Abstract
Shiga toxin (stx) transduction in various food matrices has been evaluated with lysogens of Stx phages. stx transduction events were observed for many phages under appropriate conditions. Transduction did not occur at low pH and low temperatures. A total of 103 to 104 CFU ml−1 was the minimal amount of donor and recipient strains necessary to generate transductants.
Shiga toxin-producing E. coli (STEC) strains are emergent food-borne pathogens (6, 11, 18, 27, 34). E. coli O157:H7 and other serotypes have been implicated in human diseases (18, 27, 34). The cardinal cause of virulence of STEC is the production of Shiga toxins (Stx) (6, 18). stx genes are carried in the genomes of temperate phages (15, 26), which are efficient vectors for the transfer of stx and play an important role in the evolution of new pathogens (31, 38). Stx phages can be induced from the lysogenic strain (19, 20, 41). As a result of prophage induction, bacterial host cells lyse and release free phage particles that can infect other bacteria and transduce the stx gene (2, 9, 17, 22, 28, 33, 35).
Although STEC strains are food-borne pathogens (8, 10, 11, 32, 36), there are no data on the potential transduction that could occur in food. Some food treatments can increase the rate of induction of prophages and the number of transduction events (3). In order to guarantee food quality, it is necessary to evaluate whether transduction by Stx phages can occur in food and the conditions that could favor stx transfer in food samples.
Sixty-two strains (Table 1) were tested for susceptibility to Stx phages. From this strain collection, tetracycline-resistant (Tcr) and chloramphenicol-susceptible (Cms) recipients were selected. Two nalidixic acid-resistant (Nalr) laboratory strains were also used as recipients: E. coli strain WG5 (ATCC 700078) (4) and strain C600Nal, generated in our laboratory. The donor strains used (Table 1) were E. coli lysogens harboring Stx phages with a modified stx::cat gene (28, 29, 30).
TABLE 1.
Bacterial strains used in this study
| Bacteria | Strain | No. of strains | Source | Genotypea | Antibiotic susceptibility | Reference(s) or source |
|---|---|---|---|---|---|---|
| Donor strains | ||||||
| E. coli K-12 strain C600 | LysφA9Cm | 1 | Laboratory lysogen | Δstx2::cat | Cmr Nals Tcs | 29 |
| E. coli K-12 strain C600 | Lys933WCm | 1 | Laboratory lysogen | Δstx2::cat | Cmr Nals Tcs | 29 |
| E. coli K-12 strain DH5α | LysφA557Cm | 1 | Laboratory lysogen | Δstx2::cat | Cmr Nals Tcs | 29 |
| E. coli K-12 strain DH5α | LysφVTB55Cm | 1 | Laboratory lysogen | Δstx2::cat | Cmr Nals Tcs | 29 |
| E. coli K-12 strain DH5α | LysφA312Cm | 1 | Laboratory lysogen | Δstx2::cat | Cmr Nals Tcs | 29 |
| E. coli K-12 strain DH5α | LysφA549Cm | 1 | Laboratory lysogen | Δstx2::cat | Cmr Nals Tcs | 29 |
| E. coli K-12 strain DH5α | LysφA534Cm | 1 | Laboratory lysogen | Δstx2::cat | Cmr Nals Tcs | 29 |
| E. coli K-12 strain C600 | C600φ3538 | 1 | Laboratory lysogen | Δstx2::cat | Cmr Nals Tcs | 28 |
| Recipient strains | ||||||
| E. coli | O157:H7 | 35 | Cattle and environment | stx1−, stx2+, and variable (+ or −) for eae and EHEC hly | Tcr Cms | 14, 22 |
| E. coli | Non-O157:H7 | 18 | Cattle, sheep, and food | stx1−, stx2+, and variable (+ or −) for eae and EHEC hly | Tcr Cms | 14, 22 |
| E. coli | Non-O157:H7 | 4 | Human | stx1−, stx2−, eae−, EHEC hly− | Tcr Cms | This study |
| Klebsiella pneumoniae | CF55 | 1 | Environment | Tcr Cms | This study | |
| Shigella flexneri | 2 | Human | Tcr Cms | 25 | ||
| Shigella sonnei | 2 | Human | Tcr Cms | 25 | ||
| E. coli K-12 | C600Nal | 1 | Laboratory | Nalr Cms | This study | |
| E. coli K-12 | WG5 | 1 | ATCC 700078 | Nalr Cms | 4 |
EHEC, enterohemorrhagic Escherichia coli.
