Abstract
5′-AMP-activated protein kinase (AMPK) is a metabolic fuel sensor that monitors cellular energy charge, while the vasculature is important for maintaining cellular energy homeostasis. Mice with muscle-specific inactive AMPK (AMPK DN) were used to investigate if AMPK regulates skeletal muscle capillarization and the angiogenic responses to exercise. Two hours of the AMP analogue AICAR (1.0 g kg−1) or systemic hypoxia (6% O2) increased vascular endothelial growth factor (VEGF) mRNA in wild-type (WT), but not in AMPK DN mice. In contrast, the increase in VEGF mRNA with acute exercise (1 h at 20 m min−1, 10% gradient) was greater in AMPK DN compared to WT mice. Nuclear run-on assay demonstrated that exercise increased VEGF transcription, while hypoxia decreased VEGF transcription. There was no difference in VEGF transcription between WT and AMPK DN. There was a strong correlation between VEGF transcription and VEGF mRNA at rest and with exercise. Resting capillarization was lower in AMPK DN compared to WT. Wheel running (28 days) increased capillarization and this response was AMPK independent. Significant correlations between VEGF protein and muscle capillarization are consistent with VEGF being an important determinant of skeletal muscle capillarization. These data are to our knowledge the first to demonstrate in skeletal muscle in vivo that: (1) AMPK is necessary for hypoxia-induced VEGF mRNA stabilization, (2) acute exercise increases VEGF transcription, (3) inhibition of AMPK augments the VEGF mRNA response to acute exercise, and (4) AMPK regulates basal VEGF expression and capillarization, but is not necessary for exercise-induced angiogenesis.
The ability to respond to physiological stressors, such as reduced intracellular energy charge, is critical for cell survival. 5′-AMP-activated protein kinase (AMPK) is a heterotrimeric enzyme, consisting of a catalytic subunit (α) and two regulatory subunits (β and γ), that acts as a metabolic fuel gauge to monitor cellular energy charge. Upon activation, AMPK decreases metabolic flux through energy-consuming pathways (i.e. fatty acid synthesis) and initiates energy-conserving/producing processes (i.e. fatty acid oxidation) (Winder & Hardie, 1999). The increase in AMPK activity, in response to elevated AMP : ATP ratios, is accomplished through several interrelated mechanisms (reviewed in Hardie, 2004). Both allosteric binding of AMP (Hardie et al. 1998) and phosphorylation by an upstream AMPK kinase (Sakamoto et al. 2005) contribute to increase AMPK activity. Metabolic or environmental stressors that are known to activate AMPK include exercise (Winder & Hardie, 1996; Hutber et al. 1997; Vavvas et al. 1997) and hypoxia (Hayashi et al. 2000; Gonzalez et al. 2004). It is also well established that AMPK can be activated pharmacologically with the AMP analogue 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) (Henin et al. 1996; Merrill et al. 1997; Russell et al. 1999; Musi et al. 2001). Although AMPK is traditionally known as a metabolic regulatory enzyme, a growing body of literature suggests that AMPK also plays other important physiological roles.
It has been hypothesized that the vasculature is important for maintaining cellular energy homeostasis, such that the skeletal muscle capillaries are vital for the delivery of substrates (i.e. oxygen, carbohydrates and fatty acids) to muscle for ATP synthesis (Adair, 2005). It has been proposed that skeletal muscle capillarization can be regulated by metabolic demand and oxidative capacity (Hepple et al. 1997; Frisbee, 2005). Angiogenesis, an expansion of capillaries from existing capillaries, is one of the many adaptations resulting from chronic exercise training that may aid in alleviating metabolic stress caused from repeated bouts of exercise (Hepple et al. 1997; Richardson et al. 2000); however, the mechanisms that regulate exercise-induced angiogenesis are not fully understood.
Vascular endothelial growth factor (VEGF) is a predominantly endothelial cell-specific mitogen that is an important regulator of basal capillary maintenance and angiogenesis (Ferrara, 1999). Inhibition of endogenous VEGF production reduces basal skeletal muscle capillarization 64% (Tang et al. 2004a), induces skeletal muscle cell apoptosis (Tang et al. 2004a), and inhibits skeletal muscle angiogenesis in response to exercise training (Wagner et al. 2005). It is well established that skeletal muscle VEGF expression is increased in response to acute hypoxia (Minchenko et al. 1994; Breen et al. 1996; Gavin et al. 2006) and exercise (Breen et al. 1996; Gustafsson et al. 1999; Gavin et al. 2006); however, the mechanisms that regulate skeletal muscle VEGF expression remain to be elucidated.
Ouchi et al. demonstrated that AICAR enhances capillarization and VEGF mRNA and protein in ischaemic muscle tissue (Ouchi et al. 2005). Additionally, inactivation of AMPK reduces skeletal muscle blood flow (indicative of lower vascularization) and VEGF expression in response to ischaemia (Ouchi et al. 2005). It is unknown if AMPK regulates exercise-induced VEGF expression or angiogenesis in skeletal muscle. In the present study, a mouse model that overexpresses muscle-specific inactive (dominant negative) AMPK was used to investigate if AMPK regulates skeletal muscle capillarization and the angiogenic responses to exercise.
Methods
Animal use
All animal protocols and procedures were approved by the East Carolina University Animal Use and Care Committee. Female transgenic mice overexpressing an inactive AMPK α2 catalytic subunit (termed AMPK dominant negative; AMPK DN) on a C57BL/6 background were used throughout (a kind gift from Morris J. Birnbaum, MD, Howard Hughes Medical Institute, University of Pennsylvania School of Medicine). The AMPK α2 mutation is driven by the muscle creatine kinase (MCK) promoter, thus expression of the AMPK DN transgene is limited to skeletal muscle, and to a much lesser extent, cardiac muscle. Overexpression of the inactive AMPK α2 subunit is sufficient to out-compete binding of endogenous wild-type AMPK α1 and α2 subunits for the AMPK β and γ subunits, thus eliminating essentially all AMPK activity in skeletal muscle of AMPK DN transgenic mice (Mu et al. 2001). AMPK DN mice were identified by PCR analysis of isolated tail DNA by use of primers specific for the mutated AMPK α2 gene and confirmed by Western blot analysis. AMPK DN and wild-type (WT) littermate mice were housed in a temperature-controlled environment (21°C) with a 12 : 12 h light : dark cycle and provided food and water ad libitum. All mice were anaesthetized by isofluorane inhalation and killed by cervical dislocation at the times indicated. Total number of mice used for these experiments was WT, n = 70; AMPK DN, n = 89.
Experimental design
AMPK, AICAR and VEGF mRNA
To confirm that AMPK regulates VEGF mRNA expression in skeletal muscle in vivo, WT (n = 7) and AMPK DN (n = 6) mice were given an intraperitoneal injection of the pharmacological activator of AMPK, AICAR (1.0 g (kg body wt)−1; Toronto Research Chemicals Inc., North York, Ontario, Canada). WT (n = 6) and AMPK DN (n = 3) littermates, given a comparable volume of saline, were used as controls. Two hours after injection, the gastrocnemius muscles were rapidly excised, frozen in liquid nitrogen, and stored at −80°C until analysis of VEGF mRNA.
AMPK, hypoxia and VEGF mRNA
Because the VEGF mRNA response to exercise has been proposed to involve hypoxia (Wagner, 2001), we investigated if AMPK regulates the VEGF mRNA response to acute systemic hypoxia by subjecting WT (n = 11) and AMPK DN (n = 14) mice to 2 h of normobaric systemic hypoxia (6% O2) by substituting nitrogen for ambient oxygen with standard pressure and constant flow rate. A gas analyser (SensorMedics, Anaheim, CA, USA) was used to continuously monitor oxygen concentrations. This level of hypoxia was chosen because both VEGF mRNA (Gavin et al. 2006) and hypoxia-inducible factor-1α (HIF-1α; an important transcriptional regulator of VEGF) (Stroka et al. 2001) increase in response to 6% O2 in skeletal muscle of C57BL/6 mice. Immediately following hypoxic exposure, gastrocnemius muscles were rapidly excised, frozen in liquid nitrogen, and stored at −80°C until analysis of VEGF mRNA. Resting WT (n = 16) and AMPK DN (n = 24) littermates breathing normal room air were used as controls.
