Abstract
We tested the hypothesis that a greater activation of fast-twitch (FT) fibres during dynamic exercise leads to a higher muscle oxygen uptake (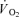 ) and energy turnover as well as a slower muscle
) and energy turnover as well as a slower muscle 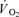 on-kinetics. Subjects performed one-legged knee-extensor exercise for 10 min at an intensity of 30 W without (CON) and with (CUR) arterial injections of the non-depolarizing neuromuscular blocking agent cisatracurium. In CUR, creatine phosphate (CP) was unaltered in slow twitch (ST) fibres and decreased (P < 0.05) by 28% in FT fibres, whereas in CON, CP decreased (P < 0.05) by 33% and 23% in ST and FT fibres, respectively. From 127 s of exercise, muscle
on-kinetics. Subjects performed one-legged knee-extensor exercise for 10 min at an intensity of 30 W without (CON) and with (CUR) arterial injections of the non-depolarizing neuromuscular blocking agent cisatracurium. In CUR, creatine phosphate (CP) was unaltered in slow twitch (ST) fibres and decreased (P < 0.05) by 28% in FT fibres, whereas in CON, CP decreased (P < 0.05) by 33% and 23% in ST and FT fibres, respectively. From 127 s of exercise, muscle 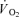 was higher (P < 0.05) in CUR compared to CON (425 ± 25 (±s.e.m.) versus 332 ± 30 ml min−1) and remained higher (P < 0.05) throughout exercise. Using monoexponential fitting, the time constant of the exercise-induced muscle
was higher (P < 0.05) in CUR compared to CON (425 ± 25 (±s.e.m.) versus 332 ± 30 ml min−1) and remained higher (P < 0.05) throughout exercise. Using monoexponential fitting, the time constant of the exercise-induced muscle 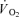 response was slower (P < 0.05) in CUR than in CON (55 ± 6 versus 33 ± 5 s). During CUR and CON, muscle homogenate CP was lowered (P < 0.05) by 32 and 35%, respectively, and also muscle lactate production was similar in CUR and CON (37.8 ± 4.1 versus 35.2 ± 6.2 mmol). Estimated total muscle ATP turnover was 19% higher (P < 0.05) in CUR than in CON (1196 ± 90 versus 1011 ± 59 mmol) and true mechanical efficiency was lower (P < 0.05) in CUR than in CON (26.2 ± 2.0 versus 30.9 ± 1.5%). In conclusion, the present findings provide evidence that FT fibres are less efficient than ST fibres in vivo at a contraction frequency of 1 Hz, and that the muscle
response was slower (P < 0.05) in CUR than in CON (55 ± 6 versus 33 ± 5 s). During CUR and CON, muscle homogenate CP was lowered (P < 0.05) by 32 and 35%, respectively, and also muscle lactate production was similar in CUR and CON (37.8 ± 4.1 versus 35.2 ± 6.2 mmol). Estimated total muscle ATP turnover was 19% higher (P < 0.05) in CUR than in CON (1196 ± 90 versus 1011 ± 59 mmol) and true mechanical efficiency was lower (P < 0.05) in CUR than in CON (26.2 ± 2.0 versus 30.9 ± 1.5%). In conclusion, the present findings provide evidence that FT fibres are less efficient than ST fibres in vivo at a contraction frequency of 1 Hz, and that the muscle 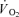 kinetics is slowed by FT fibre activation.
kinetics is slowed by FT fibre activation.
Several in vitro studies have provided evidence that fast-twitch (FT) muscle fibres are less economical than slow-twitch (ST) muscle fibres (Crow & Kushmerick, 1982; Barclay, 1996; Jackman & Willis, 1996), at least at low-to-moderate contraction velocities (di Prampero et al. 1988). There is also some experimental support of fibre type specific differences in oxygen uptake (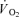 ) and mechanical efficiency during dynamic exercise in humans (Coyle et al. 1992; Barstow et al. 1996; Krustrup et al. 2004a,b). Firstly, cross-sectional studies have demonstrated a negative correlation between the fraction of FT fibres and efficiency (Coyle et al. 1992). Secondly, it has been observed that muscle
) and mechanical efficiency during dynamic exercise in humans (Coyle et al. 1992; Barstow et al. 1996; Krustrup et al. 2004a,b). Firstly, cross-sectional studies have demonstrated a negative correlation between the fraction of FT fibres and efficiency (Coyle et al. 1992). Secondly, it has been observed that muscle 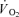 and energy turnover increase disproportionally at high exercise intensities (Bangsbo et al. 1993; Zoladz et al. 1995; Krustrup et al. 2003). An association between FT fibre recruitment and a progressive increase in
and energy turnover increase disproportionally at high exercise intensities (Bangsbo et al. 1993; Zoladz et al. 1995; Krustrup et al. 2003). An association between FT fibre recruitment and a progressive increase in 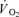 (slow component) during intense constant load exercise has also been observed in some (Russell et al. 2002; Burnley et al. 2002; Krustrup et al. 2004a,b) but not all studies (Viitasalo et al. 1985) using EMG or single fibre CP content measurement to determine fibre type recruitment. Krustrup et al. (2004b) used glycogen depletion of ST fibres by long-term cycle exercise the day prior to the experiment, and demonstrated an elevated FT fibre recruitment and a 7% increase in pulmonary
(slow component) during intense constant load exercise has also been observed in some (Russell et al. 2002; Burnley et al. 2002; Krustrup et al. 2004a,b) but not all studies (Viitasalo et al. 1985) using EMG or single fibre CP content measurement to determine fibre type recruitment. Krustrup et al. (2004b) used glycogen depletion of ST fibres by long-term cycle exercise the day prior to the experiment, and demonstrated an elevated FT fibre recruitment and a 7% increase in pulmonary 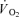 during low-intensity submaximal bicycle exercise. However, there is still a lack of direct evidence that muscle energy turnover differs between fibre types during dynamic exercise. It has also been speculated that FT fibres have a slower rise in
during low-intensity submaximal bicycle exercise. However, there is still a lack of direct evidence that muscle energy turnover differs between fibre types during dynamic exercise. It has also been speculated that FT fibres have a slower rise in 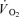 than ST fibres, and that the larger recruitment of FT fibres at high intensity work is the cause of the slowed
than ST fibres, and that the larger recruitment of FT fibres at high intensity work is the cause of the slowed 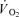 kinetics compared to low intensity exercise (Barstow et al. 1996; Krustrup et al. 2004c). However, it remains unclear whether the oxygen kinetics of FT fibres is slower than for ST fibres.
kinetics compared to low intensity exercise (Barstow et al. 1996; Krustrup et al. 2004c). However, it remains unclear whether the oxygen kinetics of FT fibres is slower than for ST fibres.
One approach to studying these phenomena is to use a non-depolarizing neuromuscular blockading agent (traditionally tubocurine) which affects preferentially ST fibres as observed in both rodents (Paton & Zaimis, 1951) and humans (Bonde-Petersen et al. 1973). Bonde-Petersen et al. (1973) studied three male subjects and found that the glycogen depletion determined by PAS staining was more pronounced in FT fibres during long-term moderate intensity cycle exercise with administration of tubocurarine. A more detailed evaluation of fibre type recruitment during exercise is to use metabolic measures in single muscle fibres from biopsies obtained at rest and immediately after exercise (Söderlund & Hultman, 1992; Krustrup et al. 2004a,b, 2008). In the 1960s, Asmussen et al. (1965) gave tubocurine to three subjects performing submaximal cycling exercise and observed an 11% increase in pulmonary 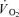 . More than two decades later Galbo et al. (1987) infused tubocurine during cycling and observed that during maximal exercise with curarization the pulmonary oxygen uptake was almost twice as high as during a control bout at the same absolute intensity. In that experiment tubocurine was provided by intravenous injections and led to considerable movements of the upper body, which was likely to contribute to the higher oxygen uptake, making a direct comparison difficult. In addition,
. More than two decades later Galbo et al. (1987) infused tubocurine during cycling and observed that during maximal exercise with curarization the pulmonary oxygen uptake was almost twice as high as during a control bout at the same absolute intensity. In that experiment tubocurine was provided by intravenous injections and led to considerable movements of the upper body, which was likely to contribute to the higher oxygen uptake, making a direct comparison difficult. In addition, 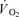 in the first phase of exercise was not measured. Cisatracurium is a substance that has a similar effect on the muscle as curare and tubocurine, but less influence on heart rate. Furthermore, to study the direct effect of neuromuscular blockade on muscle
in the first phase of exercise was not measured. Cisatracurium is a substance that has a similar effect on the muscle as curare and tubocurine, but less influence on heart rate. Furthermore, to study the direct effect of neuromuscular blockade on muscle 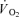 and energy turnover, small doses of cisatracurium can be infused in the artery feeding the contracting muscles thereby causing a minimal central disturbance.
and energy turnover, small doses of cisatracurium can be infused in the artery feeding the contracting muscles thereby causing a minimal central disturbance.
