Abstract
The survival of bacteria exposed to toxic compounds is a multifactorial phenomenon, involving well-known molecular mechanisms of resistance but also less-well-understood mechanisms of tolerance that need to be clarified. In particular, the contribution of biofilm formation to survival in the presence of toxic compounds, such as nickel, was investigated in this study. We found that a subinhibitory concentration of nickel leads Escherichia coli bacteria to change their lifestyle, developing biofilm structures rather than growing as free-floating cells. Interestingly, whereas nickel and magnesium both alter the global cell surface charge, only nickel promotes biofilm formation in our system. Genetic evidence indicates that biofilm formation induced by nickel is mediated by the transcriptional induction of the adhesive curli-encoding genes. Biofilm formation induced by nickel does not rely on efflux mechanisms using the RcnA pump, as these require a higher concentration of nickel to be activated. Our results demonstrate that the nickel-induced biofilm formation in E. coli is an adaptational process, occurring through a transcriptional effect on genes coding for adherence structures. The biofilm lifestyle is obviously a selective advantage in the presence of nickel, but the means by which it improves bacterial survival needs to be investigated.
Nickel is not well known to the public, since the major use of this metal is in the preparation of alloys. Nickel alloys are known for their superior resistance to both heat and corrosion. These properties make nickel useful in the chemical industry as well as in food processing (storage tanks, piping, etc.). Nickel alloys are also commonly used in medical devices (pacemakers, orthopedic implants, needles, surgical instruments, etc.). One of the major corrosion products of stainless steel is nickel, and bacteria in the surrounding media encounter these metal ions. Nickel alloys are claimed to ensure that the product or implant remains uncontaminated (26). However, increasing evidence shows that bacteria can adhere to any kind of material to develop biofilms, with detrimental consequences. The development of a biofilm displaying multiresistance to antibiotics, detergents, and sanitizers is a cause of growing concern for hospitals and industrial plants (20, 60).
Although Escherichia coli is the best-characterized living microorganism nowadays, current models of biofilm formation come mostly from experimental evidence using Pseudomonas aeruginosa, and this phenomenon is described as a multistep process. Reversible physicochemical interactions between bacteria and surfaces allow the initial bacterial attachment in a primary phase. Extracellular polymers, such as fimbriae or capsules, would then allow stable, irreversible molecular and cellular interactions to occur between bacteria and the surfaces. Depending on the extracellular polymers produced, bacterial growth results in a mushroom-shaped structure or a flat biofilm (32-34).
Bacterial survival in the presence of toxic compounds is dependent on resistance and tolerance mechanisms. Bacterial resistance is thought to be achieved using five main strategies: (i) efflux systems, (ii) sequestration of the toxic compound, (iii) reduction of bacterial membrane permeability, (iv) enzymatic alteration to a less toxic form, and (v) reduction in the sensitivity of cellular targets (9). These resistance mechanisms allow bacterial growth to occur even at high concentrations of an antibiotic or metal. In contrast to resistance, some cells are able to survive attack by metals or antibiotics without expressing or using resistance mechanisms, but these cells, referred to as persister or dormant cells, are not able to divide (39). Such persister cells were first described in planktonic culture (5) but have been recently characterized in biofilms (24, 25, 54). Poor penetration of antimicrobial agents in the biofilm polymeric matrix and specific metabolic changes in biofilm cells are also thought to be key elements of tolerance. The variety of potential mechanisms implicated in biofilm resistance/tolerance to antimicrobial agents has been recently reviewed (10, 16), but a consensus explanation for the enhanced antimicrobial resistance/tolerance of a biofilm has not yet been demonstrated.
After Pseudomonas and Staphylococcus, E. coli has been the most studied species in terms of biofilms and survival in reaction to metals and antibiotics. Planktonic E. coli bacteria can export excess metal by efflux systems, such as RcnA, a nickel and cobalt exporter (51), but no data on the expression of this pump in a biofilm have been published. E. coli is a human commensal or pathogenic bacterium that is able to colonize a wide range of habitats, from water to the different organs of its host. This bacterium is able to exist for a long time in the environment, preferentially in biofilms. Lack of data relating to sessile E. coli is mainly explained by the difficulty of cultivating biofilms from E. coli laboratory strains. Indeed, most of the K-12 strains do not express many adherence factors. Involvement of the curli in biofilm formation on inert surfaces has been discovered by characterizing a curli-expressing adherent mutant derived from the MG1655 laboratory strain (18, 48). Curli are produced by the majority of the enterotoxic and enterohemorrhagic E. coli strains, including O157:H7, and are regarded as a major virulence factor for pathogenic bacteria (4). The study of the regulation of curli genes in biofilms has led us to propose a model in which they are produced throughout the formation of a biofilm (31, 47). Curli are of particular interest since they are involved in attachment both on eukaryotic surfaces (2, 22) and on abiotic surfaces (glass, sand, plastic, etc.) (7, 38, 58). The genes necessary for curli formation are clustered in two divergently transcribed operons. The csgBA operon carries the structural genes. The csgDEFG operon encodes the transcriptional activator, CsgD, of the csgBA operon and three curli assembly factors. Complex regulatory pathways involving several two-component systems have been shown to control curli gene expression (18, 31, 37).
Numerous studies dealing with the biocide resistance of biofilms highlight the multifactorial aspect of the phenomenon. This work investigates the contribution of the biofilm lifestyle to survival of E. coli K-12 strains that produce curli in the presence of the heavy metal nickel. Because this strain is able to form thick biofilms (58), it was used as a model of environmental and medical E. coli strains that produce curli (4). Increasing amounts of soluble nickel ions were used to test the early physiological effects of this metal on the adherence and survival of bacteria on polystyrene and stainless steel. Subinhibitory concentrations of nickel promote the formation of very thick biofilms. This biofilm promoter effect does not rely on the modification of physicochemical cell surface properties by nickel. Surprisingly, this effect also seems to be independent of the efflux pump RcnA. Instead the biofilm promoter effect of nickel results from the drastic upregulation of the curli. We describe here, for the first time, the induction of biofilm formation by a subinhibitory concentration of nickel via the transcriptional activation of curli genes.
MATERIALS AND METHODS
Bacterial strains and media.
For this study, we used the E. coli K-12 derivative MG1655 ompR234 (PHL818) and derivatives of this strain (Table 1). This strain produces thick biofilms as a consequence of the expression of curli (47). Bacterial cells were grown in 4× diluted Luria-Bertani broth (LB/4) supplemented with 0.1% mannitol. LB was diluted in order to reduce osmolarity and promote bacterial adherence. Bacteria were grown at 30°C or 37°C. Planktonic growth of the cells was carried out in shaken tubes or Erlenmeyer flasks at 50 rpm or 130 rpm for 24 h. When necessary, ampicillin (100 μg/ml), chloramphenicol (20 μg/ml), kanamycin (50 μg/ml), gentamicin (10 μg/ml), or spectinomycin (100 μg/ml), purchased from Sigma, was added.
TABLE 1.