AMPK, acute exercise and VEGF mRNA
To investigate if AMPK regulates the VEGF mRNA response to acute aerobic exercise, WT and AMPK DN mice were exercised on a rodent treadmill (Stanhope, Davis, CA, USA) for 60 min at 20 m min−1, 10% gradient (55% of maximal treadmill running speed). Preliminary studies revealed no difference in maximal treadmill running speed (36.0 ± 1.4 versus 35.8 ± 1.0 m min−1) or time to exhaustion (18.0 ± 1.0 versus 17.6 ± 0.7 min) between WT (n = 4) and AMPK DN (n = 6) mice, respectively. Therefore, WT and AMPK DN mice were exercised at the same absolute intensity for the current studies. We previously reported that the VEGF mRNA response peaks 1 h after completion of the 60 min of 20 m min−1, 10% gradient treadmill exercise bout in C57BL/6 mice (Gavin et al. 2006); therefore, gastrocnemius and plantaris muscles were harvested 1 h post exercise (WT; n = 7 and AMPK DN; n = 7) for the analysis of VEGF mRNA. The same set of control WT (n = 16) and AMPK DN (n = 24) littermate mice from ‘AMPK, hypoxia and VEGF mRNA’ were used as controls for the acute exercise experiments.
AMPK, hypoxia, exercise and VEGF transcription
To investigate if the increase in VEGF mRNA expression in response to hypoxia and to exercise in skeletal muscle is the result of increased transcriptional activity, WT and AMPK DN mice underwent 2 h of hypoxia (WT n = 4; AMPK DN n = 6) or 60 min of aerobic exercise (WT n = 3; AMPK DN n = 3) as described above. Immediately after the respective treatments, gastrocnemius muscles were rapidly excised and prepared for nuclear isolation. Resting WT (n = 6) and AMPK DN (n = 7) littermates, breathing room air, were used as controls. Due to the large amount of starting material required for this analysis, nuclear isolation was performed only from gastrocnemius muscle.
AMPK, exercise-induced angiogenesis and VEGF protein
To determine if AMPK regulates exercise-induced angiogenesis and VEGF protein expression, 8-week-old WT (n = 8) and AMPK DN (n = 10) mice were housed individually in cages and allowed free access to a rodent running wheel (12 cm diameter) for 28 consecutive days (‘exercise trained’, ET). Increased capillarization has been reported within 7 days of voluntary wheel running in skeletal muscle of C57BL/6 mice (Waters et al. 2004). A bicycle computer (Sigma Sport, Neustadt, Germany) was connected to the running wheel and distance, time and speed were recorded daily. Running wheels were locked after the 28th day of voluntary wheel running and mice were killed at least 24 h after to ensure that the acute effects of the last exercise bout did not influence the analysis of VEGF protein expression. Twelve-week-old female WT (n = 8) and AMPK DN (n = 9) mice (individually housed since 8 weeks of age in a cage with locked running wheels) were used as controls (‘untrained’, UT). Gastrocnemius and plantaris muscles were harvested and muscles from one limb were analysed for muscle morphometry and capillarization and the contralateral limb for VEGF protein.
RNA isolation and real-time PCR
Total cellular RNA was isolated using commercial RNA isolation columns (RNeasy Fibrous Tissue Kit; Qiagen, Valencia, CA, USA). RNA was quantified with RiboGreen reagent (Molecular Probes, Eugene, OR, USA) and stored at −80°C until reverse transcription. Reverse transcription of both total RNA samples and nascent RNA from nuclear run-on reactions was performed from 1 μg of total RNA sample and 50 μl of nascent RNA from nuclear run-on reactions using the High Capacity cDNA Archive Kit (Applied Biosystems (AB), Foster City, CA, USA). VEGF mRNA from muscle samples and transcription assays was determined using real-time PCR, as previously described (Gavin et al. 2006). A 5′FAM-, 3′TAMRA-labelled TaqMan Gene Expression Assay (primer/probe set) designed to detect all splice variants of mouse VEGF (AB; p/n Mm00437304_m1) was used and normalized to cyclophilin A (cycA). The cycA forward/reverse primers (Fwd: CATCTCCGACTGTGGACAACTC; Rev: CTGAGCTACAGAAGGAATGGTTTG) and 5′VIC-, 3′TAMRA-labelled probe (TTCTTTGACTTGCGGGCATTTTACCCA) were designed by Primer Express 2.0 design software (AB) using specific BLAST sequences found in a mouse genome database (NM_008907; Entrez, NIH, Bethesda, MD, USA) and synthesized by Applied Biosystems. Primer/probe concentrations were optimized and amplification efficiency was verified prior to experimentation (data not shown). Amplicon product size was confirmed by gel electrophoresis using a 2.0% agarose gel stained by ethidium bromide and visualized using ultraviolet light (data not shown). PCR reaction mixtures (20 μl volume) contained 8 μl of diluted cDNA template (20 ng), 1 μl each of 20X VEGF Gene Expression Assay and 20X cycA assay set (300 nm primer, 150 nm probe concentrations), and 10 μl 2X Universal PCR Master Mix (AB). PCR reactions were performed in triplicate on the Applied Biosystems Prism 7300 sequence detection system per manufacturer's instructions: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. CycA mRNA expression was not affected by AICAR, hypoxia, exercise, or transgenic mutation (data not shown), making cycA a valid endogenous control gene for these studies (Neurath et al. 2006).
Nuclear isolation and nuclear run-on assay
Nuclei were isolated from gastrocnemius muscles using a method previously described by Hildebrandt & Neufer (2000). Muscle tissue (∼200 mg) was rapidly excised, chilled in 35 ml of ice-cold buffer A (15 mm Hepes pH 7.5, 60 mm KCl, 5 mm each of EDTA and EGTA, 3 mg ml−1 bovine serum albumin (BSA), 300 mm sucrose, 1 mm dithiothreitol (DTT), 0.15 mm spermidine, 0.5 mm spermine, 2 μg ml−1 leupeptin, and 0.5 mm phenylmethylsulphonyl floride (PMSF)), thoroughly minced, rotated at 4°C for 5 min, and homogenized for 20 s (Polytron PT10/35, Kinematica AG, Switzerland). Samples were allowed to settle on ice for 5 min and then centrifuged at 700 g for 10 min at 4°C. Crude nuclear pellets were resuspended in 10 ml of ice-cold buffer B (15 mm Hepes pH 7.5, 60 mm KCl, 0.1 mm each of EDTA and EGTA, 3 mg ml−1 BSA, 300 mm sucrose, 0.5% Triton X-100, 1 mm DTT, 0.15 mm spermidine, 0.5 mm spermine, 2 μg ml−1 leupeptin, and 0.5 mm PMSF), filtered through pre-wetted cheesecloth, and repelleted (700 g, 10 min, 4°C). Crude nuclear pellets were resuspended in 10 ml of ice-cold buffer C (15 mm Hepes pH 7.5, 60 mm KCl, 5 mm magnesium acetate, 0.1 mm each of EDTA and EGTA, 3 mg ml−1 BSA, 300 mm sucrose, 1 mm DTT, 0.15 mm spermidine, 0.5 mm spermine, 2 μg ml−1 leupeptin, and 0.5 mm PMSF) and repelleted (700 g, 10 min, 4°C). Crude nuclear preparations were resuspended in 11 ml of ice-cold buffer C/Percoll density medium (Amersham; average density = 1.07 g ml−1) and separated from contractile filaments by density gradient centrifugation at 27 000 g for 15 min at 4°C. Purified nuclei were washed in 10 ml of ice-cold buffer C and repelleted (700 g, 10 min, 4°C). Final intact nuclei were resuspended in 200 μl of ice-cold storage buffer (75 mm Hepes pH 7.5, 60 mm KCl, 15 mm NaCl, 5 mm magnesium acetate, 0.1 mm each of EDTA and EGTA, 40% glycerol, 1 mm DTT, 0.15 mm spermidine, 0.5 mm spermine, and 2 μg ml−1 each of leupeptin and aprotinin) and stored at −80°C until analysis of VEGF transcription and genomic DNA isolation. Relative VEGF transcription at rest and in response to acute systemic hypoxia and acute systemic exercise was determined by a real-time PCR-based nuclear run-on technique adapted from previously described reports (Hildebrandt & Neufer, 2000; Pilegaard et al. 2000). Nuclear run-on reactions were performed using 160 μl of nuclear preparation with 2X reaction buffer (20% glycerol, 10 mm MgCl2, 100 mm KCl, 4.5 mm DTT, 1.2 mm ATP, 0.6 mm each of CTP, GTP and UTP, 0.5 mm spermidine, 0.15 mm spermine, and 80 U ml−1 RNase inhibitor) for 20 min at 21°C. Following the reaction, nuclei were lysed with 10X SET buffer (100 mm Tris pH 8.0, 50 mm EDTA pH 8.0, and 5% SDS) and vortexing for 10 s.
Genomic DNA isolation
In order to normalize VEGF transcription and determine the amount of starting nuclear material in each sample for the nuclear run-on reaction, genomic DNA was isolated from 25 μl of nuclear preparation of each sample using commercial DNA isolation columns (DNeasy Tissue Kit, Qiagen). Genomic DNA was quantified using PicoGreen reagent (Molecular Probes). VEGF transcription was normalized to genomic DNA content for each sample.
