Abstract
The readily releasable pool of vesicles (RRP) varies in size during synaptic activity and is replenished by recruitment from the reserve pool as well as vesicle retrieval after fusion. To investigate which of these steps is rate limiting in supplying vesicles to the RRP, we measured the effects of changes in temperature in cultured hippocampal neurons, where higher average rates of release can be maintained as the temperature is increased. Using a pHluorin-based reporter of exocytosis and endocytosis (sypHy), we find that changes in temperature between 25°C and 35°C do not significantly alter the rate of recruitment from the reserve pool. In contrast, the time constant of endocytosis fell from ∼17 s at 25°C to ∼10 s at 35°C (Q10= 1.7), while the time constant of vesicle reacidification fell from ∼5.5 s to ∼1 s (Q10= 5.5). A kinetic model of the vesicle cycle constructed using measured parameters was found to describe variations in vesicle release rate observed during long trains of spikes as well as recovery from synaptic depression after bursts of activity. These results indicate that endocytosis operating with time constants of 10–15 s is the rate-limiting process determining replenishment of the RRP during long-term activity. A fast mode of vesicle retrieval could not be detected at any temperature, nor was it necessary to invoke such a mechanism to account for use-dependent changes in synaptic release probability.
The strength of a synaptic connection depends on the number of vesicles available to fuse in response to an action potential (del Castillo & Katz, 1954). The size of this readily releasable pool (RRP) is dependent on several processes in the presynaptic terminal, including the recent history of vesicle release, recruitment from the reserve pool (Fernández-Alfonso & Ryan, 2004; Micheva & Smith, 2005; Gaffield et al. 2006) and priming for efficient fusion (Ashery et al. 2000; Nofal et al. 2007). On the longer term, it is essential that the store of vesicles within the terminal be maintained by retrieval from the surface followed by refilling with neurotransmitter (Heuser & Reese, 1973). It is not clear which of these steps in the vesicle cycle limits the rate at which the RRP is refilled during ongoing activity.
One model proposes that vesicles at the active zone are retrieved intact within ∼1 s of fusion by a process of ‘kiss-and-run’, after which they immediately dock again rather than mixing in with the reserve pool distributed further within the terminal (Pyle et al. 2000; Harata et al. 2006; He et al. 2006; Zhang et al. 2007). But the existence of ‘kiss-and-run’ has been difficult to establish and recent evidence indicates that the physiologically relevant mode of retrieval in hippocampal synapses is clathrin-mediated endocytosis (CME) operating with a time constant of ∼15 s at room temperature (Granseth et al. 2006; Balaji & Ryan, 2007; Chen et al. 2008). A number of observations also indicate that retrieval does not necessarily occur at the active zone: vesicular proteins are mobile in the surface of the bouton and axon after fusion (Sankaranarayanan & Ryan, 2000; Li & Murthy, 2001) and clathrin tracks these movements (Granseth et al. 2006). A second model proposes that the immediate source of vesicles replenishing the active zone is the reserve pool, which is itself maintained by endocytosis from the cell surface (Betz & Bewick, 1993; Ryan et al. 1993; Zucker & Regehr, 2002). But can this model account for variations in synaptic strength if endocytosis occurs on the time-scale of CME? It has been suggested that CME is too slow to account for the maintenance of the RRP (Pyle et al. 2000; Harata et al. 2001, 2006; Sara et al. 2002; He et al. 2006), but there has not so far been any quantitative justification of this idea.
An important aspect of this problem is the identity of the process that is rate limiting in supplying vesicles to the RRP during ongoing activity. Recent work indicates that this process is relatively temperature sensitive, because higher rates of vesicle release can be maintained at physiological temperature compared to room temperature (Pyott & Rosenmund, 2002; Yang et al. 2005; Klyachko & Stevens, 2006; Kushmerick et al. 2006). Vesicle retrieval from the cell surface becomes quicker when the temperature is increased (Fernández-Alfonso & Ryan, 2004; Micheva & Smith, 2005), but can this account for the higher rate of release? To answer this question, the temperature dependence of other steps in the vesicle cycle must also be assessed and integrated into a quantitative model. It is particularly important to understand how quickly vesicles are recruited from the reserve pool to the RRP, but different investigators have obtained opposing results: Fernández-Alfonso & Ryan (2004) report slower kinetics as the temperature is increased, while Micheva & Smith (2005) report faster.
To understand the processes controlling the supply and release of synaptic vesicles, we have measured the effects of temperature on individual steps in the vesicle cycle, and then on the performance of the whole cycle during ongoing activity. Using cultured hippocampal neurons, we demonstrate that vesicle endocytosis and subsequent reacidification are accelerated by increasing the temperature from 25°C to 35°C, while vesicle release probability and recruitment of reserve vesicles are not significantly affected. These results indicate that the supply of vesicles to the RRP is rate-limited by an endocytic mechanism operating with a time constant of 10–15 s when hippocampal synapses are highly active. Previous work indicates that this mechanism is clathrin-mediated endocytosis (Granseth et al. 2006). A faster mode of endocytosis was not observed at any temperature, nor was it necessary to invoke such a mechanism to account for changes in the size of the RRP during and after activity.
Methods
Primary cultures of hippocampal neurons
Pregnant Sprague–Dawley rats were anaesthetized by pentobarbital (50 mg kg−1 intraperitoneally) at gestational day 18, following which the rat was killed by cervical dislocation, in accordance with the guidelines laid down by the Home Office and as previously described by Granseth et al. (2006). Fetuses were removed and decapitated, and the hippocampi were dissected free. Cells were plated on 16 mm coverslips at a density of 200 000–250 000 cells ml−1 to obtain a low-density neuronal network growing on an astrocyte monolayer. Cells were transfected after 11 days in vitro using Lipofectamine 2000 in minimum essential medium (MEM) according to the manufacturer's instructions (Invitrogen, Paisley, UK).
Imaging synaptic activity
To monitor different steps in the vesicle cycle we used sypHy, a reporter in which the pH-sensitive super-ecliptic pHluorin is fused to the intravesicular side of the vesicle protein synaptophysin (Granseth et al. 2006). SypHy operates similarly to synaptopHluorin (Miesenböck et al. 1998), but provides improved signal-to-noise because it is localized better to synaptic vesicles reducing the background fluorescence. Neurons were imaged after 14 days in vitro using a Photometrics (Tucson, AZ, USA) Cascade 512B camera mounted on an inverted Nikon (Kawasaki, Japan) Diaphot 200 microscope with a × 40, 1.3 NA, oil immersion objective. Images were captured at a depth of 16-bits using IPLab (BD Biosciences, Rockville, MD, USA). Cells were superperfused with a pH 7.4 buffer containing (in mm): 136 NaCl, 2.5 KCl, 10 Hepes, 1.3 MgCl2, 10 glucose, 2 CaCl2, 0.01 CNQX and 0.05 dl-2-amino-5-phosphonovalerate (dl-APV). In the pH 5.3 buffer, Mes replaced Hepes. Chemicals were obtained from Sigma Aldrich (St Louis, MO, USA) and receptor antagonists from Tocris Cookson (Bristol, UK). Bafilomycin A was obtained from Calbiochem (Merck, Darmstadt, Germany). The temperature was regulated by a TC-344B temperature controller, acting on a heated PH-1 platform and SF-28 in-line solution heater (Warner Instruments, Hamden, CT, USA).
A 100 W xenon arc lamp was used for illumination. In order to minimize photo-bleaching the light was attenuated × 4–8 using neutral density filters, except for single action potential (AP) experiments that were performed without attenuation. A Uniblitz VMM-D3 shutter (Vincent Associates, Rochester, NY, USA) was used to restrict illumination to periods when the camera was actively acquiring images.
Action potentials (APs) were evoked by field stimulation (20 mA, 1 ms pulses) in a custom-built chamber with two parallel platinum wires 5 mm apart (Royle et al. 2008). Rapid changes in the superfusing buffer were performed with a VC-77SP fast-step laminar flow perfusion system (Warner Instruments). The temperature of the superfusing buffers was controlled by a custom-built coil heating-device fitted to the bath capillary.
