Abstract
Vibrio vulnificus is a heterogeneous bacterial species that comprises virulent and avirulent strains from environmental and clinical sources that have been grouped into three biotypes. To validate the typing methods proposed to distinguish clinical from environmental isolates, we performed phenotypic (API 20E, API 20NE, and BIOLOG tests) and genetic (ribotyping and DNA polymorphism at several loci) studies with a large strain collection representing different biotypes, origins, and host ranges. No phenotypic method was useful for biotyping or grouping strains with regard to the origin of an isolate, and only the BIOLOG system was reliable for identifying the strains at the species level. DNA polymorphisms divided the population into three major profiles. Profile 1 strains were vcg type C, 16S rRNA type B, and vvh type 1 and included most of the biotype 1 human septicemic isolates; profile 2 strains were vcg type E, 16S rRNA type A, and vvh type 2 and included all biotype 2 isolates together with biotype 1 isolates from fish and water and some human isolates; and profile 3 strains were vcg type E, 16S rRNA type AB, and vvh type 2 and included biotype 3 strains. Ribotyping divided the species into two groups: one group that included profile 1 biotype 1 isolates and one group that included isolates of all three biotypes with the three profiles described above. In conclusion, no genotyping system was able to distinguish either clinical strains from environmental strains or biogroups within the species V. vulnificus, which suggests that new typing methodologies useful for public health have to be developed for this species.
Vibrio vulnificus is an aquatic bacterial species that produces infections in fish and humans (30, 31, 36). Although human infections are relatively uncommon, they can be life threatening in patients with chronic or immunocompromising diseases (32, 36). The main transmission routes of human vibriosis are consumption of raw or undercooked shellfish and exposure of open wounds or sores to seawater (32, 36). The mortality rate due to primary septicemia after contaminated shellfish consumption is approximately 50%, and the mortality rate resulting from reported wound infections is 25% (4, 20).
This species is phenotypically and serologically heterogeneous (9, 17, 38). Originally, it was divided into two biotypes, one virulent for humans and one virulent for fish (38). In early studies, negative results for indole production, ornithine decarboxylase activity, acid production from mannitol and sorbitol, and growth at 42°C, as well as serological specificity, allowed investigators to distinguish the first fish isolates (biotype 2 serovar E) from human isolates (9, 38). However, this simple scheme of intraspecific classification lost its utility when more strains were isolated from fish vibriosis worldwide (19, 21, 24). These new isolates differed serologically and phenotypically from the isolates initially studied (21, 24) and were grouped in two additional serovars (serovars A and I) using the same serotyping system (21; C. Amaro, unpublished results). Interestingly, biotype 2 serovar E was also isolated from human infections, usually after manipulation of diseased fish, which increased the diversity of isolates able to infect humans (1). In addition to this biotype 2 heterogeneity, a third biotype was described in 1999 in Israel (11). To date, this biotype includes only isolates from wound infections initiated by handling spiny fish (11, 16). These isolates were immunologically identical to each other but distinguishable from biotype 2 serovars using the same serotyping system (12). At least four additional serovars can be found using this serotyping system among biotype 1 isolates, although they have not been fully characterized (2; C. Amaro, unpublished data).
Due to the public health importance of this species and the difficulties in rapidly differentiating the strains with human virulence potential, several typing systems have been developed. The main genotyping systems are based on differences in the sequences of some loci, such as 16S rRNA, hemolysin (vvhA) genes, or the vcg (virulence-correlated gene) locus, which divide V. vulnificus populations in two genotypes, one primarily associated with environmental isolates and the other primarily associated with clinical isolates (7, 28, 33, 35). Most of the typing techniques described have been used for V. vulnificus, including randomly amplified polymorphic DNA analysis, repetitive extragenic palindromic PCR, and ribotyping, among others (5, 15, 23, 37, 39). These techniques are able to distinguish some specific groups within V. vulnificus; however, the majority of studies were performed with strain collections biased toward biotype 1, since they included few or no biotype 2 and 3 strains, and toward North America, since the majority of the isolates were from that geographical region (14, 37).
The main objective of our study was to validate the usefulness of two typing methodologies (ribotyping and polymorphisms at selected loci) with a wide collection of strains of the different biotypes from different sources and geographic regions, whose biochemical diversity was also analyzed by different methodologies (API 20E, API 20NE, and BIOLOG).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
A total of 111 V. vulnificus strains of different biotypes from different sources and regions were used in this study (Table 1). The strains were maintained both as lyophilized stocks at room temperature (25°C) and as frozen stocks at −80°C in marine broth (Difco) plus 20% (vol/vol) glycerol. Strains were grown in Luria-Bertani broth or on Luria-Bertani agar containing 1% (wt/vol) (total concentration) NaCl at 28°C for 24 h.
TABLE 1.
Strains used in this study and some of their properties
| Strain | Origin | Country and year of isolation | Biotype | Multiplexa | DNA polymorphismb
|
Ribogroup (Rt)c | |||
|---|---|---|---|---|---|---|---|---|---|
| vcg type | vvhA type | 16S rRNA type | Profile | ||||||
