Abstract
Although the performance capabilities of muscle differ during shortening and lengthening contractions, realization of these differences during functional tasks depends on the characteristics of the activation signal discharged from the spinal cord. Fundamentally, the control strategy must differ during the two anisometric contractions due to the lesser force that each motor unit exerts during a shortening contraction and the greater difficulty associated with decreasing force to match a prescribed trajectory during a lengthening contraction. The activation characteristics of motor units during submaximal contractions depend on the details of the task being performed. Indexes of the strategy encoded in the descending command, such as coactivation of antagonist muscles and motor unit synchronization, indicate differences in cortical output for the two types of anisometric contractions. Furthermore, the augmented feedback from peripheral sensory receptors during lengthening contractions appears to be suppressed by centrally and peripherally mediated presynaptic inhibition of Ia afferents, which may also explain the depression of voluntary activation that occurs during maximal lengthening contractions. Although modulation of the activation during shortening and lengthening contractions involves both supraspinal and spinal mechanisms, the association with differences in performance cannot be determined without more careful attention to the details of the task.
Although the performance capacity of muscle differs for shortening and lengthening contractions (Katz, 1939; Edman, 1988; Morgan et al. 2000), the realization of this potential depends on the characteristics of the activation signal discharged from the spinal cord. The purpose of this topical review is to compare the activation signals for shortening and lengthening contractions and to indicate how the differences influence performance during submaximal and maximal contractions. The review discusses why there should be a difference in the control strategies used by the nervous system for these two types of contractions, describes the findings on motor unit activity and the underlying adjustments in synaptic input to the motor neurone pool during submaximal contractions, and compares the activation signals during maximal contractions.
Control strategies
The functional distinction between shortening and lengthening contractions is simply whether the muscle fibres shorten or lengthen as the activated muscle exerts a force against a load. Knowing when fibres shorten or lengthen during a contraction, however, is not trivial. Because muscle comprises an in-series arrangement of contractile proteins and connective tissue between its attachments to the skeleton, changes in whole muscle length may not correspond to changes in muscle fibre length. Accordingly, measurements with ultrasonography have indicated that some actions can involve an increase in whole muscle length with no change in muscle fibre length (Ishikawa et al. 2005; Kawakami & Fukunaga, 2006). This review focuses on the neural control of contractions that involve either a shortening or a lengthening of the muscle fibres.
Whereas shortening contractions are used solely to displace a load, lengthening contractions can be used either to resist an imposed load or to control the displacement of a load. One distinction between the two lengthening-contraction behaviours is the extent to which the activation signal is modulated during the action. The intensity of the activation signal changes minimally when resisting an imposed load, in contrast to the amount that it can vary when a load is being displaced. Examples of lengthening contractions that are used to resist an imposed load include maximal isokinetic actions and the braking of rapid movements. Isokinetic actions require muscles to perform work against a torque motor that maintains a constant angular velocity of the limb over a specified range of motion (Kawakami & Fukunaga, 2006). When performing maximal isokinetic actions at moderate-to-fast speeds, there is little modulation in EMG amplitude and, presumably, the underlying amount of motor unit activity (Fig. 1A). Maximal lengthening contractions against a torque motor therefore require an individual to resist the lengthening action imposed by the torque motor by sustaining high levels of motor unit activity over the prescribed range of motion.
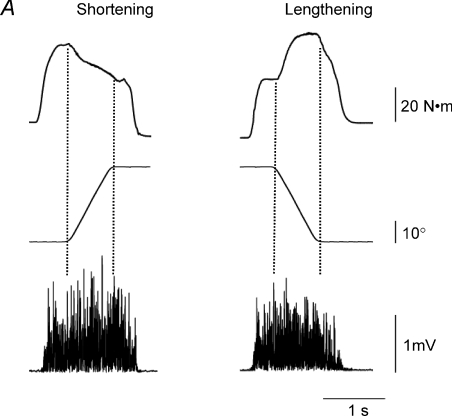
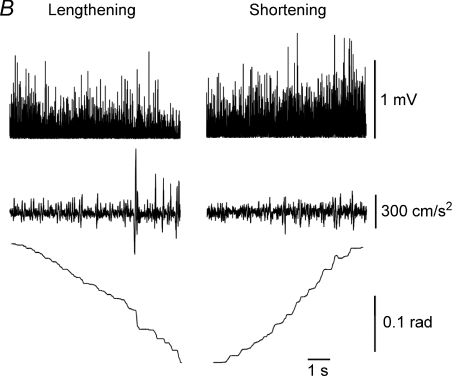
Figure 1. Examples of shortening and lengthening contractions.
A, a subject performed maximal shortening and lengthening contractions with the dorsiflexor muscles against a torque motor. The range of motion for the two isokinetic actions was 30 deg and the angular velocity 50 deg s−1. Both contractions were preceded by a maximal isometric contraction, which enabled the motivated subjects to achieve the same EMG amplitude during the two contractions (Baudry et al. 2007). The vertical dotted lines indicate the beginning and the end of the movement in each condition. The amplitude of the surface EMG recordings for tibialis anterior was slightly less (∼10%) when the muscle resisted the torque motor during the lengthening contraction than during the shortening contraction. The torque produced by the dorsiflexor muscles was greater during the lengthening contraction and changed in opposite directions during the shortening and lengthening contractions. Data provided by Dr Stéphane Baudry. B, a subject lifted and lowered an inertial load that was 15% of the maximal load with a hand muscle (first dorsal interosseus). The figure shows an 8 s lengthening contraction (adduction of the finger) to lower the load and a 7 s shortening contraction (abduction of the finger) to lift the load at a target velocity of 0.03 rad−1 over a 0.18 rad (∼10 deg) range of motion. Each contraction was preceded by a 0.5 s isometric contraction. The traces (top to bottom) indicate the rectified surface EMG for first dorsal interosseus, acceleration of the index finger in the plane (abduction–adduction) of the movement, and the displacement about the metacarpophalangeal joint. Note the gradual decrease in EMG amplitude and the greater fluctuations in acceleration during the lengthening contraction compared with the shortening contraction. Adapted from Christou et al. (2003); used with permission.
Similarly, rapid movements that involve either stopping the movement at a specific location or reversing the direction of displacement are realized with minimal modulation of the activation signal during the lengthening contraction (Corcos et al. 1989; Garland et al. 1996; d’Avella et al. 2006). A classic example of such an action is the stretch-shorten cycle. The stretch-shorten cycle is a common feature of many movements and comprises an initial increase in whole muscle length that is followed immediately by a shortening of the muscle, such as occurs with the leg extensor muscles in the stance phase of running (Nicol et al. 2006). The stretch-shorten cycle can increase the amount of positive work performed and power produced by the muscle during the shortening contraction by enabling a preceding increase in muscle length (Cavagna & Citterio, 1974; Kawakami et al. 2002). However, the stretch is so rapid that the intensity of the activation signal must be established prior to the action.