Luria-Bertani (LB) agar or broth was used for routine bacterial propagation. Chromocult coliform agar (Merck, Darmstadt, Germany) was used for the evaluation of background flora and for the detection of transductants. When necessary, media were supplemented with Cm (25 μg ml−1), Tc (25 μg ml−1), and Nal (100 μg ml−1) (Sigma-Aldrich, Steinheim, Germany).
Food samples were purchased from local retailers. Liquid samples were commercial bottled water and ultra-high-temperature-processed whole milk. Some additional experiments included 100% pure orange juice (pH 3.7). Twenty-five milliliters of a liquid sample was used for the transduction experiments. Solid samples used were salad and ground beef. Salad was a commercially prepared mixture of frisée lettuce, corn salad, and radicchio (red chicory). The beef used was freshly minced on request at local supermarkets. Twenty-five grams of minced beef or salad was diluted 1:4 (wt/vol) in one-quarter-strength Ringer's solution. The mixture was placed in stomacher bags with filters (Afora, Barcelona, Spain) and then homogenated for 1 min in a stomacher (Masticator; IUL Instruments GmbH, Königswinter, Germany) for the transduction experiments.
To prevent the interference of the present background flora and phages infecting E. coli with the transduction experiments, 25 ml or g of a sample was tested for the presence of phages infecting E. coli strains WG5, C600Nal, and DH5α (coliphages) by double-agar layer methods as described previously (4) and for antibiotic-resistant flora using Chromocult agar with antibiotics (Tc/Cm or Nal/Cm).
The food samples were also analyzed for the presence of stx2 before the experiments. To this end, 25 ml or g of sample was used for DNA extraction with the QIAamp DNA blood mini kit (Qiagen, Valencia, CA). The presence of the stx2 gene was examined by PCR, as described below.
One milliliter of donor strain culture (lysogen carrying an stx::cat phage) (Table 1) and 1 ml of the recipient strain culture (Nalr Cms or Tcr Cms) (Table 1) were added to each sample and homogenized. The inoculated samples were then statically incubated at the corresponding temperature for 18 h. To detect transductants, the samples were 10-fold diluted and 0.1 ml was plated onto Chromocult agar plus antibiotics (Nal plus Cm or Tc plus Cm) and incubated overnight at 37°C. Those colonies grown on Chromocult agar plus antibiotics showing the same color as the recipient were considered Stx transductants. At least 10 colonies from each experiment were restreaked onto a new plate of Chromocult agar plus antibiotics and confirmed by PCR and sequencing.
Variations of this protocol, including different incubation temperatures (37, 30, 25, 22, 15, and 4°C) and lower pH, were assayed. The minimal amounts of the recipient and donor strains required to produce transductants were also evaluated.
PCRs were performed with primer pair S2Aup/3Cm3pKD3 and primer pair Cm5/Cm3 (29) to confirm the presence of recombinant phages in the suspected lysogens. Primer pair UP378/LP378 (24) was used to detect the presence of the stx2 gene in the samples. The identity of each PCR product was confirmed in duplicate by sequencing. Nucleotide sequence analyses were performed with the Wisconsin package version 10.2 (Genetics Computer Group [GCG], Madison, WI). BLAST analyses were done with the tools available on the NCBI webpage (http://www.ncbi.nlm.nih.gov).