E.coli K-12 strains and plasmids used in this study
| Strain or plasmid | Relevant genotype | Source or reference |
|---|---|---|
| Strains | ||
| MG1655 | λ− F− | Laboratory collection |
| PHL818 | MG1655 ompR234 malT::Tn10 | 47 |
| PHL881 | E. coli isolated from percutaneous transhepatic catheter | 18 |
| PHL1087 | PHL818 csgD::uidA-kan | 47 |
| PHL1137 | PHL818 csgA::uidA-kan | This study |
| PHL1273 | PHL818/p127 | This study |
| PHL1359 | PHL818/pMP2444 | This study |
| PHL1370 | PHL818/p157 | This study |
| PHL1374 | PHL818 rcnA::uidA-kan | This study |
| PHL1563 | PHL818 flu::cm | This study |
| ZK2692 | W3110 Δ(argF-lac) U169 flu::cm | 14 |
| Plasmids | ||
| pCSG4 | pUC19 with a 3.5-kb HindIII fragment containing the csg intergenic region; Ampr | 44 |
| pPROBE-gfp[LVA] | Gene fusion vector, pBBR1 replicon, GFP-destabilized variant LVA; Kanr | 43 |
| pPROBE-OT′ | Gene fusion vector, pBBR1 replicon, GFP reporter gene; Spcr | 43 |
| p127 | pPROBE-gfp[LVA] carrying the gfp[LVA] coding sequence under the control of the csgBA promoter; Kanr | This study |
| p157 | pPROBE OT′ carrying the gfp coding sequence under the control of the rcnA promoter; Spcr | This study |
| pMP2444 | pBBR1MCS-5 containing the enhanced gfp coding sequence under the control of the lac promoter | 6 |
Genetic methods.
The rcnA::uidA-kan mutation was transferred from strain ARY023 (51) to PHL818 by phage P1 vir transduction, which was carried out as described by Miller (42). Kanamycin-resistant colonies were selected and tested for nickel resistance as described previously (51). The flu::cm mutation was transferred from strain ZK2692 (14) to PHL818 by phage P1 vir transduction, which was carried out as described above. Chloramphenicol-resistant colonies were selected and tested for nickel resistance as described previously (51). Transformations of E. coli cells with plasmids, described in Table 1, were performed as described in reference 11.
Construction of the csgB::gfp and rcnA::gfp fusions.
A 757-bp fragment, containing the csg intergenic region, was amplified by PCR with plasmid pCSG4 DNA (44) as a template and two primers (5′-TCTAGACATTAAACATGATGAAACCCCGC-3′ and 5′-GAGCTCCATGTTGTCACCCTGGACCTGGTC-3′) overlapping the csgBA and csgDEFG regulatory regions and containing XbaI and SacI cutting sites, respectively. The PCR fragment was first cloned in pGEM-T (Promega) to make pGEM-T-100/101. The 757-bp XbaI-SacI fragment was cloned into the corresponding sites of the pPROBE-gfp[LVA] vector (43), producing p127 described in Table 1. To construct the rcnA::gfp fusion, a 541-bp fragment containing the upstream region of rcnA was isolated from plasmid pAR020 (51) after digestion with EcoRI and PvuII. This fragment was inserted between the EcoRI and SmaI sites of vector pPROBE-OT′ (43), resulting in plasmid p157. The plasmids used in this report are listed in Table 1.
Nickel and magnesium susceptibility testing.
Stock solutions of nickel chloride (NiCl2; Merck) and magnesium chloride (MgCl2; Prolabo) were made up to a concentration of 50 mM of the metal cation in ultrapure water (Millipore). The filtered solutions were stored at 4°C. Working solutions (ranging from 100 to 300 μM of the metal cations) were prepared extemporaneously in LB/4. Free-living and sessile cell biomass was estimated by measurements of the optical density at 600 nm (OD600). In each set of experiments, the OD600 value measured from the culture without nickel or magnesium was taken as the 100% value to normalize the data (arbitrary units [AU]). Growth in the presence of nickel or magnesium is expressed as a percentage of the growth without these metals.
Fluorescence assay.
The transcriptional activity of the lac or csg promoter was estimated from measurements of the green fluorescent protein (GFP) fluorescence of cells grown in LB/4 medium containing a range of added nickel concentrations. Fluorescence measurements were made on a Kontron SMF25 fluorimeter with an excitation wavelength of 490 nm and an emission wavelength of 510 nm, after determination of the OD600 of the cultures. The fluorescence values of three independently grown cultures were averaged and normalized according to the density of the culture. Fluorescence was expressed as relative AU.
TEM observations.
For transmission electron microscopy (TEM), cell cultures were prepared in flasks containing LB/4 medium supplemented with 0.1% mannitol for 24 h at 30°C and 50 rpm. Cells were carefully washed with 1 mM EDTA in order to remove excess nickel and allowed to sediment at 4°C. Supernatants were carefully eliminated. Five microliters of sedimented cells was carefully recovered and deposited on carbon-coated 200-mesh grids. After fixation with osmium tetroxide, cell staining was performed using 1% phosphotungstic acid and the grids were observed with a Philips CM120 transmission electron microscope.
β-Glucuronidase assay.
β-Glucuronidase activity in toluene-treated samples was measured by spectrophotometrically monitoring the hydrolysis of p-nitrophenyl-β-d-glucuronide into p-nitrophenol at 405 nm (3). Specific activity was measured and expressed as units per milligram of bacterial dry weight, where 1 U corresponds to 1 nmol of product liberated per mg of total bacterial dry weight.
Static biofilm formation on polystyrene plates.
The wells of 24-well plates or petri dishes were filled with 2 and 20 ml, respectively, of the appropriate medium and inoculated with 106 cells of an overnight culture. Free-living and sessile bacteria were collected as described in references 18 and 49. Scraping, pipetting up and down, and vortexing for 20 s resulted in disruption of cell clumps, as monitored by microscopy. Free-living and sessile cell biomass was estimated by OD600 measurements. A minimum of three assays were performed in each experiment. Petri dishes were used to collect a sufficient amount of bacteria to calculate the specific activity when assaying gene fusions in a high-nickel environment.
Static biofilm formation on stainless steel coupons.
Biofilms were grown on AISI 304 stainless steel (Goodfellow, Cambridge Science Park, United Kingdom). Prior to the biofilm experiments, the solid surfaces were soaked for 10 min at 50°C in a 2% (vol/vol) solution of a commercial surfactant, RBS 35 (Société des Traitements Chimiques de Surface, Lambersart, France), rinsed for 10 min in Purit deionized water (Purit, France) at 50°C, and then rinsed five times in 500 ml of Purit deionized water at room temperature. The cleaned surfaces were finally sterilized by autoclaving for 20 min at 121°C. Overnight cultures of MG1655 ompR234 derivatives were diluted to a concentration of 1 × 106 CFU·ml−1. Twenty milliliters of the bacterial suspension was transferred to petri dishes containing the sterile solid surface of stainless steel. After 2 h of contact, the slides were rinsed with LB/4 to remove the nonadherent bacteria. Adherent cells were finally incubated at 30°C in 20 ml of LB/4 for 24 h.
Biofilm quantification by laser confocal imaging.
Biofilm quantification was assessed directly on the solid surfaces by confocal microscopy and image analysis. Twenty-four-hour-old biofilms were observed under a SP2 AOBS Leica confocal laser microscope (Leica Microsystem, France, at the MIMA2 microscopic platform). GFP was excited at 488 nm, and the bacterial fluorescence was collected in the range 500 to 600 nm. A Leica ×63/1.4 N.A. oil immersion objective was used for acquiring images in scanning mode. The overall three-dimensional structures of the biofilms were scanned from the solid surface to the interface with the growth medium, using a step of 1 μm. Quantification of biofilm biovolumes (μm3) was extracted from confocal z-stacks with the PHLIP MATLAB routine, developed by J. Xaviere for biofilm structure analysis (http://www.itqb.unl.pt:1141/∼webpages/phlip/phlip-ml/). Three-dimensional projections were performed with IMARIS software (Bitplane, Zürich, Switzerland).
Bacterial cell EM.