Lengthening contractions that involve modulation of the activation signal (Fig. 1B) are accomplished by controlling the number of motor units that are activated and the rate at which the recruited motor units discharge action potentials. When the task involves varying muscle force to match an intended profile, the control strategy must accommodate the different rise and decay times of the forces generated by the activated motor units (Nardone & Schieppati, 1988). For example, a gradual increase in force during an isometric contraction requires that the activation of the motor units be aligned so that the summed rise times match the desired trajectory (Fig. 2). Conversely, a targeted decrease in force requires that the decay times be matched, which involves predicting the time course of the contractile events that define the decay rate. Thus, the increase and decrease in force during an isometric contraction require different control strategies, which contributes to the greater difficulty that some individuals experience when decreasing force during an isometric contraction (Semmler et al. 2002; Kimura et al. 2003).
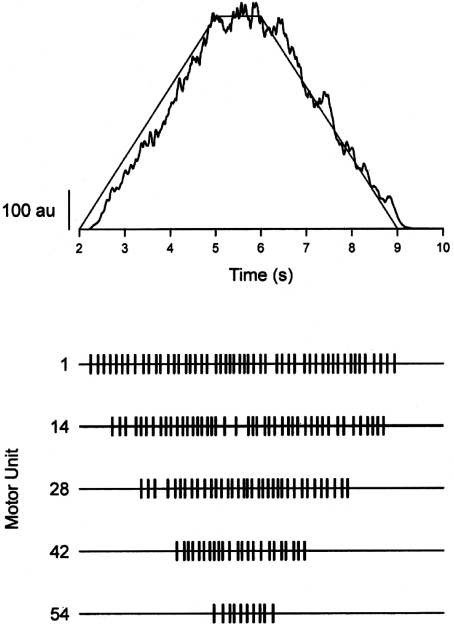
Figure 2. Simulated motor unit activity to produce an increase and decrease in isometric force.
Computer simulation of the motor unit forces required to match a template that comprised a gradual increase in force, a brief sustained force at 2% MVC force, and a gradual decrease in force. The model of motor unit recruitment and rate coding for the isometric contraction was similar to the one reported by Barry et al. (2007). The force template was approximated with ramp increases and decreases in an excitation signal that recruited and derecruited 54 motor units during the task. The timing of action potential discharges by four representative motor units (1, 14, 28, and 42) that were active during the ramp-up and ramp-down phases are indicated as tick marks in the lower traces. The mean discharge rate of these four motor units was 7.37 pulses s−1 during the ramp increase in force and 7.17 pulses s−1 during the ramp decrease in force, and the corresponding coefficients of variation for interspike interval were 29.9% and 22.4%, respectively. In contrast to the similar rate coding characteristics during the two phases of the task, the derecruitment of motor units during the ramp decrease in force was more variable than the recruitment during the ramp increase in force; the mean ±s.d. difference in the force at which consecutive motor units were recruited was 0.038 ± 0.053% MVC force compared with 0.031 ± 0.207% MVC force when the motor units were derecruited. The standard deviation of the force about the ramp change in force (detrended force) was 13.9 a.u. during the ramp increase in force and 22.4 a.u. during the decrease. Generated by Mark Jesunathadas.
The different control strategies required for increasing and decreasing force during an isometric contraction are further compounded by the influence of load compliance and changes in muscle length during anisometric contractions (Gottlieb, 1996; Christou & Carlton, 2002). When an individual performs an isometric contraction and exerts a force against a rigid restraint, for example, the inputs received by the motor neurones differ from when the same muscle force is used to support an inertial load (Akazawa et al. 1983; Hulliger et al. 1985; Doemges & Rack, 1992; Buchanan & Lloyd, 1995; De Serres et al. 2002). The control strategy is further complicated when the task involves a movement (Burnett et al. 2000; Christou & Carlton, 2002; Christou et al. 2003), as indicated by an increase in the gain of the stretch reflex (Doemges & Rack, 1992) and greater coherence at 6–12 Hz in the discharge of action potentials by pairs of motor units (Kakuda et al. 1999). Despite the increase in the gain of the stretch reflex and augmented feedback from muscle spindles (Burke et al. 1978), the amplitude of the stretch reflex is depressed during movement and especially when the task involves lengthening contractions, which suggests the involvement of central pathways to control the inflow of sensory information from the periphery (Nakazawa et al. 1997, 1998; Bawa & Sinkjær, 1999).
Taken together, these studies establish that differences in the descending input and peripheral afferent feedback to the motor neurone pool during shortening and lengthening contractions must be managed by the nervous system to accommodate the constraints associated with the required force profile for each task.
Submaximal contractions
Two factors confound the association between motor unit activity and muscle force during anisometric contractions. First, the greater intrinsic force capacity of the muscle fibres during lengthening contractions (Katz, 1939; Edman, 1988; Morgan et al. 2000) means that less motor unit activity is required to achieve a specific absolute force compared with that needed during a shortening contraction. Second, muscle torque must be greater than the load torque during shortening contractions, whereas it is required to be less than the load torque during lengthening contractions. As a consequence of these attributes, less motor unit activity is required to displace a submaximal load with a lengthening contraction than with a shortening contraction (Figs 1B and 3).
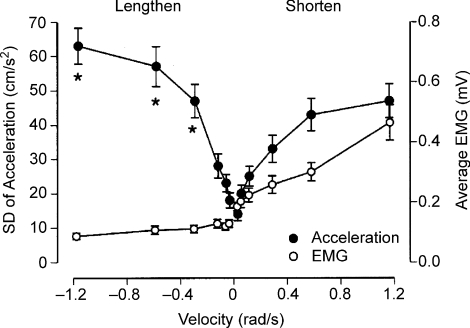
Figure 3. Influence of movement velocity on acceleration and EMG during shortening and lengthening contractions.
Index finger acceleration and the average rectified EMG for first dorsal interosseus during lengthening and shortening contractions. Values are means ±s.e.m. of the standard deviation (s.d.) for six targeted velocities. The s.d. of acceleration during the three fastest lengthening contractions was significantly greater (*) than that during the three fastest shortening contractions. EMG amplitude during the shortening contractions was greater than that during the lengthening contractions. Reproduced from Christou et al. (2003) with permission.
Motor unit activity
A critical question in this field is whether submaximal lengthening contractions are controlled by a scaled-down version of the activation signal used for shortening contractions, or do they require a unique activation signal? In general, activation of the few hundred motor neurones that innervate an average muscle depends on the interaction between the net synaptic input they receive and the distribution of intrinsic properties across the population of motor neurones (Heckman & Enoka, 2004). Because the net synaptic input does differ during shortening and lengthening contractions, the possibility exists for a qualitative difference in the activation signal during the two anisometric contractions.
The contributions of a motor unit to muscle force are characterized by the force at which it is recruited and the range over which it can vary discharge rate during a voluntary contraction. Although the recruitment order of motor units is quite consistent during voluntary contractions, it can be altered when muscle is activated with electrical stimulation (Feiereisen et al. 1997), when sensory feedback is manipulated (Stephens et al. 1978), and when a muscle contributes to multiple functions (van Zuylen et al. 1988). Although the results have been equivocal, some studies have also found that the recruitment order of motor units differs during shortening and lengthening contractions (Nardone et al. 1989; Howell et al. 1995), which would require that the synaptic inputs to the motor neurone pool, either from descending or peripheral sources, differ during the two anisometric contractions.
