Abstract
Yersinia enterocolitica is a food-borne pathogen with the ability to grow at cold temperatures and tolerate high osmolarity. The bacterium tolerates osmotic stress by intracellular accumulation of osmolytes, such as betaine. The proP gene and proU operon of Y. enterocolitica were sequenced, and single (ProP− ProU+ and ProP+ ProU−) and double (ProP− ProU−) mutants were generated. Upon exposure to osmotic or chill stress, the single and double mutants demonstrated a reduction in betaine uptake compared to that in the wild type, suggesting that proP and proU play a role in betaine uptake during osmotic and chill stress responses of Y. enterocolitica.
Yersinia enterocolitica is a gram-negative food-borne pathogen, with swine serving as a major reservoir for human pathogenic strains (4). A characteristic feature of Y. enterocolitica is its ability to survive and proliferate under conditions of cold temperatures (2, 17) and elevated osmolarity (5% sodium chloride) (3, 27). These properties of the pathogen are significant from a food safety perspective because cold storage (refrigeration and freezing) and high osmolarity (desiccation and addition of salt) are among the most common methods of food preservation. The psychrotrophic nature of Y. enterocolitica is known, and several research publications have examined the mechanisms underlying the low-temperature survival of this pathogen (1, 9, 18, 19). However, while the ability of Y. enterocolitica to tolerate high salt concentrations is well documented, studies investigating the osmotolerance mechanisms of Y. enterocolitica are limited.
Bacterial cells are required to maintain an intracellular osmotic pressure greater than that of the growth medium in order to generate cell turgor, the driving force for cell extension, growth, and division (7). Therefore, bacteria must be able to maintain turgor when exposed to variations in the osmotic pressure of the surrounding medium. Y. enterocolitica responds to elevated osmolarity in the environment by intracellular accumulation of osmolytes, such as betaine, dimethylglycine, and carnitine (20). It was found that betaine was the major osmolyte accumulated by Y. enterocolitica when grown in a nutritionally rich medium. Betaine (trimethylglycine or glycine betaine) is a quaternary amine found abundantly in plants and meat (25, 29). Betaine is one of the most common and effective osmolytes accumulated by gram-negative bacteria, such as Escherichia coli and Salmonella spp., in response to an osmotic shock (26). Although the biochemical and genetic bases of betaine transport in gram-negative bacteria are elucidated by the characterization of two genetically distinct betaine uptake systems, ProP and ProU (5, 6, 16), no information is available on the molecular systems responsible for betaine uptake in Y. enterocolitica in response to hyperosmotic stress. In gram-positive bacteria, such as Bacillus subtilis and Listeria monocytogenes, in addition to osmotic stress accumulation, betaine is also accumulated through a membrane transporter (Gbu) in response to chill stress. Y. enterocolitica also accumulates betaine in response to chill stress at 4°C (20), indicating the potential presence of an osmotic stress-induced transporter protein that can also function as a betaine transporter during chill stress. The objective of this study was to identify and characterize proP and proU of Y. enterocolitica involved in the uptake of betaine during osmotic and chill stress.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The bacterial strains and plasmids used in this study are listed in Table 1. All bacteriological media were purchased from Difco, Becton Dickinson (Sparks, MD). Y. enterocolitica and E. coli were routinely grown in Luria-Bertani (LB) broth at 30°C. LB agar was used as the solid medium for the growth of E. coli strains harboring the various plasmids. Yersinia selective agar was used as the selective growth medium for Y. enterocolitica. Antibiotics were added at the following levels when required: streptomycin (1 mg/ml), chloramphenicol (30 μg/ml), kanamycin (50 μg/ml), and ampicillin (100 μg/ml). For blue-white selection, isopropyl thiogalactoside (IPTG; final concentration of 0.1 mM) and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; 50 μg/ml) were added to LB agar plates.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| Strains | ||
| Y. enterocolitica | ||
| 9610 | ProP+ ProU+ | ATCC |
| 9610P | ProP− ProU+ | This study |
| 9610U | ProP+ ProU− | This study |
| 9610PU | ProP− ProU− | This study |
| E. coli | ||
| SM10(λpir) | thi thr tonA lacY supE recA::RP4-2-Te::Mu Kmr | 8 |
| JM109 | recA1 endA1 gyrA96 thi-1 hsdR17 (rK− mK+) relA1 supE44 Δ(lac-proAB)[F′ traD36 proAB lacIqZΔM15] | Promega |
| Plasmids | ||
| pGEM-T Easy | PCR product cloning vector; Apr | Promega |
| pACYC184 | General cloning vector; Cmr | New England Biolabs |
| pKAS46 | Suicide vector; Apr Kmr oriR6K rpsL+ | 30 |
| pGEMproP | 1,668-bp proP fragment inserted into pGEM-T Easy | This study |
| pGEMproV | 1,494-bp proV fragment inserted into pGEM-T Easy | This study |
| pGEMproW | 870-bp proW fragment inserted into pGEM-T Easy | This study |
| pGEMproX | 968-bp proV fragment inserted into pGEM-T Easy | This study |
| pPCAT | 931-bp cat inserted into pGEMproP | This study |
| pVCAT | 931-bp cat inserted into pGEMproV | This study |
| pKAP | proP::cat inserted into pKAS vector | This study |
| pKAV | proV::cat inserted into pKAS vector | This study |
DNA manipulations and analyses.
