Abstract
Tailoring steps are often important for the activity of mature antibiotics. Here, we report that novel decarboxylated FR-008/candicidin derivatives were obtained from the P450 monooxygenase gene fscP mutant of Streptomyces sp. strain FR-008. The toxicity of decarboxylated FR-008/candicidin derivatives has been shown to be greatly reduced compared to that of wild-type FR-008/candicidin.
Polyene compounds constitute a large group of effective clinical antifungal antibiotics (3). Polyene antibiotics have severe side effects in patients due to their affinity for sterols in the human body and are especially toxic to the kidney. Therefore, it is important to develop polyene derivatives with inherently reduced toxicity. It is a common phenomenon that the completed polyketide chains are released from the polyketide synthase and undergo further modifications after cyclization, called tailoring steps, including glycosylation, hydroxylation, carboxylation, and epoxylation (2, 4, 6, 13).
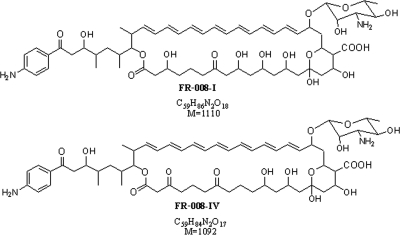
FR-008/candicidin (Fig. 1) is useful for the treatment of Trichomonas vaginalis infection and has been used for treating moniliform colpitis (8, 10, 12, 15). In addition, FR-008/candicidin can kill mosquito larvae (16), prevent benign prostatic hyperplasia, and has a proven effect on cholesterol and bile acid metabolism (11, 14).
FIG. 1.
Chemical structures of FR-008/candicidin. M, molecular weight.
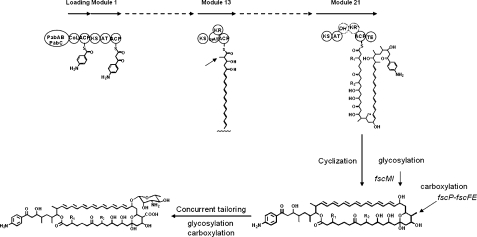
FscP encoded by the FR-008/candicidin gene cluster is highly homologous with its counterparts AmphN, NysN, and PimG of the other polyene pathways (1, 2, 4-8, 13). FscP and FscFE (carrying the electron transfer of ferredoxin in the P450 system) represent a P450 monooxygenase system that is postulated to be responsible for the oxidation of the methyl branch into a carboxyl group, presumably introduced in the 13th elongation step by the methylmalonate-specific AT13 of FscD (Fig. 2). It also remains ambiguous whether an additional oxidoreductase is required to convert a methyl branch into a carboxyl group. Our objective was to inactivate the fscP gene as an initial step toward understanding its functional role(s).
FIG. 2.
Proposed metabolic pathway of FR-008/candicidin.
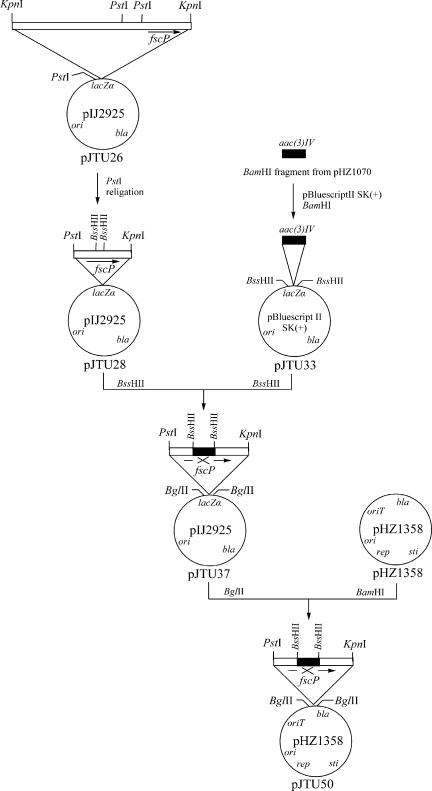
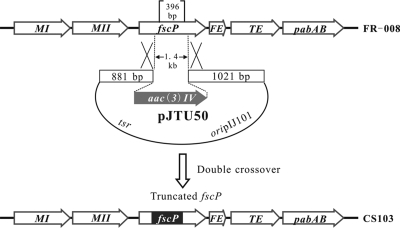
Our initial attempt to generate an in-frame deletion of the fscP gene resulted in a strain with poor sporulation and low antibiotic yield. We then generated a construct for the targeted replacement of a specific BssHII fragment inside fscP, with an apramycin resistance gene on the chromosome of Streptomyces sp. strain FR-008 (Fig. 3 and 4). We successfully obtained an fscP deletion mutant, CS103, producing novel FR-008/candicidin derivatives.
