Abstract
A rod-shaped gram-positive anaerobic bacterium, strain HE8, was isolated from human feces. The isolate was able to convert the isoflavones daidzein and genistein to equol and 5-hydroxy-equol, respectively. Based on phenotypic and phylogenetic analyses, strain HE8 is described as a new species, Slackia isoflavoniconvertens.
Daidzein, genistein, and their corresponding glycosides belong to the most common isoflavones present in the human diet. The isoflavones have been proposed to prevent hormone-dependent and age-related diseases including cancer, osteoporosis, menopausal symptoms, and cardiovascular diseases (2, 27, 33, 39). The effects of isoflavones may be mediated by binding to the estrogen receptors, by inhibition of enzymes, or by their antioxidative properties (5, 10, 11, 29). The conversion of isoflavones by intestinal bacteria may have an impact on their biological effects. For example, the bacterial metabolite equol shows a greater affinity for the estrogen receptor β than does its precursor daidzein (14, 22). The ability to form equol varies greatly among human subjects, which may be explained by differences in the gut microbiota composition (1, 26, 28). A number of bacteria involved in the formation of equol from daidzein have been isolated from animals and humans, most of them only recently (8, 16, 17, 20, 21, 32, 36, 37, 40). With regard to humans, the complete conversion of daidzein to equol has been observed exclusively with Adlercreutzia equolifaciens and strain DZE (13, 16). The formation of the analogue metabolite from genistein, 5-hydroxy-equol, was reported so far only for the mouse intestinal strain Mt1B8 (17) and the human intestinal strain DZE (13). In this study, we isolated a human intestinal bacterium capable of transforming daidzein to equol and genistein to 5-hydroxy-equol.
Isolation.
Strain HE8 was isolated from the feces of a healthy 37-year-old woman, whose intestinal microbiota was capable of converting daidzein to equol. To assay equol formation, 1 g (wet weight) of feces was resuspended in 5 ml brain heart infusion (BHI) broth (Roth, Karlsruhe, Germany) and 200 μl of the resulting fecal suspension was used to inoculate 5 ml BHI broth containing 190 μM daidzein (Acros Organics, Geel, Belgium). Daidzein was completely converted to 122 μM equol within 22 h of incubation. For isolation of strain HE8, 1 g (wet weight) of a freshly voided fecal sample was resuspended in 5 ml BHI broth. Serial dilutions of this fecal suspension were prepared and incubated in 10 ml BHI broth containing 100 μM daidzein in the presence of tetracycline (10 μg ml−1; Roth, Karlsruhe, Germany) for 72 h at 37°C. The addition of tetracycline to the medium inhibited the growth of a large proportion of bacterial community members without affecting the conversion of daidzein to equol. Both the preparation and the incubation of microbial cultures were carried out under strict anoxic conditions as described elsewhere (17). From the highest dilution containing equol-forming bacteria, serial dilutions were repeatedly prepared until a pure bacterial culture was obtained. The ability of cultures to form equol from daidzein was tested by high-pressure liquid chromatography/diode array detector analysis as described previously (17).
Phenotypic characterization.
Strain HE8 was a strictly anaerobic, rod-shaped bacterium, which appeared gram positive after staining and in the KOH test (7, 18). The cells occurred in pairs or short chains. The Schaeffer-Fulton stain did not reveal any endospores (30). Colonies grown on BHI agar (Oxoid, Wesel, Germany) or Columbia agar (bioMérieux, Marcy l'Etoile, France) after 48 h of incubation at 37°C were 1 mm in diameter, smooth, and translucent. Strain HE8 was asaccharolytic and capable of utilizing arginine. The isolate showed negative reactions for catalase, oxidase, and indole (Bactident; Merck, Darmstadt, Germany). Further biochemical characteristics of strain HE8 are given below in the species description and in Table 1. The tests were performed using the API 20A, API Rapid ID 32A, API ZYM, and Vitek (ANI card) systems (bioMérieux, Marcy l'Etoile, France) according to the manufacturer's instructions with cells grown for 48 h on Columbia agar. In parallel, the type strains of the phylogenetically closest relatives Slackia faecicanis (DSM 17537), Slackia heliotrinireducens (DSM 20476) (German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany), and Slackia exigua (CCUG 44588) (Culture Collection, University of Göteborg, Sweden) were analyzed (Table 1). Similar to S. faecicanis but in contrast to S. exigua and S. heliotrinireducens, strain HE8 showed negative results for the majority of tests, with positive reactions being obtained only for arginine dihydrolase, acid phosphatase, esterase (C4), esterase lipase (C8), and naphthol-AS-BI-phosphohydrolase.