Image sequences were imported to IgorPro (Wavemetrics, Lake Oswego, OR, USA) and analysed using custom-written scripts. Square regions of interests (ROIs) measuring 4.8 μm × 4.8 μm were positioned on synapses identified by a > 2 s.d. sypHy fluorescence increase to 40 APs at 20 Hz compared to baseline noise. To subtract the local background fluorescence the intensity of a ROI displaced in the x- or y-direction was used. Normalization of traces, when performed, used this corrected baseline for individual synapses prior to any averaging. All traces were visually inspected before being included in averages. In all graphs, error bars are ±s.e.m.
A kinetic model of the synaptic vesicle cycle
Vesicle cycling was modelled as coupled and sequential first-order differential equations as illustrated in Fig. 6A, using IgorPro. During continuous AP firing at 20 Hz the vesicle pools changed with time as:
| (1) |
| (2a) |
| (2b) |
| (3) |
| (4) |
The rate constants for the recruitment of vesicles from the reserve pool (krecr= 0.074 s−1 at 25°C; krecr= 0.079 s−1 at 35°C) were obtained experimentally (Fig. 4). We found that the model required a vesicle-priming step to appropriately describe the experimental data (Fig. 5). The rate constant for priming vesicles in the RRP (RRPn) to become release-ready vesicles (RRPp), was taken as kprim= 0.8 s−1 at 25°C according to measurements in chromaffin cells made by Ashery et al. (2000). This value was doubled for 35°C (kprim= 1.6 s−1) since Dinkelacker et al. (2000) report a temperature coefficient (Q10) of 2.3 for vesicles to achieve release competence in these cells. Rate constants of vesicle exocytosis during 20 Hz AP firing were calculated from experimentally obtained values for vesicular release probability (Prv) at the two temperatures (Fig. 3, kexo= 11.5 s−1 at 25°C; kexo= 8.6 s−1 at 35°C). Prv is equal to the mean number of vesicles released with 1 AP when a single vesicle is primed at the onset of the train (Hanse & Gustafsson, 2001). To minimize the number of parameters, the model does not include short-term enhancement of transmitter release, such as facilitation or augmentation, or asynchronous release. Experimentally obtained rate constants for endocytosis (kendo, Fig. 1) and reacidification (kreacid, Fig. 2) was used for 25°C (kendo= 0.057 s−1, kreac= 0.18 s−1) and 35°C (kendo= 0.097 s−1, kreac= 0.97 s−1). The newly endocytosed vesicles were assumed to mix at random to the reserve and the readily releasable pool with 9 vesicles out of 10 being caught in the reserve pool (Schikorski & Stevens, 2001; Rizzoli & Betz, 2004).
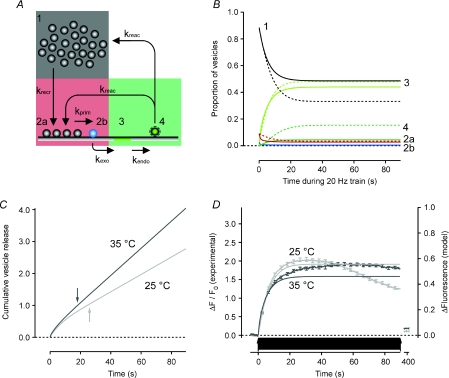
Figure 6. Expansion of the model to include endocytosis accounts for maintenance of the RRP during a train of APs at 20 Hz.
A, schematic illustration of the model. Vesicles are recruited from the reserve pool (1) to the RRP (2). Vesicles in the RRP become available for release after a priming reaction (2a and 2b) and are exocytosed to release transmitter (3) as in Fig. 5. Vesicle membrane at the surface is endocytosed (4) and will re-supply the RRP (10%) and the reserve pool (90%) after the pH gradient has been re-established. B, predictions of the model. Hatched lines are for 25°C (krecr= 0.074, kprim= 0.8, kexo= 11.5, kendo= 0.057 and kreac= 0.18 s−1) and continuous lines for 35°C (krecr= 0.079, kprim= 1.6, kexo= 8.6, kendo= 0.097 and kreac= 0.97 s−1). C, cumulative vesicle release rate at 25°C (light grey trace) and 35°C (dark grey trace) from the model. Note that the total pool of vesicles will be released several times at both temperatures. Arrows indicate time points when the total pool has been turned over once. D, predicted fluorescence intensity change (right axis) and measured sypHy fluorescence change (left axis) at 25°C (n = 430; light grey) and after 4 s at 35°C (n = 423; dark grey). Note that even though the steady state release at 35°C is higher than at 25°C the predicted fluorescence intensity is lower at the higher temperature, in line with the experimental data. Also note that the recorded fluorescence intensity at 25°C cannot maintain the expected steady state soon after the total vesicle pool has been turned over once (arrow in C).
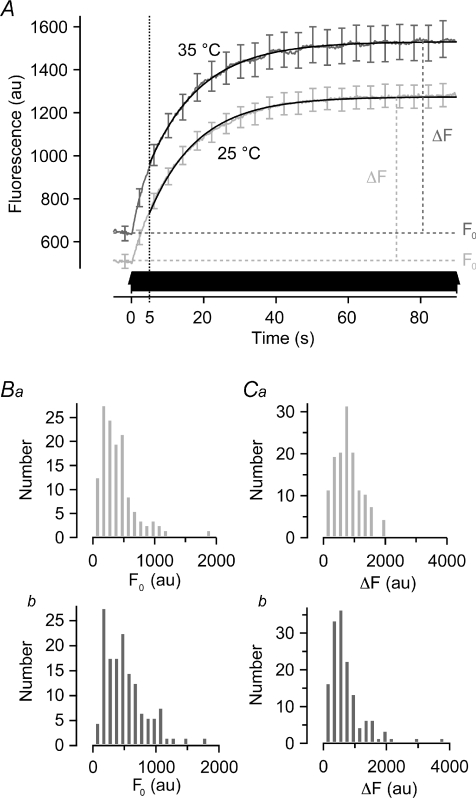
Figure 4. The time course of vesicle recruitment from the reserve pool.
A, reacidification was blocked by pre-incubation with bafilomycin (2.0 μm) and the first round of release measured in response to a train of 1800 APs at 20 Hz (arrows). Average fluorescence traces at 25°C (light grey, n = 145) and 35°C (dark grey, n = 161) could be described by exponential functions. Fits originate 5 s into the stimulation (hatched line), after release of the RRP. The time constant of recruitment and release was 13.5 ± 0.1 s at 25°C and 12.6 ± 1.5 s at 35°C. Error bars are s.e.m.B, histograms of baseline fluorescence intensity preceding stimulation (F0) at 25°C (Ba) and 35°C (Bb). No significant difference as judged by Kolmogorov–Smirnov test (P = 0.24). C, histograms of increase in fluorescence intensity from baseline to steady state during sustained AP firing (ΔF) at 25°C (Ca) and 35°C (Cb). No significant difference as judged by Kolmogorov–Smirnov test (P = 0.40).