FIG. 3.
Schematic of the cloning strategy.
FIG. 4.
Deletion of fscP by insertion of an apramycin resistance gene [aac(3)IV] into the genome of wild-type Streptomyces sp. strain FR-008.
We next constructed an expression plasmid carrying fscP-fscFE and introduced it into CS103. CS103, complemented in this way, restored normal FR-008/candicidin production to a level similar to that of the wild-type Streptomyces sp. strain FR-008 (data not shown), which unambiguously confirmed that the deficiency of this specific P450 monooxygenase system accounted for the nonproducing phenotypes in CS103. As an additional control, CS103 was not complemented by the fscTE gene lying immediately downstream of fscP-fscFE, since FR-008/candicidin production was not recovered and no other phenotypic changes were observed, confirming that the recovered FR-008/candicidin production is fscP specific.
Although no FR-008/candicidin was detected by high-performance liquid chromatography analysis, we observed new peaks that possibly represent FR-008/candicidin derivatives in CS103. However, the production levels were only 1% of the level observed with the wild-type producer. By optimizing the production of the novel decarboxylated FR-008/candicidin during submerged fermentation of CS103, the production levels of the same producer improved from 3.33 μg/ml to an optimized level of 60 μg/ml in the 30-liter fermentor (see the supplemental material). This represents a successful combination of the modern genetic breeding of the antibiotic producer with the immediate improvement by traditional fermentation technology.
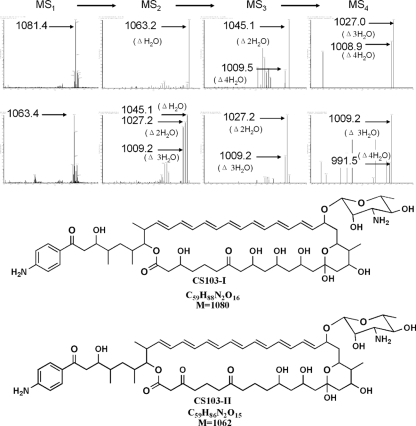
Analysis of the novel polyene fraction by electrospray ionization mass spectrometry (ESI-MS) revealed two major products showing absorption spectra characteristic of a heptaene macrolide, with calculated molecular weights of 1,080.5 and 1,062.5, respectively (Fig. 5). The molecular weights of these compounds correspond to the decarboxylated FR-008-I (molecular weight, 1,110.5) and FR-008-IV (molecular weight, 1,092.5), respectively, with a difference in weight of 30 (the difference between the methyl group and the carboxyl group). The two compounds with the same molecular weight, 1,062.5, seem to be isomers (possibly indicating the coexistence of a six-membered cyclic ketalic ring structure with the non-six-membered ketalic ring structure).
FIG. 5.
Analysis of the polyene produced by the fscP-interrupted mutant of Streptomyces sp. strain FR-008 by EIS-MS and MSn. CS103-I, 1,080.5 observed molecular weight (M + H = 1,081.5, M + Na = 1,103.5, M + K = 1,119.5); CS103-II, 1,062.5 observed molecular weight (M + H = 1,063.5, M + Na = 1,085.5, M + K = 1,101.5). Characterized loss of a carboxyl (—COO) moiety could not be detected by MSn analysis. The EIS-MS1/MS2/MS3/MS4 fragmentation data for each successive step relevant to each of the indicated structures were amplified to show m/z changes after the loss of specific groups [(H2O)n]. M, molecular weight.