The results indicate that the background flora present in the different food matrices did not generally interfere in the detection of the possible transductants because no growth was observed or because the background colonies (white) were distinguishable from the transductants (blue, pink, or violet). Coliphages and the stx2 gene were not detected in any of the samples used. The controls performed with spiked samples indicated that no inhibitors potentially present in the food samples were responsible for the negative results. The detection limit of the PCR assay was 5.101 to 102 stx2 copies ml−1 of sample.
The protocol allowed the generation of transductants in milk, water, beef, and salad. The transductants were resistant to both antibiotics (Nalr Cmr or Tcr Cmr), had the same color as the recipient strain, and presented the stx::cat fragment, as confirmed by PCR and sequencing. The controls performed with only donor or with only recipient strains in the samples did not generate transductants.
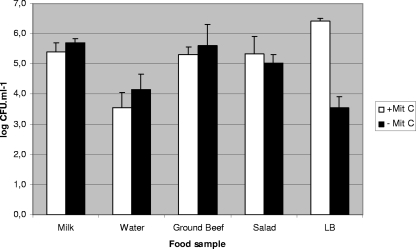
Since mitomycin C (0.5 μg ml−1) is commonly used to induce stx::cat phages from their lysogens (28, 29), it was used in the preliminary assays. Nevertheless, similar levels of transduction in samples treated with or without mitomycin C were observed (Fig. 1). Considering these results and since mitomycin C is not expected to be naturally present in food samples, the experiments presented were performed without mitomycin C induction.
FIG. 1.
Comparison of the log CFU ml−1 of transductants generated with or without mitomycin C (+Mit C and −Mit C, respectively) induction with strain LysφA557Cm as the donor strain and WG5 as the recipient strain. Results are the average of three independent experiments performed under identical conditions.
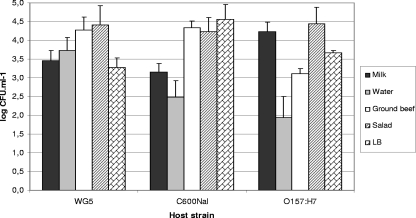
Transductants were generated with several phages assayed and in various food samples. Since the generation of transductants occurred after 18 h of incubation without antibiotic selection, the results of the generation of transductants can only be considered qualitatively. In spite of this, some repetitive levels of transductants obtained in successive replicas allowed us to present some quantitative results, for example, the values obtained when phage 933WCm was transduced from its lysogen to three E. coli host strains (WG5, C600Nal, and O157:H7 strain 278 [Tcr]) (Fig. 2).
FIG. 2.
Comparison of the log CFU ml−1 of the transductants generated with Lys933WCm as the donor strain and three recipient strains (WG5, C600Nal, and O157:H7-278) in the different food samples. Values were obtained after mitomycin C induction. Results are the average of three independent experiments performed under identical conditions.
These first studies were performed under conditions that guarantee transduction, although such conditions may not be found outside the laboratory. These optimal conditions were later modified in an attempt to emulate the environments to which food samples may be exposed outside the laboratory. In some of these “nonoptimal” situations, transductants were also generated, and it is to these conditions that attention must be paid for the risk they may represent.
Transductants were not generated in orange juice at pH 3.7, which we attributed to the low pH. To evaluate this, the pH of the samples was increased to 6.0 by the addition of 0.1 M NaOH. In this case, transductants were generated using LysφA557Cm and C600φ3538 as donors and E. coli WG5 and C600Nal as recipients. To confirm whether the survival of the recipient strain was the critical point, WG5 was then preconditioned at lower pH values in buffered LB medium. The lowest pH that allowed the growth of the recipient was 5.0. Transductants were then obtained. The survival of the donor strain does not appear to be relevant, probably because the phage particles, once induced from the donor, survive long enough at low pH to infect the recipient cells. The pH growth range of STEC is between 4.4 to 9.0, although some strains grow at lower values (<4.0) (12). The low number of transductants generated at low pH, together with the range of pH survival of STEC and other E. coli at low pH, makes transduction unlikely in acidic environments. Low pH could therefore be considered a potential treatment to prevent transduction.
