Abstract
Recombinant engineering using Red recombinase-based approaches offers efficient and rapid approaches to deletion and modification of genes. Here we describe a novel application of Red recombinant engineering that enables direct manipulation of chromosomal loci by electroporation with short synthetic DNA molecules. We demonstrate the use of this approach for the generation of scarless in-frame deletions in chromosomal genes of Salmonella enterica. Furthermore, we describe rapid site-directed mutagenesis within bacterial chromosomes without any requirement for cloning and mutating genes in vitro or for reintroducing mutant alleles into the chromosome. This approach can be expected to facilitate mutational analysis in S. enterica and in other bacterial species able to support Red-mediated recombination.
Functional genomics of bacteria frequently requires gene manipulations, such as in-frame deletion, site-directed mutagenesis, or insertion of foreign sequences. Various approaches are available to perform such manipulations with cloned genes in vitro. However, the generation of strains with mutations in chromosomal genes remains the “gold standard” for functional analyses. Current approaches are tedious and time-consuming since construction of mutant alleles in vitro and replacement of wild-type alleles by mutant alleles are required. Recent advances in molecular genetics of enterobacteria allow rapid and high-throughput approaches for the generation of chromosomal in-frame deletions (1). Expression of the phage λ recombinase Red allows homologous recombination of short (40-nucleotide) homologous sequences into the chromosome of Salmonella (3, 19). This has enabled rapid gene deletions and fusions using linear DNA constructs generated by PCR (3, 6). It has also been shown that Red recombinase can mediate recombination of synthetic oligonucleotides (4), and this approach has been utilized for recombinant engineering of bacterial artificial chromosomes (18) or viral genomes (14).
Allelic exchange in wild-type bacteria requires the use of positively selectable markers. Usually, antibiotic resistance cassettes are used for gene disruption. However, insertion of such cassettes is not compatible with in-frame deletions. For the generation of scarless chromosomal mutations, two systems of interest are (i) positive selection of tetracycline sensitivity (9) and (ii) use of homing endonuclease digestion for positive selection (16).
Here we report a novel approach that allows direct mutagenesis of chromosomal genes by electroporation with short synthetic DNA fragments and Red recombinase-mediated recombination.
MATERIALS AND METHODS
Construction of tetAR insertions.
Strains and plasmids used in this study are listed in Table 1. Salmonella enterica serovar Typhimurium NCTC12023 was used as the target strain for mutagenesis. To insert a gene cassette consisting of tetA and tetR into the target gene for mutagenesis, the tetAR fragment was amplified from genomic DNA of a strain harboring a Tn10dTc insertion using a high-fidelity polymerase mixture (MBI Fermentas) and 60-mer oligonucleotides complementary to tetAR and the target gene.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| S. enterica serovar Typhimurium strains | ||
| NCTC121023 | Wild type | NCTC, Colindale, United Kingdom |
| EB231 | phsA::Tn10dTc | B. C. Berks, Oxford, United Kingdom |
| MvP599 | ΔsiiE::FRT | 8 |
| MvP812 | ΔsiiF::FRT | 8 |
| MvP781 | ΔsiiE6540-12493::FRT (ΔIg21-40) | 5 |
| MvP990 | ΔsiiE6540-9541::FRT (ΔIg21-30) | 5 |
| MvP991 | ΔsiiE6540-8038::FRT (ΔIg21-25) | 5 |
| tetAR insertion strains | ||
| MvP1220 | siiE6540::tetAR | This study |
| MvP1287 | siiF1506::tetAR | This study |
| siiE deletion strains | ||
| MvP1222 | ΔsiiE6541-12492 (ΔIg21-40) | This study |
| MvP1226 | ΔsiiE6541-9540 (ΔIg21-30) | This study |
| MvP1227 | ΔsiiE6541-8037 (ΔIg21-25) | This study |
| Site-directed mutagenesis strains | ||
| MvP1311 | siiF(G500E) | This study |
| MvP1312 | siiF(K506L) | This study |
| MvP1313 | siiF SacI | This study |
| Plasmids | ||
| pKD46 | Red-expressing plasmid, temperature sensitive | 3 |
| pWSK29 | Low-copy-number vector, Ampr | 17 |
| p3223 | Complementation of siiF | 5 |
The PCR product was purified and used for electroporation of S. enterica serovar Typhimurium harboring pKD46 that was made competent and induced for expression of Red recombinase as described previously (6). After electroporation the cell suspension was plated onto LB plates containing tetracycline (20 μg ml−1) and incubated overnight at 37°C. Clones resistant to tetracycline were selected, and proper insertion of the tetAR cassette was confirmed by colony PCR with oligonucleotides specific for tetA and the target gene for the mutagenesis (Fig. 1 and Table 2). The resulting Tetr clones were also tested for growth inhibition on Bochner-Maloy plates (11) for 24 h at 42°C. This selection procedure is not as effective as conventional selection by antibiotics. We observed different degrees of growth inhibition by tetracycline for clones obtained using a mutagenesis approach. Clones with the highest sensitivity on Bochner-Maloy plates were selected for further mutagenesis. The medium composition, detailed step-by-step protocols, and notes are provided in the supplemental material.