TABLE 1.
Selected characteristics of strain HE8 and the species of the genus Slackiaa
| Characteristic | Strain HE8 | S. faecicanis | S. exigua | S. heliotrinireducens |
|---|---|---|---|---|
| Source | Human feces | Dog feces | Human oral lesions | Sheep rumen |
| Cell shape | Rods | Rods | Rods | Cocci |
| Gram stain | + | + | + | + |
| G+C content of DNA (mol%) | 58.5 | 61.2 | 60-64 | 61.0 |
| 16S rRNA identity to strain HE8 (1,339 bp) (%) | − | 90.9 | 91.4 | 90.6 |
| DNA-DNA homology to strain HE8 (%) | − | 28.8 ± 5.0 | 17.8 ± 0.8 | 21.6 ± 4.2 |
| Daidzein conversion | + | − | + | − |
| Equol formation from daidzein | + | − | − | − |
| Genistein conversion | + | − | + | − |
| 5-Hydroxy-equol formation from genistein | + | − | − | − |
| Arginine dihydrolase | + | + | + | + |
| Alkaline phosphatase | − | − | − | − |
| Acid phosphatase | + | + | + | − |
| Arginine arylamidase | − | − | + | + |
| Proline arylamidase | − | − | + | + |
| Leucyl glycine arylamidase | − | − | wb | + |
| Phenylalanine arylamidase | − | − | + | + |
| Leucine arylamidase | − | − | + | + |
| Tyrosine arylamidase | − | − | + | + |
| Alanine arylamidase | − | − | + | + |
| Glycine arylamidase | − | − | + | + |
| Histidine arylamidase | − | − | + | + |
| Serine arylamidase | − | − | + | + |
| Valine arylamidase | − | − | + | + |
| Cystine arylamidase | − | − | w | − |
| Esterase (C4) | + | + | + | + |
| Esterase lipase (C8) | + | w | + | w |
| Naphthol-AS-BI-phosphohydrolase | + | w | + | − |
Isoflavone conversion.
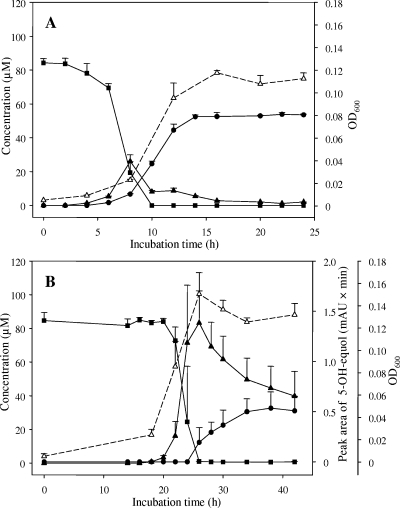
Strain HE8 transformed 84 μM daidzein within 10 h of incubation in BHI broth at 37°C completely (Fig. 1A). The daidzein conversion started after 2 h of incubation with formation of the intermediate dihydrodaidzein, immediately followed by equol formation. The maximal concentration of dihydrodaidzein (26 μM) was detected after 8 h of incubation. The end product equol was formed at a maximal concentration of 52 μM after 14 h. Compared to daidzein, genistein was converted by strain HE8 more slowly under identical conditions. Only after 20 h of incubation did the transformation of genistein start concurrently with the growth of the culture (Fig. 1B). Hereafter, genistein was completely converted within 6 h to dihydrogenistein (83 μM), which was partially transformed to 5-hydroxy-equol. After 42 h of incubation, 40 μM of dihydrogenistein was still present in the supernatant. The conversion experiments and the analyses of metabolites were done as described previously (17). Using the same conditions, the ability of the phylogenetically related Slackia species to convert daidzein and genistein was tested. S. faecicanis and S. heliotrinireducens did not transform any of the isoflavones within 96 h. Only S. exigua completely converted daidzein and genistein (50 μM each), respectively, within 96 h. Whereas O-desmethylangolensin was formed from daidzein (16), no metabolites were detected following genistein degradation.