| CECT 4869 | Diseased eel | Belgium, 1990 | 1 | BT1/3 | E | 2 | A | 2 | AI (1) |
| CG106 | Oyster | Taiwan, 1993 | 1 | BT1/3 | C | 1 | B | 1 | AI (2) |
| CECT 5168 | Human blood | United States | 1 | BT1/3 | C | 1 | B | 1 | AI (3) |
| N87 | Human blood | Japan, 1987 | 1 | BT1/3 | C | 1 | B | 1 | AI (3) |
| YJ106 | Human blood | Taiwan | 1 | BT1/3 | C | 1 | B | 1 | AI (3) |
| CECT 5167 | Human blood | Japan | 1 | BT1/3 | C | 1 | B | 1 | AI (4) |
| MLT 362 | Oyster | United States | 1 | BT1/3 | C | 1 | B | 1 | AI (5) |
| VV 425 | Oyster | United States | 1 | BT1/3 | E | 1 | A | Atypical | AI (6) |
| ATCC 33816 | Human blood | United States | 1 | BT1/3 | C | 1 | B | 1 | AI (7) |
| CG110 | Seawater | Taiwan, 1993 | 1 | BT1/3 | C | 1 | B | 1 | AI (7) |
| CG118 | Seawater | Taiwan, 1993 | 1 | BT1/3 | C | 1 | B | 1 | AI (8) |
| E4 | Oyster | United States | 1 | BT1/3 | C | 1 | B | 1 | AI (9) |
| CG111 | Seawater | Taiwan, 1993 | 1 | BT1/3 | C | 1 | B | 1 | AI (10) |
| MLT 364 | Oyster | United States | 1 | BT1/3 | C | 1 | B | 1 | AI (11) |
| VV 1003 | Oyster | United States | 1 | BT1/3 | C | 1 | B | 1 | AI (11) |
| 95-8-7 | Diseased eel | Denmark, 1995 | 2 | BT2-nonSerE | E | 2 | A | 2 | AI (11) |
| CS9133 | Human blood | South Korea | 1 | BT1/3 | C | 1 | B | 1 | AI (12) |
| CECT 4608 | Eel farm water | Spain, 1990 | 1 | BT1/3 | C | 1 | B | 1 | AII (13) |
| KH03 | Human blood | Japan, 2003 | 1 | BT1/3 | C | 1 | B | 1 | AII (14) |
| CECT 4862 | Diseased eel | Japan, 1979 | 2 | BT2-SerE | E | 2 | A | 2 | AII (14) |
| CECT 5164 | Human blood | United States | 1 | BT1/3 | C | 2 | B | Atypical | AII (15) |
| Riu-3 | Seawater | Spain, 2003 | 1 | BT1/3 | E | 2 | A | 2 | AII (16) |
| Riu-1 | Seawater | Spain, 2003 | 1 | BT1/3 | E | 2 | AB | Atypical | AII (16) |
| 94385 | Leg wound | Spain, 2001 | 1 | BT1/3 | E | 2 | B | Atypical | AII (17) |
| V4 | Human blood | Australia | 1 | BT1/3 | C | 1 | B | 1 | AII (18) |
| PD-2-52 | Eel tank water | Spain, 2003 | 2 | BT2-nonSerE | E | 2 | A | 2 | BI (19) |
| PD-2-58 | Eel tank water | Spain, 2003 | 2 | BT2-nonSerE | E | 2 | A | 2 | BI (20) |
| CECT 4917 | Diseased eel | Spain, 1997 | 2 | BT2-SerE | E | 2 | A | 2 | BI (21) |
| CECT 4998 | Diseased eel | Spain, 1997 | 2 | BT2-SerE | E | 2 | A | 2 | BI (21) |
| JE | Oyster | United States | 1 | BT1/3 | E | 2 | B | Atypical | BI (21) |
| CECT 5165 | Seawater | United States | 1 | BT1/3 | E | 2 | A | 2 | BI (22) |
| A2 | Diseased eel | Spain, 2000 | 1 | BT1/3 | E | 2 | A | 2 | BI (23) |
| An4 | Diseased eel | Spain, 2000 | 1 | BT1/3 | E | 2 | A | 2 | BI (23) |
| An5 | Diseased eel | Spain, 2000 | 1 | BT1/3 | E | 2 | A | 2 | BI (23) |
| An6 | Diseased eel | Spain, 2000 | 1 | BT1/3 | E | 2 | A | 2 | BI (23) |
| CECT 4606 | Eel tank water | Spain, 1990 | 1 | BT1/3 | E | 2 | A | 2 | BI (23) |
| PD-1 | Eel tank water | Spain, 2001 | 1 | BT1/3 | E | 2 | A | 2 | BI (23) |
| PD-12 | Eel tank water | Spain, 2001 | 1 | BT1/3 | E | 2 | A | 2 | BI (23) |
| PD-3 | Eel tank water | Spain, 2001 | 1 | BT1/3 | E | 2 | A | 2 | BI (23) |
| PD-5 | Eel tank water | Spain, 2001 | 1 | BT1/3 | E | 2 | A | 2 | BI (23) |
| V1 | Eel tank water | Spain, 2001 | 1 | BT1/3 | E | 2 | A | 2 | BI (23) |
| CECT 4605 | Diseased eel | Spain, 1990 | 2 | BT2-SerE | E | 2 | A | 2 | BI (23) |
| 11028 | Human disease | Israel, 1996 | 3 | BT1/3 | E | 1 | AB | 3 | BI (24) |
| 162 | Human disease | Israel, 1997 | 3 | BT1/3 | E | 1 | AB | 3 | BI (24) |
| 97 | Human disease | Israel, 1997 | 3 | BT1/3 | E | 1 | AB | 3 | BI (24) |
| vv12 | Human disease | Israel, 1996 | 3 | BT1/3 | E | 1 | AB | 3 | BI (24) |
| vv32 | Human disease | Israel | 3 | BT1/3 | E | 1 | AB | 3 | BI (24) |
| CECT 5169 | Human blood | United States | 1 | BT1/3 | C | 1 | B | 1 | BII (25) |
| 94-9-119 | Human disease | Denmark, 1994 | 1 | BT1/3 | E | 2 | A | 2 | BII (25) |
| CECT 4867 | Unknown | Unknown | 1 | BT1/3 | E | 2 | A | 2 | BII (25) |
| YN03 | Human blood | Japan, 2003 | 1 | BT1/3 | E | 2 | A | 2 | BII (25) |
| 535 | Diseased eel | Sweden | 2 | BT2-nonSerE | E | 2 | A | 2 | BII (25) |
| 536 | Diseased eel | Sweden | 2 | BT2-nonSerE | E | 2 | A | 2 | BII (25) |
| 960426-1/4C | Diseased eel | Denmark, 1996 | 2 | BT2-nonSerE | E | 2 | A | 2 | BII (25) |
| 960717-1/2F | Diseased eel | Denmark, 1996 | 2 | BT2-nonSerE | E | 2 | A | 2 | BII (25) |
| A10 | Diseased eel | Spain, 2002 | 2 | BT2-nonSerE | E | 2 | A | 2 | BII (25) |
| A11 | Diseased eel | Spain, 2002 | 2 | BT2-nonSerE | E | 2 | A | 2 | BII (25) |
| A13 | Diseased eel | Spain, 2002 | 2 | BT2-nonSerE | E | 2 | A | 2 | BII (25) |
| A14 | Diseased eel | Spain, 2002 | 2 | BT2-nonSerE | E | 2 | A | 2 | BII (25) |
| CECT 5198 | Diseased eel | Spain, 1999 | 2 | BT2-nonSerE | E | 2 | A | 2 | BII (25) |
| CECT 5689 | Diseased eel | Spain, 2002 | 2 | BT2-nonSerE | E | 2 | A | 2 | BII (25) |
| CECT 5768 | Diseased eel | Spain, 2001 | 2 | BT2-nonSerE | E | 2 | A | 2 | BII (25) |
| CECT 5769 | Diseased eel | Spain, 2002 | 2 | BT2-nonSerE | E | 2 | A | 2 | BII (25) |
| 90-2-11 | Diseased eel | Denmark, 1990 | 2 | BT2-SerE | E | 2 | A | 2 | BII (25) |
| 94-8-112 | Wound infection | Denmark, 1994 | 2 | BT2-SerE | E | 2 | A | 2 | BII (25) |
| 94-9-123 | Seawater | Denmark, 1994 | 2 | BT2-SerE | E | 2 | A | 2 | BII (25) |
| C1 | Healthy eel | Spain, 2003 | 2 | BT2-SerE | E | 2 | A | 2 | BII (25) |
| CECT 4602 | Diseased eel | Spain, 1990 | 2 | BT2-SerE | E | 2 | A | 2 | BII (25) |
| CECT 4603 | Diseased eel | Spain, 1990 | 2 | BT2-SerE | E | 2 | A | 2 | BII (25) |
| CECT 4604 | Diseased eel | Spain, 1990 | 2 | BT2-SerE | E | 2 | A | 2 | BII (25) |
| CECT 4864 | Diseased eel | Spain, 1994 | 2 | BT2-SerE | E | 2 | A | 2 | BII (25) |
| CECT 4868 | Diseased eel | Norway, 1990 | 2 | BT2-SerE | E | 2 | A | 2 | BII (25) |
| CECT 4870 | Diseased eel | Sweden, 1991 | 2 | BT2-SerE | E | 2 | A | 2 | BII (25) |
| CECT 5762 | Healthy eel | Spain, 2002 | 2 | BT2-SerE | E | 2 | A | 2 | BII (25) |
| CECT 898 | Diseased eel | Japan, 1979 | 2 | BT2-SerE | E | 2 | A | 2 | BII (25) |
| CIP 81.90 | Human blood | France, 1981 | 2 | BT2-SerE | E | 2 | A | 2 | BII (25) |