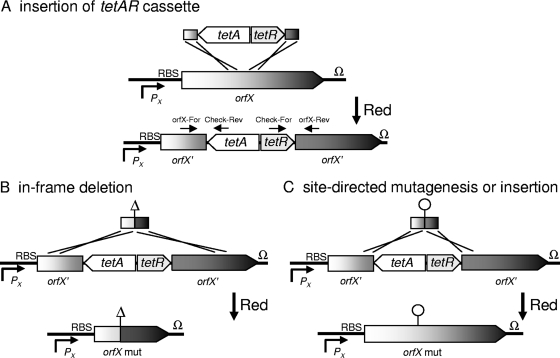
FIG. 1.
Rationale of the oligonucleotide-based recombinant engineering approach. In the first step, the tetAR cassette is inserted using Red recombinase-mediated recombination into the target locus, and Tetr strains are selected (A). Proper insertion of the tetAR cassette is confirmed by colony PCR using primer TetAR-Del-Check-For (Check-For) and a target gene-specific primer (orfX-Rev) or primer TetAR-Del-Check-Rev (Check-Rev) and a target gene-specific primer (orfX-For). In the second step, confirmed clones are transformed with the Red recombinase-expressing plasmid pKD46, and subsequently 80- to 88-bp DNA fragments are introduced by electroporation to generate in-frame deletions (B) or site-directed mutation or insertion of codons (C). Tets clones are selected on Bochner-Maloy plates. Finally, the mutations introduced by recombination are confirmed by colony PCR using primers flanking the deletion or by restriction analysis, as described in the legend to Fig. 3. RBS, ribosome binding site.
TABLE 2.
Oligonucleotides used in this study
| Oligonucleotide | Sequence |
|---|---|
| Deletions in siiE | |
| SiiE-Del-Ig21-Tet-For | 5′-CGGCACGAAAGAGGTGCTGACGGCCACCAAAGACGCGACCTTAAGACCCACTTTCACA-3′ |
| SiiE-Del-Ig21-Tet-Rev | 5′-CATCTGCCCATGTGCCGGTCGGTGTCACGCTCCAGTTGCCCTAAGCACTTGTCTCCTG-3′ |
| SiiE-Del6541-12492-For | 5′-CGGCACGAAAGAGGTGCTGCTGACGGCCACCAAAGACGCGACCGGCGAATGGAGTTGGACGCCGCCATCAGTATTAGCGCCAG-3′ |
| SiiE-Del6541-12492-Rev | 5′-CTGGCGCTAATACTGATGGCGGCGTCCAACTCCATTCGCCGGTCGCGTCTTTGGTGGCCGTCAGCACCTCTTTCGTGCCG-3′ |
| SiiE-Del6541-9540-For | 5′-CGGCACGAAAGAGGTGCTGACGGCCACCAAAGACGCGACCGACGGCTGGCGCTATCGACCGGATTCTGCTTTGGCGGACG-3′ |
| SiiE-Del6541-9540-Rev | 5′-CGTCCGCCAAAGCAGAATCCGGTCGATAGCGCCAGCCGTCGGTCGCGTCTTTGGTGGCCGTCAGCACCTCTTTCGTGCCG-3′ |
| SiiE-Del6541-8037-For | 5′-CGGCACGAAAGAGGTGCTGACGGCCACCAAAGACGCGACCGGGATCTGGGATTACACCTGGCCGAAAGATGTGACCGACG-3′ |
| SiiE-Del6541-8037-Rev | 5′-CGTCGGTCACATCTTTCGGCCAGGTGTAATCCCAGATCCCGGTCGCGTCTTTGGTGGCCGTCAGCACCTCTTTCGTGCCG-3′ |
| Site-directed mutagenesis of siiF | |
| SiiF502-503-TetAR-For | 5′-CATACCTGCGGGGCAACGTGTCGCGGTGGTAGGCGAATGCTTAAGACCCACTTTCACAtt-3′ |
| SiiF502-503-TetAR-Rev | 5′-GTAGCCAGATAGCATTCCCAGTAATGAGCTTTTTCCTGCTCCCTAAGCACTTGTCTCCTG-3′ |
| SiiF-TetSwap-G500E-For | 5′-GACATACCTGCGGGGCAACGTGTCGCGGTGGTAGaaGAATGCGGAGCCGGAAAAAGCTCATTACTGGGAATGCTATCTGGCTAC-3′ |