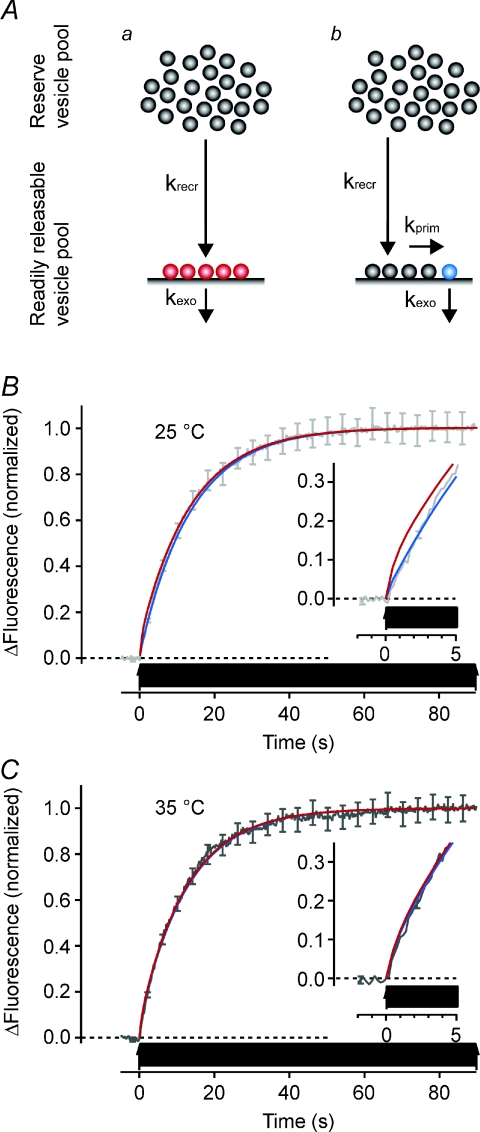
Figure 5. A simple model that uses measured parameters to account for the time course of release.
A, two models of the exocytic limb of the synaptic vesicle cycle where synaptic vesicles are recruited to the readily releasable pool (RRP) from the reserve pool with rate constant krecr. During AP firing at 20 Hz primed vesicles will be released with a rate constant kexo defined by the release probability (see text). Aa, all vesicles in the RRP are available for immediate release by an AP. Ab, a subset of the vesicles in the RRP are made available for release with priming rate constant kprim. B, model predictions at 25°C superimposed on recorded fluorescence trace normalized to a final intensity of 1. The parameter kexo is 2.0 s−1 when all vesicles in the RRP can be released (Aa, red trace) and 11.5 s−1 when priming is considered to be a factor (Ab, blue trace). krecr is 0.074 s−1 at both instances while kprim is 0.8 s−1. Inset shows the initial 5 s at a higher resolution. Note that the model without priming poorly describes how the fluorescence trace develops in the first 5 s. C, model predictions at 35°C with corresponding fluorescence trace. In the model with all RRP vesicles being releasable (Aa, red trace), kexo is 1.6 s−1 while in the model with priming (Ab, blue trace) kexo is 8.6 s−1, krecr is 0.079 s−1 and kprim is 1.6 s−1. Error bars are s.e.m.
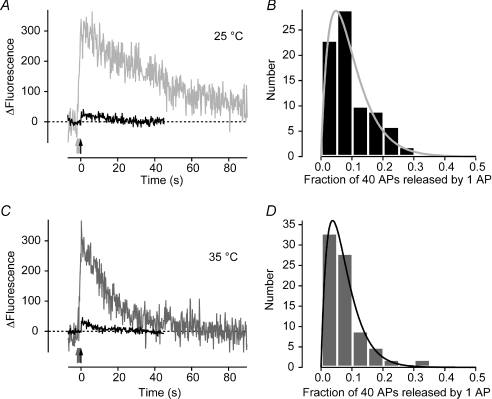
Figure 3. Release probability does not change significantly between 25° C and 35 °C.
A and B, fractional release at 25°C. Sample traces from a single synapse (A); the average response to a single AP is shown in black (n = 19) and the response to 40 APs at 20 Hz in grey. The ratio between the two peaks provides a measure of the intrinsic release probability of a vesicle in the readily releasable pool (RRP). Intrinsic release probabilities from 79 synapses are plotted in the histogram in B, the average value is 0.095 ± 0.008. Continuous grey line is a γ distribution with shape parameter constrained to 2, giving the scale parameter 0.048 ± 0.002. C and D, fractional release at 35°C. Sample traces are shown in C and histogram of intrinsic release probabilities in D. The average was 0.076 ± 0.008. Black line is a γ distribution with shape parameter constrained to 2 and scale parameter 0.037 ± 0.001. No significant difference between the two temperatures as judged by Kolmogorov–Smirnov test (P = 0.59).
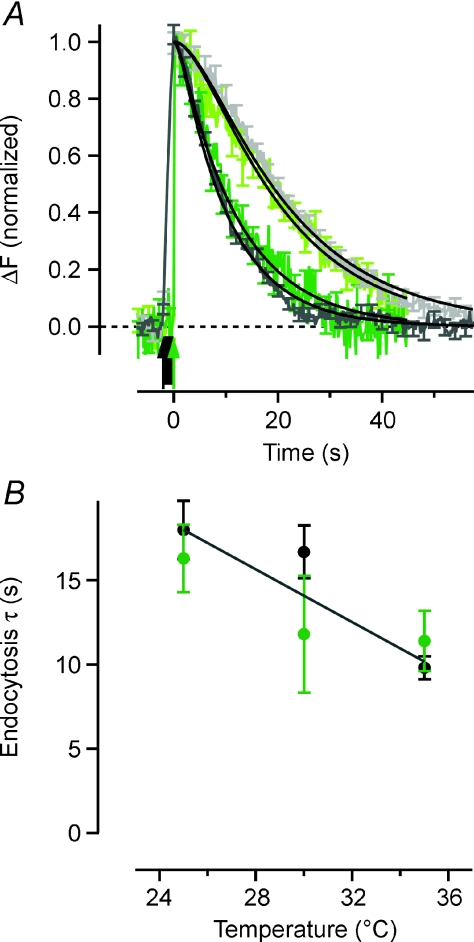
Figure 1. The temperature dependence of synaptic vesicle endocytosis.
A, averaged sypHy traces obtained in response to 40 action potentials (APs) delivered at 20 Hz or a single AP (arrows), normalized to the peak. Light grey trace is 40 APs at 25°C (n = 147) and dark grey is 40 APs at 35°C (n = 194). Light green trace is 1 AP at 25°C (n = 1480) and dark green is 1 AP at 35°C (n = 1512). Black lines are best fits of a model where synaptic vesicle endocytosis followed by reacidification is described by two sequential and irreversible reactions with first order kinetics (F(t) =F0[kr(1 – exp(−ket) –ke(1 – exp(−krt)]/(kr–ke)). With rate constants of reacidification (kr) of 0.18 s−1 at 25°C and 0.97 s−1 at 35°C, the rate constants of endocytosis (ke) are 0.056 ± 0.004 s−1 and 0.102 ± 0.007 s−1, respectively, for 40 APs and 0.070 ± 0.008 s−1 and 0.089 ± 0.012 s−1 for 1 AP. B, the temperature dependence of endocytosis plotted as time constants (τe= 1/ke). Black markers are for 40 APs and green markers for single APs. Black line is best-fit linear regression with slope –0.78 (Pearson's r2= 0.84).
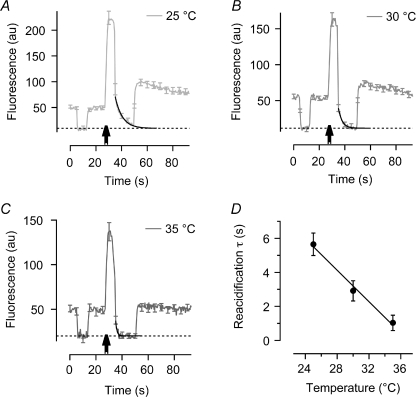
Figure 2. Vesicle acidification is strongly temperature dependent.
A–C, average fluorescence traces at 25°C (n = 91), 30°C (n = 83) and 35°C (n = 56). The extracellular buffer is changed twice from pH 7.4 to 5.3 and back, 12.5 s before and 5 s after 80 APs at 40 Hz (arrows). The first buffer change gives the baseline when the sypHy at the surface is quenched (hatched line). The second buffer change traps vesicles that have been endocytosed and are in the process of reacidifying. Exponential fits to the fluorescence decay of these trapped vesicles give the rate that the vesicular proton pumps restore the pH at the different temperatures (black lines). D, plotting time constants of reacidification over a temperature range from 25°C to 35°C. continuous black line is best-fit linear regression with slope of –0.45 (Pearson's r2= 0.94).