| G83 | Fish | South Korea | 1 | BT1/3 | E | 1 | B | Atypical | BII (25) |
| VV 352 | Seawater | United States | 1 | BT1/3 | E | 1 | A | Atypical | BII (25) |
| MLT 406 | Seawater | United States | 1 | BT1/3 | E | 2 | A | 2 | BII (26) |
| 95-8-6 | Diseased eel | Denmark, 1995 | 2 | BT2-nonSerE | E | 2 | A | 2 | BII (26) |
| CECT 529T | Human blood | United States | 1 | BT1/3 | E | 1 | A | Atypical | BII (26) |
| CECT 4174 | Diseased eel | Japan, 1979 | 2 | BT2-SerE | E | 2 | A | 2 | BII (27) |
| CG100 | Oyster | Taiwan, 1993 | 1 | BT1/3 | C | 1 | B | 1 | BII (28) |
| L49 | Brackish water | Japan | 1 | BT1/3 | E | 2 | A | 2 | BII (29) |
| CECT 4607 | Diseased eel | Spain, 1992 | 2 | BT2-SerE | E | 2 | A | 2 | BII (30) |
| CECT 4999 | Diseased eel | Spain, 1999 | 2 | BT2-SerE | E | 2 | A | 2 | BII (30) |
| PD-2-66 | Eel tank water | Spain, 2003 | 1 | BT1/3 | E | 2 | B | Atypical | BII (30) |
| CECT 4601 | Diseased eel | Spain, 1989 | 2 | BT2-SerE | E | 2 | A | 2 | BII (31) |
| 94-9-130 | Seawater | Denmark, 1994 | 1 | BT1/3 | E | 2 | A | 2 | BII (32) |
| CECT 7029 | Diseased eel | Denmark, 2004 | 2 | BT2-nonSerE | E | 2 | A | 2 | BII (32) |
| CECT 7030 | Diseased eel | Denmark, 2004 | 2 | BT2-nonSerE | E | 2 | A | 2 | BII (32) |
| 95-8-162 | Diseased eel | Denmark, 1995 | 2 | BT2-nonSerE | E | 2 | A | 2 | BII (33) |
| CECT 4863 | Leg wound | United States | 2 | BT2-SerE | E | 2 | A | 2 | BII (34) |
| CECT 897 | Diseased eel | Japan, 1979 | 2 | BT2-SerE | E | 2 | A | 2 | BII (35) |
| 95-8-161 | Diseased eel | Denmark, 1995 | 2 | BT2-nonSerE | E | 2 | A | 2 | BII (36) |
| CECT 5343 | Diseased eel | Spain, 2000 | 2 | BT2-nonSerE | E | 2 | A | 2 | BII (36) |
| MLT404 | Seawater | United States | 1 | BT1/3 | E | 2 | A | 2 | BII (37) |
| CECT 4865 | Diseased shrimp | Taiwan | 2 | BT2-SerE | E | 2 | A | 2 | BII (38) |
| CECT 5139 | Diseased eel | Spain, 1998 | 2 | BT2-SerE | E | 2 | A | 2 | BII (38) |
| CECT 4866 | Human blood | Australia | 2 | BT2-SerE | E | 2 | A | 2 | BII (39) |
| UE516 | Diseased Japanese eel | Taiwan | 2 | BT2-SerE | E | 2 | A | 2 | BII (40) |
| 94-9-118 | Human disease | Denmark, 1994 | 1 | BT1/3 | E | 2 | A | 2 | B (41) |
| 534 | Diseased eel | Sweden | 1 | BT1/3 | E | 2 | A | 2 | B (42) |
| PD-2-47 | Eel tank water | Spain, 2003 | 2 | BT2-SerE | E | 2 | A | 2 | BIII (43) |
| PD-2-51 | Eel tank water | Spain, 2003 | 2 | BT2-SerE | E | 2 | A | 2 | BIII (43) |
| CECT 5763 | Eel tank water | Spain, 2002 | 2 | BT2-SerE | E | 2 | A | 2 | BIII (44) |
| PD-2-50 | Eel tank water | Spain, 2003 | 2 | BT2-SerE | E | 2 | A | 2 | BIII (45) |
| PD-2-55 | Eel tank water | Spain, 2003 | 2 | BT2-SerE | E | 2 | A | 2 | BIII (45) |
| Riu-2 | Seawater | Spain, 2003 | 2 | BT2-SerE | E | 2 | A | 2 | BIII (46) |
| PD-2-56 | Eel tank water | Spain, 2003 | 2 | BT2-SerE | E | 2 | A | 2 | BIII (47) |
| CECT 5166 | Wound infection | United States | 1 | BT1/3 | E | 2 | B | Atypical | NDd |
BT1/3, biotype 1 or 3; BT2-nonSerE, biotype 2 and not serovar E; BT2-SerE, biotype 2 serovar E.
Results of the DNA polymorphism study (see Materials and Methods for details).
See Fig. 2. Rt, ribopattern.
ND, not determined.
Phenotypic analysis. (i) Biotyping.
The biotypes of the strains were confirmed by multiplex PCR (34). This method allows identification at the species level and at the same time discrimination between biotypes 1/3 and 2 and, within biotype 2, discrimination of serovar E, the zoonotic serovar. The biotype 3 strains used in this study were previously biotyped (11).
(ii) API 20E and API 20NE analysis.
API 20E and API 20NE test kits (bioMerieux) were used according to the manufacturer's directions, with incubation of the strips at 28°C. Bacterial suspensions in saline solution or in AUX medium plus NaCl at a final concentration 1% (wt/vol) were used as the inocula for API 20E and API 20NE kits, respectively (10). Examination of the strips was conducted after 24 and 48 h. API profiles were compared using API DataBase version 4.0 for API 20E strips and version 6.0 for API 20NE strips (APILAB Software, version 3.3.3, Apilab Plus; bioMerieux).
(iii) BIOLOG analysis.
BIOLOG-GN MicroPlates (BIOLOG) were used to evaluate substrate utilization patterns of the strains. The cells were streaked on BIOLOG Universal Growth agar (Oxoid) supplemented with 5% sheep erythrocytes (BUG-S) and incubated for 24 h at 28°C. Wells of a plate were inoculated with 150 μl of bacterial suspensions adjusted to the appropriate density in saline solution. The inoculated microplates were incubated at 28°C for 24 and 48 h and analyzed using a BIOLOG Microstation reader. Test results were obtained and identification (BIOLOG Microlog 6.01 database) was performed using BIOLOG MicroLog 3 software (BIOLOG), applying the automatic threshold option. Differences in the use of carbon substrates between the different groups were analyzed using the chi-square test function at α = 0.05, employing SPSS 14.0 for Windows. Bionumerics software version 4.0 (Applied Maths) was used to cluster the strains based on their substrate utilization patterns using the unweighted-pair group method using arithmetic average (UPGMA) and two different similarity coefficients, the Jaccard and simple matching coefficients.
Genetic fingerprinting. (i) DNA sequence polymorphisms.
The polymorphisms at selected loci were determined by PCR analysis of all V. vulnificus strains. Differentiation between the described alleles of the hemolysin gene (vvhA) and the 16S rRNA gene was performed under conditions described elsewhere (35). vcg typing for the environmental (type E) or clinical (type C) genotype was performed as described by Rosche et al. (33). In all assays, ca. 250 ng of DNA per 25 μl of reaction mixture was amplified using the high-fidelity Expand PCR system (Roche Diagnostics) in a TC-312 thermal cycler (Techne). The existence of an association between polymorphism and group (biotype, origin, or serovar) was calculated using the Pearson chi-square test function at α = 0.05, employing SPSS 14.0 for Windows.
