Abstract
The gram-positive bacterium Listeria monocytogenes is a food-borne pathogen of both public health and food safety significance. It possesses three small, highly homologous protein members of the cold shock protein (Csp) family. We used gene expression analysis and a set of mutants with single, double, and triple deletions of the csp genes to evaluate the roles of CspA, CspB, and CspD in the cold and osmotic (NaCl) stress adaptation responses of L. monocytogenes. All three Csps are dispensable for growth at optimal temperature (37°C). These proteins are, however, required for efficient cold and osmotic stress tolerance of this bacterium. The hierarchies of their functional importance differ, depending on the environmental stress conditions: CspA>CspD>CspB in response to cold stress versus CspD>CspA/CspB in response to NaCl salt osmotic stress. The fact that Csps are promoting L. monocytogenes adaptation against both cold and NaCl stress has significant implications in view of practical food microbial control measures. The combined or sequential exposure of L. monocytogenes cells to these two stresses in food environments might inadvertently induce cross-protection responses.
Listeria monocytogenes is a gram-positive food-borne pathogen of public health concern as well as a food safety challenge. This pathogen, primarily encountered through contaminated ready-to-eat foods, infects high-risk individuals, such as immunocompromised adults, pregnant women, and neonates, and leads to listeriosis, a rare but highly invasive disease with severe clinical signs and relatively high mortality (20 to 30%) (32, 35). The ubiquity of these organisms in nature and in a wide range of food-related environments means that they are a considerable microbial control challenge in food production (9). Moreover, L. monocytogenes organisms efficiently adapt and sometimes proliferate, despite exposure to low temperatures, low pH, and elevated salt (NaCl) concentrations, conditions used in preserving ready-to-eat food products (10, 24, 36). Improvement of food safety measures taken against this pathogen will depend on further insights gained into molecular cell response mechanisms underlying the various stress resistance phenotypes displayed by these organisms.
The cold shock protein (Csp) family consists of small, highly conserved, and structurally related nucleic acid binding proteins that presumably have important roles in regulation of various microbial physiological processes (8). These proteins are widely distributed among prokaryotes, including L. monocytogenes, and are often encoded through differentially regulated multiple gene families per organism (16, 29, 38). Csps are thought to serve as nucleic acid chaperones that bind RNA and DNA and thus may facilitate the control of processes such as replication, transcription, and translation within bacterial cells (8). To date, the multiple-homolog nature and functional variability of Csps are mostly derived from studies conducted with Escherichia coli and Bacillus subtilis. Nine Csps (CspA to CspI) are found in E. coli, and five (CspA, CspB, CspE, CspG, and CspI) have been linked to modulation of cold adaptation functions (12, 23, 26, 38). The CspC and CspE proteins have been implicated in chromosomal condensation and cell division and in the regulation of RpoS and UspA stress response proteins (2, 17, 19, 30, 43). Furthermore, the CspD protein of this organism has been suggested to regulate nutrient and stationary-phase stress adaptation responses (42). In B. subtilis, three Csps (CspA, CspB, and CspD) have been described and have been associated with regulation of both normal growth and cold and stationary-phase stress adaptation responses (14, 39). There is, however, limited knowledge regarding the function of Csps found in various psychrotolerant food-borne pathogens (25, 27, 40). An improved understanding of the role of these proteins in the normal growth and stress adaptation of L. monocytogenes is of particular interest, given the challenges that these organisms pose to food safety. Three Csp family proteins (CspA, CspB, and CspD) are found within the sequenced genomes of L. monocytogenes, but their functions are not yet understood (11). The present study was therefore conducted to gain further insights into Csp involvement in the normal growth and stress adaptation responses of this bacterium. To achieve this, csp gene expression patterns and the growth phenotypes of csp gene family deletion mutants were evaluated in response to optimal temperature (37°C), cold stress (4 and 10°C), and elevated NaCl salt concentration conditions.
MATERIALS AND METHODS
Bacterial strains, plasmids, and general techniques.
The wild-type and mutant bacterial strains, as well as the different plasmid vectors, used in this study are listed in Table 1. The clinical reference strain L. monocytogenes EGD-e (11) and its derivatives carrying various csp gene family deletion mutants were used. The E. coli host for all plasmid construction and propagation steps was the XL-1 Blue strain (3). The plasmids pKSV7 (32) and pPL2 (21) were employed for genetic disruption and complementation, respectively. Bacterial genomic DNA was isolated with the DNeasy blood and tissue kit (Qiagen). Plasmids were prepared using the plasmid midikit (Qiagen). PCR amplification was performed using the FastStart high-fidelity PCR system (Roche Molecular Diagnostics GmbH, Penzburg, Germany). Restriction enzymes, T4 DNA ligase, and Taq DNA polymerase were used in accordance with the protocols of the supplier (Roche). PCR products and DNA restriction fragments were cut and purified from agarose gels using the mini elute gel extraction kit (Qiagen). Oligonucleotides were all ordered from Microsynth AG (Balgach, Switzerland). DNA sequencing was also performed at Microsynth AG. Bacterial strains were grown using brain heart infusion (BHI) (Oxoid) or Luria-Bertani (LB) (Difco Laboratories) broth or chemically defined minimal media (DM) (31). Osmolarity in salt-supplemented DM (DMS; 2.2% NaCl) and BHI plus 3% NaCl media was adjusted by addition of NaCl to the desired levels.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype | Genetic deletion | Reference or source |
|---|---|---|---|
| Strains | |||
| EGD-e | Wild type (1/2a) | None | 11 |
| ΔcspA | ΔcspA | 153-bp in-frame cspA deletion | This study |
| ΔcspB | ΔcspB | 147-bp in-frame cspB deletion | This study |
| ΔcspD | ΔcspD | 147-bp in-frame cspD deletion | This study |
| ΔcspAB | ΔcspAB | cspA and cspB in-frame deletions | This study |
| ΔcspAD | ΔcspAD | cspA and cspD in-frame deletions | This study |
| ΔcspABD | ΔcspABD | cspA, cspB, and cspD in-frame deletions | This study |
| Plasmids | |||
| pKSV7 | 33 | ||
| pKSV7-ΔcspA | This study | ||
| pKSV7-ΔcspB | This study | ||
| pKSV7-ΔcspD | This study | ||
| pPL2 | 21 | ||
| pPL2-cspA | This study | ||
| pPL2-cspD | This study |
Genetic deletion and complementation.
