Abstract
The main aim of the study was to investigate whether neurones in the ipsilateral red nucleus (NR) affect hindlimb motoneurones. Intracellular records from motoneurones revealed that both EPSPs and IPSPs were evoked in them via ipsilaterally located premotor interneurones by stimulation of the ipsilateral NR in deeply anaesthetized cats in which only ipsilaterally descending tract fibres were left intact. When only contralaterally descending tract fibres were left intact, EPSPs mediated by excitatory commissural interneurones were evoked by NR stimuli alone while IPSPs mediated by inhibitory commissural interneurones required joint stimulation of the ipsilateral NR and of the medial longitudinal fascicle (MLF, i.e. reticulospinal tract fibres). Control experiments led to the conclusion that if any inadvertently coactivated axons of neurones from the contralateral NR contributed to these PSPs, their effect was minor. Another aim of the study was to investigate whether ipsilateral actions of NR neurones, pyramidal tract (PT) neurones and reticulospinal tract neurones descending in the MLF on hindlimb motoneurones are evoked via common spinal relay neurones. Mutual facilitation of these synaptic actions as well as of synaptic actions from the contralateral NR and contralateral PT neurones showed that they are to a great extent mediated via the same spinal neurones. A more effective activation of these neurones by not only ipsilateral corticospinal and reticulospinal but also rubrospinal tract neurones may thus contribute to the recovery of motor functions after injuries of the contralateral corticospinal tract neurones. No evidence was found for mediation of early PT actions via NR neurones.
Previous studies revealed that pyramidal tract (PT) neurones may affect not only contralateral but also ipsilateral hindlimb motoneurones in the cat (Edgley et al. 2004; Jankowska et al. 2006; Stecina & Jankowska, 2007). In the experiments reported here we investigated whether this might also be the case for neurones in the red nucleus (NR), another major descending motor system with crossed actions.
The rationale for this study has been twofold. First, several morphological studies have demonstrated that NR neurones have not only contralaterally but also ipsilaterally located target cells despite a general consensus that projections and actions of NR neurones are crossed (for references see, e.g. Brodal, 1981; Nathan & Smith, 1982). Using retrograde transport of markers injected into the spinal cord, neurones were labelled not only in the contralateral but also in the ipsilateral red nucleus: in the magnocellular part of the ipsilateral red nucleus in macaque (after injections of fast blue at the C6 segment; see Fig. 2 in Burman et al. (2000), and in both the magnocellular and the parvicellular parts in cats (Hayes & Rustioni, 1981; see, however, Warren et al. 2008) and rats (Shieh et al. 1983); they were labelled by HRP injected in the lumbar as well as in the cervical segments. Some neurones in the ipsilateral NR were also labelled by retrograde transneuronal transport of rabies virus injected to single hindlimb muscles in the rat (Ruigrok et al. 2008). Ipsilateral projections to the spinal cord and the brainstem were also detected using anterograde transport of markers from the red nucleus. In the rat, 10–28% of the total number of PHA-L boutons labelled in the spinal cord were found ipsilaterally (Antal et al. 1992); they were seen at all levels, including lumbar segments, in laminae V–VII. In the cat, anterograde transport of leucine demonstrated ipsilateral projections to the lateral parts of the intermediate zone in cervical segments (Holstege, 1987). Anterograde labelling with biotinylated dextran amine (BDA) revealed projections to the medial part of the ipsilateral reticular formation in the rat (Yasui et al. 2001) and with WGA-HRP to the contralateral parvicellular part of the reticular nucleus in the cat (Robinson et al. 1987). Provided that neurones contacted in these regions projected to the spinal cord, or activated reticulospinal neurones, they might thus relay some of the indirect ipsilateral actions of NR neurones.
Secondly, indications for ipsilateral actions of rubral neurones were also obtained in physiological studies. Endo et al. (1975) reported IPSPs in cat ipsilateral soleus motoneurones and EPSPs mixed with IPSPs in medial gastrocnemius and some flexor motoneurones, both at undefined but rather long latencies. Rho et al. (1999) found that weak electrical stimuli applied in the red nucleus are followed by enhancement of activity of not only contralateral but also ipsilateral forelimb muscles during both swing and stance phases of locomotion. Lavoie & Drew (2002) noted also ‘periods of phasic discharges temporally coincident with the swing phase of the ipsilateral limb in some NR neurones’.
The probability of detecting ipsilateral actions of rubral neurones therefore appeared to be fairly high, even though the conditions of analysing effects of selectively stimulated ipsilaterally projecting NR neurones are not favourable because of the risk of coactivation of other neurones inherent to centrally applied stimuli.
We also considered the possibility that ipsilaterally acting NR neurones might operate as relay neurones of ipsilateral actions of PT neurones, in parallel with the previously demonstrated relay neurones of these actions: reticulospinal (RS) neurones (Edgley et al. 2004; Jankowska et al. 2006; Stecina & Jankowska, 2007) and some spinal interneurones (Edgley et al. 2004; Jankowska & Stecina, 2007; Stecina et al. 2008). Morphological studies have demonstrated projections from the sensori-motor cortical areas to neurones in the ipsilateral NR (Rinvik & Walberg, 1963; Mabuchi & Kusama, 1966; Tsukahara & Kosaka, 1966; King et al. 1972; Padel et al. 1973; Brown, 1974; Tsukahara et al. 1975; Jeneskog & Padel, 1983; Massion, 1988); for review of earlier studies see (Massion, 1967). The projections were originally concluded to be from slowly conducting, at about 20 m s−1, corticospinal and corticorubral neurones (Tsukahara et al. 1968, 1975, 1983; Tsukahara & Kosaka, 1968), which might restrict the contribution of NR neurones to only late PT actions on motoneurones. However, Canedo & Towe (1986) found twice as many collaterals of fast than of slow conducting PT fibres within the NR, which would allow NR neurones to relay both early and late PT actions. Alternatively, NR neurones could be activated predominantly by corticorubral neurones and combined actions of NR and corticospinal neurones be evoked via common spinal or reticular relay neurones.
Methods
Preparation
The experiments were performed on six deeply anaesthetized cats weighing 2.5–3.3 kg. All experimental procedures were approved by the local Ethics Committee and followed NIH and EU guidelines for animal care. Anaesthesia induced with sodium pentobarbital (40–44 mg kg−1, i.p.) was maintained with intermittent doses of α-chloralose (Rhône-Poulenc Santé, France; 5 mg kg−1, i.v.). Additional doses of α-chloralose were administered every 1–2 h up to about 25 mg kg−1, then every 2–3 h (up to about 55 mg kg−1, i.v.) or when prompted by increases in the continuously monitored blood pressure or heart rate, or if the pupils dilated. Neuromuscular transmission was blocked by pancuronium bromide (Pavulon, Organon, Sweden; about 0.2 mg kg−1 h−1i.v.) and the animals were artificially ventilated. Mean blood pressure was kept at 100–130 mmHg and the end-tidal concentration of CO2 at about 4% by adjusting the parameters of artificial ventilation and the rate of a continuous infusion of a bicarbonate buffer solution with 5% glucose (1–2 ml h−1 kg−1). Core body temperature was kept at about 38°C by servo-controlled infrared lamps. The experiments were terminated by a lethal dose of anaesthetic and formalin perfusion resulting in cardiac arrest. Atropine (0.05–0.2 mg kg−1i.m.) and dexamethasone (1 mg kg−1i.m.; Oradexon, Organon, the Netherlands) were given at the beginning of the surgery in two experiments. The effectiveness of synaptic transmission was increased by intravenous application of 4-AP in doses of 0.1–0.2 mg kg−1, i.v. (in 4 cats) but the reported effects were also seen prior to 4-AP administration albeit less frequently.
The spinal cord was exposed by laminectomy from the fourth to the seventh lumbar (L4–L7) segments and at the level of the low thoracic (Th11–Th13) and upper cervical (C3 or C4) segments. One half of the spinal cord was transected at the Th12–13 level about 1–2 h before the recording began. The hemisection was made on the right side in three experiments in which effects of stimuli applied within the left and right NR were tested on motoneurones and on the left side in two, but in all these experiments the motoneurones were loctated located contralaterally as well as ipsilaterally with respect to the hemisection (see Fig. 1). The hemisection was performed after having opened the dura mater on one side, dissected away the dorsal columns and exposed the central canal under a dissection microscope. The lateral and ventral funiculi on either the right or the left side were then torn apart intrapially with watchmaker's forceps over a distance of about 2–3 mm until the midline was reached. The gap between the transected funiculi was filled with a small piece of gelfoam to keep them separated. The completeness of the hemisection was verified after formalin perfusion and additional post-fixation by splitting the two halves of the spinal cord about 1–2 cm away from the level of the hemisection and checking that no parts of the lesioned half were attached to the intact half within the area of the hemisection. In order to verify that no damage to the remaining part of the spinal cord occurred (e.g. by pressure), descending volleys evoked by stimulation of the MLF and the contralateral NR were recorded both rostral and caudal of the hemisection and from the lumbar segments.
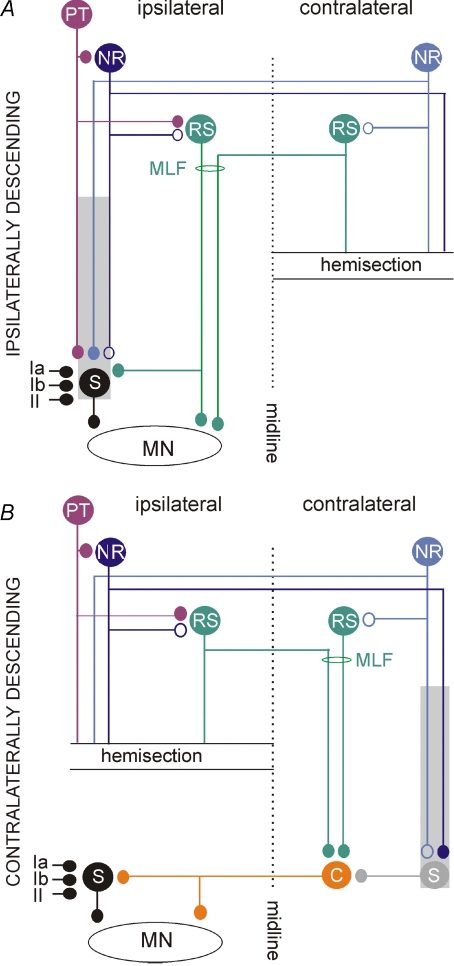
Figure 1. Ipsilaterally and contralaterally descending neuronal pathways hypothesized to relay synaptic actions from the ipsilateral red nucleus to hindlimb motoneurones.
Pathways via ipsilaterally (with respect to motoneurones) descending rubrospinal (NR), reticulospinal (RS) and pyramidal tract (PT) neurones with ipsilaterally located spinal relay neurones (S) represented by interneurones with input from group Ia, Ib and II afferents. The shaded area indicates that the spinal relay neurones might also include interneurones and propriospinal neurones spread over the whole length of the spinal cord. B, pathways via contralaterally descending NR and RS neurones and contralaterally located spinal neurones represented by commissural interneurones (C), which cross to the opposite side of the spinal cord, and interneurones, which might activate them; the interneurones might include both segmental interneurones and propriospinal neurones in various segments as indicated by shading. These pathways would thus involve double crossing, first at a supraspinal level and then at a segmental level. In both A and B small filled circles indicate established synaptic connections and the open circles indicate the hypothesized ones. By hemisecting the spinal cord at a low thoracic level we restricted synaptic actions evoked from the ipsilateral NR to those mediated by uncrossed ipsilaterally descending pathways in A and by double crossed contralaterally descending pathways in B.
