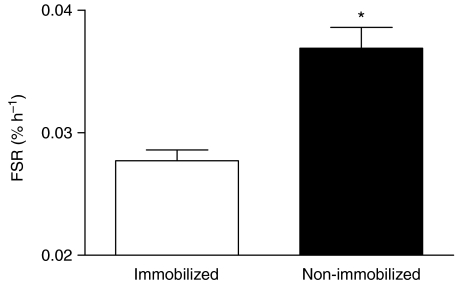
There were no differences between the fasted-state MPS rates in the muscles of individuals treated similarly with immobilization and the low or high dose infusion; we therefore pooled the data for all 12 participants (Fig. 4). Immobilization caused a reduction in post-absorptive MPS (27 ± 7%; Fig. 4, P < 0.001).
Figure 4. Pooled (from both low and high infused groups) resting fasted myofibrillar protein fractional synthetic rate (FSR).
*Significantly different from immobilized (P < 0.001).
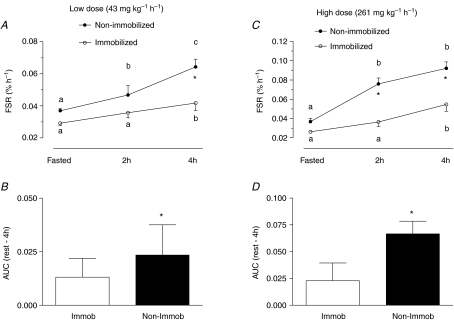
Infusion of low dose amino acids stimulated MPS in the non-immobilized leg at 2 h and induced a further rise at 4 h (Fig. 5A). The rise in MPS was blunted at 2 h in the immobilized leg but was significantly elevated at 4 h versus fasted values. However, at 4 h MPS remained significantly less in the muscle of the immobilized compared to the non-immobilized leg (Fig. 5A).
Figure 5. Myofibrillar fractional synthetic rate (FSR) and aggregate response of FSR indicated by area under the curve (AUC).
Fasted and fed state (2 h and 4 h) myofibrillar fractional synthetic rate (FSR) at low (A) and high (C) dose infusions. *Significantly different from non-immobilized (P < 0.001). Aggregate response of FSR indicated by area under the curve (AUC) for the low (B) and high (D) dose infusions. Means with different letters are significantly different from each other (P < 0.05). *Significantly different from immobilized (P < 0.001).
At the high dose amino acid infusion, the results resembled those at the low dose, but were exacerbated. At both 2 h and 4 h MPS in the non-immobilized leg was significantly higher than that in the immobilized leg (Fig. 5C). As a measure of the cumulative incorporation over the 4 h of infusion, we calculated the area under the FSR–time curves for both the immobilized and non-immobilized legs. The areas under the curve were significantly lower in the immobilized than in the non-immobilized leg at both doses (Fig. 5B and D).
Signalling proteins
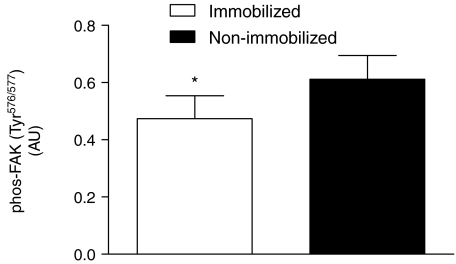
Focal adhesion kinase phosphorylation was found to be reduced (−23 ± 6%, P < 0.05; Fig. 6) in the immobilized state compared to the non-immobilized leg; these results were unaffected by feeding (data not shown).
Figure 6. Phosphorylation (Tyr576/577) of focal adhesion kinase (FAK) pooled from rested fasted immobilized (N = 12) and non-immobilized (N = 12) legs.
*Significantly different (P < 0.05) from immobilized.