Thus, the purpose of the present study was to test the hypothesis that a change in fibre type recruitment, created by arterial infusion of the non-depolarizing neuromuscular blocking agent cisatracurium leads to a higher muscle 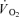 and energy turnover as well as a slower muscle
and energy turnover as well as a slower muscle 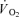 on-kinetics during moderate-intensity knee-extensor exercise. In addition, to evaluate muscle fibre type recruitment with and without non-depolarizing neuromuscular blockade, single muscle fibre metabolic measurements were performed in biopsies obtained at rest and immediately after exercise.
on-kinetics during moderate-intensity knee-extensor exercise. In addition, to evaluate muscle fibre type recruitment with and without non-depolarizing neuromuscular blockade, single muscle fibre metabolic measurements were performed in biopsies obtained at rest and immediately after exercise.
Methods
Subjects
Eight healthy male subjects at an age of 20–24 years, with a mean height of 183 (range: 169–185) cm, a mean body mass of 80.8 (59.9–89.2) kg, a fat percentage of 20.7 (11.0–24.7) and a maximal pulmonary 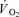 of 48.3 (41.4–54.7) ml min−1 kg−1 participated in the experiment. The subjects were untrained or recreationally active and none trained for competition. The subjects were fully informed of the risks and discomforts associated with the experimental procedures and all provided written consent. The study was carried out in accordance with the guidelines contained in the Declaration of Helsinki and was approved by the local Ethics Committee (KF 01-255671).
of 48.3 (41.4–54.7) ml min−1 kg−1 participated in the experiment. The subjects were untrained or recreationally active and none trained for competition. The subjects were fully informed of the risks and discomforts associated with the experimental procedures and all provided written consent. The study was carried out in accordance with the guidelines contained in the Declaration of Helsinki and was approved by the local Ethics Committee (KF 01-255671).
Exercise model
Subjects performed dynamic one-legged knee-extensor exercise in a supine position on an ergometer that permitted the exercise to be confined to the quadriceps muscle (Andersen et al. 1985). The subjects had several visits to the laboratory in order to become familiarized with the exercise protocol. After at least three familiarization sessions, the subjects performed an incremental exercise test in order to determine the maximal power of the knee extensors, which was 67 ± 4 (50–84) W.
Experimental procedures
Subject preparation
The subjects arrived at the laboratory at 08.00 h after consuming a standard breakfast including fruit juice and cereal. In the supine position a catheter was placed, under local anaesthesia, into the femoral artery of the left experimental leg with the tip positioned ∼2 cm proximal to the inguinal ligament. This catheter was used for collection of arterial blood samples and for injections of curare. A second catheter for collection of venous blood samples was placed in the femoral vein of the left leg with the tip positioned 2 cm distal to the inguinal ligament. A thermistor (Edslab, T.D. Probe, 94-030-2.5F, Baxter A/S, Allerod, Denmark) for measurement of blood temperature was advanced ∼8 cm beyond the tip of the catheter for the calculation of thigh blood flow. An area over one thigh was anaesthetized (1 ml of 20 mg ml−1 lidocaine) and prepared with two skin incisions for subsequent extraction of four needle biopsy samples from m. vastus lateralis (Bergström, 1962).
Experimental protocol
The exercise protocol included a 10 min warm up period with low-intensity exercise (15–20 W). After 30 min of rest, the subjects performed a 10-min bout of exercise at 30 W (CON) with a target kicking frequency of 60 kicks min−1. Subjects then rested for 45 min and performed a 10-min low-intensity warm-up including injections of the neuromuscular non-depolarizing agent cisatracurium (GlaxoPharma, Gladsaxe, Denmark). After another 30 min rest period, a 10-min bout of 30 W exercise at a target frequency of 60 kicks min−1 was carried out with injections of cisatracurium (CUR). A total of 0.2 mg L−1 thigh volume was infused during the warm-up as well as during the 30 W exercise. Twenty per cent of the dose was infused 4 min prior to the exercise, another 20% of the dose 2 min prior to the exercise and 10% after 1.5, 3, 4.5, 6, 7.5 and 9 min, respectively. The cisatracurium injections were performed during the warm-up period to ensure an optimal distribution of the neuromuscular blocking agent in the knee-extensors of the experimental leg and to fine-tune the dose used during CUR. The cisatracurium dose used during CUR was adjusted downwards for four of eight subjects (10–30%), to ensure that the subjects were able to perform exercise at the required work load. For one subject, the load was adjusted to 22 W after the initial warm-up. The average kicking frequency for CON and CUR was 61 and 60 kicks min−1, respectively, with a corresponding average power output of 29 and 28 W.
Blood was drawn from the femoral artery and vein at rest, during passive exercise and frequently during the initial phase of exercise, i.e. arterial samples were attained after −5, 0 and 5 and 10 s and venous samples were obtained after 6, 9, 12, 15 and 18 s. For the rapid initial sampling of venous blood a rag of stop-cocks was used (Bangsbo et al. 2000). Further arterial and venous samples were collected at ∼0.5, 1, 1.5, 2, 3, 6 and 10-min of exercise.
Blood flow was measured at rest, during passive exercise and from 15 to 30, 40–55, 120–135, 185–200, 370–385 and 565–580 s in CON and CUR. An occlusion cuff placed below the knee was inflated (240 mmHg) 30 s prior to exercise and remained inflated throughout exercise in order to avoid contribution of blood from the lower leg.
Muscle biopsies were obtained 5 min prior to CON and CUR as well as immediately after CON and CUR. The biopsies were taken from the medial portion of m. vastus lateralis at a depth of ∼3 cm. After exercise in CON and CUR, the leg was abruptly stopped and the muscle biopsy was obtained within 5 s of cessation of exercise and frozen in liquid nitrogen within another 5 s. This procedure was used to minimize CP changes related to recovery processes and freezing procedures (Teeter et al. 1969; Söderlund & Hultman, 1986). For 15 s immediately prior to the onset of each of the exercise bouts, the leg was passively moved in order to accelerate the ergometer flywheel and ensure a constant power from the onset of exercise.
Measurements and analyses
Thigh blood flow measurements
Thigh blood flow was measured by the constant infusion thermodilution technique (Andersen & Saltin, 1985) as modified by González-Alonso et al. 2000). Briefly, venous and infusate temperatures were measured continuously before and during ice-cold saline infusion (10–15 s) at a rate of 120 ml min−1 to achieve a drop in venous blood temperature of ∼0.6–2°C. Resting blood flow measurements were made with an infusion rate of ∼30 ml min−1 for 30–45 s. Venous temperature was measured with the thermistor positioned through the venous catheter. Infusate temperature (0–4°C) was measured at the site of entry to the catheter (Edslab flow-through thermister). Venous blood temperature and saline infusate temperatures were recorded at 400 Hz analog-to-digital sampling rate (Powerlab 16 s data acquisition system, Chart v4.13 software, ADInstruments, Sydney, Australia) onto the hard drive of a computer.
Blood analyses
Arterial and venous blood samples were immediately analysed for 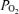 , O2 saturation and haemoglobin (ABL510, Radiometer, Copenhagen, Denmark) from which O2 content was calculated. For the determination of blood lactate and glucose (YSI 2300, Yellow Spring Instruments, Yellow Springs, OH, USA), 200 μl of whole blood was haemolysed within 10 s of sampling by adding to 200 μl of buffer (YSI; 0.5% Triton X-100) (Foxdal et al. 1992).
, O2 saturation and haemoglobin (ABL510, Radiometer, Copenhagen, Denmark) from which O2 content was calculated. For the determination of blood lactate and glucose (YSI 2300, Yellow Spring Instruments, Yellow Springs, OH, USA), 200 μl of whole blood was haemolysed within 10 s of sampling by adding to 200 μl of buffer (YSI; 0.5% Triton X-100) (Foxdal et al. 1992).