Assays at a range of temperatures, to which samples had been previously acclimated, showed the consistent generation of transductants at 37°C and most of the phages at 30°C and 25°C. At 15°C and 22°C, transductants were generated for some donor strains but not for others. Finally, transductants were not generated at 4°C. This could be due to the inhibition of DNA injection at low temperatures, as reported for phage T4 at 4°C (21) or for phage T5, for which the low temperatures rendered the recipient cell membranes rigid, thus preventing injection (13).
The recipient strains of different serotypes that showed the required antibiotic susceptibility (Tcr Cms) were selected (Table 1) to assay transduction in milk and beef. Sensitivities of 18.2% to the 933WCm phage for milk and 9% for beef were observed in the selected strains (Table 2). Our results indicated a low range of potential recipients, although our studies were limited, due to the selection of the strains attending their antibiotic susceptibilities and to a reduced set of phages.
TABLE 2.
Transduction from lysogen Lys Φ933WCm to different E. coli strains and other Enterobacteriaceae in milk and ground beef
| Bacterium | Origin | No. of strains | Genotype | Milk | Beef |
|---|---|---|---|---|---|
| E. coli O111 | Human | 1 | stx2 negative | − | − |
| E. coli O127 | Human | 1 | stx2 negative | − | − |
| E. coli O26 | Human | 2 | stx2 negative | − | − |
| E. coli O4:H4 | Cattle | 1 | stx2 | − | − |
| E. coli O6:H49 | Beef meat | 1 | stx2 | − | − |
| E. coli O8:H9 | Sheep | 1 | stx2 | − | − |
| E. coli O8:H21 | Beef meat | 1 | stx2 | − | − |
| E. coli O15:H16 | Cattle | 1 | stx2 | + | − |
| E. coli O21:H21 | Beef meat | 1 | stx2 | + | − |
| E. coli O64:H34 | Cattle | 2 | stx2 | − | − |
| E. coli O70:H- | Cattle | 2 | stx2 | − | − |
| E. coli O91:H- | Sheep | 2 | stx2 | − | − |
| E. coli O113:H21 | Cattle | 2 | stx2, EHEC hly− | − | − |
| E. coli O113:H- | Cattle | 1 | stx2 | − | − |
| E. coli O174:H- | Cattle | 1 | stx2 | − | − |
| E. coli O162:H7 | Cattle | 1 | stx2 | + | + |
| E. coli O171:H25 | Cattle | 1 | stx2 | + | + |
| E. coli O157:H7 | Cattle | 30 | stx2, eae, EHEC hly | + (4 strains) | + (2 strains) |
| E. coli O157:H7 | Environment | 5 | stx2, eae, EHEC hly | − | − |
| Shigella sonnei | Human | 2 | + | + | |
| Shigella flexneri | Human | 2 | − | − | |
| Klebsiella pneumoniae | Environment | 1 | − | − | |
| E. coli C600Nal | Laboratory | 1 | + | + |
Strain WG5 was selected as the best recipient to evaluate the minimal amounts of the recipient and donor strains necessary to generate transductants at 37°C. Differences were observed between the three donor strains used. To calculate the threshold of each donor and recipient bacterium necessary for transduction to occur, the results (Table 3) should be interpreted as follows: if 10X is the value of the donor strain while 10Y is the value of WG5, then X + Y should be between 8 and 10 for C600φ3538, more than 10 for Lysφ557Cm, and 14 for Lys933WCm to transduce WG5 in the solid samples. For the liquid samples, the threshold was generally lower. For phages C600φ3538 and Lys933WCm, X + Y was 8 and 13, respectively, and for Lysφ557Cm, it was 6 to 7 in water and 10 in milk. Transductants were not obtained when the recipient strain value was lower than 102 CFU ml−1. The controls were performed at different times to rule out the possibility that the donor and recipient strains may have grown during the process.