We then carried out a mass fragmentation study of the above-mentioned compounds produced by CS103 (Fig. 5). Under the conditions described in our previous studies, the characteristic fragmentation pattern showed the loss of a carboxyl (—COO) moiety (mass, −44). Under the same conditions, we could not detect such a fragmentation pattern (mass, −44) (Fig. 5). Furthermore, the nuclear magnetic resonance spectrum of CS103-I (see Table S4 in the supplemental material) revealed no carboxyl group. This suggests that CS103-I and CS103-II do not harbor a carboxyl group, in agreement with their mass spectra as decarboxylated FR-008/candicidin derivatives.
The inhibitory activity of the novel decarboxylated FR-008/candicidin to Saccharomyces cerevisiae Y029 was compared in parallel with the inhibition zone displayed by the wild-type FR-008/candicidin compound. CS103 showed a MIC of 0.00312 to 0.00625 μg/ml, whereas FR-008/candicidin showed a MIC of 0.00039 to 0.00078 μg/ml. The antifungal activity of decarboxylated FR-008/candicidin is about 5 to 10 times lower than that displayed by the wild-type FR-008/candicidin.
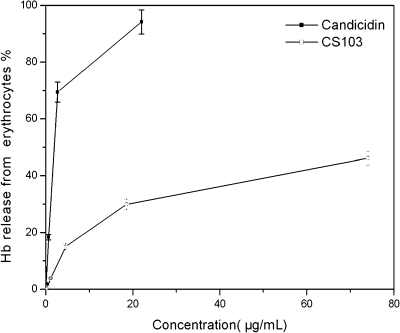
We also measured the toxicity displayed by the decarboxylated candicidin produced by CS103. FR-008/candicidin dispersed in dimethyl sulfoxide caused 50% hemolysis of rabbit erythrocytes at a concentration of ca. 1.5 μg/ml. However, at the highest concentration (74 μg/ml), decarboxylated FR-008/candicidin caused less than 50% hemolysis (Fig. 6), which is approximately 50 times lower than that for FR-008/candicidin.
FIG. 6.
Dose-response curves obtained for hemolysis of rabbit erythrocytes. Hb, hemoglobin.
Modification of the exocyclic carboxyl group of amphotericin B is known to bring about a substantial reduction in its toxicity (9). Interestingly, decarboxylated amphotericin B derivatives lacking exocyclic carboxyl groups also were one to five times less active and 10 to 33 times less hemolytic than amphotericin B (7).
The role of the fscP gene in FR-008/candicidin biosynthesis may be as a dioxydase for the introduction of two oxygen atoms into an unactivated methyl group in the macrolide antibiotics modification process, in agreement with the conclusion of Carmody et al. (7). It has been proven that no hydroxylation tailoring exists in FR-008/candicidin biosynthesis (17). When glycosylation with GDP-mycosamine was abolished by targeted disruption of the fscMI gene in CS101, the carboxyl group still formed (8). However, when exocyclic carboxylation was disrupted by targeted inactivation of the fscP cytochrome P450 gene, glycosylation still occurred in CS103. We thus conclude that there is no strict order for the tailoring steps in FR-008/candicidin biosynthesis.
Meanwhile, we are attempting to measure the effect of the new derivative compounds on cholesterol and bile acid metabolism (14), in comparison with the effect of wild-type FR-008/candicidin for the further evaluation of its other biological activities and/or potential pharmaceutical applications (Fig. 2).
Supplementary Material
Acknowledgments
We thank Qianjin Kang for nuclear magnetic resonance data analysis and many valuable comments and Jennifer Jocz for her critical reading of our manuscript.
We thank the National Science Foundation of China, the Ministry of Science and Technology (973 and 863 programs), and the Science and Technology Committee of Shanghai Municipality (grant 07DZ19503-4) for research support.