The initial conditions for the integration of the differential equations were based on a worst-case scenario where the total number of vesicles that can be recruited from the reserve pool is only 40 (Murthy & Stevens, 1999; Harata et al. 2001). The RRP has 5 vesicles (Schikorski & Stevens, 2001) where one is primed for release at the onset (Hanse & Gustafsson, 2001).
Without a priming step, for experimental conditions where endocytic recycling of vesicles did not affect the recordings (Fig. 4), the vesicle pools changed with time as:
| (1) |
| (2) |
| (3) |
Since all 5 vesicles in the RRP can be released kexo will be different (kexo= 2.0 s−1 at 25°C; kexo= 1.6 s−1 at 35°C). All other parameters are the same as for the model that had a priming step.
Results
We begin by measuring the effects of changes in temperature on key steps in the synaptic vesicle cycle in cultured hippocampal neurons. We then incorporate these measurements into a simple kinetic model and compare its predictions with changes in synaptic strength measured during and after activity that depletes the RRP.
Retrieval of synaptic vesicles is more efficient at physiological temperatures
To measure the speed of endocytosis at the synapse we used sypHy, a reporter in which the pH-sensitive super-ecliptic pHluorin is fused to the vesicle protein synaptophysin (Miesenböck et al. 1998; Granseth et al. 2006). When the vesicle fuses with the cell surface the loss of protons causes a 20-fold increase in fluorescence of the pHluorin, which becomes quenched again when the vesicle is internalized and reacidified (Sankaranarayanan & Ryan, 2000; Granseth et al. 2006; Voglmaier et al. 2006). Figure 1A compares sypHy responses to a train of 40 APs delivered at 20 Hz at 25°C (light grey trace) and 35°C (dark grey). The signal recovers to baseline more rapidly at the higher temperature, indicating an acceleration of endocytosis. To estimate the rate constant, one must take into account the fact that the fluorescence decay depends on two sequential processes: endocytosis of the vesicle followed by its reacidification by the proton pump (Atluri & Ryan, 2006). The continuous lines show fits of a model where each of these processes is irreversible and occurs with first order kinetics (Granseth et al. 2006). Using the rates of reacidification measured at these different temperatures (see below), the time constants of endocytosis (τendo) were estimated to be 17.9 ± 1.7 s (n = 147) at 25°C and 9.8 ± 0.7 s (n = 194) at 35°C.
When vesicle fusion was evoked by single APs, the rate of endocytosis was very similar to that observed after short trains (Fig. 1A): τendo= 16.4 ± 2.0 s at 25°C (n = 79; light green trace) and 11.4 ± 1.8 s at 35°C (n = 80; dark green). The rate of vesicle retrieval was therefore constant over a range of stimulus strengths, in agreement with recent reports (Granseth et al. 2006; Balaji & Ryan, 2007). We took the weighted means of the estimates from these two different stimuli to calculate the Q10: with τendo= 17.4 s at 25°C and 10.3 s at 35°C, the Q10 was 1.7. Notably, a single mechanism of vesicle retrieval was sufficient to account for the decline in fluorescence and a second, faster mechanism of retrieval could not be detected, strongly arguing against any significant component of ‘kiss-and-run’. Experiments using siRNA and over-expression of dominant negative constructs both indicate that the physiologically relevant retrieval process in hippocampal synapses is CME (Granseth et al. 2006), so the Q10 of 1.7 suggests that the rate-limiting step in CME has a relatively low activation energy. It has been proposed that this step might be diffusion of one or more key components involved in CME (Teng & Wilkinson, 2000; Fernández-Alfonso & Ryan, 2004), although this would be expected to display a Q10 of 1.3.
Reacidification of synaptic vesicles is strongly temperature dependent
The lumen of a synaptic vesicle (pH ∼5.5) is much more acidic than the cytoplasm (pH ∼7.2) and this pH gradient is created and maintained by a proton pump in the vesicle membrane (Harlos et al. 1984). This pump, a V-type ATPase, displayed a very strong temperature dependence when the speed of reacidification was measured using a ‘vesicle trapping’ approach (Atluri & Ryan, 2006; Granseth et al. 2006). An example record obtained at 25°C is shown in Fig. 2A. First, the pH of the extracellular buffer was reduced from pH 7.4 to 5.3 to obtain the baseline level of fluorescence when all sypHy on the surface is quenched (hatched line). After returning to pH 7.4, a stimulus of 80 APs at 40 Hz was delivered (arrowed) and 5 s later the pH was again returned to 5.3 to quench any vesicles still on the surface. The remaining fluorescence represents vesicles retrieved at pH 7.4 during the 5 s delay, and the quenching of this fluorescence could be described as a single exponential function (black line). Figure 2A–C shows that the rate at which the vesicular proton pump acidifies the vesicle interior accelerates as the temperature is increased from 25°C to 35°C. The time constant (τreac) was 5.65 ± 0.66 s (n = 91) at 25°C, falling to 1.03 ± 0.34 s (n = 56) at 35°C, revealing a Q10 of 5.5 (Fig. 2D).
The results in Fig. 2 demonstrate that vesicle acidification occurs in about 1 s at physiological temperatures, promptly providing the electrochemical gradient for the proton-driven transporters that concentrate neurotransmitter within the vesicle. This measure therefore provides an upper limit for the speed of refilling: the actual speed of refilling once the proton gradient is established remains to be determined (Bellocchio et al. 2000).
Vesicle release probability is weakly temperature dependent
Taken together, the results in Figs 1 and 2 indicate that the endocytic limb of the vesicle cycle is relatively strongly temperature dependent. We then went on to investigate the effects of temperature on the exocytic portion of the synaptic vesicle cycle. First, we looked for potential changes in release probability by averaging the sypHy responses to single APs (bold line in Fig. 3A). The peak amplitude (ΔF1AP) is directly proportional to the average number of vesicles released by each AP (m) at this synapse. The value of m might be obtained by dividing ΔF1AP by the quantal size (ΔFq) (del Castillo & Katz, 1954), but photo-bleaching prevents the exhaustive experiments required for reliable measurements of ΔFq at individual synapses. We therefore took an alternative approach based on the common approximation that a hippocampal synapse completely releases its store of readily releasable vesicles during a 40 AP train at 20 Hz (Murthy & Stevens, 1999; Schikorski & Stevens, 2001). Dividing the average response amplitude for a single AP with the amplitude attained with 40 APs (ΔFRRP; grey trace in Fig. 3A) gives the average intrinsic release probability for a vesicle in the RRP as Prv=ΔF1AP/ΔFRRP (Granseth et al. 2006). The distribution of Prv followed γ-function characteristics at both 25°C and 35°C (Fig. 3B and D) and the average at 35°C was 0.076 ± 0.007 (n = 80), a slight reduction compared to the value of 0.095 ± 0.008 (n = 79) at 25°C (although not statistically significant: P = 0.59, Kolmogorov–Smirnov test). The average number of vesicles in the RRP is 4.6 (Murthy & Stevens, 1999; Schikorski & Stevens, 2001), so we estimate of the mean number of vesicles released by an AP to be 0.35 ± 0.04 at 35°C and 0.44 ± 0.04 at 25°C.
Temperature has a weak effect on vesicle recruitment
The next aspect of exocytosis that we investigated was the recruitment of vesicles for release during ongoing activity. The RRP is consumed within about 2 s when a hippocampal synapse is stimulated at 20 Hz (Murthy & Stevens, 1999; Schikorski & Stevens, 2001), but exocytosis continues if the train is prolonged because new vesicles can be recruited from the reserve pool within the bouton to the RRP at the active zone (Ryan & Smith, 1995; Murthy & Stevens, 1999; Harata et al. 2001; Schikorski & Stevens, 2001; Gaffield et al. 2006). To investigate how temperature affects vesicle recruitment, we applied stimulus trains lasting 90 s (1800 APs, 20 Hz) and monitored the increase in sypHy fluorescence while reacidification was blocked by the proton pump inhibitor bafilomycin (2 μm). Under these conditions, vesicles remain fluorescent after they fuse with the cell surface, so the sypHy trace provides a cumulative record of vesicle fusion. This record is independent of endocytosis and any further rounds of release that a vesicle might undergo, and so reflects the exocytic limb of the vesicle cycle in isolation. In Fig. 4 we see that the maximum fluorescence intensity change (ΔF) was very similar at 25°C and 35°C (P = 0.40, Kolmogorov–Smirnov test), indicating that the total number of releasable vesicles was constant, in agreement with the measurements of Fernández-Alfonso & Ryan (2004).