FIG. 1.
Time course of daidzein and genistein conversion by strain HE8. (A) Daidzein (▪) was transformed via dihydrodaidzein (▴) to equol (•). Cell growth is indicated by the optical density at 600 nm (OD600) (▵) (y axis on the right). (B) Genistein (▪) was transformed via dihydrogenistein (▴) to 5-hydroxy-equol (•). Values of 5-hydroxy-equol are expressed as peak areas (first y axis on the right). Cell growth is indicated by OD600 (▵) (second y axis on the right). The symbols indicate the means of triplicate experiments. The error bars indicate standard deviations.
While formation of equol from daidzein via dihydrodaidzein is catalyzed by several bacterial species (16, 17, 20, 21, 40), the corresponding conversion of genistein to 5-hydroxy-equol has been demonstrated for only two strains, Mt1B8 and DZE (13, 17). This may be due to the fact that genistein transformation has not been investigated. The enrichment of the intermediate dihydrogenistein in the course of genistein conversion by strain HE8 was reported previously for strain Mt1B8 (17). However, the initial growth inhibition by genistein occurred only with strain HE8. The ability of strain HE8 to convert daidzein and genistein in the stationary growth phase was tested as described previously for strain Mt1B8 (17). Similarly to strain Mt1B8, strain HE8 hardly converted daidzein and genistein when the isoflavones were added in the stationary growth phase to cells grown in their absence. The conversion rates (standard deviations) were 0.16 (±0.09) μmol h−1 mg protein−1 for daidzein and 0.20 (±0.09) μmol h−1 mg protein−1 for genistein. When the cells were grown in the presence of daidzein, the conversion rate of daidzein, added during the early stationary phase, increased 10-fold to 1.55 (±0.01) μmol h−1 mg protein−1. Similarly, the rate of genistein conversion in the stationary phase increased fivefold to 1.06 (±0.21) μmol h−1 mg protein−1, when bacteria were grown with genistein. The conversion of genistein in the stationary phase was even 12-fold increased to 2.40 (±1.86) μmol h−1 mg protein−1, when strain HE8 had been grown in the presence of daidzein. The findings obtained for both strain Mt1B8 and strain HE8 suggest that the isoflavone conversion in other equol-forming intestinal bacteria is also inducible.
Phylogenetic affiliation.
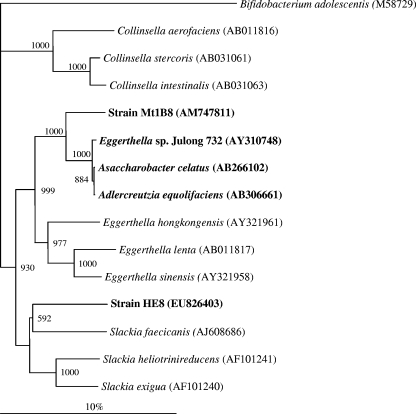
The genomic DNA of strain HE8 was isolated (FastDNA Spin kit; MP Biomedicals, Heidelberg, Germany), and the 16S rRNA genes were almost completely amplified with the primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1401R (5′-CGGTGTGTACAAGACCC-3′) (4, 23) using the following PCR program: 4 min at 94°C; 35 cycles of 1 min at 94°C, 1 min at 50°C, and 2 min at 72°C; and finally 10 min at 72°C. The composition of the PCR mixture and the purification of the resulting amplicon were described previously (4). The purified PCR products were sequenced (Eurofins MWG Operon, Martinsried, Germany), and the nucleotide sequences were edited manually using the ContigExpress function of the Vector NTI Suite 9 software package (Invitrogen, Carlsbad, CA). Sequences encoding the 16S rRNA of organisms related to strain HE8 were obtained using the BLAST service of the NCBI website (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Sequence alignment using the Bioedit software (Ibis Biosciences, Carlsbad, CA) revealed 91% identity of the 16S rRNA gene sequence (1,339 bp) of strain HE8 with the closest described relatives, Slackia exigua isolated from human oral lesions (91.4%), Slackia faecicanis from dog feces (90.9%), and Slackia heliotrinireducens from sheep rumen (90.6%) (15, 35). Lower values of sequence identity were observed for strain HE8 and the equol-forming species Adlercreutzia equolifaciens (90.2%), Eggerthella sp. strain Julong 732 (89.9%) and strain DZE (87.6%) from human feces, Asaccharobacter celatus (90.1%) from rat cecum, and strain Mt1B8 from mouse intestine (88.8%), all of which affiliate within the Coriobacteriaceae (13, 16, 17, 20, 21, 36). All of these bacterial strains produce equol from daidzein, with the exception of Eggerthella sp. strain Julong 732, which produces equol from dihydrodaidzein. The phylogenetic tree, generated by the neighbor-joining method using the software Clustal X 1.7 (12) and TreeView 1.0 (24), shows that strain HE8 together with the other known Slackia species forms a distinct cluster within the Coriobacteriaceae (Fig. 2). Hybridization of DNA from strain HE8 with DNA from the Slackia species revealed 17.8 to 28.8% similarity (Table 1). The DNA G+C content of strain HE8 was 58.5 mol%. These analyses were done at the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany) according to standard methods (3, 6, 9, 19, 31, 34).
