Abstract
A marine roseophage RDJLΦ1 lytically infecting Roseobacter denitrificans OCh114 was isolated and characterized. RDJLΦ1 can package several host cellular proteins into its virions, and its DNA is refractory to several commonly used restriction enzymes. This paper presents the first report of a bacteriophage isolated from the aerobic anoxygenic phototrophic bacteria.
Aerobic anoxygenic phototrophic bacteria (AAPB) are considered to play a unique role in global oceanic carbon and energy cycles (17, 18). Roseobacter denitrificans OCh114, the first bacterium discovered to have the aerobic anoxygenic phototrophic feature (14, 31), is the most studied model strain of an AAPB (43). In addition, R. denitrificans OCh114 is a member of the Roseobacter lineage, which is one of the dominant lineages in the marine environment (7, 37).
In recent years, great attention has been given to the ecological dynamics and genetic diversity of AAPB (4, 15, 17-19, 30, 38) and to the Roseobacter lineage (7, 37); however, little is known about the viruses that infect AAPB. As is well known, viruses are the most abundant biological entities in the sea (13, 33), and most of them are phages responsible for a substantial portion of the bacterial mortality there (3, 39). They can structure the microbial community and influence the processes and biogeochemical cycles of the microbial food web. Meanwhile, lateral gene transfer by viral infection also promotes the community's evolutionary processes (5, 13, 20, 23, 34, 39, 40). Therefore, studies on marine phages, especially those infecting environmentally important microorganisms such as AAPB and roseobacters, are of great ecological significance. However, so far, no AAPB phage, and only one roseophage, are reported (29).
To set up a host-phage system belonging to the Roseobacter and AAPB group, R. denitrificans OCh114 was employed as the host to select phages. Surface-water samples (100 liters) were collected from the South China Sea (17.597°N, 116.029°E) and then concentrated following a protocol described by Chen et al. (10). The final viral concentrate was used to screen phages using the double-agar layer method (1). As a result, a phage provisionally named RDJLΦ1 that could form small, clear, round plaques (ca. 3 mm in diameter) on the bacterial lawn was obtained and then purified using the CsCl density gradient ultracentrifugation method as previously described by Chen et al. (11).
A transmission electron micrograph (Fig. 1) of the purified phage negatively stained with 2% phosphotungstic acid shows that RDJLΦ1 has an isometric head (ca. 69 nm in diameter) and a long, flexible, noncontractile tail (ca. 170 nm long and ca. 9 nm wide) and thus should be a member of the Siphoviridae family.
FIG. 1.
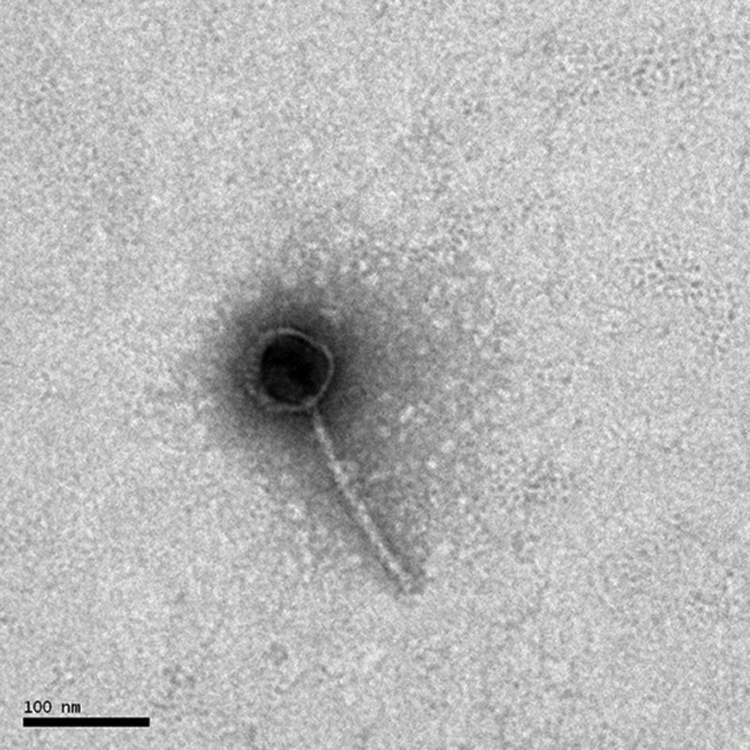
Electron micrograph of RDJLΦ1.