Genomic DNA from Y. enterocolitica was isolated by using an AquaPure genomic DNA isolation kit (Bio-Rad, Hercules, CA). Plasmid DNA from E. coli was extracted by using a QIAprep spin miniprep kit (Qiagen, Valencia, CA). Restriction endonuclease digestions, ligations, and transformations were performed as per standard protocols or the manufacturer's instructions.
Cloning and sequencing of proP, proV, proW, and proX of Y. enterocolitica.
Sequence analysis of the whole genome of Y. pestis provided by the Sanger Institute, Cambridge, United Kingdom (21), revealed the presence of a putative proP gene and proU operon. The proU operon is comprised of three open reading frames (ORFs), proV, proW, and proX. We hypothesized the presence of homologous genes in Y. enterocolitica. The primers for proP and individual ORFs of the proU operon were selected from the genome sequence of Y. pestis (GenBank accession no. AL590842.1) and synthesized commercially (IDT DNA, Coralville, IA). Primer pairs YEPROPFO1-YEPROPRO1, YEPROVFO1-YEPROVRO1, YEPROWFI1-YEPROWRI1, and YEPROXFI1-YEPROXRI1 were used to amplify putative proP (1,668 bp), proV (1,494 bp), proW (870 bp), and proX (968 bp) genes, respectively, of Y. enterocolitica ATCC 9160. The nucleotide sequences of the primers used in this study are provided in Table 2. The amplified PCR products corresponding to the proP, proV, proW, and proX genes were gel extracted, cloned separately into the pGEM-T Easy vector (Promega Corp, Madison, WI) by utilizing the TA cloning strategy, and transformed into competent E. coli (JM109) cells (Promega) to obtain the recombinant plasmids pGEMproP, pGEMproV, pGEMproW, and pGEMproX. The presence of proP, proV, proW, and proX inserts in the recombinant white colonies was confirmed by restriction digestion and sequencing of the extracted plasmid DNA. The sequences generated were analyzed by using Sequencher 4.1.4 (Gene Codes Corporation, Ann Arbor, MI), BPROM (http://www.softberry.com), ClustalW (EMBnet), and Blastn (NCBI).
TABLE 2.
Primers used for PCR and sequencing
| Primer | Sequence | Description |
|---|---|---|
| YEPROPFO1 | ATGCTGAAGTGGTAGCGG | Amplification of proP |
| YEPROPRO1 | TCGGGTCAAGCAGAAGGTC | Amplification of proP |
| YEPROVFO1 | AGAAGCAACCTCATGACTCG | Amplification of proV |
| YEPROVRO1 | AGGGTTGTCTCAGATTCTCATTGTG | Amplification of proV |
| YEPROWFI1 | ATACCAGCGGCCAACGCCT | Amplification of proW |
| YEPROWRI1 | TCAGCCTGATGGACCCGTT | Amplification of proW |
| YEPROXFI1 | CAGCCATCAAAGGTTGACTG | Amplification of proX |
| YEPROXRI1 | GGGAAAATTATGCGTAAGACCG | Amplification of proX |
| CATFASIS1 | CAGGTGCGATCGCTATCACTTATTCAGGCGTA | Amplification of cat with AsiS1 |
| CATRASIS1 | CTCGTGCGATCGCTAAATACCTGTGACGGAAG | Amplification of cat with AsiS1 |
| CATFBSU361 | CACGTCCTCAGGTATCACTTATTCAGGCGTAGC | Amplification of cat with Bsu361 |
| CATRBSU361 | CACGTCCTCAGGTAAATACCTGTGACGGAAGA | Amplification of cat with Bsu361 |
Construction of Y. enterocolitica transporter mutant strains. (i) Creation of Y. enterocolitica proP and proU mutants.
A proP mutant of Y. enterocolitica was created by integrating an insertionally mutated copy of the gene into the chromosomal DNA of Y. enterocolitica ATCC 9610 by using the streptomycin-sensitive (Strs), ampicillin-resistant (Ampr), kanamycin-resistant (Kanr) suicide vector pKAS46 (24). The recombinant pGEM-T Easy plasmid designated pGEMproP (described above) and containing the whole proP insert was restriction digested with Bsu36I and ligated with a ∼930-bp chloramphenicol acetyltransferase (cat) cassette. The cat cassette coding for chloramphenicol resistance was amplified from the plasmid pACYC184 (New England Biolabs, Beverly, MA) by PCR using primers CATFBsu36I and CATRBsu36I, designed with restriction sites for the enzyme Bsu36I at their ends. The resulting plasmid, pGEMproP::CAT, designated pPCAT, was digested with NotI to excise the proP::cat fragment. This fragment was ligated into the unique NotI site of the suicide vector pKAS46 to yield the plasmid pKAP. This suicide vector, pKAP, containing the mutated copy of the proP gene, was transformed into E. coli SM10(λpir) and then mobilized into streptomycin-resistant Y. enterocolitica (wild type) by conjugation. Chromosomal proP mutants of Y. enterocolitica showing successful double-crossover events were selected on LB agar containing 30 μg/ml of chloramphenicol and 1 mg/ml streptomycin by following established procedures (24). This proP mutant strain (ProP− ProU+) was designated 9610P.