A number of peripheral nerves were transected and dissected free. These included the left and right quadriceps (Q) and sartorius (Sart) branches of the femoral nerve, which were mounted in subcutaneous cuff electrodes; and the left and right gastrocnemius–soleus (GS), left posterior biceps and semitendinosus (PBST), anterior biceps and semimembranosus (ABSM), plantaris (PL) and deep peroneal (DP) nerves (where the DP nerve included the extensor digitorum longus and tibialis anterior nerve branches), which were mounted on hook electrodes in a paraffin oil pool.
Stimulation and recording
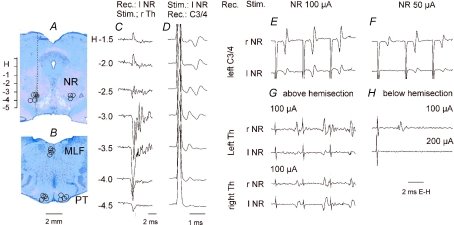
Tungsten electrodes were placed in either one or both red nuclei, at Horsley-Clarke's coordinates anterior 3–4 (at the level of the oculomotor nerve), lateral 2–2.5 and horizontal −3–3.5. The final placement of these electrodes was guided by recording antidromic field potentials evoked by stimulation of the contralateral lateral funiculus at the T12 level (Fig. 2C) and by recording descending volleys following stimuli applied within the NR (Fig. 2D) as described originally by Hongo et al. (1969a). The descending volleys were recorded using silver ball electrodes in contact with the surface of the dorsal columns or at the border between the dorsal columns and the left lateral funiculus at the level of the C3/4 and lumbar segments and from the lateral funiculus (contralateral to the stimulation side) at the Th13 level. Descending volleys from the ipsilateral NR were recorded only to monitor the placement of the stimulating electrode and to define the safe range of stimulus intensities. They could not be used to relate synaptic actions evoked from the ipsilateral NR to the timing of action potentials in ipsilaterally descending axons because these were undetectable below the level of the hemisection (see Methodological problems and Fig. 2G and H).
Figure 2. Location of the stimulating electrodes and descending rubrospinal volleys.
A and B, reconstruction of the stimulation sites in the red nuclei (NR) and in the pyramidal tracts (PT) in five experiments in which effects of NR stimulation were tested on motoneurones. They are displayed on representative brainstem sections in the plane of the inserted electrodes. Stimulation sites in the medial longitudinal fascicle (MLF) are projected on the same sections of the brainstem as the PT stimulation sites although they were a few millimetres more caudally. Stimulation sites to the left show those ipsilateral to the left side motoneurones recorded from in preparations with either ipsilateral or contralateral descending tracts intact. Stimulation sites to the right are contralateral with respect to the same motoneurones. The circles indicate location of the electrolytic marking lesions. The filled circle is for the data in C and D. The scale to the left of A shows Horsley-Clarke's horizontal coordinates. C, antidromic field potentials recorded along the electrode track indicated in A; they were evoked by 500 μA applied to the contralateral lateral funiculi. D, descending volleys recorded from the dorsal columns at the third cervical (C3) level when the stimuli (100 μA) were applied at the indicated depths. Note that the first one was evoked from locations within which antidromic field potentials were recorded (H-3 to -4) and from just outside the dorsal and ventral borders of the NR (H-2.5 and -4.5). The second one would correspond to synaptic activation of rubrospinal neurones but also of other neurones at unknown locations and/or destination activated from H-1 to -2.5. E and F, comparison of descending volleys evoked by 100 and 50 μA triple stimuli applied in the right and left NR at the locations at which maximal antidromic field potentials were evoked by Th stimuli as in C; these volleys were recorded from the border between the dorsal columns and the left lateral funiculus at a C3/4 level. Note that both were evoked at similar latencies as indirect volleys in D and that both were temporally facilitated. G and H, comparison of descending volleys evoked from the left and right NR above and below hemisection of the spinal cord made on the right side; they were recorded in parallel with descending volleys illustrated in E and F. Note that above the hemisection the volleys were evoked from both the left and right NR (being larger contralaterally) while below the hemisection they were evoked only from the right (contralateral) NR. Note also that they were evoked in a preparation in which only indirect volleys were evoked from either ipasilateral or contralateral NR and that both their latency and their temporal facilitation characterize them as evoked indirectly. All of the records are with the negativity up and with shock artefacts truncated.
Tungsten electrodes were also inserted in the left and right medullary PTs at the level of the superior olive and in the MLF (left or right depending on the side of the hemisection) at the level of the inferior olive (at Horsley-Clarkes horizontal levels from about −5 to −6 and from about −10 to −11, respectively). The electrodes were inserted through the cerebellum (at an angle of 35 deg, with the tip directed rostrally). All stimulation electrodes were left at the sites from which descending volleys recorded at the level of the C3/4 and Th segments were evoked at threshold stimulus intensities of 20 μA or less. The stimulation sites were marked with electrolytic lesions and verified histologically on transverse sections of the brainstem, cut in the plane of insertion of the electrodes using a freezing microtome and counterstained with cresyl violet (Fig. 2A and B). Henceforward all the stimulation sites and lesions on the side of location of motoneurones recorded from will be referred to as ipsilateral and those on the opposite side as contralateral.
For activation of the rubrospinal, corticospinal and reticulospinal tract fibres, trains of four or five constant current cathodal stimuli of 0.2 ms duration stimuli at 250 or 330 Hz were used. They were of ≤ 100 μA for NR, 100–150 μA for PT and 100–200 μA for the MLF. As shown previously, no current spread to the other PT has been found when PT stimuli were ≤ 150 μA (see, e.g. Jankowska et al. 2006). No current spread has either been found from the PT electrode to the MLF fibres, which was estimated by comparing descending C3/4 and Th volleys evoked from the MLF, from within the PTs and from the areas dorsal to the PTs. The risk of inadvertent activation of axons of contralateral NR neurones after they have crossed the midline by stimuli applied in the ventral part of the ipsilateral NR was estimated to be negligible when intensity of the stimuli did not exceed 100 μA (see below). No attempts were made to differentiate between effects of stimuli applied within the ipsilateral and contralateral MLF as even submaximal stimuli would encroach upon fibres on the other side of the midline. Peripheral nerves were stimulated with constant voltage stimuli at intensities expressed in multiples of threshold (T) for the activation of the most excitable fibres.
Glass micropipettes filled with 2 m solution of potassium citrate (2–5 MΩ) were used for intracellular recording from α-motoneurones identified by antidromic activation following stimulation of a muscle nerve. In three experiments motoneurones were recorded at both the left and the right sides.
Analysis
Both original data and averages of 10–40 single records (with the time resolution of 30 μs per address) were stored on-line using software for sampling and analysis developed by E. Eide, T. Holmström and N. Pihlgren (Göteborg University). The latencies of the postsynaptic potentials evoked by stimulation of the NR, PT and MLF were measured from the stimuli that were responsible for these potentials and, whenever possible, from the descending volleys.
Data are expressed as means ±s.e.m. Differences between data sets were assessed for statistical significance by using Student's t test for paired or unpaired samples, ANOVA for repeated measures and the Chi square test. Changes in PSPs evoked from the PTs, MLF and/or peripheral nerves following conditioning stimulation of the NR were estimated by comparing the latencies and the areas of the control and conditioned averaged PSPs. Mean changes were calculated from the mean averaged control areas and difference between the control and conditioned areas.
Methodological problems
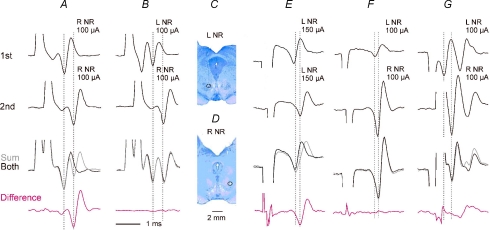
Methodological problems related to the use of electrical stimulation of the NR for studies of its functions in the cat were extensively discussed by Hongo et al. (1969a) and Baldissera et al. (1972a,1972b) and in the rat by Al-Izki et al. (2008). The main problem concerns the risk of spread of current to axons of neurones from the contralateral NR, after they had passed the ventral tegmental decussation and are on their way to join the rubrospinal tract in the lateral part of the medulla (see, e.g. Fig. 1 in Courville, 1966). The risk was estimated to be highest from within the most ventral and caudal parts of the NR, or at electrode positions ventral and medial to its boundaries (Baldissera et al. 1972b). Effects of stimuli applied at three such histologically verified electrode positions were therefore not included in those reported in the present study. Tests were also made for collision between C3/4 descending volleys evoked by stimulation of the left and right NR. To this end stimuli were applied through electrodes inserted to the two nuclei either one at a time or together, at time intervals (0.25–3.5 ms) at which the second stimulus applied to the same fibres would be ineffective because of the refractory period after the first stimulus. The sum of effects of two stimuli applied to the same fibres one at a time would then be larger than the effect of application of these stimuli when one followed the other. As illustrated in Fig. 3A and E, the difference between them (bottom traces) and the volleys evoked by the second stimulus were practically the same, showing that the majority, if not all fibres stimulated by the second stimulus were activated by the first stimulus. However, when the first stimulus was applied to the left NR and the second stimulus to the right NR (Fig. 3B, F and G) no fibres appeared to be stimulated twice as there was practically no difference between effects of these stimuli when they were applied separately or jointly, at time periods during which the volleys were evoked. The collision test was repeated at several stimulus intensities to define the critical intensity above which there were indications for spread of current to axons of neurones from the contralateral NR. Such a critical intensity was at 90, 100, 100 and 110 μA in the four experiments in which it was verified, restricting the stimulation intensity to be used to ≤ 100 μA. The collision was tested either on indirectly evoked volleys (Fig. 3B and F) or on direct components of these volleys (Fig. 3G) at 0.3–0.4 ms intervals between the stimuli. It should nevertheless be considered that the collision test might not be sufficiently sensitive to exclude spread of current to all of the crossed fibres. This test was therefore supplemented by other tests described in Results.
Figure 3. Interactions between descending volleys evoked by stimulation of the red nuclei on both sides.