To understand if the virion of RDJLΦ1 contains lipids, we tested the phage sensitivity to chloroform as described by Alonso et al. (2). As a result, no decrease was observed in the viable titer of RDJLΦ1 when mixed with chloroform (data not shown). The resistance to chloroform indicated that RDJLΦ1 is a lipid-free phage.
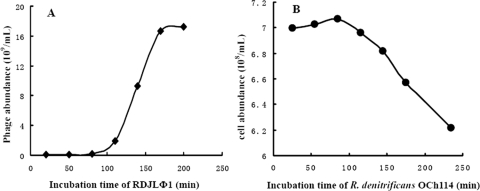
Using the double-agar layer method (1), the host range of RDJLΦ1 was tested with twenty-four available bacterial strains. As shown in Table 1, only R. denitrificans OCh114 was susceptible to RDJLΦ1, indicating the relatively narrow host range of this phage. Next, the one-step growth curve of RDJLΦ1 was measured using a modification of the method of Wu et al. (42). Bacteriophages and the exponentially growing R. denitrificans OCh114 culture, at a multiplicity of infection of approximately 0.1, were incubated for 20 min at 4°C to allow phage adsorption. The mixture was then centrifuged, and the pelleted cells were resuspended in 30 ml of the autoclaved medium containing (liter−1 of 0.22-μm-filtered seawater) 1.0 g of yeast extract and 1.0 g of peptone. Samples were taken at 20- to 30-min intervals and immediately fixed with glutaraldehyde (2.5% final concentration) prior to enumeration. The host and phages stained with SYBR gold were enumerated using epifluorescence microscopy (9). Finally, based on the growth curves of RDJLΦ1 and its host (Fig. 2A and B), the burst size of RDJLΦ1 was estimated to be ca. 203, and the latent period was ca. 80 min. We have to point out that the burst size of an isolated host-phage system is consistently larger, and the latent period is shorter than those occurring in the natural environment, since the growth characteristics of a phage are influenced by the nutritional or metabolic status of the host (6, 39).
TABLE 1.
Bacterial strains used in this study and their susceptibility to RDJLΦ1
| Species | Straina | Origin | AAPBb | Susceptibilityc |
|---|---|---|---|---|
| Roseobacter denitrificans | OCh114* or DSM 7001* | Seaweed, Japan | + | + |
| Roseobacter sp. | JL1366 | South China Sea | − | − |
| Roseobacter litoralis | DSM 6996* | Seaweed, Japan | + | − |
| Sulfitobacter sp. | EE 36** | Salt marsh on the coast, United States | − | − |
| Sulfitobacter sp. | JL1353 | South China Sea | − | − |
| Erythrobacter longus | DSM 6997* | Seaweed, Japan | + | − |
| Erythrobacter sp. | JL475 | South China Sea | + | − |
| Erythrobacter sp. | JL359 | South China Sea | + | − |
| Erythrobacter litoralis | DSM 8509* | Cyanobacterial mat, The Netherlands | + | − |
| Erythrobacter sp. | JL1350 | South China Sea | − | − |
| Dinoroseobacter sp. | JL1447 | South China Sea | + | − |
| Citromicrobium sp. | JL354 | South China Sea | + | − |
| Citromicrobium sp. | JL1351 | South China Sea | − | − |
| Antarctobacter sp. | JL351 | South China Sea | + | − |
| Stappia sp. | JL1358 | South China Sea | − | − |
| Cytophaga sp. | JL1362 | South China Sea | − | − |
| Alteromonas sp. | JL1357 | South China Sea | − | − |
| Nocardioides sp. | JL1369 | South China Sea | − | − |
| Alcanivorax sp. | JL1378 | South China Sea | − | − |
| Furvibacter sp. | JL1383 | South China Sea | − | − |
| Micrococcus sp. | JL1389 | South China Sea | − | − |
| Pseudoalteromonas sp. | JL1391 | South China Sea | − | − |
| Acinetobacter sp. | JL1404 | South China Sea | − | − |
| Vibrio sp. | JL1405 | South China Sea | − | − |
Strains with an asterisk were purchased from DSMZ (the German Resource Center for Biological Material), Germany. The strain with double asterisks was supplied by Feng Chen at the Center of Marine Biotechnology, University of Maryland Biotechnology Institute, MD. All other strains were isolated in N. Jiao's laboratory from samples taken from the South China Sea.