A set of csp gene deletion mutants was created in the EGD-e strain background. The splicing-by-overlap extension (SOE) PCR protocol was used for nonpolar deletions of the csp gene family (18). The ΔcspA, ΔcspB, and ΔcspD constructs were created by PCR amplification of EGD-e DNA templates by using the various SOE primers listed in Table 2. The SOE PCR products were, thereafter, cloned into the pKSV7 plasmid by using the incorporated restriction enzyme sites. The pKSV7-ΔcspA, pKSV7-ΔcspB, and pKSV7-ΔcspD plasmid constructs were confirmed by DNA sequencing. The deletion of the targeted genes through homologous recombination and allelic exchange was facilitated by following previously described protocols with some modifications (4). Briefly, L. monocytogenes cells were transformed with the plasmid DNA constructs as previously described (28). Transformants were selected on LB plates in the presence of chloramphenicol (10 μg/ml) after 48 to 72 h of incubation at 30°C. Integration mutants were enriched for 3 to 6 generations at 42°C in 10 ml BHI broth plus chloramphenicol (10 μg/ml). The integrated strains were grown on BHI agar plates in the presence of chloramphenicol (10 μg/ml) for 24 to 48 h at 42°C. Colonies of integrants were confirmed after colony PCR analysis with a primer combination that bound to defined regions in the pKSV7 plasmid and EGD-e genomic DNA. Plasmid excision was facilitated through growth of the integrants in BHI broth for 3 to 50 generations. During this period, samples were drawn at every third generation and plated onto BHI agar plates. The colonies were thereafter plated onto BHI plates with and without antibiotic to identify chloramphenicol-sensitive colonies in which the plasmid had been excised. PCR analysis and subsequent DNA sequencing of the targeted regions confirmed deletion mutants. The ΔcspA, ΔcspB, and ΔcspD single-deletion mutant strains were generated initially. These strains were subsequently used in a second round of mutagenesis to target a second csp gene to create the ΔcspAB, ΔcspAD, and ΔcspBD double-deletion mutants. Finally, the cspA gene was targeted in the ΔcspBD strain, generating the ΔcspABD triple-deletion mutant strain. Defective phenotypes of ΔcspA and ΔcspD strains were complemented using a pPL2 plasmid-based system as described previously (21). DNA fragments consisting of the complete cspA and cspD genes, as well as their entire upstream sequence between them and the preceding gene, were amplified from the EGD-e DNA templates and subsequently cloned between the PstI and SmaI restriction sites of the pPL2 vector to create the pPL2-cspA and pPL2-cspD complementation plasmids. Upstream sequences included the predicted promoter elements and ribosome binding sites preceding these two genes. These constructs were confirmed by DNA sequencing and were subsequently used to transform the ΔcspA and ΔcspD strains, respectively. Colonies of transformants obtained on LB agar plates in the presence of chloramphenicol (10 μg/ml) were analyzed for proper integration of pPL2-cspA and pPL2-cspD constructs into the PSA prophage integration site by PCR analysis using the previously described NC16/PL95 primer set (21).
TABLE 2.
Oligonucleotide primers used in this studyf
| Primer | Sequenceg |
|---|---|
| SOE-P1-CspA-A | GGAATTCCGGAAAACAAACAAGTGACGCTa |
| SOE-P1-CspA-B | TGCTTGAGGTCCGCGACCTTGTTCCATGTTCATGTTCCT |
| SOE-P1-CspA-C | AGGAACATGGAACAAGGTCGCGGACCTCAAGCAGCTe |
| SOE-P1-CspA-D | ACGCGTCGACCACCATCACCGATTATCGGAATAb |
| SOE-P2-CspB-A | TCCCCCGGGGCGTCCGGCAAGTGTGAATTGGTTAGTGAGCGAGc |
| SOE-P2-CspB-B | TGCTTGTGGGCCACGAACTGTACCTGTTTGCATATTTCACAA |
| SOE-P2-CspB-C | AACAGGTACAGTTCGTGGCCCACAAGCAGAAAAAGe |
| SOE-P2-CspB-D | TCCCCCGGG CGCACTGGAATACAAGCTGGGACTATGCCCGCTTTc |
| SOE-P3-CspD-A | TCCCCCGGGCCACCATTTTTGTTACAGAGGAAGGAc |
| SOE-P3-CspD-B | CAATGCAAAAT GGAAAGTACGCGGCGCTCAAG CAG |
| SOE-P3-CspD-C | CTGCTTGAGCGCCGCGTACTTTCCCATTTTGCATTGAAATAAATCCe |
| SOE-P3-CspD-D | TCCCCCGGGCGCACTGGAATACAAGCTGGGACTATGCCCGCTTTc |
| CspA-comp-P1 | AAAACTGCAG CTGATTTAATCGCACTTAGAGAAAATTAATCAd |
| CspA-comp-P2 | TCCCCCGGG TTACGCTTTTTGAACGTTAGCTGCTTc |
| CspA-fw | AACATGGAACAAGGTACAG* |
| CspA-rv | GTTGGCCTTCTTCAACG* |
| CspB-fw | CAAACAGGTACAGTTAAATGGTTTA* |
| CspB-rv | ACGATTTCAAATTCAACGCTTTGA* |
| CspD-fw | TACGGTTTTATCGAATCAGAC* |
| CspD-rv | ACGTTAGCTGCTTGAG* |
| 16S rRNA-fw | CTTCCGCAATGGACGAAAGT* |
| 16S rRNA-rv | CTCATCGTTTACGGCGTG* |
The EcoRI restriction site incorporated in this primer to facilitate cloning is underlined.