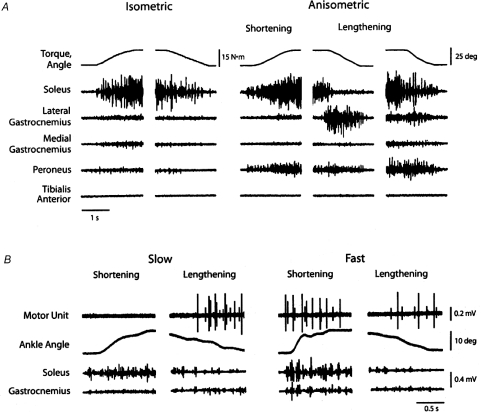
A seminal study on recruitment order during lengthening contractions recorded the discharge of single motor units in soleus, lateral gastrocnemius and medial gastrocnemius as seated subjects performed isometric and anisometric contractions with the plantarflexor muscles (Nardone et al. 1989). The anisometric contractions involved lifting and lowering an inertial load to match a prescribed trajectory. In contrast to the strategy used to increase and decrease torque during the isometric contractions, the distribution of EMG activity across the involved muscles changed when lifting and lowering the load with shortening and lengthening contractions, respectively (Fig. 4A). The change in strategy indicated that the lengthening contraction was not simply the converse of the shortening contraction (Nardone & Schieppati, 1988).
Figure 4. Activation of the triceps surae muscles during isometric and anisometric contractions.
The subject matched the rate of change in torque during the isometric contractions and the rate of change in ankle angle during the anisometric contractions. A, the shortening contraction and the increase in torque during the isometric contraction involved a comparable increase in soleus EMG, but a minor difference in the involvement of other muscles. In contrast, the distribution of EMG activity during the lengthening contraction differed from that observed when decreasing force during the isometric contraction. Furthermore, subjects used various strategies during the lengthening contraction, as indicated by the two columns on the right. Reproduced from Nardone & Schieppati (1988). B, motor units in lateral gastrocnemius (top trace) within the recording volume of the electrode were selectively recruited during slow lengthening contractions and during a fast shortening contraction. Reproduced from Nardone et al. (1989).
From a sample of 99 motor units, Nardone et al. (1989) found that 15% of the units in soleus and 50% of the units in the two gastrocnemii were recruited only during lengthening contractions (Fig. 4B). These motor units invariably had a high recruitment threshold during an isometric contraction. When the task involved a shortening–isometric-lengthening sequence of contractions, the recruitment of these units during the lengthening contraction was accompanied by the derecruitment of other units that were active during the shortening and isometric contractions (Fig. 4B). Significantly, the selective recruitment of high-threshold motor units during the lengthening contraction only occurred when dictated by the target angular velocity. Nardone et al. (1989) proposed that the more rapid relaxation of the force for the high-threshold motor units enabled the subjects to match the torque to the prescribed trajectory more accurately during faster lengthening contractions. They suggested that such a change in strategy might be accomplished by a selective reduction in net excitation to low-threshold motor units so that the desired decrease in force can be achieved more readily.
Other studies that have examined recruitment order during anisometric contractions, however, have found no difference between shortening and lengthening contractions either when lifting an inertial load (Garland et al. 1996; Søgaard et al. 1996; Laidlaw et al. 2000; Stotz & Bawa, 2001) or pushing against a torque motor (Stotz & Bawa, 2001; Pasquet et al. 2006). These findings indicate that recruitment order does not consistently vary during lengthening contractions, especially when the task does not require matching the displacement to a target trajectory. The evidence seems to suggest therefore that the recruitment order of motor units is usually similar during shortening and lengthening contractions but that it may vary when the task requires the individual to perform a relatively fast lengthening contraction that matches a prescribed trajectory (Nardone et al. 1989).
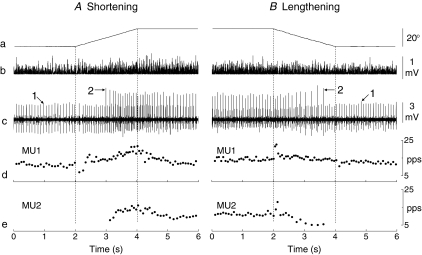
The influence of the task requirements on the recruitment of motor units during anisometric contractions also extends to the modulation of discharge rate. Because the net muscle torque must exceed the load torque during shortening contractions and be less than the load torque during lengthening contractions, motor units are derecruited and discharge rate decreases when lowering an inertial load with a lengthening contraction (Laidlaw et al. 2000; Stotz & Bawa, 2001; Del Valle & Thomas, 2005; Pasquet et al. 2006). The discharge characteristics of motor units during shortening and lengthening contractions that involve resisting a torque motor again depend on the task requirements. When individuals performed slow shortening and lengthening contractions with a similar change in fascicle length in tibialis anterior (Fig. 5), for example, the shortening contraction was characterized by greater modulation of discharge rate than during the lengthening contraction and the recruitment of additional motor units that were derecruited during the lengthening contraction (Pasquet et al. 2006).
Figure 5. Modulation of discharge rate during anisometric contractions.
The discharge and recruitment patterns of two motor units (MU1 and MU2) in tibialis anterior during an initial isometric contraction and subsequent shortening (A) and lengthening (B) contractions. The vertical dotted lines indicate the beginning and the end of each movement. The traces indicate angular ankle displacement (a), rectified surface (b) and intramuscular (c) EMG of the tibialis anterior, and instantaneous discharge rate of MU1 (d) and MU2 (e). MU2 was recruited during the shortening contraction at an ankle angle of 1 deg dorsiflexion and derecruited during the lengthening contraction at a more extended ankle joint angle (8 deg plantarflexion). At the transition from the initial isometric contraction to the anisometric contraction, there was either a transient decrease (shortening contraction) or increase (lengthening contraction) in discharge rate due to an unloading reflex or stretch reflex, respectively (d and e). Subsequently, there was greater modulation of discharge rate for both motor units during the shortening contraction than during the lengthening contraction. Reproduced from Pasquet et al. (2006) with permission.
Adjustments in synaptic input
The differences in motor unit recruitment and discharge characteristics during shortening and lengthening contractions require modulation of either the descending command or the sensory feedback to the motor neurone pool (Nielsen, 2004). A consistent finding in studies on the neural control of anisometric contractions is the reduced amplitude of responses evoked by supraspinal and peripheral input during lengthening contractions. The reduction has been observed for motor evoked potentials elicited by transcranial magnetic and electrical stimulation (Abbruzzese et al. 1994; Sekiguchi et al. 2003b), Hoffmann (H) reflexes (Romanò & Schieppati, 1987; Abbruzzese et al. 1994; Nordlund et al. 2002; Duclay & Martin, 2005), and stretch reflexes (Nakazawa et al. 1997) when EMG was matched in both contraction types. Because transcranial electrical stimulation is likely to activate the axons of cortical neurones whereas transcranial magnetic stimulations activates the neurones transynaptically (Rothwell, 1997), the decrease in the amplitude of the responses evoked by both supraspinal and peripheral (H reflex) stimuli during lengthening contractions has been attributed to spinal mechanisms (Abbruzzese et al. 1994).