Single fibre analyses
After storage at −80°C, ∼40 mg w.w. muscle tissue was freeze dried and used for metabolic analyses in single muscle fibres. From each biopsy, 75 single muscle fibres were manually dissected under a low power microscope. Three pieces of each fibre fragment were cut off and attached to three separate glass plates in drops of distilled water. After evaporation of the water, a triple identification of fibre type (ST or FT) was performed by incubation for myofibrillar ATPase activity at pH 9.4, after preincubation at pH 10.3 (Essén et al. 1975). With alkaline preincubation the ST and FT fibres stain light and dark, respectively. The remainder of each fibre fragment was weighed on a quartz-fibre fish-pole balance (Lowry & Passonneau, 1972). The quartz-fibre was calibrated before and after the weighing procedure by a specthrophotometrical determination of the weight of p-nitrophenol crystals that had been weighed on the balance. From each biopsy, 13 (7–18) ST fibre fragments and 15 (6–21) FT fibre fragments weighing 1–4 μg were used for a luminometric determination of CP in single fibres with the firefly luciferase method (Wibom et al. 1991). Briefly, each fibre fragment was extracted in 200 μl trichloroacetic acid (2.5%) followed by neutralization in 20 μl 2.2 m KHCO3. A 50 μl aliquot of the extract was then added to a 925 μl sucrose buffer containing d-luceferin. The assay was then carried out by the use of a luminometer (1251, Bio Orbit Oy, Turku, Finland) fitted with a temperature-controlled, 25-position sample carousel and three automatic dispensing units that were connected to cuvettes containing 10 μm ATP standard, 50 μm ATP substrate and 10 μg ml−1 creatine kinase, respectively. In step 1 of the assay, sample (25 μl) was added to the ATP monitoring reagent, and the light emission corresponding to the sample ATP concentration was measured. In steps 2 and 3, ADP substrate (15 μl) and CK (10 μl) were added. The light emission was measured before the addition of CK and after the completion of the CK reaction. Finally, in step 4, ATP (10 μl) for internal standardization was added, and the subsequent increase in signal recorded.
Previous studies from our laboratory have shown that the coefficient of variance (CV) for duplicate determinations of CP is 5.7% when using the same fibre fragment and 7.0% when using two separate fibre fragments from the same fibre (Krustrup et al. 2004d). A total of 28 biopsies were used for single fibre analyses, i.e. seven biopsies taken prior to CON, after CON, before CUR and after CUR.
Muscle homogenate analyses
Another 20–25 mg w.w. muscle tissue from each biopsy was used for muscle homogenate metabolite analyses. The frozen samples for biochemical analyses were weighed before and after freeze-drying to determine water content. After freeze-drying, the muscle samples were dissected free of blood, fat and connective tissue and about 2 mg d.w. muscle tissue was extracted in a solution of ice-cold 3 m perchloric acid. The extract was kept in an ice bath for 30 min while being agitated with a vortex mixer and then centrifuged (10 000 g). The supernatant was neutralized to pH 7.0 with ice-cold 2 m KHCO3 after which it was centrifuged (10 000 g), pipetted and stored at −80°C until analysed for lactate and creatine phosphate (CP) by fluorometric assays (Ratio-2 filter fluorometer, Optical Technology Devices Inc., Valhalla, NY, USA) (Lowry & Passonneau, 1972). Thus, lactate was determined in a series of enzymatic reactions in a glycylglycine buffer (0.3 m, pH 9.9, containing 1.75 mm glutamic acid and 0.3 mm NAD+). After addition of the metabolite extract, the NADH was determined fluorometrically at 340 nm before and 120 min after addition of lactate dehydrogenase and glutamic-pyruvic transaminase to a final concentration of 500 and 2000 U l−1, respectively, allowing the transformation of lactate and NAD to pyruvate and NADH, and pyruvate and glutamate to alanine and oxalogluterate. The net formation of NADH was used to determine lactate concentration after standardizing with a lactate standard.
CP was determined in a series of enzymatic reactions in a Tris buffer (0.1 m, pH 8.1, containing 1 mm glucose, 1 mm MgCl2, 0.5 mm AMP, 0.12 mm ADP, 5 μm di-adenosine pentaphosphate, 60 μm of NADP, 140 U l−1 of glucose 6-phosphate (G6P) dehydrogenase, 140 U l−1 hexokinase) and a small amount of dithiothreitol. After addition of the metabolite extract time was allowed for a complete rundown of ATP by a reaction of ATP + glucose to ADP and G6P by hexokinase and G6P + NADP to 6-phosphoglucono-δ-lactone + NADPH by G6P-dehydrogenase. The NADPH was then determined fluorometrically at 340 nm before and 30 min after addition of creatine kinase allowing the formation of CrP and ADP to ATP and creatin and further to NADPH, which again was determined. The net formation of NADPH was used to determine the CP concentration after standardizing with a CP standard.
Another 2 mg d.w. muscle tissue was extracted in 1 m HCl and hydrolysed at 100°C for 3 h and the glycogen content was determined by the hexokinase method (Lowry & Passoneau, 1972).
Muscle fibre types
Approximately 30 mg w.w. of the resting muscle biopsy was mounted in an embedding medium (OCT Compound Tissue-Tek, Sakura Finetek, Zoeterwoude, the Netherlands), frozen in isopentane that was precooled to the freezing point and stored at −80°C, until analysed histochemically for fibre type distribution. Five serial 10 μm thick sections were cut at −20°C and incubated for myofibrillar ATPase reactions at pH 9.4 after preincubation at pH 4.3, 4.6 and 10.3 (Brooke & Kaiser, 1970). Based on the myofibrillar ATP staining, individual fibres were classified under light microscopy as ST, FTa or FTx. The distribution of ST, FTa and FTx fibres in m. vastus lateralis was 51.7 ± 4.8% (33.3–64.7), 30.9 ± 4.4% (17.6–48.9) and 17.5 ± 1.6% (9.7–24.1), respectively.
Thigh volume and quadriceps muscle mass
The thigh volume and mass of the quadriceps femoris muscle of the experimental leg were estimated by anthropometry with measurements of thigh length, circumference and skin fold thickness (Jones & Pearson, 1969) and corrected based on a comparison between MR-scan and anthropometric determinations (Krustrup et al. 2004d). The thigh volume of the experimental leg was 8.9 ± 0.4 (6.9–10.2) L and the quadriceps muscle mass was 2.44 ± 0.10 (1.96–2.75) kg.
Calculations
Thigh 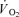 and lactate release were calculated by multiplying blood flow with arterial–venous O2 difference and venous–arterial lactate difference, respectively. A continuous blood flow curve was constructed for each subject by linear interpolation of the measured blood flow data points to obtain time-matched values of blood flow with the blood variables. Total
and lactate release were calculated by multiplying blood flow with arterial–venous O2 difference and venous–arterial lactate difference, respectively. A continuous blood flow curve was constructed for each subject by linear interpolation of the measured blood flow data points to obtain time-matched values of blood flow with the blood variables. Total 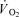 and thigh lactate release were calculated as the area under the uptake–release curve. Thigh
and thigh lactate release were calculated as the area under the uptake–release curve. Thigh 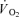 was converted to aerobic ATP production by a factor of 4.5 ml O2 per mmol ATP. This factor implies a mole volume of 22.4 l per mol of oxygen (O2) and a P/O ratio of 2.5 mmol ATP (mmol O)−1 (Hinkle & Yu, 1979). Thigh
was converted to aerobic ATP production by a factor of 4.5 ml O2 per mmol ATP. This factor implies a mole volume of 22.4 l per mol of oxygen (O2) and a P/O ratio of 2.5 mmol ATP (mmol O)−1 (Hinkle & Yu, 1979). Thigh 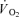 was converted into kJ by multiplying by 20.5 kJ l−1 O2 assuming an RQ of 0.9 during the exercise (Kiens et al. 1993). Muscle anaerobic ATP production was calculated as Δ muscle CP + 3/2 Δ muscle lactate + 3/2 lactate release. Anaerobic energy turnover was determined from the net change in reactant levels and average values of energy produced in each of the reactions determined in vitro. ΔH values of 55 and 67 kJ mol−1 of ATP produced were used for the creatine kinase reaction and glycolysis leading to lactate formation, respectively (Walsh & Woledge, 1970; Curtin & Woledge, 1978). Muscle ATP production (mmol ATP) and the energy turnover were determined as the sum of aerobic and anaerobic ATP production and energy turnover, respectively. Metabolic efficiency was calculated as the ratio between the total ATP production (mmol ATP) and the total work performed (J). Gross efficiency was calculated as the ratio between the external power and total energy turnover. Moreover, the ‘true’ mechanical efficiency was estimated as the total power divided by total energy turnover. The total power was estimated as the sum of external power output and the internal work that is related to the gravitational and inertial forces acting on the lower leg (17 W at 60 r.p.m.; Ferguson et al. 2000). The muscle
was converted into kJ by multiplying by 20.5 kJ l−1 O2 assuming an RQ of 0.9 during the exercise (Kiens et al. 1993). Muscle anaerobic ATP production was calculated as Δ muscle CP + 3/2 Δ muscle lactate + 3/2 lactate release. Anaerobic energy turnover was determined from the net change in reactant levels and average values of energy produced in each of the reactions determined in vitro. ΔH values of 55 and 67 kJ mol−1 of ATP produced were used for the creatine kinase reaction and glycolysis leading to lactate formation, respectively (Walsh & Woledge, 1970; Curtin & Woledge, 1978). Muscle ATP production (mmol ATP) and the energy turnover were determined as the sum of aerobic and anaerobic ATP production and energy turnover, respectively. Metabolic efficiency was calculated as the ratio between the total ATP production (mmol ATP) and the total work performed (J). Gross efficiency was calculated as the ratio between the external power and total energy turnover. Moreover, the ‘true’ mechanical efficiency was estimated as the total power divided by total energy turnover. The total power was estimated as the sum of external power output and the internal work that is related to the gravitational and inertial forces acting on the lower leg (17 W at 60 r.p.m.; Ferguson et al. 2000). The muscle 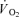 data were modelled from the onset of exercise as shown in eqn (1), using the computer software MathCad (PTC, Needham, MA, USA).
