Abstract
The hyperpolarization-activated current (Ih) is an inward current activated by hyperpolarization from the resting potential and is an important modulator of action potential firing frequency in many excitable cells. Four hyperpolarization-activated, cyclic nucleotide-modulated subunits, HCN1–4, can form Ih ion channels. In the present study we investigated the function of Ih in primary somatosensory neurons. Neuronal firing in response to current injection was promoted by elevating intracellular cAMP levels and inhibited by blockers of Ih, suggesting that Ih plays a critical role in modulating firing frequency. The properties of Ih in three size classes of sensory neurons were next investigated. In large neurons Ih was fast activating and insensitive to elevations in cAMP, consistent with expression of HCN1. Ih was ablated in most large neurons in HCN1−/− mice. In small neurons a slower activating, cAMP-sensitive Ih was observed, as expected for expression of HCN2 and/or HCN4. Consistent with this, Ih in small neurons was unchanged in HCN1−/− mice. In a neuropathic pain model HCN1−/− mice exhibited substantially less cold allodynia than wild-type littermates, suggesting an important role for HCN1 in neuropathic pain. This work shows that Ih is an important modulator of action potential generation in somatosensory neurons.
The intensity of sensation in all sensory systems is determined mainly by the frequency of action potential firing in afferent nerve fibres. This generalization also holds true for sensory afferents responding to painful stimuli, named nociceptors by Sherrington (1906). Nociceptors are unique amongst sensory receptors, though, in that the sensitivity of the nerve terminals to noxious stimuli is enhanced in inflammatory hyperalgesia, which is caused by the release of pro-inflammatory mediators such as bradykinin, nerve growth factor (NGF) and prostaglandin E2 (PGE2). Pro-inflammatory mediators increase the frequency of action potentials elicited by a noxious stimulus by two distinct mechanisms: by increasing the inward current activated by a noxious stimulus, or by increasing the frequency of action potentials elicited by a given inward current. We focus here on the second process.
Three mechanisms have been proposed to explain the modulation of action potential generation by pro-inflammatory mediators. The threshold for activation of the voltage-sensitive sodium channel NaV1.8 is shifted in the negative direction by phosphorylation by PKA and/or PKC (Gold et al. 1996;, 1998; England et al. 1996; Fitzgerald et al. 1999). This mechanism seems unlikely to offer a complete explanation of either inflammatory hyperalgesia or neuropathic pain, however, because in NaV1.8−/− mice inflammatory hyperalgesia is reduced but not abolished, and neuropathic pain is unaffected (Akopian et al. 1999; Kerr et al. 2001). Secondly, a voltage-sensitive K+ current is inhibited by activation of PKA (Nicol et al. 1997; Evans et al. 1999; Jiang et al. 2003). Finally, the voltage dependence of activation of the hyperpolarization-activated inward current, Ih, is shifted to less negative membrane potentials (Ingram & Williams, 1994, 1996).
Ih was first studied in cardiac pacemaker cells (DiFrancesco & Ojeda, 1980; DiFrancesco, 1993). Ih is activated by hyperpolarization from the resting potential, rather than by depolarization as for other ion channels. The activation range of Ih is shifted in the positive direction by a direct binding of adenosine 3′,5′-cyclic monophosphate (cAMP) to a cyclic-nucleotide binding region located in the C-terminal cytoplasmic domain (DiFrancesco & Tortora, 1991; Kaupp & Seifert, 2001), thereby increasing the inward current activated by hyperpolarization. Ih is also expressed in many neurons, where its presence can modulate the resting potential and the action potential firing frequency (Pape & McCormick, 1989; Pape, 1996; Ludwig et al. 2003; Nolan et al. 2003; Chan et al. 2004).
Four ion channel subunits responsible for Ih have been cloned and are termed hyperpolarization-activated, cyclic nucleotide-modulated (HCN1–4) channels (reviewed by Kaupp & Seifert, 2001). HCN channels expressed as homomers differ in two main respects: the activation time constants are in the order of HCN1 < HCN2 < HCN3 < HCN4; and HCN2 and HCN4 are strongly modulated by elevations in cAMP, with the midpoint of the voltage-activation curve shifted in the positive direction by 12–20 mV, whereas HCN1 and HCN3 channels show little sensitivity to cAMP. These differences can be used as a guide to determining the molecular makeup of Ih in dorsal root ganglion (DRG) neurons.
Here we studied the properties of Ih in primary sensory neurons, the role of Ih in the initiation of action potential firing, and the role of one isoform, HCN1, in inflammatory and neuropathic pain. Initial studies were carried out in sensory neurons from neonatal rat, and we then confirmed that Ih had similar properties in sensory neurons from adult mice. We found that Ih plays a critical role in the enhanced generation of action potentials caused by PGE2. We show that Ih has different properties in large non-nociceptive neurons, which express a fast, cAMP-insensitive Ih consistent with expression of HCN1, and in small nociceptive neurons, which express a slower, cAMP-sensitive Ih. Ih was found to be absent in large neurons from HCN1−/− mice. Finally, the role played by HCN1 in inflammatory and neuropathic pain in vivo was investigated by comparing thermal and mechanical thresholds in HCN1+/+ and HCN1−/− mice.
Methods
Animal experiments
All experiments performed on animals were carried out in accordance with the Animal Scientific Procedures Act of the United Kingdom (Section 1, revised 1986) and were monitored by the Home Office. Permission to carry out the procedures as described below was granted by an institutional ethical review committee.
Cell culture
Rat dorsal root ganglion (DRG) neurons
DRG neurons were isolated from neonatal Wistar rats (3–7 days old) killed by cervical dislocation followed by decapitation. DRG neurons were prepared as previously described (Cesare & McNaughton, 1996). Briefly, 20–30 ganglia were isolated from the full length of the spinal column following removal of the spinal cord. After incubation in collagenase (12.5 mg ml−1) for 1 h at 37°C, ganglia were mechanically triturated with fire-polished glass Pasteur pipettes. The cell suspension was centrifuged and re-suspended in Dulbecco's modified Eagle's medium (DMEM, Gibco) supplemented with 50 u ml−1 penicillin and 0.05 mg ml−1 streptomycin (Invitrogen), 1%l-glutamine (Invitrogen), 10% fetal bovine serum (FBS, Gibco), 50 ng ml−1 nerve growth factor (NGF, Promega) and 1.25 μg ml−1 cytosine β-d-arabinofuranoside (Ara-C, Sigma). DRG neurons were plated onto 35 mm Petri dishes previously coated with poly l-lysine (20 μg ml−1, Sigma). After 24 h the medium was replaced with DMEM supplemented with 1% N-2 supplement Invitrogen, 50 ng ml−1 NGF and 0.5 μg ml−1 Ara-C. All electrophysiology recordings were made within 48 h of dissociation. DRG neurons were classified based on diameter of somata as follows: small (< 25 μm), medium (25–35 μm) and large (> 35 μm).
Mouse DRG neurons
DRG neurons were isolated as above from HCN1+/+ and HCN1−/− adult mice killed by cervical dislocation and prepared using a standard enzymatic dissociation procedure. Briefly, ganglia were incubated in papain (20 units ml−1, Sigma) for 20 min at 37°C followed by collagenase/dispase (5 mg ml−1, Invitrogen, and 12 mg ml−1, Roche, respectively) for 20 min at 37°C. Ganglia were then mechanically triturated. The resultant cell suspension was layered onto 25% Percoll in L15 (Sigma) and was centrifuged at 1030 g for 8 min. Neurons were re-suspended in F12 media (Gibco) supplemented with 10% heat-inactivated horse serum (Invitrogen), 100 u ml−1 penicillin and 0.1 mg ml−1 streptomycin (Sigma) and 50 ng ml−1 NGF (Invitrogen). DRG neurons were plated onto 13 mm cover-slips previously coated with poly l-lysine (50 μg ml−1, Sigma) and laminin (30 μg ml−1, Invitrogen). All electrophysiological recordings were made within 48 h of dissociation. Mouse DRG neurons were classified based on diameter of somata as follows: small (< 20 μm), medium (20–30 μm) and large (> 30 μm).
Electrophysiology
Solutions
Extracellular solution contained (in mm): 140 NaCl, 1.8 CaCl2, 1 MgCl2, 4 KCl, 10 Hepes, 4 d(+)glucose, adjusted to pH 7.4 with NaOH, osmolarity 300–310 mosmol l−1. Pipettes were filled with an intracellular solution containing (in mm): 140 KCl, 1.6 MgCl2, 2 EGTA, 2.5 MgATP, 0.5 Li2GTP, 10 Hepes and adjusted to pH 7.3 with KOH, osmolarity 310–315 mosmol l−1. Cells were continuously perfused with extracellular solution using a gravity-fed perfusion system.
Current-clamp and voltage-clamp recordings
Current-clamp and voltage-clamp experiments were performed using an Axopatch 200B patch-clamp amplifier (MDS, CA, USA). Pipettes, resistance 3–6 MΩ, were pulled from Blaubrand 100 μl borosilicate glass micropipettes (Scientific Laboratory Supplies) using Brown-Flaming P-80 and P-97 horizontal micropipette pullers (Sutter Instruments) and in some instances were fire-polished to improve sealing. All experiments were begun in voltage-clamp mode. Pipette capacitance transients were cancelled while in cell-attached mode and whole-cell capacitance (10–40 pF) and series resistance (70–80%) were compensated once whole-cell mode had been attained. Pipette liquid junction potentials were calculated to be −3.7 mV using pCLAMP software and all values of membrane potential have been corrected using this value. Whole-cell membrane currents and voltages were filtered at 1 kHz and sampled at 10 kHz. Data were acquired and analysed using either pCLAMP 9.0 software (MDS, CA, USA) or Signal 2.16 (Cambridge Electronic Design). All recordings were made at 22–24°C.