+, an AAPB; −, not an AAPB.
+, cell lysis; −, no effect.
FIG. 2.
One-step growth curve of RDJLΦ1 (A) and the growth curve of the infected R. denitrificans OCh114 (B).
To determine the nature of the RDJLΦ1 nucleic acid, we extracted the phage genome following the procedures described by Jamalludeen et al. (16) and treated it with RNase A and DNase I separately. The results showed that the phage genome was insensitive to RNase A, but completely degraded by DNase I (data not shown), indicating that RDJLΦ1 is a DNA phage.
Then the phage DNA was subjected to digestion by six different restriction enzymes, including AfaI, HhaI, HaeIII, TaqI, XbaI, and EcoRI. Of these enzymes, only AfaI, TaqI, and EcoRI could digest the phage DNA (data not shown). The susceptibility of the phage DNA to restriction digestion further indicated that the RDJLΦ1 genome is a double-stranded DNA molecule. However, the phage DNA was resistant to the other three enzymes (HhaI, HaeIII, and XbaI).
Many phages have been reported to be refractory to restriction digestion (28, 32, 42). The resistance to restriction digestion is considered a phage development during the endless arms race between the phage and its host (25, 36). Some bacteria can use their restriction-modification systems to fight against phages by cutting the exogenous phage DNA into pieces (8, 26). Correspondingly, to escape digestion by the restriction-modification system, phages can evolve several strategies to protect themselves, such as losing restriction sites, the incorporation of unusual bases within the phage DNA, and encoding methyltransferase to modify specific nucleotides within the recognition site (25).
In order to obtain some phage DNA sequences for investigating the resistance mechanism of RDJLΦ1 to several restriction enzymes, two decamer primers (P1 [5′-GGG TAA CGC C-3′] and P2 [5′-AAC GGG CAG A-3′]) were applied to the randomly amplified polymorphic DNA (RAPD) PCR of the phage genome (32, 41). RAPD PCR was performed with a total volume of 50 μl containing 35.5 μl of distilled water, 5 μl of 10× PCR buffer, 4 μl of deoxynucleoside triphosphate mixture, 50 pmol of primer, 0.5 μg template DNA, and 0.5 μl of Taq DNA polymerase under the following conditions: 94°C for 5 min, followed by 35 cycles of 94°C for 5 s, 36°C for 45 s, and 72°C for 1.3 min and finally an extension step at 72°C for 5 min.
Cloning and sequencing of the RAPD PCR products revealed a 1,651-bp phage DNA sequence (GenBank accession no. FJ169484) with G+C content of 58.7%. Using DNAssist software (27), an in silico digestion of this phage DNA fragment was performed with those restriction enzymes unable to cut the phage genome as described above. Interestingly, besides XbaI, both HhaI and HaeIII were found to have their specific cleavage sites within this DNA fragment (HhaI, 9 sites; HaeIII, 14 sites). This suggested that the phage genome may contain some modified DNA bases which conferred upon RDJLΦ1 resistance to these commonly used restriction enzymes. In the RDJLΦ1-host system, although the host genome was found to contain the R/M system (type I) genes (35), whose translated products are potent to be involved in the defense against phages, RDJLΦ1 still can infect R. denitrificans OCh114 without any restriction, probably due to the modification of its DNA.
By using the online GeneMark.hmm programs (22), the 1,651-bp DNA fragment obtained was predicted to contain two complete genes, provisionally named N1 (215 to 616 [402 bp]) and N2 (621 to 941 [321 bp]). Then the deduced amino acid sequences of N1 and N2 were used as the query in a BLAST search of the nonredundant GenBank protein database. The results showed that the most significant matches (i.e., the most homologous proteins) of N1 and N2 were both from a marine phage, ΦJL001, indicating a close relationship between phages RDJLΦ1 and ΦJL001. ΦJL001 is a marine double-stranded DNA siphophage infecting a sponge-associated alphaproteobacterium (21). While different from RDJLΦ1, which has a high lytic ability, ΦJL001 is a temperate phage with some pseudolysogenic characteristics (21).