Averaged (n = 10) descending volleys recorded from the cord dorsum at the level of the C3/4 segments following stimulation of the left (L) and the right (R) red nucleus (NR). A, effects of two stimuli 0.4 ms apart, applied through the same electrode, separately (1st, 2nd), or jointly (both, black trace), the sum of potentials evoked by the 1st and 2nd stimulus (grey trace) and the difference between the sum of the volleys evoked by the separate stimuli and the volleys evoked by their joint application. Dotted lines indicate the onset of indirect components of the volleys, which were in this case larger than the direct. Note that the difference and the volley evoked by the 2nd stimulus alone were practically identical, showing that when this stimulus followed the 1st stimulus it did not add to its effects because all the fibres activated by the 1st stimulus were during refractory period. B, as in A but when the two stimuli were applied on the left and right side, respectively. Note that the direct volley evoked by stimuli applied within the left NR was larger than that evoked from the right NR but the time intervals between indirect volleys evoked from the two nuclei were the same as in A. In this case there were no indications that the same fibres were excited by the two stimuli, or at least that the number of such fibres was very small, because there was hardly any difference between the sum of separate effects of these stimuli and the effects of their joint application. C and D, marking lesions indicating location of the stimulating electrodes in the left and right NR from which the volleys in A and B were evoked. When the collision test was repeated for stimuli applied at different depths on the left side, the first stimulus was found to activate fibres that were refractory at the time of application of the 2nd stimulus only when it was applied 1 mm deeper. E and F, as in A and B but from another preparation in which only indirect volleys were evoked from either the left or the right NR. G, as in B but in a preparation in which both direct and indirect volleys were evoked from the left and right NR. The stimuli were timed such that the direct volley from the right NR was delayed with respect to the direct volley from the left NR; dotted lines indicate the onset of these direct volleys. Note that during the period corresponding to the second direct volley, the sum of the records (grey) was not larger than the volley (black) induced by joint stimulation of the two nuclei even if there were differences in the indirect components which might reflect activation of neurones that were not activated directly. The records are with the negativity up. Shock artefacts have been truncated.
The second methodological problem was related to the way the rubrospinal neurones are activated by electrical stimuli, directly or trans-synaptically (Baldissera et al. 1972b). Descending volleys evoked from within either the contralatral or the ipsilateral NR often showed two components illustrated in Figs 2 and 3. At the C3/4 level the earlier components were evoked at latencies 0.6–0.7 ms from the onset of the stimulus and the second components at latencies 1.2–1.4 ms. The first of these components is attributable to direct and the second one to indirect activation of NR neurones and the proportion of neurones activated in these two ways may vary depending on the location of the stimulating electrode. Similar amplitudes of the two components, as in Fig. 3B (L NR) and G (L NR) indicate that similar proportions of neurones in the stimulated nucleus were activated directly and indirectly, no NR neurones being likely to be activated both directly and indirectly (Baldissera et al. 1972b). Larger later components of volleys in Fig. 3A show that in this particular experiment a larger proportion of neurones was activated indirectly than directly, while the opposite was true for volleys illustrated in Fig. 3G (R NR). The indirect activation secondary to stimulation of axons of interposito-rubral neurones was found to be particularly potent (Baldissera et al. 1972b) but stimulation of any axons providing input to NR neurones might contribute to it.
Descending volleys recorded from the cord dorsum, or from the surface of the contralateral lateral funiculus at a thoracic level are usually much smaller, and those at lumbar levels even smaller and their two components are less distinct. In the rat, as in the cat, two components of the descending volleys about 0.8 ms apart were recorded from the contralateral lateral funiculus while the second component was absent in recordings from the ipsilateral funiculus (at the C6 level; Al-Izki et al. 2008). Two sources of origin of the ipsilaterally recorded volleys were therefore considered: that the ipsilaterally recorded volleys might be secondary to direct activation of the axons of neurones from the contralateral NR, or of ipsilaterally projecting neurones. Both of these explanations would apply to the results of experiments in the rat but descending volleys recorded from the ipsilateral lateral funiculus in the cat (at a Th level) seem to be somewhat at variance with what was found in the rat, because they showed features of both directly and indirectly induced volleys. Latencies of the earliest components of some of these ipsilaterally recorded volleys (close to the latencies of antidromic activation of neurones in the NR) were as required for directly induced volleys. In favour of the indirect origin of later components of the same or of other volleys were the following: about 0.5 ms longer latencies, temporal facilitation (Fig. 2G) and also their appearance in preparations in which only indirect volleys were recorded from the C3/4 levels, as in the experiment illustrated in Fig. 2E–H.
Records in Fig. 2H show that descending volleys following stimulation of the ipsilateral NR disappeared below the level of the contralateral hemisection at a thoracic level, suggesting a third interpretation of the ipsilateral volleys in addition to the two interpretations proposed by Al-Izki et al. (2008). As these volleys were recorded a few millimetres rostral (Fig. 2G) but not caudal (Fig. 2H) to the hemisection, they might also be considered as reflecting activity in contralaterally rather than ipsilaterally descending rubrospinal tract fibres, as in other cases of volleys recorded at a distance (see, e.g. Jankowska et al. 2003). Direct volleys recorded ipsilaterally in the intact spinal cord might thus be indeed due to activation of crossed rubrospinal tract fibres, but descending in the contralateral lateral funiculus and originating in the ipsilateral and not contralateral NR. If so, one may consider that activation of a very small number of ipsilaterally descending rubrospinal neurones is not associated with distinct volleys and may not be expected at a lumbar level.
No evidence for activation of reticulospinal or crossed corticospinal tract fibres following stimuli applied in the NR was found below the cervical enlargement by Baldissera et al. (1972b), which might also apply to uncrossed PT fibres. As an additional means to differentiate between synaptic actions evoked via NR neurones and via other neurones activated by collaterals of the same presynaptic fibres, we compared effects of stimuli applied within, dorsal and ventral to the NR, considering that stronger synaptic actions evoked from within the NR would be more likely to be mediated by NR than by other neurones. The same test was also used to estimate the risk of spread of current to areas of origin of the tecto-spinal and tecto-reticulo-spinal pathways (Appelberg & Jeneskog, 1972; Appelberg et al. 1982) by stimuli applied in the NR, which was our third methodological problem. No synaptic actions were found from these areas in hindlimb motoneurones (Hongo et al. 1969a,b; Baldissera et al. 1972a). However, this was again ascertained for contralateral motoneurones and ipsilateral motoneurones might differ in this respect. Since the involved regions of the mesencephalic tegmentum neighbour the NR, we examined whether the same effects would be evoked from just dorsal to the NR as from within the NR. Lack of such effects allowed those from within the NR to be considered as reasonably selective. It will also be noted that only longer latency descending volleys were evoked from regions dorsal to the NR (Fig. 2D) where they could be secondary to stimulation of axons of reticulospinal or reticulo-reticular neurones (Mitani et al. 1988a,b; Matsuyama et al. 1993).
Results
Postsynaptic potentials evoked from the ipsilateral NR
In preparations in which only the ipsilateral half of the spinal cord remained intact (see Fig. 1A), EPSPs from the ipsilateral NR were found in 37% and IPSPs in 29% of motoneurones tested in five preparations (Table 1). Examples of these PSPs are shown in Fig. 4A–C, G and H. In preparations in which the ipsilateral half of the spinal cord was transected and only the contralateral half was intact (see Fig. 1B) EPSPs from the ipsilateral NR were found in 38% of motoneurones but IPSPs in none. No major differences have been found between synaptic actions evoked in flexor and extensor motoneurones (represented by PBST and DP and by GS, Pl, FDL and Q, respectively).
Table 1.
Comparison of proportions of motoneurones with EPSPs and IPSPs evoked from the ipsilateral and contralateral NR
| 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|
| Pathways | Test | Total no | Ipsi NR | Contra NR |
| Ipsilaterally descending | EPSPs | 38 | 37% | 53% |
| Contralaterally descending | EPSPs | 37 | 38% | 19% |
| Ipsilaterally descending | IPSPs | 38 | 29% | 42% |
| Contralaterally descending | IPSPs | 37 | 0% | 0% |
Columns: 1, pathways via which facilitation was evoked; 2, tested PSPs; 3, total numbers of motoneurones tested; 4 and 5, percentages of motoneurones in which PSPs were evoked by stimulation (trains of 4–5 stimuli, at 100 μA) of the ipsilateral (4) or the contralateral (5) NR. Ipsilateral and contralateral denote nuclei and tracts with respect to motoneurones. No significant differences were found between percentages of motoneurones affected by stimulation of ipsilateral and contralateral NR via either pathway (Chi-square tests).
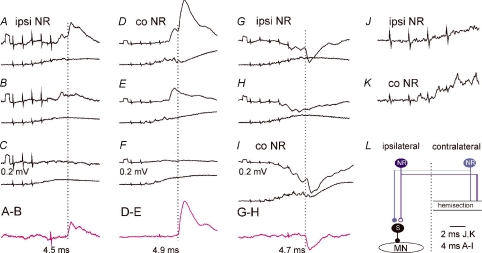
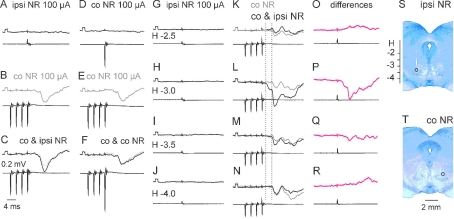
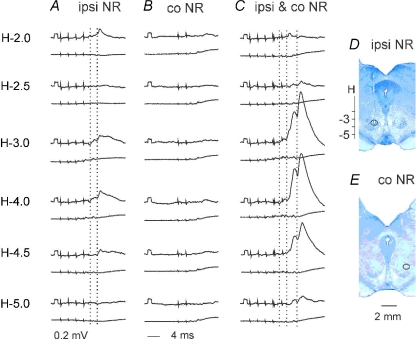
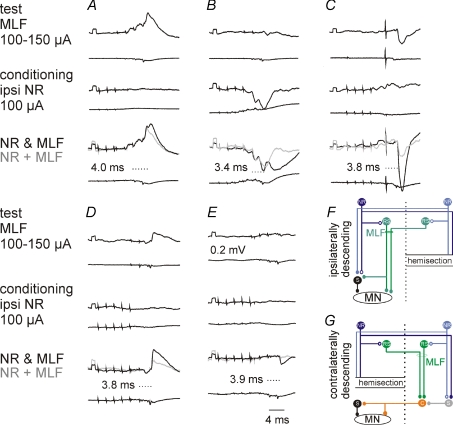
Figure 4. Comparison of EPSPs and IPSPs evoked from the ipsilateral and contralateral NR via ipsilaterally descending pathways.
A–I, intracellular records from motoneurones (upper traces) and cord dorsum potentials (lower traces) from the surface of the spinal cord a few millimetres rostral or caudal of the location of the motoneurones. In A–F and G–I are EPSPs from a DP and IPSPs from a GS motoneurone evoked by stimulation of the ipsilateral (ipsi) or contralateral (co) NR (100 μA) in a preparation with only ipsilaterally descending pathways left intact. Rectangular pulses at the beginning of the traces are calibration pulses (0.2 mV). Bottom traces in C, F and H show results of subtractions (A – B, D – E and G – H) that allow visualizing PSPs evoked by the last stimulus in a given train; their latencies (dotted lines) measured from the last stimulus are indicated by the values. NR was stimulated at sites shown in Fig. 3. J and K, expanded records of cord dorsum potentials in B and D (4× vertically; 2× horizontally). L, simplified diagram of the neuronal networks in Fig. 1 tested in the illustrated experiments. In this and the following figures, negativity is down in intracellular records and up in records from cord dorsum.
Effects of stimuli applied in the ipsilateral and contralateral NR were compared in about one half of the motoneurones in which any PSPs were evoked. As shown in Table 1, the proportions of motoneurones with EPSPs and IPSPs evoked from the ipsilateral and contralateral NR via either ipsilaterally or contralaterally descending pathways differed, with a tendency for more frequently evoked crossed rubrospinal actions, even though no statistically significant differences were found between them. Table 1 also shows that no inhibitory commissural neurones were activated by stimuli applied within either ipsilateral or contralateral NR alone via contralaterally descending pathways, as no IPSPs were evoked under our experimental conditions.