| SiiF-TetSwap-G500E-Rev | 5′-GTAGCCAGATAGCATTCCCAGTAATGAGCTTTTTCCGGCTCCGCATTCTTCTACCACCGCGACACGTTGCCCCGCAGGTATGTC-3′ |
| SiiF-TetSwap-K506L-For | 5′-CATACCTGCGGGGCAACGTGTCGCGGTGGTAGGCGAATGCGGAGCCGGATTAAGCTCATTACTGGGAATGCTATCTGGCTACCTTT-3′ |
| SiiF-TetSwap-K506L-Rev | 5′-AAAGGTAGCCAGATAGCATTCCCAGTAATGAGCTTAATCCGGCTCCGCATTCGCCTACCACCGCGACACGTTGCCCCGCAGGTATG-3′ |
| SiiF-TetSwap-SacI-For | 5′-CATACCTGCGGGGCAACGTGTCGCGGTGGTAGGCGAATGCGGAGCCGGAAAGAGCTCATTACTGGGAATGCTATCTGGCTACCT-3′ |
| SiiF-TetSwap-SacI-Rev | 5′-AGGTAGCCAGATAGCATTCCCAGTAATGAGCTCTTTCCGGCTCCGCATTCGCCTACCACCGCGACACGTTGCCCCGCAGGTATG-3′ |
| Control primers | |
| TetAR-Red-Check-For | 5′-GATCAAGAGCATCAAGTCGC-3′ |
| TetAR-Red-Check-Rev | 5′-TCAGCAAGGTGCTTTACAGG-3′ |
Generation of double-stranded DNA for electroporation.
Oligonucleotides were synthesized and purified using high-performance liquid chromatography by Thermo Scientific (Ulm, Germany). Lyophilized oligonucleotides were resuspended in distilled H2O at a concentration of 500 pmol ml−1. A mixture (total volume, 20 μl) containing equal amounts of the forward and reverse oligonucleotides was prepared in a “Safelock” Eppendorf tube. The tube was incubated in a water bath at 95°C for 15 min, and the water bath was allowed to slowly cool to room temperature overnight. Proper annealing was verified by gel electrophoresis of diluted samples of the mixture on 2% agarose gels.
For electroporation, 2 to 3 μl of the annealed oligonucleotides or various dilutions of the mixture were used to transform pKD46-containing Tetr target strains to a Tets phenotype. After electroporation, various dilutions of the cell suspension were plated onto freshly prepared Bochner-Maloy plates and incubated for 24 h at 42°C. Tets clones were selected and streak purified on Bochner-Maloy plates with incubation for 24 h at 42°C. Selection at 42°C was necessary to obtain the strongest possible growth inhibition of Tetr clones. Purified clones were streaked in parallel onto LB plates without an antibiotic or containing tetracycline (20 μg ml−1). Clones were further confirmed by colony PCR of the mutated loci. A detailed step-by-step protocol and further practical notes are provided in the supplemental material.
Sequence analyses.
DNA sequencing and restriction analyses were performed with PCR products obtained by colony PCR of selected clones.
Analyses of SiiE synthesis and secretion.