The SalI restriction site incorporated in this primer to facilitate cloning is underlined.
The SmaI restriction site incorporated in this primer to facilitate cloning is underlined.
The PstI restriction site incorporated in this primer to facilitate cloning is underlined.
The regions complementary to SOE-P1-CspA-B, SOE-P1-CspB-B, and SOE-P1-CspD-B primers are in italics.
Oligonucleotides were synthesized at Microsynth AG (Balgach, Switzerland). Primers were designed using the LightCycler probe and primer design software (Roche Molecular Diagnostics GmBH, Penzburg, Germany).
*, quantitative real-time RT-PCR primer.
Growth conditions.
Stationary phase inocula (109 CFU/ml) of wild-type and mutant strains were prepared by growth of single colonies in 10 ml BHI overnight (16 to 18 h) in a shaking incubator (220 rpm) at 37°C. The stationary-phase inocula were serially diluted 10-fold to 105 CFU/ml in 0.85% NaCl-peptone. Thereafter, aliquots of 100 μl were used for inoculation of 10 ml BHI, DM, or DMS, resulting in 103 CFU/ml starter cultures. Growth evaluation in BHI and DM at different temperatures involved preparation of three duplicate sets from each strain, which were subsequently incubated at 37, 10, and 4°C, without shaking. For growth experiments in DMS (DM plus 2.2% NaCl), 10-ml duplicate cultures were inoculated and similarly incubated at 37°C. The growth kinetics in each sample was monitored by standard colony counting at defined time intervals. The results presented in this study depict means and standard deviations of three independent experimental runs.
Cold and NaCl stress adaptation experiments.
For cold stress exposure, 10-ml BHI stationary-phase cultures of the wild-type EGD-e strain were prepared by overnight growth at 37°C. The stationary-phase cultures were centrifuged (4,000 × g for 5 min), and the pellets were resuspended in 10 ml fresh BHI and divided into two 5-ml aliquots, which were habituated to 4°C and 37°C by 2 h of incubation. Thereafter, 1.5-ml samples were drawn, and total RNA templates were isolated as outlined below. For NaCl stress exposure, stationary-phase inocula, prepared as described above, were used to inoculate 10 ml of BHI or BHI plus 3% NaCl at 103 CFU/ml. These cultures were then incubated without shaking at 37°C for 10 (BHI) and 15 (BHI-NaCl) h to reach the late log phase, which was confirmed by colony counting in each case. At this point, 1.5-ml aliquots were drawn and directly processed for total RNA as outlined below or frozen at −20°C until processed at a later date.
Total RNA extraction.
The RNA extraction procedure that was employed combined rapid mechanical lysis and column-based RNA purification. The 1.5-ml aliquots from each culture were centrifuged at 4°C (cold-adapted cells) or room temperature (non-cold-adapted cells) for 5 min at 4,000 × g. The supernatants were discarded, and pellets were resuspended in 0.5 ml lysis buffer provided in the RNeasy plus minikit (Qiagen). The mixtures were transferred onto the lysing bead matrix in MagNA lyser tubes (Roche Molecular Diagnostics GmbH, Penzburg, Germany). Bacterial cells were mechanically disrupted using the MagNA lyser instrument (6,500 rpm for 60 s). Total RNA was purified from the lysates on RNA binding columns according to the RNeasy plus minikit protocol and included two DNA removal steps. The cell lysates were passed through a genomic DNA removal column, and an on-column DNase I digestion step was performed. Total RNA was eluted using 30 μl of RNase-free water, and the yield was determined by measuring absorption at 260 nm using the Nanodrop ND1000 (Nanodrop Instruments, DE). RNA template integrity was further analyzed under UV light after denaturing agarose gel electrophoresis and ethidium bromide staining.
Reverse transcription.
Reverse transcription (RT) was performed using the Quantitect RT kit (Qiagen). Three hundred nanograms of total RNA from each sample were converted into cDNA in 20-μl reaction mixtures. Similar amounts of total RNA from each sample were also subjected to the cDNA synthesis reaction without the inclusion of the reverse transcriptase enzyme as controls. These were subsequently used as “minus RT” controls to assess potential residual DNA contamination in total RNA templates.
Real-time PCR and quantification of gene expression.
Primers used in this study are listed in Table 2. The real-time PCRs were performed in the LightCycler 480 instrument (Roche Molecular Diagnostics, Rotkreuz, Switzerland). The reactions were performed in a final reaction volume of 10 μl: 2.5 μl (3.75 ng) cDNA (1:10 dilution of cDNA generated as described above); 2.5 μl (0.4 μM) of gene-specific forward and reverse primer mix; 5 μl of 2× LightCycler 480 SYBR green I master mix. The real-time PCR run protocol was comprised of preincubation (4 min at 95°C), 40 amplification cycles (10 s at 95°C; 20 s at 56°C; 20 s at 72°C; 5 s at 80°C with a single fluorescent measurement); and melting curve (65 to 97°C at 2.2°C/s plus continuous fluorescent measurement). Primers and reaction conditions were optimized for specificity and target amplification, with efficiencies of 90 to 100%. Transcript levels were determined using the LightCycler 480 relative quantification software (Roche Molecular Diagnostics). Initially a reference gene validation under the different experimental conditions was performed as previously described (37), and the 16S rRNA transcripts were established as the most suitable internal control reference gene for relative mRNA quantification in our study. Based on this, we quantified csp gene transcripts relative to the 16S rRNA transcript level of the same sample.
Statistical analysis.
Statistical analysis was performed using the Stat View 4.02 (Abacus Concepts Inc., Berkeley, CA) program. The bacterial colony counts were converted into log CFU/ml followed by calculation of the means and standard deviations. The statistical significances of differences in the bacterial colony counts between the wild type and the csp mutants were evaluated using one-way analysis of variance at a significance level (α) of 0.05. The comparative data derived from quantitative gene expression analysis were analyzed using Student's t test, and differences with P values of <0.05 were considered to be statistically significant.