Morphometric and morphological analysis
Gastrocnemius and plantaris muscles were mounted in an OCT–tragacanth gum mixture, frozen in liquid nitrogen-cooled isopentane, and stored at −80°C until processing for the measurement of muscle morphometry and capillarization. Muscle tissue was sectioned to a thickness of 10 μm on a cryostat, transferred to slides, and kept at −20°C until fixation. Sections were stained for capillaries using a modified ATPase method (Rosenblatt), which simultaneously provides fibre typing and capillary visualization (Rosenblatt et al. 1987).
The gastrocnemius is a predominantly fast glycolytic muscle and the mid-belly, white region accounts for a large proportion of the total mass of the gastrocnemius (Burkholder et al. 1994); therefore, only the mid-belly, white region of the gastrocnemius was analysed for muscle morphometry and morphology. Mid-belly muscle sections from gastrocnemius and plantaris were viewed under a light microscope (Nikon 400) and a digital image taken of the superficial, white region of the gastrocnemius and the mid-belly of the plantaris (Nikon Coolpix 990) as previously described (Gavin et al. 2004). Data were collected from muscle sections by an investigator who was blinded to treatment groups. Capillaries were quantified manually from the digital image on individual fibres. The following indexes were measured: (1) the number of capillaries around a fibre (capillary contacts, CC), (2) the capillary-to-fibre ratio on an individual fibre basis (C/Fi), and (3) the number of fibres sharing each capillary (sharing factor). Capillary density (CD) was calculated by using the fibre as the reference space. Capillary-to-fibre perimeter exchange index (CFPE) was calculated as an estimate of the capillary-to-fibre surface area (Hepple et al. 1997). Quantification of the capillary supply was performed on at least 50 fibres by randomly selecting fibres in an artifact-free region. Fibre cross-sectional area (FCSA) and perimeter were measured with the image-analysis system and commercial software (SigmaScan, Jandel Scientific), calibrated to transform the number of pixels (viewed on a computer monitor) into micrometres.
Protein isolation and analysis
Protein was isolated from approximately 30 mg of pulverized gastrocnemius muscle and from whole plantaris muscle. Muscle tissue was homogenized (Polytron) in 300 μl of RIPA buffer (1X PBS, 1% Igepal, 0.5% sodium deoxycholate and 0.1% SDS with protease and phosphatase (I and II) inhibitors (Sigma)). Homogenates were centrifuged at 10 000 g for 15 min at 4°C to remove insoluble proteins and fibrous tissue and protein content for each sample was determined using bicinchoninic acid (BCA protein assay; Pierce-Rockford, IL, USA).
VEGF protein was purified by heparin-binding affinity chromatography then analysed by Western blot. Protein (200 μg) from muscle homogenates, diluted in 750 μl of RIPA buffer with protease inhibitors, was rotated overnight at 4°C with 40 μl of a 50% heparin–agarose bead slurry (Sigma; H-6508). Beads were pelleted by centrifugation at 5000 g for 2 min and the supernatant was discarded. Beads were washed twice in 750 μl of RIPA buffer and beads were repelleted by centrifugation (5000 g for 2 min) after each wash. Wash buffer was discarded and beads were resuspended in 20 μl of 3X Laemmli buffer. Samples were boiled for 10 min, vortexed and centrifuged (5000 g for 30 s). Samples were separated by SDS-PAGE on 12.5% gels then transferred to PVDF membranes. Membranes were blocked in 5% non-fat dry milk (NFDM) for 1 h at room temperature then incubated with VEGF primary antibody (Santa Cruz; 1 : 1000 dilution in 5% NFDM) for 1 h at room temperature. Immunoblots were incubated with horseradish peroxidase-conjugated secondary antibody (Santa Cruz; 1 : 10 000 dilution in 5% NFDM) for 1 h at room temperature. Immunoblots were detected using ECL + Western Blotting Detection reagent (Amersham) on autoradiography film (Midwest Scientific, St Louis, MO, USA). Autoradiographs were quantified by densitometry using GelPro Analyser 4.0 (Media Cybernetics, Silver Spring, MD, USA).
Statistical analysis
A two-way ANOVA was used to analyse muscle morphology/morphometry and VEGF protein, mRNA and transcription data. Following a significant F ratio, Fisher's LSD post hoc analyses were performed. For all other variables, Student's unpaired t tests were used to compare differences between WT and AMPK DN. Linear regression was performed to identify associations between selected variables. Data are represented as mean ±s.e.m. and statistical significance was set at P ≤ 0.05.
Results
AMPK and skeletal muscle VEGF mRNA in vivo
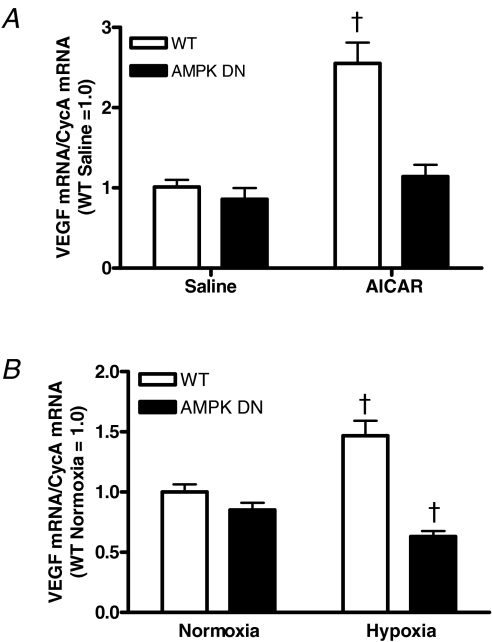
We investigated if AMPK can regulate skeletal muscle VEGF mRNA expression in vivo. Acute administration of AICAR significantly increased VEGF mRNA in the gastrocnemius of WT, but not in AMPK DN mice (Fig. 1A). These data demonstrate that activation of AMPK increases skeletal muscle VEGF mRNA expression in vivo and that the AMPK DN transgenic model used in the current report is an appropriate model to investigate AMPK regulation of skeletal muscle VEGF expression.
Figure 1. Gastrocnemius VEGF mRNA in WT and AMPK DN mice after acute AICAR (1 g kg−1) administration (A) or exposure to acute systemic hypoxia (6% O2) (B).
AICAR increased VEGF mRNA in WT, but not AMPK DN mice. Acute hypoxia increased VEGF mRNA in WT and slightly but significantly decreased VEGF mRNA in AMPK DN mice. Data are mean ±s.e.m. WT saline, n = 6; AMPK DN saline, n = 3; WT AICAR, n = 7; AMPK DN AICAR, n = 6. WT normoxia, n = 16; AMPK DN normoxia, n = 24; WT hypoxia, n = 11; AMPK DN hypoxia, n = 14. †Significantly different from all other groups.
Given that systemic hypoxia increases both AMPK and VEGF and hypoxia has been proposed as a potential regulator of exercise-induced VEGF, we investigated if AMPK regulates the skeletal muscle VEGF mRNA response to hypoxia. In WT mice, acute hypoxic exposure increased gastrocnemius VEGF mRNA compared to normoxia. In AMPK DN mice by contrast, acute hypoxic exposure decreased VEGF mRNA compared to normoxia (Fig. 1B). These findings suggest that hypoxia increases skeletal muscle VEGF mRNA through an AMPK-dependent mechanism.
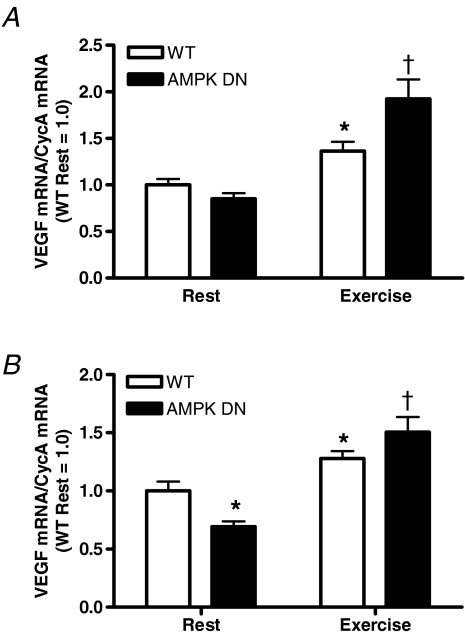
To investigate AMPK regulation of the VEGF response to acute exercise, VEGF mRNA was measured in WT and AMPK DN mice after an acute treadmill exercise bout. Contrary to our hypothesis, the exercise-induced increase in VEGF mRNA was greater in the gastrocnemius (Fig. 2A) and plantaris (Fig. 2B) muscles of AMPK DN compared to WT. Also in the plantaris, resting VEGF mRNA was lower in AMPK DN compared to WT.