(ii) Automated ribotyping.
Ribotyping of the V. vulnificus isolates was carried out with the Riboprinter system (Qualicon Inc.). The assay was performed under conditions recommended by the manufacturer using HindIII (Roche) at 400 U μl−1. Riboprinter patterns were partially processed by the Riboprinter system software in order to reduce background noise and to normalize the band positions using DNA size standards as references. The normalized patterns were then exported for further analysis as .txt files and imported into the Bionumerics software (version 4.0; Applied Maths) using LoadSamples script (DuPont Qualicon). Clustering analysis was performed by UPGMA based on the Dice coefficient for band matching, with a position tolerance and an optimization setting of 1%. Bands for band matching were assigned automatically and manually edited if necessary.
RESULTS
Phenotypic analysis. (i) API 20E and API 20NE analysis.
A total of 25 different API 20E and API 20NE profiles were obtained for the V. vulnificus collection (see Tables S1 and S2 in the supplemental material). In the case of the API 20E system, only 60% of the strains were correctly identified as V. vulnificus, and the percentage was 20% for biotype 3 isolates. The remaining strains gave a mixed profile or were misidentified as Burkholderia cepacia or Vibrio parahaemolyticus. Three main profiles were detected for biotype 1 isolates (5346105 [19.6%], 5146105 [15.7%], and 5346005 [17.6%]), two main profiles were detected for biotype 2 serovar E isolates (5006005 [29.4%] and 5206005 [23.5%]), two main profiles were detected for biotype 2 non-serovar E isolates (5146105 [38%] and 5346105 [33%]), and one main profile was detected for biotype 3 isolates (4146004 [40%]) (see Table S1 in the supplemental material). None of the strains was correctly identified as V. vulnificus with the API 20NE system. Instead, most of the isolates were identified at the genus level as Aeromonas or Vibrio, and the species Aeromonas hydrophila and Vibrio cholerae were the most frequent options (see Table S2 in the supplemental material). The main profile exhibited by biotype 1 isolates was 7476745 (43.1%), the main profile exhibited biotype 2 serovar E isolates was 5472745 (73.6%), the main profile exhibited by biotype 2 non-serovar E isolates was 7476745 (61.9%), and the main profile exhibited by biotype 3 strains was 7062745 (40%).
(ii) BIOLOG GN2 plates.
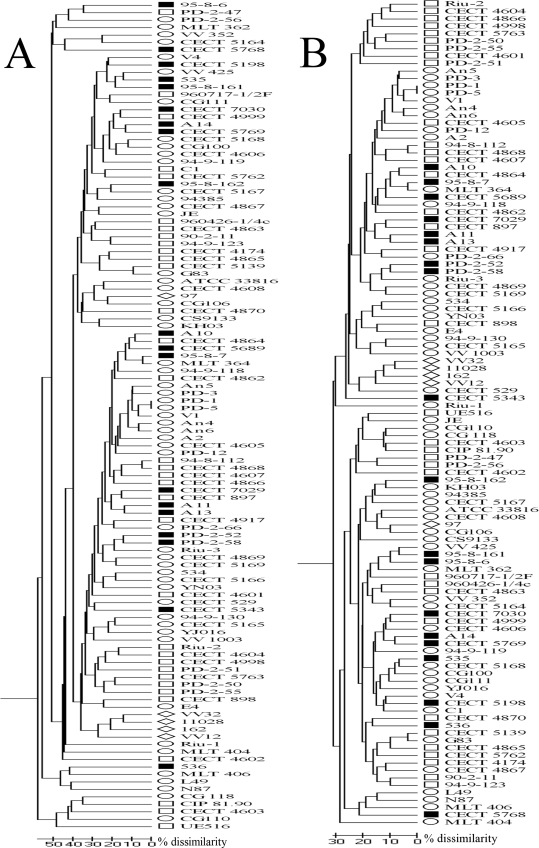
The BIOLOG system correctly identified 84% of the 111 isolates studied. Eight of the low-discrimination identifications (7% of the strains) were listed as V. vulnificus as the first option, although with low probability. The carbon sources that V. vulnificus was able to oxidize are shown in Table 2. On average, 32 carbon substrates were utilized. V. vulnificus strains did not use amines or carboxylic acids, with the exception of d-glucoronic and succinic acid, which were metabolized by more than the 80% of the strains examined (Table 2). There was no specific profile (expressed as the carbon sources utilized by an isolate) that could be assigned to a particular group of strains. In fact, only two strains, isolated from the same water sample (PD-1 and PD-5), used the same carbon sources. In addition, cluster analysis performed by applying either the Jaccard index, which takes into account the similarity based on the number of positive coincidences, or simple matching, which takes into account the positive and negative coincidences, revealed that most of the groups of strains were not related to a common biotype, serovar, or origin (Fig. 1). Such variability in the use of carbon sources caused difficulties in attempts to establish a reliable test that could be used for discriminating biotypes or serovars. Nevertheless, we found statistical differences for the differential use of some carbon sources between groups (Table 2). Some examples are the use of the cellobiose and gentibiose, which was negative for the biotype 3 isolates but positive for the rest of the strains tested. The biotype 2 strains were able to oxidize β-methyl-d-glucoside; however, no biotype 3 and few biotype 1 isolates were able to use this carbon source.
TABLE 2.