Prior to the electrophoretic mobility (EM) measurements, bacteria were centrifuged at 2,000 × g for 10 min and rinsed twice in 5 ml of 15 mM sodium chloride at pH 6 to reach a concentration of 1 × 107 CFU·ml−1. Bacterial suspensions were then incubated for half an hour at room temperature in the presence of 100 μM of nickel chloride, 100 μM of magnesium chloride, or without metal. Electrophoretic measurements were taken in an electric field of 50 V with a Laser Zetameter (CAD Instrumentation, France). Results are based on an automated video analysis of about 300 particles for each measurement. Each experiment was performed in duplicate on two independently prepared cultures.
Statistical analysis.
The values for each experiment are the mean values of results from three experiments unless otherwise stated. The error bars in figures indicate standard deviation. Analyses of variance were performed with Statgraphics Plus 4.1 (Manugistics, Rockville, MD).
RESULTS
Dual effect of nickel on biofilm growth.
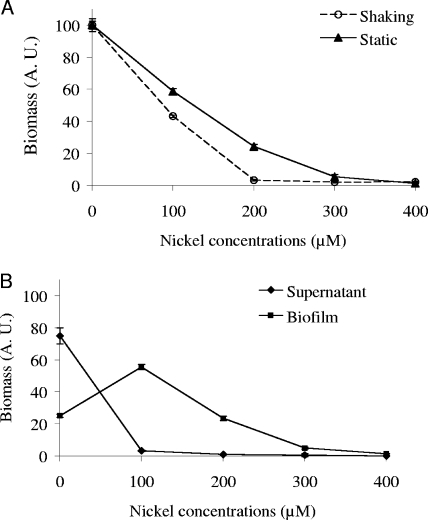
As expected, an increasing amount of nickel is toxic for E. coli cells cultured under static conditions as well as in shaken cultures (Fig. 1A). Compared to the bacterial growth in the absence of metal, estimated from the OD600 measurement, 40% of biomass was formed in the presence of 100 μM of nickel in agitated cultures versus 60% in static cultures. Moreover, no growth was observed at 200 μM of nickel in agitated cultures, whereas the inhibitory concentration was 300 μM under static conditions. The toxicity of nickel was therefore significantly less severe in static cultures. A closer insight indicates a complex phenomenon beyond global nickel toxicity in such cultures. Bacteria grown under static conditions were separated into adherent and free-floating subpopulations. In this case, an opposite effect of nickel was observed in the two subpopulations (Fig. 1B). The global loss of 40% of the total bacterial population in the presence of 100 μM of nickel in static cultures (Fig. 1A, triangles) results mostly from the disappearance of planktonic cells, and not from a decrease of the biofilm biomass. On the contrary, biofilm biomass increased twofold in the presence of 100 μM of nickel compared to the biofilm grown in the absence of this metal (Fig. 1B). Generation time reached 1.07 ± 0.06 h when the strain was grown without nickel compared to 1.10 ± 0.14 h during growth with 100 μM of nickel (37°C, 130 rpm). This nickel concentration did not alter significantly the growth rate of E. coli MG1655 ompR234 (Mann-Whitney test, P < 0.05). We conclude that a subinhibitory concentration of nickel causes the bacteria to develop inside a biofilm rather than growing as free-floating cells.
FIG. 1.
(A) Inhibition of MG1655 ompR234 (PHL818) growth by increasing the concentration of nickel. The OD600 was measured either in cultures that had been shaken (open circles) at 130 rpm or in static cultures (dark triangles), as described in Materials and Methods. The incubation period was 24 h, at 30°C, in LB/4 medium in the presence of increasing nickel concentrations ranging from 0 to 400 μM. (B) Enhancing effect of the low concentration of nickel on biofilm formation on polystyrene. Differential growth of MG1655 ompR234 (PHL818) in supernatant (dark diamonds) and biofilm (dark squares) fractions was estimated from the OD600 in petri dish cultures, as described in Materials and Methods. The OD600 without nickel reached about 1.3 in the supernatant and 0.3 in the biofilm. The addition of supernatant and biofilm fractions resulted in the curve of static growth shown in panel A.
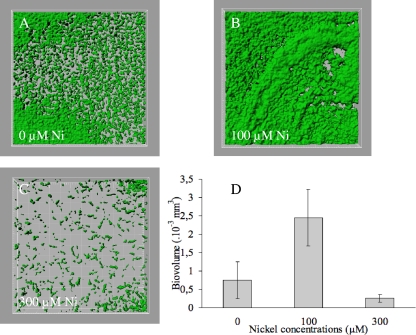
To quantify this phenomenon by confocal microscopy and to test another abiotic surface, biofilms were grown on steel coupons from E. coli MG1655 ompR234, expressing a lac-gfp fusion from a low-copy plasmid, in the presence of an increasing amount of nickel. Immobilized cells growing on the surface of the coupons were visualized by confocal microscopy. In LB/4 and in the absence of nickel, bacteria developed only a poor biofilm (Fig. 2A), suggesting that LB/4 does not support efficient curli production. However, a thicker and heterogeneous biofilm was formed in the presence of 100 μM of nickel (Fig. 2B). Only scattered colonies were observed at a sublethal concentration of 300 μM nickel (Fig. 2C). The resulting biovolumes were statistically compared using analysis of variance, and this revealed a significant increase in biovolume observed in the presence of 100 μM of nickel (Fig. 2D; P < 0.05). This method indicates a 3.2-fold increase of the biomass of the biofilm grown in the presence of a low concentration of nickel (100 μM) and confirms the results obtained with OD600 measurements on polystyrene. To check that the expression of the lac-gfp fusion was not affected by nickel addition to the growth medium, fluorescence measurements of agitated cultures supplemented with 0, 100, and 300 μM nickel were performed. A constant value of 1.2 U of fluorescence/OD600 was routinely obtained under all conditions tested (data not shown).
FIG. 2.
A low concentration of nickel promotes biofilm formation on stainless steel coupons. Biofilms, formed by the GFP-labeled strain MG1655 ompR234 (PHL1359), were observed under a confocal microscope after 24 h of growth, as described in Materials and Methods. (A) Uniform biofilm grown without nickel. (B) Irregular three-dimensional structured biofilm grown in the presence of 100 μM of nickel. (C) Microcolonies grown in the presence of 300 μM of nickel. (D) Mean biovolumes calculated from three z-stacks made on each coupon. Variance analysis was performed using Statgraphics (Manugistic, Inc., Rockville, MD). The P value reflects the probability that the mean of the biovolumes with exposure to nickel is equal to the mean of the biovolumes without exposure (P < 0.0001).
Biofilm formation induced by nickel is not related to bacterial surface charge.
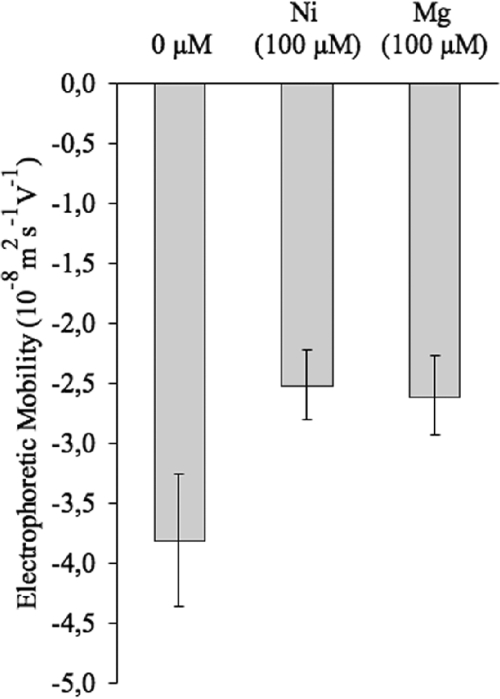
The adhesion behavior of microbial cells has been shown to depend on cell surface physicochemical properties and chemical composition. Cations are known to modify the electrical charges of the bacterial surface, often altering the bacterial adhesion (19, 41, 53). To gain information about the global electrical charge of the bacterial cell surface, we assayed the electrophoretic mobility of the cells grown in the absence or presence of metal (57). The presence of nickel in the culture medium decreased the electronegativity of the cell (Fig. 3; P < 0.05). To determine if the biofilm enhancer effect of nickel observed could be attributed to a general property of cations, the influence of another cation was assayed under the same culture conditions. The metal magnesium was chosen because of its chemical proximity to nickel. The presence of magnesium in the culture medium decreased the bacterial cell electronegativity to the same extent as nickel (Fig. 3; P < 0.05). The presence of magnesium at a concentration of 100 μM in the culture medium does not enhance the biofilm formation (P > 0.05; data not shown). Therefore, the biofilm formation induced by nickel does not simply rely on a modification of the bacterial surface charge but rather on a physiological effect.