Smaller proportions of motoneurones in which PSPs were evoked from the ipsilateral than from the contralateral NR were associated with several times smaller amplitudes of these PSPs (cf. Figs 4A–C, D–F, 4G–H and I). Amplitudes of ipsilaterally evoked PSPs were of about 0.2–0.3 mV following the first effective stimulus (usually third or fourth in the train) although PSPs added by successive stimuli were up to about 1 mV. Less effective stimuli would be compatible with lower synaptic efficacy and/or effects of smaller numbers of ipsilaterally descending rubrospinal tract fibres reaching the last order interneurones on either the same or on the opposite side to that of the location of the motoneurones (see Fig. 1A and B).
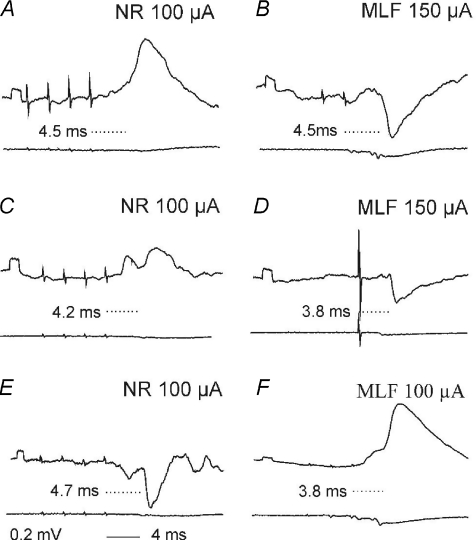
In order to evaluate a relative contribution of uncrossed and inadvertently stimulated crossed rubrospinal fibres to PSPs evoked from the ipsilateral NR, we made additional tests illustrated in Figs 5 and 6. Figure 5A–F shows that single stimuli applied in the ipsilateral NR were much more effective in facilitating PSPs evoked by a train of stimuli applied within the contralateral NR than when an additional stimulus was added to this train. This is indicated by a much more marked difference between the test and conditioned IPSPs in C than in F. As the single stimuli were applied simultaneously with the last stimulus, these observations are in favour of stimulation of distinct rather than the same fibres in the two nuclei.
Figure 5. Facilitation of IPSPs evoked from the contralateral NR by additional stimuli applied in the ipsilateral but not in the contralateral NR.
Intracellular records from a PBST motoneurone (A–F) and a DP motoneurone (G–R) (upper traces) and from cord dorsum in the C3 segment (lower traces) in the experiment illustrated in Fig. 3K–N. A and D, effects of single stimuli in the contralateral (co) and ipsilateral (ipsi) NR at locations (H-3.5) indicated in S and T. B and E, IPSPs evoked by a train of stimuli applied in the contralateral NR at the same depth as in D (at a latency of 5.7 ms from the last stimulus and 2.4 and 1.7 ms from the first and second components of the descending volleys recorded from the L7 segment, and hence most likely disynaptically or trisynaptically. C and F, IPSPs evoked by joint actions of these stimuli and of additional stimuli applied within the ipsilateral NR (C) and the contralateral NR (F). G–N, effects of single stimuli applied at the indicated depths above, within and below the ipsilateral NR, when they were applied alone (G–J) or added to the train of stimuli applied in the contralateral NR (K–N; black traces) at the same depth as in B which by themselves evoked responses in grey. The single stimuli were applied simultaneously with the 4th contralateral stimulus. O–R, differences between the grey and black records in K–N. S and T, stimulation sites at which the stimuli were applied (after hemisection on the right side). Note that the facilitation from the ipsilateral NR was evoked from 2 sites within the NR but not from more dorsal or more ventral sites 0.5 mm away. Note also that the facilitation evoked from H-3 was so effective that it enhanced effects of not only the fourth but also of the third contralateral stimulus; both were evoked at the same latencies of 4.5 ms from the stimuli, their onsets being indicated by the two vertical dotted lines. Diagram L in Fig. 4 applies to Fig. 5.
Figure 6. Comparison of EPSPs evoked from within and from outside of the ipsilateral NR and facilitation of EPSPs evoked from the ipsilateral and contralateral NR.
A–C, records from a DP motoneurone (upper traces) in the same experiment as those in Fig. 3 and from the cord dorsum in the L6 segment (lower traces). In A are examples of EPSPs evoked by a train of 100 μA stimuli applied in the ipsilateral NR along the electrode track shown in D, at the depths given to the left. The EPSPs were evoked at latencies of 4.5 ms from the effective stimuli, and 1.6 ms from the first components of the descending volleys; the onset of EPSPs evoked by the 3rd and 4th stimuli is indicated by the vertical dotted lines. Records in B show only marginal effects of double stimuli applied in the contralateral NR at a site indicated in E. Records in C show that large EPSPs were evoked when the conditioning stimuli were applied within the ipsilateral NR, but not when they were applied more dorsally or more ventrally. The facilitation involved EPSPs evoked by the 4th ipsilateral stimuli (second dotted line) and the 2nd contralateral stimuli (third dotted line). The hemisection was on the right side. Diagram L in Fig. 4 applies to Fig. 6.
Similar results were obtained in 10 motoneurones in two experiments whether the interactions were tested on facilitation of EPSPs (n = 2) or of IPSPs (n = 8). Records in Fig. 5G–R show furthermore that such facilitation was much more potent when single stimuli were applied within the ipsilateral NR than ventral to it where the probability of spread of current to the crossed rubrospinal axons would be higher. This is indicated by a considerable increase in amplitude of IPSPs in Fig. 5L and M and by the size of the differences between conditioned and unconditioned IPSPs in Fig. 5P and Q. In fact, facilitation was more potent about 1 mm than 0.5 mm dorsal to the ventral border of the nucleus because it involved not only IPSPs evoked by the 4th stimuli (at the level of the second dotted line in Fig. 5L and M) but also by the 3rd stimuli (first dotted line in Fig. 5L) that were occasionally seen also in other motoneurones (see, e.g. Fig. 5B and E). Another example of much more potent mutual facilitation of effects evoked from within than from outside NR nuclei is shown in Fig. 6, in this case for EPSPs.
These results lead to two conclusions. First, most of the fibres excited by stimuli applied in the ipsilateral and contralateral NR were distinct. Second, major effects of ipsilateral stimuli on hindlimb motoneurones seen under our experimental conditions may be attributed to ipsilaterally projecting NR neurones, rather than to inadvertently stimulated other axons (see Discussion).
Could synaptic actions of ipsilateral corticospinal neurones on motoneurones be relayed by rubrospinal neurones?
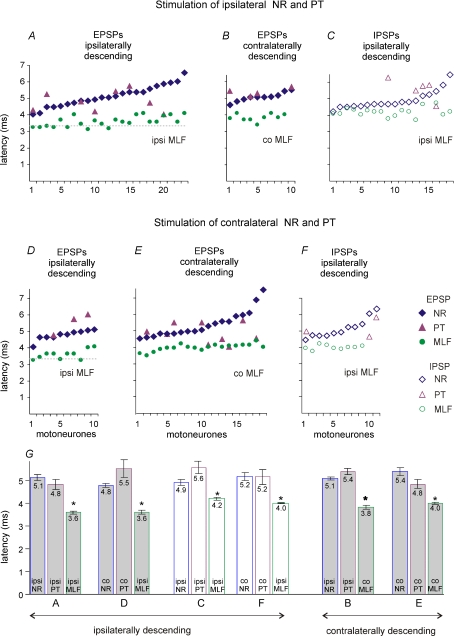
In order to answer this question we compared timing of PSPs evoked from the ipsilateral NR and the ipsilateral PT. Plots in Fig. 7A and C show that latencies of EPSPs and IPSPs evoked from the ipsilateral NR (diamonds) ranged between 4.2 and 6.3 ms. The shortest latencies were 4–4.5 ms, i.e. 0.5–1.5 ms shorter than the shortest latencies of trisynaptic actions from the ipsilateral PT (evoked at latencies within 4.5–5.5 ms range (Stecina & Jankowska, 2007). If NR neurones which evoked the earliest PSPs were activated by PT neurones, then they could, theoretically, relay some of the trisynaptic actions from the ipsilateral PT. However, latencies of the majority of PSPs which were evoked in the same motoneurones (triangles) do not support this possibility. In all but three of these motoneurones latencies of PSPs evoked from the PT were less than 0.5 ms longer, the same, or even shorter than latencies of PSPs evoked from the NR while an additional synaptic delay in NR would require at least 1 ms, especially as the conduction distance along rubrospinal fibres would be a few millimetres longer than along corticospinal fibres and might therefore involve a somewhat longer conduction time. This was also true for EPSPs and IPSPs evoked via contralaterally (Fig. 7B) descending pathways. Similar time relationships were found for EPSPs and IPSPs evoked from the contralateral NR (Fig. 7D–F).
Figure 7. Comparison of latencies of EPSPs and IPSPs evoked from the ipsilateral and contralateral NR, PT and MLF.
Minimal latencies of EPSPs and IPSPs evoked by the 4th or 5th stimulus in the train of stimuli measured from stimulus artefacts. In A–C are data for motoneurones in which EPSPs or IPSPs were evoked from the ipsilateral NR, ipsilateral PT and ipsilateral or contralateral MLF. They are ranked in an increasing order for PSPs evoked from the NR with corresponding latencies of PSPs from the MLF and from the PT in the same motoneurones. In D–F are data for motoneurones in which EPSPs or IPSPs were evoked from the contralateral NR, contralateral PT and ipsilateral or contralateral MLF plotted in the same way. Horizontal dotted lines in A and D indicate mean latencies of monosynaptic EPSPs from the MLF. G, mean latencies (±s.e.m.) of EPSPs and IPSPs evoked from the ipsilateral (ipsi) and contralateral (co) NR, PT and MLF plotted in A–F. The PSPs evoked by stimulation of the MLF (either ipsilateral or contralateral to the motoneurones) had significantly shorter latencies than those evoked by NR or PT stimulation as indicated by * (t test, P < 0.005). However, no statistically significant differences were found between the mean latencies of any PSPs evoked by the ipsilaterally or the contralaterally descending pathways, or between those evoked by stimulation in the ipsilateral or in the contralateral sites (ANOVA).
Are synaptic actions of ipsilateral corticospinal and rubrospinal neurones on motoneurones mediated via the same relay neurones and, if so, which?
In order to investigate whether PSPs evoked from the PT and those evoked from the NR were relayed by the same neurones, mutual facilitation of synaptic actions of PT and NR neurones on motoneurones was tested in a number of combinations. The results of these tests are illustrated in Fig. 8 and summarized in Table 2. They revealed mutual facilitation of both EPSPs (Fig. 8A, B and D) and IPSPs (Fig. 8C and E), although EPSPs were facilitated in a higher percentage of the motoneurones. The degree of the facilitation evoked via ipsilaterally (Fig. 8D and E) and contralaterally (Fig. 8A–C) descending pathways was found to be generally comparable (column 5 in Table 2).
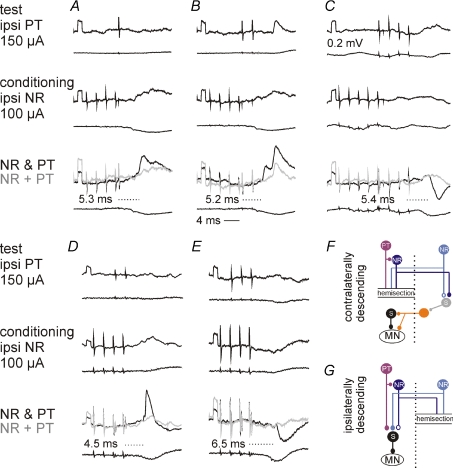
Figure 8. Ipsilateral NR neurones enhance ipsilateral actions of pyramidal tract neurones via ipsilaterally and contralaterally descending pathways.