Carbon sources used by V. vulnificus strains
| Carbon source | Type | Growtha
|
|||||
|---|---|---|---|---|---|---|---|
| Species (n = 111) | Biotype 1 (n = 51) | Biotype 2
|
Biotype 3 (n = 5) | ||||
| Total (n = 55) | Serovar E (n = 36) | Non-serovar E (n = 19) | |||||
| Dextrin | Polymer | + | + | + | + | + | + |
| Glycogen | Polymer | + | + | + | + | + | + |
| α-d-Glucose | Carbohydrate | + | + | + | + | + | + |
| d-Trehalose | Carbohydrate | + | + | + | + | + | + |
| N-Acetyl-d-glucosamine | Carbohydrate | + | + | + | + | + | + |
| d-Fructose | Carbohydrate | + | + | + | + | + | + |
| l-Asparagine | Amino acid | + | + | + | + | + | + |
| Glucose 6-phosphate | Phosphorylated chemical | + | + | + | + | (+) | + |
| Tween 80 | Polymer | + | + | + | + | + | (+) |
| l-Glutamic acid | Amino acid | + | + | (+) | (+) | + | + |
| Inosine | Aromatic chemical | + | + | (+) | (+) | + | + |
| Maltose | Carbohydrate | (+) | (+) | + | + | (+) | + |
| d-Gluconic acid | Carboxylic acid | (+) | + | (+) | (+) | + | + |
| Cellobioseb,c | Carbohydrate | (+) | (+) | + | + | + | − |
| d-Mannose | Carbohydrate | (+) | (+) | (+) | (+) | (+) | (+) |
| Methylpyruvate | Ester | (+) | + | (+) | (+) | + | (+) |
| l-Aspartic acid | Amino acid | (+) | + | (+) | (+) | (+) | + |
| Gentiobioseb,c | Carbohydrate | (+) | (+) | + | + | (+) | − |
| Tween 40 | Polymer | (+) | (+) | (+) | (+) | (+) | + |
| Succinic acid | Amide | (+) | (+) | (+) | (+) | (+) | + |
| Glucose 1-phosphate | Phosphorylated chemical | (+) | (+) | (+) | (+) | (+) | (+) |
| Monomethylsuccinate | Ester | (+) | (+) | V (73) | V (69) | (+) | + |
| l-Alanylglycine | Amino acid | V (74) | V (69) | (+) | (+) | (+) | V (60) |
| Glycyl-l-aspartic acidc | Amino acid | V (73) | V (59) | (+) | (+) | (+) | (+) |
| N-Acetyl-d-galactosamine | Carbohydrate | V (72) | V (67) | (+) | V (72) | (+) | (+) |
| Uridined | Aromatic chemical | V (72) | (+) | V (69) | V (59) | (+) | (+) |
| l-Alanine | Amino acid | V (66) | V (63) | V (69) | V (72) | V (63) | V (60) |
| β-Methyl-d-glucosideb,c | Carbohydrate | V (59) | V (37) | (+) | (+) | (+) | − |
| dl-Lactic acidb,c,d | Carboxylic acid | V (59) | V (73) | V (44) | V (33) | V (63) | (+) |
| Glycerol | Alcohol | V (57) | V (59) | V (55) | V (56) | V (53) | V (60) |
| d-Galactose | Carbohydrate | V (54) | V (57) | V (55) | V (47) | V (68) | V (20) |
| Bromosuccinic acid | Brominated chemical | V (51) | V (53) | V (47) | V (44) | V (53) | (+) |
| dl-α-Glycerolphosphated | Phosphorylated chemical | V (48) | V (49) | V (46) | V (56) | V (26) | V (60) |
| l-Proline | Amino acid | V (47) | V (43) | V (47) | V (47) | V (47) | (+) |
| Acetic acid | Carboxylic acid | V (38) | V (45) | V (35) | V (36) | V (32) | − |
| l-Threonine | Amino acid | V (38) | V (45) | V (35) | V (31) | V (42) | − |
| d-Mannitolc,d | Carbohydrate | V (36) | V (39) | V (36) | − | (+) | − |
| d-Psicose | Carbohydrate | V (35) | V (31) | V (38) | V (44) | V (26) | V (40) |
| l-Serineb | Amino acid | V (34) | V (43) | V (29) | V (28) | V (32) | − |
| d-Glucuronic acidd | Carboxylic acid | V (33) | V (28) | V (38) | V (28) | V (58) | V (40) |
| α-Ketoglutaric acidd | Carboxylic acid | V (32) | V (39) | V (27) | V (22) | V (37) | − |
| Glucuronamide | Amide | V (32) | V (29) | V (33) | V (25) | V (47) | V (40) |
| Glycyl-l-glutamic acid | Amino acid | V (32) | V (28) | V (35) | V (33) | V (37) | V (40) |
| α-d-Lactoseb,c | Carbohydrate | V (31) | V (16) | V (47) | V (50) | V (43) | − |
| Thymidineb,c | Aromatic chemical | V (31) | V (41) | V (18) | − | V (32) | V (60) |
| Alaninamide | Amide | − | V (22) | V (22) | − | V (37) | − |
| ρ-Hydroxyphenylacetic acid | Carboxylic acid | − | V (25) | V (16) | V (22) | − | − |
| d-Alanine | Amino acid | − | VV (22) | − | − | V (16) | − |
| Propionic acidb | Carboxylic acid | − | − | V (20) | V (19) | V (21) | − |
+, positive reaction for ≥90% of the isolates; (+), positive reaction for 75 to 89% of the isolates; V, positive reaction for 11 to 74% of the isolates (the number in parentheses is the percentage of positive isolates); −, positive reaction for ≤10% of the isolates.
Carbon source for which there are significant statistical differences (P < 0.05) in its use between the three biotypes.
Carbon source for which there are significant statistical differences (P < 0.05) in its use between the four groups (biotype 1, biotype 2 serovar E, biotype 2 non-serovar E, and biotype 3).
Carbon source for which there are significant statistical differences (P < 0.05) in its use between the serovar E isolates and the rest of the biotype 2 isolates.
FIG. 1.
Dendrograms based on UPGMA analysis of the BIOLOG results obtained for the V. vulnificus collection using the Jaccard (A) or simple matching (B) similarity coefficient. The scale bars indicate the percentage of dissimilarity. Biotype 1 strains are indicated by open ellipses, biotype 2 serovar E strains are indicated by open rectangles, biotype 2 non-serovar E strains are indicated by filled rectangles, and biotype 3 strains are indicated by open diamonds.
Genetic diversity observed with DNA polymorphism locus typing.
Results of the multiplex PCR (34) analysis are shown in Table 1. The allelic distribution among environmental, human, and fish V. vulnificus isolates for the three biotypes is shown in Table 3. Biotype 1 strains from oysters and human blood predominantly were vcg type C, whereas the biotype 1 strains from fish and nonsepticemic human infections and most of the biotype 1 isolates from water, together with biotype 2 and 3 isolates regardless of their origin, were vcg type E. We detected vvhA gene type 1 in biotype 1 strains from oysters and human septicemia together with biotype 3 strains from human bacteremia, whereas biotype 1 strains from fish and human wounds and all biotype 2 strains, irrespective of their origin, were vvhA type 2. In contrast to the vcg results, we observed more variability in the vvhA typing results with the water isolates of biotype 1. These isolates, together with those from human wounds, also showed variable results for the 16S rRNA gene polymorphisms, while fish biotype 1 and biotype 2 isolates, regardless of their origin, were type A. Oyster and human blood biotype 1 isolates were type B, and biotype 3 isolates were type AB. Thus, three main genotypic profiles were found among the collection of V. vulnificus isolates. Profile 1 consisted of genotype vcg type C, 16S rRNA type B, and vvh type 1 and was exhibited by biotype 1 strains from human septicemia and oysters. Profile 2 consisted of genotype vcg type E, 16S rRNA type A, and vvh type 2 and was exhibited by biotype 2 isolates, regardless of their origin, and by biotype 1 isolates from fish and water and some human isolates. Profile 3 consisted of genotype vcg type E, 16S rRNA type AB, and vvh type 2 and was exhibited only by biotype 3 strains. No specific profile was found for water and human nonblood isolates of biotype 1, which showed variable results. Some atypical profiles were also found, such as the profile showed by environmental biotype 1 isolate Riu-1, which was positive for both types of 16S rRNA (as were the biotype 3 strains) and possessed hemolysin type 2.
TABLE 3.
Distribution of genotypes and genotypic profiles among V. vulnificus biotypes according to strain origin
| Biotype | Origin | n | % with genotypea:
|
% with genotypic profileb:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| vcg type C | vcg type E | vvh type 1 | vvh type 2 | rRNA type A | rRNA type B | rRNA type AB | 1 | 2 | 3 | Atypical | |||
| 1 | Water | 19 | 21 | 79 | 26 | 74 | 68 | 26 | 5 | 21 | 63 | 0 | 16 |
| Oyster | 8 | 75 | 25 | 87 | 13 | 13 | 87 | 0 | 75 | 0 | 0 | 25 | |
| Fish | 6 | 0 | 100 | 17 | 83 | 83 | 17 | 0 | 0 | 83 | 0 | 17 | |
| Human blood | 12 | 83 | 17 | 83 | 17 | 17 | 83 | 0 | 75 | 8 | 0 | 16 | |
| Human (not blood) | 4 | 0 | 100 | 0 | 100 | 50 | 50 | 0 | 0 | 50 | 0 | 50 | |
| 2 | Water | 10 | 0 | 100 | 0 | 100 | 100 | 0 | 0 | 0 | 100 | 0 | 0 |
| Healthy fish | 2 | 0 | 100 | 0 | 100 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | |
| Diseased fish | 38 | 0 | 100 | 0 | 100 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | |
| Diseased shrimp | 1 | 0 | 100 | 0 | 100 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | |
| Human blood | 2 | 0 | 100 | 0 | 100 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | |
| Human (not blood) | 2 | 0 | 100 | 0 | 100 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | |
| 3 | Bacteremia | 5 | 0 | 100 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 100 | 0 |
Percentage of isolates with positive PCR results.