The components of the ProU osmotolerance system are encoded by an operon containing three ORFs: the proV, proW, and proX genes. The proU operon was mutated by inserting a cat cassette in proV, the first ORF of the operon, using procedures similar to those followed for mutating the proP gene. Briefly, similar to the suicide vector pKAP (described above) for the proP gene, a suicide vector pKAV, containing the mutated copy of the proV gene, was generated and used to create the chromosomal proV mutant of Y. enterocolitica. The proV insertional mutation by cat cassette resulted in the proU insertional mutation in Y. enterocolitica. This proU mutant strain (ProP+ ProU−) was designated 9610U.
(ii) Creation of a double mutant of Y. enterocolitica with mutations in proP and the proU operon.
A double-mutant strain with mutations in proP and the proU operon was constructed by replacing the wild-type copies of the respective genes with insertionally inactivated copies. This was accomplished by mobilizing pKAP, containing an insertionally inactivated copy of proP, into the streptomycin-resistant Y. enterocolitica proU mutant (9610U) by conjugation, as described above. Here chloramphenicol selection was not effective since the recipient (9610U) was already chloramphenicol resistant due to the presence of the cat cassette in its nonfunctional proU copy. Therefore, the transconjugants with single-crossover events were selected on yersinia selective agar plates containing 100 μg/ml of ampicillin and 100 μg/ml of kanamycin, since pKAP is ampicillin and kanamycin resistant. The second crossover event could lead to a desired double mutant or reversion back to the original recipient, 9610U, when plated on LB agar containing 30 μg/ml of chloramphenicol and 1 mg/ml streptomycin. Therefore, numerous colonies were subjected to rapid colony PCR after the second recombination selection, to identify the desired double mutant. The double mutants were also checked for the excision of plasmid pKAP by plating for ampicillin and kanamycin sensitivity. This proP proU mutant strain (ProP− ProU−) was designated 9610PU. The insertion of the cat cassette in the single and double mutants was confirmed by PCR, Southern hybridization, and sequencing (data not shown).
Growth measurements.
To assess the ability of the wild-type and mutant strains of Y. enterocolitica to use betaine as an osmoprotectant, cells were grown in modified minimal A (MMA) medium (20). Bacteria were grown in MMA medium for 18 h at 30°C and subsequently inoculated (2%, vol/vol) into 250-ml Erlenmeyer flasks containing 100 ml of MMA medium with or without 2.5% NaCl or MMA medium with 2.5% NaCl that was supplemented with 1 mM betaine. The cultures were incubated at 30°C or 4°C, and growth was monitored spectrophotometrically at 600 nm. The results are reported as the averages of the results for triplicate samples. The experiments were repeated at least three times.
Betaine uptake.
To measure the ability of the wild-type and mutant strains of Y. enterocolitica to accumulate betaine, log-phase cells grown in LB at 30°C were harvested by centrifugation (13,000 × g for 2 min), washed twice, and resuspended in MMA medium to an optical density at 600 nm of ca. 1.0. The cells were energized by the addition of glucose (5 mM), and where indicated, 2.5% sodium chloride was also added. After 20 min of incubation at 30°C, the assays were initiated by the addition of [14C]betaine (final concentration, 1 mM; 65 μCi/mmol) (American Radiolabeled Chemicals, Inc., St. Louis, MO). The mixtures were incubated separately at 30°C for 0, 20, 40, and 60 min or at 4°C for 0, 30, 60, and 120 min, and 1-ml samples were removed and centrifuged through silicon oil, as described by Kashket and Barker (12). Radioactivity was measured by scintillation counting, and betaine uptake was calculated per milligram of total cellular protein. The results were reported as the average of the results for triplicate samples. The experiments were repeated three times. Statistical analysis was performed by using the mixed-model analysis of variance procedure of Statistical Analysis software (SAS Institute, Inc., Cary, NC).
Nucleotide sequence accession numbers.
The nucleotide sequences obtained for the Y. enterocolitica proP, proV, proW, and proX genes were deposited in the GenBank database (NCBI) under the accession numbers AY605256, AY570522, AY605257, and AY570523, respectively.
RESULTS
Sequence analysis of the proP gene and proU operon.
A 1,668-bp fragment of Y. enterocolitica chromosomal DNA containing the proP gene along with its flanking sequences was amplified by using primers complementary to the respective regions conserved in Y. pestis. The putative ORF as predicted by the ORF finder (NCBI) was 1,368 bp long, extending from base 188 to base 1,555 of the cloned fragment. Alignment and sequence comparison of the complete proP sequence (1,368 bp) from Y. enterocolitica ATCC 9610 with those of Y. pestis and Yersinia pseudotuberculosis revealed a sequence identity of 83%. Comparison of the deduced amino acid sequence of the Y. enterocolitica glycine/proline permease (ProP protein) with the putative ProP proteins of Y. pestis and Y. pseudotuberculosis (see Fig. S1 in the supplemental material) revealed that the proteins share an identity of 90% and a sequence conservation of 94% at the amino acid level. Further, ProP showed a 53% sequence conservation with E. coli ProP, or proline permease.