Intracellular records from four motoneurones in 3 cats: Q (A and B), Sart (C), PBST (D), and Pl (E) and records from the cord dorsum in the L6 segment. Upper and middle panels: effects of stimulation of the ipsilateral PT alone and of the ipsilateral NR alone. Lower panels: effects of joint stimulation of the NR and PT (black traces) and the sums of records in upper and middle panels (grey traces). Note differences between them. Dotted lines and values in bottom panels give latencies of the facilitated components from the effective PT stimulus. The records are from preparations with only the contralaterally (A–C) or only the ipsilaterally (D–E) descending pathways left intact, with the corresponding simplified diagrams of the neuronal networks in F and G.
Table 2.
Comparison of facilitation of EPSPs and IPSPs from the ipsilateral PT by stimuli applied in the ipsilateral NR, evoked via ipsilaterally and contralaterally descending pathways
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|
| Pathways | Test | Motoneurones tested (Total number) | Motoneurones facilitated (%) | Facilitation mean ±s.e.m. (mV) | Facilitation cond/test (%) | Facilitated PSP latency (ms) |
| Ipsilaterally descending | EPSPs | 37 | 59% | 0.26 ± 0.04 | 158 ± 11% (6) | 5.4 ± 0.1* |
| Ipsi NR & ipsi PT | IPSPs | 37 | 27% | 0.37 ± 0.08 | 189 ± 7% (3) | 6.2 ± 0.3 |
| Contralaterally descending | EPSPs | 23 | 39% | 0.22 ± 0.03 | 373% & 116% | 6.0 ± 0.4 |
| Ipsi NR & ipsi PT | IPSPs | 23 | 13% | 0.35 ± 0.06 | — | 8.7 ± 2.3 |
1, pathways via which facilitation was evoked; 2, tested PSPs; 3, total numbers of motoneurones tested; 4, percentages of motoneurones in which PSPs were facilitated; 5, mean amplitude of the facilitated components of the PSPs in mV; data for all motoneurones tested. 6, relative increases in test PSPs in percentage of control levels (calculated for motoneurones in which PSPs were evoked by the test stimuli alone with the numbers of motoneurones in parantheses). 7, latencies of the facilitated components measured from the last PT stimulus. No statistically significant differences were found between the degree of facilitation evoked via ipsilaterally or contralaterally descending pathways (amplitudes of the facilitated components in column 5 or their latencies in column 7 but differences in proportions of motoneurones in which it was found were not statistically significant.
As ipsilateral actions of PT neurones on motoneurones are relayed by both reticulospinal neurones and spinal interneurones (Edgley et al. 2004; Jankowska & Stecina, 2007; Stecina & Jankowska, 2007; Stecina et al. 2008; see diagrams in Fig. 1), facilitation of these actions by NR neurones might have occurred at the level of either only one or of both of these neurones. The following experiments were therefore made in order to investigate these two possibilities.
Mutual facilitation of ipsilateral PT and NR actions at the level of reticulospinal neurones would require that at least some actions evoked by NR stimulation were mediated by reticulospinal neurones. We compared therefore latencies of PSPs evoked from the NR and from the MLF to verify whether PSPs from the NR were evoked at latencies compatible with an additional synaptic delay. As shown in Fig. 7, latencies of EPSPs evoked from the NR exceeded latencies of EPSPs evoked from the MLF by 1.1–1.8 ms, as required for such a delay. In all individual motoneurones (Fig. 7A and B) this delay was evident and differences between their mean values were statistically significant. In contrast, latencies of IPSPs evoked from the NR were often less than 0.5 ms longer than of those from the MLF (Fig. 7C). The differences between them were accordingly too short to allow mediation of IPSPs from NR by reticulospinal neurones. In addition, latencies of IPSPs were similar to, or even shorter than, latencies of EPSPs evoked from the NR (cf. Fig. 7A and C). However, they should be longer if IPSPs were to be mediated by both RS neurones and inhibitory premotor interneurones, as no direct inhibitory actions of RS neurones on hindlimb motoneurones have been found. Similar latencies of IPSPs and of EPSPs would thus indicate that the IPSPs were relayed by inhibitory premotor interneurones directly excited by rubrospinal neurones rather than via reticulospinal neurones.
Mutual facilitation of ipsilateral PT and NR actions at the level of spinal interneurones could occur on several subpopulations of these neurones. It could in particular occur on interneurones coexcited by muscle afferents and PT fibres (Stecina et al. 2008), in view of previously demonstrated projections of neurones in the contralateral NR on such interneurones (Hongo et al. 1972; Davies & Edgley, 1994). Such similar actions are illustrated in Fig. 4 and summarized in Fig. 7 and Table 1. Mutual facilitation of synaptic actions from the ipsilateral and contralateral NR is in addition illustrated in Figs 5 and 6.
Whether interneurones coexcited by ipsilateral PT and NR neurones were also coexcited by reticulospinal tract fibres required separate tests. We verified therefore whether mutual facilitation occurred between synaptic actions from not only the ipsilateral NR and PT but also from the ipsilateral NR and MLF. Figure 9 shows examples of such facilitation for both EPSPs (Fig. 9A and D) and IPSPs (Fig. 9B, C and E). This figure and data in Table 3 show also that the facilitation occurred when only ipsilaterally (Fig. 9A–C) or only contralaterally (Fig. 9D and E) descending pathways were left intact. The facilitation should thus involve both ipsilaterally and contralaterally located spinal interneurones previously identified as mediating reticulospinal actions (Jankowska et al. 2003; Cabaj et al. 2006). However, facilitation of IPSPs was found to be evoked in a greater proportion of motoneurones via ipsilaterally than via contralaterally descending pathways. IPSPs evoked by joint stimulation of the MLF and either ipsilateral or contralateral NR via contralaterally descending pathways would thus be most likely mediated by inhibitory commissural interneurones even if NR stimuli alone did not suffice to activate them. Data in Tables 1 and 3 would thus not be in variance.
Figure 9. Ipsilateral NR neurones enhance ipsilateral actions evoked from the MLF via ipsilaterally and contralaterally descending pathways.
Upper traces, intracellular records from four motoneurones in 2 cats: DP (A), GS (B and D), PBST (C) and Sart (E). Lower traces, records from the cord dorsum in the L6 or L7 segment. In each column the records show effects of stimulation of the ipsilateral (A, B and C) or contralateral (D and E) MLF alone, of the ipsilateral NR alone and of the NR and the MLF together, with the sum of effects evoked by separate stimuli in grey. The latencies of the facilitated components shown in bottom panels are from the last MLF stimulus. The records are from preparations with only the ipsilaterally (A–C) or only the contralaterally (D and E) descending pathways left intact, with the corresponding simplified diagrams of the neuronal networks in F and G.
Table 3.
Mutual facilitation of synaptic actions evoked from the ipsilateral MLF and ipsilateral or contralateral NR
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|
| Pathways | Test | Motoneurones tested (total number) | Motoneurones facilitated (%) | Facilitation mean ±s.e.m. (mV) | Facilitation cond/test (%) | Facilitated PSP latency (ms) |
| Ipsilaterally descending | EPSPs | 53 | 38% | 1.38 ± 0.13 | 170 ± 12% (19) | 3.86 ± 0.07 |
| Ipsi NR & ipsi MLF | IPSPs | 53 | 55% | 2.09 ± 0.20* | 286 ± 22% (29) | 4.09 ± 0.06* |
| Contralaterally descending | EPSPs | 21 | 100% | 1.26 ± 0.11 | 199 ± 20% (19) | 3.88 ± 0.03 |
| Ipsi NR & contra MLF | IPSPs | 21 | 10% | 4.0 | 129% (1) | 3.9 & 4.29 |
| Ipsilaterally descending | EPSPs | 7 | 71% | 1.97 ± 0.61 | 270 ± 141% (3) | 3.93 ± 0.32 |
| Co NR & ipsi MLF | IPSPs | 7 | 57% | 2.75 ± 0.89 | 283 ± 62% (5) | 3.98 ± 0.11 |
| Contralaterally descending | EPSPs | 21 | 81% | 2.07 ± 0.28 | 231 ± 21% (16) | 4.03 ± 0.05 |
| Co NR & contra MLF | IPSPs | 21 | 24% | 4.60 ± 2.67 | 354 ± 74% (3) | 4.39 ± 0.34 |
Format as for data in Table 2. 1, pathways via which facilitation was evoked; 2, tested PSPs; 3, total numbers of motoneurones tested; 4, percentages of motoneurones in which the facilitation was found; 5, mean amplitude of the facilitated components of the PSPs in mV (data for all motoneurones); 6, relative increases in test PSPs in percentage (when applicable, with the numbers of motoneurones tested in the parentheses). 7, latencies of the facilitated components from the onset of the 2nd or 3rd stimulus delivered to the MLF. As in the case of facilitation between PT and NR actions described above, both the increase in the area (in percentage) of the test PSPs, when these were originally present as in Fig. 9A, B and D, and the peak amplitude of the facilitated components in mV were used as the measure of the facilitation. Only the latter measure could be used for PSPs that were originally lacking but appeared when the test stimuli were preceded by the conditioning stimuli, as IPSPs in Fig. 9C and E. Statistically significant differences between latencies of EPSPs and IPSPs are indicated by * (t test).
Despite the frequent occurrence of mutual facilitation of synaptic actions evoked from the ipsilateral NR and the MLF, our results also provided evidence that some of their relay neurones are separate. This was indicated by synaptic actions evoked from the ipsilateral NR that were not associated with similar actions from the MLF (n = 2), or were associated with different actions (n = 9) in the same motoneurone. Examples of such different actions are shown in Fig. 10, with EPSPs evoked from the NR but IPSPs from the MLF (Fig. 10A–D), or IPSPs from the NR but EPSPs from the MLF (Fig. 10E and F). Different actions from NR than from the MLF were seen in 4 out of 5 preparations but were more frequently evoked via ipsilaterally descending (n = 10) than via contralaterally descending pathways (n = 1). Opposite effects were also found in a few motoneurones in which stimuli applied in the ipsilateral NR facilitated EPSPs from the PT but IPSPs from the MLF or IPSPs from PT but EPSPs from the MLF.
Figure 10. Examples of opposite synaptic actions from the ipsilateral NR and from the MLF.
Upper traces, intracellular records from two PBST motoneurones (A and B) and a DP motoneurone (C). They are from 3 preparations in which only ipsilaterally descending pathways were left intact. Lower traces are from the cord dorsum. Values between the records give the latencies from the last stimuli; they correspond to segmental latencies from the NR volleys of 1.5–1.7 ms and from the MLF volleys of 1–1.5 ms.