Bioinformatics.
The Vector NTI Advance 10.3 program (Invitrogen) was used for DNA and protein sequence manipulations. Protein motif search and hairpin predictions were performed using the DNASIS MaxVersion 2.6 (Miraibio, San Francisco, CA). Sequence homologies and protein sequence alignments were investigated using the BLASTn and BLASTp (NCBI) programs and multiple sequence alignment with hierarchical clustering (7).
RESULTS
Comparative nucleotide and amino acid sequence analysis of L. monocytogenes Csps.
Nucleotide sequence comparison of Csps in the EGD-e strain revealed high levels of sequence similarity, with identities ranging from 77 to 84% (data not shown). All the csp open reading frames in this organism are preceded by relatively long stretches of 5′ untranslated leader regions (5 ′UTR) ranging from 198 bp (cspA) to 363 bp (cspD), a situation which is similar to what has previously been described for some csp genes of other bacterial species (1, 34). These long 5′ UTR regions were predicted to be rich in RNA hairpin loop secondary structures (DNASIS MaxVersion 2.6 program; Miraibio, San Francisco, CA), which might have implications for the regulation of csp gene expression in this organism. The amino acid sequence comparison matrix of L. monocytogenes EGD-e Csps, and the homologs of E. coli (CspAEc) and B. subtilis (CspBBs) are presented in Table 3. As shown, L. monocytogenes CspA, CspB, and CspD also share high levels of amino acid sequence identity (67 to 73%). These proteins are also closely related to the B. subtilis (72 to 80%) and E. coli (58 to 62%) homologs. In fact, L. monocytogenes CspA and CspB are more closely related to B. subtilis CspBBs (74 to 80%) than to the other Csps of this organism. An amino acid sequence alignment further highlights general sequence conservation and the canonical nucleic acid binding motifs, RNP-1 and RNP-2 (Fig. 1). Although some amino acid substitutions are found within the RNP-1 and RNP-2 motifs, these are conserved. As shown, a tyrosine-to-phenylalanine substitution occurs in L. monocytogenes CspB RNP-1 and an isoleucine-to-valine substitution in L. monocytogenes CspB and CspD RNP-2 motifs, compared to the motifs of E. coli and B. subtilis homologs.
TABLE 3.
Amino acid sequence comparison matrix of L. monocytogenes Csps, E. coli CspA, and B. subtilis CspBa
| Csp | Sequence identity (%)
|
||||
|---|---|---|---|---|---|
| CspALm | CspBLm | CspDLm | CspAEc | CspBBs | |
| CspALm | 100 | ||||
| CspBLm | 73 | 100 | |||
| CspDLm | 73 | 67 | 100 | ||
| CspAEc | 62 | 59 | 58 | 100 | |
| CspBBs | 74 | 80 | 72 | 61 | 100 |
Lm, L. monocytogenes; Ec, E. coli; Bs, B. subtilis.
FIG. 1.
Amino acid sequence alignment of L. monocytogenes Csps (CspALm, CspBLm, and CspDLm) and the E. coli (CspAEc) and B. subtilis (CspBBs) homologs. The sequences were aligned using the program for multiple sequence alignment with hierarchical clustering (6). Dots represent identical amino acids in all five Csp homologs, and dashes represent the gaps placed in the sequences by the alignment program to optimize the alignment. The highly conserved nucleic acid binding motifs (RNP-1 and RNP-2) of cold shock domain proteins are highlighted in bold and underlined.
Cold stress-associated induction of csp gene expression.
The effect of L. monocytogenes cold adaptation on the expression pattern of its csp gene family was examined. To achieve this, stationary-phase L. monocytogenes EGD-e cells originally grown at 37°C were adapted to cold stress conditions by incubation for 2 h at 4°C. As a control, an equal aliquot of the same EGD-e culture was also adapted to optimal temperature conditions by similar incubation for 2 h at 37°C. Total RNA isolated from these samples was used to determine the level of csp gene family (cspA, cspB, and cspD) transcripts in quantitative RT-PCR assays. A summary of the relative csp gene expression ratios at 4 and 37°C, as well as cold stress-dependent induction, are presented in Table 4. As shown, there is low constitutive expression of cspA, cspB, and cspD transcripts in EGD-e cells held at 37°C, but all csp gene transcripts are significantly induced (P < 0.05) in cold-adapted EGD-e cells at 4°C. Under these conditions, mean cold stress-dependent inductions of 23-fold, 4.5-fold, and 7.4-fold were observed for cspA, cpsB, and cspD transcripts, respectively.
TABLE 4.
Relative csp gene expression induction of cold stress-adapted stationary-phase EGD-e cells
| Gene | Relative gene expression ratio ata:
|
Fold induction at 4°C vs 37°Cb | |
|---|---|---|---|
| 37°C | 4°C | ||
| cspA | 0.02 ± 0.01 | 0.46 ± 0.20 | 23 |
| cspB | 0.37 ± 0.20 | 1.48 ± 0.30 | 4 |
| cspD | 0.05 ± 0.04 | 0.37 ± 0.10 | 7.4 |
The levels of csp gene transcripts are expressed relative to the levels of 16S rRNA reference gene transcripts.
The fold inductions in csp gene expression ratios of cold-adapted cells at 4°C are relative to those of control cells adapted to the optimal temperature (37°C).
NaCl stress-dependent induction of cspA and cspD gene transcripts.
Next, the influence of EGD-e strain growth under NaCl stress on csp gene family expression was investigated. For this purpose, late log phase EGD-e cells grown in BHI plus 3% NaCl and controls that had been similarly grown in regular BHI were compared. The csp gene expression results from these studies are presented in Table 5. As shown, both cspA and cspD transcripts were also significantly enhanced (P < 0.05) in NaCl stress-adapted EGD-e cells compared to the control of regular BHI-grown EGD-e cells. In contrast, there was no statistically significant increase associated with cspB transcripts (P > 0.05) in NaCl stress-adapted EGD-e cells (Table 5). The mean NaCl stress-dependent inductions achieved were 2.8-fold and 4.4-fold for cspA and cspD transcripts, respectively.