Some evidence, however, suggests that cortical output differs during shortening and lengthening contractions. Despite a smaller EMG during lengthening contractions, for example, the amplitude of the movement-related cortical potential derived from the electroencephalogram was greater during a lengthening contraction than a shortening contraction when subjects lowered and raised, respectively, an inertial load (10% of body weight) to match a prescribed trajectory with the elbow flexor muscles (Fang et al. 2001). A similar difference was also evident during maximal isokinetic actions as the amplitude and distribution of the planning-and-execution component of the movement-related cortical potential was greater for maximal lengthening contractions when the elbow flexor muscles pushed against a torque motor (Fang et al. 2004). The relative difference in the cortical potential between the shortening and lengthening contractions with the elbow flexor muscles was greater for the maximal contractions compared with the submaximal ones. Although movement-related cortical potentials provide a relatively crude measure of the output from the brain, the changes suggest that the brain is more involved in the preparation, planning and execution of the movement and with the processing of sensory input during lengthening contractions compared with shortening contractions.
The shift in cortical activity during the two types of anisometric contractions may contribute to the differences that have been observed in indexes of the strategy encoded in the descending command, such as coactivation of the antagonist muscle and motor unit synchronization. For example, Sekiguchi et al. 2003a) evoked H reflexes in soleus as subjects slowly lifted and lowered an inertial load with the dorsiflexor muscles to match a target trajectory. Stimulus intensity was varied across trials to determine the recruitment curves for the H reflex and M wave (direct motor response) during the shortening and lengthening contractions. The slope of the ascending phase of the recruitment curve for the H reflex relative to the corresponding slope for the M wave, which is an index of the responsiveness of the motor neurone pool to input from Ia afferents, was greater during the lengthening contractions relative to the shortening contractions, despite similar background EMGs. Because the excitability of the motor neurone pool innervating the antagonist muscle is depressed by reciprocal inhibition from the agonist muscle during these actions (Crone & Nielsen, 1994; Pyndt et al. 2003), the greater responsiveness during the lengthening contractions may be due to a lower reciprocal inhibition from the agonist to the antagonist muscles than occurs during shortening contractions. However, Pasquet et al. (2006) did not observe any difference in coactivation when individuals performed slow shortening and lengthening contractions with the dorsiflexor muscles against a torque motor.
Consistent with the influence of targeted displacements of inertial loads on H reflexes during anisometric contractions, Semmler et al. (2002) found that the level of motor unit synchronization was greater during slow lengthening contractions compared with slow shortening contractions when subjects lifted and lowered an inertial load (∼5% MVC force) to match a target trajectory with the first dorsal interosseus muscle. The strength of synchronization was 50% greater during the lengthening contractions and the amount of low-frequency (2–12 Hz) motor unit coherence was less during the shortening contractions. These findings indicate that the relative amount of common input to the motor neurone pool from descending pathways differs during shortening and lengthening contractions.
In parallel with the change in descending input to the motor neurone pool during shortening and lengthening contractions, there are also changes in sensory feedback from the periphery. Notably, there is an increase in the amount of feedback from muscle spindles during lengthening contractions (Burke et al. 1978; Hulliger et al. 1985). Despite the augmented feedback from Ia afferents, however, the amplitude of both the H reflex and the stretch reflex is depressed during lengthening contractions, even when the EMG level is matched during the two anisometric contractions (Romanò & Schieppati, 1987). Therefore, the attenuation of the motor neurone response to muscle spindle input during lengthening contractions is mainly attributed either to centrally and peripherally mediated presynaptic inhibition of the Ia afferents (Romanò & Schieppati, 1987; Pasquet et al. 2006) or to homosynaptic postactivation depression (Hultborn et al. 1996); the latter mechanism, however, is less evident during a contraction than at rest (Pinniger et al. 2001; Stein et al. 2007). In contrast, Petersen et al. (2007) found that the probability of discharge by single motor units in tibialis anterior in response to electrical stimulation of the Ia afferents did not change during shortening and lengthening contractions, despite an increase in spindle discharge during muscle lengthening when motor unit discharge rate was kept constant. Because the probability of discharge depends on both pre- and postsynaptic mechanisms, and discharge rate was held constant, they concluded that presynaptic inhibition did not differ during shortening and lengthening contractions. However, the task used by Petersen et al. (2007) involved maintaining a constant discharge rate of an identified motor unit in tibialis anterior while pushing against a torque motor, which differs substantially from the modulation of discharge rate that occurs when an individual displaces an inertial load to match a prescribed trajectory.
Despite the observations that synaptic input delivered to motor neurones can differ during shortening and lengthening contractions, the lack of attention to the association between the adjustments in synaptic input and the task demands precludes an understanding of how the changes in motor output are achieved. There is no evidence, for example, on the adjustments that might cause a change in the recruitment order of motor units during lengthening contractions. Even when the adjustments are probed during tasks that do involve a change in strategy, however, the measurements often provide limited insight on the function of the entire motor unit pool. This difficulty is evident in the study of Nardone & Schieppati (1988) on the strategies used by individuals to lower an inertial load with plantarflexor muscles (Fig. 4A). Although two distinct strategies for the lengthening contractions were identified, all participants experienced a similar depression of H-reflex amplitude in soleus and lateral gastrocnemius despite a difference in the relative EMG amplitude in these muscles. Because the H reflex typically involves low-threshold motor units and these units receive greater amounts of presynaptic and recurrent inhibition (Pierrot-Deseilligny & Burke, 2005), the measurement was not sensitive enough to differences that might exist across all of the involved motor units.
Although the synaptic input received by the motor neurone pool can differ during shortening and lengthening contractions, there is a lack of information about the specificity of the adjustments that produce the observed changes in motor output during the two anisometric contractions.
Maximal contractions
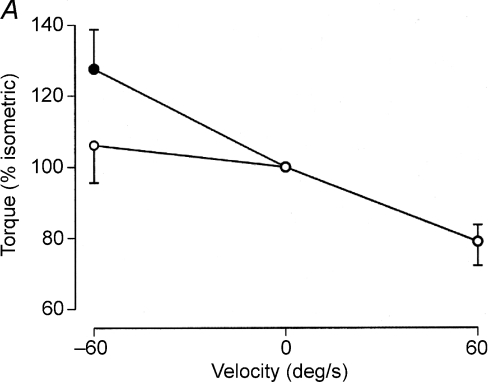
In the performance of maximal shortening and lengthening contractions, the debate has focused on whether or not the voluntary activation is sufficient to elicit the maximal force capacity of the muscle. Whereas the peak force that can be evoked from isolated fibres and whole muscle is 50–80% greater when lengthening contractions are performed on the plateau or descending limb of the length–tension relation than during isometric contractions (Katz, 1939; Edman, 1988; Morgan et al. 2000), the peak muscle force achieved during maximal voluntary contractions is usually either similar during the two anisometric contractions or only modestly greater (< 40%) during lengthening contractions compared with slow shortening contractions (Westing et al. 1991; Amiridis et al. 1996; Kellis & Baltzopoulos, 1998; Aagaard et al. 2000; Seger & Thorstensson, 2000; Pasquet et al. 2000; Babault et al. 2001; Klass et al. 2005).