data were modelled from the onset of exercise as shown in eqn (1), using the computer software MathCad (PTC, Needham, MA, USA).
| (1) |
where: 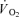 (t) represents the absolute
(t) represents the absolute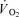 at a given time t;
at a given time t; 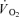 baseline represents the
baseline represents the 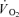 in the baseline period; Ap, Tdp, and τp represent the amplitude, time delay and time constant, respectively, describing the increase in
in the baseline period; Ap, Tdp, and τp represent the amplitude, time delay and time constant, respectively, describing the increase in 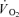 above baseline.
above baseline.
Statistics
Data were analysed using a two-factor (condition × time) repeated measure analysis of variance (ANOVA), with significance set at P < 0.05. Significant interactions and main effects were subsequently analysed using a Newman–Keuls post hoc test. Differences in the muscle homogenate metabolite changes, single fibre CP changes and 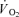 kinetics variables during CON and CUR were evaluated by Student's paired t test. The single ST and FT fibre data were averaged for each individual before statistical evaluation. Data are presented as means ± standard error of the mean (s.e.m.), unless otherwise stated.
kinetics variables during CON and CUR were evaluated by Student's paired t test. The single ST and FT fibre data were averaged for each individual before statistical evaluation. Data are presented as means ± standard error of the mean (s.e.m.), unless otherwise stated.
Results
CP in single fibres
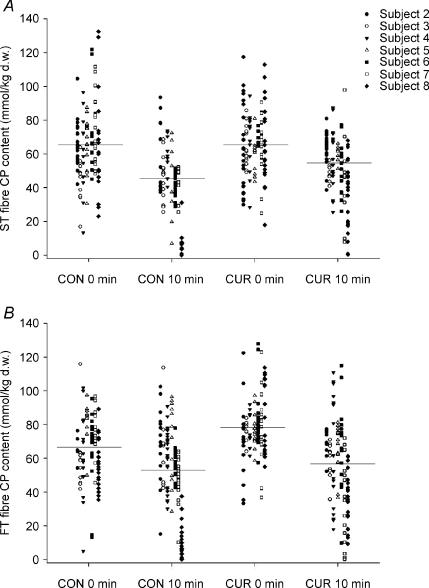
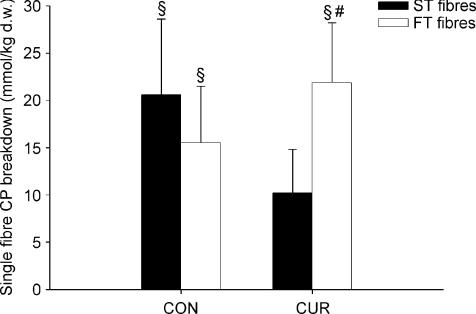
During exercise in CUR, CP was not significantly altered in ST fibres, whereas it decreased (P < 0.05) by 28% in FT fibres (Figs 1 and 2). In CON, CP decreased (P < 0.05) during exercise by 33% and 23% in ST and FT fibres, respectively, and CP was lower (P < 0.05) in ST than in FT fibres after exercise (43.0 ± 6.4 versus 52.0 ± 7.8 mmol (kg d.w.)−1) (Fig. 1).
Figure 1. Single fibre CP content in ST fibres (A) and FT fibres (B) before and immediately after 10-min of knee-extension exercise at 30 W with (CUR) and without (CON) arterial injections of a neuromuscular blocking agent.
Individual values (n = 7) are presented.
Figure 2. Average CP utilization in ST fibres (filled bars) and FT fibres (open bars) during 10-min of knee-extension exercise at 30 W with (CUR) and without (CON) arterial injections of a neuromuscular blocking agent.
Values are means ±s.e.m. (n = 7). #Significant different (P < 0.05) from ST fibres. §Significant (P < 0.05) CP utilization during exercise.
Thigh blood flow
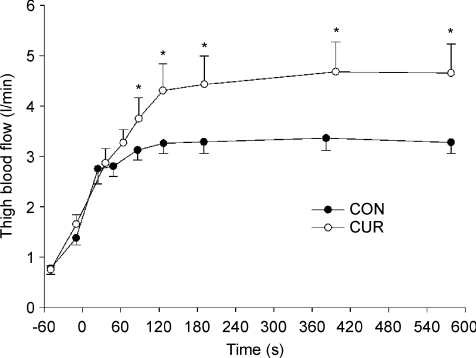
At rest, during passive exercise and during the first minute of exercise, thigh blood flow was not different between CUR and CON (Fig. 3). However, after 86 s of exercise, thigh blood flow was 20% higher (P < 0.05) in CUR than in CON (3.75 ± 0.41 versus 3.12 ± 0.20 l min−1) and it remained higher throughout the 10-min exercise bout, being 32% higher (P < 0.05) in CUR than in CON after 126 s of exercise and 42% higher (P < 0.05) at the end of exercise (4.65 ± 0.58 versus 3.27 ± 0.22 l min−1) (Fig. 3).
Figure 3. Thigh blood flow before and during 10-min of knee-extension exercise at 30 W with (CUR; ^) and without (CON; •) arterial injections of a neuromuscular blocking agent.
Values are means ±s.e.m. (n = 8). *CUR significantly P < 0.05 different from CON.
Thigh oxygen uptake
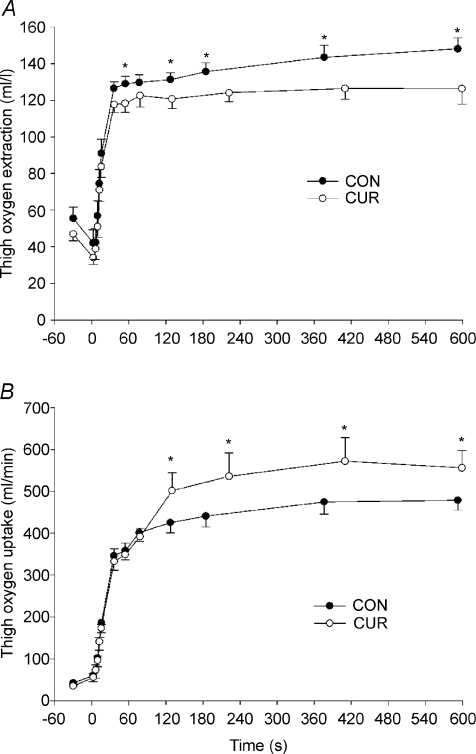
At rest, during passive exercise and during the first 15 s of exercise, thigh oxygen extraction (a-v O2diff) was not different between CUR and CON (Fig. 4A). After 36 s, a-v O2diff was 7% lower (P < 0.05) in CUR than in CON (118 ± 4 versus 127 ± 4 ml l−1) and it remained lower (P < 0.05) throughout the exercise, being 15% lower at the end of exercise (126 ± 8 versus 148 ± 6 ml l−1) (Fig. 4A).
Figure 4. Thigh oxygen extraction (A) and oxygen uptake (B) before and during 10-min of knee-extension exercise at 30 W with (CUR; ^) and without (CON; •) arterial injections of a neuromuscular blocking agent.
Values are means ±s.e.m. (n = 8). *CUR significantly P < 0.05 different from CON.