To characterize the proteome of RDJLΦ1, the phage proteins were extracted following the procedure of Cho et al. (12) and separated by electrophoresis in 12.5% Tris-glycine-sodium dodecyl sulfate polyacrylamide gel with a 3% stacking gel. The visualized protein bands were then analyzed via matrix-assisted laser desorption ionization-time of flight mass spectrometry and tandem mass spectrometry. A protein score of more than 75 and at least 4 matched peptides per protein were set as the threshold for positive identification. The results show that RDJLΦ1 contains at least 12 proteins (Fig. 3). Surprisingly, two of them were successfully identified as cellular proteins of R. denitrificans OCh114 (Table 2, bands F and I), and they accounted for a large proportion of the phage proteome. Before it was analyzed, phage RDJLΦ1 had been extensively purified by filtration and density gradient ultracentrifugation and verified to be free of cellular organelles by electron microscopy (data not shown); hence, it is unlikely that the phages were contaminated with cellular proteins. Finding cellular proteins within RDJLΦ1 is unusual, for it is commonly considered that small viruses lacking envelopes have limited or no capacity to package host proteins (24). We are still unclear as to whether or not the two cellular proteins can play functional roles in the virion of RDJLΦ1 or are just passenger proteins accidentally packaged due to their location and high abundance. Further studies will be required to clarify the functions and assembly mechanisms of these cellular proteins within RDJLΦ1.
FIG. 3.
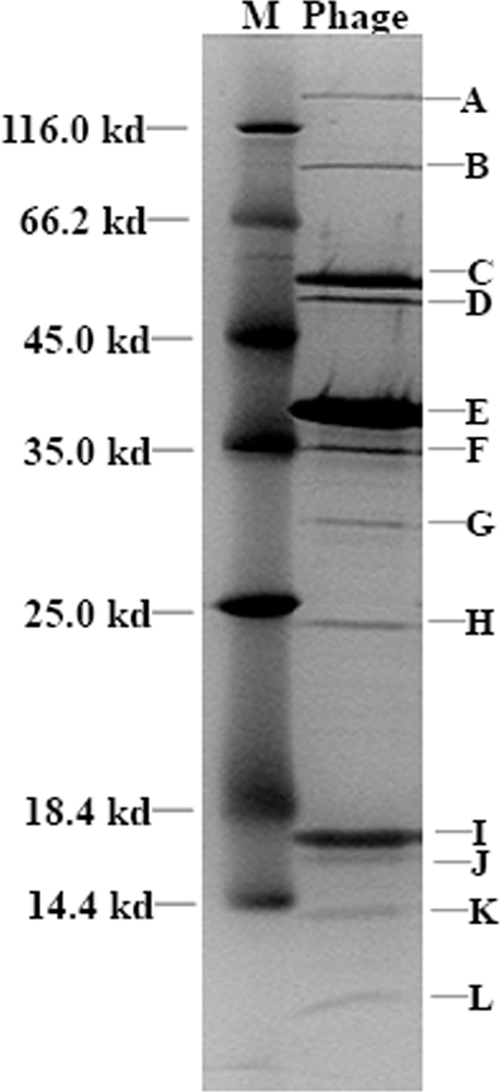
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the structural proteins of RDJLΦ1. The molecular masses (M) of the standard proteins are indicated on the left. The letters on the right indicate the bands.
TABLE 2.
Phage proteins identified using matrix-assisted laser desorption ionization-time of flight tandem mass spectrometry
| Band | Protein | Score | No. of matched peptides | Molecular weight | pI | NCBI accession no. | Origin |
|---|---|---|---|---|---|---|---|
| F | Outer membrane porin | 647 | 8 | 32,923.5 | 3.84 | gi 110677464 | R. denitrificans OCh114 |
| I | 50S ribosomal protein L14 | 142 | 4 | 13,531.5 | 10.74 | gi 110678738 | R. denitrificans OCh114 |
| J | 50S ribosomal protein L22 | 94 | 2 | 14,241.8 | 10.28 | gi 110678726 | R. denitrificans OCh114 |
| L | Hypothetical protein RD1_3847 | 213 | 1 | 9,112.6 | 6.14 | gi 110680988 | R. denitrificans OCh114 |
In addition, except for the phage protein bands J and L, which were identified as putative, the cellular 50S ribosomal protein L22, and protein RD1_3847 with less than four peptides matched, the other eight phage proteins are all novel proteins with no reliable homologous matches in the NCBI database, although all of them had high-quality MS spectra.