The increase of sypHy fluorescence is the final step in a series of sequential reactions: an individual vesicle must be recruited before it is exocytosed and it is the slowest step that determines the rate of vesicle release during ongoing activity. It is clear that this rate-limiting step is the mobilization of vesicles from the reserve pool to the RRP (Ryan & Smith, 1995; Poskanzer & Davis, 2004; Micheva & Smith, 2005). When single exponential functions were fitted to the traces beginning 5 s after the start of the stimulus train, a point in time well beyond when the vesicles originally in the RRP have been consumed, the time constants were 13.5 ± 0.1 s (n = 181) at 25°C and 12.6 ± 1.5 s (n = 169) at 35°C. The accelerated recruitment at 35°C compared to 25°C was not statistically significant (P = 0.54, Student's t test). The speed of recruitment to the RRP therefore appeared not to be dependent on temperature in this range.
A kinetic model predicts an increased vesicle release rate with increased temperature
The results described above indicate that an increase in temperature from 25°C to 35°C significantly accelerates the endocytic limb of the vesicle cycle (retrieval and reacidification), but has very little effect on the exocytic limb (recruitment of vesicles from the reserve pool and release probability). To investigate the consequences of accelerated endocytosis during ongoing activity we constructed a simple kinetic model comprising exocytosis, endocytosis and refilling of the RRP during AP firing.
The model was built in two steps; first the exocytic part was tested against data obtained when sypHy was used to measure exocytosis independently of endocytosis (Fig. 5). Then the endocytic limb of the vesicle cycle was added (Fig. 6, below). The model comprises two populations of vesicles: the RRP and reserve pool (Fig. 5A). The relative proportions were estimated from sypHy traces monitoring exocytosis when vesicle reacidification was blocked with bafilomycin (Fig. 5B). Assuming that the RRP is released in the first 2 s of a 20 Hz train (Murthy & Stevens, 1999; Pyle et al. 2000; Schikorski & Stevens, 2001), we found that 11% of all releasable vesicles were in the RRP when the synapse was at rest (Fig. 5B and C), setting one of the initial conditions for testing the model. Two versions were subsequently tested against the data: one where all vesicles in the RRP can be exocytosed when an AP arrives at the presynaptic bouton (Fig. 5Aa) and another where only a subpopulation of the RRP is primed and release-competent (Fig. 5Ab). In both instances there are five vesicles in the RRP (Murthy & Stevens, 1999; Schikorski & Stevens, 2001) while in latter case only one of these are primed for release at the onset of an AP train (Hanse & Gustafsson, 2001).
During a train at 20 Hz, vesicles are released from the RRP with a rate constant kexo calculated from Prv, providing values of 2.0 s−1 and 1.6 s−1 at 25°C and 35°C if all vesicles in the RRP can be released, and 11.5 s−1 and 8.6 s−1 when a priming step is required. The rate constants for vesicle priming used for the modelling were kprim= 0.8 s−1 at 25°C and 1.6 s−1 at 25°C, which are measurements made in chromaffin cells (Ashery et al. 2000; Dinkelacker et al. 2000). Although the Ca2+-regulated release of catecholamines in these cells does not occur at morphologically defined synapses, chromaffin cells share much of the molecular machinery for vesicle fusion with neurons in the CNS (Neher, 2006). The practical reason for using these data is that the exocytic process in chromaffin cells has been dissected with exceptional detail, providing quantitative measurements that are not available for any classical synapse. In both models, with or without a priming step, the RRP receives new vesicles from the reserve pool with the previously measured rate constants krecr 0.074 s−1 at 25°C and 0.079 s−1 at 35°C. The version of the model in which all vesicles in the RRP are immediately available clearly exaggerates vesicle release during the first 5 s of AP firing at 25°C (Fig. 5B inset). We therefore made a slight modification of the model, incorporating a priming step preceding the release of vesicles from the RRP. Both schemes provided a good description of the increase in fluorescence elicited by a train of APs at 35°C (Fig. 5C).
Next, the endocytic limb of the vesicle cycle was added to the model with the rate constants of retrieval measured in Fig. 1 and vesicle reacidification measured in Fig. 2. The complete scheme is depicted in Fig. 6A with the reserve pool of vesicles in grey, the RRP in red and vesicles on the surface or retrieved but not yet acidified in green (this being the population that generates the increase in fluorescence measured with sypHy). Figure 6B shows the changes in the proportion of vesicles in these different states during a prolonged train at 20 Hz, with continuous lines depicting the prediction at 35°C and dashed lines at 25°C. The output from the synapse, plotted as the cumulative release of the total releasable vesicle pool is plotted in Fig. 6C. The steady-state release at 35°C corresponds to 4.3% of the total releasable pool per second, compared to 2.7% at 25°C. This is 1.9 vesicles s−1 and 1.2 vesicles s−1, respectively, under the model conditions with a total pool of 45 vesicles. At both temperatures this means that vesicle release is depressed; however, at 35°C the synapse can sustain 27% of the initial release rate while at 25°C it can only muster 14%. A similar degree of synaptic depression has been measured electrophysiologically in this type of synapse (Pyott & Rosenmund, 2002; Sara et al. 2002; Klyachko & Stevens, 2006). We conclude that vesicle retrieval with τendo= 10–15 s is sufficient to account for the supply of vesicles to the RRP during prolonged stimulation.
The model predictions for the sypHy fluorescence correspond well to data recorded during intact vesicle recycling at both temperatures (Fig. 6D) during the first 30 s of activity. At 25°C, however, the steady state is not maintained after the total releasable pool of vesicles has been turned over once (25 s, indicated by light grey arrow in Fig. 6C). The likely reason is that bulk retrieval of vesicle membrane slows the clathrin-mediated budding of synaptic vesicles at this temperature (Teng & Wilkinson, 2000; Voglmaier et al. 2006; Heerssen et al. 2008). The experimental measurements in Figs 1–5 and modelling in Fig. 6 together indicate that endocytosis is the rate-limiting step determining the temperature dependence of release during prolonged high-frequency stimulation.
Recovery from depletion
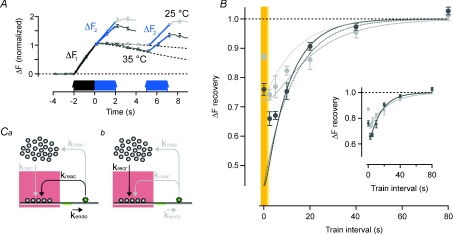
A key property of synapses is the ability to recover from synaptic depression caused by depletion of the RRP (Zucker & Regehr, 2002). We therefore investigated if the model could provide insights into the vesicular pathways active during and after short trains of APs delivered in pairs to probe paired-burst depression (PBD). First, the RRP was depleted by 40 APs at 20 Hz, and the initial rate of fluorescence increase was used to estimate the rate of vesicle release (ΔF1 in Fig. 7A). Then the same stimulus was applied after a variable delay, and the initial rate of fluorescence increase measured again (ΔF2, Fig. 7A). At short intervals, ΔF2 was depressed relative to ΔF1 due to depletion of the RRP. The magnitude of the depression measured 2.5 s after the first stimulus train was 26% at 25°C and 44% at 35°C, while recovery from depression occurred with time constants of 23 s and 15 s, respectively (Fig. 7B, inset).