At rest, during passive exercise and during the first 76 s of exercise, thigh oxygen uptake (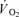 ) was not different between CUR and CON (76 s: 346 ± 35 and 332 ± 30 ml min−1; Fig. 4B). After 127 s of exercise, thigh
) was not different between CUR and CON (76 s: 346 ± 35 and 332 ± 30 ml min−1; Fig. 4B). After 127 s of exercise, thigh 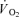 was 18% higher (P < 0.05) in CUR compared to CON (425 ± 25 versus 332 ± 30 ml min−1) and remained higher (P < 0.05) throughout exercise, being 16% higher (P < 0.05) at the end of exercise (556 ± 42 versus 479 ± 23 ml min−1) (Fig. 4B). The total
was 18% higher (P < 0.05) in CUR compared to CON (425 ± 25 versus 332 ± 30 ml min−1) and remained higher (P < 0.05) throughout exercise, being 16% higher (P < 0.05) at the end of exercise (556 ± 42 versus 479 ± 23 ml min−1) (Fig. 4B). The total 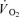 was 20 ± 9% higher (P < 0.05) in CUR than in CON (5.05 ± 0.42 versus 4.23 ± 0.26 l). From 2 to 10-min of exercise,
was 20 ± 9% higher (P < 0.05) in CUR than in CON (5.05 ± 0.42 versus 4.23 ± 0.26 l). From 2 to 10-min of exercise, 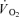 was 23 ± 10% higher (P < 0.05) in CUR than in CON (4.38 ± 0.39 versus 3.58 ± 0.22 l).
was 23 ± 10% higher (P < 0.05) in CUR than in CON (4.38 ± 0.39 versus 3.58 ± 0.22 l).
Thigh oxygen uptake kinetics
The baseline of thigh 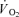 was not different between CUR and CON (38 ± 5 versus 43 ± 8 ml min−1; NS). The time constant (τp) of the thigh
was not different between CUR and CON (38 ± 5 versus 43 ± 8 ml min−1; NS). The time constant (τp) of the thigh 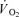 on-kinetics was 55 ± 6 s in CUR, which was longer (P < 0.05) than in CON (33 ± 5 s). The time delay of the fundamental component was slightly shorter (P < 0.05) in CUR than in CON (1 ± 1 versus 4 ± 1 s). The amplitude of the fundamental thigh
on-kinetics was 55 ± 6 s in CUR, which was longer (P < 0.05) than in CON (33 ± 5 s). The time delay of the fundamental component was slightly shorter (P < 0.05) in CUR than in CON (1 ± 1 versus 4 ± 1 s). The amplitude of the fundamental thigh 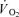 response was 519 ± 50 ml min−1 during CUR, which tended (P = 0.07) to be higher than in CON (418 ± 28 ml min−1).
response was 519 ± 50 ml min−1 during CUR, which tended (P = 0.07) to be higher than in CON (418 ± 28 ml min−1).
Muscle CP utilization
Muscle homogenate metabolite concentrations before and after CUR and CON are presented in Table 1. Muscle creatine phosphate (CP) was ∼96 and 86 mmol (kg d.w.)−1 prior to CUR and CON, respectively, and decreased (P < 0.05) by 32 and 35% during exercise in CUR and CON, respectively. Thus, the net CP utilization during exercise was similar in CUR and CON (30.4 ± 7.1 and 30.0 ± 8.4 mmol (kg d.w.)−1, respectively).
Table 1.
Muscle CP, lactate and glycogen before and immediately after 10-min of knee-extensor exercise with (CUR) and without (CON) femoral arterial injections of a neuromuscular blocking agent
| CON |
CUR |
|||
|---|---|---|---|---|
| 0 min | 10-min | 0 min | 10-min | |
| Muscle CP (mmol (kg d.w.)−1) | 86.0 ± 3.1 | 56.0 ± 10.1* | 95.8 ± 3.0 | 65.5 ± 7.1* |
| Muscle lactate (mmol (kg d.w.)−1) | 5.2 ± 1.3 | 21.4 ± 8.9* | 4.4 ± I.0 | 19.7 ± 5.5* |
| Muscle glycogen (mmol (kg d.w.)−1) | 432 ± 33 | 384 ± 40 | 386 ± 46 | 332 ± 49 |
Values are means ±s.e.m. (n = 8). *Significantly different (P < 0.05) from pre-exercise values.
Thigh lactate production
Muscle lactate was ∼5 and 4 mmol (kg d.w.)−1 prior to CUR and CON, respectively, and it increased (P < 0.05) about 4-fold during exercise (Table 1). No significant difference was observed in net muscle lactate accumulation between CUR and CON (15.2 ± 4.9 and 16.2 ± 8.1 mmol (kg d.w.)−1, respectively).
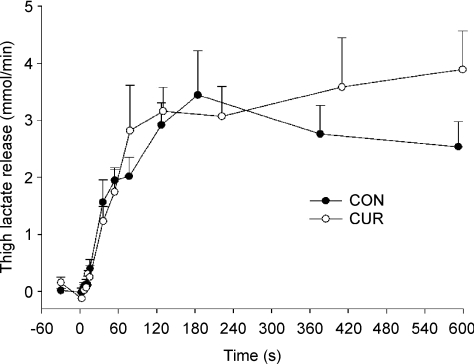
No net lactate release from the thigh was measured before and during the first 15 s of exercise, after which it increased (P < 0.05) to 1.2 ± 0.3, 3.1 ± 0.5 and 3.9 ± 0.7 mmol min−1 after 36 s, 3 min and 10-min of exercise in CUR, respectively, which was not different from CON (1.6 ± 0.4, 2.9 ± 0.4 and 2.5 ± 0.4 mmol min−1, respectively; Fig. 5). The total net lactate release was not different between CUR and CON during the 10-min exercise bout (29.1 ± 3.1 and 26.2 ± 2.7 mmol, respectively) or from 2 to 10-min (25.1 ± 2.7 and 22.8 ± 2.6 mmol, respectively). Net thigh lactate production was 37.8 ± 4.1 and 35.2 ± 6.2 mmol in CUR and CON, respectively (P > 0.05).
Figure 5. Thigh lactate release before and during 10-min of knee-extension exercise at 30 W with (CUR; ^) and without (CON; •) arterial injections of a neuromuscular blocking agent.
Values are means ±s.e.m. (n = 8).
Muscle glycogen utilization
No difference in net utilization of muscle glycogen was observed between CUR and CON (54 ± 16 and 47 ± 24 mmol (kg d.w.)−1, respectively) (Table 1).
Total muscle energy turnover
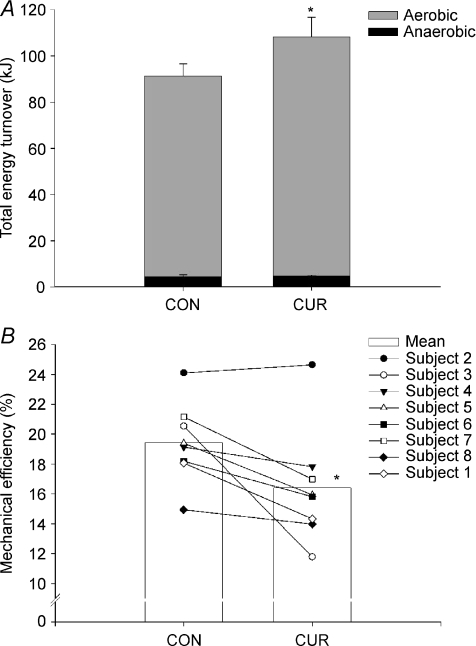
The estimated aerobic ATP turnover for the quadriceps muscle during exercise was 1121 ± 93 mmol in CUR, which was 20% higher (P < 0.05) than in CON (940 ± 58 mmol). The total anaerobic ATP production, based on the sum of net CP breakdown and lactate production, was not significantly different between CUR and CON (74 ± 4 versus 70 ± 12 mmol). Thus, the total estimated ATP turnover (sum of aerobic and anaerobic ATP turnover) was 1196 ± 90 mmol in CUR, which was 19% higher (P < 0.05) than in CON (1011 ± 59 mmol). Similarly, the total energy turnover of 108.2 ± 8.4 kJ in CUR was 19% higher (P < 0.05) than in CON (91.2 ± 5.4 kJ) (Fig. 6A). In CON and CUR, aerobic ATP turnover accounted for 95 ± 1% (90–97) and 95 ± 1% (92–98) of total ATP turnover, respectively (Fig. 6A).
Figure 6. Total energy turnover (A) and mechanical efficiency (B) during 10-min of knee-extension exercise at 30 W with (CUR) and without (CON) arterial injections of a neuromuscular blocking agent.
Individual values (n = 8) as well as means ±s.e.m. are presented. *CUR significantly different (P < 0.05) from CON.
Mechanical efficiency
Gross efficiency was 16.4 ± 1.4% in CUR, which was lower (P < 0.05) than in CON (19.4 ± 0.9%) (Fig. 6B). The true mechanical efficiency, including internal work, was 26.2 ± 2.0% in CUR, which was lower (P < 0.05) than in CON (30.9 ± 1.5%). The total power output per ATP production was 23.7 ± 1.8 J (mmol ATP)−1 in CUR, with values being 21 ± 8% higher (P < 0.05) in CON (27.9 ± 1.3 J (mmol ATP)−1). The mechanical efficiency in CUR (r =−0.50, P < 0.05) and CON (r =−0.45, P < 0.05) correlated with the relative fraction of FTx fibres, whereas mechanical efficiency was not correlated with the relative fraction of ST fibres.