FIG. 3.
Electrophoretic mobility of MG1655 ompR234 (PHL818) in the presence of nickel or magnesium. Similar changes in bacterial surface electronegativity are induced by nickel and magnesium. Electrophoretic mobility of PHL818 was measured after half an hour of contact with 100 μM of nickel or magnesium, as described in Materials and Methods.
Biofilm formation induced by nickel does not rely on the nickel-efflux pump RcnA.
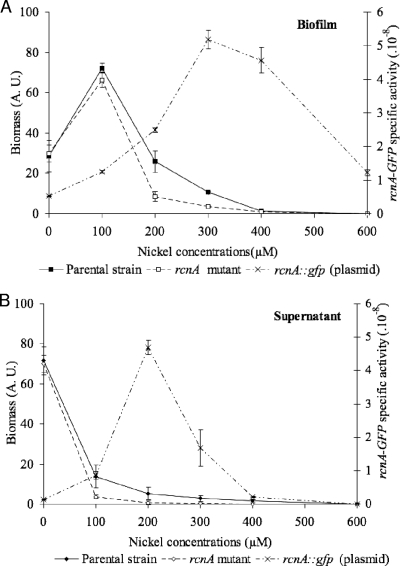
Since E. coli has been shown to resist high concentrations of nickel by using the inducible efflux pump RcnA (48), we first determined whether the enhancing effect of nickel on E. coli biofilms was related to this nickel-resistance mechanism. To test the implication of the Ni/Co efflux pump RcnA in the formation of biofilms in a nickel rich medium, the RcnA-defective MG1655 ompR234 mutant PHL1374 was constructed by transducing the rcnA::uidA-KanR cassette from strain ARY023 (48). The rcnA mutation significantly impairs the growth of bacteria in the presence of nickel, whether it is in the biofilm or in the supernatant fractions, as compared to the parental strain (Fig. 4). More surprising for this strain, we confirmed that the presence of 100 μM of nickel in the growth medium promotes biofilm formation, whereas it strongly impairs the growth of free-living cells (compare Fig. 4A and B), as previously observed for the parental strain (Fig. 1 and 4).
FIG. 4.
The nickel promoter effect in MG1655 ompR234 PHL818 biofilm formation does not rely on the nickel efflux pump RcnA. (A) Biofilm fractions. (B) Planktonic fractions. Growth of MG1655 ompR234 (PHL818; closed symbols) and its rcnA mutant (PHL1374; open symbols) was estimated from the OD600 in supernatant and biofilm fractions in 24-well polystyrene plates. The fluorescence of PHL818 harboring the rncA::gfp fusion (PHL1370) was measured in supernatant and biofilm fractions using a spectrofluorimeter, as described in Materials and Methods. Specific activity for GFP fluorescence (dashed lines) was calculated as follows: (GFP fluorescence - initial fluorescence)/OD600. If OD600 values of one of the fractions were low (below 0.1 U), assays were repeated in petri dishes to collect larger volumes of culture, leading to a more precise quantification of bacterial growth.
The transcriptional level of rcnA in the parental background PHL818 was estimated from the activity of an rcnA-gfp fusion carried by the low-copy plasmid p157. The rcnA-gfp fusion activity in a biofilm is indeed more active at a high concentration of nickel (Fig. 4A), suggesting that the efflux pump RcnA operates mainly in the presence of a high concentration of nickel. These results show that biofilm formation induced by a low concentration of nickel does not result from a selective advantage conferred by the presence of the pump. Data obtained from planktonic cultures confirm that high transcription of the efflux pump gene is observed under harsh conditions (less than 20% of cell survival; Fig. 4B).
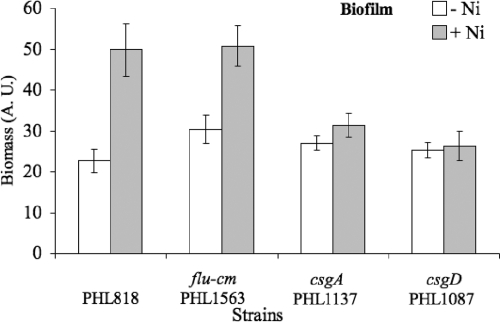
Biofilm formation induced by nickel is mediated by curli.
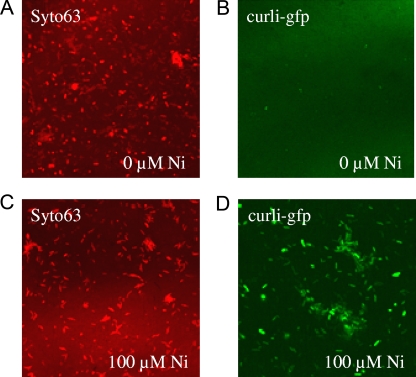
Another possibility to explain biofilm formation induced by nickel is that nickel molecules are able to modify the bacterial physiology in a way that increases the adherence properties of each cell. Antigen 43 (Ag43) is a surface-displayed autotransporter protein able to mediate autoaggregation and biofilm formation of E. coli. Mutation of the flu gene encoding Ag43 did not affect biofilm formation induced by nickel (Fig. 5). Since curli are one of the main adherence factors in E. coli strain MG1655 ompR234 (58), we tested the hypothesis that nickel could mediate biofilm formation induced by nickel by increasing curli production. First, the effect of a subinhibitory concentration of nickel was tested on non-curli-producing strains PHL1137 (csgA) and PHL1087 (csgD). Biofilm formation induced by nickel was significantly reduced (Fig. 5; P < 0.05), indicating that both genes are required to develop thick biofilms. Moreover, Fig. 5 shows that neither Ag43 nor curli are involved in the poor adherence (20%) occurring in LB/4. Poor adherence obtained in plain BL/4 probably results from the production of another adherence structure (exopolysaccharide or adhesin). To measure the transcription activity of the curli promoter, we inserted a reporter gene coding for a GFP with a short half-life (1) under the control of the promoter csgBA. This construct was introduced into the MG1655 ompR234 strain. To avoid problems with the number of copies and stability of the plasmids, the csgB-gfp[LVA] fusion was initially inserted as a monocopy at the trp locus of the MG1655 ompR234 chromosome. However, the degree of expression of the fusion remained at the limit of the detection level, irrespective of the culture medium used (data not shown). Thus, the level of transcription of the curli promoter makes it difficult to work with a chromosomal monocopy linked to a reporter gene coding for GFP with a reduced half-life. To overcome this problem, we introduced into MG1655 ompR234 the csgB::gfp[LVA] fusion on a stable plasmid with a low copy number (p127), giving rise to strain PHL1273. To determine if transcriptional induction of curli by nickel occurs during biofilm formation, the level and localization of the csgB-gfp[LVA] fusion activity were analyzed using confocal scanning laser microscopy on biofilms grown on a steel coupon, as described in Materials and Methods. For that purpose, bacteria were allowed to develop as a biofilm on stainless steel coupons for 24 h. Nickel was then added to a final concentration of 100 μM, and the biofilm was allowed to grow for an additional 3 h. Visualization of the whole biofilm was performed with Syto63 DNA staining (Fig. 6A and C), and GFP expression, driven from the curli promoter, was also assayed (Fig. 6B and D). With the same microscope settings, the bright GFP expression detected in the presence of nickel (Fig. 6D) was no longer detected in the absence of the metal (Fig. 6B). These results show that supplementation of the growth media with 100 μM of nickel quickly overinduces the expression of curli in the biofilm. From this experiment, we conclude that a 3-h contact with nickel was not long enough to modify the structure of the biofilm (compare Fig. 6A and C) but was sufficient to cause a striking increase in curli transcription (compare Fig. 6B and D). Longer exposure to subinhibitory concentrations of nickel results in biofilm thickening (Fig. 2B), very likely as a result of curli production following curli gene transcriptional activation.