FIG. 2.
Neighbor-joining tree based on 16S rRNA gene sequences (1,339 bp) showing the phylogenetic position of the newly isolated strain HE8 among closely related members of the Coriobacteriaceae. The species with equol-forming ability are given in bold. The accession numbers of the 16S rRNA gene sequences are given in parentheses. The numbers at the branch points are bootstrap values based on 1,000 samplings. The bar indicates 10% estimated sequence divergence.
Based on the main phenotypic characteristics and phylogenetic analyses, strain HE8 is a member of the genus Slackia within the Coriobacteriaceae. Whereas the new isolate can be phenotypically readily distinguished from S. exigua and S. heliotrinireducens, it shows a high degree of similarity to S. faecicanis in a large number of phenotypical traits (Table 1). However, in contrast to strain HE8, S. faecicanis did not transform daidzein or genistein. The 9% 16S rRNA divergence from the three currently recognized Slackia species and DNA-DNA hybridization values of ≤29% indicate that strain HE8 represents a new species (38), for which we propose the name Slackia isoflavoniconvertens. Beside Adlercreutzia equolifaciens, the newly isolated bacterium represents the second thoroughly characterized equol-forming species isolated from humans. The identification of bacteria responsible for equol formation in humans is an important step toward the elucidation of the observed interindividual differences and to a better understanding of the efficacy of ingested daidzein. Beside equol formation, the new species could also contribute to disease prevention in humans by its ability to convert genistein to 5-hydroxy-equol, which may be expected to show properties similar to those of equol.
Description of Slackia isoflavoniconvertens sp. nov.
Slackia isoflavoniconvertens (i.so.fla.vo.ni.con.ver′tens. N.L. neut. n. isoflavonum, isoflavone; L. part. adj. convertens, converting; isoflavoniconvertens, isoflavone converting). Cells are gram-positive short rods (2.4 × 0.4 μm) occurring in pairs or short chains and do not form endospores. Colonies grown on Columbia agar after 48 h of incubation at 37°C are 1 mm in diameter, smooth, and translucent. Strict anaerobe. Growth is stimulated by 1% arginine. Indole is not produced. Nitrate is not reduced. Gelatin and esculin are not hydrolyzed. Acid is not produced from glucose, lactose, mannitol, saccharose, maltose, xylose, arabinose, cellobiose, mannose, melezitose, raffinose, rhamnose, trehalose, sorbitol, glycerol, or salicin. Positive reactions are obtained for arginine dihydrolase, esterase (C4), esterase lipase (C8), acid phosphatase, and naphthol-AS-BI-phosphohydrolase. Negative results are obtained for catalase, oxidase, urease, α-glucosidase, β-glucosidase, α-galactosidase, β-galactosidase, β-glucuronidase, α-arabinosidase, α-mannosidase, α-fucosidase, arginine arylamidase, N-acetyl-β-glucosaminidase, β-galactosidase-6-phosphate, glutamic acid decarboxylase, alkaline phosphatase, lipase (C14), trypsin, α-chymotrypsin, alanine arylamidase, cystine arylamidase, glycine arylamidase, glutamyl glutamic acid arylamidase, histidine arylamidase, leucine arylamidase, leucyl glycine arylamidase, phenylalanine arylamidase, proline arylamidase, pyroglutamic acid arylamidase, serine arylamidase, tyrosine arylamidase, and valine arylamidase. It is capable of converting the isoflavones daidzein and genistein. The G+C content of the DNA is 58.5 mol%. The type strain, HE8 (=DSM 22006), was isolated from human feces.