Figure 2. VEGF mRNA response to acute exercise (20 m min−1 for 60 min) in gastrocnemius (A) and plantaris (B) muscles of WT and AMPK DN mice.
Exercise increased VEGF mRNA in gastrocnemius and plantaris. In both the gastrocnemius and plantaris the increase was greater in AMPK DN compared to WT. In the plantaris, resting VEGF mRNA was lower in AMPK DN compared to WT. Data are mean ±s.e.m. WT rest, n = 16; AMPK DN rest, n = 24; WT exercise, n = 7; AMPK DN exercise, n = 7. *Significantly different from WT rest. †Significantly different from all other groups.
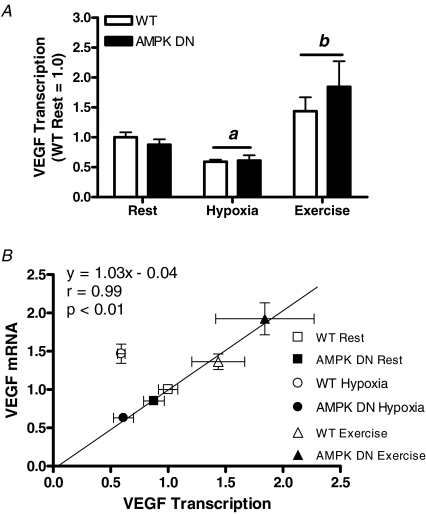
To further investigate AMPK regulation of VEGF, VEGF transcription was measured in the basal state (resting normoxia), as well as in response to acute systemic hypoxia or to acute exercise. Exposure to 2 h of systemic hypoxia reduced VEGF transcription in gastrocnemius of WT and AMPK DN mice, but acute exercise increased VEGF transcription, compared to rest (Fig. 3A). There was no effect of transgene on VEGF transcription in any condition. Given that systemic hypoxia increases skeletal muscle VEGF mRNA (in spite of reduced transcription with hypoxia) and that the VEGF mRNA response to hypoxia is reduced in AMPK DN mice, these findings suggest that AMPK regulates VEGF mRNA stabilization and not transcription.
Figure 3. Gastrocnemius VEGF transcription at rest and in response to acute systemic hypoxia or to acute exercise (A) and VEGF transcription plotted versus VEGF mRNA content (B).
Hypoxia decreased VEGF transcription, while exercise increased VEGF transcription. There was no effect of genotype on the VEGF transcription response to hypoxia or exercise. When transcription is plotted versus VEGF content, it is visually apparent that, except for WT hypoxia, there is an association between VEGF transcription and VEGF mRNA content. Linear regression, excluding WT hypoxia, revealed a strong correlation (r = 0.99; P < 0.01) between VEGF transcription and VEGF mRNA content, suggesting that transcription is important for basal and exercise-induced VEGF mRNA regulation, while stabilization is important for VEGF mRNA regulation in systemic hypoxia. Data are mean ±s.e.m. WT rest, n = 6; AMPK DN rest, n = 7; WT hypoxia, n = 4; AMPK DN hypoxia, n = 6; WT exercise, n = 3; AMPK DN exercise, n = 3. a, main effect of hypoxia. b, main effect of exercise.
To identify if VEGF transcription is associated with VEGF mRNA content in skeletal muscle, VEGF transcription was plotted versus VEGF mRNA content in WT and AMPK DN mice in resting normoxia and in response to acute systemic hypoxia or to acute exercise. It is visually evident that, except for the response to hypoxia in WT mice, there is an association between VEGF transcription and VEGF mRNA content (Fig. 3B). Linear regression, when performed excluding WT hypoxia, revealed a significant correlation (r = 0.99) between VEGF transcription and VEGF mRNA content with a slope no different than 1.0 and a y-intercept slope no different than 0; suggesting that basal and exercise-induced regulation of VEGF occurs predominantly through changes in VEGF transcription.
AMPK and exercise-induced angiogenesis
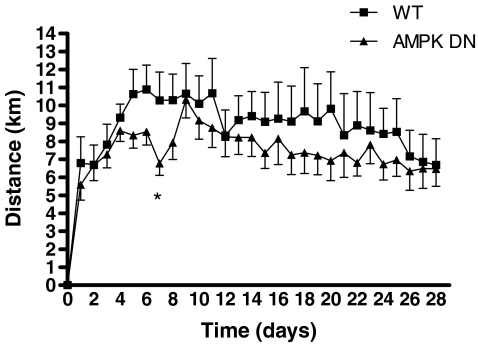
To investigate if AMPK regulates exercise-induced angiogenesis, WT and AMPK DN animals were exposed to a running wheel for 28 days. There was no effect of exercise training status (untrained (UT) or exercise trained (ET)) on body weight; however, body weight was lower in AMPK DN compared to WT. Exercise training increased plantaris mass, regardless of transgene, but not gastrocnemius mass (Table 1). Daily average running distance was not statistically different on each day of the training between WT and AMPK DN except on day 7, where AMPK DN ran less than WT (Fig. 4). When analysed for area under the curve, there was no difference between WT and AMPK DN. In addition, there were no differences in total distance, total time, daily time, or daily speed between WT and AMPK DN mice over the 28 days of voluntary wheel running (Table 2).
Table 1.
Body weight, muscle mass and muscle mass/body weight of untrained and exercise-trained WT and AMPK DN mice
| Untrained | Exercise trained | P value | |||||
|---|---|---|---|---|---|---|---|
| WT (n = 8) | AMPK DN (n = 9) | WT (n = 8) | AMPK DN (n = 10) | Interaction | Transgene | Exercise | |
| Body weight (g) | 22.4 ± 0.5 | 21.3 ± 0.5 | 23.0 ± 0.4 | 22.1 ± 0.4 | 0.76 | 0.04 | 0.13 |
| Muscle mass (mg) | |||||||
| Gastrocnemius | 101.5 ± 2.6 | 97.0 ± 2.9 | 98.7 ± 2.3 | 96.0 ± 1.3 | 0.69 | 0.12 | 0.40 |
| Plantaris | 13.4 ± 0.3 | 13.1 ± 0.3 | 14.9 ± 0.4 | 14.4 ± 0.3 | 0.89 | 0.23 | <0.01 |
| Muscle mass/body weight (mg g−1) | |||||||
| Gastrocnemius | 4.53 ± 0.07 | 4.34 ± 0.10 | 4.41 ± 0.07 | 4.34 ± 0.05 | 0.40 | 0.09 | 0.39 |
| Plantaris | 0.60 ± 0.01 | 0.59 ± 0.01 | 0.67 ± 0.02 | 0.65 ± 0.02 | 0.96 | 0.38 | <0.01 |
Data are mean ±s.e.m.
Figure 4. Voluntary wheel running distance in WT and AMPK DN mice over the 28 day programme.
Running distance was lower only on day 7 in AMPK DN, compared to WT. Total running distance over the 28 day running programme was not different between WT and AMPK DN mice. Data are mean ±s.e.m. WT, n = 8; AMPK DN, n = 10. *Significantly different from WT.
Table 2.
Voluntary wheel running over 28 days in WT and AMPK DN mice
| WT (n = 8) | AMPK DN (n = 10) | P value | |
|---|---|---|---|
| Total | |||
| Distance (km) | 251 ± 39 | 212 ± 21 | 0.37 |
| Time (h) | 138 ± 16 | 118 ± 10 | 0.28 |
| Daily | |||
| Distance (km) | 8.96 ± 1.39 | 7.57 ± 0.74 | 0.37 |
| Time (h) | 4.9 ± 0.6 | 4.2 ± 0.4 | 0.28 |
| Speed (m min−1) | 29.4 ± 1.9 | 29.7 ± 0.8 | 0.87 |
Total, the total distance and time of voluntary wheel running over the 28 day training period. Daily, the average daily distance, time and speed of voluntary wheel running over the 28 day training period. Data are mean ± s.e.m.
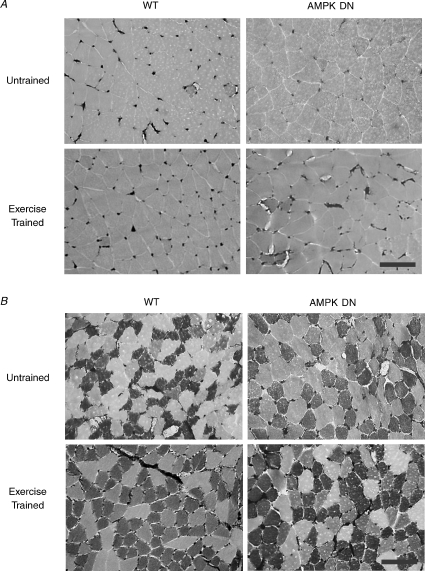
Gastrocnemius and plantaris muscles were analysed for capillarization and morphometry in UT and ET WT and AMPK DN mice. In gastrocnemius muscle (Table 3), both CC and C/Fi were lower in untrained AMPK DN compared to WT mice. Exercise training increased CC and C/Fi in AMPK DN, but not in WT such that neither CC nor C/Fi was different between WT and AMPK DN mice after training. Exercise training increased CFPE independent of transgene, while there were no effects of transgene or exercise on FCSA, fibre perimeter, or CD. As anticipated, the mid-belly portion of the gastrocnemius was composed entirely of type IIb fibres in both WT and AMPK DN mice and fibre-type composition did not change with exercise training (Fig. 5A).