TABLE 5.
Relative csp gene expression induction of BHI-NaCl stress-adapted log phase EGD-e cells
| Gene | Relative gene expression ratio ina:
|
Fold induction in BHI-NaCl vs BHIb | |
|---|---|---|---|
| BHI | BHI-3% NaCl | ||
| cspA | 0.06 ± 0.01 | 0.17 ± 0.02 | 2.8 |
| cspB | 0.56 ± 0.31 | 0.83 ± 0.35 | NSc |
| cspD | 0.15 ± 0.01 | 0.66 ± 0.32 | 4.4 |
The levels of csp gene transcripts are expressed relative to the levels of 16S rRNA reference gene transcripts.
The fold inductions in the csp gene expression ratios of BHI-NaCl-grown cells are relative to those of regular BHI-grown controls.
The gene expression induction differences between stress- and non-stress-exposed control cells were not statistically significant (NS) (P > 0.05).
Creation of cspA deletion mutants in L. monocytogenes EGD-e.
To gain more insights into the phenotypic contributions of Csps to L. monocytogenes growth under different conditions, various deletion mutants were created in the EGD-e strain (Table 1). The deletions of the targeted Csps were all performed in frame and verified through PCR and DNA sequence analysis of the targeted DNA regions (data not shown). Furthermore, quantitative RT-PCR assays with gene-specific primer pairs confirmed that transcripts corresponding to targeted cspA genes were also absent in the relevant mutant strains (data not shown). The phenotypic restoration in ΔcspA and ΔcspD strains was accomplished using pPL2-cspA and pPL2-cspD plasmid constructs. This process restored deleted cspA and cspD genes by integration into the PSA prophage attB site of the L. monocytogenes EGD-e chromosome in respective mutant strains.
Csps are required for efficient L. monocytogenes growth under cold stress.
The growth kinetics of the wild type and the various cspA deletion mutant strains are summarized in Table 6. Representative growth curves depicting the growth of the wild type and selected cspA deletion mutants are presented in Fig. 2. As shown, an individual cspA, cspB or cspD gene deletion resulted in no detectable growth phenotype differences from the parent wild-type strain at 37°C in rich (BHI) and minimal (DM) nutrient conditions (Fig. 2A and B). Similarly, double deletions as well as triple deletions of all known csp genes produced no detectable growth phenotype defects at 37°C, as summarized by Table 6. Meanwhile, the growth analysis at 4°C showed that cspB deletion also had no influence, while deletion of cspA or cspD impairs L. monocytogenes growth under cold stress (Fig. 2C and D). In fact, the ΔcspA strain completely failed to grow at 4°C and 10°C in BHI and DM. In support of a causal link, the cold growth phenotype of the ΔcspA strain was successfully restored by a pPL2-cspA complementation (Fig. 2E). The cold growth phenotype in the ΔcspD strain was only significantly poor (P < 0.05) compared to that of the wild type at 4°C, but not at 10°C (Fig. 2C and D and Table 6). The cold-sensitive phenotype of this mutant was primarily observed with the defined minimal nutrient conditions of DM. But it was less pronounced within the rich complex nutrient background in BHI (Fig. 2C and D). Similarly, growth analysis of double (ΔcspAB, ΔcspAD, and ΔcspBD) and triple (ΔcspABD) Csp deletion strains further confirmed that a cspA or cspD gene deletion compromises the cold growth phenotypes of L. monocytogenes EGD-e (Table 6), while cspB gene functions seemed dispensable as long as the cspA and cspD genes were retained in this bacterium. This hypothesis was confirmed by cold growth analysis of a ΔcspDB mutant strain in DM at 4°C (Fig. 2F). As shown, growth in this mutant strain is further compromised by cspB deletion, compared to growth of the ΔcspD or ΔcspB strain (Fig. 2F). Thus, loss of cspB gene functions, combined with a cspD deletion, also leads to impaired cold growth of EGD-e cells, suggesting some minimal CspB protein contribution to cold adaptation functions of this organism.
TABLE 6.
Overview of csp gene deletion mutant growth phenotypes at 37, 10, and 4°C
| Strain | Growth phenotype at indicated temperature ina:
|
|||||
|---|---|---|---|---|---|---|
| BHI
|
DM
|
|||||
| 37°C | 10°C | 4°C | 37°C | 10°C | 4°C | |
| Wild type | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| ΔcspA | ++++ | − | − | ++++ | − | − |
| ΔcspB | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| ΔcspD | ++++ | ++++ | +++ | ++++ | ++++ | ++ |
| ΔcspAB | ++++ | − | − | ++++ | − | − |
| ΔcspAD | ++++ | − | − | ++++ | − | − |
| ΔcspBD | ++++ | ++++ | +++ | ++++ | ++++ | + |
| ΔcspABD | ++++ | − | − | ++++ | − | − |
The growth phenotype of each mutant strain is expressed relative to the wild-type strain. ++++ to +, most to least growth, respectively; −, no growth.
FIG. 2.
Growth of wild-type EGD-e and various csp gene family deletion mutants of this strain. (A to D) Growth kinetics of the wild-type, ΔcspA, ΔcspB, and ΔcspD strains in BHI and DM at optimal (37°C) and cold stress (4°C) temperatures. (E) Growth of wild-type, ΔcspA, and pPL2-cspA-complemented ΔcspA strains at 10°C in BHI. (F) Growth kinetics of ΔcspB, ΔcspD, and ΔcspBD at 4°C in DM. The results represent the means (± standard deviations) from duplicates of three independent experimental runs.