SiiE was detected in cell lysates of various S. enterica serovar Typhimurium strains (5) and quantification of SiiE secretion into the culture supernatant by various strains by an enzyme-linked immunosorbent assay (ELISA) (7) was performed as previously described.
RESULTS AND DISCUSSION
Rationale for oligonucleotide-based recombineering.
Homologous recombination mediated by the Red recombinase is a highly efficient process that has allowed generation of deletions and gene fusions in various microbial organisms. Flanking regions as short as 40 bp are sufficient to enable Red-mediated homologous recombination in Escherichia coli, S. enterica, and various other bacteria. We speculated that this specific feature might allow the use of short synthetic DNA for recombinant engineering of target genes directly in their genomic context.
We devised a two-step procedure for construction of in-frame deletions and site-directed mutagenesis (Fig. 1). In the first step, a cassette consisting of the tetAR genes was introduced into the target locus for mutagenesis by Red-mediated recombination (Fig. 1A). The resulting clones were selected by virtue of their resistance to tetracycline, and the proper position of the tetAR cassette was confirmed by colony PCR. In the second step, confirmed clones were electroporated with pKD46 and made competent for electroporation again. For electroporation, we used short synthetic DNA fragments that consisted of two complementary oligonucleotides (80 to 88 nucleotides) that were allowed to form double strands prior to transformation. The design of these oligonucleotides allowed generation of scarless in-frame deletions (Fig. 1B) and site-directed mutagenesis (Fig. 1C), as well as insertion of foreign sequences (not shown).
Generation of scarless in-frame deletions in the Salmonella chromosome.
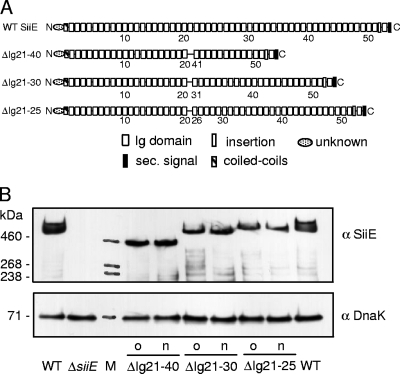
To test this approach for the generation of scarless in-frame chromosomal deletions, we selected target genes within Salmonella pathogenicity island 4 (SPI4), a virulence locus of S. enterica that is essential for the adhesion to and invasion of polarized cells by S. enterica serovar Typhimurium (5). SPI4 contains a 17-kb gene encoding the giant adhesin SiiE and genes for a cognate type I secretion system consisting of SiiC, SiiD, and SiiF (8). The large size and repetitive nature of siiE make expression from episomal elements and genetic manipulation extremely difficult. We have previously performed Red-mediated mutagenesis to generate in-frame deletions in siiE of 200 to 1,984 codons and demonstrated that there is a correlation between the length of SiiE and its function as an adhesin (5). However, generation of in-frame deletions as described by Datsenko and Wanner (3) results in insertion of a 27-codon “scar” sequence that may interfere with the function of the target in an unpredictable manner. In the work described here, we generated a set of in-frame deletion alleles of siiE by replacing the tetAR cassette by oligonucleotides (Fig. 2A). For this purpose, a strain harboring a tetAR cassette in siiE was electroporated with short synthetic double-stranded DNA fragments (80 to 88 bp) with flanks complementary to the flanks of the deletion in the chromosomal gene (Fig. 1B). Red-mediated homologous recombination was induced and led to replacement of the tetAR cassette by the synthetic DNA fragment. Recombinant clones were selected by inhibition of Tetr clones by fusaric acid on Bochner-Maloy plates as previously described (9). The transformation usually resulted in a large number (range, 101 to >102) of colonies on selection plates. In contrast to other published approaches (4), the transformation with single-stranded oligonucleotides did not yield recombinants in our setting. Tets clones were further characterized by colony PCR to scan for the deletions introduced. About 33% of the Tets clones yielded PCR products of the predicted size and were further confirmed by DNA sequencing. Positive clones were analyzed for the expression of mutant alleles of siiE, and all clones confirmed by PCR synthesized SiiE, as detected by ELISA and Western blotting (data not shown). The molecular weights of scarless SiiE variants in strains generated by oligonucleotide-mediated recombinant engineering were comparable to those of molecules in previously generated mutant strains containing the “Red scar” (Fig. 2B). It should be noted that the small difference in molecular mass (about 2.9 kDa) due to the presence or absence of the “Red scar” could not be resolved by the sodium dodecyl sulfate- polyacrylamide gel electrophoresis analysis used here. The characteristics of the strains with scarless in-frame deletions in siiE with respect to adhesion to and invasion of polarized cells were in line with our previous observations (data not shown).