Percentage of isolates with each profile. See the text for a description of the profiles.
Automated ribotyping.
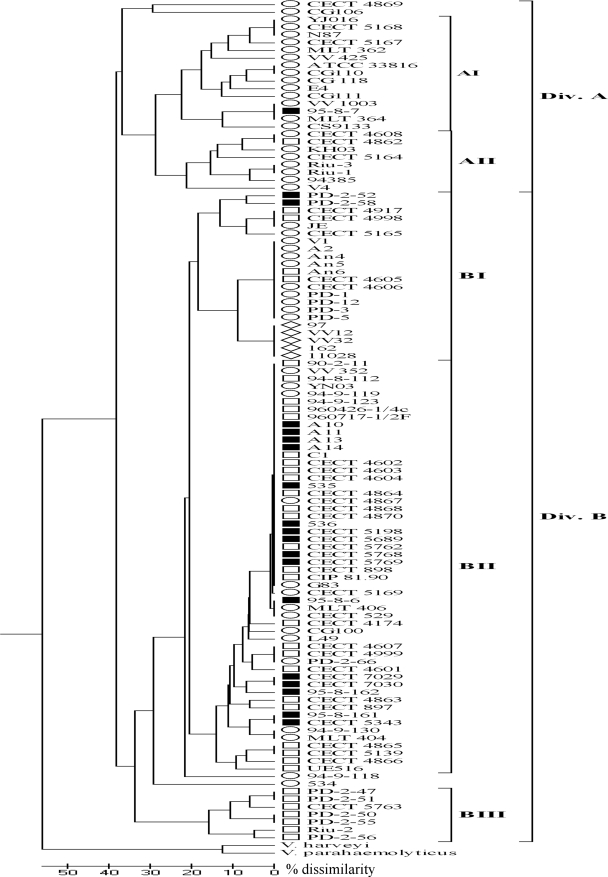
Figure 2 shows the dendrogram obtained from the normalized ribotypes (Rt) after UPGMA clustering. The Riboprinter generated some bands with low intensity, especially above 15 kb, that probably corresponded to undigested DNA as they were not reproducible when selected strains were ribotyped a second time. These bands, together with those at <1 kb, were not taken into account in the ribotyping cluster analysis. Forty-seven Rt were distinguished among the 111 V. vulnificus strains (Table 1), which grouped at a similarity level of 62%. We included two strains of other Vibrio species as outgroups, V. harveyi and V. parahaemolyticus, which were clearly different from the strains of V. vulnificus and which grouped at 45% similarity (Fig. 2). Two main groups of V. vulnificus strains could be distinguished. Division A included 92% of the biotype 1 strains and the majority of human blood (70%) and oyster (75%) isolates with profile 1, whereas division B included some biotype 1 strains from human wounds, fish, and water with profile 2 and the majority of biotype 2 (96%) and 3 (100%) isolates with profiles 2 and 3, respectively. These two divisions could be subdivided into five groups (ribogroups AI, AII, BI, BII, and BIII) based on ≥80% similarity, even though five strains (CECT 4869, CG106, V4, 94-9-118, and 534) did not cluster with other strains (Fig. 2). All of the strains with profile 1 were clustered in ribogroups AI and AII, except for a strain from human septicemia (CECT 5169) and an environmental strain (CG100). Ribogroups AI, AII, and BIII included strains with a unique biotype; ribogroups AI and AII included biotype 1 strains, mostly from humans, and ribogroup BIII included biotype 2 and serovar E strains isolated from an unusual source, brackish water of the estuary of the River Ebro (Mediterranean Sea). The rest of the biotype 2 strains were included in ribogroup BII, and most of them exhibited the same Rt pattern (Rt 25) regardless of the serovar, while all the strains of biotype 3 were grouped in ribogroup BI, which also showed a unique Rt (Rt 24).
FIG. 2.
Dendrogram based on UPGMA cluster analysis of the ribotypes obtained in this study. The scale bar indicates the percentage of dissimilarity. Divisions A and B and the five ribogroups are shown. The symbols are the same as those used in Fig. 1.
DISCUSSION
In order to validate the genotyping systems designed for differentiating clinical from environmental V. vulnificus isolates, a selection of these systems was used with a large collection of strains of different biotypes and serovars recovered from sources worldwide. The phenotypic diversity of the collection was analyzed first with three miniaturized bacterial identification systems, whose usefulness for identification of V. vulnificus at the specific or intraspecific level was evaluated in parallel. The BIOLOG system was the most effective system, giving 84% correctly identified strains. The BIOLOG results showed that V. vulnificus has the ability to oxidize a great variety of carbon sources. This species is also highly heterogeneous, since almost every isolate had a unique profile. The percentage of correctly identified strains was 60% when the API 20E system was used and 0% with the API 20NE system. These results are in agreement with previous reports on the doubtful usefulness of both API systems for identification of clinical and environmental V. vulnificus isolates (10, 17, 18, 29), although they are still being used, mostly for clinical diagnosis. Based on these results, the BIOLOG system is the most adequate system for V. vulnificus identification at the species level, and the other two systems, especially the API 20NE system, should not be used unless the databases are updated with the profiles found in the present work. When the utility of the three systems for intraspecific classification was considered, none of them was able to distinguish biotype 2 non-serovar E (serovar A/I) isolates from biotype 1 isolates, although several API 20E and API 20NE profiles were found to be specific for biotype 2 serovar E and biotype 3 strains (see Tables S1 and S2 in the supplemental material). Inclusion of these profiles in the API database would facilitate correct identification of more V. vulnificus isolates and in some cases subclassification into biotype 2 serovar E or biotype 3. In general, however, the profiles would not allow discrimination of other biotypes or groups. Despite finding several tests in the BIOLOG system that revealed significant differences between groups, we found that using these tests was not adequate for good discrimination. Only the combination of negative results for cellobiose and gentibiose breakdown allowed allocation of isolates to biotype 3.