TABLE 3.
Generation of transductants using different values for the donor strains (lysogens of phages) and the recipient strain (E. coli WG5) in the food samples without mitomycin C inductiona
| Donor strain and no. of CFU (10X) | No. of recipient strain CFU (10Y) | Threshold (X + Y)b | Presence of transductants ind:
|
||||
|---|---|---|---|---|---|---|---|
| Water | Milk | Beef | Salad | LB medium | |||
| C600φ3538 | |||||||
| 108 | 108 | 16 | +b | + | + | + | + |
| 106 | 14 | + | + | + | + | + | |
| 104 | 12 | + | + | + | +/− | + | |
| 102 | 10 | − | − | − | − | + | |
| 107 | 107 | 14 | + | + | + | + | + |
| 106 | 106 | 12 | + | + | + | + | + |
| 105 | 105 | 10 | + | + | + | + | + |
| 104 | 104 | 8 | + | + | +/− | +/− | + |
| 103 | 103 | 6-7c | +/− | − | − | − | +/− |
| 102 | 102 | 4-5c | − | − | − | − | − |
| LysφA557Cm | |||||||
| 107 | 106 | 13 | + | + | + | + | + |
| 104 | 11 | + | + | + | + | ||
| 102 | 9 | − | +/− | − | − | + | |
| 106 | 108 | 14 | + | + | + | + | + |
| 106 | 12 | + | + | + | + | + | |
| 105 | 105 | 10 | + | + | +/− | − | + |
| 104 | 104 | 8 | + | +/− | − | − | + |
| 103 | 103 | 6-7c | + | +/− | − | − | +/− |
| 102 | 102 | 4-5c | − | − | − | − | − |
| 101 | 101 | 2-3c | − | − | − | ||
| Lys933WCm | |||||||
| 107 | 107 | 14 | + | + | + | + | + |
| 106 | 13 | + | + | − | − | + | |
| 105 | 12 | − | − | − | − | ||
| 106 | 107 | 13 | + | + | + | + | + |
| 106 | 12 | − | − | − | − | ||
Numbers of CFU ml−1 or g−1 of sample were measured for the donor and recipient strains.
The threshold necessary for the generation of transductants was estimated and is expressed as the sum of X + Y, where X is the exponential value for the donor strain and Y is the exponential value for the recipient strain.
When using lower values of donor and recipient cells, the X + Y calculation varies with the replicas and is expressed as a range between two log units.
+, transductants were generated, unless otherwise indicated, at >103 CFU ml−1 or g−1 of sample; +/−, some replicas positive and some negative; −, no transductants.
The higher threshold observed for the solid samples can be explained since a solid sample is a more complex and heterogeneous matrix, with an irregular distribution of the cells that reduces the phage-bacteria encounters necessary for infection to occur. The results for the liquid samples are similar to those reported elsewhere for replicating E. coli and a specific bacteriophage (39) and also for coliphages in water environments (23).
The levels of donor and recipient strains needed to generate transductants are not expected to be found in a food sample available to consumers, and so transduction is unlikely to occur in such samples. The low prevalence of O157 found in food samples (11, 12) indicates that transduction may be rare, although the prevalence of STEC in food (1) indicates that this possibility cannot be ruled out if some break in the control of the food chain happens (7, 16). The transduction of stx to recipient strains present in the background flora could be the main risk for the consumer.