Discussion
The results of this study provide evidence that stimuli applied in the ipsilateral NR affect hindlimb motoneurones by evoking in them short latency EPSPs and IPSPs and that they facilitate excitation and inhibition of motoneurones by corticospinal and reticulospinal neurones. However, in view of methodological problems related to centrally applied electrical stimuli, several issues had to be considered before allowing us to attribute the reported ipsilateral actions to neurones in the NR. As indicated in Methods and Results, the major issue was the risk of spread of current to axons of neurones located outside the stimulated nucleus, in particular to axons of neurones in the contralateral NR after they crossed the midline. None of the control experiments of this study conclusively excluded it by itself, but each increased the confidence that the reported effects were evoked by ipsilaterally acting NR neurones rather than by inadvertently stimulated axons of other neurones. (i) The collision tests illustrated in Fig. 3 did not reveal spread of current from the ipsilateral NR to the crossed rubrospinal axons, as judged by lack of effects of its stimulation on descending volleys evoked from the contralateral NR. Nevertheless, a contribution of some crossed rubrospinal fibres to these volleys could not be excluded by these tests. (ii) The tests illustrated in Fig. 5, in which effects of stimuli applied within the ipsilateral and contralateral NR were evaluated from their postsynaptic actions, similarly failed to reveal a contribution of the crossed rubrospinal axons but could not exclude that some added to actions of the uncrossed axons. (iii) Similar EPSPs and IPSPs were evoked from the dorsal and ventral parts of the ipsilateral NR and much smaller, or negligible PSPs were evoked from areas just outside the borders of the NR, either ventral or dorsal to it, as illustrated in Figs 5 and 6. Therefore we consider it unlikely that these PSPs were evoked by spread of current to neurones or fibres outside rather than inside NR, including crossed rubrospinal axons running ventral to its borders (see, e.g. Hinman & Carpenter, 1959; Courville, 1966). (iv) Consequences of spread of current to the neighbouring mesencephalic tegmentum could be obviated because no PSPs at similarly short latencies were found to be evoked from the areas just adjacent to the ipsilateral NR. PSPs were only evoked from more dorsal areas within which axons of reticulospinal, or reticulo-reticular neurones (Mitani et al. 1988a,b; Matsuyama et al. 1993) could have been stimulated. These observations thus extend observations of Baldissera et al. (1972a) who reported that stimuli applied in the contralateral mesencephalic tegmentum failed to evoke any short latency actions on α-motoneurones in the lumbosacral enlargement. (v) Effects of unavoidable activation of fibres providing input to neurones in the NR were concluded to be without major consequences within the lumbosacral enlargement because no discharges were found in descending tract fibres by stimuli applied in the NR after transection of rubrospinal tract fibres in the lateral part of the medulla (Baldissera et al. 1972b; see, however, Appelberg & Jeneskog, 1972; Appelberg et al. 1982). This was demonstrated for fibres descending contralaterally and may or may not apply to the ipsilateral ones. However, at least the probability that reticulospinal tract fibres excited by spread of current would mediate EPSPs evoked from the NR would be low because latencies of EPSPs evoked from the NR were always longer than of EPSPs evoked by stimulation of the MLF.
Taken together the results of both the present and the previous control tests, lead to the conclusion that effects of stimuli applied in this study in the ipsilateral NR were primarily due to effects of neurones in this nucleus.
Comparison of crossed and uncrossed actions from NR
Records of synaptic potentials evoked in motoneurones, illustrated in Figs 4, 6, 8, 9 and 10 consistently show that they were very small, and together with those of Endo et al. (1975) indicate much weaker ipsilateral than contralateral actions. On the basis of these records they might be considered as marginal and we would not like to give the impression that we overestimate them. However, even though effects of ipsilateral NR neurones on motoneurones are weak, this does not exclude the possibility that their effects on interneurones relaying NR actions are far from marginal. In fact all the tests illustrated in Figs 5, 6 8 and 9 show that stimuli applied in ipsilateral NR potently increase effects of stimulation of other neuronal systems (see below). Even though morphological studies indicate that not more than some 10% of rubrospinal neurones project ipsilaterally (see Introduction), their terminals were found within several segments and Holstege, 1987) came to the conclusion ‘that every ipsilaterally descending fibre had several branches’ in the intermediate zone. By providing input to premotor interneurones in parallel with other sources of input to them, ipsilaterally descending NR neurones may thus be instrumental in bringing them to suprathreshold levels and assist in their activation. As shown in Results (Fig. 5) amplitudes of PSPs evoked from the contralateral NR were sometimes doubled when stimulation of this nucleus was combined with stimulation of the ipsilateral nucleus.
Under conditions when premotor interneurones are highly efficiently activated by other neuronal systems, contribution of uncrossed actions of NR neurones may be functionally insignificant. However, their relative contribution may increase when crossed corticospinal and/or rubrospinal pathways are damaged, especially if the density of synaptic contacts of uncrossed NR neurones is increased by sprouting during the recovery period. As shown in Results and discussed in the next sections, this could occur at the level of both supraspinal and spinal neurones.
Integration of synaptic actions on ipsilateral hindlimb motoneurones evoked by pyramidal tract neurones and by neurones in the red nucleus and in the reticular formation
Considering that NR neurones might relay PSPs evoked by PT neurones, we paid particular attention to the latencies of EPSPs and IPSPs evoked by NR stimuli. These latencies should not exceed 3.7–4.3 ms to allow NR neurones to contribute to the earliest components of PSPs of PT origin evoked in hindlimb motoneurones that were found to be 4.7–5.3 ms (see Fig. 3A in Stecina & Jankowska (2007) because activation of rubrospinal neurones by PT fibres should require at least 1 ms. The additional 1 ms delay should include about 0.5–0.6 ms for conduction time along PT fibres and their collaterals, about 0.3–0.4 ms of synaptic delay, and 0.3–0.4 ms between the onset of slow rising EPSPs of PT origin induced in NR neurones and generation of action potentials in these neurones (Tsukahara et al. 1968; Tsukahara et al. 1975). However, EPSPs and IPSPs evoked at latencies of 3.7–4.3 ms were found only exceptionally. Latencies of PSPs from the NR most often overlapped with, or exceeded latencies of PSPs of PT origin evoked in the same motoneurones and inspection of data in Fig. 7A shows that this would be often true even if latencies of some PSPs evoked by NR stimuli were overestimated by 0.7 ms (i.e. if they were secondary to indirect rather than to direct activation of NR neurones and related to the second rather than to the first of the two descending volleys following these stimuli (Baldissera et al. 1972b). Thus our results do not support the possibility that NR neurones relay the earliest trisynaptically evoked EPSPs and IPSPs of PT origin.
Ipsilateral NR neurones may nevertheless importantly contribute to ipsilateral actions of PT neurones by providing input to neurones that mediate PT actions. The evidence for this is threefold. First, we have found that PSPs evoked by weak PT stimuli may critically depend on their association with NR stimuli, both applied ipsilaterally. This was demonstrated by mutual facilitation of NR and PT actions, especially when no PSPs were evoked by either NR or PT stimuli alone but appeared on their joint application. Amplitudes of these PSPs were low under our experimental conditions, but PSPs of PT origin were often doubled or tripled by NR conditioning stimulation. Second, our results suggest that some synaptic actions of not only PT neurones but also NR neurones might be relayed by reticulospinal neurones and that input from NR neurones might increase the probability of activation of reticulospinal neurones by PT neurones. This is indicated by observations that EPSPs (although not IPSPs) of NR origin are properly delayed with respect to EPSPs evoked from the MLF and that effects of stimuli applied within the NR and within the MLF potently facilitate each other. Third, our results indicate that NR neurones may assist in activation of not only reticulospinal but also of spinal relay neurones of PT neurones. The evidence for this is mutual facilitation of PSPs evoked by NR and PT stimuli at too short latencies to be compatible with their mediation by reticulospinal neurones.
Similar latencies of synaptic actions evoked by NR and PT stimuli together with a mutual facilitation of these actions might raise the question whether collaterals of PT fibres responsible for these actions were not activated from within NR. No evidence for this was found by Baldissera et al. (1972b) for PT fibres reaching lumbosacral enlargement and our observations are more consistent with synaptic actions mediated via the same relay neurones than with stimulation of the same fibres. As indicated above, latencies of PSPs evoked by NR stimuli were similar, but also shorter or longer than latencies of PSPs evoked by PT stimuli. Furthermore, facilitation of synaptic actions of PT and NR stimuli often occurred when the two stimuli were applied within very short intervals (see Fig. 8A and D) at which one of these would coincide with the refractory period after the other; the facilitation would thus be unlikely due to stimulation of the same fibres.
Morphologically demonstrated ipsilateral projections of NR neurones to the reticular formation (see Introduction) indicate that NR neurones might activate reticular neurones which act on ipsilateral motoneurones either directly (if they are reticulospinal and synapse with motoneurones), or indirectly (via other reticular neurones and/or spinal interneurones; for references see Jankowska et al. 2003).
As outlined in the results, our data are consistent with the possibility that some reticulospinal neurones mediate rubral actions for short latency EPSPs but not IPSPs. As shown in Fig. 7, latencies of EPSPs evoked from the NR were generally 1–2 ms longer than latencies of EPSPs from the MLF, this difference being as needed for one or two additional synaptic delays and conduction time along most likely much slower conducting axon collaterals, while differences between latencies of IPSPs evoked from the NR and the MLF were much smaller. Inconsistent with the mediation of the earliest IPSPs from the NR by RS neurones is also that latencies of the majority of IPSPs evoked from either the ipsilateral NR or the contralateral NR overlapped with latencies of EPSPs and that mean latencies of the IPSPs from the ipsilateral NR were in fact shorter than of EPSPs. In view of previous evidence that RS neurones projecting to the lumbosacral enlargement are excitatory (Grillner et al. 1968; Peterson et al. 1979) they should evoke IPSPs in motoneurones via inhibitory premotor interneurones, i.e. with an additional synaptic delay. For these reasons the most plausible explanation of the enhancement of disynaptic IPSPs evoked from the MLF by stimuli applied in the ipsilateral NR would be facilitation of activation of premotor inhibitory interneurones activated by reticulospinal neurones (Takakusaki et al. 1989, 2003; Davies & Edgley, 1994; Cabaj et al. 2006). Facilitation of disynaptic IPSPs from the MLF should have occurred on ipsilaterally located inhibitory premotor interneurones via ipsilaterally descending pathways (Fig. 1A) and on inhibitory commissural interneurones via contralaterally descending pathways (Fig. 1B), both monosynaptically activated by reticulospinal neurones. However, effects of activation of inhibitory commissural interneurones were found only very rarely and only by combined actions of MLF and NR stimuli (Table 3) but not by NR stimuli alone (Table 1). Facilitation of disynaptic EPSPs could on the other hand have occurred at the level of either ipsilaterally located premotor interneurones or excitatory commissural interneurones excited by reticulospinal neurones. It could also occur at the level of reticulospinal neurones activated by axon collaterals of fibres stimulated within the MLF (see Fig. 1 in Edgley et al. 2004 and Fig. 6 in Jankowska et al. 2006), if NR neurones provided input to them.