Previous studies have proposed various genetic methods to distinguish strains of this species with human-pathogenic potential (14, 28, 33, 35). We performed an analysis of three of these methods, the 16S rRNA, vvhA, and vcg methods, with our V. vulnificus collection. According to previous reports, most V. vulnificus human isolates should be vcg type C, vvhA type 1, and 16S rRNA type B (genotypic profile 1 in our study), and most environmental isolates should be vcg type E, vvhA type 2, and 16S rRNA type A (genotypic profile 2 in our study). However, we were able to establish an association only between genotypic profile 1 and human isolates for biotype 1 strains from septicemic cases, regardless of their geographical origin. The remaining biotype 1 human isolates mostly exhibited genotype profile 2, like the majority of the environmental biotype 1 isolates, except those from oysters. Our results for oyster isolates are opposite the results obtained by other workers (22, 28, 33) and could be due to the inclusion of isolates from Asia, where a major proportion of 16S rRNA type B has been reported (25). In previous studies variation between ratios of 16S rRNA type A to rRNA type B were observed for different sampling points or water temperatures (22, 27, 39). For the rest of the isolates, we found an association between profile and biotype rather than an association between profile and origin of the isolate. Thus, all biotype 2 strains, regardless of their source (human, fish, or water), exhibited profile 2, and all biotype 3 strains, all from human bacteremia, exhibited profile 3. The variability in profiles observed among biotype 1 strains can be attributed to the greater genetic variability of this biotype. Genetic characterization of our V. vulnificus collection performed by ribotyping confirmed this observation. Ribotyping is a general technique also used in epidemiological studies of V. vulnificus (3, 5, 6, 9, 19, 23). We selected HindIII to perform the DNA digestion since it has been reported to provide the best discrimination between biotypes (9, 18). A common pattern was observed for all V. vulnificus strains, and a group of bands between 2 and 3 kb was absent in the profiles of the other vibrios examined. These bands were also observed in previous studies with manual protocols (9, 18, 22). The strains were grouped on the basis of their similarities in ribopatterns into two divisions and five groups. As expected, biotype 1 strains were found in almost every group and subgroup. Nevertheless, the biotype 1 strains that exhibited profile 1 were located mostly in division A, while the second division included all the biotype 2 and 3 strains together with additional biotype 1 strains from environmental sources and wound infections. In this division, the major genotypic profiles were profiles 2 and 3. Interestingly, ribogroup BI was comprised of all biotype 3 isolates that were closely related to environmental biotype 1 strains from sites related to fish farms. This result supports the previous hypothesis concerning the origin of biotype 3 as a clone associated with tilapia culture in Israel that recently emerged (13).
The eel-pathogenic strains were located in division B, with a major Rt (Rt 25) that included strains of the different serovars as well as other biotype 1 strains isolated mainly from the environment or from human wounds. This result also supports the hypothesis that biotype 2 strains could have been emerged from biotype 1 strains present in the environment. Recently, it has been shown that eel virulence relies on a 68-kb plasmid that can be transmitted between strains by conjugation with the aid of a conjugative plasmid (26). In this scenario, biotype 2 strains could have evolved independently by acquisition of the virulence plasmid by different clones of biotype 1 strains in the environment. Only one of these clones (serovar E) could be phenotypically distinguished from the rest of the biotypes, while the rest (serovars A and I) could not be distinguished. Additional studies based on multilocus sequence analysis with biotype 2 strains of different serovars and from different sources are needed to confirm this hypothesis.
Ribotyping has been used for differentiating clinical and environmental V. vulnificus isolates and biotypes, and several correlations between ribopatterns and geographic origin have been found (5, 6, 8). Our results, however, suggest that this technique may be useful for revealing genetic relationships among V. vulnificus isolates, but it is not likely to be useful for rapid identification of strains with public health interest.
In conclusion, the results obtained in the present work demonstrate that the species V. vulnificus is highly heterogeneous and that most of the diversity is present in biotype 1. Biotype 2 and 3 strains, in contrast, are more homogeneous, even though biotype 2 is serologically and phenotypically heterogeneous. There is a need for methods capable of rapid, sensitive, and accurate identification of the strains dangerous for public health. The DNA polymorphisms studied have been proposed for routine monitoring of the quality of seafood and water, but our results suggest that their use could eliminate samples containing strains with human-pathogenic potential, such as biotype 2 serovar E and biotype 3 strains. It is clear, therefore, that new genetic markers with epidemiological potential need to be found to clearly differentiate V. vulnificus.
Supplementary Material
Acknowledgments
This work was financed by grants AGL2005-04688 (cofinanced with FEDER funds), AGL2008-03977/ACU, and PET2005-0053 from the Spanish Ministry for Education and Science and by grant MTKD-CT-2004-0145019 from the European Union.
We thank Sergi Ferrer for advice concerning the Bionumerics software.
Footnotes
Published ahead of print on 9 January 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Amaro, C., and E. G. Biosca. 1996. Vibrio vulnificus biotype 2, pathogenic for eels, is also an opportunistic pathogen for humans. Appl. Environ. Microbiol. 62:1454-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amaro, C., E. G. Biosca, B. Fouz, and E. Garay. 1992. Electrophoretic analysis of heterogeneous lipopolysaccharides from various strains of Vibrio vulnificus biotypes 1 and 2 by silver staining and immunoblotting. Curr. Microbiol. 25:99-104. [DOI] [PubMed] [Google Scholar]
- 3.Amaro, C., L. I. Hor, E. Marco-Noales, T. Bosque, B. Fouz, and E. Alcaide. 1999. Isolation of Vibrio vulnificus serovar E from aquatic habitats in Taiwan. Appl. Environ. Microbiol. 65:1352-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anonymous. 1993. Vibrio vulnificus infections associated with raw oyster consumption—Florida, 1981-1992. MMWR Morb. Mortal. Wkly. Rep. 42:405-407. [PubMed] [Google Scholar]
- 5.Arias, C. R., M. J. Pujalte, E. Garay, and R. Aznar. 1998. Genetic relatedness among environmental, clinical, and diseased-eel Vibrio vulnificus isolates from different geographic regions by ribotyping and randomly amplified polymorphic DNA PCR. Appl. Environ. Microbiol. 64:3403-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arias, C. R., L. Verdonck, J. Swings, E. Garay, and R. Aznar. 1997. Intraspecific differentiation of Vibrio vulnificus biotypes by amplified fragment length polymorphism and ribotyping. Appl. Environ. Microbiol. 63:2600-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aznar, R., W. Ludwig, R. I. Amann, and K.-H. Schleifer. 1994. Sequence determination of rRNA genes of pathogenic Vibrio species and whole-cell identification of Vibrio vulnificus with rRNA-targeted oligonucleotide probes. Int. J. Syst. Bacteriol. 44:330-337. [DOI] [PubMed] [Google Scholar]
- 8.Aznar, R., W. Ludwig, and K.-H. Schleifer. 1993. Ribotyping and randomly amplified polymorphic DNA analysis of Vibrio vulnificus. Syst. Appl. Microbiol. 16:303-309. [Google Scholar]