Table 3.
Skeletal muscle morphology and capillarization in the gastrocnemius in untrained and exercise-trained muscle of WT and AMPK DN mice
| Untrained | Exercise trained | P value | |||||
|---|---|---|---|---|---|---|---|
| WT (n = 8) | AMPK DN (n = 9) | WT (n = 8) | AMPK DN (n = 10) | Interaction | Transgene | Exercise | |
| Capillary contacts | 4.03 ± 0.17 | 3.09 ± 0.23 † | 4.12 ± 0.37 | 4.25 ± 0.20 | 0.03 | — | — |
| Capillary-to-fibre ratio | 1.35 ± 0.07 | 1.04 ± 0.09 † | 1.42 ± 0.12 | 1.46 ± 0.07 | 0.05 | — | — |
| Capillary density (capillaries mm−2) | 708 ± 68 | 605 ± 94 | 796 ± 95 | 699 ± 25 | 0.97 | 0.19 | 0.22 |
| CFPE (capillaries (1000 mm)−1) | 7.15 ± 0.45 | 5.86 ± 0.65 | 7.68 ± 0.63 | 7.44 ± 0.27 | 0.31 | 0.15 | 0.05 |
| Fibre area (μm2) | 2167 ± 152 | 2113 ± 203 | 2207 ± 195 | 2356 ± 118 | 0.55 | 0.78 | 0.41 |
| Fibre perimeter (μm) | 194 ± 7 | 187 ± 9 | 193 ± 10 | 201 ± 4 | 0.33 | 0.94 | 0.42 |
| Fibre-type composition (%) | |||||||
| Type IIb | 100 | 100 | 100 | 100 | |||
Data are mean ± s.e.m. CFPE, capillary-to-fibre perimeter exchange index.
Significantly different from all other groups.
Figure 5. Representative photomicrographs of gastrocnemius (A) and plantaris (B) muscle sections from untrained and exercise-trained WT and AMPK DN mice stained for capillaries and fibre type.
Capillaries appear as dark-stained regions between fibres. Type IIb fibres are shown by the lightest stain, type IIa fibres by the darkest stain, and type I fibres are intermediate. Scale bar, 100 μm.
In plantaris muscle (Table 4), CD and CFPE were lower in AMPK DN compared to WT independent of training status. Exercise training increased CC, C/Fi and CFPE independent of transgene. Exercise training increased FCSA and fibre perimeter in WT, but not in AMPK DN, while there was no difference in FCSA or fibre perimeter between untrained WT and AMPK DN mice. In untrained plantaris muscle, AMPK DN contained fewer type IIa fibres and tended to have more type IIb fibres (P = 0.08), compared to WT. Exercise training increased the percentage of type IIa fibres and decreased the percentage of type IIb fibres independent of transgene (Fig. 5B).
Table 4.
Skeletal muscle morphology and capillarization in the plantaris in untrained and exercise-trained muscle of WT and AMPK DN mice
| Untrained | Exercise trained | P value | |||||
|---|---|---|---|---|---|---|---|
| WT (n = 8) | AMPK DN (n = 9) | WT (n = 8) | AMPK DN (n = 10) | Interaction | Transgene | Exercise | |
| Capillary contacts | 4.46 ± 0.14 | 4.10 ± 0.28 | 5.22 ± 0.21 | 5.10 ± 0.24 | 0.60 | 0.30 | <0.01 |
| Capillary-to-fibre ratio | 1.58 ± 0.06 | 1.41 ± 0.11 | 1.94 ± 0.09 | 1.88 ± 0.11 | 0.59 | 0.26 | <0.01 |
| Capillary density (capillaries mm−2) | 1425 ± 104 | 1063 ± 73 | 1230 ± 106 | 1218 ± 90 | 0.08 | 0.05 | 0.83 |
| CFPE (capillaries (1000 mm)−1) | 11.17 ± 0.49 | 9.13 ± 0.62 | 11.50 ± 0.56 | 11.18 ± 0.30 | 0.09 | 0.02 | 0.02 |
| Fibre area (μm2) | 1187 ± 52 | 1446 ± 88 | 1783 ± 142 * | 1575 ± 128 | 0.05 | — | — |
| Fibre perimeter (μm) | 140 ± 3 | 153 ± 4 | 172 ± 8* | 161 ± 7 | 0.05 | — | — |
| Fibre-type composition (%) | |||||||
| Type I | 21.1 ± 2.5 | 21.5 ± 3.7 | 22.1 ± 1.1 | 25.1 ± 1.6 | 0.60 | 0.51 | 0.37 |
| Type IIa | 43.4 ± 2.7 | 33.3 ± 2.6* | 55.1 ± 4.1*# | 59.0 ± 2.6*# | 0.03 | — | — |
| Type IIb | 35.5 ± 3.4 | 45.2 ± 5.0 | 22.8 ± 4.3*# | 15.9 ± 1.6*# | 0.04 | — | — |
Mean ± s.e.m. CFPE, capillary-to-fibre perimeter exchange index.
Significantly different from untrained WT.
Significantly different from untrained AMPK DN.
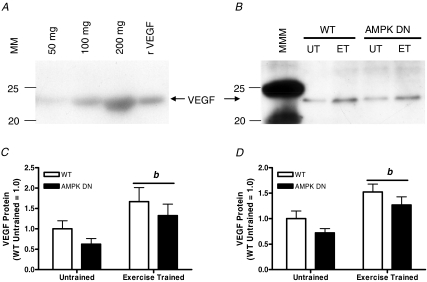
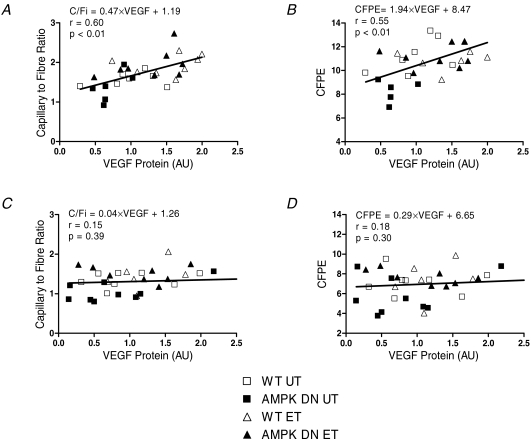
To investigate if AMPK regulates the VEGF response to exercise training, VEGF protein was measured from the gastrocnemius and plantaris muscles in UT and ET mice. Exercise increased VEGF protein in the gastrocnemius (Fig. 6C) and plantaris (Fig. 6D), independent of transgene. VEGF protein tended to be lower (P = 0.08) in the plantaris of AMPK DN compared to WT, independent of training. Linear regression was performed to identify the potential importance of VEGF protein in regulating skeletal muscle capillarization. In plantaris muscle, significant correlations between VEGF protein and C/Fi (Fig. 7A) and VEGF protein and CFPE (Fig. 7B) were identified. While in gastrocnemius muscle, there was no association between VEGF protein and C/Fi (Fig. 7C) or VEGF protein and CFPE (Fig. 7D). These findings suggest that VEGF is an important determinant of skeletal muscle capillarization in plantaris muscle.
Figure 6. VEGF protein expression in gastrocnemius and plantaris muscles of WT and AMPK DN mice at control (Untrained; UT) and after 28 days of voluntary wheel running (Exercise Trained; ET).
A, increasing amounts of protein from gastrocnemius muscle homogenates, as well as recombinant mouse VEGF (rVEGF), were pre-incubated with heparin beads and then a Western blot was performed under reducing conditions. B, representative Western blot for VEGF protein in gastrocnemius muscle homogenates. C and D, quantitative analysis of VEGF protein expression in gastrocnemius (C) and plantaris (D). VEGF protein was normalized to WT untrained for each muscle. Exercise training increased VEGF protein in WT and AMPK DN mice in the gastrocnemius and plantaris. In the plantaris, VEGF protein tended (P = 0.08) to be lower in AMPK DN compared to WT mice. Data are mean ±s.e.m. MM, molecular mass (kDa), MMM, molecular mass marker. WT untrained, n = 8; AMPK DN untrained, n = 9; WT exercise trained, n = 8; AMPK DN exercise trained, n = 10. b, main effect of exercise.