The discrepancy in the relative forces during the evoked and voluntary protocols is often ascribed to an activation signal that cannot realize the force capacity of muscle during maximal lengthening contractions. When individuals perform maximal isokinetic actions, for example, EMG amplitude is often greater during shortening contractions than during lengthening contractions (Tesch et al. 1990; Westing et al. 1991; Amiridis et al. 1996; Kellis & Baltzopoulos, 1998; Aagaard et al. 2000; Komi et al. 2000) and voluntary activation is depressed during lengthening contractions (Amiridis et al. 1996; Babault et al. 2001; Beltman et al. 2004). Consistent with this deficit, Beltman et al. (2004) reported a voluntary activation level of 79 ± 8% during a maximal lengthening contraction with quadriceps femoris compared with a level of 92 ± 3% during a maximal shortening contraction (Fig. 6A). Furthermore, the distribution of the ratio of phosphocreatine to creatine in muscle fibres obtained from vastus lateralis was shifted less after 10 maximal lengthening contractions compared with 10 maximal shortening contractions, which is consistent with a lower activation of the muscle during the lengthening contractions.
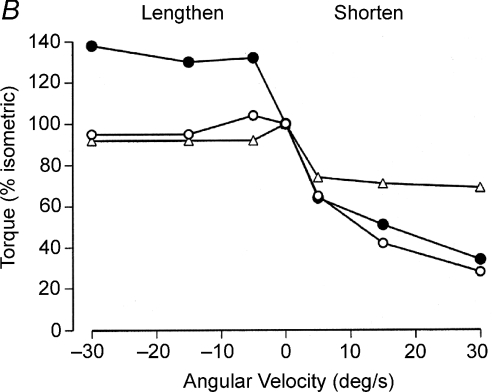
Figure 6. Examples of the depression in muscle activation during lengthening contractions.
A, as subjects performed maximal isometric and anisometric contractions with the knee extensors, three electric stimuli (200 μs pulses at 300 Hz; 162 ± 26 mA) were applied to the femoral nerve and the evoked torque was measured. When normalized to the torque produced during maximal isometric contractions, the voluntary (○) and evoked (•) torques were similar during maximal shortening contractions (60 deg s−1) but the evoked torque was significantly greater than the voluntary torque during the maximal lengthening contraction. The difference in the two torques during the lengthening contractions suggests that the voluntary activation was less than adequate. Data from Beltman et al. (2004) used with permission. B, the torque produced during maximal (Δ) and submaximal (○) lengthening contractions with the plantarflexor muscles (mainly soleus) was similar to the torque achieved during isometric contractions. In contrast, electrical stimulation of the muscles to produce an isometric torque of 30% MVC force resulted in a significant increase in torque when a torque motor lengthened the activated muscles (•) at three slow velocities. Thus, the greater relative torque during the evoked contractions suggests that the level of activation was depressed during the voluntary lengthening contractions. Data from Pinniger et al. (2000) reproduced with kind permission from Springer Science+Business Media: European Journal of Applied Physiology, Tension regulation during lengthening and shortening actions of the human soleus muscle, vol. 375, 2000, p. 379, Pinniger GJ, Steele JR, Thorstensson A & Cresswell AG, Fig. 4.
The adaptations elicited by physical training seem to be consistent with a deficit in voluntary activation during maximal lengthening contractions (Hortobágyi et al. 1996a,b, 2001; Aagaard et al. 2000; Duclay et al. 2008). When Amiridis et al. (1996) compared the torque produced during maximal lengthening contractions with and without superimposed electrical stimulation, the peak torque achieved during the anisometric contractions with the knee extensors increased for sedentary individuals but not for elite athletes. Similarly, Aagaard et al. (2000) found that 14 weeks of strength training the knee extensors increased both the peak torque and EMG amplitude during maximal isokinetic actions but EMG amplitude increased to a greater extent during lengthening compared with shortening contractions. Caution is necessary in interpreting differences in EMG amplitude (Farina et al. 2004; Keenan et al. 2005), however, because the greatest EMG amplitudes both before and after training occurred during fast shortening contractions when muscle torque was the least. Nonetheless, these results are usually interpreted as an increased neural drive in the descending corticospinal pathways during voluntary activation of the muscle (Aagaard et al. 2002; Duclay et al. 2008).
A common explanation for the lesser voluntary activation during maximal lengthening contractions is the existence of a tension-limiting mechanism to minimize possible damage to the muscle and its associated connective tissues (Westing et al. 1991; Amiridis et al. 1996; Aagaard et al. 2000; Pinniger et al. 2000; Seger & Thorstensson, 2000; Del Valle & Thomas, 2005). Proponents of this protective strategy suggest that inhibitory feedback from sensory receptors, especially Golgi tendon organs, depresses the responsiveness of the motor neurone pool to incoming descending inputs (Westing et al. 1991; Aagaard et al. 2000). When normalized to the corresponding isometric force, however, the force–velocity relation during both maximal and submaximal (30% MVC) lengthening contractions is similar in the soleus muscle (Fig. 6B), which suggests that the inhibition of the activation during lengthening contraction may not be influenced by a tension-related feedback mechanism. Furthermore, the depression in EMG that occurs during a lengthening contraction was established during a preceding maximal isometric contraction prior to the change in muscle length (Grabiner & Owings, 2002) and the muscle damage caused by lengthening contractions depends on muscle length and not muscle tension (Talbot & Morgan, 1998). At least some of the reduction in activation during lengthening contractions therefore must be produced by descending signals, such as a decline in output from the motor cortex or an increase in presynaptic inhibition of facilitation from the periphery (Pinniger et al. 2000; Fang et al. 2001; Duclay & Martin, 2005).
Taken together, the activation of muscle seems to be depressed during maximal lengthening contractions, at least when these contractions are performed by average individuals on isokinetic dynamometers. How well this observation generalizes to other tasks and the relative significance of the candidate mechanisms remains to be determined.
Conclusion
When a task involves submaximal contractions to either lift an inertial load or push against an imposed load, the amount of motor unit activity differs during shortening and lengthening contractions. Due to the greater force capacity of muscle during lengthening contractions, fewer motor units are recruited and discharge rate is lower during lengthening contractions compared with shortening contractions. Although the synaptic input delivered to the motor neurone pool by descending pathways and peripheral afferents can differ during shortening and lengthening contractions, too little attention has been afforded the task demands to identify the specific adjustments responsible for differences in motor performance. Similarly, the mechanisms underlying the depression of voluntary activation that has been observed in several muscles during maximal lengthening contractions performed on an isokinetic dynamometer are yet to be identified, and the extent to which this observation generalizes to other tasks is unknown. These comparisons indicate that there is a critical need for studies that can explain differences in the performance of shortening and lengthening contractions in terms of the specific adjustments used by the nervous system to control the activity of the involved motor neurones.