Discussion
The major finding of the present was that muscle oxygen uptake and energy turnover were elevated and that the muscle oxygen uptake kinetics was slower during submaximal continuous exercise, with partial neuromuscular blockade. The determination of metabolites in single fibres furthermore revealed that a low intra-arterial dose of cisatracurium reduced the recruitment of ST fibres. Thus, the present findings support the hypothesis that the muscle energy turnover for a given work rate is higher for FT fibres than for ST fibres and that FT fibres have slower oxygen uptake kinetics.
The muscle oxygen uptake was about 20% higher with partial neuromuscular blockade than in the control situation. This difference is more pronounced than the observed increase in pulmonary oxygen uptake using curarization (11%, Asmussen et al. 1965) or prior ST-fibre glycogen depletion (7%, Krustrup et al. 2004a) to change fibre type recruitment. On the other hand, the 20% difference was smaller than observed by Galbo et al. (1987), where a two-fold increase was observed in pulmonary oxygen uptake during maximal cycling in the tubocurarine trial compared to exercise at the same intensity in a control condition. In that experiment, however, tubocurine was provided by intravenous injections and led to considerable movements of the upper body, which are likely to have contributed to the higher oxygen uptake, making a direct comparison difficult. Changes in variables related to anaerobic energy production were the same in CUR and CON. Thus, net muscle CP utilization and lactate accumulation as well as lactate release from the muscle were equal in CON and CUR. A similar muscle glycogen breakdown during CUR and CON supports that the rate of glycolysis was not different in the two situations. With anaerobic energy turnover being the same in the two situations the total energy turnover was 19% higher in CUR than CON. Correspondingly the gross efficiency was 16.4% in CUR and 19.4% in the control trial, which is similar to a value of 19.1% found in a comparable knee-extensor exercise study using a protocol with 10-min of exercise at 37 W (Ferguson et al. 2001). In CUR, mechanical efficiency was lower than 16% for 5 of 8 subjects (Fig. 6), which is among the lowest reported values during knee-extensor exercise.
The difference in energy production between CON and CUR was about 20%, which is smaller than the observed difference in energy turnover between ST and FT fibres in in vitro studies (Crow & Kushmerick, 1982; Jackman & Willis, 1996). However, it represents a minimum difference since the single fibre analysis of CP revealed that a substantial number of FT fibres contributed to the work in the control situation, which is in accordance with recent observations from our laboratory using moderate intensity knee-extensor exercise (Krustrup et al. 2008). The individual data presented in Fig. 1 show that the FT fibre CP content decreased by > 15 mmol (kg d.w.)−1 for subjects 3, 7 and 8 during the control trial. It should also be emphasized that the FT fibre CP content may have decreased even more in the rectus femoris muscle than in the biopsied muscle portion, as the m. vastus lateralis activity during moderate intensity knee-extensor exercise is somewhat lower than the rectus femoris involvement and slightly lower than the average response of the entire m. quadriceps femoris (Krustrup et al. 2008). In addition, the single fibre analyses revealed that some subjects had a partial recruitment of their ST fibre pool despite the neuromuscular blockade, as can be seen from the 20 and 30 mmol (kg d.w.)−1 decrease in ST fibre CP content in the curare trial for subjects 7 and 8. However, the observed decrease in ST fibre CP content during the curare trial was lower than during the control, also for subjects 7 and 8 (10 and 30 mmol (kg d.w.)−1, respectively). Together, it is clear from the present results that less ST and more FT fibres were recruited with cisatracurium administration compared to control and also that the blockade in humans may not be as specific as observed in animals (Paton & Zaimis, 1951). The difference in energy turnover between ST and FT fibres in the present study compared to in vitro studies may also be related to the way of activating the fibres. In in vitro studies electrical stimulation is used to promote rhythmic or sustained static contractions, which is different from the dynamic exercise with a contraction cycle of 1 Hz used in the present study. Another important aspect is that in vitro studies have shown a marked variation in the difference in energy turnover between ST and FT fibres depending on the stimulation rate and contraction frequency. For example, Di Prampero et al. (1988) observed that the energy turnover was only larger in FT fibres than in ST fibres at contraction velocities below 2 fibre lengths per second. The contraction velocity used in the present study was 135 deg s−1 or ∼20% of the maximal kicking velocity (Aagaard et al. 1994), which has been suggested to be within the optimal range of ST fibres and suboptimal for FT fibres (di Prampero et al. 1988; Barclay, 1996).
In vitro studies have demonstrated that the rate of glycolysis and lactate accumulation are considerably greater (100%) in FT fibres compared to ST fibres when stimulated at a given frequency (Sawka et al. 1981). In pooled ST and FT fibres from muscle biopsies obtained from humans after intense dynamic exercise the difference was less (40%; Tesch et al. 1978; Greenhaff et al. 1994) and no fibre type specific differences were observed in lactate accumulation during submaximal cycle exercise (Jacobs & Kaiser, 1982). During intense exercise in humans, FT fibres have also been shown to have a faster breakdown of CP than ST fibres (Söderlund & Hultman, 1992; Greenhaff et al. 1994; Casey et al. 1996). Apparently the FT fibres have a greater capacity to produce anaerobic energy and a higher rate of anaerobic energy turnover during the first part of intense exercise compared to ST fibres. However, the present findings suggest that during submaximal activation the anaerobic energy production in humans is not higher in FT fibres.
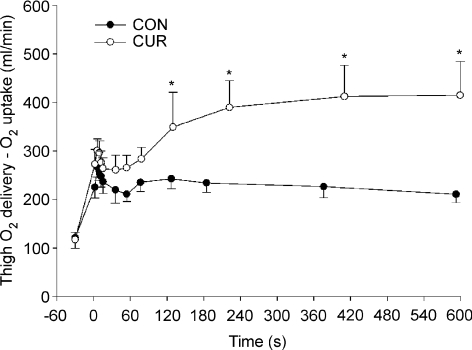
The hypothesis that the oxygen uptake kinetics would be slower after partial neuromuscular blockade was confirmed since the mean response time was significant longer in CUR than in CON, supporting the suggestion that FT fibres have slower oxygen kinetics than ST fibres during dynamic exercise in humans (Barstow et al. 1996). In the study by Barstow et al. (1996) it was observed that subjects with predominantly ST fibres had a much faster oxygen uptake kinetics than subjects with few ST fibres. However, in that study the recruitment of fibres during exercise was unclear and it was not possible to provide evidence of a causal relationship between FT fibre activation and slow oxygen uptake kinetics. The present finding of a longer time constant of the oxygen uptake response when more FT fibres were recruited can explain why Krustrup et al. (2004c) found that muscle oxygen kinetics was faster at a low intensity compared to a high submaximal intensity where there probably was a significantly greater recruitment of FT fibres. In that study it was also observed that the difference between the low and high intensity exercise disappeared after an 8-week training period, which led to a 20% increase in the number of capillaries surrounding FT fibres and a marked increase in the muscle activity of CS and HAD (Krustrup et al. 2004c; Jensen et al. 2004). It may be that the FT fibres by high intensity training improve their ability to extract oxygen in the initial phase of exercise and the results of the present study may have been different if the subjects had been more trained. In support of this suggestion is the observation that the two most trained subjects had a minimal difference in oxygen uptake on-kinetics between CUR and CON. The finding that the oxygen kinetics was associated with the recruitment of muscle fibres suggests that the oxygen utilization in the initial phase of exercise is not limited by oxygen delivery. This notion is supported by the finding that both in CON and CUR the oxygen provided but not used was > 260 ml min−1 in the first phase of exercise and that it was greater in CUR after 127 s of exercise (Fig. 7). These observations are in agreement with findings in animal studies (Grassi et al. 1998) and other human studies (Bangsbo et al. 2000), but not with studies using intermittent static handgrip exercise and knee-extensor exercise under conditions with restricted oxygen delivery (Hughson et al. 1996; MacDonald et al. 1998), where it has been proposed that oxygen availability was a limiting factor of the muscle respiration during exercise.
Figure 7. Difference between thigh oxygen delivery (thigh blood flow × arterial O2 content) and thigh oxygen uptake (thigh blood flow × oxygen extraction) before and during 10-min of knee-extension exercise at 30 W with (CUR; ^) and without (CON;•) arterial injections of a neuromuscular blocking agent.
Values are means ±s.e.m. (n = 8). *CUR significantly P < 0.05 different from CON.