Three more fragments of Y. enterocolitica chromosomal DNA, 1,494 bp, 870 bp, and 968 bp in size, encompassing the complete coding sequence of the proV gene and partial coding sequences of proW and proX, respectively, were also amplified by using primers complementary to the regions conserved in Y. pestis. In Y. pestis, proV, proW, and proX form the ORFs that comprise the proU operon. Sequence analysis of the 1,494-bp fragment of proV revealed a 1,200-bp ORF, extending from base 182 to base 1,381 of the cloned fragment. Alignment and sequence comparison of the complete 1,200-bp ORF with those of Y. pestis and Y. pseudotuberculosis revealed a sequence homology of 87%. The deduced amino acid sequence of the ProV protein shared an identity of 95% and a sequence conservation of 99% with the ProV proteins from Y. pestis and Y. pseudotuberculosis (see Fig. S2 in the supplemental material). The partial coding sequences of proW and proX share homologies of 83 and 86%, respectively, with those of Y. pestis and Y. pseudotuberculosis at the nucleic acid level. Further, the partially deduced amino acid sequences of both the ProW and ProX proteins revealed identities and sequence conservation of more than 95% with their counterpart proteins in Y. pestis and Y. pseudotuberculosis. Comparison of the protein sequences of the products of the three ORFs with those of the E. coli K12 genome revealed a high conservation of 83% at the amino acid level to the betaine transporter belonging to the ABC (ATP binding cassette) super family of membrane proteins.
Betaine accumulation in osmotically stressed Y. enterocolitica.
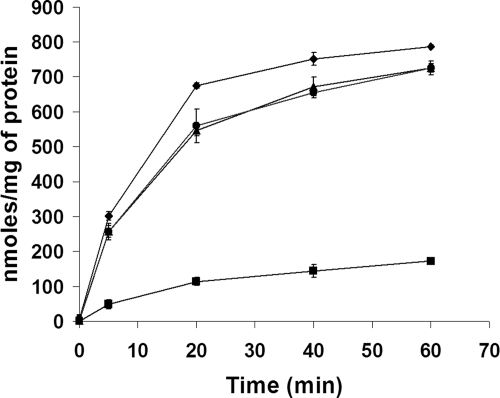
In the absence of sodium chloride, the bacterial cells were not subjected to any osmotic stress and both the wild-type and mutant strains of Y. enterocolitica did not accumulate any betaine (data not shown). Figure 1 depicts the accumulation of betaine by Y. enterocolitica in the presence of 2.5% sodium chloride at 30°C. The wild-type strain, containing the fully functional proP and proU, started accumulating betaine as early as 5 min in the presence of sodium chloride. It was found to accumulate ∼800 nmol of betaine per mg of protein by 60 min of incubation. The single (9610P or 9610U) and double (9610PU) mutants accumulated ∼725 and ∼170 nmol betaine/mg of protein, respectively, following 60 min of hyperosmotic challenge. Although the inactivation of proP or proU resulted in only a small reduction (8% at 60 min of incubation) of betaine accumulation in the single mutants compared to that in the wild type, there was a significant reduction of 80% in betaine accumulation in the double mutant, where both transporters involved in the osmolyte transport were inactivated. However, there was no significant difference (P > 0.05) in betaine accumulation in the single-mutant strains, 9610P and 9610U.
FIG. 1.
Accumulation of betaine, in response to osmotic stress (2.5% sodium chloride) at 30°C, by Y. enterocolitica. Cell suspensions containing [14C]betaine (1 mM) were incubated for 0, 20, 40, and 60 min. At appropriate time points, 1 ml of cell suspension was centrifuged and the pellet was subjected to scintillation counting to calculate betaine accumulation. Betaine accumulation was normalized per mg of total cellular protein. Error bars reflect standard errors of the means. ♦, 9610 (ProP+ ProU+); ▴, 9610P (ProP− ProU+); •, 9610U (ProP+ ProU−); ▪, 9610PU (ProP− ProU−).
Betaine accumulation in chill-stressed Y. enterocolitica.