Figure 7. The recovery of a partially depleted RRP parallels the kinetics of endocytic retrieval of vesicles.
A, traces are average fluorescence at 25°C (light grey, n = 351 and 381) and 35°C (dark grey n = 390 and 388). A first train of 40 APs at 20 Hz (black arrows) was used to estimate the RRP size. This stimulation will also deplete it of releasable vesicles. The recovery of the RRP was probed by a second 40 AP train at various time intervals to the first (blue arrows), traces for intervals of 0 and 5 s are shown. Vesicle release was estimated from linear curve fits to the rising fluorescence trace during stimulation (ΔF). Hatched lines are projected decay of fluorescence used for correction when the rising phase of the second stimulation was superimposed on the decaying part of the first. B, graph showing the time course of recovery of the RRP. Light grey is at 25°C and dark grey at 35°C. Each data point from 145 to 390 synapses. Note that y-axis starts from 0.45. Continuous lines are from a model where the RRP recovers from a depleted state at t = 0 s (26% at 25°C and 44% at 35°C, predicted from the model in Fig. 6B). The recovery of the RRP was modelled either with the endocytic pathway being the only source of vesicles (Ca, continuous lines) or recruitment of reserve vesicles being the only source (Cb, dotted lines). Data points from an uninterrupted 80 AP train are highlighted on a yellow background. Inset shows single exponential curve fits to the same data, time constants are 23.1 s at 25°C and 15.3 s at 35°C, respectively. C, schematic illustrations of model conditions during intervals without APs with the RRP being replenished by endocytosis (a) or recruitment from the reserve pool (b).
Why is recovery from depression accelerated at higher temperatures? The experiments in Figs 1–5 indicate that recruitment of vesicles from the reserve pool is not significantly dependent on temperature, and that the endocytic limb of the vesicle cycle is much more sensitive to temperature than the exocytic limb. It therefore seemed most likely that accelerated recovery from depression at higher temperatures reflected the acceleration of endocytosis. This idea was tested quantitatively using the models shown in Fig. 7C, the predictions of which are shown by the continuous lines in Fig. 7B (light grey 25°C and dark grey 35°C). We tested two alternative scenarios. In the first, the RRP is refilled by newly retrieved vesicles (Fig. 7Ca, plotted as continuous lines in Fig. 7B). In the second, vesicles are recruited from the reserve pool (Fig. 7Cb, plotted as dashed lines in Fig. 7B). Both models provide an adequate description of the basic time course of recovery from depression, although the assumption of direct recovery into the RRP provides a better description of recovery with longer intervals at both temperatures.
The models in Fig. 7C failed to account for a notable and surprising feature of PBD: the extent of depression was not immediately expressed at the maximum level, but gradually increased for a few seconds after a burst of 40 APs (Fig. 7B, data points highlighted on a yellow background). Which process is not accounted for by the models in Fig. 7C? The most obvious is asynchronous release of vesicles (Zucker & Regehr, 2002): the model assumes vesicles are only released while APs are fired. We did not incorporate asynchronous release into the model because of the lack of kinetic information about this process and because the objective was to make a quantitative test of the role of endocytosis. Short-term enhancement of transmitter release, such as facilitation, augmentation and potentiation, which is not included in the model either, could also be of some importance for reducing the PBD at this time-scale (Zucker & Regehr, 2002).
Recovery of the RRP was dependent on endocytosis
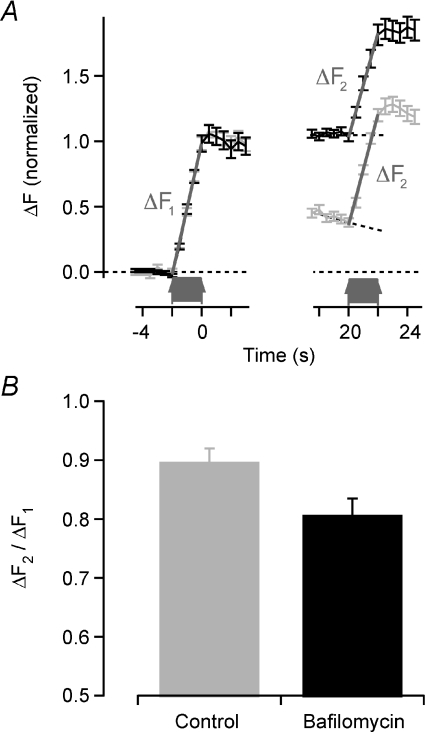
The results presented above indicate that replenishment of the RRP is dependent on endocytosis, even after relatively weak stimuli that release only a fraction of all vesicles in the bouton. To test further how far endocytosis contributed to recovery from depression we carried out experiments using the same dual-stimulation protocol as in Fig. 7, but in the presence of bafilomycin. This inhibitor of the proton pump causes all vesicles released by the first train of 40 APs to remain bright so that they do not contribute any further increase in fluorescence if exocytosed again during the second train (Fig. 8). PBD was measured at 25°C, when endocytosis occurs with a time constant of ∼15 s, and the second train was delivered at a delay of 20 s. Under control conditions, the RRP recovered by 89.0 ± 3.0%, but this fell to 80.7 ± 2.8% when the contribution of retrieved vesicles was blocked by bafilomycin, a difference that was significant at a confidence level of P < 0.05. In other words, vesicles retrieved after the first train of 40 APs contributed about 9% of the vesicles refilling the RRP. This result is consistent with two of the basic assumptions used in our model: retrieved vesicles mix randomly through the reserve and readily releasable pools, with 10% of these in the RRP.
Figure 8. Endocytic retrieval of synaptic vesicles is important for the recovery after 40 APs.
A, normalized fluorescence intensity for 93 synapses pre-incubated with medium containing 2 μm bafilomycin to block vesicle reacidification after endocytosis (grey) and 129 controls (black). All experiments performed at 25°C. The synapses were stimulated twice with 40 APs at 20 Hz with an interval of 20 s (arrows). The first train depletes the RRP and the second train probes its recovery during the interval. ΔF indicates linear curve fits to quantify the responses. Hatched lines are baselines used for correction of control responses being superimposed on the decaying fluorescence of the previous train. B, recovery of depression during 20 s in bafilomycin (black) and control (grey) conditions. Bafilomycin significantly reduced the extent of recovery (P = 0.044, Student's t test). Note that y-axis starts from 0.5. Error bars are s.e.m.
Discussion
Using the fluorescent vesicle probe sypHy in cultured hippocampal neurons, we investigated the temperature dependence of key events in the synaptic vesicle cycle. The recycling steps, endocytosis followed by reacidification, were significantly accelerated by increasing the temperature from 25°C to 35°C. In contrast, the vesicle release probability and recruitment from the reserve pool were very weakly dependent on temperature. The kinetic data we obtained were introduced into a model revealing that a single endocytic pathway can account for changes in synaptic strength caused by short and long bursts of activity and the modulation of these processes by changes in temperature. This pathway operated with τ= 10 s at 35°C, and is the likely molecular process limiting replenishment of the RRP during long-term activity. We could not measure a faster mechanism of retrieval, nor was it necessary to invoke such a mechanism to account for changes in synaptic strength reflecting changes in the number of readily releasable vesicles.
Vesicle release
We found that increasing temperature from 25°C to 35°C had little effect on Prv in hippocampal synapses: the mean value fell by about 20%, but this was not statistically significant (Fig. 3). These results are similar to those of Pyott & Rosenmund (2002), who found that an increase in temperature over this range reduced release probability in hippocampal synapses by about 30%. We do not believe that this estimate of the change in Prv is likely to be affected by estimating the RRP using a train of 40 APs at 20 Hz. At room temperature, this stimulus reliably releases about the same number of vesicles as are found morphologically docked to the active zone (Schikorski & Stevens, 2001). We cannot rule out the possibility that there may also be some recruitment of vesicles to the RRP during a 2 s train, but we do not believe that this is likely to pose a significant problem since recruitment from the reserve pool is barely accelerated by an increase in temperature, nor do we see any significant change in the total number of releasable vesicles (Fig. 4; see also Fernández-Alfonso & Ryan, 2004).