L. monocytogenes Csps contribute to efficient cellular growth under NaCl stress.
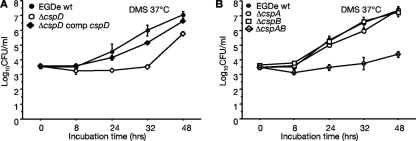
The growth responses of the wild type and various cspA deletion mutants were also investigated in DM plus 2.2% NaCl (DMS). The loss of cspD gene function significantly compromises the NaCl stress tolerance capacity of EGD-e cells (Fig. 3A). The growth of the ΔcspD in DMS was significantly slower than that of the wild-type strain. This was despite the fact that both strains grew similarly in regular DM (Fig. 2B). Phenotypic defects of ΔcspD growth in DMS were partly restored through pPL2-cspD-based genetic complementation (Fig. 3A). On the other hand, individual cspA and cspB gene deletions did not influence EGD-e growth phenotypes in DMS (Fig. 3B and Table 7). A double deletion of these two genes, however, significantly reduced growth in DMS (Fig. 3B). The loss of cspD gene function in cspA/D (ΔcspAD) and cspB/D (ΔcspBD) double-deletion mutants was also confirmed to impair growth phenotypes of EGD-e under NaCl stress conditions of DMS (Table 7). A cspA/B/D triple-deletion mutant also leads to complete growth failure of EGD-e cells in DMS, indicating that at least one Csp must be retained for L. monocytogenes growth at high NaCl salt concentrations.
FIG. 3.
Growth of the wild-type EGD-e and various csp gene family deletion mutants under NaCl stress in DMS. Growth kinetics in DMS of wild-type, ΔcspD, and pPL2-cspD-complemented ΔcspD strains (A) and wild-type, ΔcspA, ΔcspB, and ΔcspAB strains (B). The results are means (± standard deviations) from duplicates of three independent experimental runs.
TABLE 7.
Overview of Csp deletion mutant growth phenotypes in DM and DMS1
| Strain | Growth phenotype ina:
|
|
|---|---|---|
| DM | DMS | |
| Wild type | ++++ | ++++ |
| ΔcspA | ++++ | ++++ |
| ΔcspB | ++++ | ++++ |
| ΔcspD | ++++ | ++ |
| ΔcspAB | ++++ | ++ |
| ΔcspAD | ++++ | ++ |
| ΔcspBD | ++++ | ++ |
| ΔcspABD | ++++ | − |
The growth phenotype of each mutant strain is expressed relative to the wild-type strain. ++++ to ++, most to least growth, respectively; −, no growth.
DISCUSSION
Csp family proteins have previously been linked to regulation of both normal growth and stress adaptation processes against cold and nutrient starvation as well as to promotion of survival stationary-phase cells in some organisms (13-15, 41, 42). In cold-adapted L. monocytogenes cells, the induction of Csp-like proteins, as well as cspA gene transcripts, was previously documented (5, 40). The current study was conducted in order to expand this knowledge and to explore the Csps’ contribution in other stress responses of this organism. In contrast to B. subtilis, in which at least one csp gene is essential for viability (14), none of the three csp genes is essential for L. monocytogenes viability. In fact, a mutant carrying deletions of all three csp genes could be generated without any discernable defects in the growth phenotype at 37°C, even under the defined minimal nutrient conditions of DM. It thus appears that functions encoded by the L. monocytogenes Csp genes are not critical requirements for viability or efficient growth under optimal temperature conditions. To the contrary, a hierarchy (cspA>cspD>cspB) was established in csp gene importance for cold adaptation and L. monocytogenes growth at low temperatures (4 and 10°C). The deletion of the cspA gene completely abolishes cold growth, and cspD deletion leads to significantly reduced cold (4°C) growth efficiency of this organism. The functions of the cspB gene on the other hand are largely dispensable. There were no cold growth defects associated with the deletion of this gene, as long as the cpsA or cspD gene was retained.
The potential role of Csps in osmotic stress adaptation so far stems from the fact that expression of the cspC gene in Bordetella bronchiseptica is significantly induced following exposure of this organism to 2 M NaCl (34). We show here that direct growth of L. monocytogenes EGD-e under NaCl stress in BHI-NaCl leads to significant induction of cspA and cspD gene expression, although the overall NaCl stress-associated csp inductions appeared lower compared to those in cold-adapted cells. The induction trend observed of cspD>cspA was consistent with the subsequent trend observed in the stress sensitivity (ΔcspD>ΔcspA or ΔcspB) of the csp gene deletion mutants. In fact, a single deletion of cspA or cspB gave no discernable NaCl stress growth phenotypes in DMS cultures. The ΔcspAB double-deletion mutant was, however, also significantly impaired during growth under similar NaCl stress conditions. It also seems that at least one csp gene is required for L. monocytogenes growth at higher NaCl concentrations. A ΔcspABD triple-deletion mutant completely failed to grow under NaCl stress conditions in DMS cultures.
In this study, we have presented both gene expression and stress growth phenotypic evaluation data suggesting that L. monocytogenes Csps are functionally required for efficient cold and osmotic stress adaptation responses in this bacterium, although the precise nature of Csp involvement in such stress adaptation mechanisms still remains to be investigated. Key cold stress challenges include negatively supercoiled DNA and stabilized RNA secondary structures. These impair cellular replication, transcription, and translation processes. Therefore, increased Csp synthesis and activity at low temperatures provides DNA and RNA chaperone functions (8, 29), which are needed in cold-exposed L. monocytogenes cells to help resolve these nucleic acid structural hurdles. The Csp functional contributions to NaCl osmotic stress adaptation in L. monocytogenes are not yet clear. One possibility is that Csp chaperones might also promote the increased production of sodium ion extrusion transporter proteins through their effects in facilitating transcription and translation processes. This might enhance protection of L. monocytogenes cells from NaCl toxicity through increased extrusion of intracellular sodium ions. Alternatively, as observed in eukaryotic cells, high cellular NaCl concentration might lead to increased levels of cellular DNA damage in L. monocytogenes cells (20). It is thus plausible that Csps through their DNA chaperone activity facilitate repair of NaCl stress-damaged DNA and promote L. monocytogenes under NaCl-associated osmotic stress.