Figure 7. Relationship between VEGF protein and skeletal muscle capillarization in gastrocnemius and plantaris of untrained and exercise-trained WT and AMPK DN mice.
Greater VEGF protein is associated with greater capillary-to-fibre ratio (A) and greater capillary-to-fibre perimeter exchange index (B) in plantaris. VEGF protein is not associated with capillary-to-fibre ratio (C) or capillary-to-fibre perimeter exchange index (D) in gastrocnemius. UT, untrained; ET, exercise trained; CFPE, capillary-to-fibre perimeter exchange index; AU, arbitrary units.
Discussion
In the present study, a mouse model with inactive skeletal muscle AMPK was used to investigate the role of AMPK in the regulation of skeletal muscle angiogenesis and VEGF expression in response to exercise. The main finding of this investigation was that AMPK is not necessary for the angiogenic response to exercise, but is important for basal skeletal muscle capillarization and VEGF expression. Additionally, regulation of basal and exercise-induced VEGF mRNA in skeletal muscle is predominantly due to VEGF transcription and not mRNA stabilization.
AMPK and skeletal muscle capillarization
It has been proposed that the vascular supply is important for maintaining cellular energy homeostasis (Adair, 2005) and that skeletal muscle capillarization is, in turn, regulated by metabolic demand (Hepple et al. 1997; Frisbee, 2005). AMPK is a metabolic fuel sensor that alters substrate utilization during periods of energy imbalance such as occurs during exercise. Since (1) AICAR enhances the development of collateral circulation in response to ischaemia (Ouchi et al. 2005), and (2) inactivation of AMPK reduces resting skeletal muscle blood flow (an indication of lower vascularization) (Ouchi et al. 2005), we sought to determine if AMPK regulates the angiogenic response to exercise in skeletal muscle. Surprisingly, it was observed that inactivation of AMPK did not alter the angiogenic response to exercise. Taken together, these data suggest that the mechanisms responsible for physiological (exercise) and pathological (ischaemia) muscle angiogenesis are different and that unique intracellular signalling pathways may regulate these different responses.
While much remains to be discovered concerning the mechanisms that regulate skeletal muscle capillarization, in the last decade considerable evidence has demonstrated that VEGF is essential in determining skeletal muscle capillarization. It has been shown that blocking endogenous VEGF production reduces basal skeletal muscle capillarization 64% (Tang et al. 2004a) and inhibits skeletal muscle angiogenesis in response to exercise training (Wagner et al. 2005). Here, it was found that higher VEGF protein is associated with greater capillarization in plantaris muscle (Fig. 7A and B). In addition, VEGF mRNA and protein were approximately 20–30% lower in resting, control muscle of AMPK DN mice compared to WT mice, suggesting that AMPK is responsible in part for the regulation of resting VEGF expression. Consistent with VEGF being important for the regulation of skeletal muscle capillarization, our results demonstrate that inactivation of AMPK lowers basal skeletal muscle capillarization. Since the AMPK DN transgenic mice used in the current report are essentially devoid of AMPK activity in skeletal muscle (Mu et al. 2001), our data suggest an important role for AMPK in the regulation of basal VEGF expression and capillarization in skeletal muscle in vivo.
AMPK and VEGF regulation
Lee et al. (2003) were the first to demonstrate that AMPK regulates VEGF expression in cancer cells. It is well established that AICAR increases AMPK activity in skeletal muscle (Merrill et al. 1997; Musi et al. 2001). Furthermore, inhibition of AMPK completely abolishes AICAR-induced increases in VEGF mRNA in muscle cells in vitro (Ouchi et al. 2005). Consistent with these findings, AICAR increased VEGF mRNA in skeletal muscle of WT mice, but not in AMPK DN mice (Fig. 1A), thereby supporting the notion that AMPK is important for skeletal muscle VEGF expression in vivo.
Hypoxia and exercise increase both skeletal muscle VEGF expression (Minchenko et al. 1994; Breen et al. 1996; Gustafsson et al. 1999; Gavin et al. 2006) and AMPK activity (Winder & Hardie, 1996; Hutber et al. 1997; Vavvas et al. 1997; Hayashi et al. 2000; Gonzalez et al. 2004). It has been suggested that hypoxia may be responsible for increases in VEGF with acute exercise (Wagner, 2001). Therefore, we investigated AMPK regulation of the individual VEGF responses to systemic hypoxia and exercise in skeletal muscle. While hypoxia increased VEGF mRNA only in WT mice (Fig. 1B), VEGF transcription was reduced in both WT and AMPK DN mice (Fig. 3A). Given that hypoxia is thought to be responsible for increases in VEGF mRNA with exercise and based on our finding that inactivation of AMPK abolishes the VEGF mRNA response to hypoxia, we hypothesized that inactivation of AMPK would attenuate the VEGF mRNA response to exercise as well. Contrary to this hypothesis, it was observed that the VEGF mRNA response to acute exercise was greater in AMPK DN mice compared to WT mice (Fig. 2), suggesting that inhibition of AMPK augments the VEGF mRNA response to exercise. This response in AMPK DN mice may be a consequence of reduced capillarization resulting in diminished nutrient and oxygen delivery to exercising muscle. Additionally, exercise increased VEGF transcription in both WT and AMPK DN mice (Fig. 3A). To the best of our knowledge, this is the first study to report that: (1) acute exercise increases VEGF transcription in skeletal muscle; and (2) AMPK is not necessary for exercise-induced VEGF expression in skeletal muscle. While it is important to acknowledge that several other mechanisms activated by systemic exercise could be contributing to compensate for the lack of skeletal muscle AMPK activity in these animals, our data suggest that AMPK is not essential for the VEGF mRNA response to acute exercise.
The regulation of VEGF expression is thought to occur predominantly at the level of mRNA (Tang et al. 2004b), such that both transcription and mRNA stabilization may contribute to regulate VEGF mRNA in response to hypoxia and to exercise (Tang et al. 2002). In an attempt to further understand the regulation of VEGF mRNA in skeletal muscle, VEGF mRNA was plotted against VEGF transcription for gastrocnemius muscles of WT and AMPK DN mice at rest and in response to acute systemic hypoxia or to acute exercise (Fig. 3B). Interestingly, VEGF mRNA levels correlate with VEGF transcription rates in WT and AMPK DN mice for all conditions (P < 0.01), except for WT hypoxia. Consistent with others that report hypoxia-induced increases in VEGF expression are the results of mRNA stabilization (Dibbens et al. 1999; Ouchi et al. 2005); our data in skeletal muscle suggest that: (1) increased VEGF mRNA in response to acute systemic hypoxia is the result of mRNA stabilization and not increased transcription, and (2) hypoxia-induced stabilization of VEGF mRNA is AMPK dependent. Taken together, these data support our conclusions that VEGF expression at rest and in response to exercise is transcriptionally regulated, but in acute systemic hypoxia VEGF is regulated via mRNA stabilization.
Hypoxia-inducible factor-1 (HIF-1) is thought to be an important transcriptional regulator of VEGF expression in response to low oxygen stress. Ameln et al. (2005) demonstrated that exercise stabilizes and induces the nuclear translocation of HIF-1α and studies from cancer cells demonstrate that AMPK is necessary for HIF-1-dependent transcriptional activity of VEGF (Lee et al. 2003; Neurath et al. 2006). However, abolishing HIF-1α does not alter skeletal muscle VEGF mRNA levels (Mason et al. 2004). In fact, untrained HIF-1α null mice display a similar muscle phenotype to that of wild-type mice after exercise training (i.e. increased capillarization), a consequence the authors attributed to elevated AMPK activity in resting HIF-1α null muscle (Mason et al. 2007). Consistent with this, our results argue against the transcriptional regulation of VEGF via AMPK and HIF-1 in skeletal muscle in response to exercise and suggest that systemic hypoxia and systemic exercise function through different mechanisms to increase VEGF mRNA in skeletal muscle in vivo. It is well documented that VEGF is essential for the maintenance and expansion of skeletal muscle capillarization (Tang et al. 2004a; Wagner et al. 2005); however, taken together, these results question the importance of HIF-1α for exercise-induced VEGF expression and angiogenesis in skeletal muscle.
AMPK, exercise and fibre-type transitions
Voluntary wheel running is an appropriate model to investigate adaptations to exercise training as increases in skeletal muscle capillarization have been reported within 7 days of running in C57BL/6 mice (Waters et al. 2004). No significant differences were observed in: (1) speed or time to exhaustion with treadmill running or (2) distance or time over the 28 days of voluntary wheel running between WT and AMPK DN mice (Table 2); however, previous studies have reported decreased exercise tolerance and voluntary activity levels in AMPK DN mice (Mu et al. 2001, 2003). The authors partly attributed the exercise intolerance in AMPK DN mice to reduced muscle glycogen. While muscle glycogen was reduced ∼25% in AMPK DN mice (data not shown), in our hands, the AMPK DN mice displayed no significant exercise intolerance, despite having lower basal capillarization (an important determinant of 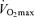 ; Ingjer, 1978).