Nucleotide sequence accession number.
The 16S rRNA gene sequence of strain HE8 has been deposited in the GenBank nucleotide sequence database under accession number EU826403.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (grant number BR 2269/3-1).
Footnotes
Published ahead of print on 9 January 2009.
REFERENCES
- 1.Atkinson, C., C. L. Frankenfeld, and J. W. Lampe. 2005. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Exp. Biol. Med. (Maywood) 230:155-170. [DOI] [PubMed] [Google Scholar]
- 2.Birt, D. F., S. Hendrich, and W. Wang. 2001. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol. Ther. 90:157-177. [DOI] [PubMed] [Google Scholar]
- 3.Cashion, P., M. A. Holder-Franklin, J. McCully, and M. Franklin. 1977. A rapid method for the base ratio determination of bacterial DNA. Anal. Biochem. 81:461-466. [DOI] [PubMed] [Google Scholar]
- 4.Clavel, T., G. Henderson, C. A. Alpert, C. Philippe, L. Rigottier-Gois, J. Dore, and M. Blaut. 2005. Intestinal bacterial communities that produce active estrogen-like compounds enterodiol and enterolactone in humans. Appl. Environ. Microbiol. 71:6077-6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cos, P., T. De Bruyne, S. Apers, D. Vanden Berghe, L. Pieters, and A. J. Vlietinck. 2003. Phytoestrogens: recent developments. Planta Med. 69:589-599. [DOI] [PubMed] [Google Scholar]
- 6.De Ley, J., H. Cattoir, and A. Reynaerts. 1970. The quantitative measurement of DNA hybridization from renaturation rates. Eur. J. Biochem. 12:133-142. [DOI] [PubMed] [Google Scholar]
- 7.Gregersen, T. 1978. Rapid method for distinction of gram-negative from gram-positive bacteria. Eur. J. Appl. Microbiol. Biotechnol. 5:123-127. [Google Scholar]
- 8.Hur, H. G., J. O. Lay, Jr., R. D. Beger, J. P. Freeman, and F. Rafii. 2000. Isolation of human intestinal bacteria metabolizing the natural isoflavone glycosides daidzin and genistin. Arch. Microbiol. 174:422-428. [DOI] [PubMed] [Google Scholar]
- 9.Huss, V. A. R., H. Festl, and K. H. Schleifer. 1983. Studies on the spectrophotometric determination of DNA hybridization from renaturation rates. Syst. Appl. Microbiol. 4:184-192. [DOI] [PubMed] [Google Scholar]
- 10.Hwang, C. S., H. S. Kwak, H. J. Lim, S. H. Lee, Y. S. Kang, T. B. Choe, H. G. Hur, and K. O. Han. 2006. Isoflavone metabolites and their in vitro dual functions: they can act as an estrogenic agonist or antagonist depending on the estrogen concentration. J. Steroid Biochem. Mol. Biol. 101:246-253. [DOI] [PubMed] [Google Scholar]
- 11.Jackman, K. A., O. L. Woodman, and C. G. Sobey. 2007. Isoflavones, equol and cardiovascular disease: pharmacological and therapeutic insights. Curr. Med. Chem. 14:2824-2830. [DOI] [PubMed] [Google Scholar]
- 12.Jeanmougin, F., J. D. Thompson, M. Gouy, D. G. Higgins, and T. J. Gibson. 1998. Multiple sequence alignment with Clustal X. Trends Biochem. Sci. 23:403-405. [DOI] [PubMed] [Google Scholar]
- 13.Jin, J. S., T. Nishihata, N. Kakiuchi, and M. Hattori. 2008. Biotransformation of C-glucosylisoflavone puerarin to estrogenic (3S)-equol in co-culture of two human intestinal bacteria. Biol. Pharm. Bull. 31:1621-1625. [DOI] [PubMed] [Google Scholar]