The fact that Csps seem to promote L. monocytogenes adaptation against both cold and NaCl stress also has significant implications in view of practical food microbial control measures. The combined or sequential exposure of L. monocytogenes cells to these two stresses in food environments might inadvertently induce cross-protection responses. Cold stress induced by low temperature may inadvertently cross-protect cells against NaCl stress due to induction of cspA, cspD, and cspB gene expression. Conversely, exposure to higher NaCl salt concentrations in some foods before cooling can also adapt cells to grow at low temperatures due to induction of cspA and cspD gene functions in response to NaCl stress. A similar phenomenon was recently described for the spoilage bacterium Shewanella putrefaciens (22) and for an lmo1078 gene transposon deletion mutant of L. monocytogenes (6). A mutant in this gene, which encodes a putative UDP-glucose phosphorylase enzyme, also exhibited cold and NaCl salt stress sensitivity.
Acknowledgments
B. Schmid was funded by a research grant from the University of Zurich.
Footnotes
Published ahead of print on 16 January 2009.
REFERENCES
- 1.Bae, W., P. G. Jones, and M. Inouye. 1997. CspA, the major cold shock protein of Escherichia coli, negatively regulates its own gene expression. J. Bacteriol. 179:7081-7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bae, W., S. Phadtare, K. Severinov, and M. Inouye. 1999. Characterization of Escherichia coli cspE, whose product negatively regulates transcription of cspA, the gene for the major cold shock protein. Mol. Microbiol. 31:1429-1441. [DOI] [PubMed] [Google Scholar]
- 3.Bullock, W. O., J. M. Fernandez, and J. M. Short. 1987. XL1-blue: a high efficiency plasmid transforming recA Escherichia coli strain with β-galactosidase selection. BioTechniques 5:376-379. [Google Scholar]
- 4.Camilli, A., L. G. Tilney, and D. A. Portnoy. 1993. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol. Microbiol. 8:143-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan, Y. C., S. Raengpradub, K. J. Boor, and M. Wiedmann. 2007. Microarray-based characterization of the Listeria monocytogenes cold regulon in log- and stationary-phase cells. Appl. Environ. Microbiol. 73:6484-6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chassaing, D., and F. Auvray. 2007. The lmo1078 gene encoding a putative UDP-glucose pyrophosphorylase is involved in growth of Listeria monocytogenes at low temperature. FEMS Microbiol. Lett. 275:31-37. [DOI] [PubMed] [Google Scholar]
- 7.Corpet, F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16:10881-10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ermolenko, D. N., and G. I. Makhatadze. 2002. Bacterial cold-shock proteins. Cell. Mol. Life Sci. 59:1902-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farber, J. M., and P. I. Peterkin. 1991. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55:476-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gandhi, M., and M. L. Chikindas. 2007. Listeria: a foodborne pathogen that knows how to survive. Int. J. Food Microbiol. 113:1-15. [DOI] [PubMed] [Google Scholar]
- 11.Glaser, P., L. Frangeul, C. Buchrieser, C. Rusniok, A. Amend, F. Baquero, P. Berche, H. Bloecker, P. Brandt, T. Chakraborty, A. Charbit, F. Chetouani, E. Couve, A. de Daruvar, P. Dehoux, E. Domann, G. Dominguez-Bernal, E. Duchaud, L. Durant, O. Dussurget, K. D. Entian, H. Fsihi, F. Garcia-del Portillo, P. Garrido, L. Gautier, W. Goebel, N. Gomez-Lopez, T. Hain, J. Hauf, D. Jackson, L. M. Jones, U. Kaerst, J. Kreft, M. Kuhn, F. Kunst, G. Kurapkat, E. Madueno, A. Maitournam, J. M. Vicente, E. Ng, H. Nedjari, G. Nordsiek, S. Novella, B. de Pablos, J. C. Perez-Diaz, R. Purcell, B. Remmel, M. Rose, T. Schlueter, N. Simoes, A. Tierrez, J. A. Vazquez-Boland, H. Voss, J. Wehland, and P. Cossart. 2001. Comparative genomics of Listeria species. Science 294:849-852. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein, J., N. S. Pollitt, and M. Inouye. 1990. Major cold shock protein of Escherichia coli. Proc. Natl. Acad. Sci. USA 87:283-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graumann, P., K. Schroder, R. Schmid, and M. A. Marahiel. 1996. Cold shock stress-induced proteins in Bacillus subtilis. J. Bacteriol. 178:4611-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graumann, P., T. M. Wendrich, M. H. Weber, K. Schroder, and M. A. Marahiel. 1997. A family of cold shock proteins in Bacillus subtilis is essential for cellular growth and for efficient protein synthesis at optimal and low temperatures. Mol. Microbiol. 25:741-756. [DOI] [PubMed] [Google Scholar]
- 15.Graumann, P. L., and M. A. Marahiel. 1999. Cold shock proteins CspB and CspC are major stationary-phase-induced proteins in Bacillus subtilis. Arch. Microbiol. 171:135-138. [DOI] [PubMed] [Google Scholar]
- 16.Graumann, P. L., and M. A. Marahiel. 1998. A superfamily of proteins that contain the cold-shock domain. Trends Biochem. Sci. 23:286-290. [DOI] [PubMed] [Google Scholar]
- 17.Harrington, E. W., and N. J. Trun. 1997. Unfolding of the bacterial nucleoid both in vivo and in vitro as a result of exposure to camphor. J. Bacteriol. 179:2435-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horton, R. M., Z. L. Cai, S. N. Ho, and L. R. Pease. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. BioTechniques 8:528-535. [PubMed] [Google Scholar]