Footnotes
Published ahead of print on 9 January 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Aparicio, J. F., P. Caffrey, J. A. Gil, and S. B. Zotchev. 2003. Polyene antibiotic biosynthesis gene clusters. Appl. Microbiol. Biotechnol. 61:179-188. [DOI] [PubMed] [Google Scholar]
- 2.Aparicio, J. F., R. Fouces, M. V. Mendes, N. Olivera, and J. F. Martin. 2000. A complex multienzyme system encoded by five polyketide synthase genes is involved in the biosynthesis of the 26-membered polyene macrolide pimaricin in Streptomyces natalensis. Chem. Biol. 7:895-905. [DOI] [PubMed] [Google Scholar]
- 3.Bolard, J. 1986. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim. Biophys. Acta 864:257-304. [DOI] [PubMed] [Google Scholar]
- 4.Brautaset, T., O. N. Sekurova, H. Sletta, T. E. Ellingsen, A. R. Strøm, S. Valla, and S. B. Zotchev. 2000. Biosynthesis of the polyene antifungal antibiotic nystatin in Streptomyces noursei ATCC 11455: analysis of the gene cluster and deduction of the biosynthetic pathway. Chem. Biol. 7:395-403. [DOI] [PubMed] [Google Scholar]
- 5.Caffrey, P., J. F. Aparicio, F. Malpartida, and S. B. Zotchev. 2008. Biosynthetic engineering of polyene macrolides towards generation of improved antifungal and antiparasitic agents. Curr. Top. Med. Chem. 8:639-653. [DOI] [PubMed] [Google Scholar]
- 6.Caffrey, P., S. Lynch, E. Flood, S. Finnan, and M. Oliynyk. 2001. Amphotericin biosynthesis in Streptomyces nodosus: deductions from analysis of polyketide synthase and late genes. Chem. Biol. 8:713-723. [DOI] [PubMed] [Google Scholar]
- 7.Carmody, M., B. Murphy, B. Byrne, P. Power, D. Rai, B. Rawlings, and P. Caffrey. 2005. Biosynthesis of amphotericin derivatives lacking exocyclic carboxyl groups. J. Biol. Chem. 280:34420-34426. [DOI] [PubMed] [Google Scholar]
- 8.Chen, S., X. Huang, X. Zhou, L. Bai, J. He, K. J. Jeong, S. Y. Lee, and Z. Deng. 2003. Organizational and mutational analysis of a complete FR-008/candicidin gene cluster encoding a structurally related polyene complex. Chem. Biol. 10:1065-1076. [DOI] [PubMed] [Google Scholar]
- 9.Cheron, M., B. Cybulska, J. Mazerski, J. Grzybowska, A. Czerwinski, and E. Borowski. 1988. Quantitative structure-activity relationships in amphotericin B derivatives. Biochem. Pharmacol. 37:827-836. [DOI] [PubMed] [Google Scholar]
- 10.Gil, J. A., and J. F. Martin. 1995. Polyene antibiotics, p. 551-575. In W. R. Strohl (ed.), Biotechnology of antibiotics. M. Dekker, New York, NY.
- 11.Gordon, H. W., and C. P. Schaffner. 1968. The effect of polyene macrolides on the prostate gland and canine prostatic hyperplasia. Proc. Natl. Acad. Sci. USA 60:1201-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu, C. M., L. E. McDaniel, and C. P. Schaffner. 1972. Studies on candicidin biogenesis. J. Antibiot. (Tokyo) 25:116-121. [DOI] [PubMed] [Google Scholar]
- 13.Mendes, M. V., E. Recio, R. Fouces, R. Luiten, J. F. Martin, and J. F. Aparicio. 2001. Engineered biosynthesis of novel polyenes: a pimaricin derivative produced by targeted gene disruption in Streptomyces natalensis. Chem. Biol. 8:635-644. [DOI] [PubMed] [Google Scholar]
- 14.Singhal, A. K., E. H. Mosbach, and C. P. Schaffner. 1981. Effect of candicidin on cholesterol and bile acid metabolism in the rat. Lipids 16:423-426. [DOI] [PubMed] [Google Scholar]
- 15.Waksman, S. A., H. A. Lechevalier, and C. P. Schaffner. 1965. Candicidin and other polyenic antifungal antibiotics. Bull. W. H. O. 33:219-226. [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan, D., and Q. Zhou. 1990. The killing activity to mosquito larvae of a new antibiotic produced by FR-008, an intra-specific fusant of S. hygroscopicus var. yingchengensis. J. Huazhong Agric. Univ. 2:209. [Google Scholar]
- 17.Zhou, Y., J. Li, J. Zhu, S. Chen, L. Bai, X. Zhou, H. Wu, and Z. Deng. 2008. Incomplete beta-ketone processing as a mechanism for polyene structural variation in the FR-008/candicidin complex. Chem. Biol. 15:629-638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.