Spinal neurones relaying ipsilateral NR actions remain to be identified, considering that they may include propriospinal neurones located anywhere between the upper cervical and lower thoracic segments as well as segmental interneurones. The most extensively investigated propriospinal neurones, those located in the C3 and C4 spinal segments, are coexcited by contralateral PT and NR neurones and ipsilateral RS neurones (for review see Lundberg, 1999; Alstermark & Isa, 2002). Segmental interneurones likely to mediate ipsilateral NR actions should include interneurones with input from the contralateral PT and the contralateral NR (Tsukahara et al. 1968; Lundberg, 1975, 1979; Kostyuk & Vasilenko, 1978) because mutual facilitation of actions from either the ipsilateral or contralateral PT and from either the ipsilateral or contralateral NR (see Figs 6 and 7) on motoneurones indicates that these interneurones are coexcited by PT and NR neurones from both sides. The same neurones would also be coexcited by RS fibres and by group I afferents and/or group II afferents (Hongo et al. 1969b, 1972; Davies & Edgley, 1994; Cabaj et al. 2006). Ipsilaterally descending rubrospinal tract fibres would thus increase the probability of activation of these neurones by any other peripheral or descending sources of input to them. It is therefore of particular interest that activation of not only PT neurones and NR neurones but also neurones in the ipsilateral (Buford & Davidson, 2004) or both ipsilateral and contralateral (Schepens & Drew, 2006) pontomedullary reticular formation in ‘behaving animals’ is closely movement related. There is also growing evidence that not only ipsilaterally located segmental interneurones and propriospinal neurones (see, e.g. Alstermark & Lundberg, 1992; Blagovechtchenski et al. 2000) but also commissural interneurones (Blagoveshchenskii et al. 2005) contribute to voluntary movements.
Functional consequences of ipsilateral actions from the red nucleus
Involvement of neurones in not only contralateral but also ipsilateral red nucleus in centrally initiated movements may be of particular importance for the recovery of motor functions after injuries of corticospinal neurones. As indicated by results of this study as well as of previous studies of ipsilateral PT actions and of connections between PT neurones and ipsilateral NR neurones, ipsilateral actions of PT neurones may be strengthened by NR neurones and by their ipsilateral actions. Both may be furthermore strengthened by temporal and spatial facilitation on any of their shared relay neurones. In view of the previously documented high plasticity of cortico-rubral connections (Tsukahara et al. 1975; Murakami et al. 1977) relative contribution of PT neurones to activation of NR neurones might be also enhanced and the degree to which NR neurones relay actions of ipsilateral PT neurones increased. Our observations do not provide support for a major contribution of NR-relayed PSPs to early actions of PT neurones, but later actions may be non-negligible for motor recovery, especially when enhanced. Of particular interest in this context might be changes in time characteristics of EPSPs evoked by cortico-rubral neurones in the denervated NR neurones (Tsukahara et al. 1975, 1983) and that individual neurones in the NR draw input from large cortical areas (1 through 5) and from both the forelimb and hindlimb representation areas in addition to their somatotopically organized main input (Jeneskog & Padel, 1983). The reorganization of the cortical neuronal systems after stroke or other central injuries, with the increasing involvement of other than the motor area during voluntary movements (for reviews see, e.g. Hallett, 2001; Cauraugh & Summers, 2005; Gerloff et al. 2006) might thus be associated with an increase in the efficiency of activation of rubral neurones from these areas and in the increased involvement of these neurones in centrally initiated movements. It might also be reflected in a more efficient modulation of activity in other neuronal systems including centrally initiated locomotion (Rho et al. 1999; Lavoie & Drew, 2002). In keeping with the results of the present study, weak stimuli applied in NR enhanced amplitude and duration of EMG activity in both contralateral and ipsilateral hindlimb muscles but, in contrast to effects of stimulation of the motor cortex, did not influence the timing of the locomotor pattern (Rho et al. 1999). This could be related to the fact that activation of NR neurones on one side does not activate inhibitory commissural interneurones which coordinate the timing of locomotor activity on both sides of the body.
The contribution of ipsilateral actions of NR neurones to motor recovery after injuries of contralateral corticospinal and/or contralateral NR neurones might differ depending on the kind and the site of the injury. The contribution of ipsilateral NR neurones should be more important under conditions when spinal projections of both ipsilaterally and contralaterally projecting NR neurones are intact because, as reported, at least some actions of ipsilateral and contralateral NR neurones are relayed by the same spinal neurones. Injuries of neuronal systems providing input to NR neurones would likewise decrease the probability of activation of these neurones. Any re-organization of the ipsilateral corticospinal output following a unilateral cortical injury, including stronger actions on rubrospinal and reticulospinal neurones would on the other hand be beneficial because stronger activation of reticulospinal neurones could by itself assist in replacing missing actions of injured contralaterally projecting corticospinal neurones and also favour joint actions of ipsilateral rubral and reticulospinal neurones on the same premotor neurones. It is also conceivable that factors assisting the reorganization of descending neuronal systems at spinal levels might increase the probability of contribution of ipsilateral NR neurones to recovery of motor functions after central injuries. Particularly important might be the sprouting of uncrossed axons of NR neurones at spinal levels and the concomitant increase in efficacy of synaptic transmission between these neurones and their target cells. For recent references on such possibilities see, e.g. Bareyre et al. (2002), Maier & Schwab (2006), Schwab et al. (2006).
Acknowledgments
We wish to thank Mrs Rauni Larsson for her invaluable assistance, Dr Mary Pauline Galea for participation in a control experiment and Drs Jennifer Kornelsen and Ingela Hammar for helpful discussions and comments on the manuscript. The study was supported by grants from NINDS/NIH (R01 NS040863) and the Swedish Research Council (15393–01A).
References
- Al-Izki S, Kirkwood PA, Lemon RN, Enriquez Denton M. Electrophysiological actions of the rubrospinal tract in the anaesthetised rat. Exp Neurol. 2008;212:118–131. doi: 10.1016/j.expneurol.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Isa T. Premotoneuronal and direct corticomotoneuronal control in the cat and macaque monkey. Adv Exp Med Biol. 2002;508:281–297. doi: 10.1007/978-1-4615-0713-0_34. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Lundberg A. The C3–C4 propriospinal system: target-reaching and food-taking. In: Jami L, Pierrot-Deseilligny E, Zytnicki D, editors. Muscle Afferents and Spinal Control of Movement. Oxford: Pergamon Press; 1992. pp. 327–354. [Google Scholar]
- Antal M, Sholomenko GN, Moschovakis AK, Storm-Mathisen J, Heizmann CW, Hunziker W. The termination pattern and postsynaptic targets of rubrospinal fibers in the rat spinal cord: a light and electron microscopic study. J Comp Neurol. 1992;325:22–37. doi: 10.1002/cne.903250103. [DOI] [PubMed] [Google Scholar]
- Appelberg B, Hulliger M, Johansson H, Sojka P. An intracellular study of rubrospinal and rubro-bulbospinal control of lumbar gamma-motoneurones. Acta Physiol Scand. 1982;116:377–386. doi: 10.1111/j.1748-1716.1982.tb07155.x. [DOI] [PubMed] [Google Scholar]
- Appelberg B, Jeneskog T. Mesencephalic fusimotor control. Exp Brain Res. 1972;15:97–112. doi: 10.1007/BF00234960. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Lundberg A, Udo M. Activity evoked from the mesencephalic tegmentum in descending pathways other than the rubrospinal tract. Exp Brain Res. 1972a;15:133–150. doi: 10.1007/BF00235578. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Lundberg A, Udo M. Stimulation of pre- and postsynaptic elements in the red nucleus. Exp Brain Res. 1972b;15:151–167. doi: 10.1007/BF00235579. [DOI] [PubMed] [Google Scholar]
- Bareyre FM, Haudenschild B, Schwab ME. Long-lasting sprouting and gene expression changes induced by the monoclonal antibody IN-1 in the adult spinal cord. J Neurosci. 2002;22:7097–7110. doi: 10.1523/JNEUROSCI.22-16-07097.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagovechtchenski E, Pettersson LG, Perfiliev S, Krasnochokova E, Lundberg A. Control of digits via C3–C4 propriospinal neurones in cats; recovery after lesions. Neurosci Res. 2000;38:103–107. doi: 10.1016/s0168-0102(00)00147-4. [DOI] [PubMed] [Google Scholar]
- Blagoveshchenskii ED, Pettersson LG, Perfil’ev SN. Control of fine movements mediated by propriospinal neurons. Neurosci Behav Physiol. 2005;35:299–304. [PubMed] [Google Scholar]
- Brodal A. Neurological Anatomy in Relation to Clinical Medicine. Oxford: Oxford University Press; 1981. [Google Scholar]
- Brown LT. Rubrospinal projections in the rat. J Comp Neurol. 1974;154:169–187. doi: 10.1002/cne.901540205. [DOI] [PubMed] [Google Scholar]
- Buford JA, Davidson AG. Movement-related and preparatory activity in the reticulospinal system of the monkey. Exp Brain Res. 2004;159:284–300. doi: 10.1007/s00221-004-1956-4. [DOI] [PubMed] [Google Scholar]
- Burman K, Darian-Smith C, Darian-Smith I. Macaque red nucleus: origins of spinal and olivary projections and terminations of cortical inputs. J Comp Neurol. 2000;423:179–196. doi: 10.1002/1096-9861(20000724)423:2<179::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Cabaj A, Stecina K, Jankowska E. Same spinal interneurons mediate reflex actions of group Ib and II afferents and crossed reticulospinal actions. J Neurophysiol. 2006;95:3911–3922. doi: 10.1152/jn.01262.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canedo A, Towe AL. Pattern of pyramidal tract collateralization to medial thalamus, lateral hypothalamus and red nucleus in the cat. Exp Brain Res. 1986;61:585–596. doi: 10.1007/BF00237585. [DOI] [PubMed] [Google Scholar]
- Cauraugh JH, Summers JJ. Neural plasticity and bilateral movements: a rehabilitation approach for chronic stroke. Prog Neurobiol. 2005;75:309–320. doi: 10.1016/j.pneurobio.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Courville J. Rubrobulbar fibres to the facial nucleus and the lateral reticular nucleus (nucleus of the lateral funiculus). An experimental study in the cat with silver impregnation methods. Brain Res. 1966;1:317–337. doi: 10.1016/0006-8993(66)90125-9. [DOI] [PubMed] [Google Scholar]