Food products can be subjected to conditions that reduce the growth of some bacteria but prolong their survival. In these conditions, bacterial populations suffer stress (37), which can increase the induction of prophages to their lytic cycle and therefore increase the number of free phage particles (5). Certain treatments used for the processing of food or water samples, such as chlorination (24), thermal treatment (24), radiation with 60Co (40), or high hydrostatic pressure (3) fail to inactivate Stx phages. Indeed, some of these treatments may even increase the induction of the lytic cycle of certain Stx phages, which will persist after the application of the treatment (3). Further studies to evaluate the occurrence of free Stx phages in food matrices, especially in cattle products, are required. Attending to our results, transduction in food does not seem to be a high-risk way of stx transmission, although it can take place under the appropriate conditions and should not be discounted.
Acknowledgments
We thank J. Blanco, G. Prats, and F. Ribas for providing some of the strains used in this study.
This study was supported by the Autonomous Government of Catalonia (Generalitat de Catalunya) (2005SGR00592), by the Xarxa de Recerca en Biotecnologia (XRB), by the Spanish Ministry of Education and Science (AGL200601566/ALI), and by grant 01Kl 07128 (Food-Borne Zoonotic Infections of Humans) from the German Federal Ministry of Education and Research (BMBF). L.I. is a recipient of a grant from the Spanish Ministry of Education and Science (FPI 20060054361).
Footnotes
Published ahead of print on 23 January 2009.
REFERENCES
- 1.Acheson, D. W. 2000. How does Escherichia coli O157:H7 testing in meat compare with what we are seeing clinically? J. Food Prot. 63:819-821. [DOI] [PubMed] [Google Scholar]
- 2.Acheson, D. W., J. Reidl, X. Zahang, G. T. Keusch, J. J. Mekelanos, and M. K. Waldor. 1998. In vivo transduction with Shiga toxin 1-encoding phage. Infect. Immun. 66:4496-4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aertsen, A., D. Faster, and C. W. Michiels. 2005. Induction of Shiga toxin-converting prophage in Escherichia coli by high hydrostatic pressure. Appl. Environ. Microbiol. 71:1155-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anonymous. 2000. ISO 10705-2: water quality. Detection and enumeration of bacteriophages—part 2: enumeration of somatic coliphages. International Organisation for Standardisation, Geneva, Switzerland.
- 5.Brabban, A. D., E. Hite, and T. R. Callaway. 2005. Evolution of foodborne pathogens via temperate bacteriophage-mediated gene transfer. Foodborne Pathog. Dis. 2:287-303. [DOI] [PubMed] [Google Scholar]
- 6.Caprioli, A., S. Morabito, H. Brugère, and E. Oswald. 2005. Enterohaemorrhagic Escherichia coli: emerging issues on virulence and modes of transmission. Vet. Res. 36:289-311. [DOI] [PubMed] [Google Scholar]
- 7.Chua, D., K. Goh, R. A. Saftner, and A. A. Bhagwat. 2008. Fresh-cut lettuce in modified atmosphere packages stored at improper temperatures supports enterohemorrhagic E. coli isolates to survive gastric acid challenge. J. Food Sci. 73:M148-M153. [DOI] [PubMed] [Google Scholar]
- 8.Cody, S. H., M. K. Glynn, J. A. Farrar, K. L. Cairns, P. M. Griffin, J. Kobayashi, M. Fyfe, R. Hoffman, A. S. King, J. H. Lewis, B. Swaminathan, R. G. Bryant, and D. J. Vugia. 1999. An outbreak of Escherichia coli O157:H7 infection from unpasteurized commercial apple juice. Ann. Intern. Med. 130:202-209. [DOI] [PubMed] [Google Scholar]
- 9.Cornick, N. A., A. F. Helgerson, V. Mai, J. M. Ritchie, and D. W. Acheson. 2006. In vivo transduction of an Stx-encoding phage in ruminants. Appl. Environ. Microbiol. 72:5086-5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dev, V. J., M. Main, and I. Gould. 1991. Waterborne outbreak of Escherichia coli O157. Lancet 337:1412. [DOI] [PubMed] [Google Scholar]
- 11.Erickson, M. C., and M. P. Doyle. 2007. Food as a vehicle for transmission of Shiga toxin-producing Escherichia coli. J. Food Prot. 70:2426-2449. [DOI] [PubMed] [Google Scholar]
- 12.European Commission. 2003. Opinion of the scientific committee on veterinary measures relating to public health on verotoxigenic E. coli (VTEC) in foodstuffs. Health and Consumer Protection Directorate-General, European Commission, Brussels, Belgium. http://ec.europa.eu/food/fs/sc/scv/outcome_en.html.