- 9.Biosca, E. G., C. Amaro, J. L. Larsen, and K. Pedersen. 1997. Phenotypic and genotypic characterization of Vibrio vulnificus: proposal for the substitution of the subspecific taxon biotype for serovar. Appl. Environ. Microbiol. 63:1460-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biosca, E. G., E. Garay, and C. Amaro. 1993. Evaluation of the API 20E system for identification and discrimination of Vibrio vulnificus biotypes 1 and 2. J. Fish Dis. 16:72-82. [Google Scholar]
- 11.Bisharat, N., V. Agmon, R. Finkelstein, R. Raz, G. Ben-Dror, L. Lerner, S. Soboh, R. Colodner, D. N. Cameron, D. L. Wykstra, D. L. Swerdlow, J. J. Farmer III, and the Israel Vibrio Study Group. 1999. Clinical, epidemiological, and microbiological features of Vibrio vulnificus biogroup 3 causing outbreaks of wound infection and bacteraemia in Israel. Lancet 354:1421-1424. [DOI] [PubMed] [Google Scholar]
- 12.Bisharat, N., C. Amaro, B. Fouz, A. Llorens, and D. I. Cohen. 2007. Serological and molecular characteristics of Vibrio vulnificus biotype 3: evidence for high clonality. Microbiology 153:847-856. [DOI] [PubMed] [Google Scholar]
- 13.Bisharat, N., D. I. Cohen, R. M. Harding, D. Falush, D. W. Crook, T. Peto, and M. C. Maiden. 2005. Hybrid Vibrio vulnificus. Emerg. Infect. Dis. 11:30-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chatzidaki-Livanis, M., M. A. Hubbard, K. Gordon, V. J. Harwood, and A. C. Wright. 2006. Genetic distinctions among clinical and environmental strains of Vibrio vulnificus. Appl. Environ. Microbiol. 72:6136-6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chatzidaki-Livanis, M., M. K. Jones, and A. C. Wright. 2006. Genetic variation in the Vibrio vulnificus group 1 capsular polysaccharide operon. J. Bacteriol. 188:1987-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colodner, R., B. Chazan, J. Kopelowitz, Y. Keness, and R. Raz. 2002. Unusual portal of entry of Vibrio vulnificus: evidence of its prolonged survival on the skin. Clin. Infect. Dis. 34:714-715. [DOI] [PubMed] [Google Scholar]
- 17.Colodner, R., R. Raz, I. Meir, T. Lazarovich, L. Lerner, J. Kopelowitz, Y. Keness, W. Sakran, S. Ken-Dror, and N. Bisharat. 2004. Identification of the emerging pathogen Vibrio vulnificus biotype 3 by commercially available phenotypic methods. J. Clin. Microbiol. 42:4137-4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalsgaard, A., I. Dalsgaard, L. Høi, and J. L. Larsen. 1996. Comparison of a commercial biochemical kit and an oligonucleotide probe for identification of environmental isolates of Vibrio vulnificus. Lett. Appl. Microbiol. 22:184-188. [DOI] [PubMed] [Google Scholar]
- 19.Dalsgaard, A., N. Frimodt-Moller, B. Bruun, L. Høi, and J. L. Larsen. 1996. Clinical manifestations and molecular epidemiology of Vibrio vulnificus infections in Denmark. Eur. J. Clin. Microbiol. Infect. Dis. 15:227-232. [DOI] [PubMed] [Google Scholar]
- 20.Dechet, A. M., N. Koram, S. Jain, and J. Painter. 2005. Wound infections: an important cause of Vibrio morbidity and mortality. United States, 1997-2003, abstr. P-016, p. 71. 54th Epidemic Intelligence Service Conference, Atlanta, GA. Centers for Disease Control and Prevention, Atlanta, GA.
- 21.Fouz, B., J. L. Larsen, and C. Amaro. 2006. Vibrio vulnificus serovar A: an emerging pathogen in European anguilliculture. J. Fish Dis. 29:285-291. [DOI] [PubMed] [Google Scholar]
- 22.Gordon, K. V., M. C. Vickery, A. DePaola, C. Staley, and V. J. Harwood. 2008. Real-time PCR assay for quantification and differentiation of Vibrio vulnificus strains in oysters and water. Appl. Environ. Microbiol. 74:1704-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Høi, L., A. Dalsgaard, J. L. Larsen, J. M. Warner, and J. D. Oliver. 1997. Comparison of ribotyping and randomly amplified polymorphic DNA PCR for characterization of Vibrio vulnificus. Appl. Environ. Microbiol. 63:1674-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Høi, L., I. Dalsgaard, A. DePaola, R. J. Siebeling, and A. Dalsgaard. 1998. Heterogeneity among isolates of Vibrio vulnificus recovered from eels (Anguilla anguilla) in Denmark. Appl. Environ. Microbiol. 64:4676-4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim, M. S., and H. D. Jeong. 2001. Development of 16S rRNA targeted PCR methods for the detection and differentiation of Vibrio vulnificus in marine environments. Aquaculture 193:199-211. [Google Scholar]
- 26.Lee, C.-T., C. Amaro, K.-M. Wu, E. Valiente, Y.-F. Chang, S.-F. Tsai, C.-H. Chang, and L.-I. Hor. 2008. A common virulence plasmid in biotype 2 Vibrio vulnificus and its dissemination aided by a conjugal plasmid. J. Bacteriol. 190:1638-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin, M., and J. Schwartz. 2003. Seasonal shifts in population structure of Vibrio vulnificus in an estuarine environment as revealed by partial 16S ribosomal DNA sequencing. FEMS Microbiol. Ecol. 45:23-27. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson, W. B., R. N. Paranjype, A. DePaola, and M. S. Strom. 2003. Sequence polymorphism of the 16S rRNA gene of Vibrio vulnificus is a possible indicator of strain virulence. J. Clin. Microbiol. 41:442-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Hara, C. M., C. M. Sowers, C. A. Bopp, S. B. Duda, and N. A. Strockbine. 2003. Accuracy of six commercially available systems for identification of members of the family Vibrionaceae. J. Clin. Microbiol. 41:5654-5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliver, J. D. 2006. Vibrio vulnificus, p. 349-366. In F. L. Thompson, B. Austin, and J. Swings (ed.), Biology of vibrios. American Society for Microbiology Press, Washington, DC.
- 31.Oliver, J. D. 2006. Vibrio vulnificus, p. 253-276. In S. Belkin and R. R. Colwell (ed.), Oceans and health: pathogens in the marine environment. Springer Science, New York, NY.
- 32.Oliver, J. D. 2005. Wound infections caused by Vibrio vulnificus and other marine bacteria. Epidemiol. Infect. 133:383-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosche, T. M., Y. Yano, and J. D. Oliver. 2005. A rapid and simple PCR analysis indicates there are two subgroups of Vibrio vulnificus which correlate with clinical or environmental isolation. Microbiol. Immunol. 49:381-389. [DOI] [PubMed] [Google Scholar]
- 34.Sanjuán, E., and C. Amaro. 2007. Multiplex PCR assay for detection of Vibrio vulnificus biotype 2 and simultaneous discrimination of serovar E strains. Appl. Environ. Microbiol. 73:2029-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Senoh, M., S. Miyoshi, K. Okamoto, B. Fouz, C. Amaro, and S. Shinoda. 2005. The cytotoxin-hemolysin genes of human and eel pathogenic Vibrio vulnificus strains: comparison of nucleotide sequences and application to the genetic grouping. Microbiol. Immunol. 49:513-519. [DOI] [PubMed] [Google Scholar]
- 36.Strom, M. S., and R. N. Paranjpye. 2000. Epidemiology and pathogenesis of Vibrio vulnificus. Microbes Infect. 2:177-188. [DOI] [PubMed] [Google Scholar]
- 37.Tamplin, M. L., J. K. Jackson, C. Buchrieser, R. L. Murphree, K. M. Portier, V. Gangar, L. G. Miller, and C. W. Kaspar. 1996. Pulsed-field gel electrophoresis and ribotype profiles of clinical and environmental Vibrio vulnificus isolates. Appl. Environ. Microbiol. 62:3572-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tison, D. L., M. Nishibuchi, J. D. Greenwood, and R. J. Seidler. 1982. Vibrio vulnificus biogroup 2: new biogroup pathogenic for eels. Appl. Environ. Microbiol. 44:640-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vickery, M. C. L., W. B. Nilsson, M.S. Strom, J. L. Nordstrom, and A. DePaola. 2007. A real-time PCR assay for the rapid determination of 16S rRNA genotype in Vibrio vulnificus. J. Microbiol. Methods 68:376-384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.