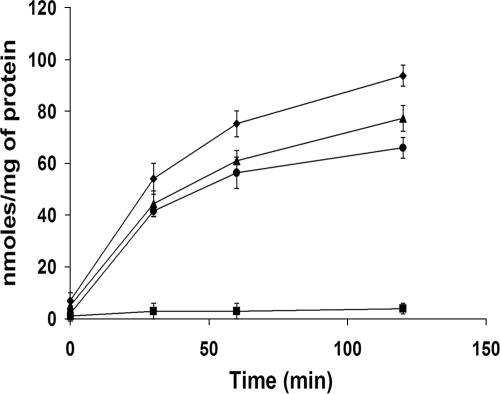
Since it is known that betaine is accumulated in response to chill stress in some psychrotrophic bacteria, such as L. monocytogenes, betaine accumulation by the wild-type and mutant strains of Y. enterocolitica was studied to determine if inactivation of any of the betaine transporters would have an effect on the accumulation of the osmolyte during chill stress (4°C). Both the wild type and the single mutants followed a pattern of betaine accumulation during chill stress that was similar to that seen under osmotic stress. At the end of 120 min of cold stress, the mean betaine levels accumulated by the wild-type and single-mutant strains were approximately 90 and 60 to 70 nmol/mg protein, respectively (Fig. 2). However, the double-mutant strain (9610PU) accumulated only negligible levels of betaine and showed a 97% reduction compared to the accumulation in the wild-type strain at the end of 120 min of chill stress. Although betaine accumulation declined by ∼25% when only proP or proU was inactivated, there was no significant difference in the osmolyte uptake of the two single mutants.
FIG. 2.
Accumulation of betaine, in response to chill stress at 4°C, by Y. enterocolitica. Cell suspensions containing [14C]betaine (1 mM) were incubated for 0, 30, 60, and 120 min. At appropriate time points, 1 ml of cell suspension was centrifuged and the pellet was subjected to scintillation counting to calculate betaine accumulation. Betaine accumulation was normalized per mg of total cellular protein. Error bars reflect standard errors of the means. ♦, 9610 (ProP+ ProU+); ▴, 9610P (ProP− ProU+); •, 9610U (ProP+ ProU−); ▪, 9610PU (ProP− ProU−).
Effect of betaine on growth of Y. enterocolitica under cold and osmotic stress.
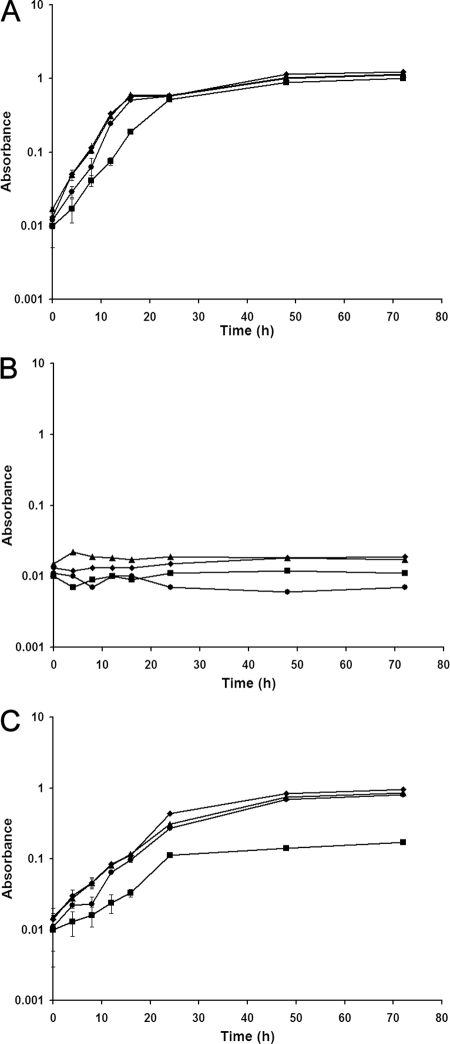
To provide evidence on the ability of exogenous betaine to enter the cell through the transporters (ProP and ProU) and protect the cell against osmotic stress, wild-type and mutant strains of Y. enterocolitica were grown in MMA medium containing betaine with or without added sodium chloride and incubated at 30°C. In the absence of osmotic stress, i.e., when no salt was added to the MMA medium, there was no significant difference between the growth rates of the wild-type and the different mutant strains (Fig. 3A). However, the double mutant (9610PU) demonstrated a slightly extended lag compared to the growth rates of the wild-type and the single mutants (9610P or 9610U) (Fig. 3A). No visible growth was observed when the wild-type and mutant strains were subjected to an osmotic stress of 2.5% NaCl at 30°C in MMA medium over a period of 72 h in the absence of betaine (Fig. 3B). However, when betaine (1 mM) was added to MMA medium containing 2.5% NaCl, growth was observed in the different strains of Y. enterocolitica (Fig. 3C). Although there was no significant difference between the growth rates of the single mutants and the wild type, a marked reduction (P < 0.05) in the growth of the double mutant was observed.
FIG. 3.
Growth, measured by absorbance at an optical density of 600 nm, of wild-type and mutant strains of Y. enterocolitica at 30°C in MMA medium (A), in MMA medium containing 2.5% sodium chloride (B), and in MMA medium containing 2.5% sodium chloride and 1 mM betaine (C). Error bars reflect standard errors of the means. ♦, 9610 (ProP+ ProU+); ▴, 9610P (ProP− ProU+); •, 9610U (ProP+ ProU−); ▪, 9610PU (ProP− ProU−).