It might be expected that an increase in temperature would increase release probability because the calcium currents in neurons display a Q10 of ∼1.5 (van Lunteren et al. 1993). But an increase in temperature also causes the AP duration to become shorter and, at the calyx of Held, this leads to a net decrease in calcium entry measured at the presynaptic terminal (Borst & Sakmann, 1998). A small decrease in release probability with increasing temperature is therefore consistent with the effects on calcium influx triggered by an action potential.
Vesicle recruitment
The speed at which vesicles were recruited to the RRP from the reserve pool was little affected by temperature. If a reaction involving synapsin phosphorylation, liberating the vesicles from the cytoskeleton, was rate limiting, a Q10 closer to 2 than 1 would be expected (Llinás et al. 1991; Chi et al. 2003). The lack of temperature dependence is consistent with the random diffusion of the liberated vesicles to the active zone being the rate-limiting step. When studying the vesicle mobility at hippocampal synapses by tracking single vesicles stained with FM-dyes, the diffusion coefficient is very low (D = 0.0005 μm2 s−1, Jordan et al. 2005; Lemke & Klingauf, 2005). It will increase two- to fourfold in a subpopulation of vesicles when the synapses are stimulated (Lemke & Klingauf, 2005) but this is still far from the predicted value for free diffusion in aqueous medium (D = 0.15 μm2 s−1). The relatively low mobility of the vesicles at this type of synapse has been attributed to the liberated vesicles being hindered by vesicles still bound to the cytoskeleton (Gaffield et al. 2006).
Although the speed at which reserve vesicles are liberated from the cytoskeleton might increase with temperature, we and other investigators (Fernández-Alfonso & Ryan, 2004) do not see an increase in the total number of vesicles that can be recruited, and this might be related to the observation that the recruitment step itself is not strongly temperature dependent (Fig. 4). It is notable that Micheva & Smith (2005) have reached a different conclusion by photo-converting an FM-dye and electron microscopy. After increasing the temperature they saw an increase in both the total number of vesicles and speed of mobilization during intense AP firing. These different conclusions may reflect differences in methods for assessing vesicle release and recycling and perhaps the need for fixation before photo-conversion. Genetically encoded pHluorins have the advantage of allowing dynamic measurements of vesicle release and retrieval in living neurons.
Vesicle recycling
Previous studies have shown that vesicle recycling becomes more efficient when the temperature is increased (Fernández-Alfonso & Ryan, 2004; Micheva & Smith, 2005). The present study builds upon this knowledge and provides quantitative data on both the speed of endocytosis and the reacidification step that is essential for the vesicles to re-load with transmitter substance (Bellocchio et al. 2000). The speed of endocytosis that we measure here (τ= 10 s) is in close agreement with other studies identifying clathrin-mediated endocytosis at the synapse (Teng & Wilkinson, 2000; Evergren et al. 2004; Dickman et al. 2005; Jockusch et al. 2005; Granseth et al. 2006). CME has generally been considered to be ‘slow’, but the results we present demonstrate that it is sufficient to maintain vesicle release at a reasonable level at 25°C and even more efficiently when the temperature is increased to 35°C. CME is not, however, fast enough to prevent the accumulation of membrane at the surface of the bouton during prolonged AP firing at high rates (Fig. 6). It seems likely that bulk endocytosis occurs under these conditions (Takei et al. 1996; Voglmaier et al. 2006; Heerssen et al. 2008). Nevertheless, our results support a clathrin-mediated budding mechanism for the production of vesicles from the large endocytosed compartments being the rate-limiting step in the supply of vesicles to the RRP and reserve vesicle pool (Takei et al. 1996; Heerssen et al. 2008).
Acknowledgments
The authors are grateful to Dr Aude Derevier for help with cell cultures. B.G. was supported by a Career Development Fellowship from the MRC and the Swedish Research Council, and L.L. is supported by a Programme Grant from the Wellcome Trust.
References
- Ashery U, Varoqueaux F, Voets T, Betz A, Thakur P, Koch H, Neher E, Brose N, Rettig J. Munc13-1 acts as a priming factor for large dense-core vesicles in bovine chromaffin cells. EMBO J. 2000;19:3586–3596. doi: 10.1093/emboj/19.14.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atluri PP, Ryan TA. Kinetics of synaptic vesicle reacidification at hippocampal nerve terminals. J Neurosci. 2006;26:2313–2320. doi: 10.1523/JNEUROSCI.4425-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji J, Ryan TA. Single-vesicle imaging reveals that synaptic vesicle exocytosis and endocytosis are coupled by a single stochastic mode. Proc Natl Acad Sci U S A. 2007;104:20576–20581. doi: 10.1073/pnas.0707574105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocchio EE, Reimer RJ, Fremeau RTJ, Edwards RH. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;289:957–960. doi: 10.1126/science.289.5481.957. [DOI] [PubMed] [Google Scholar]
- Betz WJ, Bewick GS. Optical monitoring of transmitter release and synaptic vesicle recycling at the frog neuromuscular junction. J Physiol. 1993;460:287–309. doi: 10.1113/jphysiol.1993.sp019472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst JG, Sakmann B. Calcium current during a single action potential in a large presynaptic terminal of the rat brainstem. J Physiol. 1998;506:143–157. doi: 10.1111/j.1469-7793.1998.143bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Barg S, Almers W. Release of the styryl dyes from single synaptic vesicles in hippocampal neurons. J Neurosci. 2008;28:1894–1903. doi: 10.1523/JNEUROSCI.4518-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi P, Greengard P, Ryan TA. Synaptic vesicle mobilization is regulated by distinct synapsin I phosphorylation pathways at different frequencies. Neuron. 2003;38:69–78. doi: 10.1016/s0896-6273(03)00151-x. [DOI] [PubMed] [Google Scholar]
- del Castillo J, Katz B. Quantal components of the end-plate potential. J Physiol. 1954;124:560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman DK, Horne JA, Meinertzhagen IA, Schwartz TL. A slowed classical pathway rather than kiss-and-run mediates endocytosis at synapses lacking synaptojanin and endophilin. Cell. 2005;123:521–533. doi: 10.1016/j.cell.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Dinkelacker V, Voets T, Neher E, Moser T. The readily releasable pool of vesicles in chromaffin cells is replenished in a temperature dependent manner and transiently overfills at 37°C. J Neurosci. 2000;20:8377–8383. doi: 10.1523/JNEUROSCI.20-22-08377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evergren E, Marcucci M, Tomilin N, Löw P, Slepnev V, Andersson F, Gad H, Brodin L, De Camilli P, Shupliakov O. Amphiphysin is a component of clathrin coats formed during synaptic vesicle recycling at the lamprey giant synapse. Traffic. 2004;5:514–528. doi: 10.1111/j.1398-9219.2004.00198.x. [DOI] [PubMed] [Google Scholar]
- Fernández-Alfonso T, Ryan TA. The kinetics of synaptic vesicle pool depletion at CNS synaptic terminals. Neuron. 2004;41:943–953. doi: 10.1016/s0896-6273(04)00113-8. [DOI] [PubMed] [Google Scholar]
- Gaffield MA, Rizzoli SO, Betz WJ. Mobility of synaptic vesicles in different pools in resting and stimulated frog motor nerve terminals. Neuron. 2006;51:317–325. doi: 10.1016/j.neuron.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Granseth B, Odermatt B, Royle SJ, Lagnado L. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron. 2006;51:773–786. doi: 10.1016/j.neuron.2006.08.029. [DOI] [PubMed] [Google Scholar]