- 14.Kinjo, J., R. Tsuchihashi, K. Morito, T. Hirose, T. Aomori, T. Nagao, H. Okabe, T. Nohara, and Y. Masamune. 2004. Interactions of phytoestrogens with estrogen receptors α and β (III). Estrogenic activities of soy isoflavone aglycones and their metabolites isolated from human urine. Biol. Pharm. Bull. 27:185-188. [DOI] [PubMed] [Google Scholar]
- 15.Lawson, P. A., H. L. Greetham, G. R. Gibson, C. Giffard, E. Falsen, and M. D. Collins. 2005. Slackia faecicanis sp. nov., isolated from canine faeces. Int. J. Syst. Evol. Microbiol. 55:1243-1246. [DOI] [PubMed] [Google Scholar]
- 16.Maruo, T., M. Sakamoto, C. Ito, T. Toda, and Y. Benno. 2008. Adlercreutzia equolifaciens gen. nov., sp. nov., an equol-producing bacterium isolated from human faeces, and emended description of the genus Eggerthella. Int. J. Syst. Evol. Microbiol. 58:1221-1227. [DOI] [PubMed] [Google Scholar]
- 17.Matthies, A., T. Clavel, M. Gütschow, W. Engst, D. Haller, M. Blaut, and A. Braune. 2008. Conversion of daidzein and genistein by a newly isolated anaerobic bacterium from mouse intestine. Appl. Environ. Microbiol. 74:4847-4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKay, G. S. 1970. Gram stain modified to improve colour contrast. J. Clin. Pathol. 23:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mesbah, M., U. Premachandran, and W. B. Whitman. 1989. Precise measurement of the G+C content of deoxyribonucleic acid by high-performance liquid chromatography. Int. J. Syst. Bacteriol. 39:159-167. [Google Scholar]
- 20.Minamida, K., K. Ota, M. Nishimukai, M. Tanaka, A. Abe, T. Sone, F. Tomita, H. Hara, and K. Asano. 2008. Asaccharobacter celatus gen. nov., sp. nov., isolated from rat caecum. Int. J. Syst. Evol. Microbiol. 58:1238-1240. [DOI] [PubMed] [Google Scholar]
- 21.Minamida, K., M. Tanaka, A. Abe, T. Sone, F. Tomita, H. Hara, and K. Asano. 2006. Production of equol from daidzein by gram-positive rod-shaped bacterium isolated from rat intestine. J. Biosci. Bioeng. 102:247-250. [DOI] [PubMed] [Google Scholar]
- 22.Morito, K., T. Hirose, J. Kinjo, T. Hirakawa, M. Okawa, T. Nohara, S. Ogawa, S. Inoue, M. Muramatsu, and Y. Masamune. 2001. Interaction of phytoestrogens with estrogen receptors α and β. Biol. Pharm. Bull. 24:351-356. [DOI] [PubMed] [Google Scholar]
- 23.Nubel, U., B. Engelen, A. Felske, J. Snaidr, A. Wieshuber, R. I. Amann, W. Ludwig, and H. Backhaus. 1996. Sequence heterogeneities of genes encoding 16S rRNAs in Paenibacillus polymyxa detected by temperature gradient gel electrophoresis. J. Bacteriol. 178:5636-5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page, R. D. 1996. TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 25.Poco, S. E., Jr., F. Nakazawa, T. Ikeda, M. Sato, T. Sato, and E. Hoshino. 1996. Eubacterium exiguum sp. nov., isolated from human oral lesions. Int. J. Syst. Bacteriol. 46:1120-1124. [DOI] [PubMed] [Google Scholar]
- 26.Rafii, F., C. Davis, M. Park, T. M. Heinze, and R. D. Beger. 2003. Variations in metabolism of the soy isoflavonoid daidzein by human intestinal microfloras from different individuals. Arch. Microbiol. 180:11-16. [DOI] [PubMed] [Google Scholar]