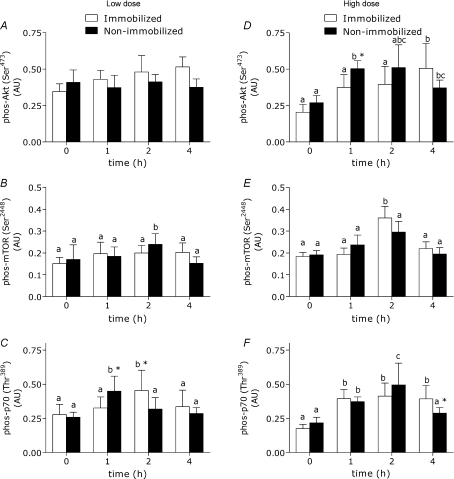
Proteins of the Akt–mTOR–p70S6k pathway were examined and found to remain unchanged in content between immobilized and non-immobilized legs (see online Fig. 2 Supplementary data). At the high dose amino acid infusion, changes in phosphorylation of Akt were greater (P < 0.05) 1 h into the infusion in the non-immobilized leg (Fig. 7D; P < 0.05). Low dose amino acid infusion induced little notable change in Akt or mTOR phosphorylation (Fig. 7A and E). Low dose AA infusion did result in differential phosphorylation of p70S6k, however, with increases in phosphorylation on this protein being greater (P < 0.05) at 1 h in the non-immobilized leg than the immobilized, a trend that was reversed at 2 h of infusion (Fig. 7C).
Figure 7. Phosphorylation (phos) of Akt, mTOR and p70S6k (normalized to α-actin) in immobilized and non-immobilized legs at a low dose (43 mg kg−1 h−1; panels on left) and a high dose (261 mg kg−1 h−1; panels on right).
*Significantly different from the opposite condition at the same time point (P < 0.05), means with different letters are significantly different across time (P < 0.05). Values are means + s.e.m.
Phosphorylation of eEF2 showed little change with either feeding or immobilization (see Fig. 1 Supplementary data). Akt-mediated inhibitory phosphorylation of GSK3-β at serine 9 increased in the non-immobilized leg by 4 h of feeding at the high dose (see Fig. 1 Supplementary data). Neither immobilization (P = 0.3) nor amino acid infusion (P = 0.09) changed the muscle content of polyubiquitinated proteins (see Fig. 3 Supplementary data).
Discussion
We have discovered, for the first time in human subjects, that immobilization appears to cause a deficit in the protein synthetic response of muscle to increased availability of amino acids. We have also confirmed the results of others (Gibson et al. 1987; Ferrando et al. 1996; Paddon-Jones et al. 2006; de Boer et al. 2007), that immobilization reduces post-absorptive muscle protein synthesis (in our case by 27%). Provision of amino acids at both low and high doses resulted in a delayed and lower net response (i.e. area under the curve) of MPS in the immobilized compared to the non-immobilized leg. Thus, the effect of feeding is not simply an extension of the lower fasted-state synthetic rate but is an example of what has been previously described as anabolic resistance (Cuthbertson et al. 2005), which is observable in a wide variety of clinical and subclinical states of slow muscle wasting (Rennie & Wilkes, 2005). In anabolic resistance, the normal anabolic response to amino acids is less sensitive and has a lower capacity with the probable result that fed-state gains in protein, which normally balance fasted-state losses (Rennie et al. 2004; Phillips, 2004), are less. Over time, such a state would result in declines in muscle mass, but as a result of a lower post-absorptive and fed-state protein accretion and not elevated proteolysis. Calculations presented elsewhere (de Boer et al. 2007) indicate that the observed reductions in post-absorptive muscle protein synthesis (Gibson et al. 1987; Ferrando et al. 1996; Paddon-Jones et al. 2006; de Boer et al. 2007) in periods over 10 days in combination with predicted estimates, confirmed here, of the extent of reduction in fed-state MPS would be enough to account for most, if not all, of the immobilization-induced decline in muscle protein mass. Indeed, Biolo et al. (2004) noted that the reduction in fed-state net protein deposition in subjects after 14 days bed rest could be completely accounted for by a suppression in amino acid-induced stimulation of whole-body protein synthesis whereas post-absorptive and fed-state whole-body proteolysis were not elevated. Our results showing a lack of elevation in polyubiquitinated muscle protein suggest no increased protein flux through the ATP-dependent polyubiqutin proteasome pathway. Thus, it appears that, in contrast to data obtained from studies in small animals (Gomes et al. 2001; Lecker et al. 2004, 2006; Sacheck et al. 2007), elevated proteolysis probably cannot be the primary mechanism underpinning the reduction in human muscle protein mass resulting from disuse. In fact, given the reported declines in post-absorptive (Gibson et al. 1987; Ferrando et al. 1996; Paddon-Jones et al. 2006; de Boer et al. 2007) and fed-state (current results) MPS, if proteolysis were even moderately elevated, then muscle mass would decline by more than 65% with ∼40 days of disuse. Adams et al. (2003) showed, using multiple models of disuse, however, that the decline in muscle CSA with unweighting eventually reaches a nadir.