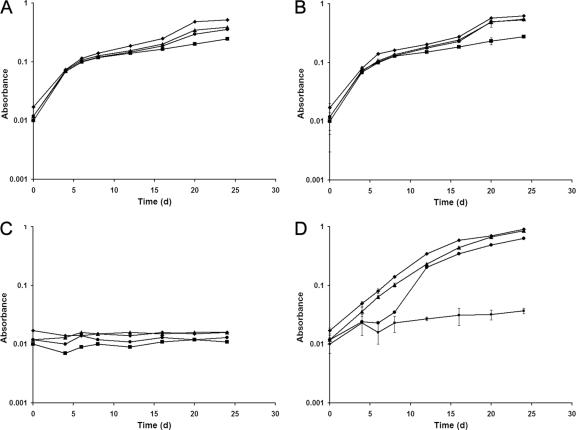
The effect of exogenous betaine on the growth of the wild-type and mutant strains of Y. enterocolitica when subjected to chill stress (4°C) and osmotic stress (2.5% sodium chloride) was studied by incubating the bacteria in MMA medium with or without betaine. When the cells were subjected only to chill stress, there was no difference in the growth rates of the wild-type and mutant strains regardless of the absence (Fig. 4A) or presence of 1 mM betaine (Fig. 4B). However, if the cells were subjected to an osmotic stress in addition to chill stress, the presence of betaine significantly enhanced the growth of the wild type and the single mutants over that of the double mutant (Fig. 4D) in comparison to the results under similar growth conditions in the absence of betaine (Fig. 4C). The double mutant (9610PU) failed to grow significantly and demonstrated a substantial reduction in its growth rate even in the presence of betaine (Fig. 4D).
FIG. 4.
Growth, measured by absorbance at an optical density of 600 nm, of wild-type and mutant strains of Y. enterocolitica at 4°C in MMA medium (A), in MMA medium containing 1 mM betaine (B), in MMA medium containing 2.5% sodium chloride (C), and in MMA medium containing 2.5% sodium chloride and 1 mM betaine (D). Error bars reflect standard errors of the means. ♦, 9610 (ProP+ ProU+); ▴, 9610P (ProP− ProU+); •, 9610U (ProP+ ProU−); ▪, 9610PU (ProP− ProU−).
DISCUSSION
ProP and ProU are the two major transporters responsible for the uptake of osmolytes in gram-negative enteric bacteria (10). ProP is a single-component, proton-driven transport system mediating the transport of a wide variety of osmoprotectants structurally related to proline and betaine (23). ProP-mediated import of compatible solutes is greatly enhanced in highly osmotic conditions as a result of increased proP expression and stimulated transport activity (13). ProU, the second osmoprotectant transporter of E. coli, is a binding protein-dependent transport system and a member of the ABC super family of transporters. The ProU system consists of a membrane-associated ATPase (ProV), the integral membrane protein ProW, and the periplasmic substrate-binding protein ProX. ProU is a high-affinity transport system for betaine (28).
Our DNA manipulations of Y. enterocolitica bacteria resulted in identifying proP and the individual ORFs of the proU operon, namely proV, proW, and proX. As evident from the gene sequence analysis, these genetic loci are highly conserved (more than 90% homologous) among the three major species of Yersinia, Y. pestis, Y. pseudotuberculosis, and Y. enterocolitica. After sequencing and identification of the aforementioned genetic loci, we characterized their function by insertional inactivation of the proP gene and the proU operon. A similar approach was followed by Fraser et al. (8), who characterized the carnitine uptake system opuC operon in L. monocytogenes by insertionally inactivating the first ORF, opuCA, located in the opuC operon.
The phenomenon of osmotic stress-induced betaine accumulation has been observed in several other osmoregulation studies, involving E. coli, L. monocytogenes, and Y. enterocolitica (15, 20, 22). As observed in our study, a similar level of betaine accumulation by a wild-type strain of Y. enterocolitica (9610) under comparable conditions was previously reported by Park and coworkers (20). In our study, although the single-mutant strains showed reduced betaine uptake compared to that of the wild type, there was no difference between them in osmolyte uptake; only the double-mutant strain exhibited a significant limitation in betaine uptake (Fig. 1). Similar to the results for betaine uptake, the single mutants had growth characteristics identical to that of the wild type when osmotically stressed in the presence of betaine at 30°C (Fig. 3C). However, the double mutant (9610PU) demonstrated a noticeable decrease in its growth rate and could not reach the cell density of the wild-type or single-mutant strains throughout the duration of the experiment (Fig. 3C). These results suggest that both proP and proU are involved in betaine uptake and that the inactivation of one of the betaine uptake systems is compensated by osmolyte uptake through the other system under the given conditions of osmotic stress.
A limited number of studies have investigated the effects on bacterial growth of mutations involving multiple osmolyte uptake systems. For example, Haardt et al. (11), while studying the influence of betaine on the growth of E. coli K12 osmolyte uptake mutants BK32 (ProP− ProU+), MKH17 (ProP+ ProU−), and MKH13 (ProP− ProU−) in MMA medium containing 0.3 M NaCl, observed that the double mutant did not grow well in the high-osmolarity medium. Moreover, they found that the single mutants had similar growth patterns and were able to grow at relatively higher rates than the double-mutant strain, MKH13. Likewise, it was also observed for Salmonella enterica serovar Typhimurium that strains carrying a mutation in both proP and proU were severely restricted in betaine uptake, whereas the single-mutant strains exhibited only a minimal reduction in the uptake of this osmolyte (6).