- 27.Scalbert, A., C. Manach, C. Morand, C. Remesy, and L. Jimenez. 2005. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 45:287-306. [DOI] [PubMed] [Google Scholar]
- 28.Setchell, K. D., N. M. Brown, and E. Lydeking-Olsen. 2002. The clinical importance of the metabolite equol—a clue to the effectiveness of soy and its isoflavones. J. Nutr. 132:3577-3584. [DOI] [PubMed] [Google Scholar]
- 29.Setchell, K. D., and A. Cassidy. 1999. Dietary isoflavones: biological effects and relevance to human health. J. Nutr. 129:758S-767S. [DOI] [PubMed] [Google Scholar]
- 30.Schaeffer, A. B., and M. Fulton. 1933. A simplified method of staining endospores. Science 77:194. [DOI] [PubMed] [Google Scholar]
- 31.Tamaoka, J., and K. Komagata. 1984. Determination of DNA base composition by reversed-phase high-performance liquid chromatography. FEMS Microbiol. Lett. 25:125-128. [Google Scholar]
- 32.Tamura, M., T. Tsushida, and K. Shinohara. 2007. Isolation of an isoflavone-metabolizing, Clostridium-like bacterium, strain TM-40, from human faeces. Anaerobe 13:32-35. [DOI] [PubMed] [Google Scholar]
- 33.Usui, T. 2006. Pharmaceutical prospects of phytoestrogens. Endocr. J. 53:7-20. [DOI] [PubMed] [Google Scholar]
- 34.Visuvanathan, S., M. T. Moss, J. L. Stanford, J. Hermon-Taylor, and J. McFadden. 1989. Simple enzymic method for isolation of DNA from diverse bacteria. J. Microbiol. Methods 10:59-64. [Google Scholar]
- 35.Wade, W. G., J. Downes, D. Dymock, S. J. Hiom, A. J. Weightman, F. E. Dewhirst, B. J. Paster, N. Tzellas, and B. Coleman. 1999. The family Coriobacteriaceae: reclassification of Eubacterium exiguum (Poco et al. 1996) and Peptostreptococcus heliotrinreducens (Lanigan 1976) as Slackia exigua gen. nov., comb. nov. and Slackia heliotrinireducens gen. nov., comb. nov., and Eubacterium lentum (Prevot 1938) as Eggerthella lenta gen. nov., comb. nov. Int. J. Syst. Bacteriol. 49:595-600. [DOI] [PubMed] [Google Scholar]
- 36.Wang, X. L., H. G. Hur, J. H. Lee, K. T. Kim, and S. I. Kim. 2005. Enantioselective synthesis of S-equol from dihydrodaidzein by a newly isolated anaerobic human intestinal bacterium. Appl. Environ. Microbiol. 71:214-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, X. L., K. H. Shin, H. G. Hur, and S. I. Kim. 2005. Enhanced biosynthesis of dihydrodaidzein and dihydrogenistein by a newly isolated bovine rumen anaerobic bacterium. J. Biotechnol. 115:261-269. [DOI] [PubMed] [Google Scholar]
- 38.Wayne, L. G., D. J. Brenner, R. R. Colwell, P. A. D. Grimont, O. Kandler, M. I. Krichevsky, L. H. Moore, W. E. C. Moore, R. G. E. Murray, E. Stackebrandt, M. Starr, and H. G. Trüper. 1987. Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. Int. J. Syst. Bacteriol. 37:463-464. [Google Scholar]
- 39.Williamson, G., and C. Manach. 2005. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 81:243S-255S. [DOI] [PubMed] [Google Scholar]
- 40.Yu, Z. T., W. Yao, and W. Y. Zhu. 2008. Isolation and identification of equol-producing bacterial strains from cultures of pig faeces. FEMS Microbiol. Lett. 282:73-80. [DOI] [PubMed] [Google Scholar]