The situation may be different early in immobilization. There is some evidence of increased muscle expression of mRNA and protein for proteolytic enzymes early on after limb immobilization and spinal cord injury (Urso et al. 2006, 2007). Increased (∼3-fold) mRNA expression of MuRF-1 (but no change in MAFbx or tripeptidyl peptidase II mRNA) has also been observed (de Boer et al. 2007). The lack of robust measurements of muscle protein breakdown by gold standard methods (e.g. stable isotope dilution across the immobilized leg of tracer amino acids) means, however, that we are still uncertain as to what happens in the early time period following immobilization. Recently indirect evidence for increased protein breakdown has been inferred from a comparison of measures of interstitial 3-methyl histidine derived from dialysis procedures in muscles from immobilized and non-immobilized legs (Tesch et al. 2008). Although there are a number of methodological difficulties with this approach, these data have been interpreted to suggest that in the short term (72 h), immobilization may induce a rise in interstitial 3-methyl histidine. Nevertheless, a disproportionate loss of the myofibrillar fraction or specific myofibrillar proteins (myosin and actin) from the vastus lateralis was not observed in a recent study with 35 day unilateral lower limb suspension (Haus et al. 2007). We and others (Paddon-Jones et al. 2001) did note increased intracellular concentrations of some amino acids (Table 1) with immobilization or bed rest, which could reflect a decreased demand for substrates to support synthesis. Intracellular amino acid concentrations increased in both legs during the high rate infusion, however, suggesting that uptake into the muscle is not impaired with immobilization.
Based on our work we propose that interventions to counter disuse atrophy over the medium to longer term would be most effective if they targeted the decline in protein synthesis rather than trying to offset increased proteolysis. An obvious intervention that has been repeatedly shown to be successful in countering disuse atrophy is resistance-based exercise (Carrithers et al. 2002; Alkner & Tesch, 2004; Haus et al. 2007) or combinations of resistance and aerobic-based exercise (Trappe et al. 2007, 2008). Our data indicate that amino acid or protein provision alone, without activity, would not ameliorate loss of muscle mass with disuse, and indeed several chronic bed rest studies have confirmed this proposition (Trappe et al. 2007, 2008; Brooks et al. 2008). By contrast, Paddon-Jones found that 16.5 g per day of crystalline essential amino acids (equivalent to ∼40 g of high quality protein) and glucose (30 g) consumed thrice daily was able to attenuate the decline in leg lean mass (Paddon-Jones et al. 2004) even with hypercortisolaemia (Paddon-Jones et al. 2005). However, given the failure of extra nutrition to maintain muscle mass during bed rest (Trappe et al. 2007, 2008; Brooks et al. 2008), the blunting of the whole-body protein synthetic response to hyperaminoacidaemia after 14 days of bed rest (Biolo et al. 2004), and our findings of anabolic resistance after acutely infusing quantities of amino acids greater than those used by Paddon-Jones et al. (2004, 2005), it is difficult to understand how an essential amino acid supplement was able to retain its capacity to repeatedly trigger a robust anabolic response throughout a 4 week period of bed rest. Whereas protein/amino acid supplementation has not been tested in an immobilization model, it is hard to imagine that an effect would be observed in a model of local atrophy when the majority of bed rest studies have failed to detect a benefit. Interestingly, net balance data indicated that the control group in Paddon-Jones et al. (2004) was in a mild catabolic state from the onset of bed rest, bringing into question its suitability. On the other hand, our subjects maintained their typical diets. Total body and lean mass did not change; therefore it is unlikely that they were in a general catabolic state, and yet they lost mass locally. We acknowledge that continuous amino acid infusion is not representative of the typical ‘bolus’ plasma response of oral feeding, and this difference may be raised as a reason for the divergent findings. We also noted, in contrast to Bohe et al. (2001), that MPS did not become refractory to high aminoacidaemia. In all likelihood this is due to the fact we did not achieve plateau in amino acid concentration until 3–4 h into the infusion versus a plateau at ∼30 min (J. Bohe and M. J. Rennie, unpublished data). However, hyperaminoacidaemia by direct infusion is normally a robust stimulator of synthesis, and yet it failed to elicit an equivalent response in the disused leg.