Despite the fact that interactions between a phage and host in culture would more or less differ from those occurring in the natural environment, setting up of the host-phage system for different microbial communities is ecologically meaningful and useful practically. The present study has opened a window to interactions between AAPB and their phages. We believe that with more and more phages isolated, more host-phage systems will be built up. Information from such models would contribute to our understanding of the ecological dynamics of AAPB as well as the origin of the phototrophic function of AAPB. In addition, studies on RDJLΦ1 will expand our knowledge concerning the roseophages.
Acknowledgments
This work was supported by the MOST projects (2007CB815904 and 2006BAC11B04), SOA project (200805068), NSFC project (40632013), and MOE key project (704029).
We thank Chun-xiao Huang for assistance in the laboratory and Feng Chen for the gift of two bacterial cultures. John Hodgkiss is thanked for his help with English.
Footnotes
Published ahead of print on 9 January 2009.
REFERENCES
- 1.Adams, M. 1959. Assay of phage by agar layer method, p. 450-454. In Bacteriophages. Interscience Publishers, Inc., New York, NY.
- 2.Alonso, M. D., J. Rodriguez, and J. J. Borrego. 2002. Characterization of marine bacteriophages isolated from the Alboran Sea (Western Mediterranean). J. Plankton Res. 24:1079-1087. [Google Scholar]
- 3.Angly, F. E., B. Felts, M. Breitbart, P. Salamon, R. A. Edwards, C. Carlson, A. M. Chan, M. Haynes, S. Kelley, H. Liu, J. M. Mahaffy, J. E. Mueller, J. Nulton, R. Olson, R. Parsons, S. Rayhawk, C. A. Suttle, and F. Rohwer. 2006. The marine viromes of four oceanic regions. PLoS Biol. 4:2121-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beja, O., M. T. Suzuki, J. F. Heidelberg, W. C. Nelson, C. M. Preston, T. Hamada, J. A. Eisen, C. M. Fraser, and E. F. DeLong. 2002. Unsuspected diversity among marine aerobic anoxygenic phototrophs. Nature 415:630-633. [DOI] [PubMed] [Google Scholar]
- 5.Bohannan, B. J. M., and R. E. Lenski. 2000. Linking genetic change to community evolution: insights from studies of bacteria and bacteriophage. Ecol. Lett. 3:362-377. [Google Scholar]
- 6.Børsheim, K. Y. 1993. Native marine bacteriophages. FEMS Microbiol. Lett. 102:141-159. [Google Scholar]
- 7.Buchan, A., J. M. Gonzalez, and M. A. Moran. 2005. Overview of the marine Roseobacter lineage. Appl. Environ. Microbiol. 71:5665-5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burrus, V., C. Bontemps, B. Decaris, and G. Guedon. 2001. Characterization of a novel type II restriction-modification system, Sth368I, encoded by the integrative element ICESt1 of Streptococcus thermophilus CNRZ368. Appl. Environ. Microbiol. 67:1522-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, F., J.-R. Lu, B. J. Binder, Y.-C. Liu, and R. E. Hodson. 2001. Application of digital image analysis and flow cytometry to enumerate marine viruses stained with SYBR gold. Appl. Environ. Microbiol. 67:539-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, F., C. Suttle, and S. Short. 1996. Genetic diversity in marine algal virus communities as revealed by sequence analysis of DNA polymerase genes. Appl. Environ. Microbiol. 62:2869-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, F., K. Wang. J. Stewart, and R. Belas. 2006. Induction of multiple prophages from a marine bacterium: a genomic approach. Appl. Environ. Microbiol. 72:4995-5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho, H. H., H. H. Park, J. O. Kim, and T. J. Choi. 2002. Isolation and characterization of Chlorella viruses from freshwater sources in Korea. Mol. Cells 14:168-176. [PubMed] [Google Scholar]