References
- Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulson P. Neural adaptation to resistance training: changes in evoked V-wave and H-reflex responses. J Appl Physiol. 2002;92:2309–2318. doi: 10.1152/japplphysiol.01185.2001. [DOI] [PubMed] [Google Scholar]
- Aagaard P, Simonsen EB, Andersen JL, Magnusson SP, Halkjær-Kristensen J, Dyhre-Poulsen P. Neural inhibition during maximal eccentric and concentric quadriceps contraction: effects of resistance training. J Appl Physiol. 2000;89:2249–2257. doi: 10.1152/jappl.2000.89.6.2249. [DOI] [PubMed] [Google Scholar]
- Abbruzzese G, Morena M, Spadavecchia L, Schieppati M. Response of arm flexor muscles to magnetic and electrical and brain stimulation during shortening and lengthening tasks in man. J Physiol. 1994;481:499–507. doi: 10.1113/jphysiol.1994.sp020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akazawa K, Milner TE, Stein RB. Modulation of reflex EMG and stiffness in response to stretch of a human finger muscle. J Neurophysiol. 1983;49:16–27. doi: 10.1152/jn.1983.49.1.16. [DOI] [PubMed] [Google Scholar]
- Amiridis IG, Martin A, Morlon B, Martin L, Cometti G, Pousson M, Van Hoecke J. Co-activation and tension-regulating phenomena during isokinetic knee extension in sedentary and highly skilled humans. Eur J Appl Physiol. 1996;73:149–156. doi: 10.1007/BF00262824. [DOI] [PubMed] [Google Scholar]
- Babault N, Pousson M, Ballay Y, Van Hoecke J. Activation of human quadriceps femoris during isometric, concentric, and eccentric contractions. J Appl Physiol. 2001;91:2628–2634. doi: 10.1152/jappl.2001.91.6.2628. [DOI] [PubMed] [Google Scholar]
- Barry BK, Pascoe MA, Jesunathadas M, Enoka RM. Rate coding is compressed but variability is unaltered for motor units in hand muscle of old adults. J Neurophysiol. 2007;97:3206–3218. doi: 10.1152/jn.01280.2006. [DOI] [PubMed] [Google Scholar]
- Baudry S, Klass M, Pasquet B, Duchateau J. Age-related fatigability of the ankle dorsiflexor muscles during concentric and eccentric contractions. Eur J Appl Physiol. 2007;100:515–525. doi: 10.1007/s00421-006-0206-9. [DOI] [PubMed] [Google Scholar]
- Bawa P, Sinkjær T. Reduced short and long latency reflexes during voluntary tracking movement of the human wrist joint. Acta Physiol Scand. 1999;167:241–246. doi: 10.1046/j.1365-201x.1999.00608.x. [DOI] [PubMed] [Google Scholar]
- Beltman JGM, Sargeant AJ, van Mechelen W, de Haan A. Voluntary activation level and muscle fiber recruitment of human quadriceps during lengthening contractions. J Appl Physiol. 2004;97:619–626. doi: 10.1152/japplphysiol.01202.2003. [DOI] [PubMed] [Google Scholar]
- Buchanan TS, Lloyd DG. Muscle activity is different for humans performing static tasks which require force control and position control. Neurosci Lett. 1995;194:61–64. doi: 10.1016/0304-3940(95)11727-e. [DOI] [PubMed] [Google Scholar]
- Burke D, Hagbarth KE, Löfstedt L. Muscle spindle activity in man during shortening and lengthening contractions. J Physiol. 1978;277:131–142. doi: 10.1113/jphysiol.1978.sp012265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett RA, Laidlaw DH, Enoka RM. Coactivation of the antagonist muscle does not covary with steadiness in old adults. J Appl Physiol. 2000;89:61–71. doi: 10.1152/jappl.2000.89.1.61. [DOI] [PubMed] [Google Scholar]
- Cavagna GA, Citterio G. Effect of stretching on the elastic characteristics of the contractile component of the frog striated muscle. J Physiol. 1974;239:1–14. doi: 10.1113/jphysiol.1974.sp010552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou EA, Carlton LG. Motor output is more variable during eccentric compared with concentric contractions. Med Sci Sports Exerc. 2002;34:1772–1778. doi: 10.1097/00005768-200211000-00013. [DOI] [PubMed] [Google Scholar]
- Christou EA, Shinohara M, Enoka RM. Fluctuations in acceleration during voluntary contractions lead to greater impairment of movement accuracy in older adults. J Appl Physiol. 2003;95:373–384. doi: 10.1152/japplphysiol.00060.2003. [DOI] [PubMed] [Google Scholar]
- Corcos DM, Gottlieb GL, Agarwal GC. Organizing principles for single-joint movements. II. A speed-sensitive strategy. J Neurophysiol. 1989;62:358–368. doi: 10.1152/jn.1989.62.2.358. [DOI] [PubMed] [Google Scholar]
- Crone C, Nielsen J. Central control of disynaptic reciprocal inhibition in humans. Acta Physiol Scand. 1994;152:351–363. doi: 10.1111/j.1748-1716.1994.tb09817.x. [DOI] [PubMed] [Google Scholar]
- d’Avella A, Portone A, Fernandez L, Lacquaniti F. Control of fast-reaching movements by muscle synergy combinations. J Neurosci. 2006;26:7791–7810. doi: 10.1523/JNEUROSCI.0830-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Serres SJ, Bennett DJ, Stein RB. Stretch reflex gain in cat triceps surae in muscles with compliant loads. J Physiol. 2002;545:1027–1040. doi: 10.1113/jphysiol.2002.027177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle A, Thomas CK. Firing rates of motor units during strong dynamic contractions. Muscle Nerve. 2005;32:316–325. doi: 10.1002/mus.20371. [DOI] [PubMed] [Google Scholar]
- Doemges F, Rack PMH. Changes in the stretch reflex of the human first dorsal interosseous muscle during different tasks. J Physiol. 1992;447:563–573. doi: 10.1113/jphysiol.1992.sp019018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclay J, Martin A. Evoked H-reflex and V-wave responses during maximal isometric, concentric, and eccentric muscle contraction. J Neurophysiol. 2005;94:3555–3562. doi: 10.1152/jn.00348.2005. [DOI] [PubMed] [Google Scholar]
- Duclay J, Martin A, Robbe A, Pousson M. Spinal reflex plasticity during maximal dynamic contractions after eccentric training. Med Sci Sports Exerc. 2008;40:722–734. doi: 10.1249/MSS.0b013e31816184dc. [DOI] [PubMed] [Google Scholar]
- Edman KAP. Double-hyperbolic force-velocity relation in frog muscle fibres. J Physiol. 1988;404:301–321. doi: 10.1113/jphysiol.1988.sp017291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Siemionow V, Sahgal V, Xiong F, Yue GH. Greater movement-related cortical potential during human eccentric versus concentric muscle contractions. J Neurophysiol. 2001;86:1764–1772. doi: 10.1152/jn.2001.86.4.1764. [DOI] [PubMed] [Google Scholar]