Phosphorylation of FAK had been reported to be reduced with hindlimb suspension in rodents and increased with synergist ablation (Gordon et al. 2001) or chronic overload in avian muscle (Fluck et al. 1999). Surprisingly, there are few reports of FAK or other proteins of the focal adhesion complex (paxillin, serum response factor) in differing loading states in human skeletal muscle. FAK phosphorylation was reported to be reduced with immobilization (de Boer et al. 2007). FAK has been proposed to be a mechanically sensitive protein (Fluck et al. 1999; Gordon et al. 2001), and thus may be a functional link between the loading of the muscle and a dampened protein synthetic response. We did not note, as we reasoned we might, a strong link between the responsiveness of the Akt–mTOR–p70S6 pathway to feeding. Other mechanically responsive integrins such as α7A/B-integrin, β1D-integrin and muscle agrin all appear to be altered with inactivity (Anastasi et al. 2006) and are perhaps worthy of further study.
In an attempt to understand the expected differences in MPS between the immobilized and non-immobilized limbs, we measured the phosphorylation of candidate signalling molecules known to be involved with the activation of protein synthesis in humans in response to feeding and/or altered loading (Kimball et al. 2002; Karlsson et al. 2004; Kimball & Jefferson, 2006; Dreyer et al. 2006; Eliasson et al. 2006; Fujita et al. 2007). We had hypothesized that the reduction in sensitivity of MPS to AA provision with immobilization would be accompanied by a broad reduction in phosphorylation (presumably as an indication of activity) of sites responsible for activation/deactivation of proteins that ‘turn on’ protein synthesis. Regrettably, little of the data we obtained can provide much insight into a potential mechanism of how immobilization might reduce the response of MPS to amino acid provision. We did see some evidence of a delayed phosphorylation of Akt at a high dose AA infusion and a similar phenomenon with p70S6k at the low dose, but the significance of these findings is unclear. Insulin increased during the high dose infusion, so the delay in Akt responsiveness may reflect some degree of inactivity-induced insulin resistance. Short-term (7 day) immobilization is sufficient to reduce insulin action on leg glucose uptake (Richter et al. 1989). Given that many of the signalling proteins are modulators of translational initiation, it is possible that we missed an early and transient divergence in the signalling response. Muscle protein synthesis is not up-regulated in response to amino acids until an hour after the onset of hyperaminoacidaemia (Bohe et al. 2001), but we are not aware of any information on the signalling response in humans within the first hour of feeding. It may be that there is no ‘master switch’ or regulator of protein synthesis but instead substantial redundancy and synergism (Proud et al. 2001; Averous & Proud, 2006). Furthermore, the suitability of using translational signalling factor phosphorylation as a surrogate of changes in human MPS is questionable. For example, the effects of amino acids and insulin on signalling and protein turnover have revealed a disconnect between insulin-induced increases in phosphorylation of Akt and p70s6k and MPS rates (Greenhaff et al. 2008). Nonetheless, several recent reports indicate that other signalling proteins may play greater regulatory roles in influencing protein synthesis than those we measured. For example, a recently identified site on eIF2Bɛ, Ser525, has been shown to be critical for the activation of this protein in response to AA (Wang & Proud, 2008). Similarly, sites on 4E-BP1 (Wang et al. 2005) as well as eukaryotic elongation factor-2 (eEF2) (Smith & Proud, 2008) also appear to be critically important for regulation of protein synthesis in response to provision of AA. It will be important to define the ‘crosstalk’ between mechanically responsive proteins and regulatory sites on critically important signalling proteins.