; Ingjer, 1978).
Exercise training induces fibre-type transitions from a more glycolytic phenotype to a more oxidative phenotype. More specifically, type IIb fibres transition to type IIa fibres, but type IIa fibres do not typically transition to type I fibres with normal exercise training (Luginbuhl et al. 1984). Consistent with our data, Waters et al. (2004) reported significant fibre-type transitions with voluntary wheel running in plantaris muscle of C57BL/6 mice. Since the AMPK DN transgene is driven by the MCK promoter, which is preferentially active in fast, type II muscle fibres (Chin et al. 1998), we chose to analyse only the gastrocnemius and plantaris muscles for these studies. AMPK has been proposed as a major mediator of skeletal muscle plasticity (Nielsen et al. 2003). Röckl et al. (2007) indicated that AMPK is important for exercise-induced fibre-type transformations in triceps brachii muscle. We also observed fibre-type transitions to a more oxidative phenotype (IIb → IIa) with exercise training, but there was no difference in fibre-type transition between WT and AMPK DN. Interestingly, our results demonstrate that plantaris muscle of AMPK DN mice displays a faster, less oxidative phenotype in the untrained state, compared to WT mice. Chronic AICAR administration increases (Winder et al. 2000) and inactivation of AMPK decreases (Jorgensen et al. 2007) mitochondrial enzyme content in muscle. AMPK activates peroxisome proliferator-activated receptor (PPAR) and PPAR coactivator-1α (PGC-1α) (Lee et al. 2006), important factors for the control of mitochondrial biogenesis. It is possible that lack of AMPK activation of PPAR and PGC-1α may play a role in the faster, less oxidative phenotype in untrained plantaris muscle of AMPK DN mice. Our data suggest that AMPK is not necessary for exercise-induced fibre-type transitions, but does appear to be important for determining basal fibre-type composition. Consistent with this notion, Jorgensen et al. (2007) concluded that AMPK may not be essential for metabolic adaptations to exercise training, but AMPK is important for basal expression of mitochondrial enzymes.
The Rosenblatt technique is limited to the detection of type I, IIa and IIb fibres and does not identify IIx fibres. When mATPase histochemical and immunohistochemical identification of fibres types are compared, type IIx fibres are identified as IIa (Gorza, 1990). Thus, the findings in the current report may overstate the percentage of IIa fibres. It should be noted though that in mice, types IIa and IIx fibres demonstrate similar oxidative capacities as measured by succinate dehydrogenase (SDH) activity (Gorza, 1990) and thus the resultant phenotypes should be similar regardless of the identification of fibres as type IIa or IIx.
In conclusion, we have demonstrated that in skeletal muscle in vivo: (1) AMPK regulates the maintenance of basal capillarization, but not exercise-induced angiogenesis; (2) AMPK regulates basal VEGF expression; and (3) regulation of basal and exercise-induced VEGF mRNA is predominantly through the regulation of mRNA transcription and not stabilization. Taken together, these results suggest that AMPK plays an important role in basal VEGF expression, capillarization, and fibre-type composition; but AMPK is not necessary for the angiogenic responses to exercise training in skeletal muscle.
Acknowledgments
We would like to thank Morris Birnbaum, MD for providing the AMPK DN transgenic mice. We also wish to thank G. Lynis Dohm, PhD, Scott Gordon, PhD, and David Thomson, PhD for their intellectual and technical insight and Kathryn Verbanac, PhD, P. Darrell Neufer, PhD, Ed Tapscott, and Brandon Thompson for technical assistance. This research was supported by NIA AG-21891 (T.P.G.), AHA Mid-Atlantic 0465415U (T.P.G.), and Gatorade Sports Science Institute Graduate Student Grant (K.A.Z.).
References
- Adair TH. Growth regulation of the vascular system: an emerging role for adenosine. Am J Physiol Regul Integr Comp Physiol. 2005;289:R283–R296. doi: 10.1152/ajpregu.00840.2004. [DOI] [PubMed] [Google Scholar]
- Ameln H, Gustafsson T, Sundberg CJ, Okamoto K, Jansson E, Poellinger L, Makino Y. Physiological activation of hypoxia inducible factor-1 in human skeletal muscle. FASEB J. 2005;19:1009–1011. doi: 10.1096/fj.04-2304fje. [DOI] [PubMed] [Google Scholar]
- Breen EC, Johnson EC, Wagner H, Tseng HM, Sung LA, Wagner PD. Angiogenic growth factor mRNA responses in muscle to a single bout of exercise. J Appl Physiol. 1996;81:355–361. doi: 10.1152/jappl.1996.81.1.355. [DOI] [PubMed] [Google Scholar]
- Burkholder TJ, Fingado B, Baron S, Lieber RL. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J Morph. 1994;221:177–190. doi: 10.1002/jmor.1052210207. [DOI] [PubMed] [Google Scholar]
- Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM, Wu H, Zhu W, Bassel-Duby R, Williams RS. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 1998;12:2499–2509. doi: 10.1101/gad.12.16.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibbens JA, Miller DL, Damert A, Risau W, Vadas MA, Goodall GJ. Hypoxic regulation of vascular endothelial growth factor mRNA stability requires the cooperation of multiple RNA elements. Mol Biol Cell. 1999;10:907–919. doi: 10.1091/mbc.10.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med. 1999;77:527–543. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- Frisbee JC. Reduced nitric oxide bioavailability contributes to skeletal muscle microvessel rarefaction in the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol. 2005;289:R307–R316. doi: 10.1152/ajpregu.00114.2005. [DOI] [PubMed] [Google Scholar]
- Gavin TP, Robinson CB, Yeager RC, England JA, Nifong LW, Hickner RC. Angiogenic growth factor response to acute systemic exercise in human skeletal muscle. J Appl Physiol. 2004;96:19–24. doi: 10.1152/japplphysiol.00748.2003. [DOI] [PubMed] [Google Scholar]
- Gavin TP, Westerkamp LM, Zwetsloot KA. Soleus, plantaris and gastrocnemius VEGF mRNA responses to hypoxia and exercise are preserved in aged compared with young female C57BL/6 mice. Acta Physiol (Oxf) 2006;188:113–121. doi: 10.1111/j.1748-1716.2006.01609.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez AA, Kumar R, Mulligan JD, Davis AJ, Saupe KW. Effects of aging on cardiac and skeletal muscle AMPK activity: basal activity, allosteric activation, and response to in vivo hypoxemia in mice. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1270–R1275. doi: 10.1152/ajpregu.00409.2004. [DOI] [PubMed] [Google Scholar]
- Gorza L. Identification of a novel type 2 fiber population in mammalian skeletal muscle by combined use of histochemical myosin ATPase and anti-myosin monoclonal antibodies. J Histochem Cytochem. 1990;38:257–265. doi: 10.1177/38.2.2137154. [DOI] [PubMed] [Google Scholar]
- Gustafsson T, Puntschart A, Kaijser L, Sundberg CJ, Johnson E, Jansson E. Exercise-induced expression of angiogenic-related transcription and growth factors in human skeletal muscle. Am J Physiol Heart Circ Physiol. 1999;276:H679–H685. doi: 10.1152/ajpheart.1999.276.2.H679. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated protein kinase: a key system mediating metabolic responses to exercise. Med Sci Sports Exerc. 2004;36:28–34. doi: 10.1249/01.MSS.0000106171.38299.64. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Hirshman MF, Fujii N, Habinowski SA, Witters LA, Goodyear LJ. Metabolic stress and altered glucose transport: activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes. 2000;49:527–531. doi: 10.2337/diabetes.49.4.527. [DOI] [PubMed] [Google Scholar]
- Henin N, Vincent MF, Van Den Berghe G. Stimulation of rat liver AMP-activated protein kinase by AMP analogues. Biochim Biophys Acta. 1996;1290:197–203. doi: 10.1016/0304-4165(96)00021-9. [DOI] [PubMed] [Google Scholar]
-
Hepple RT, Mackinnon SL, Goodman JM, Thomas SG, Plyley MJ. Resistance and aerobic training in older men: effects on
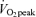 and the capillary supply to skeletal muscle. J Appl Physiol. 1997;82:1305–1310. doi: 10.1152/jappl.1997.82.4.1305. [DOI] [PubMed] [Google Scholar]