- 13.Filali Maltouf, A., and B. Labedan. 1983. Host cell metabolic energy is not required for injection of bacteriophage T5 DNA. J. Bacteriol. 153:124-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.García-Aljaro, C., X. Bonjoch, and A. R. Blanch. 2005. Combined use of an immunomagnetic separation method and immunoblotting for the enumeration and isolation of E. coli O157 in wastewaters. J. Appl. Microbiol. 98:589-597. [DOI] [PubMed] [Google Scholar]
- 15.Herold, S., K. Karch, and H. Schmidt. 2004. Shiga-toxin-converting bacteriophages. Genomes in motion. Int. J. Med. Microbiol. 294:115-121. [DOI] [PubMed] [Google Scholar]
- 16.Hoyle, B. 2008. Rise in food-borne outbreaks from leafy greens proves puzzling. Microbe 6:269-270. [Google Scholar]
- 17.James, C. E., K. N. Stanley, H. E. Allison, H. J. Flint, C. S. Stewart, R. J. Sharp, J. R. Saunders, and A. J. McCarthy. 2001. Lytic and lysogenic infection of diverse Escherichia coli and Shigella strains with a verocytotoxigenic bacteriophage. Appl. Environ. Microbiol. 67:4335-4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaper, J. B., J. P. Nataro, and H. L. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123-140. [DOI] [PubMed] [Google Scholar]
- 19.Kimmitt, P. T., C. R. Harwood, and M. R. Barer. 2000. Toxin gene expression by Shiga toxin-producing Escherichia coli: the role of antibiotics and the bacterial SOS response. Emerg. Infect. Dis. 6:458-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohler, B., H. Karch, and H. Schmidt. 2000. Antibacterials that are used as growth promoters in animal husbandry can affect the production of Shiga-toxin-2-converting bacteriophages and Shiga toxin 2 from Escherichia coli strains. Microbiology 146:1085-1090. [DOI] [PubMed] [Google Scholar]
- 21.Labedan, B., and E. B. Goldberg. 1979. Requirement for membrane potential in injection of phage T4 DNA. Proc. Natl. Acad. Sci. USA 76:4669-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muniesa, M., J. E. Blanco, M. De Simon, R. Serra-Moreno, A. R. Blanch, and J. Jofre. 2004. Diversity of stx2-converting bacteriophages induced from Shiga-toxin-producing Escherichia coli strains isolated from cattle. Microbiology 150:2959-2971. [DOI] [PubMed] [Google Scholar]
- 23.Muniesa, M., and J. Jofre. 2004. Factors influencing the replication of somatic coliphages in the water environment. Antonie van Leeuwenhoek 86:65-76. [DOI] [PubMed] [Google Scholar]
- 24.Muniesa, M., F. Lucena, and J. Jofre. 1999. Comparative survival of free Shiga toxin 2-encoding phages and Escherichia coli strains outside the gut. Appl. Environ. Microbiol. 5:5615-5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muniesa, M., R. Serra-Moreno, and J. Jofre. 2004. Free Shiga toxin bacteriophages isolated from sewage showed diversity although the stx genes appeared conserved. Environ. Microbiol. 6:716-725. [DOI] [PubMed] [Google Scholar]
- 26.O'Brien, A. D., J. W. Newland, S. F. Miller, R. K. Holmes, H. W. Smith, and S. B. Formal. 1984. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science 226:694-696. [DOI] [PubMed] [Google Scholar]