In summary, our data indicate that immobilized skeletal muscle exhibits a decrease in responsiveness of MPS to amino acids across a wide range of amino acid concentrations. Provision of very high concentrations of AA failed to return MPS to that seen in the non-immobilized muscle. This was associated with a marked reduction in the phosphorylation of the putative tension-sensing protein FAK in the immobilized leg, but with no striking evidence of decreased signalling in the Akt–mTOR–p70S6 pathway. Further work is clearly needed to confirm the possible interactions of FAK and the molecular regulators of muscle protein turnover. In particular, the elements which reduce the activation of protein synthesis in the immobilized state need to be identified.
Acknowledgments
This work was supported by a Collaborative Health Research Program award with grants from the Canadian Institutes of Health Research (CHIR) and the National Science and Engineering Research Council (NSERC) of Canada to S.M.P. and from UK BBSRC and EC EXEGENESIS to M.J.R. E.I.G. was supported by a CIHR Doctoral award. We thank the subjects for their patience and compliance with our protocols.
Supplemental material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2008.160333/DC1
References
- Adams GR, Caiozzo VJ, Baldwin KM. Skeletal muscle unweighting: spaceflight and ground-based models. J Appl Physiol. 2003;95:2185–2201. doi: 10.1152/japplphysiol.00346.2003. [DOI] [PubMed] [Google Scholar]
- Alkner BA, Tesch PA. Efficacy of a gravity-independent resistance exercise device as a countermeasure to muscle atrophy during 29-day bed rest. Acta Physiol Scand. 2004;181:345–357. doi: 10.1111/j.1365-201X.2004.01293.x. [DOI] [PubMed] [Google Scholar]
- Anastasi G, Cutroneo G, Santoro G, Arco A, Rizzo G, Trommino C, Bramanti P, Soscia L, Favaloro A. Integrins, muscle agrin and sarcoglycans during muscular inactivity conditions: an immunohistochemical study. Eur J Histochem. 2006;50:327–336. [PubMed] [Google Scholar]
- Averous J, Proud CG. When translation meets transformation: the mTOR story. Oncogene. 2006;25:6423–6435. doi: 10.1038/sj.onc.1209887. [DOI] [PubMed] [Google Scholar]
- Biolo G, Ciocchi B, Lebenstedt M, Barazzoni R, Zanetti M, Platen P, Heer M, Guarnieri G. Short-term bed rest impairs amino acid-induced protein anabolism in humans. J Physiol. 2004;558:381–388. doi: 10.1113/jphysiol.2004.066365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohe J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol. 2001;532:575–579. doi: 10.1111/j.1469-7793.2001.0575f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohe J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose–response study. J Physiol. 2003;552:315–324. doi: 10.1113/jphysiol.2003.050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks N, Cloutier GJ, Cadena SM, Layne JE, Nelsen CA, Freed AM, Roubenoff R, Castaneda-Sceppa C. Resistance training and timed essential amino acids protect against the loss of muscle mass and strength during 28 days of bed rest and energy deficit. J Appl Physiol. 2008;105:241–248. doi: 10.1152/japplphysiol.01346.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrithers JA, Tesch PA, Trieschmann J, Ekberg A, Trappe TA. Skeletal muscle protein composition following 5 weeks of ULLS and resistance exercise countermeasures. J Gravit Physiol. 2002;9:155–156. [PubMed] [Google Scholar]