FIG. 5.
Involvement of curli in the biofilm formation induced by nickel. PHL818 and PHL818 mutants, defective for Ag43 (PHL1563) or curli production (PHL1087 and PHL1137), were assayed for adherence in LB/4 without (light gray) or with 100 μM of nickel (dark gray), at 30°C, in 24-well plates, as described in Materials and Methods. For clarity of the figure, biofilm growth only is shown; growth in the supernatant fraction can be calculated as follows: 100% − biofilm fractions. The values are means for two independent experiments of quadruple cultures; the error bars indicate standard deviations.
FIG. 6.
Induction of curli transcription by nickel in biofilms. Biofilms of the strain PHL1273 were grown for 24 h in LB/4, at 30°C, on stainless steel coupons under static conditions. An additional 3 h of incubation was performed in the absence (A and B) or presence (C and D) of 100 μM of nickel. Detection of the living cells was carried out using the fluorescent dye Syto63 (A and C). The GFP fluorescence driven from the curli promoter in the biofilm was compared with (D) and without (B) nickel, with the same microscope settings.
Subinhibitory concentration of nickel activates the expression of csg genes.
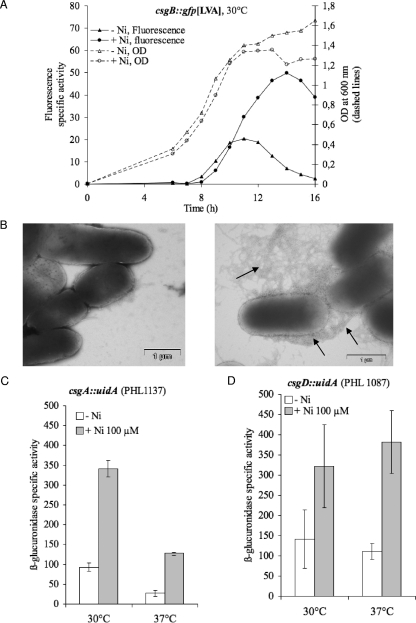
In a liquid batch culture in LB/4, without nickel, the level of expression of the csgB-gfp[LVA] fusion increased slightly at the end of the exponential phase, but only a low level of expression was observed (Fig. 7A). Supplementation with nickel from the beginning of the growth experiment leads to a striking increase in csgB-gfp[LVA] expression from entry into the stationary phase (Fig. 7A). The induction of curli production by nickel was confirmed using direct observation by electron microscopy. Strain PHL818, grown in either the absence of nickel or the presence of 100 μM nickel chloride, was observed after negative staining. The global observation under both conditions confirmed that cell aggregates are larger and more numerous in the presence of nickel. A close-up of bacterial cells (Fig. 7B) shows clearly that curli are scarce in the absence of nickel, whereas a bushy curli coat is observed when growth occurred with nickel. These microscopic observations are in direct correlation with the csgA::gfp assay. This was further confirmed by the use of the csgA-uidA fusion, where the presence of 100 μM of nickel in the medium leads to a 3.7-fold-increase in csgB expression (Fig. 7C; P < 0.05). We checked that addition of magnesium at a concentration of 100 μM had no effect on the expression of curli genes (data not shown). Since magnesium does not enhance biofilm formation (see above) or csgB transcription, we conclude that a low concentration of nickel activates the transcription of csgBA genes in a specific manner.
FIG. 7.
Overinduction of curli transcription by nickel in batch culture. (A) csgB-gfp[LVA] expression under conditions favorable for expression of curli. Planktonic cultures of PHL1273 were grown at 30°C in the presence (open triangles) or absence (open circles) of 100 μM of nickel. Bacterial growth was estimated from the OD600 (dashed lines). Specific activity for GFP fluorescence (solid lines) was calculated as follows: (GFP fluorescence − initial fluorescence)/OD600. Three independent experiments were performed. Results of a typical experiment are presented. (B) TEM observation of the induction of curli production by nickel. Strain PHL818 was grown in the absence (left panel) or presence (right panel) of 100 μM NiCl2. The arrows point to curli structure. (C) csgA::uidA expression. The expression level of the fusion csgA::uidA was compared in the late exponential phase under conditions favorable for curli expression (30°C; left) and conditions unfavorable for curli expression (37°C; right). Strain PHL1137 was grown in planktonic culture in standard medium (white bars) or in the presence of 100 μM of nickel (dark bars). Specific β-glucuronidase activity was calculated as described in Materials and Methods. The values are means for triplicate cultures; the error bars indicate standard deviations. (D) csgD::uidA expression. Strain PHL1087 was grown in standard medium (white bars) or in the presence of 100 μM of nickel (dark bars). Other conditions are as described in the legend to panel C.
In E. coli K-12, the expression of curli genes is restricted to temperatures below 30°C under microaerophilic conditions. To evaluate the impact of nickel on the known environmental regulations of curli, the expression of curli gene fusions in the presence of nickel was tested at 37°C with vigorous shaking. As expected, poor expression was observed in standard medium at 37°C, but a subinhibitory concentration of nickel drastically enhanced the expression of the csgA-uidA fusions (Fig. 7C; P < 0.05). Similar results were obtained with csgB-gfp[LVA] (data not shown).
Adherence assays demonstrate that a functional CsgD protein is required to obtain the biofilm promoter effect (see above). To test if nickel also affects the transcription of this main activator, a csgD::uidA fusion was assayed in the presence of nickel at a concentration of 100 μM. Figure 7D shows that nickel increased the csgD expression to the same extent under favorable (30°C) and unfavorable (37°C) conditions (P < 0.05). Taken together, our results show that biofilm formation induced by nickel is mediated by the activation of curli genes from transcriptional activation of the csgD activator gene. Moreover, nickel seems to be able to bypass the usual environmental regulation of csgD in the PHL818 strain.
DISCUSSION
Increased biofilm formation by strain MG1655 ompR234, in the presence of a moderate concentration of nickel, was observed on polystyrene and stainless steel coupons. This phenomenon is also observed with the other curli-expressing E. coli strain, MC4100 ompR234 PHL644, and also with the parental MG1655 strain and clinical isolates such as PHL881 (data not shown). Quitting planktonic life to grow a thick biofilm could be a common response of E. coli to the presence of a subinhibitory concentration of nickel in the medium.