In current-clamp experiments action potential firing in small rat DRG neurons was elicited by a series of depolarizing current pulses (5 s duration) from 0 to 200 pA in 50 pA step increments. Ih was identified in these neurons by the presence of voltage ‘sag’ of greater than 10% following injection of a hyperpolarizing current pulse of 500 pA (see Fig. 1A), and neurons expressing Ih were chosen for further testing. The fast current-clamp mode was employed in order to minimize artifacts which can be introduced into recordings of rapidly changing voltage signals (Magistretti et al. 1998). In the fast current-clamp mode the 10–90% step response rise time is 10 μs with cell parameters similar to those of small DRG neurons (Axopatch 200B manual). The most rapidly changing signal in the present experiments is the rising phase of the action potential, which in small DRG neurons has a rate of rise ∼40 V s−1 (see Fig. 1D). At the point of fastest rate of rise of the action potential, 40 mV positive to the resting potential, the maximum voltage error introduced by the 10 μs delay will be ca 1%. The error will be less for other more slowly changing voltage signals. We conclude that negligible distortion of the voltage signal will be introduced.
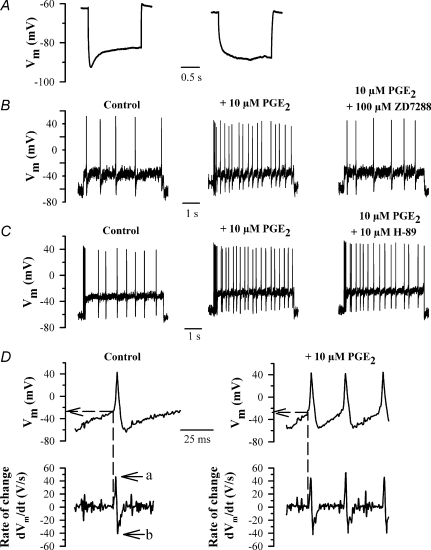
Figure 1. Enhancement by PGE2 of action potential firing frequency in small DRG neurons.
A, time-dependent rectification or voltage ‘sag’ of membrane potential in small (< 25 μm diameter) rat DRG neurons. Representative traces of membrane potential following injection of a 500 pA hyperpolarizing current pulse for 2 s, showing a neuron expressing Ih (left) or not expressing Ih (right). A neuron was considered to express Ih if the amplitude of the voltage sag exceeded 10% of the initial peak voltage excursion. B, representative traces showing action potential firing produced by a 100 pA depolarizing current in control conditions (left), in the presence of 10 μm PGE2 (centre), and in the presence of 10 μm PGE2 plus 100 μm ZD7288 (right). C, same as B but in control (left), in the presence of 10 μm PGE2 (centre) and in the presence of 10 μm PGE2 plus 10 μm H-89 (right). Data in B and C were obtained from two different neurons. D, upper panels: action potentials, taken from traces in B, at an expanded time scale, in control conditions (left) and in the presence of 10 μm PGE2 (right). Lower panels show the 1st derivative (dVm/dt) of the voltage trace shown above. Arrows show maximum rate of rise (‘a’), maximum rate of fall (‘b’).
Ih was studied in voltage-clamp recordings in rat and mouse DRG neurons using a series of depolarizing and hyperpolarizing step voltage pulses. Rat DRG neurons were subjected to voltage-clamp steps from a holding potential of −60 mV to between −20 and −160 mV for 2 s in 10 mV increments, followed by a final step to −160 mV for 1 s. A similar hyperpolarizing voltage protocol was used to activate Ih in mouse HCN1+/+ and HCN1−/− DRG neurons, except that mouse neurons were less able to withstand extreme hyperpolarization and so were pulsed to a maximum membrane potential of −140 mV.
Ih currents recorded in voltage-clamp mode were found to be contaminated by a rapidly decaying inward current (Scroggs et al. 1994; Yagi & Sumino, 1998; Komagiri & Kitamura, 2003). To remove this interfering current and to reveal ‘pure’Ih, a second series of identical voltage steps was imposed following inhibition of Ih using the fast-acting and reversible Ih blocker CsCl (5 mm) and these traces were then subtracted from the raw traces to give Cs-subtracted traces (Mo & Davis, 1997; Southan et al. 2000) (see Fig. 3). This procedure was adopted for all traces apart from those shown in Fig. 5.
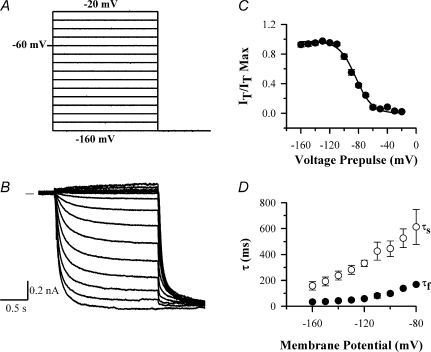
Figure 3. Voltage-clamp traces of Ih recorded from a large neuron using the caesium-subtraction technique.
A, voltage protocol; Ih was activated from a holding potential of −60 mV in 2 s pulses from −20 mV to −160 mV in 10 mV increments, followed by a final step to −160 mV. B, Cs-subtracted traces, following subtraction of current traces in which Ih had been completely inhibited by application of 5 mm CsCl. Zero current level shown by horizontal line in this and other traces. C, activation of Ih as a function of membrane voltage, obtained from the magnitude of the Ih tail current at the start of the −160 mV test potential. Trace fitted to points with parameters V½=−81.4 ± 1.3 mV, slope factor s = 11.6 ± 1.0 mV. D, time constants as a function of membrane voltage. Ih current traces were fitted with sum of two exponentials as described in Methods to obtain a fast (τf, •) and slow time constant (τs, ○).
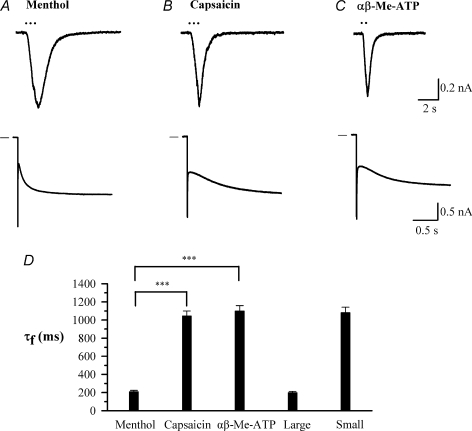
Figure 5. A subpopulation of small rat DRG neurons expresses TRPM8 and has fast-activating Ih.
A–C, sample inward currents activated by a 1 s exposure to menthol (50 μm), capsaicin (1 μm) and α,β-methyl ATP (αβ-Me-ATP; 10 μm) in three different small neurons (< 25 μm); and Ih activated in the same neurons by a voltage step from −60 mV to −90 mV. The cell responding to menthol did not respond to either capsaicin or αβ-Me-ATP. D, time constants of the fast component of Ih in each class of neuron compared with the overall time constants for large (> 35 μm) and small (< 25 μm) neurons. ***P < 0.001 for the comparisons indicated. Note that data in this figure were obtained without the use of the Cs-subtraction technique, and values of time constants are therefore not directly comparable with those given in other figures which were all obtained using Cs-subtraction.
We investigated whether the standard 2 s pulse was adequate to allow Ih current to reach steady state at all membrane potentials by comparing activation curves obtained with a variable-length pulse protocol (cf. Altomare et al. 2003) in which pulse lengths up to 20 s in length were imposed. For small HCN1+/+ mouse neurons, in which activation of Ih is slowest, the extended pulse length gave a less steep activation curve and a value of V½ shifted to more positive potentials by 6 mV. The extended pulse protocol was, however, protracted and its use was impractical when several activation curves needed to be determined in quick succession in different solutions. We also note that shifts in voltage-activation curves, a main focus of this paper, are much less affected than the absolute positions of voltage-activation curves by incomplete activation of Ih.
The voltage dependence of activation and therefore the mid-point potential (V½) of Ih was obtained from tail currents derived upon a final step to either −160 mV (rat) or −140 mV (mouse). Activation curves were fitted with a Boltzmann equation of the following form:
where Vm is the membrane potential of the prepulse, V½ is the membrane potential at which Ih is half-activated, k is the slope factor, It is the current amplitude of the tail current recorded for a given prepulse and It(max) is the maximum current amplitude of the tail current. Values of V½ were found to stabilize within 5 min of attaining whole-cell mode and all recordings were made after at least this interval. The rate of channel activation was found to be well fitted in all cases with a double exponential function (Santoro et al. 2000). Time constants were determined by fitting currents using pCLAMP or Signal software with a double-exponential function of the form:
where Ih(t) is the amplitude of the current at time t and Af and As are the initial amplitudes of the fast (τf) and slow (τs) activation time constant components, respectively.
Generation of HCN1−/− mice
HCN1 conditional knockout mice were generated by homologous recombination in embryonic stem cells. This work was carried out for us by Lexicon Pharmaceuticals, Inc. Exon1, encoding the cytoplasmic amino tail of the HCN1 protein, was flanked with LoxP sites (see Fig. S1A in online Supplemental material). A phosphoglycerate kinase (PGK) promoter-driven neomycin resistance (Neo) gene was inserted between exon1 and the downstream LoxP site. Homologous recombination was confirmed by Southern blot analysis (Supplemental Fig. S1B). Using a 3′ external probe, a 20 kb EcoR I fragment could be detected in wild-type mice, whereas mice carrying both wild-type and the targeted allele gave rise to the expected 20 kb and 7 kb fragments. HCN1 knockout mice (HCN1−/−) were subsequently generated by mating mice carrying the LoxP-targeted allele (flox mice) with a global deleter strain, expressing Cre under the cytomegalovirus (CMV) promoter (Jackson Labs). Deletion of exon1 was confirmed by PCR on genomic DNA from tail biopsies (Supplemental Fig. S1B). HCN1−/− mice were viable, fertile and obtained in the expected Mendelian ratios. To confirm the absence of HCN1 mRNA, RT-PCR experiments were performed on RNA isolated from a range of tissues from both knockout and wild-type littermate mice. Primers spanning exon1–2, 2–3 and 4–5 were used to detect HCN1 mRNA. Whereas in wild-type mice HCN1 transcripts were present in brain, eye, tongue and heart (Supplemental Fig. S2B; only RT-PCR exon2–3 is shown), no transcripts could be detected in tissues of the HCN1−/− mice. An N-terminal HCN1 antibody detected a 96 kDa protein in wild-type brain and spleen (not in heart, eye and tongue) that was not present in the HCN1−/− protein samples (Supplemental Fig. S2A).