FIG. 2.
Oligonucleotide-based recombineering of SPI4-encoded SiiE. (A) Schematic representation of the domain organization of siiE encoding a giant nonfimbrial adhesin in S. enterica serovar Typhimurium. Oligonucleotide-mediated recombinant engineering was performed to delete regions encoding the 5, 10, or 20 immunoglobulin (Ig) domain as indicated. Deletions between the Ig repeats were in frame without introduction of a scar or additional alterations. WT, wild type; sec., secretion. (B) Synthesis of SiiE and derivatives by Salmonella wild-type and various mutant strains harboring deletions of the entire siiE gene or in-frame deletions of the 5, 10, or 20 Ig domains. Bacterial cultures were subcultured in LB for 3.5 h. Equal amounts of cells, adjusted by using optical density at 600 nm, were harvested, and proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on gradient gels. Proteins were transferred onto nitrocellulose membranes, and SiiE was detected by incubation with antiserum against SiiE (α SiiE). As loading controls, Western blots were probed with antiserum against DnaK (StressGen) (α DnaK). The sizes of SiiE variants from mutant strains (o) were described previously (5) and strains generated in this study (n) were compared. Lane M contained high-molecular-weight protein standards.
Rapid site-directed mutagenesis of chromosomal genes.
We next investigated if Red-mediated replacement of the tetAR cassette by oligonucleotides can be used for site-directed mutagenesis directly in the chromosome of the target strain. Here, the tetAR cassette was replaced by recombination with a short synthetic DNA fragment that introduced silent mutations and changes in the amino acid sequence (Fig. 1C). siiF was selected as a target gene that encodes the putative ATPase and energizer of the type I secretion system for secretion of SiiE (5, 12). SiiF contains an ATP-binding cassette (ABC) motif, and previous work on the putative homologue in the type I secretion system for hemolysin secretion in E. coli showed that single amino acid exchanges in “Walker box A” of the ABC are sufficient to eliminate the function of the transport ATPase and to prevent secretion (10).
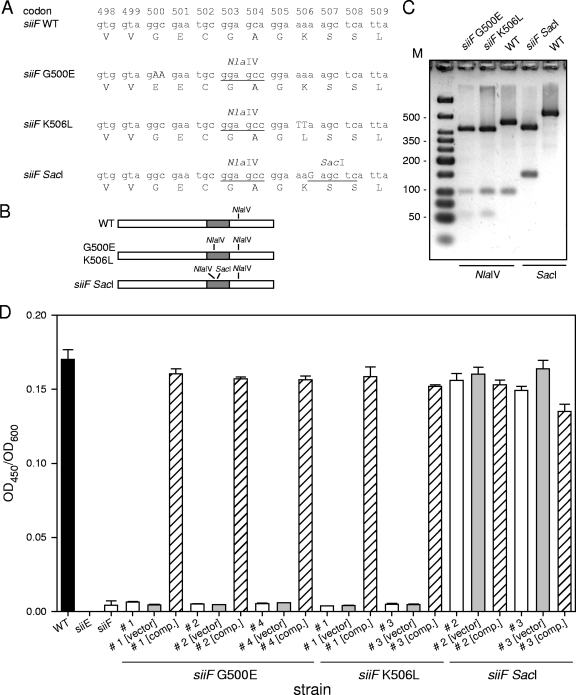
For mutagenesis of siiF, we inserted the tetAR cassette between codons 502 and 503 of siiF. Oligonucleotides were designed that introduced a silent mutation resulting in a new restriction site for NlaIV, as well as G500E or K506L exchange (Fig. 3A). For control mutagenesis, we designed oligonucleotides that introduced the NlaIV site, and a new SacI site within the ABC motif was used as an additional silent mutation (Fig. 3A). Since no change in the amino acid sequence was introduced, we expected that the resulting mutant strain would have a wild-type phenotype with respect to secretion of SiiE.