- 13.Fuhrman, J. A. 1999. Marine viruses and their biogeochemical and ecological effects. Nature 399:541-548. [DOI] [PubMed] [Google Scholar]
- 14.Harashima, K., and H. Nakada. 1983. Carotenoids and ubiquinone in aerobically grown cells of an aerobic photosynthetic bacterium, Erythrobacter species OCh 114. Agric. Biol. Chem. 47:1057-1063. [Google Scholar]
- 15.Hu, Y. H., H. L. Du, N. Z. Jiao, and Y. H. Zeng. 2006. Abundant presence of the gamma-like proteobacterial pufM gene in oxic seawater. FEMS Microbiol. Lett. 263:200-206. [DOI] [PubMed] [Google Scholar]
- 16.Jamalludeen, N., A. M. Kropinski, R. P. Johnson, E. Lingohr, J. Harel, and C. L. Gyles. 2008. Complete genomic sequence of bacteriophage φEcoM-GJ1, a novel phage that has myovirus morphology and a podovirus-like RNA polymerase. Appl. Environ. Microbiol. 74:516-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiao, N. Z., Y. Zhang, Y. H. Zeng, N. Hong, R. L. Liu, F. Chen, and P. X. Wang. 2007. Distinct distribution pattern of abundance and diversity of aerobic anoxygenic phototrophic bacteria in the global ocean. Environ. Microbiol. 9:3091-3099. [DOI] [PubMed] [Google Scholar]
- 18.Kolber, Z. S., F. G. Plumley, A. S. Lang, J. T. Beatty, R. E. Blankenship, C. L. VanDover, C. Vetriani, M. Koblizek, C. Rathgeber, and P. G. Falkowski. 2001. Contribution of aerobic photoheterotrophic bacteria to the carbon cycle in the ocean. Science 292:2492-2495. [DOI] [PubMed] [Google Scholar]
- 19.Lami, R., M. T. Cottrell, J. Ras, O. Ulloa, I. Obernosterer, H. Claustre, D. L. Kirchman, and P. Lebaron. 2007. High abundances of aerobic anoxygenic photosynthetic bacteria in the South Pacific Ocean. Appl. Environ. Microbiol. 73:4198-4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindell, D., J. D. Jaffe, M. L. Coleman, M. E. Futschik, I. M. Axmann, T. Rector, G. Kettler, M. B. Sullivan, R. Steen, W. R. Hess, G. M. Church, and S. W. Chisholm. 2007. Genome-wide expression dynamics of a marine virus and host reveal features of co-evolution. Nature 449:83-86. [DOI] [PubMed] [Google Scholar]
- 21.Lohr, J. E., F. Chen, and R. T. Hill. 2005. Genomic analysis of bacteriophage ΦJL001: insights into its interaction with a sponge-associated alpha-proteobacterium. Appl. Environ. Microbiol. 71:1598-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lukashin, A., and M. Borodovsky. 1998. GeneMark.hmm: new solutions for gene finding. Nucleic Acids Res. 26:1107-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mann, N. H. 2005. The third age of phage. PLoS Biol. 3:753-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maxwell, K. L., and L. Frappier. 2007. Viral proteomics. Microbiol. Mol. Biol. Rev. 71:398-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moineau, S., S. Pandian, and T. Klaenhammer. 1993. Restriction/modification systems and restriction endonucleases are more effective on lactococcal bacteriophages that have emerged recently in the dairy industry. Appl. Environ. Microbiol. 59:197-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Sullivan, D., R. P. Ross, D. P. Twomey, G. F. Fitzgerald, C. Hill, and A. Coffey. 2001. Naturally occurring lactococcal plasmid pAH90 links bacteriophage resistance and mobility functions to a food-grade selectable marker. Appl. Environ. Microbiol. 67:929-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patterton, H. G., and S. Graves. 2000. DNAssist: the integrated editing and analysis of molecular biology sequences in Windows. Bioinformatics 16:652-653. [DOI] [PubMed] [Google Scholar]