For both electrophysiological and behavioural experiments heterozygous HCN1+/− mice were produced by back-crossing onto the parental strain for six generations and were mated. HCN1+/+ and HCN1−/− littermates were identified from PCR on genomic DNA from tail biopsies and were used for experiments.
Behavioural studies
Animals were allowed free access to food and water and were kept in a 12 h on, 12 h off light–dark cycle. All behavioural experiments were performed in a temperature-controlled environment (24 ± 1°C) with humidity of 45–55%. Adult mice of both sexes were used, weighing between 20 and 30 g and age matched. In all cases mice were given 1 h to habituate to the test environment before experimentation commenced.
Inflammatory pain
Inflammatory pain was induced by the administration of 0.75 nmol PGE2 in 20 μl saline solution into the plantar surface of the hind paw. Saline was injected in control animals. Latencies of paw withdrawal from a mechanical and heat stimulus (see below) were recorded at 0.5, 1, 2 and 24 h after injection.
Partial sciatic nerve ligation (Seltzer model)
Partial sciatic nerve ligation (PNL) was performed under isoflurane anaesthesia (administered at 3% and maintained at 2%). We followed the description of the Seltzer model (Seltzer et al. 1990) as first described in mice (Malmberg & Basbaum, 1998). Briefly, the sciatic nerve was exposed at the upper thigh level. The dorsal third to half of the sciatic nerve was tightly ligated at a site distal to nerve trifurcation with a 5-0 black nylon suture (Harvard Apparatus). In sham-operated mice, the nerve was exposed but not ligated.
Mechanical
Response to mechanical stimuli was determined using a dynamic plantar aesthesiometer (Ugo Basile, Comerio, Italy). Mice were placed on a wire mesh floor in individual compartments. A 2.5 g force was applied to the plantar surface of the hind paw and latency of paw withdrawal was recorded. Mice were tested 3 times with 3 min intervals between each test.
Heat
Response to noxious heat stimuli was determined using Hargreaves’ plantar test (Ugo Basile, Comerio, Italy). Mice were placed on a glass floor in individual boxes. A radiant heat source was applied to the plantar surface of the hind paw and the latency of paw withdrawal was recorded. Mice were tested 3 times with 3 min intervals between each test.
Cold
Cold allodynia was determined using the acetone drop technique as first described by Carlton et al. (1994) and Choi et al. (1994). A 10 μl drop of acetone was applied to the plantar surface of the hind paw by forming a drop at the end of a polyethylene tube attached to a syringe. The number of flinches and licks within a 1 min period was recorded. Mice were tested 5 times with a 3 min interval between each test. Mean number of flinches and licks was calculated.
Data analysis
All data shown are mean ±s.e.m. Tests for statistical differences were carried out using either paired or unpaired Student's t tests or one-way ANOVA, as appropriate. The criterion for statistical significance was a value of P < 0.05.
Results
Role of Ih in action potential firing
We initially investigated the role of Ih in the modulation of action potential firing in response to a constant-current stimulus in neonatal rat DRG neurons. We focused on small neurons (< 25 μm cell body diameter), of which the majority are nociceptors (Fang et al. 2005). We identified neurons expressing Ih by the presence of a voltage ‘sag’ in the membrane potential following a hyperpolarizing current step (see Fig. 1A). Ih was identified in 43/92 small rat neurons on this criterion, or 46.7%, in good agreement with the proportion identified as expressing Ih in voltage-clamp experiments (see below). Action potential firing was evoked using 5 s depolarizing current pulses. In all but one of the 43 small DRG neurons exhibiting Ih we observed repetitive action potential firing in response to current injection (Fig. 1B). The action potential frequency increased monotonically with increasing stimulus strength (Fig. 2A). By contrast, all large neurons (> 35 μm, 10/10) exhibited Ih, but fired only a single action potential at all strengths of injected current.
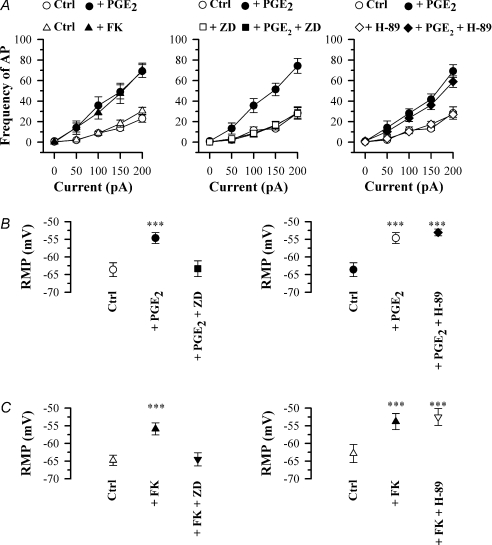
Figure 2. Effect of PGE2, forskolin (FK), ZD7288 (ZD) and H-89 on action potential firing in small (< 25 μm) rat DRG neurons.
A, frequency of AP firing in response to increasing injected current strength in the following conditions: left panel, control conditions (○) and in 10 μm PGE2 (•, n = 19), or (in a separate series of experiments) in control (Δ) and in 50 μm forskolin (FK) (▲, n = 11); centre panel, control (○), 10 μm PGE2 (•), 100 μm ZD7288 (□), 10 μm PGE2 and 100 μm ZD7288 (▪, n = 13); right panel, control (○), 10 μm PGE2 (•),10 μm H-89 (◊), 10 μm PGE2 and 10 μm H-89 (♦, n = 6). B, effect on mean resting membrane potential (RMP) of 10 μm PGE2 alone and with 100 μm ZD7288 (left panels, n = 6) and alone and with 10 μm H-89 (right panels, n = 5). C, similar series of experiments in which 50 μm forskolin (FK) was used in place of PGE2 (left panels, n = 6; right panels, n = 5). Significance levels compared to control: ***P < 0.001.
The physiological pro-inflammatory mediator PGE2 has been shown to sensitize nociceptive neurons by increasing their excitability (Gold et al. 1996; England et al. 1996), an effect which depends on increases in cAMP (Lopshire & Nicol, 1998). We confirmed that exposure of small Ih-expressing DRG neurons to PGE2 (10 μm, 4 min) enhanced their excitability, as shown by an increase in action potential frequency elicited by a current pulse (Fig. 1B). The increase in firing frequency was present over the whole range of test stimuli used (Fig. 2A), and can be attributed to a significantly increased rate of depolarization in the pacemaker region between action potentials (see Fig. 1D). We also observed a significant depolarizing shift in the resting membrane potential, from −63.6 ± 2.0 to −54.7 ± 1.5 mV (P < 0.001, n = 19; Fig. 2B). The adenylate cyclase activator forskolin (50 μm) increased action potential frequency in a similar manner to PGE2 (Fig. 2A) and also depolarized the resting membrane potential, from −64.8 ± 1.4 to −55.9 ± 1.7 mV (P < 0.001, n = 11; Fig. 2C). In contrast, in large neurons (> 35 μm, 10/10) application of the pro-inflammatory mediator PGE2 or elevating cAMP levels using forskolin failed to have any effect on action potential firing, and nor was there any effect on resting membrane potential (−62.4 ± 1.7 mV in the absence of PGE2 and −63.4 ± 1.5 mV in the presence of PGE2, 10 μm, 4 min; n = 10, P > 0.05).
If PGE2 acts to cause sensitization of nociceptors through a lowering of the voltage threshold or an increase in the amplitude of NaV1.8 then an effect on the action potential threshold or the rate of rise of the action potential (respectively) would be expected. In a similar way, an inhibition of voltage-dependent potassium currents by PGE2 (Nicol et al. 1997) should cause a decrease in the rate of fall of the repolarization phase of the action potential. We estimated these parameters by calculating the first derivative of membrane potential, dVm/dt (Fig. 1D). The value of dVm/dt is positive during the interval between action potentials, and abruptly increases as the action potential upstroke begins (see Fig. 1D). We estimated the action potential threshold from the membrane potential at the moment when dVm/dt attains its minimum value immediately before the action potential upstroke (dashed lines in Fig. 1D). Prior to exposure to PGE2 the action potential threshold was −34.7 ± 1.4 mV, while after PGE2 application it was −33.8 ± 1.5 mV (P > 0.05, difference not significant, n = 19; Table 1). Forskolin also failed to have any significant effect on action potential threshold (Table 1). Furthermore, neither the maximum rate of rise nor the maximum rate of fall of the action potential (‘a’ and ‘b’ in Fig. 1D) was significantly affected by either PGE2 or forskolin (Table 1). These results argue against a major effect of increases in cAMP on either the voltage-dependent Na+ or K+ currents.
Table 1.
Effect of PGE2 (10 μm), forskolin (50 μm), ZD7288 (100 μm) and H-89 (10 μm) on parameters of action potentials in small rat DRG neurons (see Figs 1 and 2)
| Condition | AP threshold (mV) | Rate of rise dVm/dt (V s−1) | Rate of fall dVm/dt (V s−1) |
|---|---|---|---|
| Ctrl | −34.7 ± 1.4 | 41.4 ± 0.7 | −41.2 ± 2.0 |
| + PGE2 | −33.8 ± 1.5 | 41.9 ± 2.5 | −40.3 ± 0.6 |
| + PGE2+ ZD7288 | −34.1 ± 1.3 | 41.9 ± 0.9 | −40.6 ± 1.4 |
| + PGE2+ H-89 | −34.1 ± 0.7 | 41.1 ± 1.2 | −41.2 ± 0.7 |
| Ctrl | −33.9 ± 0.8 | 42.2 ± 1.4 | −42.4 ± 0.9 |
| + FK | −33.3 ± 0.5 | 43.0 ± 1.8 | −41.3 ± 1.3 |
| + FK + ZD7288 | −33.8 ± 0.9 | 42.2 ± 1.4 | −41.8 ± 0.8 |
| + FK + H-89 | −34.5 ± 1.2 | 41.9 ± 2.7 | −40.6 ± 1.4 |
None of the three parameters in the table was significantly affected by any of these treatments. Number of neurons n = 5–6 for all measurements.