- Cuthbertson DJ, Babraj JA, Smith K, Wilkes E, Fedele MJ, Esser K, Rennie MJ. Anabolic signalling and protein synthesis in human skeletal muscle after dynamic shortening or lengthening exercise. Am J Physiol Endocrinol Metab. 2006;290:E731–E738. doi: 10.1152/ajpendo.00415.2005. [DOI] [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- de Boer MD, Selby A, Atherton P, Smith K, Seynnes OR, Maganaris CN, Maffulli N, Movin T, Narici MV, Rennie MJ. The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J Physiol. 2007;585:241–251. doi: 10.1113/jphysiol.2007.142828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab. 2008;294:E392–E400. doi: 10.1152/ajpendo.00582.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576:613–624. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson J, Elfegoun T, Nilsson J, Kohnke R, Ekblom B, Blomstrand E. Maximal lengthening contractions increase p70, S6 kinase phosphorylation in human skeletal muscle in the absence of nutritional supply. Am J Physiol Endocrinol Metab. 2006;291:E1197–E1205. doi: 10.1152/ajpendo.00141.2006. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol. 1996;270:E627–E633. doi: 10.1152/ajpendo.1996.270.4.E627. [DOI] [PubMed] [Google Scholar]
- Fluck M, Carson JA, Gordon SE, Ziemiecki A, Booth FW. Focal adhesion proteins FAK and paxillin increase in hypertrophied skeletal muscle. Am J Physiol Endocrinol Metab. 1999;277:C152–C162. doi: 10.1152/ajpcell.1999.277.1.C152. [DOI] [PubMed] [Google Scholar]
- Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshizawa F, Volpi E, Rasmussen BB. Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol. 2007;582:813–823. doi: 10.1113/jphysiol.2007.134593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JN, Halliday D, Morrison WL, Stoward PJ, Hornsby GA, Watt PW, Murdoch G, Rennie MJ. Decrease in human quadriceps muscle protein turnover consequent upon leg immobilization. Clin Sci (Lond) 1987;72:503–509. doi: 10.1042/cs0720503. [DOI] [PubMed] [Google Scholar]
- Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A. 2001;98:14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SE, Fluck M, Booth FW. Selected contribution: Skeletal muscle focal adhesion kinase, paxillin, and serum response factor are loading dependent. J Appl Physiol. 2001;90:1174–1183. doi: 10.1152/jappl.2001.90.3.1174. [DOI] [PubMed] [Google Scholar]
- Greenhaff PL, Karagounis L, Peirce N, Simpson EJ, Hazell M, Layfield R, Wackerhage H, Smith K, Atherton P, Selby A, Rennie MJ. Disassociation between the effects of amino acids and insulin on signalling, ubiquitin-ligases and protein turnover in human muscle. Am J Physiol Endocrinol Metab. 2008;295:E595–E604. doi: 10.1152/ajpendo.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haus JM, Carrithers JA, Carroll CC, Tesch PA, Trappe TA. Contractile and connective tissue protein content of human skeletal muscle: Effects of 35 and 90 days of simulated microgravity and exercise countermeasures. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1722–R1727. doi: 10.1152/ajpregu.00292.2007. [DOI] [PubMed] [Google Scholar]
- Johnstone AM, Murison SD, Duncan JS, Rance KA, Speakman JR. Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. Am J Clin Nutr. 2005;82:941–948. doi: 10.1093/ajcn/82.5.941. [DOI] [PubMed] [Google Scholar]
- Karlsson HK, Nilsson PA, Nilsson J, Chibalin AV, Zierath JR, Blomstrand E. Branched-chain amino acids increase p70S6k phosphorylation in human skeletal muscle after resistance exercise. Am J Physiol Endocrinol Metab. 2004;287:E1–E7. doi: 10.1152/ajpendo.00430.2003. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Farrell PA, Jefferson LS. Role of insulin in translational control of protein synthesis in skeletal muscle by amino acids or exercise. J Appl Physiol. 2002;93:1168–1180. doi: 10.1152/japplphysiol.00221.2002. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr. 2006;136:227S–231S. doi: 10.1093/jn/136.1.227S. [DOI] [PubMed] [Google Scholar]
- Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol. 2006;17:1807–1819. doi: 10.1681/ASN.2006010083. [DOI] [PubMed] [Google Scholar]
- Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18:39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- Moore DR, Phillips SM, Babraj JA, Smith K, Rennie MJ. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab. 2005;288:E1153–E1159. doi: 10.1152/ajpendo.00387.2004. [DOI] [PubMed] [Google Scholar]
- Paddon-Jones D, Leveritt M, Lonergan A, Abernethy P. Adaptation to chronic eccentric exercise in humans: the influence of contraction velocity. Eur J Appl Physiol. 2001;85:466–471. doi: 10.1007/s004210100467. [DOI] [PubMed] [Google Scholar]
- Paddon-Jones D, Sheffield-Moore M, Cree MG, Hewlings SJ, Aarsland A, Wolfe RR, Ferrando AA. Atrophy and impaired muscle protein synthesis during prolonged inactivity and stress. J Clin Endocrinol Metab. 2006;91:4836–4841. doi: 10.1210/jc.2006-0651. [DOI] [PubMed] [Google Scholar]
- Paddon-Jones D, Sheffield-Moore M, Urban RJ, Aarsland A, Wolfe RR, Ferrando AA. The catabolic effects of prolonged inactivity and acute hypercortisolemia are offset by dietary supplementation. J Clin Endocrinol Metab. 2005;90:1453–1459. doi: 10.1210/jc.2004-1702. [DOI] [PubMed] [Google Scholar]
- Paddon-Jones D, Sheffield-Moore M, Urban RJ, Sanford AP, Aarsland A, Wolfe RR, Ferrando AA. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J Clin Endocrinol Metab. 2004;89:4351–4358. doi: 10.1210/jc.2003-032159. [DOI] [PubMed] [Google Scholar]
- Phillips SM. Protein requirements and supplementation in strength sports. Nutrition. 2004;20:689–695. doi: 10.1016/j.nut.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Proud CG. Signalling to translation: how signal transduction pathways control the protein synthetic machinery. Biochem J. 2007;403:217–234. doi: 10.1042/BJ20070024. [DOI] [PubMed] [Google Scholar]
- Proud CG, Wang X, Patel JV, Campbell LE, Kleijn M, Li W, Browne GJ. Interplay between insulin and nutrients in the regulation of translation factors. Biochem Soc Trans. 2001;29:541–547. doi: 10.1042/bst0290541. [DOI] [PubMed] [Google Scholar]
- Rennie MJ, Wackerhage H, Spangenburg EE, Booth FW. Control of the size of the human muscle mass. Annu Rev Physiol. 2004;66:799–828. doi: 10.1146/annurev.physiol.66.052102.134444. [DOI] [PubMed] [Google Scholar]
- Rennie MJ, Wilkes EA. Maintenance of the musculoskeletal mass by control of protein turnover: the concept of anabolic resistance and its relevance to the transplant recipient. Ann Transplant. 2005;10:31–34. [PubMed] [Google Scholar]
- Richter EA, Kiens B, Mizuno M, Strange S. Insulin action in human thighs after one-legged immobilization. J Appl Physiol. 1989;67:19–23. doi: 10.1152/jappl.1989.67.1.19. [DOI] [PubMed] [Google Scholar]
- Sacheck JM, Hyatt JP, Raffaello A, Jagoe RT, Roy RR, Edgerton VR, Lecker SH, Goldberg AL. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J. 2007;21:140–155. doi: 10.1096/fj.06-6604com. [DOI] [PubMed] [Google Scholar]
- Smith EM, Proud CG. cdc2-cyclin B regulates eEF2 kinase activity in a cell cycle- and amino acid-dependent manner. EMBO J. 2008;27:1005–1016. doi: 10.1038/emboj.2008.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein TP, Wade CE. Metabolic consequences of muscle disuse atrophy. J Nutr. 2005;135:1824S–1828S. doi: 10.1093/jn/135.7.1824S. [DOI] [PubMed] [Google Scholar]