and the capillary supply to skeletal muscle. J Appl Physiol. 1997;82:1305–1310. doi: 10.1152/jappl.1997.82.4.1305. [DOI] [PubMed] [Google Scholar] - Hildebrandt AL, Neufer PD. Exercise attenuates the fasting-induced transcriptional activation of metabolic genes in skeletal muscle. Am J Physiol Endocrinol Metab. 2000;278:E1078–E1086. doi: 10.1152/ajpendo.2000.278.6.E1078. [DOI] [PubMed] [Google Scholar]
- Hutber CA, Hardie DG, Winder WW. Electrical stimulation inactivates muscle acetyl-CoA carboxylase and increases AMP-activated protein kinase. Am J Physiol Endocrinol Metab. 1997;272:E262–E266. doi: 10.1152/ajpendo.1997.272.2.E262. [DOI] [PubMed] [Google Scholar]
- Ingjer F. Maximal aerobic power related to the capillary supply of the quadriceps femoris muscle in man. Acta Physiol Scand. 1978;104:238–240. doi: 10.1111/j.1748-1716.1978.tb06273.x. [DOI] [PubMed] [Google Scholar]
- Jorgensen SB, Treebak JT, Viollet B, Schjerling P, Vaulont S, Wojtaszewski JF, Richter EA. Role of AMPKα2 in basal, training-, and AICAR-induced GLUT4, hexokinase II, and mitochondrial protein expression in mouse muscle. Am J Physiol Endocrinol Metab. 2007;292:E331–E339. doi: 10.1152/ajpendo.00243.2006. [DOI] [PubMed] [Google Scholar]
- Lee M, Hwang J-T, Lee H-J, Jung S-N, Kang I, Chi S-G, Kim S-S, Ha J. AMP-activated protein kinase activity is critical for hypoxia-inducible factor-1 transcriptional activity and its target gene expression under hypoxic conditions in DU145 cells. J Biol Chem. 2003;278:39653–39661. doi: 10.1074/jbc.M306104200. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Kim M, Park HS, Kim HS, Jeon MJ, Oh KS, Koh EH, Won JC, Kim MS, Oh GT, Yoon M, Lee KU, Park JY. AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARα and PGC-1. Biochem Biophys Res Commun. 2006;340:291–295. doi: 10.1016/j.bbrc.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Luginbuhl AJ, Dudley GA, Staron RS. Fiber type changes in rat skeletal muscle after intense interval training. Histochem Cell Biol. 1984;81:55–58. doi: 10.1007/BF00495401. [DOI] [PubMed] [Google Scholar]
- Mason SD, Howlett RA, Kim MJ, Olfert IM, Hogan MC, McNulty W, Hickey RP, Wagner PD, Kahn CR, Giordano FJ, Johnson RS. Loss of skeletal muscle HIF-1α results in altered exercise endurance. PLoS Biol. 2004;2:e288. doi: 10.1371/journal.pbio.0020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason SD, Rundqvist H, Papandreou I, Duh R, McNulty WJ, Howlett RA, Olfert IM, Sundberg CJ, Denko NC, Poellinger L, Johnson RS. HIF-1α in endurance training: suppression of oxidative metabolism. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2059–R2069. doi: 10.1152/ajpregu.00335.2007. [DOI] [PubMed] [Google Scholar]
- Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol Endocrinol Metab. 1997;273:E1107–E1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- Minchenko A, Bauer T, Salceda S, Caro J. Hypoxic stimulation of vascular endothelial growth factor expression in vitro and in vivo. Lab Invest. 1994;71:374–379. [PubMed] [Google Scholar]
- Mu J, Barton ER, Birnbaum MJ. Selective suppression of AMP-activated protein kinase in skeletal muscle: update on ‘lazy mice’. Biochem Soc Trans. 2003;31:236–241. doi: 10.1042/bst0310236. [DOI] [PubMed] [Google Scholar]
- Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- Musi N, Hayashi T, Fujii N, Hirshman MF, Witters LA, Goodyear LJ. AMP-activated protein kinase activity and glucose uptake in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2001;280:E677–E684. doi: 10.1152/ajpendo.2001.280.5.E677. [DOI] [PubMed] [Google Scholar]
- Neurath KM, Keough MP, Mikkelsen T, Claffey KP. AMP-dependent protein kinase alpha 2 isoform promotes hypoxia-induced VEGF expression in human glioblastoma. Glia. 2006;53:733–743. doi: 10.1002/glia.20326. [DOI] [PubMed] [Google Scholar]
- Nielsen JN, Mustard KJ, Graham DA, Yu H, MacDonald CS, Pilegaard H, Goodyear LJ, Hardie DG, Richter EA, Wojtaszewski JF. 5′-AMP-activated protein kinase activity and subunit expression in exercise-trained human skeletal muscle. J Appl Physiol. 2003;94:631–641. doi: 10.1152/japplphysiol.00642.2002. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Shibata R, Walsh K. AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ Res. 2005;96:838–846. doi: 10.1161/01.RES.0000163633.10240.3b. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Ordway GA, Saltin B, Neufer PD. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am J Physiol Endocrinol Metab. 2000;279:E806–E814. doi: 10.1152/ajpendo.2000.279.4.E806. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Wagner H, Mudaliar SR, Saucedo E, Henry R, Wagner PD. Exercise adaptation attenuates VEGF gene expression in human skeletal muscle. Am J Physiol Heart Circ Physiol. 2000;279:H772–H778. doi: 10.1152/ajpheart.2000.279.2.H772. [DOI] [PubMed] [Google Scholar]
- Röckl KS, Hirshman MF, Brandauer J, Fujii N, Witters LA, Goodyear LJ. Skeletal muscle adaptation to exercise training: AMP-activated protein kinase mediates muscle fiber type shift. Diabetes. 2007;56:2062–2069. doi: 10.2337/db07-0255. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JD, Kuzon WM, Jr, Plyley MJ, Pynn BR, McKee NH. A histochemical method for the simultaneous demonstration of capillaries and fiber type in skeletal muscle. Stain Technol. 1987;62:85–92. doi: 10.3109/10520298709107973. [DOI] [PubMed] [Google Scholar]
- Russell RR, 3rd, Bergeron R, Shulman GI, Young LH. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol Heart Circ Physiol. 1999;277:H643–H649. doi: 10.1152/ajpheart.1999.277.2.H643. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, McCarthy A, Smith D, Green KA, Grahame Hardie D, Ashworth A, Alessi DR. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 2005;24:1810–1820. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroka DM, Burkhardt T, Desbaillets I, Wenger RH, Neil DA, Bauer C, Gassmann M, Candinas D. HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J. 2001;15:2445–2453. doi: 10.1096/fj.01-0125com. [DOI] [PubMed] [Google Scholar]
- Tang K, Breen EC, Gerber H-P, Ferrara NMA, Wagner PD. Capillary regression in vascular endothelial growth factor-deficient skeletal muscle. Physiol Genomics. 2004a;18:63–69. doi: 10.1152/physiolgenomics.00023.2004. [DOI] [PubMed] [Google Scholar]
- Tang K, Breen EC, Wagner PD. Hu protein R-mediated posttranscriptional regulation of VEGF expression in rat gastrocnemius muscle. Am J Physiol Heart Circ Physiol. 2002;283:H1497–H1504. doi: 10.1152/ajpheart.00813.2001. [DOI] [PubMed] [Google Scholar]
- Tang K, Breen EC, Wagner H, Brutsaert TD, Gassmann M, Wagner PD. HIF and VEGF relationships in response to hypoxia and sciatic nerve stimulation in rat gastrocnemius. Respir Physiol Neurobiol. 2004b;144:71–80. doi: 10.1016/j.resp.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Vavvas D, Apazidis A, Saha AK, Gamble J, Patel A, Kemp BE, Witters LA, Ruderman NB. Contraction-induced changes in acetyl-CoA carboxylase and 5′-AMP-activated kinase in skeletal muscle. J Biol Chem. 1997;272:13255–13261. doi: 10.1074/jbc.272.20.13255. [DOI] [PubMed] [Google Scholar]
- Wagner PD. Skeletal muscle angiogenesis. Adv Exp Med Biol. 2001;502:21–38. A possible role for hypoxia. [PubMed] [Google Scholar]
- Wagner PD, Mark Olfert I, Tang K, Breen EC. Muscle-targeted deletion of VEGF and exercise capacity in mice. Respir Physiol Neurobiol. 2005;151:159–166. doi: 10.1016/j.resp.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Waters RE, Rotevatn S, Li P, Annex BH, Yan Z. Voluntary running induces fiber type-specific angiogenesis in mouse skeletal muscle. Am J Physiol Cell Physiol. 2004;287:C1342–C1348. doi: 10.1152/ajpcell.00247.2004. [DOI] [PubMed] [Google Scholar]
- Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol Endocrinol Metab. 1996;270:E299–E304. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol Endocrinol Metab. 1999;277:E1–E10. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]
- Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol. 2000;88:2219–2226. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]