Figure 1B shows that the effect of PGE2 on neuronal excitability was completely reversed by the specific Ih blocker ZD7288 (Harris & Constanti, 1995). Over the whole range of currents tested, ZD7288 reversed the enhancement in action potential frequency to a level not significantly different from that before application of PGE2 (Fig. 2A). Furthermore, the resting membrane potential was hyperpolarized by ZD7288 from −54.7 ± 1.5 to −64.5 ± 1.9 mV (n = 13), the latter value being not significantly different from the resting potential before application of PGE2 (Fig. 2B). ZD7288 also caused a similar inhibition of the enhanced excitability observed when adenylate cyclase was activated directly with forskolin (not shown), and also reversed the depolarization of resting membrane potential seen following application of forskolin (Fig. 2C).
We conducted experiments in the presence of a PKA inhibitor, H-89 (10 μm), to investigate a possible contribution of signalling via PKA to the enhanced action potential firing. As shown in Figs 1C and 2A, H-89 had little effect on the enhanced excitability following application of PGE2. The resting membrane potential remained depolarized (−52.5 ± 2.4 mV, not significantly different from value of −53.7 ± 1.2 mV in PGE2 alone, n = 6, Fig. 2B) while the action potential threshold remained unchanged, as did the maximum rate of rise and maximum rate of fall of the action potential (Table 1). H-89 also had little effect on the membrane depolarization caused by forskolin (Fig. 2C). From these experiments and those above we conclude that the major action of PGE2 in enhancing action potential frequency occurs directly through increases in cAMP, and that any effect caused by PKA-dependent phosphorylation of downstream ion channel targets is small.
Dependence of Ih on neuronal size
Sensory afferent nerve fibres are classified into three main categories: large-diameter, fast-conducting myelinated Aβ-fibres, which serve a variety of mainly non-nociceptive functions; smaller and slower myelinated Aδ-fibres, some of which are nociceptive and others non-nociceptive; and the smallest and slowest unmyelinated C-fibres, the majority of which are nociceptors (Lawson, 2002). A correlation exists between cell body diameter and conduction velocity of the afferent fibre in adult rat, with Aβ-fibres having a mean cell body diameter of 38 μm, Aδ-fibres 30 μm and C-fibres 24 μm (Harper & Lawson, 1985). Cell body sizes are smaller in the neonatal rats used in the present study, so in order to classify rat neurons approximately into the three main functional classes we employed a classification as follows: large (> 35 μm), medium (25–35 μm) and small (< 25 μm).
Ih in DRG neurons was activated by imposing membrane potential steps in whole-cell voltage-clamp mode in 10 mV increments from a holding potential of −60 mV (Fig. 3A and B). The steady-state voltage dependence of Ih activation was determined from the initial amplitudes of tail currents at a final step potential of −160 mV. Activation curves were fitted with a Boltzmann equation (see Methods) to determine the midpoint of voltage activation, V½, and the slope factor, k (Fig. 3C). Time constants of current activation were determined by fitting double-exponential functions (Fig. 3D); note that two time constants are required to fit the activation of even homomeric HCN subunits in expression systems (Santoro et al. 2000). The example shown in Fig. 3 is typical of a large rat DRG neuron (> 35 μm diameter); the characteristics of Ih in other size classes of neurons are different (see below).
Ih was considered to be present in a DRG neuron if the time-dependent inward current activated by a voltage step to −160 mV (see Fig. 3B) was greater than 50 pA. On this criterion Ih was observed in 37 out of 87 small neurons (42.5%), 68 out of 101 medium neurons (67.3%) and 50 out of 50 large neurons (100%) as shown in Fig. 4A, in line with earlier reports (Scroggs et al. 1994; Yagi & Sumino, 1998). The proportion of small neurons expressing Ih was also similar on this criterion to that obtained with current injection (46.7%, see above). Fifteen out of 20 small neurons expressing Ih also expressed TRPV1 and/or P2X3, as shown by the presence of inward currents activated by capsaicin or the P2X3 receptor agonist α,β-methyl ATP (see example traces in Fig. 5), and therefore have the properties of nociceptors.
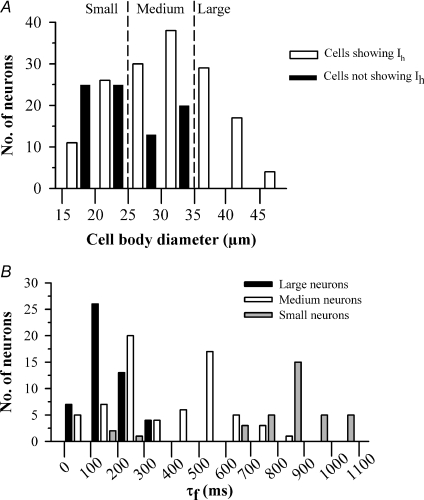
Figure 4. Properties of Ih in 3 size classes of rat DRG neurons.
A, histogram showing numbers of neurons which show Ih (amplitude of Ih at −60 mV > 50 pA, open bars) or do not show Ih (amplitude of Ih at −160 mV < 50 pA, black bars) in relation to cell body diameter. B, histogram showing τf values, obtained at a membrane potential of −90 mV, in the three classes of rat neurons: large (black bars, > 35 μm); medium (open bars, 25−35 μm); and small (grey bars, < 25 μm).
There was a marked difference in the activation time constants of Ih among the different classes of rat DRG neurons (Fig. 4B). The mean value of τf, the fast-activation time constant that accounts for 75–85% of the Ih current amplitude in all neurons, was 154.8 ± 17.1 ms (n = 50) in large neurons and 942.6 ± 80.4 ms (n = 37, P < 0.001) in small neurons at −90 mV, a more than sixfold difference (see Fig. 4B). In medium rat neurons two kinetically distinct populations of Ih were present: a fast-activating population with time constants similar to those obtained in large neurons, and a second population with slower activation of Ih (Fig. 4B). There is little overlap in the values of time constants between these two populations (see Fig. 4B) and we therefore termed them ‘medium fast’ (τf < 400 ms) and ‘medium slow’ (τf > 400 ms). No significant difference in mean cell body diameter was observed between medium fast and medium slow neurons (32.5 ± 1.3 and 31.0 ± 1.5 μm, respectively). Ih in the medium slow population was, however, significantly faster-activating than that in small neurons (Fig. 4B).
A further important point to note is that a minor population of small neurons had a fast-activating Ih indistinguishable from that seen in large neurons (Fig. 4B). This population comprised 9% of the small neurons and 2% of the total, and was readily distinguishable from the remaining 91% of small neurons, which express an Ih which activates on average 6 times more slowly (see Fig. 4B). Small neurons with fast Ih may correspond to cold-sensitive neurons, which form less than 5% of the DRG neuronal population (S. Mak, unpublished observations) and have a fast-activating Ih (Viana et al. 2002). We therefore tested for expression of the cold-sensitive ion channel TRPM8 by applying menthol, which is a specific activator of TRPM8 (McKemy et al. 2002). Figure 5 shows that small neurons with fast Ih were sensitive to menthol. These cells did not respond to the TRPV1 agonist capsaicin or the P2X3 agonist α,β-methyl ATP. The time constant of the fast-activating Ih in these menthol-sensitive small neurons was indistinguishable from that in large neurons but was highly significantly different from that in the non-menthol-sensitive small neurons (P < 0.001, Fig. 5D). Small neurons responding to capsaicin or to α,β-methyl ATP, on the other hand, had a slowly activating Ih indistinguishable from that of the overall small neuron population (Fig. 5D).
In summary, we identify four distinct neuronal populations, based on the time constants of activation of Ih and cell diameter: (1) large and medium fast cells, both of which express a similar fast-activating Ih; (2) medium slow cells, which express an Ih which activates with an intermediate time constant; (3) the majority of small cells, which express a slowly activating Ih; and (4) a minor population of small cells, which express TRPM8 and a fast-activating Ih.
Sensitivity to cAMP as a function of neuronal size
The activation of HCN2 and HCN4 as a function of membrane voltage is markedly sensitive to intracellular cAMP, while HCN1 and HCN3 are relatively insensitive. To investigate further the possible molecular basis of native Ih in sensory neurons we elevated intracellular levels of cAMP in three separate ways: by activating adenylate cyclase with forskolin; by applying PGE2; or by applying cAMP directly via the patch pipette.
Extracellular application of 50 μm forskolin for 4 min to large rat neurons had no detectable effect on the voltage sensitivity or the time constant of activation of Ih. V½ was −81.6 ± 1.6 mV in the absence and −81.4 ± 2.1 mV in the presence of forskolin (n = 10, P > 0.05), and there was no change in the voltage dependence of τf (Fig. 6A). There was also no effect on Ih in large neurons following application of 10 μm PGE2 nor when 50 μm cAMP was present in the patch pipette, as determined by calculating ΔV½ (+0.7 ± 0.9 mV, n = 10, and +0.2 ± 1.2 mV, n = 12, respectively, neither significant). A similar lack of effect of forskolin, PGE2 or intracellular cAMP on the activation of Ih was also observed in medium fast neurons (Fig. 6B and Table 2).
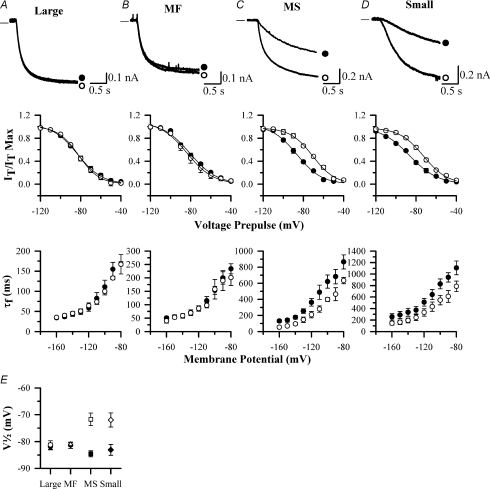
Figure 6. Effect of elevation of cAMP on properties of Ih in rat DRG neurons of different sizes.