- Tang JE, Manolakos JJ, Kujbida GW, Lysecki PJ, Moore DR, Phillips SM. Minimal whey protein with carbohydrate stimulates muscle protein synthesis following resistance exercise in trained young men. Appl Physiol Nutr Metab. 2007;32:1132–1138. doi: 10.1139/H07-076. [DOI] [PubMed] [Google Scholar]
- Tesch PA, von Walden F, Gustafsson T, Linnehan RM, Trappe TA. Skeletal muscle proteolysis in response to short-term unloading in humans. J Appl Physiol. 2008;105:902–906. doi: 10.1152/japplphysiol.90558.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trappe S, Creer A, Minchev K, Slivka D, Louis E, Luden N, Trappe T. Human soleus single muscle fiber function with exercise or nutrition countermeasures during 60 days of bed rest. Am J Physiol Regul Integr Comp Physiol. 2008;294:R939–R947. doi: 10.1152/ajpregu.00761.2007. [DOI] [PubMed] [Google Scholar]
- Trappe S, Creer A, Slivka D, Minchev K, Trappe T. Single muscle fiber function with concurrent exercise or nutrition countermeasures during 60 days of bed rest in women. J Appl Physiol. 2007;103:1242–1250. doi: 10.1152/japplphysiol.00560.2007. [DOI] [PubMed] [Google Scholar]
- Urso ML, Chen YW, Scrimgeour AG, Lee PC, Lee KF, Clarkson PM. Alterations in mRNA expression and protein products following spinal cord injury in humans. J Physiol. 2007;579:877–892. doi: 10.1113/jphysiol.2006.118042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urso ML, Scrimgeour AG, Chen YW, Thompson PD, Clarkson PM. Analysis of human skeletal muscle after 48 h immobilization reveals alterations in mRNA and protein for extracellular matrix components. J Appl Physiol. 2006;101:1136–1148. doi: 10.1152/japplphysiol.00180.2006. [DOI] [PubMed] [Google Scholar]
- Vargas R, Lang CH. Alcohol accelerates loss of muscle and impairs recovery of muscle mass resulting from disuse atrophy. Alcohol Clin Exp Res. 2008;32:128–137. doi: 10.1111/j.1530-0277.2007.00548.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Beugnet A, Murakami M, Yamanaka S, Proud CG. Distinct signaling events downstream of mTOR cooperate to mediate the effects of amino acids and insulin on initiation factor 4E-binding proteins. Mol Cell Biol. 2005;25:2558–2572. doi: 10.1128/MCB.25.7.2558-2572.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda) 2006;21:362–369. doi: 10.1152/physiol.00024.2006. [DOI] [PubMed] [Google Scholar]
- Wang X, Proud CG. A novel mechanism for the control of translation initiation by amino acids, mediated by phosphorylation of eukaryotic initiation factor 2B. Mol Cell Biol. 2008;28:1429–1442. doi: 10.1128/MCB.01512-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson SB, Kim PL, Armstrong D, Phillips SM. Addition of glutamine to essential amino acids and carbohydrate does not enhance anabolism in young human males following exercise. Appl Physiol Nutr Metab. 2006;31:518–529. doi: 10.1139/h06-028. [DOI] [PubMed] [Google Scholar]
- Wilkinson SB, Tarnopolsky MA, MacDonald MJ, Macdonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion following resistance exercise than an isonitrogenous and isoenergetic soy protein beverage. Am J Clin Nutr. 2007;85:1031–1040. doi: 10.1093/ajcn/85.4.1031. [DOI] [PubMed] [Google Scholar]
- Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84:475–482. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- Yasuda N, Glover EI, Phillips SM, Isfort RJ, Tarnopolsky MA. Sex-based differences in skeletal muscle function and morphology with short-term limb immobilization. J Appl Physiol. 2005;99:1085–1092. doi: 10.1152/japplphysiol.00247.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.