Distribution of this phenomenon among medical and environmental isolates of E. coli needs to be investigated. Among other bacterial species, adhesion of Desulfovibrio desulfuricans has been shown to be more extensive on a nickel surface and the growth rate of this bacterium was found to be positively affected by nickel (40). This growth-promoting effect was observed in our system at a lower concentration of nickel (50 μM; data not shown). The biofilm formation induced by nickel in E. coli occurs at a non-growth-promoting concentration of nickel (100 μM) and does not result from a global increase in the bacterial population. Biofilm formation by E. coli appears, instead, to be an adaptative and defensive reaction of the bacteria to the presence of nickel. Other biocides have been shown to induce biofilm formation in a wide range of bacteria. Aminoglycoside antibiotics have been shown to induce E. coli and P. aeruginosa biofilm formation (27). Powerful detergents that disrupt biological membranes, such as sodium dodecyl sulfate or bile salts, also stimulate biofilm formation in Pseudomonas aeruginosa (35), Vibrio cholerae (30), and Bacteroides fragilis (50). Therefore, we suggest that induction of the biofilm mode of growth could be the early response of bacteria to biocides, allowing the concomitant development of an antibiotic- and metal-resistant phenotype.
Our first hypothesis was that biofilm formation induced by nickel results from enhanced adhesion of bacteria to abiotic surfaces mediated by physicochemical interactions. Bacterial attachment to surfaces is, indeed, the first step in the development of a biofilm. This first step depends on the interactions between the cell surface and abiotic surfaces and is likely to be influenced by the presence of metal in the medium. The cell surface properties of E. coli cells were, therefore, characterized by measuring their electrophoretic mobility. As expected, adding nickel to the growth medium strongly modified E. coli surface charges. Adding magnesium similarly modified the cell surface charge, but no biofilm increase was then detected. Modification of physicochemical interactions probably plays a key role in early adhesion, but this could not account for biofilm formation induced by nickel. This effect appears to be mediated by the genetic regulation of sticky structures that allow for subsequent biofilm development.
The second hypothesis was tested by assaying several mutants involved in production of the adherence factors. We found that the mutations affecting curli production abolish nickel-induced biofilm formation. These results indicate that curli genes are required to promote the biofilm formation when bacteria encounter subinhibitory concentrations of nickel in the surrounding medium. The transcription of both curli operons was strikingly increased by subinhibitory concentrations of nickel at 30°C and 37°C. Upregulation of curli genes appears to be the key factor in the biofilm formation induced by nickel. Moreover, the presence of nickel in the surrounding media is able to bypass the strict thermoregulation of curli in K-12 strains. This result shows the high reactivity of the curli promoter to environmental changes, but the mechanism of nickel action has not yet been elucidated. As for many virulence factors, curli production is heavily regulated at the transcriptional level by environmental factors, such as temperature, osmolarity, oxygen concentration of the medium, pH, the presence of ethanol, and certain nutrient deficiencies (nitrogen, phosphate, and iron [reviewed in reference 37]). Our work shows, for the first time, the reactivity of curli genes to nickel.
Beyond its role as activator of the structural csgBA operon, CsgD also regulates genes involved in the synthesis of cell surface structures (cellulose, O antigen, and the determinant for biofilm formation), transport, and metabolism (reviewed in reference 23). Increased transcription of the csgD gene potentially results in significant changes in cell physiology associated with the transition from planktonic cells to biofilm. We found that nickel affects the central regulatory knot csgD, but the molecular mechanism of this activation still needs to be investigated. A number of regulators are known to affect, positively or negatively, the transcription of the csgD promoter (CpxR, Crl, H-NS, MlrA, OmpR, RpoS, and RcsB [reviewed in reference 37]), but the molecular mechanism of the csgD promoter activation by nickel remains to be elucidated. It is noteworthy that CpxA/CpxR, which negatively regulates the csgD promoter (31), belongs to the copper stimulon (61). This transduction system could also be involved in nickel sensing. We found that the adherence properties of cpxR mutants in the presence of a subinhibitory concentration of nickel were not significantly affected. Moreover, no significant effect on transcription of the csgA::gfp fusion in mutant strains could be detected (data not shown). The curli are, therefore, induced by nickel but not in a CpxAR-dependent manner. MlrA is a transcriptional activator of the csgD promoter which shares similarities with a regulatory protein of the MerR family (MerR-like regulator A) (8). MerR represents one of the five metal sensor families able to bind metal ions and allosterically regulate DNA binding and transcription of target operons (45). However, neither MlrA nor the two nickel-sensing dedicated regulators in E. coli, namely NikR (17) and RcnR (36), were shown to be involved in the induction of csgB or cgsD by nickel (our personal data). Induction of biofilm formation by sodium dodecyl sulfate and aminoglycosides was shown to involve cyclic di-GMP (c-di-GMP) signaling (27, 35). This bacterial second messenger controls lifestyle choices in bacteria, such as a commitment to sessile life or virulence (12), and it is antagonistically controlled by diguanylate cyclases (GGDEF proteins) and phosphodiesterases (EAL proteins). Recent studies point to csgD as a major target for regulation by c-di-GMP. Several of these c-di-GMP control modules (YdaM/YclR, YegE/YhjH, and YaiC/YoaD) are indeed involved in curli regulatory cascades, with CsgD representing the major signal integration node (7, 46, 59). c-di-GMP signaling could, therefore, mediate biofilm induction by nickel as a global effect of stress on E. coli bacterial cells.
A similar effect of nickel on another adherence factor has been recently observed in pathogenic E. coli. Low concentrations of nickel were shown to increase the transcription of the bundle-forming pilus (BFP) structural gene (bfpA) in enteropathogenic E. coli. This effect was specific to nickel, as compared with copper and zinc (13). Nickel influences the transcription of curli and BFP genes, which are both involved in the adherence and virulence of E. coli. A global increase of adherence, via the transcriptional regulatory mechanisms of the adherence structure, could be an early response of E. coli to nickel and more generally to metals. However, although the accumulation of copper in the cueO mutant results in cell aggregation correlated with an increased expression of cell surface adhesion Ag43 and curli (55), a strong inhibition of enteropathogenic E. coli (EPEC) adherence factors, such as BFP, EspA, and intimin, was observed in the presence of zinc (13). Metals are clearly involved in adherence, but the effect on adherence structures appears to be metal specific. Other biocides are involved in the transcriptional control of adherence structures. For example, bile salts can cause fimbrial overproduction and increase E. coli adhesion to human epithelial cells (15). The involvement of biocides in adherence may therefore be considered as a general phenomenon.
An important question to consider is why bacteria begin to grow inside a biofilm in the presence of a subinhibitory concentration of nickel. Cells in a biofilm could benefit from protection against harsh conditions as a result of higher tolerance mechanisms. In E. coli, only one nickel efflux system (RcnA) has been identified (51) and our results show that the efflux pump is not required for the formation of a biofilm in the presence of 100 μM of nickel. This efflux pump RcnA seems to be used only as an emergency system and mainly operates at higher nickel concentrations (Fig. 4). Other mechanisms of defense could be provided intrinsically by an E. coli biofilm. Curli are thought to enhance resistance to antibacterial agents, such as chlorine or quaternary ammonium sanitizer (52, 56). The amyloid property of curli supports the idea of them having a protective role (21). Formation of biofilm by a strain expressing curli may confer resistance to heavy metals by retarding metal diffusion (28, 29). Indeed, tolerance of E. coli biofilms to metals has been demonstrated to be time dependent (25).
Nickel tolerance of biofilms results most probably from the conjunction of several mechanisms that act in a time-dependent manner. We propose that increased adherence occurs as an early cell adaptation to subinhibitory concentrations of nickel and that increased tolerance inherent to the biofilm mode of growth occurs as a consequence. Special attention must therefore be given to the effect of a low concentration of nickel, and more generally of biocides, in the environment or in biomaterials, to avoid biofilm development.
Acknowledgments
We thank Sylvie Reverchon for critical reading of the manuscript and Valerie James for correction of the English. We thank G. Bloemberg and R. Kolter for their gift of strains.
Footnotes
Published ahead of print on 23 January 2009.