- Fang Y, Siemionow V, Sahgal V, Xiong F, Yue GH. Distinct brain activation patterns for human maximal voluntary eccentric and concentric muscle actions. Brain Res. 2004;1023:200–212. doi: 10.1016/j.brainres.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol. 2004;96:1486–1495. doi: 10.1152/japplphysiol.01070.2003. [DOI] [PubMed] [Google Scholar]
- Feiereisen P, Duchateau J, Hainaut K. Motor unit recruitment order during voluntary and electrically induced contractions in the tibialis anterior. Exp Brain Res. 1997;114:117–123. doi: 10.1007/pl00005610. [DOI] [PubMed] [Google Scholar]
- Garland SJ, Cooke JD, Miller KJ, Ohtsuki T, Ivanova T. Motor unit activity during human single joint movements. J Neurophysiol. 1996;76:1982–1990. doi: 10.1152/jn.1996.76.3.1982. [DOI] [PubMed] [Google Scholar]
- Gottlieb GL. On the voluntary control of compliant (inertial-viscoelastic) loads by parcellated control mechanisms. J Neurophysiol. 1996;76:3207–3229. doi: 10.1152/jn.1996.76.5.3207. [DOI] [PubMed] [Google Scholar]
- Grabiner MD, Owings TM. EMG differences between concentric and eccentric maximum voluntary contractions are evident prior to movement speed. Exp Brain Res. 2002;145:505–511. doi: 10.1007/s00221-002-1129-2. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Enoka RM. Physiology of the motor neuron and the motor unit. In: Eisen A, editor. Handbook of Clinical Neurophysiology. Vol. 4. New York: Elsevier; 2004. pp. 119–147. Clinical Neurophysiology of Motor Neuron Diseases. [Google Scholar]
- Hortobágyi T, Barrier J, Beard D, Braspennincx J, Koens P, DeVita P, Dempsey L, Lambert J. Greater initial adaptations to submaximal lengthening than maximal shortening. J Appl Physiol. 1996a;81:1677–1682. doi: 10.1152/jappl.1996.81.4.1677. [DOI] [PubMed] [Google Scholar]
- Hortobágyi T, DeVita P, Money J, Barrier J. Effects of standard and eccentric overload training in young women. Med Sci Sports Exerc. 2001;33:1206–1212. doi: 10.1097/00005768-200107000-00020. [DOI] [PubMed] [Google Scholar]
- Hortobágyi T, Hill JP, Houmard JA, Fraser DD, Lambert NH, Israel RG. Adaptative responses to muscle lengthening and shortening in humans. J Appl Physiol. 1996b;80:765–772. doi: 10.1152/jappl.1996.80.3.765. [DOI] [PubMed] [Google Scholar]
- Howell JN, Fuglevand AJ, Walsh ML, Bigland-Ritchie B. Motor unit activity during isometric and concentric-eccentric contractions of the human first dorsal interosseus muscle. J Neurophysiol. 1995;74:901–904. doi: 10.1152/jn.1995.74.2.901. [DOI] [PubMed] [Google Scholar]
- Hulliger M, Nordh E, Vallbo ÅB. Discharge in muscle spindle afferents related to direction of slow precision movements. J Physiol. 1985;362:437–453. doi: 10.1113/jphysiol.1985.sp015687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res. 1996;108:450–462. doi: 10.1007/BF00227268. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Niemelä E, Komi PV. Interaction between fascicle and tendinous tissues in short-contact stretch-shortening cycle exercise with varying eccentric intensities. J Appl Physiol. 2005;99:217–223. doi: 10.1152/japplphysiol.01352.2004. [DOI] [PubMed] [Google Scholar]
- Kakuda N, Nagaoka M, Wessberg J. Common modulation of motor unit pairs during slow wrist movement in man. J Physiol. 1999;520:929–940. doi: 10.1111/j.1469-7793.1999.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. J Physiol. 1939;96:45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y, Fukunaga T. New insights into in vivo human skeletal muscle function. Exerc Sport Sci Rev. 2006;34:16–21. doi: 10.1097/00003677-200601000-00005. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Muraoka T, Ito S, Kanehisa H, Fukunaga T. In vivo muscle-fibre behaviour during countermovement exercise in human reveals significant role for tendon elasticity. J Physiol. 2002;540:635–646. doi: 10.1113/jphysiol.2001.013459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan KG, Farina D, Maluf KS, Merletti R, Enoka RM. Influence of amplitude cancellation on the simulated surface electromyogram. J Appl Physiol. 2005;98:120–131. doi: 10.1152/japplphysiol.00894.2004. [DOI] [PubMed] [Google Scholar]
- Kellis E, Baltzopoulos V. Muscle activation differences between eccentric and concentric isokinetic exercise. Med Sci Sports Exerc. 1998;30:1616–1623. doi: 10.1097/00005768-199811000-00010. [DOI] [PubMed] [Google Scholar]
- Kimura T, Yamanaka K, Nozaki D, Nakazawa K, Miyoshi T, Akai M, Ohtsuki T. Hysteresis in corticospinal excitability during gradual muscle contraction and relaxation in humans. Exp Brain Res. 2003;152:123–132. doi: 10.1007/s00221-003-1518-1. [DOI] [PubMed] [Google Scholar]
- Klass M, Baudry S, Duchateau J. Aging does not affect voluntary activation of the ankle dorsiflexors during isometric, concentric, and eccentric contractions. J Appl Physiol. 2005;99:31–38. doi: 10.1152/japplphysiol.01426.2004. [DOI] [PubMed] [Google Scholar]
- Komi PV, Linnamo V, Silventoinen P, Sillanpää M. Force and EMG power spectrum during eccentric and concentric actions. Med Sci Sports Exerc. 2000;32:1757–1762. doi: 10.1097/00005768-200010000-00015. [DOI] [PubMed] [Google Scholar]
- Laidlaw DH, Bilodeau M, Enoka RM. Steadiness is reduced and motor unit discharge is more variable in old adults. Muscle Nerve. 2000;23:600–612. doi: 10.1002/(sici)1097-4598(200004)23:4<600::aid-mus20>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Morgan DL, Whitehead NP, Wise AK, Gregory JE, Proske U. Tension changes in the cat soleus muscle following slow stretch or shortening of the contracting muscle. J Physiol. 2000;522:503–513. doi: 10.1111/j.1469-7793.2000.t01-2-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Yamamoto SI, Yano H. Short- and long-latency reflex responses during different motor tasks in elbow flexor muscles. Exp Brain Res. 1997;116:20–28. doi: 10.1007/pl00005740. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Yano H, Satoh H, Fujisaki I. Differences in stretch reflex responses of elbow flexor muscles during shortening, lengthening and isometric contractions. Eur J Appl Physiol. 1998;77:395–400. doi: 10.1007/s004210050350. [DOI] [PubMed] [Google Scholar]