An interesting property of osmolytes, particularly betaine, is their ability to act as cryoprotectants in certain psychrotrophic bacteria, besides the osmoprotective effect. Although the exact mechanism by which betaine confers cryotolerance has not been elucidated, in L. monocytogenes during cold stress, an increased transport and accumulation of betaine from the growth medium has been observed (15). Further, a null mutation in gbu, which encodes an ATP-dependent betaine transporter in L. monocytogenes, abolishes the accumulation of betaine and impairs the mutant's growth at refrigeration temperatures (14). In Y. enterocolitica, it has been shown that chill stress stimulates the intracellular accumulation of betaine (20). These researchers observed that wild-type Y. enterocolitica (9610) accumulated betaine when subjected to a chill stress of 4°C in MMA medium containing 1 mM betaine. While we report similar betaine accumulation by the wild-type strain in response to chill stress, we also demonstrate the role of proP and proU in transporting betaine into Y. enterocolitica in response to chill stress. As seen in the case of osmotic stress-induced betaine accumulation, a significant difference in betaine uptake was only observed in the case of the double mutant, where there was a ∼97% reduction in its uptake compared to the accumulation in the wild-type strain (Fig. 4). Previously, Park et al. (20) observed that although betaine accumulation did not appear to contribute significantly to the increased cold stress survival of Y. enterocolitica at low osmolarity, the addition of a mere 1% NaCl showed that betaine conferred a greater improvement in the growth rate of chill-stressed cells. A similar growth-enhancing effect of betaine on the chill-stressed cells of L. monocytogenes only in the presence of an additional osmotic stress was also observed by Ko et al. (15). Similar to the above-described results, we also found in our study that the cryoprotective effect of betaine in the absence of an osmotic stress was minimal. The growth rates of chill-stressed wild-type and mutant strains of Y. enterocolitica in the absence of betaine (Fig. 4A) did not show any significant difference from the growth rates of the different strains of chill-stressed Y. enterocolitica in the presence of betaine (Fig. 4B). But when the bacteria were osmotically stressed, the presence of betaine in MMA medium enhanced the growth of chill-stressed wild-type and single-mutant strains significantly more than that of the double mutant (Fig. 4D).
In summary, it is clear from the results of this study that proU and proP play an important role in betaine uptake by Y. enterocolitica when the cells are subjected to cold and osmotic stresses. Our future studies will focus on the ability of proU and proP loci to act as transporters of other osmolytes, in addition to betaine, from complex environments, such as foods.
Supplementary Material
Footnotes
Published ahead of print on 29 December 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Annamalai, T., and K. Venkitanarayanan. 2005. Expression of major cold shock proteins and genes by Yersinia enterocolitica in synthetic medium and foods. J. Food Prot. 68:2454-2458. [DOI] [PubMed] [Google Scholar]
- 2.Bhaduri, S. 2006. Enrichment, isolation, and virulence of freeze-stressed plasmid-bearing virulent strains of Yersinia enterocolitica on pork. J. Food Prot. 69:1983-1985. [DOI] [PubMed] [Google Scholar]
- 3.Bhaduri, S., R. Buchanan, and J. G. Phillips. 1995. Expanded response surface model for predicting the effects of temperatures, pH, sodium chloride contents and sodium nitrite concentrations on the growth rate of Yersinia enterocolitica. J. Appl. Bacteriol. 79:163-170. [DOI] [PubMed] [Google Scholar]
- 4.Bottone, E. J. 1997. Yersinia enterocolitica: the charisma continues. Clin. Microbiol. Rev. 10:257-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cairney, J., I. R. Booth, and C. F. Higgins. 1985. Osmoregulation of gene expression in Salmonella typhimurium: proU encodes an osmotically induced betaine transport system. J. Bacteriol. 164:1224-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cairney, J., I. R. Booth, and C. F. Higgins. 1985. Salmonella typhimurium proP gene encodes a transport system for the osmoprotectant betaine. J. Bacteriol. 164:1218-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csonka, L. N. 1989. Physiological and genetic responses of bacteria to osmotic stress. Microbiol. Rev. 53:21-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser, K. R., D. Harvie, P. J. Coote, and C. P. O'Byrne. 2000. Identification and characterization of an ATP binding cassette l-carnitine transporter in Listeria monocytogenes. Appl. Environ. Microbiol. 66:4696-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goverde, R. L., J. H. Huis in't Veld, J. G. Kusters, and F. R. Mooi. 1998. The psychrotrophic bacterium Yersinia enterocolitica requires expression of pnp, the gene for polynucleotide phosphorylase, for growth at low temperature (5°C). Mol. Microbiol. 28:555-569. [DOI] [PubMed] [Google Scholar]