- Hanse E, Gustafsson B. Vesicle release probability and pre-primed pool at glutamatergic synapses in area CA1 of the rat neonatal hippocampus. J Physiol. 2001;531:481–493. doi: 10.1111/j.1469-7793.2001.0481i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harata N, Pyle JL, Aravanis AM, Mozhayeva M, Kavalali ET, Tsien RW. Limited numbers of recycling vesicles in small CNS nerve terminals: implications for neural signaling and vesicular cycling. Trends Neurosci. 2001;24:637–643. doi: 10.1016/s0166-2236(00)02030-0. [DOI] [PubMed] [Google Scholar]
- Harata NC, Choi S, Pyle JL, Aravanis AM, Tsien RW. Frequency-dependent kinetics and prevalence of kiss-and-run and reuse at hippocampal synapses studied with novel quenching methods. Neuron. 2006;49:243–256. doi: 10.1016/j.neuron.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Harlos P, Lee DA, Stadler H. Characterization of a Mg2+-ATPase and a proton pump in cholinergic synaptic vesicles from the electric organ of Torpedo marmorata. Eur J Biochem. 1984;144:441–446. doi: 10.1111/j.1432-1033.1984.tb08485.x. [DOI] [PubMed] [Google Scholar]
- He L, Wu XS, Mohan R, Wu LG. Two modes of fusion pore opening revealed by cell-attached recordings at a synapse. Nature. 2006;444:102–105. doi: 10.1038/nature05250. [DOI] [PubMed] [Google Scholar]
- Heerssen H, Fetter RD, Davis GW. Clathrin dependence of synaptic-vesicle formation at the Drosophila neuromuscular junction. Curr Biol. 2008;18:401–409. doi: 10.1016/j.cub.2008.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser JE, Reese TS. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockusch WJ, Praefcke GJ, McMahon HT, Lagnado L. Clathrin-dependent and clathrin-independent retrieval of synaptic vesicles in retinal bipolar cells. Neuron. 2005;46:869–878. doi: 10.1016/j.neuron.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Jordan R, Lemke EA, Klingauf J. Visualization of synaptic vesicle movement in intact synaptic boutons using fluorescence fluctuation spectroscopy. Biophys J. 2005;89:2091–2102. doi: 10.1529/biophysj.105.061663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyachko VA, Stevens CF. Temperature-dependent shift of balance among the components of short-term plasticity in hippocampal synapses. J Neurosci. 2006;26:6945–6957. doi: 10.1523/JNEUROSCI.1382-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick C, Renden R, von Gersdorff H. Physiological temperatures reduce the rate of vesicle pool depletion and short-term depression via an acceleration of vesicle recruitment. J Neurosci. 2006;26:1366–1377. doi: 10.1523/JNEUROSCI.3889-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke EA, Klingauf J. Single synaptic vesicle tracking in individual hippocampal boutons at rest and during synaptic activity. J Neurosci. 2005;23:11034–11044. doi: 10.1523/JNEUROSCI.2971-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Murthy VN. Visualizing postendocytic traffic of synaptic vesicles at hippocampal synapses. Neuron. 2001;31:593–605. doi: 10.1016/s0896-6273(01)00398-1. [DOI] [PubMed] [Google Scholar]
- Llinás R, Gruner JA, Sugimori M, McGuinness TL, Greengard P. Regulation by synapsin I and Ca2+-calmodulindependent protein kinase II of the transmitter release in squid giant synapse. J Physiol. 1991;436:257–282. doi: 10.1113/jphysiol.1991.sp018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheva KD, Smith SJ. Strong effects of subphysiological temperature on the function and plasticity of mammalian presynaptic terminals. J Neurosci. 2005;25:7481–7488. doi: 10.1523/JNEUROSCI.1801-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesenböck G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Stevens CF. Reversal of synaptic vesicle docking at central synapses. Nat Neurosci. 1999;2:503–507. doi: 10.1038/9149. [DOI] [PubMed] [Google Scholar]
- Neher E. A comparison between exocytic control mechanisms in adrenal chromaffin cells and a glutamatergic synapse. Pflugers Arch. 2006;453:261–268. doi: 10.1007/s00424-006-0143-9. [DOI] [PubMed] [Google Scholar]
- Nofal S, Becherer U, Hof D, Matti U, Rettig J. Primed vesicles can be distinguished from docked vesicles by analyzing their mobility. J Neurosci. 2007;27:1386–1395. doi: 10.1523/JNEUROSCI.4714-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskanzer KE, Davis GW. Mobilization and fusion of a non-recycling pool of synaptic vesicles under conditions of endocytic blockade. Neuropharmacology. 2004;47:714–723. doi: 10.1016/j.neuropharm.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Pyle JL, Kavalali ET, Piedras-Renteria ES, Tsien RW. Rapid reuse of readily releasable pool vesicles at hippocampal synapses. Neuron. 2000;28:221–231. doi: 10.1016/s0896-6273(00)00098-2. [DOI] [PubMed] [Google Scholar]
- Pyott SJ, Rosenmund C. The effects of temperature on vesicular supply and release in autaptic cultures of rat and mouse hippocampal neurons. J Physiol. 2002;539:523–535. doi: 10.1113/jphysiol.2001.013277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli SO, Betz WJ. The structural organization of the readily releasable pool of synaptic vesicles. Science. 2004;303:2037–2039. doi: 10.1126/science.1094682. [DOI] [PubMed] [Google Scholar]
- Royle SJ, Granseth B, Odermatt B, Derevier A, Lagnado L. Methods in Molecular Biology. Vol. 457. Totowa, NJ, USA: Humana Press; 2008. Imaging pHluorin-based probes at hippocampal synapses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan TA, Reuter H, Wendland B, Schweizer FE, Tsien RW, Smith SJ. The kinetics of synaptic vesicle recycling measured at single presynaptic boutons. Neuron. 1993;11:713–724. doi: 10.1016/0896-6273(93)90081-2. [DOI] [PubMed] [Google Scholar]
- Ryan TA, Smith SJ. Vesicle pool mobilization during action potential firing at hippocampal synapses. Neuron. 1995;14:983–989. doi: 10.1016/0896-6273(95)90336-4. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Ryan TA. Real-time measurements of vesicle-SNARE recycling in synapses of the central nervous system. Nat Cell Biol. 2000;2:197–204. doi: 10.1038/35008615. [DOI] [PubMed] [Google Scholar]
- Sara Y, Mozhayeva MG, Liu X, Kavalali ET. Fast vesicle recycling supports neurotransmission during sustained stimulation at hippocampal synapses. J Neurosci. 2002;22:1608–1617. doi: 10.1523/JNEUROSCI.22-05-01608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikorski T, Stevens CF. Morphological correlates of functionally defined synaptic vesicle populations. Nat Neurosci. 2001;4:391–395. doi: 10.1038/86042. [DOI] [PubMed] [Google Scholar]
- Takei K, Mundigl O, Daniell L, De Camilli P. The synaptic vesicle cycle: a single vesicle budding step involving clathrin and dynamin. J Cell Biol. 1996;133:1237–1250. doi: 10.1083/jcb.133.6.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng H, Wilkinson RS. Clathrin-mediated endocytosis near active zones in snake motor boutons. J Neurosci. 2000;20:7986–7993. doi: 10.1523/JNEUROSCI.20-21-07986.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lunteren E, Elmslie KS, Jones SW. Effects of temperature on calcium current of bullfrog sympathetic neurons. J Physiol. 1993;466:81–93. [PMC free article] [PubMed] [Google Scholar]
- Voglmaier SM, Kam K, Yang H, Fortin DL, Hua Z, Nicoll RA, Edwards RH. Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron. 2006;51:71–84. doi: 10.1016/j.neuron.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Yang XF, Ouyang Y, Kennedy BR, Rothman SM. Cooling blocks rat hippocampal neurotransmission by a presynaptic mechanism: observations using 2-photon microscopy. J Physiol. 2005;567:215–224. doi: 10.1113/jphysiol.2005.088948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Cao YQ, Tsien RW. Quantum dots provide an optical signal specific to full collapse fusion of synaptic vesicles. Proc Natl Acad Sci U S A. 2007;104:17843–17848. doi: 10.1073/pnas.0706906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]