- 27.Paton, J. C., and A. W. Paton. 1998. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 11:450-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt, H., M. Bielaszewska, and H. Karch. 1999. Transduction of enteric Escherichia coli isolates with a derivative of Shiga toxin 2-encoding bacteriophage φ3538 isolated from Escherichia coli O157:H7. Appl. Environ. Microbiol. 65:3855-3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serra-Moreno, R., S. Acosta, J.-P. Hernalsteens, J. Jofre, and M. Muniesa. 2006. Use of the lambda red recombinase system to produce recombinant prophages carrying antibiotic resistance genes. BMC Mol. Biol. 7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serra-Moreno, R., J. Jofre, and M. Muniesa. 2007. Insertion site occupancy by stx2 bacteriophages depends on the locus availability of the host strain chromosome. J. Bacteriol. 189:6645-6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serra-Moreno, R., J. Jofre, and M. Muniesa. 2008. The CI repressors of Shiga toxin-converting prophages are involved in coinfection of Escherichia coli strains, which causes a down regulation in the production of Shiga toxin 2. J. Bacteriol. 190:4722-4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh, B. R., S. B. Kulshreshtha, and K. N. Kapoor. 1995. An orange juice-borne diarrhoeal outbreak due to enterotoxigenic Escherichia coli. J. Food Sci. Technol. 32:504-506. [Google Scholar]
- 33.Strauch, E., R. Lurzand, and L. Beutin. 2001. Characterization of a Shiga toxin-encoding temperate bacteriophage of Shigella sonnei. Infect. Immun. 69:7588-7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarr, P. I., C. A. Gordon, and W. L. Chandler. 2005. Shiga toxin-producing Escherichia coli and hemolytic uraemic syndrome. Lancet 365:1073-1086. [DOI] [PubMed] [Google Scholar]
- 35.Toth, I., H. Schmidt, M. Dow, A. Malik, E. Oswald, and B. Nagy. 2003. Transduction of porcine enteropathogenic Escherichia coli with a derivative of a Shiga toxin 2-encoding bacteriophage in a porcine ligated ileal loop system. Appl. Environ. Microbiol. 69:7242-7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uhlich, G. A., J. R. Sinclair, N. G. Warren, W. A. Chmielecki, and P. Fratamico. 2008. Characterization of Shiga toxin-producing Escherichia coli isolates associated with two multistate food-borne outbreaks that occurred in 2006. Appl. Environ. Microbiol. 74:1268-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uyttendaele, M., I. Taverniers, and J. Debevere. 2001. Effect of stress induced by suboptimal growth factors on survival of Escherichia coli O157:H7. Int. J. Food Microbiol. 66:31-37. [DOI] [PubMed] [Google Scholar]
- 38.Wagner, P. L., M. N. Neely, X. Zhang, D. W. Acheson, M. K. Waldor, and D. I. Friedman. 2001. Role for a phage promoter in Shiga toxin 2 expression from a pathogenic Escherichia coli strain. J. Bacteriol. 183:2081-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiggins, B. A., and M. Alexander. 1985. Minimum bacterial density for bacteriophage replication: implications for significance of bacteriophages in natural ecosystems. Appl. Environ. Microbiol. 49:19-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto, T., S. Kojio, I. Taneike, S. Nakagawa, N. Iwakura, and N. Wakisaka-Saito. 2003. 60Co irradiation of Shiga toxin (Stx)-producing Escherichia coli induces Stx phage. FEMS Microbiol. Lett. 222:115-121. [DOI] [PubMed] [Google Scholar]
- 41.Zhang, X., A. D. McDaniel, L. E. Wolf, G. T. Keusch, and M. K. Waldor. 2000. Quinolone antibiotics induce Shiga toxin-encoding bacteriophages, toxin production, and death in mice. J. Infect. Dis. 181:664-670. [DOI] [PubMed] [Google Scholar]