- 10.Gutierrez, C., T. Abee, and I. R. Booth. 1995. Physiology of the osmotic stress response in microorganisms. Int. J. Food Microbiol. 28:233-244. [DOI] [PubMed] [Google Scholar]
- 11.Haardt, M., B. Kempf, E. Faatz, and E. Bremer. 1995. The osmoprotectant proline betaine is a major substrate for the binding-protein-dependent transport system ProU of Escherichia coli K-12. Mol. Gen. Genet. 246:783-786. [DOI] [PubMed] [Google Scholar]
- 12.Kashket, E. R., and S. L. Barker. 1977. Effects of potassium ions on the electrical and pH gradients across the membrane of Streptococcus lactis cells. J. Bacteriol. 130:1017-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kempf, B., and E. Bremer. 1998. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol. 170:319-330. [DOI] [PubMed] [Google Scholar]
- 14.Ko, R., and L. T. Smith. 1999. Identification of an ATP-driven, osmoregulated glycine betaine transport system in Listeria monocytogenes. Appl. Environ. Microbiol. 65:4040-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ko, R., L. T. Smith, and G. M. Smith. 1994. Glycine betaine confers enhanced osmotolerance and cryotolerance on Listeria monocytogenes. J. Bacteriol. 176:426-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.May, G., E. Faatz, M. Villarejo, and E. Bremer. 1986. Binding protein dependent transport of glycine betaine and its osmotic regulation in Escherichia coli K12. Mol. Gen. Genet. 205:225-233. [DOI] [PubMed] [Google Scholar]
- 17.Mollaret, H. H., and E. Thal. 1974. Yersinia, p. 330-332. In R. E. Buchanan and N. E. Gibbons (ed.), Bergey's manual of determinative bacteriology, 8th ed. Williams and Wilkins, Baltimore, MD.
- 18.Neuhaus, K., K. P. Francis, S. Rapposch, A. Görg, and S. Scherer. 1999. Pathogenic Yersinia species carry a novel, cold-inducible, major cold shock protein tandem gene duplication producing both bicistronic and monocistronic mRNA. J. Bacteriol. 181:6449-6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neuhaus, K., N. Anastasov, V. Kaberdin, K. P. Francis, V. L. Miller, and S. Scherer. 2003. The AGUAAA motif in cspA1/A2 mRNA is important for adaptation of Yersinia enterocolitica to grow at low temperature. Mol. Microbiol. 50:1629-1645. [DOI] [PubMed] [Google Scholar]
- 20.Park, S., L. T. Smith, and G. M. Smith. 1995. Role of glycine betaine and related osmolytes in osmotic stress adaptation in Yersinia enterocolitica ATCC 9610. Appl. Environ. Microbiol. 61:4378-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parkhill, J., B. W. Wren, N. R. Thomson, R. W. Titball, M. T. Holden, M. B. Prentice, M. Sebaihia, K. D. James, C. Churcher, K. L. Mungall, S. Baker, D. Basham, S. D. Bentley, K. Brooks, A. M. Cerdeno-Tarraga, T. Chillingworth, A. Cronin, R. M. Davies, P. Davis, G. Dougan, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Leather, S. Moule, P. C. Oyston, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413:523-527. [DOI] [PubMed] [Google Scholar]
- 22.Perroud, B., and D. Le Rudulier. 1985. Glycine betaine transport in Escherichia coli: osmotic modulation. J. Bacteriol. 161:393-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Racher, K. I., D. E. Culham, and J. M. Wood. 2001. Requirements for osmosensing and osmotic activation of transporter ProP from Escherichia coli. Biochemistry 40:7324-7333. [DOI] [PubMed] [Google Scholar]
- 24.Skorupski, K., and R. K. Taylor. 1996. Positive selection vectors for allelic exchange. Gene 169:47-52. [DOI] [PubMed] [Google Scholar]
- 25.Smith, L. T. 1996. Role of osmolytes in adaptation of osmotically stressed and chill-stressed Listeria monocytogenes grown in liquid media and on processed meat surfaces. Appl. Environ. Microbiol. 62:3088-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stirling, D. A., C. S. J. Hulton, L. Waddell, S. F. Park, G. S. A. B. Stewart, I. R. Booth, and C. F. Higgins. 1989. Molecular characterization of the proU loci of Salmonella typhimurium and Escherichia coli encoding osmoregulated glycine betaine transport systems. Mol. Microbiol. 3:1025-1038. [DOI] [PubMed] [Google Scholar]
- 27.Sutherland, J. P., and A. J. Bayliss. 1994. Predictive modelling of growth of Yersinia enterocolitica: the effects of temperature, pH and sodium chloride. Int. J. Food Microbiol. 21:197-215. [DOI] [PubMed] [Google Scholar]
- 28.Sutherland, L., J. Cairney, M. J. Elmore, I. R. Booth, and C. F. Higgins. 1986. Osmotic regulation of transcription: induction of the proU betaine transport gene is dependent on the intracellular potassium. J. Bacteriol. 168:805-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verheul, A., E. Glaasker, B. Poolman, and T. Abee. 1997. Betaine and l-carnitine transport by Listeria monocytogenes ScottA in response to osmotic signals. J. Bacteriol. 179:16979-16985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.