A, B, C and D show properties of activation of Ih in rat large (A), medium fast (MF, B), medium slow (MS, C) and small DRG neurons (D). Upper panels show representative current traces in response to a voltage pulse from −60 mV to −90 mV in the absence (•) and presence (○) of 50 μm FK. Middle panels show steady-state activation curves of Ih as a function of membrane voltage over the range −120 mV to −40 mV in the absence (•) and presence (○) of 50 μm FK. Lower panels show τf in absence (•) and presence (○) of 50 μm FK. E, effect of 50 μm FK on values of V½ in the four populations of rat DRG neurons shown in A–D. Absence and presence of 50 μm FK is shown by filled or open symbols, respectively. Experiments repeated n = 10–15 times in each neuronal population.
Table 2.
Comparison of parameters of activation of Ih in the four main classes of rat and mouse HCN1+/+ DRG neurons
| Large | Medium fast | Medium slow | Small | |||||
|---|---|---|---|---|---|---|---|---|
| Rat | Mouse | Rat | Mouse | Rat | Mouse | Rat | Mouse | |
| Size classes (μm) | > 35 | > 30 | 25–35 | 20–30 | 25–35 | 20–30 | < 25 | < 20 |
| V½ (mV) | −81.6 ± 1.6 | −84.3 ± 2.4 | −81.1 ± 1.6 | −86.7 ± 2.4 | −85.9 ± 1.0 | −91.4 ± 1.9 | −86.3 ± 1.6 | −88.3 ± 1.4 |
| τf (ms, at −90 mV) | 155 ± 17 | 101 ± 28 | 200 ± 56 | 178 ± 38 | 622 ± 90 | 652 ± 68 | 943 ± 80 | 830 ± 87 |
| τs (ms, at −90 mV) | 526 ± 72 | 443 ± 39 | 929 ± 109 | 727 ± 71 | 1378 ± 128 | 1352 ± 125 | 2146 ± 235 | 1616 ± 107 |
| ΔV½ (mV) FK | 0.2 ± 0.5 | 1.3 ± 0.9 | 1.9 ± 0.6 | 0.9 ± 0.5 | 14.4 ± 0.7 | 13.2 ± 1.9 | 13.8 ± 0.4 | 16.7 ± 0.5 |
All data from Cs-subtracted current traces. The value of V½ was almost unaffected by forskolin (FK, 50 μm) in large and medium fast neurons, but was shifted in the positive direction by ∼14 mV in both medium slow and small neurons (see also Fig. 6E). Similar results were also obtained in rat neurons with exposure to PGE2 (10 μm) and with cAMP (50 μm) incorporated into the patch-clamp pipette. Number of neurons n = 10–15 for each measurement.
In contrast, most small and all medium slow neurons responded with a depolarizing shift in V½ in the presence of 50 μm forskolin, from −86.3 ± 1.6 to −72.5 ± 1.8 mV (n = 11) and −85.9 ± 1.0 to −71.5 ± 1.7 mV (n = 12), respectively (Fig. 6C and D, and Table 2). There was a corresponding shift in the dependence of the time constants of activation of Ih on membrane voltage in these two classes of neurons (Fig. 6C and D). Elevations in cAMP caused by either exogenous application of PGE2 or by including cAMP in the patch pipette, also brought about quantitatively similar depolarizing shifts in the Ih voltage-activation curve in both populations of neurons (not shown). Small neurons with fast-activating Ih, by contrast, were insensitive to elevation of cAMP, with ΔV½= 0.5 ± 0.3 mV in the presence of 50 μm forskolin (n = 3). Results for the effects of forskolin on V½ in the four major classes of neurons are summarized in Fig. 6E and Table 2.
The results presented in Figs 4–6 show that large and medium-fast neurons, and a small population of TRPM8-expressing small neurons, express a fast-activating, cAMP-insensitive Ih, consistent with the expression of HCN1. Medium slow and small neurons express two variants of slower-activating Ih, both of which are sensitive to cAMP and are therefore consistent with expression of HCN2 and/or HCN4. We further investigated the HCN isoform distribution in DRG neurons by constructing a HCN1−/− mouse.
Ih in mouse HCN1+/+ and HCN1−/− DRG neurons
Adult mouse DRG neurons are smaller than rat, so a revision of the size classes was required for mouse neurons. The mean diameter of cell bodies subtending fast-conducting Aβ-fibres is 31 μm and of unmyelinated slow-conducting C-fibres is 14 μm (Yoshida & Matsuda, 1979). In a more recent study neurons binding the plant lectin IB4, which are a subpopulation of unmyelinated C-fibres (Snider & McMahon, 1998), had a mean diameter ∼17 μm (Stucky & Lewin, 1999). We therefore classified mouse neurons into three size classes: large (> 30 μm), medium (20–30 μm) and small (< 20 μm).
In mouse HCN1+/+ neurons Ih was present in 26/26 (100%) of large neurons, 47/57 (82.4%) of medium neurons and 30/68 (44.1%) of small neurons, proportions which are identical within experimental error to the corresponding data obtained for rat neurons. Values of V½ were ∼5 mV more negative than in rat (Table 2). Values and distributions of τf in the three size classes in HCN1+/+ mice were very similar to those for rat (see Fig. 7E and Table 2). A fast-activating Ih was found in large HCN1+/+ DRG neurons and in a subpopulation of medium HCN1+/+ neurons (Fig. 7A and B), which we label medium fast by analogy with rat neurons. This population was broadly similar in its properties to the corresponding population in rat, though a small number of neurons had significantly longer time constants than was found in rat (compare black bars in Figs 4B and 7E). Application of forskolin (50 μm) to large and medium-fast neurons (including the small number with longer time constants than found in rat) had no significant effect on V½ (Table 2) or activation time constant (data not shown). A second subpopulation of medium-sized HCN1+/+ neurons had intermediate activation time constants, forming a distinct group from the medium-fast neurons. This population was similar to the rat medium-slow neuronal population (Fig. 7E). The majority of small neurons had an even slower Ih (grey bars in Fig. 7E). Application of 50 μm forskolin caused 13–14 mV depolarizing shifts in the values of V½ (Table 2) and in the voltage dependence of the activation time constant (not shown) in both medium-slow and small mouse neurons. As in rat, a small subpopulation of small neurons had fast time constants (Fig. 7E), and the V½ of Ih in these neurons was insensitive to forskolin (ΔV½= 0.4 ± 0.2 mV, n = 3). These results are closely similar to the corresponding results for neonatal rat neurons, when the size difference between neonatal rat and adult mouse neurons is taken into account.
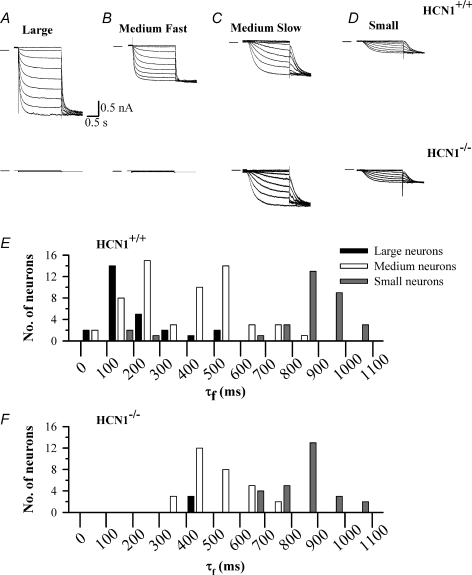
Figure 7. Recordings of Ih from mouse HCN1+/+ and HCN1−/− DRG neurons.
A, B, C and D show representative recordings of Ih in large (A), medium fast (B), medium slow (C) and small DRG neurons (D), from HCN1+/+ (upper panel) and HCN1−/− mice (lower panel). Ih was activated from a holding potential of −60 mV in 10 mV steps followed by a final step to −140 mV. E, histogram showing τf values derived at a membrane potential of −90 mV in the three classes of mouse HCN1+/+ neurons. F, similar histogram for HCN1−/− neurons.
We next examined the effect of genetic deletion of HCN1. In 9 out of 12 large DRG neurons from HCN1−/− mice no Ih could be detected (Fig. 7A, lower panel). This is in contrast to the 26 out of 26 HCN1+/+ large DRG neurons that showed a fast-activating Ih using the same voltage pulse protocol. The absence of Ih in most large HCN1−/− neurons is also reflected in an absence of fast τf values in this group of neurons (Fig. 7F). These results show that HCN1 is the only isoform contributing to the fast Ih present in the majority of large DRG neurons. In the three large neurons in which Ih was present, it was slow activating, with a mean τf of 474.5 ± 169.8 ms (n = 3), and was cAMP insensitive. A current with these properties was not recorded in any rat or HCN1+/+ mouse neuron. A likely explanation is that this current reflects expression of HCN3, which activates slowly and is insensitive to cAMP (Stieber et al. 2005), and that the presence of HCN3 is masked in HCN1+/+ neurons by coexpression of the fast-activating HCN1 (discussed further below).