- Davies HE, Edgley SA. Inputs to group II-activated midlumbar interneurones from descending motor pathways in the cat. J Physiol. 1994;479:463–473. doi: 10.1113/jphysiol.1994.sp020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Hammar I. Ipsilateral actions of feline corticospinal tract neurons on limb motoneurons. J Neurosci. 2004;24:7804–7813. doi: 10.1523/JNEUROSCI.1941-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo K, Araki T, Kawai Y. Contra- and ipsilateral cortical and rubral effects on fast and slow spinal motoneurons of the cat. Brain Res. 1975;88:91–98. doi: 10.1016/0006-8993(75)90953-1. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Bushara K, Sailer A, Wassermann EM, Chen R, Matsuoka T, Waldvogel D, Wittenberg GF, Ishii K, Cohen LG, Hallett M. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain. 2006;129:791–808. doi: 10.1093/brain/awh713. [DOI] [PubMed] [Google Scholar]
- Grillner S, Hongo T, Lund S. Reciprocal effects between two descending bulbospinal systems with monosynaptic connections to spinal motoneurones. Brain Res. 1968;10:477–480. doi: 10.1016/0006-8993(68)90221-7. [DOI] [PubMed] [Google Scholar]
- Hallett M. Plasticity of the human motor cortex and recovery from stroke. Brain Res Brain Res Rev. 2001;36:169–174. doi: 10.1016/s0165-0173(01)00092-3. [DOI] [PubMed] [Google Scholar]
- Hayes NL, Rustioni A. Descending projections from brainstem and sensorimotor cortex to spinal enlargements in the cat. Single and double retrograde tracer studies. Exp Brain Res. 1981;41:89–107. doi: 10.1007/BF00236598. [DOI] [PubMed] [Google Scholar]
- Hinman A, Carpenter MB. Efferent fiber projections of the red nucleus in the cat. J Comp Neurol. 1959;113:61–82. doi: 10.1002/cne.901130104. [DOI] [PubMed] [Google Scholar]
- Holstege G. Anatomical evidence for an ipsilateral rubrospinal pathway and for direct rubrospinal projections to motoneurons in the cat. Neurosci Lett. 1987;74:269–274. doi: 10.1016/0304-3940(87)90308-9. [DOI] [PubMed] [Google Scholar]
- Hongo T, Jankowska E, Lundberg A. The rubrospinal tract. I. Effects on alpha-motoneurones innervating hindlimb muscles in cats. Exp Brain Res. 1969a;7:344–364. doi: 10.1007/BF00237320. [DOI] [PubMed] [Google Scholar]
- Hongo T, Jankowska E, Lundberg A. The rubrospinal tract. II. Facilitation of interneuronal transmission in reflex paths to motoneurones. Exp Brain Res. 1969b;7:365–391. doi: 10.1007/BF00237321. [DOI] [PubMed] [Google Scholar]
- Hongo T, Jankowska E, Lundberg A. The rubrospinal tract. IV. Effects on interneurones. Exp Brain Res. 1972;15:54–78. doi: 10.1007/BF00234958. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Slawinska U, Maleszak K, Edgley SA. Neuronal basis of crossed actions from the reticular formation upon feline hindlimb motoneurons. J Neurosci. 2003;23:1867–1878. doi: 10.1523/JNEUROSCI.23-05-01867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Stecina K. Uncrossed actions of feline corticospinal tract neurones on lumbar interneurones evoked via ipsilaterally descending pathways. J Physiol. 2007;580:133–147. doi: 10.1113/jphysiol.2006.122739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Stecina K, Cabaj A, Pettersson L-G, Edgley SA. Neuronal relays in double crossed pathways between feline motor cortex and ipsilateral hindlimb motoneurones. J Physiol. 2006;575:527–541. doi: 10.1113/jphysiol.2006.112425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeneskog T, Padel Y. Cerebral cortical areas of origin of excitation and inhibition of rubrospinal cells in the cat. Exp Brain Res. 1983;50:309–320. doi: 10.1007/BF00239195. [DOI] [PubMed] [Google Scholar]
- King JS, Martin GF, Conner JB. A light and electron microscopic study of corticorubral projections in the opossum, Didelphis marsupialis virginiana. Brain Res. 1972;38:251–265. doi: 10.1016/0006-8993(72)90711-1. [DOI] [PubMed] [Google Scholar]
- Kostyuk PG, Vasilenko DA. Propriospinal neurones as a relay system for transmission of cortico-spinal influences. J Physiol (Paris) 1978;74:247–250. [PubMed] [Google Scholar]
- Lavoie S, Drew T. Discharge characteristics of neurons in the red nucleus during voluntary gait modifications: a comparison with the motor cortex. J Neurophysiol. 2002;88:1791–1814. doi: 10.1152/jn.2002.88.4.1791. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Control of spinal mechanisms from the brain. In: Tower DB, editor. The Basic Neurosciences. New York: Raven Press; 1975. pp. 253–265. [Google Scholar]
- Lundberg A. Integration in propiospinal motor centre controlling the forelimb in the cat. In: Asanuma H, Wilson VS, editors. Integration in the Nervous System. Tokyo, New York: Igaru-Shoin; 1979. pp. 47–65. [Google Scholar]
- Lundberg A. Descending control of forelimb movements in the cat. Brain Res Bull. 1999;50:323–324. doi: 10.1016/s0361-9230(99)00151-3. [DOI] [PubMed] [Google Scholar]
- Mabuchi M, Kusama T. The cortico-rubral projection in the cat. Brain Res. 1966;2:254–273. doi: 10.1016/0006-8993(66)90048-5. [DOI] [PubMed] [Google Scholar]
- Maier IC, Schwab ME. Sprouting, regeneration and circuit formation in the injured spinal cord: factors and activity. Philos Trans R Soc Lond B Biol Sci. 2006;361:1611–1634. doi: 10.1098/rstb.2006.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massion J. The mammalian red nucleus. Physiol Rev. 1967;47:383–436. doi: 10.1152/physrev.1967.47.3.383. [DOI] [PubMed] [Google Scholar]
- Massion J. Red nucleus: past and future. Behav Brain Res. 1988;28:1–8. doi: 10.1016/0166-4328(88)90071-x. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Kobayashi Y, Takakusaki K, Mori S, Kimura H. Termination mode and branching patterns of reticuloreticular and reticulospinal fibers of the nucleus reticularis pontis oralis in the cat: an anterograde PHA-L tracing study. Neurosci Res. 1993;17:9–21. doi: 10.1016/0168-0102(93)90024-k. [DOI] [PubMed] [Google Scholar]
- Mitani A, Ito K, Mitani Y, McCarley RW. Morphological and electrophysiological identification of gigantocellular tegmental field neurons with descending projections in the cat. I. Pons. J Comp Neurol. 1988a;268:527–545. doi: 10.1002/cne.902680405. [DOI] [PubMed] [Google Scholar]
- Mitani A, Ito K, Mitani Y, McCarley RW. Descending projections from the gigantocellular tegmental field in the cat: cells of origin and their brainstem and spinal cord trajectories. J Comp Neurol. 1988b;268:546–566. doi: 10.1002/cne.902680406. [DOI] [PubMed] [Google Scholar]
- Murakami F, Tsukahara N, Fujito Y. Properties of the synaptic transmission of the newly formed cortico-rubral synapses after lesion of the nucleus interpositus of the cerebellum. Exp Brain Res. 1977;30:245–258. doi: 10.1007/BF00237254. [DOI] [PubMed] [Google Scholar]
- Nathan PW, Smith MC. The rubrospinal and central tegmental tracts in man. Brain. 1982;105:223–269. doi: 10.1093/brain/105.2.223. [DOI] [PubMed] [Google Scholar]
- Padel Y, Smith AM, Armand J. Topography of projections from the motor cortex to rubrospinal units in the cat. Exp Brain Res. 1973;17:315–332. doi: 10.1007/BF00234669. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Pitts NG, Fukushima K. Reticulospinal connections with limb and axial motoneurons. Exp Brain Res. 1979;36:1–20. doi: 10.1007/BF00238464. [DOI] [PubMed] [Google Scholar]
- Rho MJ, Lavoie S, Drew T. Effects of red nucleus microstimulation on the locomotor pattern and timing in the intact cat: a comparison with the motor cortex. J Neurophysiol. 1999;81:2297–2315. doi: 10.1152/jn.1999.81.5.2297. [DOI] [PubMed] [Google Scholar]
- Rinvik E, Walberg F. Demonstration of a somatotopically arranged cortico-rubral projection in the cat. An experimental study with silver methods. J Comp Neurol. 1963;120:393–407. doi: 10.1002/cne.901200303. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Houk JC, Gibson AR. Limb specific connections of the cat magnocellular red nucleus. J Comp Neurol. 1987;257:553–577. doi: 10.1002/cne.902570406. [DOI] [PubMed] [Google Scholar]
- Ruigrok TJ, Pijpers A, Goedknegt-Sabel E, Coulon P. Multiple cerebellar zones are involved in the control of individual muscles: a retrograde transneuronal tracing study with rabies virus in the rat. Eur J Neurosci. 2008;28:181–200. doi: 10.1111/j.1460-9568.2008.06294.x. [DOI] [PubMed] [Google Scholar]
- Schepens B, Drew T. Descending signals from the pontomedullary reticular formation are bilateral, asymmetric, and gated during reaching movements in the cat. J Neurophysiol. 2006;96:2229–2252. doi: 10.1152/jn.00342.2006. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Brechtel K, Mueller CA, Failli V, Kaps HP, Tuli SK, Schluesener HJ. Experimental strategies to promote spinal cord regeneration – an integrative perspective. Prog Neurobiol. 2006;78:91–116. doi: 10.1016/j.pneurobio.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Shieh JY, Leong SK, Wong WC. Origin of the rubrospinal tract in neonatal, developing, and mature rats. J Comp Neurol. 1983;214:79–86. doi: 10.1002/cne.902140108. [DOI] [PubMed] [Google Scholar]
- Stecina K, Jankowska E. Uncrossed actions of feline corticospinal tract neurones on hindlimb motoneurones evoked via ipsilaterally descending pathways. J Physiol. 2007;580:119–132. doi: 10.1113/jphysiol.2006.122721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecina K, Jankowska E, Cabaj A, Pettersson L-G, Bannatyne BA JMD. Premotor interneurones contributing to actions of feline pyramidal tract neurones on ipsilateral hindlimb motoneurones. J Physiol. 2008;586:557–574. doi: 10.1113/jphysiol.2007.145466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakusaki K, Kohyama J, Matsuyama K. Medullary reticulospinal tract mediating a generalized motor inhibition in cats. III. Functional organization of spinal interneurons in the lower lumbar segments. Neuroscience. 2003;121:731–746. doi: 10.1016/s0306-4522(03)00542-6. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Ohta Y, Mori S. Single medullary reticulospinal neurons exert postsynaptic inhibitory effects via inhibitory interneurons upon alpha-motoneurons innervating cat hindlimb muscles. Exp Brain Res. 1989;74:11–23. doi: 10.1007/BF00248276. [DOI] [PubMed] [Google Scholar]
- Tsukahara N, Fujito Y, Kubota M. Specificity of the newly-formed corticorubral synapses in the kitten red nucleus. Exp Brain Res. 1983;51:45–56. doi: 10.1007/BF00236801. [DOI] [PubMed] [Google Scholar]
- Tsukahara N, Fuller DR, Brooks VB. Collateral pyramidal influences on the corticorubrospinal system. J Neurophysiol. 1968;31:467–484. doi: 10.1152/jn.1968.31.3.467. [DOI] [PubMed] [Google Scholar]
- Tsukahara N, Hultborn H, Murakami F, Fujito Y. Electrophysiological study of formation of new synapses and collateral sprouting in red nucleus neurons after partial denervation. J Neurophysiol. 1975;38:1359–1372. doi: 10.1152/jn.1975.38.6.1359. [DOI] [PubMed] [Google Scholar]
- Tsukahara N, Kosaka K. The mode of cerebral activation of red nucleus neurons. Experientia. 1966;22:193–194. doi: 10.1007/BF01897733. [DOI] [PubMed] [Google Scholar]
- Tsukahara N, Kosaka K. The mode of cerebral excitation of red nucleus neurons. Exp Brain Res. 1968;5:102–117. doi: 10.1007/BF00238700. [DOI] [PubMed] [Google Scholar]
- Warren S, Waitzman DM, May PJ. Anatomical evidence for interconnections between the central mesencephalic reticular formation and cervical spinal cord in the cat and macaque. Anat Rec (Hoboken) 2008;291:141–160. doi: 10.1002/ar.20644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui Y, Yokota S, Ono K, Tsumori T. Projections from the red nucleus to the parvicellular reticular formation and the cervical spinal cord in the rat, with special reference to innervation by branching axons. Brain Res. 2001;923:187–192. doi: 10.1016/s0006-8993(01)03196-1. [DOI] [PubMed] [Google Scholar]