- 28.Petty, N. K., I. J. Foulds, E. Pradel, J. J. Ewbank, and G. P. C. Salmond. 2006. A generalized transducing phage (phi lF3) for the genomically sequenced Serratia marcescens strain Db11: a tool for functional genomics of an opportunistic human pathogen. Microbiology 152:1701-1708. [DOI] [PubMed] [Google Scholar]
- 29.Rohwer, F., A. Segall, G. Steward, V. Seguritan, M. Breitbart, F. Wolven, and F. Azam. 2000. The complete genomic sequence of the marine phage Roseophage SIO1 shares homology with nonmarine phages. Limnol. Oceanogr. 45:408-418. [Google Scholar]
- 30.Salka, I., V. Moulisova, M. Koblizek, G. Jost, K. Jurgens, and M. Labrenz. 2008. Abundance, depth distribution, and composition of aerobic bacteriochlorophyll a-producing bacteria in four basins of the central Baltic Sea. Appl. Environ. Microbiol. 74:4398-4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shiba, T., and U. Simidu. 1982. Erythrobacter longus gen. nov., sp. nov., an aerobic bacterium which contains bacteriochlorophyll a. Int. J. Syst. Bacteriol. 32:211-217. [Google Scholar]
- 32.Shivu, M. M., B. C. Rajeeva, S. K. Girisha, I. Karunasagar, and G. Krohne. 2007. Molecular characterization of Vibrio harveyi bacteriophages isolated from aquaculture environments along the coast of India. Environ. Microbiol. 9:322-331. [DOI] [PubMed] [Google Scholar]
- 33.Suttle, C. A. 2007. Marine viruses—major players in the global ecosystem. Nat. Rev. Microbiol. 5:801-812. [DOI] [PubMed] [Google Scholar]
- 34.Suttle, C. A. 2005. Viruses in the sea. Nature 437:356-361. [DOI] [PubMed] [Google Scholar]
- 35.Swingley, W. D., S. Sadekar, S. D. Mastrian, H. J. Matthies, J. Hao, H. Ramos, C. R. Acharya, A. L. Conrad, H. L. Taylor, L. C. Dejesa, M. K. Shah, M. E. O'Huallachain, M. T. Lince, R. E. Blankenship, J. T. Beatty, and J. W. Touchman. 2007. The complete genome sequence of Roseobacter denitrificans reveals a mixotrophic rather than photosynthetic metabolism. J. Bacteriol. 189:683-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tock, M. R., and D. T. F. Dryden. 2005. The biology of restriction and anti-restriction. Curr. Opin. Microbiol. 8:466-472. [DOI] [PubMed] [Google Scholar]
- 37.Wagner-Döbler, I., and H. Biebl. 2006. Environmental biology of the marine Roseobacter lineage. Annu. Rev. Microbiol. 60:255-280. [DOI] [PubMed] [Google Scholar]
- 38.Waidner, L. A., and D. L. Kirchman. 2007. Aerobic anoxygenic phototrophic bacteria attached to particles in turbid waters of the Delaware and Chesapeake estuaries. Appl. Environ. Microbiol. 73:3936-3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinbauer, M. G. 2004. Ecology of prokaryotic viruses. FEMS Microbiol. Rev. 28:127-181. [DOI] [PubMed] [Google Scholar]
- 40.Weinbauer, M. G., and F. Rassoulzadegan. 2004. Are viruses driving microbial diversification and diversity? Environ. Microbiol. 6:1-11. [DOI] [PubMed] [Google Scholar]
- 41.Winget, D. M., and K. E. Wommack. 2008. Randomly amplified polymorphic DNA PCR as a tool for assessment of marine viral richness. Appl. Environ. Microbiol. 74:2612-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu, L.-T., S.-Y. Chang, M.-R. Yen, T.-C. Yang, and Y.-H. Tseng. 2007. Characterization of extended-host-range pseudo-T-even bacteriophage Kpp95 isolated on Klebsiella pneumoniae. Appl. Environ. Microbiol. 73:2532-2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yurkov, V. V., and J. T. Beatty. 1998. Aerobic anoxygenic phototrophic bacteria. Microbiol. Mol. Biol. Rev. 62:695-724. [DOI] [PMC free article] [PubMed] [Google Scholar]