In medium HCN1−/− neurons two main populations were present: one in which no Ih could be detected (10 out of 39 medium neurons, see Fig. 7B lower panel), and a second, and more prevalent population (29 out of 39 medium neurons, see Fig. 7C) in which a slower Ih was seen, with τf at −90 mV of 534.5 ± 109.8 ms, a value similar to that of the medium slow neurons in HCN1+/+ mice (Fig. 7F). Unlike the mouse HCN1+/+ or rat medium slow neuron population, however, this was not a single population because application of 50 μm forskolin produced two distinct results. In 10 out of 29 neurons (medium slow 1), forskolin failed to have any effect on activation of Ih (Fig. 8A), a result in contrast to the rat or mouse HCN1+/+ medium slow neurons, which all responded to forskolin with a shift in V½. The insensitivity to cAMP and the slow activation of Ih in these neurons suggests the presence of HCN3, the activity of which is not modulated by cAMP (Stieber et al. 2005). The fact that no analogous population was present in HCN1+/+ neurons suggests that HCN3 normally forms heteromers with HCN1, and that these heteromers activate rapidly and therefore contribute to the ‘medium fast’ neuronal population. In the remaining medium slow neurons (19 out of 29, medium slow 2) forskolin caused Ih to activate faster and shifted V½ from −85.3 ± 1.7 to −72.0 ± 1.2 mV (Fig. 8B), a result indistinguishable from the behaviour of mouse HCN1+/+ or rat medium slow neurons.
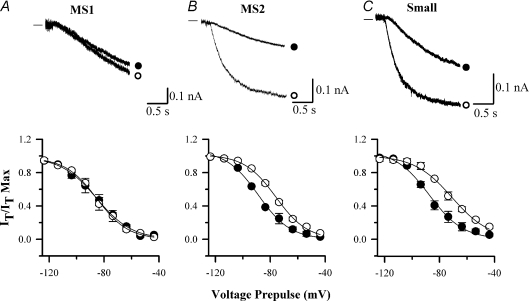
Figure 8. cAMP sensitivity of Ih in mouse HCN1−/− neurons.
A, B and C are representative recordings of Ih activation in medium slow 1 (A, MS1), medium slow 2 (B, MS2) and small DRG neurons (C) from HCN1−/− mice. Upper panel: Ih was activated by a voltage pulse from −60 mV to −90 mV in the absence (•) and presence (○) of 50 μm FK. Lower panel: activation curves of Ih as a function of membrane voltage in the absence (•) and presence (○) of 50 μm FK. Time constants in the MS1 and MS2 classes are similar but MS1 neurons are cAMP insensitive. Experiments repeated n = 10–15 times in each neuronal population.
In small neurons Ih was unaffected by the deletion of HCN1 (Fig. 7D). The proportion of neurons expressing Ih (12/26 or 46.2%) was similar to that in mouse HCN1+/+ and rat small neurons. The values of τf and V½ in mouse HCN1−/− small neurons were similar to those obtained in HCN1+/+ neurons. Application of forskolin to HCN1−/− small neurons caused a shift in V½ from −87.4 ± 1.5 to −69.6 ± 1.1 mV (n = 27, see Fig. 8C) and a corresponding shift in the voltage dependence of the values of τf (data not shown). These values are indistinguishable from those obtained in small mouse HCN1+/+ and rat neurons (Table 2), with the exception that no small neurons with fast-activating Ih were found.
In summary, these results show that the majority of large neurons, and some medium fast neurons, express only HCN1, and that therefore on deletion of HCN1 no Ih remains. The remaining large and medium-fast neurons are likely to coexpress HCN1 and HCN3, so that following deletion of HCN1 only HCN3 remains, generating a slowly activating, cAMP-insensitive Ih. Medium slow neurons and the large majority of small neurons do not express HCN1, so their properties are unaltered by deletion of HCN1. However, the subpopulation of small neurons with a fast-activating, cAMP-insensitive Ih, which therefore probably expressed HCN1, was absent in HCN1−/− mice. The menthol sensitivity of these neurons was not tested but it seems likely that this population corresponds to the small TRPM8-expressing neurons identified in rat.
Role of HCN1 in inflammatory and neuropathic pain
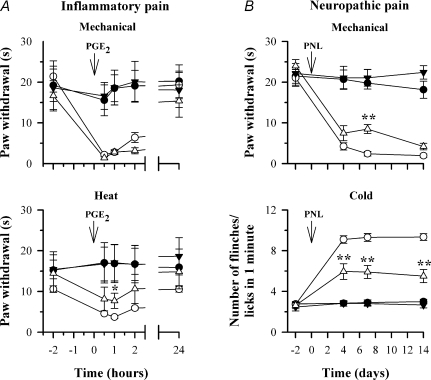
We assessed the in vivo role of HCN1 channels in hyperalgesia and neuropathic pain by comparing the responses in HCN1+/+ and HCN1−/− littermates to mechanical and thermal stimuli in both inflammatory and neuropathic pain models. Inflammatory pain was induced by injecting the pro-inflammatory mediator PGE2 into the hind paw. Figure 9A shows a reduction in paw withdrawal latency in response to mechanical and heat stimuli following intradermal injection of 0.75 nmol PGE2. The reduction was present for 0.5, 1 and 2 h following PGE2 injection and returned to baseline after 24 h. No significant difference between HCN1+/+ and HCN1−/− mice was seen in paw withdrawal latency in response to a mechanical stimulus. Heat hyperalgesia was also little affected by deletion of HCN1 (Fig. 9A).
Figure 9. Hyperalgesia and allodynia in response to mechanical and thermal stimuli in inflammatory and neuropathic pain models in HCN1+/+ and HCN1−/− mice.
A, response to mechanical (2.5 g, upper panel) and radiant heat stimuli (lower panel) in HCN1+/+ and HCN1−/− mice following PGE2-induced inflammation. Symbols show the following: •, HCN1+/+, saline; ○, HCN1+/+, PGE2; ▼, HCN1−/−, saline; ○, HCN1−/−, PGE2. Different groups of n = 5 mice used for each time series. B, response to mechanical stimuli (upper panel ordinate shows paw withdrawal time to constant 2.5 g stimulus) and cold stimuli (lower panel, ordinate shows mean number of flinches/licks in 1 min following application of a drop of acetone) in HCN1+/+ and HCN1−/− mice following partial sciatic nerve ligation (PNL). Symbols are: •, HCN1+/+, sham; ○, HCN1+/+, operated; ▼, HCN1−/−, sham; Δ, HCN1−/−, operated. Same mice used for both panels; n = 6 for sham and n = 10 for operated. Tests of significance conducted between data obtained in HCN1+/+ and HCN1−/− mice; *P < 0.05, **P < 0.01.
We implemented the partial sciatic nerve ligation model (PNL, Seltzer et al. 1990; Malmberg & Basbaum, 1998) in order to study a possible role of HCN1 in neuropathic pain. PNL caused the development of mechanical hyperalgesia and cold allodynia in both HCN1+/+ and HCN1−/− mice (Fig. 9B). These effects were observed from the first test point on day 4 and remained unchanged for the remainder of the study (14 days). Mechanical hyperalgesia was similar between HCN1+/+ and HCN1−/− mice following PNL; a significant difference seen at 7 days was not confirmed at other time points (Fig. 9B, upper panel). There was, however, a sustained and highly significant reduction in cold allodynia in HCN1−/− mice following PNL (Fig. 9B, lower panel). Cold allodynia, measured from the number of flinches or licks following application of the cooling drop of acetone, was more than halved by deletion of HCN1 and the effect persisted unchanged throughout the course of the experiment.
Discussion
The role of Ih in modulation of action potential firing in sensory neurons has received little attention, but our results show that it is a major determinant of action potential firing frequency in about half of small nociceptive neurons. An increase of intracellular cAMP levels produced two major effects, both consistent with enhanced activation of Ih: an increase in the rate of depolarization of the pacemaker potential region between action potentials, and a depolarization of the resting membrane potential. Both effects were completely reversed by the specific Ih blocker ZD7288, and neither was affected by blockade of PKA.
Voltage-clamp experiments showed that elevating cAMP levels shifted the voltage-activation curve of Ih in small nociceptive neurons, but not in large neurons, to more depolarized potentials. This shift has the effect both of increasing the amplitude of inward current carried by Ih and of increasing its rate of activation. A third important effect is the steady depolarization of the resting membrane potential caused by tonic activation of Ih, which is equivalent to a constant current injection at the resting potential.
Modulation of voltage-dependent sodium or potassium channels has to date been considered the most probable explanation for the enhanced firing of nociceptors in the presence of pro-inflammatory mediators such as PGE2. Modulation of NaV1.8 sodium channels via the cAMP/PKA pathway has been proposed to lower the activation threshold and increase the amplitude of the voltage-activated Na+ current in nociceptive neurons (Gold et al. 1996; England et al. 1996), but no effect of PGE2 or forskolin on action potential threshold or rate of rise was observed in the present experiments. There was also no effect on the rate of repolarization following an action potential, suggesting that suppression of voltage-dependent potassium conductance (Nicol et al. 1997) is unlikely to play an important role. The enhanced excitability was instead completely suppressed by ZD7288, a specific inhibitor of Ih, but was unaffected by H-89, an inhibitor of PKA. These experiments show that Ih is a major determinant of the enhanced nociceptor excitability caused by exposure to pro-inflammatory mediators.
HCN isoforms responsible for Ih
Large DRG neurons express a fast-activating Ih (Chaplan et al. 2003; Yao et al. 2003; Doan et al. 2004; Tu et al. 2004). The values of τf at −90 mV obtained in the present study in large neurons, 155 ± 17 ms, or in the medium fast class of neurons, 200 ± 56 ms, are not significantly different and are similar to the values obtained for the cloned HCN1 channel (Santoro et al. 2000; Chen et al. 2001). The lack of effect of cAMP on the voltage dependence of Ih in large and medium-fast neurons is also consistent with expression of HCN1. These findings suggest a major involvement of HCN1 in large and medium-fast neurons, but they do not exclude the possible presence of coexpressed HCN3. The absence of cAMP sensitivity makes an involvement of HCN2 or HCN4 unlikely. These results were supported by the absence of Ih in most large HCN1−/− neurons (Fig. 7), and by in situ hybridization and immunohistochemistry data (Moosmang et al. 2001; Chaplan et al. 2003) showing high levels of HCN1 mRNA and protein expression in large and medium DRG neurons. A slower, cAMP-insensitive Ih was found in some HCN1−/− large and medium-sized neurons; no current with these properties was observed in any mouse HCN1+/+ or rat neuron. This current may therefore be due to HCN3, which is more slowly activating than HCN1 and is insensitive to cAMP (Stieber et al. 2005). Thus in HCN1+/+ mice and rats we propose that HCN1 is the only isoform expressed in the majority of large and medium-fast neurons, but that HCN1 forms heteromers with HCN3 in a minority of large and medium-fast neurons.