REFERENCES
- 1.Andersen, J. B., C. Sternberg, L. K. Poulsen, S. P. Bjørn, M. Givskov, and S. Molin. 1998. New unstable variants of green fluorescent protein for studies of transient gene expression in bacteria. Appl. Environ. Microbiol. 64:2240-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105-107. [DOI] [PubMed] [Google Scholar]
- 3.Bardonnet, N., and C. Blanco. 1992. ′uidA-antibiotic-resistance cassettes for insertion mutagenesis, gene fusions and genetic constructions. FEMS Microbiol. Lett. 72:243-247. [DOI] [PubMed] [Google Scholar]
- 4.Barnhart, M. M., and M. R. Chapman. 2006. Curli biogenesis and function. Annu. Rev. Microbiol. 60:131-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bigger, J. W. 1944. Treatment of staphyloccoccal infections with penicillin by intermittent sterilisation. Lancet 244:497-500. [Google Scholar]
- 6.Bloemberg, G. V., A. H. Wijfjes, G. E. Lamers, N. Stuurman, and B. J. Lugtenberg. 2000. Simultaneous imaging of Pseudomonas fluorescens WCS365 populations expressing three different autofluorescent proteins in the rhizosphere: new perspectives for studying microbial communities. Mol. Plant-Microbe Interact. 13:1170-1176. [DOI] [PubMed] [Google Scholar]
- 7.Brombacher, E., C. Dorel, A. J. Zehnder, and P. Landini. 2003. The curli biosynthesis regulator CsgD co-ordinates the expression of both positive and negative determinants for biofilm formation in Escherichia coli. Microbiology 149:2847-2857. [DOI] [PubMed] [Google Scholar]
- 8.Brown, P. K., C. M. Dozois, C. A. Nickerson, A. Zuppardo, J. Terlonge, and R. Curtiss III. 2001. MlrA, a novel regulator of curli (AgF) and extracellular matrix synthesis by Escherichia coli and Salmonella enterica serovar Typhimurium. Mol. Microbiol. 41:349-363. [DOI] [PubMed] [Google Scholar]
- 9.Bruins, M. R., S. Kapil, and F. W. Oehme. 2000. Microbial resistance to metals in the environment. Ecotoxicol. Environ. Saf. 45:198-207. [DOI] [PubMed] [Google Scholar]
- 10.Chambless, J. D., S. M. Hunt, and P. S. Stewart. 2006. A three-dimensional computer model of four hypothetical mechanisms protecting biofilms from antimicrobials. Appl. Environ. Microbiol. 72:2005-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung, C. T., S. L. Niemela, and R. H. Miller. 1989. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc. Natl. Acad. Sci. USA 86:2172-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cotter, P. A., and S. Stibitz. 2007. c-di-GMP-mediated regulation of virulence and biofilm formation. Curr. Opin. Microbiol. 10:17-23. [DOI] [PubMed] [Google Scholar]
- 13.Crane, J. K., T. M. Naeher, I. Shulgina, C. Zhu, and E. C. Boedeker. 2007. Effect of zinc in enteropathogenic Escherichia coli infection. Infect. Immun. 75:5974-5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danese, P. N., L. A. Pratt, S. L. Dove, and R. Kolter. 2000. The outer membrane protein, antigen 43, mediates cell-to-cell interactions within Escherichia coli biofilms. Mol. Microbiol. 37:424-432. [DOI] [PubMed] [Google Scholar]
- 15.de Jesus, M. C., A. A. Urban, M. E. Marasigan, and D. E. Barnett Foster. 2005. Acid and bile-salt stress of enteropathogenic Escherichia coli enhances adhesion to epithelial cells and alters glycolipid receptor binding specificity. J. Infect. Dis. 192:1430-1440. [DOI] [PubMed] [Google Scholar]
- 16.del Pozo, J. L., and R. Patel. 2007. The challenge of treating biofilm-associated bacterial Infections. Clin. Pharmacol. Ther. 82:204-209. [DOI] [PubMed] [Google Scholar]
- 17.De Pina, K., V. Desjardin, M. A. Mandrand-Berthelot, G. Giordano, and L.-F. Wu. 1999. Isolation and characterization of the nikR gene encoding a nickel-responsive regulator in Escherichia coli. J. Bacteriol. 181:670-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorel, C., O. Vidal, C. Prigent-Combaret, I. Vallet, and P. Lejeune. 1999. Involvement of the Cpx signal transduction pathway of E. coli in biofilm formation. FEMS Microbiol. Lett. 178:169-175. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher, M. 1988. Attachment of Pseudomonas fluorescens to glass and influence of electrolytes on bacterium-substratum separation distance. J. Bacteriol. 170:2027-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fux, C. A., J. W. Costerton, P. S. Stewart, and P. Stoodley. 2005. Survival strategies of infectious biofilms. Trends Microbiol. 13:34-40. [DOI] [PubMed] [Google Scholar]
- 21.Gebbink, M. F., D. Claessen, B. Bouma, L. Dijkhuizen, and H. A. Wosten. 2005. Amyloids—a functional coat for microorganisms. Nat. Rev. Microbiol. 3:333-341. [DOI] [PubMed] [Google Scholar]
- 22.Gophna, U., M. Barlev, R. Seijffers, T. A. Oelschlager, J. Hacker, and E. Z. Ron. 2001. Curli fibers mediate internalization of Escherichia coli by eukaryotic cells. Infect. Immun. 69:2659-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gualdi, L., L. Tagliabue, and P. Landini. 2007. Biofilm formation-gene expression relay system in Escherichia coli: modulation of σS-dependent gene expression by the CsgD regulatory protein via σS protein stabilization. J. Bacteriol. 189:8034-8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrison, J. J., H. Ceri, N. J. Roper, E. A. Badry, K. M. Sproule, and R. J. Turner. 2005. Persister cells mediate tolerance to metal oxyanions in Escherichia coli. Microbiology 151:3181-3195. [DOI] [PubMed] [Google Scholar]
- 25.Harrison, J. J., R. J. Turner, and H. Ceri. 2005. Persister cells, the biofilm matrix and tolerance to metal cations in biofilm and planktonic Pseudomonas aeruginosa. Environ. Microbiol. 7:981-994. [DOI] [PubMed] [Google Scholar]
- 26.Hertel, R. F., T. Maass, and V. R. Müller. 1991. Nickel, p. 21-24. In R. F. Hertel (ed.), Environmental health criteria, vol. 108. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 27.Hoffman, L. R., D. A. D'Argenio, M. J. MacCoss, Z. Zhang, R. A. Jones, and S. I. Miller. 2005. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 436:1171-1175. [DOI] [PubMed] [Google Scholar]
- 28.Hu, Z., G. Hidalgo, P. L. Houston, A. G. Hay, M. L. Shuler, H. D. Abruña, W. C. Ghiorse, and L. W. Lion. 2005. Determination of spatial distributions of zinc and active biomass in microbial biofilms by two-photon laser scanning microscopy. Appl. Environ. Microbiol. 71:4014-4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu, Z., J. Jin, H. D. Abruna, P. L. Houston, A. G. Hay, W. C. Ghiorse, M. L. Shuler, G. Hidalgo, and L. W. Lion. 2007. Spatial distributions of copper in microbial biofilms by scanning electrochemical microscopy. Environ. Sci. Technol. 41:936-941. [DOI] [PubMed] [Google Scholar]
- 30.Hung, D. T., J. Zhu, D. Sturtevant, and J. J. Mekalanos. 2006. Bile acids stimulate biofilm formation in Vibrio cholerae. Mol. Microbiol. 59:193-201. [DOI] [PubMed] [Google Scholar]
- 31.Jubelin, G., A. Vianney, C. Beloin, J.-M. Ghigo, J.-C. Lazzaroni, P. Lejeune, and C. Dorel. 2005. CpxR/OmpR interplay regulates curli gene expression in response to osmolarity in Escherichia coli. J. Bacteriol. 187:2038-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katsikogianni, M., and Y. F. Missirlis. 2004. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur. Cells Mater. 8:37-57. [DOI] [PubMed] [Google Scholar]
- 33.Klausen, M., A. Aaes-Jorgensen, S. Molin, and T. Tolker-Nielsen. 2003. Involvement of bacterial migration in the development of complex multicellular structures in Pseudomonas aeruginosa biofilms. Mol. Microbiol. 50:61-68. [DOI] [PubMed] [Google Scholar]
- 34.Klausen, M., A. Heydorn, P. Ragas, L. Lambertsen, A. Aaes-Jorgensen, S. Molin, and T. Tolker-Nielsen. 2003. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol. Microbiol. 48:1511-1524. [DOI] [PubMed] [Google Scholar]
- 35.Klebensberger, J., K. Lautenschlager, D. Bressler, J. Wingender, and B. Philipp. 2007. Detergent-induced cell aggregation in subpopulations of Pseudomonas aeruginosa as a preadaptive survival strategy. Environ. Microbiol. 9:2247-2259. [DOI] [PubMed] [Google Scholar]
- 36.Koch, D., D. H. Nies, and G. Grass. 2007. The RcnRA (YohLM) system of Escherichia coli: a connection between nickel, cobalt and iron homeostasis. Biometals 20:759-771. [DOI] [PubMed] [Google Scholar]
- 37.Landini, P., G. Jubelin, and C. Dorel. 2006. The molecular genetics of bioadhesion and biofilm formation, p. 21-35. In A. M. Smith and J. A. Callow (ed.), Biological adhesives. Springer-Verlag, Berlin, Germany.