An interesting finding of the present study was that leg blood flow was higher in CUR than in CON. It may be related to the greater oxygen demand, since a number of studies have shown a close relationship between oxygen need and blood flow when the oxygen content of the arterial blood has been manipulated (González-Alonso et al. 2001). However it is probably not the only explanation, since the a-v O2 difference was lower in CUR than in CON after 26 s of exercise. It is unclear whether the higher blood flow is related directly to a greater activation of the FT fibres. The observation in a number of studies that there is a linear increase in blood flow from low-intensity to high-intensity exercise (e.g. Andersen & Saltin, 1985) speaks against that theory, since it has been established that low intensity exercise is performed with minimal FT fibre activity and that the relative FT fibre recruitment increases progressively with increased work load in the high-intensity exercise domain (Völlestad & Blom, 1985; Krustrup et al. 2004b). In addition, blood flow for a given oxygen uptake has actually been observed to be higher at rest and during contraction in a muscle comprising predominantly ST fibres (soleus) compared to a muscle with primarily FT fibres (white gastrocnemius) in anaesthetized rats (McDonough et al. 2005; Ferreira et al. 2006), which does not support the possibility that the higher blood flow in CON was caused by the enhanced recruitment of fast twitch fibres. However, the latter study may not be relevant for dynamic contractions in human muscles with a heterogeneous distribution of fibres of different types, with a different activation pattern and an active sympathetic system, which appears to affect more the fast twitch muscle (Laughlin & Armstrong, 1987). An alternative hypothesis for the increase in blood flow, not related to the greater energy demand, is that an excessive release of acethylcholine from the motor nerves during cisatracurium infusion is causing a greater vasodilatation, as acethylcholine has been shown to relax the smooth muscle cells. However, this has only been observed in vitro and the effect of acethylcholine in humans is unknown (Segal & Kurjiaka, 1995). Further research is needed to explore the possible causes of the elevated muscle blood flow during cisatracurium inhibition.
In summary, the present study has demonstrated that the muscle oxygen uptake and energy turnover are elevated when neuromuscular blockade is applied to block ST fibres, suggesting that FT fibres are less effective than ST fibres at a contraction frequency of 1 Hz. Furthermore the muscle oxygen uptake on-kinetics appears to be slower when more FT fibres are activated. On the other hand, the anaerobic energy production during submaximal exercise was not elevated with a greater involvement of FT fibres.
Acknowledgments
We thank the subjects for their committed participation. The excellent technical assistance of Winnie Taagerup, Ingelize Kring and late Merete Vannby is greatly appreciated. We also thank Dr Ansgar Sørensen for assistance with matemathical modelling of the 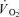 curves. The study was supported by the Danish National Research Foundation (504-14).
curves. The study was supported by the Danish National Research Foundation (504-14).
References
- Aagaard P, Trolle M, Simonsen EB, Klausen K, Bangsbo J. Moment and power generation during maximal knee extension performed at low and high speed. Eur J Appl Physiol. 1994;69:376–381. doi: 10.1007/BF00865398. [DOI] [PubMed] [Google Scholar]
- Andersen P, Adams RP, Sjøgaard G, Thorboe A, Saltin B. Dynamic knee extension as a model for the study of an isolated exercising muscle in man. J Appl Physiol. 1985;59:1647–1653. doi: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmussen E, Johansen SH, Jørgensen M, Nielsen M. On the nervous factors controlling respiration and circulation during exercise: experiments with curarization. Acta Physiol Scand. 1965;63:343–350. doi: 10.1111/j.1748-1716.1965.tb04073.x. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Krustrup P, González-Alonso J, Boushel R, Saltin B. Muscle oxygen uptake kinetics at onset of intense dynamic exercise. Am J Physiol Regul Integr Comp Physiol. 2000;279:R899–R906. doi: 10.1152/ajpregu.2000.279.3.R899. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Michalsik L, Petersen A. Accumulated O2 deficit during intense exercise and muscle characteristics of elite athletes. Int J Sports Med. 1993;14:207–213. doi: 10.1055/s-2007-1021165. [DOI] [PubMed] [Google Scholar]
- Barclay CJ. Mechanical efficiency and fatigue of fast and slow muscle of the mouse. J Physiol. 1996;497:781–794. doi: 10.1113/jphysiol.1996.sp021809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barstow TJ, Jones AM, Nguyen PH, Casabury R. Influence of muscle fiber type and pedal frequency on oxygen uptake kinetics of heavy exercise. J Appl Physiol. 1996;81:1624–1650. doi: 10.1152/jappl.1996.81.4.1642. [DOI] [PubMed] [Google Scholar]
- Bergström J. Muscle electrolytes in man. Scand J Clin Lab Invest. 1962;68(Suppl):1–101. [Google Scholar]
- Bonde-Petersen F, Gollnick PD, Hansen TJ, Hulten N, Kristensen JH, Secher N, Secher O. Glycogen depletion pattern in human muscle fiber during work under curarization (d-tubocurarine) In: Howald H, Poortmans JR, editors. Metabolic Adaptation to Prolonged Physical Exercise. Basel: Birkhauser-Verlag; 1973. pp. 422–430. [Google Scholar]
- Brooke MH, Kaiser KK. Three ‘myosine adenosine triphosphatase’ systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem. 1970;18:670–672. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- Burnley M, Doust JH, Ball D, Jones AM. Effects of prior heavy exercise on VO2 kinetics during heavy exercise are related to changes in muscle activity. J Appl Physiol. 2002;93:167–174. doi: 10.1152/japplphysiol.01217.2001. [DOI] [PubMed] [Google Scholar]
- Casey A, Constantin-Teodosiu D, Howell S, Hultman E, Greenhaff P. Metabolic response of ST and FT fibres during repeated bouts of maximal exercise in humans. Am J Physiol Endocrinol Metab. 1996;271:E38–E43. doi: 10.1152/ajpendo.1996.271.1.E38. [DOI] [PubMed] [Google Scholar]
- Coyle EF, Sidossis LS, Horowitz JF, Beltz JD. Cycling efficiency is related to percentage of Type I muscle fibers. Med Sci Sports Exerc. 1992;24:782–788. [PubMed] [Google Scholar]
- Crow MT, Kushmerick MJ. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol. 1982;79:147–166. doi: 10.1085/jgp.79.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin NA, Woledge RC. Energy changes and muscular contraction. Physiol Rev. 1978;58:690–761. doi: 10.1152/physrev.1978.58.3.690. [DOI] [PubMed] [Google Scholar]
- di Prampero PE, Boutellier U, Marguerat A. Efficiency of work performance and contraction velocity in isotonic tetani of frog sartorius. Pflugers Arch. 1988;412:455–461. doi: 10.1007/BF00582533. [DOI] [PubMed] [Google Scholar]
- Essén B, Jansson E, Henriksson J, Taylor A, Saltin B. Metabolic characteristics of fibre types in human skeletal muscle. Acta Physiol Scand. 1975;95:153–165. doi: 10.1111/j.1748-1716.1975.tb10038.x. [DOI] [PubMed] [Google Scholar]
- Ferguson RA, Aagaard P, Ball D, Sargeant AJ, Bangsbo J. Total power output generated during dynamic knee extensor exercise at different contraction frequencies. J Appl Physiol. 2000;89:1912–1918. doi: 10.1152/jappl.2000.89.5.1912. [DOI] [PubMed] [Google Scholar]
- Ferguson RA, Ball D, Krustrup P, Aagaard P, Kjær M, Sargeant AJ, Hellsten Y, Bangsbo J. Muscle oxygen uptake and energy turnover during dynamic exercise at different contraction frequencies in humans. J Physiol. 2001;536:261–271. doi: 10.1111/j.1469-7793.2001.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LF, McDonough P, Behnke BJ, Musch TI, Poole DC. Blood flow and O2 extraction as a function of O2 uptake in muscles composed of different fiber types. Respir Physiol Neurobiol. 2006;153:237–249. doi: 10.1016/j.resp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Foxdal P, Berqvist Y, Eckerbom S, Sandhagen B. Improving lactate analysis with the YSI 2300 GL: Hemolyzing blood samples makes results comparable with those for deproteinized whole blood. Clin Chem. 1992;38:2110–2114. [PubMed] [Google Scholar]