Most small neurons and about half of medium-sized neurons express a slowly activating Ih whose voltage activation is modulated by cAMP. Ih in these neurons was unaffected by deletion of HCN1 (Fig. 7, Table 2). Several lines of evidence suggest that HCN2 is likely to be a major contributor to Ih in these neurons. The activation time constant of Ih seen in these neurons resembles that of HCN2 (Santoro et al. 2000; Chen et al. 2001) rather than HCN4 (Seifert et al. 1999). In immunocytochemical and in situ hybridization studies HCN2 has been found to be strongly expressed in DRG neurons, while HCN4 is expressed at a low level (Moosmang et al. 2001; Chaplan et al. 2003; Tu et al. 2004; Matsuyoshi et al. 2006; Luo et al. 2007; Obreja et al. 2008). These considerations do not, however, explain why the time constants of Ih activation differ in these two groups of neurons (see Figs 4 and 6). HCN channels have been shown to form heteromers in expression systems (Chen et al. 2001; Ulens & Tytgat, 2001), and HCN2 may form heteromers with HCN4 in small neurons, which have the slowest time constants.
A final minor class of small neurons expresses an Ih which is fast-activating, with a time constant indistinguishable from that in large neurons, and is insensitive to cAMP. These properties are consistent with expression of HCN1, and in agreement with this no small neurons with fast-activating Ih were found in HCN1−/− mice (Fig. 7F). These small neurons with fast Ih also express the cold-sensitive ion channel TRPM8, as shown by their sensitivity to menthol, a specific agonist for TRPM8 (Fig. 5).
The role of HCN1 in inflammatory and neuropathic pain
We tested the effect of deleting HCN1 on both inflammatory hyperalgesia and neuropathic pain in vivo. We induced inflammatory hyperalgesia by injection of PGE2, which induces robust inflammatory hyperalgesia in vivo (Taiwo et al. 1989). We used PGE2 rather than a more commonly used inflammatory stimulus (such as carageenan) in order to allow direct comparisons with our in vitro studies. HCN1 is expressed in large neurons, which are often thought of as being non-nociceptive in function, but in fact a substantial proportion of the largest afferent fibres are nociceptors, amongst which are both mechanically sensitive and heat-sensitive nociceptors (20% of somatosensory neurons in rat, see Djouhri & Lawson, 2004; Fang et al. 2005). HCN1 does not appear to play a substantial role in either mechanical or heat hyperalgesia, however, because the substantial reduction in mechanical and heat threshold seen following injection of PGE2 was unchanged in HCN1−/− mice (Fig. 9A).
Neuropathic pain is a widespread clinical problem, poorly treated by current drug therapies. Cold allodynia, an unpleasant sensation in response to normally non-noxious cold stimuli, is a particular problem in some forms of cancer chemotherapy, and is often a limiting factor in patients’ ability to tolerate chemotherapy. Previous studies have suggested an important role for Ih in the initiation of action potentials in neuropathic pain (Chaplan et al. 2003). Continuous or burst firing in afferent nociceptors is likely to be a causative agent in the continuous pain, allodynia and hyperalgesia which are characteristic of neuropathic pain states (Kajander et al. 1992; Sheen & Chung, 1993).The Ih blocker ZD7288 reduces ectopic firing in sensory neurons and alleviates allodynia caused by nerve injury (Chaplan et al. 2003; Luo et al. 2007). In addition, a role for Ih in the hyper-excitability of damaged peripheral nerves has been proposed (Yao et al. 2003).
We examined a possible role for HCN1 in neuropathic pain by implementing in HCN1+/+ and HCN1−/− littermates an experimental neuropathic pain model, in which neuropathic pain is induced by partial ligation of the sciatic nerve (Seltzer et al. 1990; Malmberg & Basbaum, 1998). Robust mechanical hyperalgesia and cold allodynia were observed 4 days post-operation and persisted for > 2 weeks (Fig. 9B). Deletion of HCN1 had little effect on mechanical hyperalgesia (Fig. 9B, top panel), implying that HCN1 plays little role in the generation of action potentials in the sensory endings responsible for the mechanical hyperalgesia in neuropathic pain.
A substantial and highly significant difference was observed in cold allodynia, however, which was more than halved by deleting HCN1 at all times tested. Detection of cold stimuli is mediated by a subpopulation of small-diameter DRG neurons (Reid & Flonta, 2001), which are unusual among small neurons in that a voltage- and time-dependent inward current is rapidly activated in response to hyperpolarizing current injection (Viana et al. 2002), consistent with expression of HCN1. In the present work we have identified in both rat and mouse neurons a subpopulation of small neurons with a fast-activating, cAMP-insensitive Ih, likely to correspond to the expression of HCN1, and we demonstrated in rat that these neurons are menthol sensitive and therefore express the cold-sensitive ion channel TRPM8 (Fig. 5). Repetitive firing in this population of small, cold-sensitive neurons in response to the steady inward current induced by a cold stimulus may therefore be promoted by the presence of HCN1, and thus would provide an explanation for our observation that deletion of HCN1 substantially diminishes cold allodynia.
Acknowledgments
We thank Carlos Belmonte and Felix Viana for helpful discussions, Xuming Zhang and Esther Berrocoso for comments on the manuscript, and we thank Sue Boyce and Gillian Gourlay for assistance with the behavioural models. This work was supported by the BBSRC. The generation of HCN1−/− mice was carried out in collaboration with Lexicon Pharmaceuticals Inc.
Commercial interest
Dr Adrian Mason, a joint author of this manuscript, is an employee of Schering-Plough Corporation, currently pursuing a drug discovery programme in the area of HCN ion channels.
Supplemental material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2008.163154/DC1
References
- Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN. The tetrodotoxinresistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci. 1999;2:541–548. doi: 10.1038/9195. [DOI] [PubMed] [Google Scholar]
- Altomare C, Terragni B, Brioschi C, Milanesi R, Pagliuca C, Viscomi C, Moroni A, Baruscotti M, DiFrancesco D. Heteromeric HCN1-HCN4 channels: a comparison with native pacemaker channels from the rabbit sinoatrial node. J Physiol. 2003;549:347–359. doi: 10.1113/jphysiol.2002.027698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton SM, Lekan HA, Kim SH, Chung JM. Behavioral manifestations of an experimental model for peripheral neuropathy produced by spinal nerve ligation in the primate. Pain. 1994;56:155–166. doi: 10.1016/0304-3959(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Cesare P, McNaughton PA. A novel heat-activated current in nociceptive neurons, and its sensitization by bradykinin. Proc Natl Acad Sci U S A. 1996;93:15435–15439. doi: 10.1073/pnas.93.26.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CS, Shigemoto R, Mercer JN, Surmeier DJ. HCN2 and HCN1 channels govern the regularity of autonomous pacemaking and synaptic resetting in globus pallidus neurons. J Neurosci. 2004;24:9921–9932. doi: 10.1523/JNEUROSCI.2162-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Guo HQ, Lee DH, Luo L, Liu C, Kuei C, Velumian AA, Butler MP, Brown SM, Dubin AE. Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J Neurosci. 2003;23:1169–1178. doi: 10.1523/JNEUROSCI.23-04-01169.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Wang J, Siegelbaum SA. Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide. J Gen Physiol. 2001;117:491–504. doi: 10.1085/jgp.117.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369–376. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol. 1993;55:455–472. doi: 10.1146/annurev.ph.55.030193.002323. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D, Ojeda C. Properties of the current if in the sino-atrial node of the rabbit compared with those of the current iK2, in Purkinje fibres. J Physiol. 1980;308:353–367. doi: 10.1113/jphysiol.1980.sp013475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991;351:145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Lawson SN. Aβ-fiber nociceptive primary afferent neurons: a review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Brain Res Rev. 2004;46:131–145. doi: 10.1016/j.brainresrev.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Doan TN, Stephans K, Ramirez AN, Glazebrook PA, Andresen MC, Kunze DL. Differential distribution and function of hyperpolarization-activated channels in sensory neurons and mechanosensitive fibers. J Neurosci. 2004;24:3335–3343. doi: 10.1523/JNEUROSCI.5156-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England S, Bevan S, Docherty RJ. PGE2 modulates the tetrodotoxin-resistant sodium current in neonatal rat dorsal root ganglion neurones via the cyclic AMP–protein kinase A cascade. J Physiol. 1996;495:429–440. doi: 10.1113/jphysiol.1996.sp021604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AR, Vasko MR, Nicol GD. The cAMP transduction cascade mediates the PGE2-induced inhibition of potassium currents in rat sensory neurones. J Physiol. 1999;516:163–178. doi: 10.1111/j.1469-7793.1999.163aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, McMullan S, Lawson SN, Djouhri L. Electrophysiological differences between nociceptive and non-nociceptive dorsal root ganglion neurones in the rat in vivo. J Physiol. 2005;565:927–943. doi: 10.1113/jphysiol.2005.086199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald EM, Okuse K, Wood JN, Dolphin AC, Moss SJ. cAMP-dependent phosphorylation of the tetrodotoxin-resistant voltage-dependent sodium channel SNS. J Physiol. 