- 19.Hu, K. H., E. Liu, K. Dean, M. Gingras, W. DeGraff, and N. J. Trun. 1996. Overproduction of three genes leads to camphor resistance and chromosome condensation in Escherichia coli. Genetics 143:1521-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kültz, D., and D. Chakravarty. 2001. Hyperosmolality in the form of elevated NaCl but not urea causes DNA damage in murine kidney cells. Proc. Natl. Acad. Sci. USA 98:1999-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauer, P., M. Y. Chow, M. J. Loessner, D. A. Portnoy, and R. Calendar. 2002. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J. Bacteriol. 184:4177-4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leblanc, L., C. Leboeuf, F. Leroi, A. Hartke, and Y. Auffray. 2003. Comparison between NaCl tolerance response and acclimation to cold temperature in Shewanella putrefaciens. Curr. Microbiol. 46:157-162. [DOI] [PubMed] [Google Scholar]
- 23.Lee, S. J., A. Xie, W. Jiang, J. P. Etchegaray, P. G. Jones, and M. Inouye. 1994. Family of the major cold-shock protein, CspA (CS7.4), of Escherichia coli, whose members show a high sequence similarity with the eukaryotic Y-box binding proteins. Mol. Microbiol. 11:833-839. [DOI] [PubMed] [Google Scholar]
- 24.Lianou, A., and J. N. Sofos. 2007. A review of the incidence and transmission of Listeria monocytogenes in ready-to-eat products in retail and food service environments. J. Food Prot. 70:2172-2198. [DOI] [PubMed] [Google Scholar]
- 25.Mayr, B., T. Kaplan, S. Lechner, and S. Scherer. 1996. Identification and purification of a family of dimeric major cold shock protein homologs from the psychrotrophic Bacillus cereus WSBC 10201. J. Bacteriol. 178:2916-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakashima, K., K. Kanamaru, T. Mizuno, and K. Horikoshi. 1996. A novel member of the cspA family of genes that is induced by cold shock in Escherichia coli. J. Bacteriol. 178:2994-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neuhaus, K., K. P. Francis, S. Rapposch, A. Gorg, and S. Scherer. 1999. Pathogenic Yersinia species carry a novel, cold-inducible major cold shock protein tandem gene duplication producing both bicistronic and monocistronic mRNA. J. Bacteriol. 181:6449-6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park, S. F., and G. S. Stewart. 1990. High-efficiency transformation of Listeria monocytogenes by electroporation of penicillin-treated cells. Gene 94:129-132. [DOI] [PubMed] [Google Scholar]
- 29.Phadtare, S. 2004. Recent developments in bacterial cold-shock response. Curr. Issues Mol. Biol. 6:125-136. [PubMed] [Google Scholar]
- 30.Phadtare, S., and M. Inouye. 2001. Role of CspC and CspE in regulation of expression of RpoS and UspA, the stress response proteins in Escherichia coli. J. Bacteriol. 183:1205-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Premaratne, R. J., W. J. Lin, and E. A. Johnson. 1991. Development of an improved chemically defined minimal medium for Listeria monocytogenes. Appl. Environ. Microbiol. 57:3046-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramaswamy, V., V. M. Cresence, J. S. Rejitha, M. U. Lekshmi, K. S. Dharsana, S. P. Prasad, and H. M. Vijila. 2007. Listeria—review of epidemiology and pathogenesis. J. Microbiol. Immunol. Infect. 40:4-13. [PubMed] [Google Scholar]
- 33.Smith, K., and P. Youngman. 1992. Use of a new integrational vector to investigate compartment-specific expression of the Bacillus subtilis spoIIM gene. Biochimie 74:705-711. [DOI] [PubMed] [Google Scholar]
- 34.Stubs, D., T. M. Fuchs, B. Schneider, A. Bosserhoff, and R. Gross. 2005. Identification and regulation of cold-inducible factors of Bordetella bronchiseptica. Microbiology 151:1895-1909. [DOI] [PubMed] [Google Scholar]
- 35.Swaminathan, B., and P. Gerner-Smidt. 2007. The epidemiology of human listeriosis. Microbes Infect. 9:1236-1243. [DOI] [PubMed] [Google Scholar]
- 36.Tasara, T., and R. Stephan. 2006. Cold stress tolerance of Listeria monocytogenes: a review of molecular adaptive mechanisms and food safety implications. J. Food Prot. 69:1473-1484. [DOI] [PubMed] [Google Scholar]
- 37.Tasara, T., and R. Stephan. 2007. Evaluation of housekeeping genes in Listeria monocytogenes as potential internal control references for normalizing mRNA expression levels in stress adaptation models using real-time PCR. FEMS Microbiol. Lett. 269:265-272. [DOI] [PubMed] [Google Scholar]
- 38.Wang, N., K. Yamanaka, and M. Inouye. 1999. CspI, the ninth member of the CspA family of Escherichia coli, is induced upon cold shock. J. Bacteriol. 181:1603-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weber, M. H., and M. A. Marahiel. 2002. Coping with the cold: the cold shock response in the Gram-positive soil bacterium Bacillus subtilis. Philos. Trans. R. Soc. Lond. B 357:895-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wemekamp-Kamphuis, H. H., A. K. Karatzas, J. A. Wouters, and T. Abee. 2002. Enhanced levels of cold shock proteins in Listeria monocytogenes LO28 upon exposure to low temperature and high hydrostatic pressure. Appl. Environ. Microbiol. 68:456-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamanaka, K., L. Fang, and M. Inouye. 1998. The CspA family in Escherichia coli: multiple gene duplication for stress adaptation. Mol. Microbiol. 27:247-255. [DOI] [PubMed] [Google Scholar]
- 42.Yamanaka, K., and M. Inouye. 1997. Growth-phase-dependent expression of cspD, encoding a member of the CspA family in Escherichia coli. J. Bacteriol. 179:5126-5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamanaka, K., T. Mitani, T. Ogura, H. Niki, and S. Hiraga. 1994. Cloning, sequencing, and characterization of multicopy suppressors of a mukB mutation in Escherichia coli. Mol. Microbiol. 13:301-312. [DOI] [PubMed] [Google Scholar]