- Galbo H, Kjær M, Secher NH. Cardiovascular, ventilatory and catecholamine responses to maximal dynamic exercise in partially curarized man. J Physiol. 1987;389:557–568. doi: 10.1113/jphysiol.1987.sp016672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Quistorff B, Krustrup P, Bangsbo J, Saltin B. Heat production in human skeletal muscle at the onset of intense dynamic exercise. J Physiol. 2000;524:603–615. doi: 10.1111/j.1469-7793.2000.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Richardsson R, Saltin B. Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen. J Physiol. 2001;530:331–341. doi: 10.1111/j.1469-7793.2001.0331l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi B, Gladden B, Stary CM, Wagner PD, Hogan M. Faster adjustment of O2 delivery does not alter VO2 on-kinetics in isolated in situ canine muscle. J Appl Physiol. 1998;86:1394–1403. doi: 10.1152/jappl.1998.85.4.1394. [DOI] [PubMed] [Google Scholar]
- Greenhaff PL, Nevill ME, Söderlund K, Brodin K, Boobis LH, Williams C, Hultman E. The metabolic response of human type I and II muscle fibres during maximal treadmill sprinting. J Physiol. 1994;478:149–155. doi: 10.1113/jphysiol.1994.sp020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle PC, Yu ML. The phosphorus/oxygen ratio of mitochondrial oxidative phosphorylation. J Biol Chem. 1979;254:2450–2455. [PubMed] [Google Scholar]
- Hughson RL, Shoemaker JK, Tschakovsky ME, Kowalchuk JM. Dependence of muscle VO2 on blood flow dynamics at onset of forearm exercise. J Appl Physiol. 1996;81:1619–1626. doi: 10.1152/jappl.1996.81.4.1619. [DOI] [PubMed] [Google Scholar]
- Jackman MR, Willis WT. Characteristics of mitochondria isolated from type I and type IIb skeletal muscle. Am J Physiol Cell Physiol. 1996;270:C673–C678. doi: 10.1152/ajpcell.1996.270.2.C673. [DOI] [PubMed] [Google Scholar]
- Jacobs I, Kaiser P. Lactate in blood, mixed skeletal muscle, and FT or ST fibres during cycle exercise in man. Acta Physiol Scand. 1982;114:461–466. doi: 10.1111/j.1748-1716.1982.tb07010.x. [DOI] [PubMed] [Google Scholar]
- Jensen L, Bangsbo J, Hellsten Y. Effect of high intensity training on capillarization and presence of angiogenic factors in human skeletal muscle. J Physiol. 2004;557:571–582. doi: 10.1113/jphysiol.2003.057711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PRM, Pearson J. Anthropometric determination of leg fat and muscle plus bone volumes in young male and female adults. J Physiol. 1969;204:36P. [PubMed] [Google Scholar]
- Kiens B, Essen-Gustavsson B, Christensen NJ, Saltin B. Skeletal muscle substratexen utilization during submaximal exercise in man: effect of endurance training. J Physiol. 1993;469:459–478. doi: 10.1113/jphysiol.1993.sp019823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krustrup P, Ferguson RA, Kjær M, Bangsbo J. Heat and ATP turnover during dynamic exercise in humans: higher efficiency of anaerobic than aerobic ATP resynthesis. J Physiol. 2003;549:255–269. doi: 10.1113/jphysiol.2002.035089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krustrup P, Hellsten Y, Bangsbo J. Intense interval training enhances human skeletal muscle oxygen uptake in the initial phase of exercise at high but not at low intensities. J Physiol. 2004c;559:335–345. doi: 10.1113/jphysiol.2004.062232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krustrup P, Söderlund K, Mohr M, Bangsbo J. The slow component of oxygen uptake during intense sub-maximal exercise in man is associated with additional fibre recruitment. Pflugers Arch. 2004a;447:855–866. doi: 10.1007/s00424-003-1203-z. [DOI] [PubMed] [Google Scholar]
- Krustrup P, Söderlund K, Mohr M, Bangsbo J. Slow-twitch fiber glycogen depletion elevates moderateexercise fast-twitch fiber activity and O2 uptake. Med Sci Sports Exerc. 2004b;36:973–982. doi: 10.1249/01.mss.0000128246.20242.8b. [DOI] [PubMed] [Google Scholar]
- Krustrup P, Söderlund K, Mohr M, González-Alonso J, Bangsbo J. Recruitment of fiber types and quadriceps muscle portions during repeated, intense knee-extension exercise in humans. Pflugers Arch. 2004d;449:56–65. doi: 10.1007/s00424-004-1304-3. [DOI] [PubMed] [Google Scholar]
- Krustrup P, Söderlund K, Relu MU, Ferguson RA, Bangsbo J. Heterogeneous recruitment of quadriceps muscle portions and fibre types during moderate intensity kneeextensor exercise: effect of thigh occlusion. Scand J Med Sci Sports. 2008 doi: 10.1111/j.1600-0838.2008.00801.x. in press. DOI. 10.1111/j.1600-0838.2008.00801. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Armstrong RB. Adrenoreceptor effects on rat muscle blood flow during treadmill exercise. J Appl Physiol. 1987;62:1465–1472. doi: 10.1152/jappl.1987.62.4.1465. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV. A Flexible System of Enzymatic Analysis. New York: Academic Press; 1972. [Google Scholar]
- MacDonald MJ, Shoemaker JK, Tschakovsky ME, Hughson RL. Alveolar oxygen uptake and femoral artery blood flow dynamics in upright and supine leg exercise in humans. J Appl Physiol. 1998;85:1622–1628. doi: 10.1152/jappl.1998.85.5.1622. [DOI] [PubMed] [Google Scholar]
- McDonough P, Behnke BJ, Padilla DJ, Musch TI, Poole DC. Control of microvascular oxygen pressures in rat muscles comprised of different fibre types. J Physiol. 2005;563:903–913. doi: 10.1113/jphysiol.2004.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton WDM, Zaimis EJ. The action of d-tubocurarine and of decamenthoium on respiratory and other muscles in the cat. J Physiol. 1951;112:311–331. doi: 10.1113/jphysiol.1951.sp004531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A, Wadley G, Snow R, Giacobino JP, Muzzin P, Garnham A, Cameron-Smith D. Slow component of VO2 kinetics: the effect of training status, fibre type, UCP3 mRNA and citrate synthase activity. Int J Obes Relat Metab Disord. 2002;26:157–164. doi: 10.1038/sj.ijo.0801885. [DOI] [PubMed] [Google Scholar]
- Sawka MN, Petrofsky JS, Phillips CA. Energy cost of submaximal isometric concentrations in cat fast and slow twitch muscles. Pflugers Arch. 1981;390:164–168. doi: 10.1007/BF00590201. [DOI] [PubMed] [Google Scholar]
- Segal SS, Kurjiaka DT. Coordination of blood flow control in the resistance vasculature of skeletal muscle. Med Sci Sports Exerc. 1995;27:1158–1164. [PubMed] [Google Scholar]
- Söderlund K, Hultman E. Effects of delayed freezing on content of phosphagens in human skeletal muscle biopsy samples. J Appl Physiol. 1986;61:832–835. doi: 10.1152/jappl.1986.61.3.832. [DOI] [PubMed] [Google Scholar]
- Söderlund K, Hultman E. Energy metabolism in type I and type II human muscle fibres during short term electrical stimulation at different frequencies. Acta Physiol Scand. 1992;144:15–22. doi: 10.1111/j.1748-1716.1992.tb09262.x. [DOI] [PubMed] [Google Scholar]
- Teeter C, Carr SC, Tsai R, Briskey EJ. A cryobiopsy technique for assessing metabolite levels in skeletal muscle. Proc Soc Exp Biol Med. 1969;131:5–7. doi: 10.3181/00379727-131-33790. [DOI] [PubMed] [Google Scholar]
- Tesch P, Sjödin B, Karlsson J. Relationship between lactate accumulation, LDH activity, LDH isozyme and fibre type distribution in human skeletal muscle. Acta Physiol Scand. 1978;103:40–46. doi: 10.1111/j.1748-1716.1978.tb06188.x. [DOI] [PubMed] [Google Scholar]
- Viitasalo JT, Luhtanen P, Rahkila P, Rusko H. Electromyographic activity related to aerobic and anaerobic threshold in ergometer bicycling. Acta Physiol Scand. 1985;124:287–293. doi: 10.1111/j.1748-1716.1985.tb07663.x. [DOI] [PubMed] [Google Scholar]
- Völlestad NK, Blom PC. Effect of varying exercise intensity on glycogen depletion in human muscle fibres. Acta Physiol Scand. 1985;125:395–405. doi: 10.1111/j.1748-1716.1985.tb07735.x. [DOI] [PubMed] [Google Scholar]
- Walsh TH, Woledge RC. Heat production and chemical changes in tortoise muscle. J Physiol. 1970;206:457–469. doi: 10.1113/jphysiol.1970.sp009024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibom R, Söderlund K, Lundin A, Hultman E. A luminometric method for determination of ATP and phosphocreatine in single human skeletal muscle fibres. J Biolumin Chemilumin. 1991;6:123–129. doi: 10.1002/bio.1170060210. [DOI] [PubMed] [Google Scholar]
- Zoladz JA, Rademaker AC, Sargeant AJ. Non-linear relationship between O2 uptake and power output at high intensities of exercise in humans. J Physiol. 1995;488:211–217. doi: 10.1113/jphysiol.1995.sp020959. [DOI] [PMC free article] [PubMed] [Google Scholar]