- 38.Le Thi, T. T., C. Prigent-Combaret, C. Dorel, and P. Lejeune. 2001. First stages of biofilm formation: characterization and quantification of bacterial functions involved in colonization process. Methods Enzymol. 336:152-159. [DOI] [PubMed] [Google Scholar]
- 39.Lewis, K. 2007. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 5:48-56. [DOI] [PubMed] [Google Scholar]
- 40.Lopes, F. A., P. Morin, R. Oliveira, and L. F. Melo. 2005. The influence of nickel on the adhesion ability of Desulfovibrio desulfuricans. Colloids Surf. B 46:127-133. [DOI] [PubMed] [Google Scholar]
- 41.McEldowney, S. 1994. Effect of cadmium and zinc on attachment and detachment interactions of Pseudomonas fluorescens H2 with glass. Appl. Environ. Microbiol. 60:2759-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller, J. E. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 43.Miller, W. G., J. Leveau, and S. Lindow. 2000. Improved gfp and inaZ broad-host-range promoter-probe vectors. Mol. Plant-Microbe Interact. 13:1243-1250. [DOI] [PubMed] [Google Scholar]
- 44.Olsen, A., A. Arnqvist, M. Hammar, S. Sukupolvi, and S. Normark. 1993. The RpoS sigma factor relieves H-NS-mediated transcriptional repression of csgA, the subunit gene of fibronectin-binding curli in Escherichia coli. Mol. Microbiol. 7:523-536. [DOI] [PubMed] [Google Scholar]
- 45.Pennella, M. A., and D. P. Giedroc. 2005. Structural determinants of metal selectivity in prokaryotic metal-responsive transcriptional regulators. Biometals 18:413-428. [DOI] [PubMed] [Google Scholar]
- 46.Pesavento, C., G. Becker, N. Sommerfeldt, A. Possling, N. Tschowri, A. Mehlis, and R. Hengge. 2008. Inverse regulatory coordination of motility and curli-mediated adhesion in Escherichia coli. Genes Dev. 22:2434-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prigent-Combaret, C., E. Brombacher, O. Vidal, A. Ambert, P. Lejeune, P. Landini, and C. Dorel. 2001. Complex regulatory network controls initial adhesion and biofilm formation in Escherichia coli via regulation of the csgD gene. J. Bacteriol. 183:7213-7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prigent-Combaret, C., G. Prensier, T. T. Le Thi, O. Vidal, P. Lejeune, and C. Dorel. 2000. Developmental pathway for biofilm formation in curli-producing Escherichia coli strains: role of flagella, curli and colanic acid. Environ. Microbiol. 2:450-464. [DOI] [PubMed] [Google Scholar]
- 49.Prigent-Combaret, C., O. Vidal, C. Dorel, and P. Lejeune. 1999. Abiotic surface sensing and biofilm-dependent regulation of gene expression in Escherichia coli. J. Bacteriol. 181:5993-6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pumbwe, L., C. A. Skilbeck, V. Nakano, M. J. Avila-Campos, R. M. Piazza, and H. M. Wexler. 2007. Bile salts enhance bacterial co-aggregation, bacterial-intestinal epithelial cell adhesion, biofilm formation and antimicrobial resistance of Bacteroides fragilis. Microb. Pathog. 43:78-87. [DOI] [PubMed] [Google Scholar]
- 51.Rodrigue, A., G. Effantin, and M.-A. Mandrand-Berthelot. 2005. Identification of rcnA (yohM), a nickel and cobalt resistance gene in Escherichia coli. J. Bacteriol. 187:2912-2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ryu, J.-H., and L. R. Beuchat. 2005. Biofilm formation by Escherichia coli O157:H7 on stainless steel: effect of exopolysaccharide and curli production on its resistance to chlorine. Appl. Environ. Microbiol. 71:247-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song, B., and L. G. Leff. 2006. Influence of magnesium ions on biofilm formation by Pseudomonas fluorescens. Microbiol. Res. 161:355-361. [DOI] [PubMed] [Google Scholar]
- 54.Spoering, A. L., and K. Lewis. 2001. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J. Bacteriol. 183:6746-6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tree, J. J., G. C. Ulett, J. L. Hobman, C. Constantinidou, N. L. Brown, C. Kershaw, M. A. Schembri, M. P. Jennings, and A. G. McEwan. 2007. The multicopper oxidase (CueO) and cell aggregation in Escherichia coli. Environ. Microbiol. 9:2110-2116. [DOI] [PubMed] [Google Scholar]
- 56.Uhlich, G. A., P. H. Cooke, and E. B. Solomon. 2006. Analyses of the red-dry-rough phenotype of an Escherichia coli O157:H7 strain and its role in biofilm formation and resistance to antibacterial agents. Appl. Environ. Microbiol. 72:2564-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Loosdrecht, M. C. M., J. Lyklema, W. Norde, G. Schraa, and A. J. Zehnder. 1987. Electrophoretic mobility and hydrophobicity as a measure to predict the initial steps of bacterial adhesion. Appl. Environ. Microbiol. 53:1898-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vidal, O., R. Longin, C. Prigent-Combaret, C. Dorel, M. Hooreman, and P. Lejeune. 1998. Isolation of an Escherichia coli K-12 mutant strain able to form biofilms on inert surfaces: involvement of a new ompR allele that increases curli expression. J. Bacteriol. 180:2442-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weber, H., C. Pesavento, A. Possling, G. Tischendorf, and R. Hengge. 2006. Cyclic-di-GMP-mediated signalling within the sigma network of Escherichia coli. Mol. Microbiol. 62:1014-1034. [DOI] [PubMed] [Google Scholar]
- 60.Wright, G. D. 2007. The antibiotic resistome: the nexus of chemical and genetic diversity. Nat. Rev. Microbiol. 5:175-186. [DOI] [PubMed] [Google Scholar]
- 61.Yamamoto, K., and A. Ishihama. 2005. Transcriptional response of Escherichia coli to external copper. Mol. Microbiol. 56:215-227. [DOI] [PubMed] [Google Scholar]