1999;516:433–446. doi: 10.1111/j.1469-7793.1999.0433v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Levine JD, Correa AM. Modulation of TTX-R INa by PKC and PKA and their role in PGE2-induced sensitization of rat sensory neurons in vitro. J Neurosci. 1998;18:10345–10355. doi: 10.1523/JNEUROSCI.18-24-10345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Reichling DB, Shuster MJ, Levine JD. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc Natl Acad Sci U S A. 1996;93:1108–1112. doi: 10.1073/pnas.93.3.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol. 1985;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NC, Constanti A. Mechanism of block by ZD 7288 of the hyperpolarization-activated inward rectifying current in guinea pig substantia nigra neurons in vitro. J Neurophysiol. 1995;74:2366–2378. doi: 10.1152/jn.1995.74.6.2366. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Williams JT. Opioid inhibition of Ih via adenylyl cyclase. Neuron. 1994;13:179–186. doi: 10.1016/0896-6273(94)90468-5. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Williams JT. Modulation of the hyperpolarisation-activated current (Ih) by cyclic nucleotides in guinea-pig primary afferent neurons. J Physiol. 1996;492:97–106. doi: 10.1113/jphysiol.1996.sp021292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Zhang YH, Clark JD, Tempel BL, Nicol GD. Prostaglandin E2 inhibits the potassium current in sensory neurons from hyperalgesic Kv1.1 knockout mice. Neuroscience. 2003;119:65–72. doi: 10.1016/s0306-4522(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Kajander KC, Wakisaka S, Bennett GJ. Spontaneous discharge originates in the dorsal root ganglion at the onset of a painful peripheral neuropathy in the rat. Neurosci Lett. 1992;138:225–228. doi: 10.1016/0304-3940(92)90920-3. [DOI] [PubMed] [Google Scholar]
- Kaupp UB, Seifert R. Molecular diversity of pacemaker ion channels. Annu Rev Physiol. 2001;63:235–257. doi: 10.1146/annurev.physiol.63.1.235. [DOI] [PubMed] [Google Scholar]
- Kerr BJ, Souslova V, McMahon SB, Wood JN. A role for the TTX-resistant sodium channel Nav 1.8 in NGF-induced hyperalgesia, but not neuropathic pain. Neuroreport. 2001;12:3077–3080. doi: 10.1097/00001756-200110080-00019. [DOI] [PubMed] [Google Scholar]
- Komagiri Y, Kitamura N. Effect of intracellular dialysis of ATP on the hyperpolarization-activated cation current in rat dorsal root ganglion neurons. J Neurophysiol. 2003;90:2115–2122. doi: 10.1152/jn.00442.2003. [DOI] [PubMed] [Google Scholar]
- Lawson SN. Phenotype and function of somatic primary afferent nociceptive neurones with C-, Aδ- or Aα/β-fibres. Exp Physiol. 2002;87:239–244. doi: 10.1113/eph8702350. [DOI] [PubMed] [Google Scholar]
- Lopshire JC, Nicol GD. The cAMP transduction cascade mediates the prostaglandin E2 enhancement of the capsaicin-elicited current in rat sensory neurons: whole-cell and single-channel studies. J Neurosci. 1998;18:6081–6092. doi: 10.1523/JNEUROSCI.18-16-06081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A, Budde T, Stieber J, Moosmang S, Wahl C, Holthoff K, Langebartels A, Wotjak C, Munsch T, Zong X, Feil S, Feil R, Lancel M, Chien KR, Konnerth A, Pape HC, Biel M, Hofmann F. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J. 2003;22:216–224. doi: 10.1093/emboj/cdg032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Chang L, Brown SM, Ao H, Lee DH, Higuera ES, Dubin AE, Chaplan SR. Role of peripheral hyperpolarization-activated cyclic nucleotide-modulated channel pacemaker channels in acute and chronic pain models in the rat. Neuroscience. 2007;144:1477–1485. doi: 10.1016/j.neuroscience.2006.10.048. [DOI] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Magistretti J, Mantegazza M, de Curtis M, Wanke E. Modalities of distortion of physiological voltage signals by patch-clamp amplifiers: a modeling study. Biophys J. 1998;74:831–842. doi: 10.1016/S0006-3495(98)74007-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg AB, Basbaum AI. Partial sciatic nerve injury in the mouse as a model of neuropathic pain: behavioral and neuroanatomical correlates. Pain. 1998;76:215–222. doi: 10.1016/s0304-3959(98)00045-1. [DOI] [PubMed] [Google Scholar]
- Matsuyoshi H, Masuda N, Chancellor MB, Erickson VL, Hirao Y, de Groat WC, Wanaka A, Yoshimura N. Expression of hyperpolarization-activated cyclic nucleotide-gated cation channels in rat dorsal root ganglion neurons innervating urinary bladder. Brain Res. 2006;1119:115–123. doi: 10.1016/j.brainres.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Mo ZL, Davis RL. Heterogeneous voltage dependence of inward rectifier currents in spiral ganglion neurons. J Neurophysiol. 1997;78:3019–3027. doi: 10.1152/jn.1997.78.6.3019. [DOI] [PubMed] [Google Scholar]
- Moosmang S, Stieber J, Zong X, Biel M, Hofmann F, Ludwig A. Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues. Eur J Biochem. 2001;268:1646–1652. doi: 10.1046/j.1432-1327.2001.02036.x. [DOI] [PubMed] [Google Scholar]
- Nicol GD, Vasko MR, Evans AR. Prostaglandins suppress an outward potassium current in embryonic rat sensory neurons. J Neurophysiol. 1997;77:167–176. doi: 10.1152/jn.1997.77.1.167. [DOI] [PubMed] [Google Scholar]
- Nolan MF, Malleret G, Lee KH, Gibbs E, Dudman JT, Santoro B, Yin D, Thompson RF, Siegelbaum SA, Kandel ER, Morozov A. The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar Purkinje cells. Cell. 2003;115:551–564. doi: 10.1016/s0092-8674(03)00884-5. [DOI] [PubMed] [Google Scholar]
- Obreja O, Klusch A, Ponelies N, Schmelz M, Petersen M. A subpopulation of capsaicin-sensitive porcine dorsal root ganglion neurons is lacking hyperpolarization-activated cyclic nucleotide-gated channels. Eur J Pain. 2008;12:775–789. doi: 10.1016/j.ejpain.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Pape HC. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- Pape HC, McCormick DA. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature. 1989;340:715–718. doi: 10.1038/340715a0. [DOI] [PubMed] [Google Scholar]
- Reid G, Flonta ML. Cold current in thermoreceptive neurons. Nature. 2001;413:480. doi: 10.1038/35097164. [DOI] [PubMed] [Google Scholar]
- Santoro B, Chen S, Luthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci. 2000;20:5264–5275. doi: 10.1523/JNEUROSCI.20-14-05264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scroggs RS, Todorovic SM, Anderson EG, Fox AP. Variation in IH, IIR, and ILEAK between acutely isolated adult rat dorsal root ganglion neurons of different size. J Neurophysiol. 1994;71:271–279. doi: 10.1152/jn.1994.71.1.271. [DOI] [PubMed] [Google Scholar]
- Seifert R, Scholten A, Gauss R, Mincheva A, Lichter P, Kaupp UB. Molecular characterization of a slowly gating human hyperpolarization-activated channel predominantly expressed in thalamus, heart, and testis. Proc Natl Acad Sci U S A. 1999;96:9391–9396. doi: 10.1073/pnas.96.16.9391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- Sheen K, Chung JM. Signs of neuropathic pain depend on signals from injured nerve fibers in a rat model. Brain Res. 1993;610:62–68. doi: 10.1016/0006-8993(93)91217-g. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. The Integrative Action of the Nervous System. New York: Scribner; 1906. [Google Scholar]
- Snider WD, McMahon SB. Tackling pain at the source: New ideas about nociceptors. Neuron. 1998;20:629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- Southan AP, Morris NP, Stephens GJ, Robertson B. Hyperpolarization-activated currents in presynaptic terminals of mouse cerebellar basket cells. J Physiol. 2000;526:91–97. doi: 10.1111/j.1469-7793.2000.t01-1-00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieber J, Stockl G, Herrmann S, Hassfurth B, Hofmann F. Functional expression of the human HCN3 channel. J Biol Chem. 2005;280:34635–34643. doi: 10.1074/jbc.M502508200. [DOI] [PubMed] [Google Scholar]
- Stucky CL, Lewin GR. Isolectin B4-positive and -negative nociceptors are functionally distinct. J Neurosci. 1999;19:6497–6505. doi: 10.1523/JNEUROSCI.19-15-06497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiwo YO, Bjerknes LK, Goetzl EJ, Levine JD. Mediation of primary afferent peripheral hyperalgesia by the cAMP second messenger system. Neuroscience. 1989;32:577–580. doi: 10.1016/0306-4522(89)90280-7. [DOI] [PubMed] [Google Scholar]
- Tu H, Deng L, Sun Q, Yao L, Han JS, Wan Y. Hyperpolarization-activated, cyclic nucleotide-gated cation channels: roles in the differential electrophysiological properties of rat primary afferent neurons. J Neurosci Res. 2004;76:713–722. doi: 10.1002/jnr.20109. [DOI] [PubMed] [Google Scholar]
- Ulens C, Tytgat J. Functional heteromerization of HCN1 and HCN2 pacemaker channels. J Biol Chem. 2001;276:6069–6072. doi: 10.1074/jbc.C000738200. [DOI] [PubMed] [Google Scholar]
- Viana F, de la Peña E, Belmonte C. Specificity of cold thermotransduction is determined by differential ionic channel expression. Nat Neurosci. 2002;5:254–260. doi: 10.1038/nn809. [DOI] [PubMed] [Google Scholar]
- Yagi J, Sumino R. Inhibition of a hyperpolarization-activated current by clonidine in rat dorsal root ganglion neurons. J Neurophysiol. 1998;80:1094–1104. doi: 10.1152/jn.1998.80.3.1094. [DOI] [PubMed] [Google Scholar]
- Yao H, Donnelly DF, Ma C, LaMotte RH. Upregulation of the hyperpolarization-activated cation current after chronic compression of the dorsal root ganglion. J Neurosci. 2003;23:2069–2074. doi: 10.1523/JNEUROSCI.23-06-02069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Matsuda Y. Studies on sensory neurons of the mouse with intracellular-recording and horseradish peroxidase-injection techniques. J Neurophysiol. 1979;42:1134–1145. doi: 10.1152/jn.1979.42.4.1134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.