- Nardone A, Romanò C, Schieppati M. Selective recruitment of high-threshold human motor units during voluntary isotonic lengthening of active muscles. J Physiol. 1989;409:451–471. doi: 10.1113/jphysiol.1989.sp017507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone A, Schieppati M. Shift of activity from slow to fast muscle during voluntary lengthening contractions of the triceps surae muscles in humans. J Physiol. 1988;395:363–381. doi: 10.1113/jphysiol.1988.sp016924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol C, Avela J, Komi PV. The stretch-shortening cycle. A model to study naturally occurring neuromuscular fatigue. Sports Med. 2006;36:977–999. doi: 10.2165/00007256-200636110-00004. [DOI] [PubMed] [Google Scholar]
- Nielsen JB. Sensorimotor integration at spinal level as a basis for muscle coordination during voluntary movement in humans. J Appl Physiol. 2004;96:1961–1967. doi: 10.1152/japplphysiol.01073.2003. [DOI] [PubMed] [Google Scholar]
- Nordlund MM, Thorstensson A, Cresswell AG. Variations in the soleus H-reflex as a function of activation during controlled lengthening and shortening actions. Brain Res. 2002;952:301–307. doi: 10.1016/s0006-8993(02)03259-6. [DOI] [PubMed] [Google Scholar]
- Pasquet B, Carpentier A, Duchateau J. Specific modulation of motor unit discharge for a similar change in fascicle length during shortening and lengthening contractions in humans. J Physiol. 2006;577:753–765. doi: 10.1113/jphysiol.2006.117986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquet B, Carpentier A, Duchateau J, Hainaut K. Muscle fatigue during concentric and eccentric contractions. Muscle Nerve. 2000;23:1727–1735. doi: 10.1002/1097-4598(200011)23:11<1727::aid-mus9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Petersen NT, Butler JE, Carpenter MG, Cresswell AG. Ia-afferent input to motoneurons during shortening and lengthening muscle contractions in humans. J Appl Physiol. 2007;102:144–148. doi: 10.1152/japplphysiol.00362.2006. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Burke D. The Circuitry of the Human Spinal Cord. Cambridge, UK: Cambridge University Press; 2005. [Google Scholar]
- Pinniger GJ, Nordlund M, Steele JR, Cresswell AG. H-reflex modulation during passive lengthening and shortening of the human triceps surae. J Physiol. 2001;534:913–923. doi: 10.1111/j.1469-7793.2001.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinniger GJ, Steele JR, Thorstensson A, Cresswell AG. Tension regulation during lengthening and shortening actions of the human soleus muscle. Eur J Appl Physiol. 2000;81:375–383. doi: 10.1007/s004210050057. [DOI] [PubMed] [Google Scholar]
- Pyndt HS, Laursen M, Nielsen JB. Changes in reciprocal inhibition across the ankle joint with changes in external load and pedaling rate during bicycling. J Neurophysiol. 2003;90:3168–3177. doi: 10.1152/jn.00444.2003. [DOI] [PubMed] [Google Scholar]
- Romanò C, Schieppati M. Reflex excitability of human soleus motoneurones during voluntary shortening or lengthening contractions. J Physiol. 1987;390:271–284. doi: 10.1113/jphysiol.1987.sp016699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J Neurosci Methods. 1997;27:113–122. doi: 10.1016/s0165-0270(97)02242-5. [DOI] [PubMed] [Google Scholar]
- Seger JY, Thorstensson A. Electrically evoked eccentric and concentric torque-velocity relatonships in human knee extensor muscles. Acta Physiol Scand. 2000;169:63–69. doi: 10.1046/j.1365-201x.2000.00694.x. [DOI] [PubMed] [Google Scholar]
- Sekiguchi H, Nakazawa K, Akai M. Recruitment gain of antagonistic motoneurons is higher during lengthening contraction than during shortening contraction in man. Neurosci Lett. 2003a;342:69–72. doi: 10.1016/s0304-3940(03)00241-6. [DOI] [PubMed] [Google Scholar]
- Sekiguchi H, Nakazawa K, Suzuki S. Differences in recruitment properties of the corticospinal pathway between lengthening and shortening contractions in human soleus muscle. Brain Res. 2003b;977:169–179. doi: 10.1016/s0006-8993(03)02621-0. [DOI] [PubMed] [Google Scholar]
- Semmler JG, Kornatz KW, Dinenno DV, Zhou S, Enoka RM. Motor unit synchronisation is enhanced during slow lengthening contractions of a hand muscle. J Physiol. 2002;545:681–695. doi: 10.1113/jphysiol.2002.026948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søgaard K, Christensen H, Jensen BR, Finsen L, Sjøgaard G. Motor control and kinetics during low level concentric and eccentric contractions in man. Electroencephal Clin Neurophysiol. 1996;101:453–460. [PubMed] [Google Scholar]
- Stein RB, Estabrooks KL, McGie S, Roth MJ, Jones KE. Quantifying the effects of voluntary contraction and inter-stimulus interval on the human soleus H-reflex. Exp Brain Res. 2007;182:309–319. doi: 10.1007/s00221-007-0989-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens JA, Garnett R, Buller NP. Reversal of recruitment order of single motor units produced by cutaneous stimulation during voluntary muscle contraction in man. Nature. 1978;272:362–364. doi: 10.1038/272362a0. [DOI] [PubMed] [Google Scholar]
- Stotz PJ, Bawa P. Motor unit recruitment during lengthening contractions of human wrist flexors. Muscle Nerve. 2001;24:1535–1541. doi: 10.1002/mus.1179. [DOI] [PubMed] [Google Scholar]
- Talbot JA, Morgan DL. The effects of stretch parameters on eccentric exercise-induced damage to toad skeletal muscle. J Muscle Res Cell Motil. 1998;19:237–245. doi: 10.1023/a:1005325032106. [DOI] [PubMed] [Google Scholar]
- Tesch PA, Dudley GA, Duvoisin MR, Hather BM, Harris RT. Force and EMG signal patterns during repeated bouts of concentric and eccentric muscle actions. Acta Physiol Scand. 1990;138:263–271. doi: 10.1111/j.1748-1716.1990.tb08846.x. [DOI] [PubMed] [Google Scholar]
- van Zuylen EJ, Gielen CCAM, Denier Van Der Gon JJ. Coordination and inhomogeneous activation of human arm muscles during isometric torques. J Neurophysiol. 1988;60:1523–1548. doi: 10.1152/jn.1988.60.5.1523. [DOI] [PubMed] [Google Scholar]
- Westing SH, Cresswell AG, Thorstensson A. Muscle activation during maximal voluntary eccentric and concentric knee extension. Eur J Appl Physiol. 1991;62:104–108. doi: 10.1007/BF00626764. [DOI] [PubMed] [Google Scholar]