FIG. 3.
Site-directed mutagenesis of chromosomal siiF in S. enterica serovar Typhimurium. (A) Sequence of the target region in siiF encoding the putative ABC and design of site-directed mutations and silent mutations. All manipulations resulted in a silent mutation that generated a novel NlaIV site at codons 503 and 504. In addition, mutations G500E and K506L resulted in amino acid changes in the ABC of SiiF. Mutation siiF SacI introduced an additional SacI site without altering the amino acid sequence of the ABC. Newly introduced restriction sites are underlined, and mutations resulting in amino acid changes are indicated by capital letters. WT, wild type. (B) Predicted restriction map for a 500-bp PCR product of siiF obtained for wild-type and various mutant strains. The gray box indicates the mutagenesis target region. (C) The PCR products for fragments of siiF from wild-type and representative clones of various mutant strains were subjected to restriction analyses with NlaIV or SacI as indicated. Lane M contained a DNA size marker. (D) The secretion of SiiE by Salmonella wild-type and various mutant strains was analyzed by an ELISA for SiiE. The Salmonella wild-type strain (black bar), mutant strains with deletions of the entire siiE or siiF gene, selected clones (indicated by number signs) with site-directed mutations in siiF (open bars), and mutant strains harboring the plasmid vector pWSK29 (vector) (gray bars) or plasmid p3223 for expression of wild-type siiF (comp.) (hatched bars) were grown in LB medium, and culture supernatants were collected for quantification of SiiE by ELISA. The values for SiiE (optical density at 450 nm [OD450]) were normalized by using the culture density (optical density at 600 nm [OD600]).
Clones harboring the mutant alleles were screened by colony PCR, followed by digestion with NlaIV or SacI. We observed that 80 to 90% of the clones selected on the basis of sensitivity to tetracycline contained the newly introduced NlaIV site, as well as the additional SacI site in the case of the siiF SacI mutation (Fig. 3B and C). For each mutation selected clones were analyzed by DNA sequencing of the target region in siiF, which revealed that of the five clones analyzed, three siiE(G500E), two siiF(K506L), and all siiF SacI clones had error-free sequences with the desired mutations. Clones whose sequences were confirmed were subjected to functional analyses by quantification of the amount of secreted SiiE by ELISA (Fig. 3D). We observed that the G500E and K506L point mutations did not affect the synthesis of SiiE but eliminated secretion of the protein into the culture supernatant (Fig. 3D). The amounts of SiiE secreted by the strains with a point mutation in siiF were similar to those secreted by strain MvP812 with the entire siiF gene deleted. Introduction of the SacI site affected neither the synthesis nor the secretion of SiiE. As expected from these results, we observed that adhesion to and invasion of polarized epithelial cells were highly reduced for strains MvP1311 [siiF(G500E)] and MvP1312 [siiF(K506L)] but not for strain MvP1313 (siiF SacI) (data not shown). These virulence defects could be restored by complementation with a plasmid-borne copy of siiF. These results demonstrate that oligonucleotide-based recombinant engineering allows direct site-directed mutagenesis of chromosomal target sequences.
Concluding remarks.
The method described here allows rapid mutagenesis of genes in bacterial chromosomes by means of Red-mediated recombination. Although the method was used for manipulation of virulence genes in S. enterica, it should basically be applicable to a larger number of bacteria, provided that Red-mediated recombination can be used in the strains or species and that selectable as well as counterselectable markers are available. Our approach entirely eliminates the need for cloning target genes and for reintroduction of mutated genes into the chromosome of the target organism. Since no amplification and cloning of target genes are required, the overall error rate of this method is very low and depends mainly on the accuracy of the oligonucleotide synthesis.
Although the two-step approach makes it necessary to introduce a Red recombinase-expressing plasmid twice, the tetAR-containing strains generated in the first step can be utilized for different mutagenesis procedures at the same locus.
We applied this approach to the mutagenesis of various chromosomal genes in S. enterica. One critical parameter was the efficiency of the selection of Tets clones on Bochner-Maloy plates, which was variable between clones with insertions in various loci. An interesting alternative could be selection of Tets clones based on the increased sensitivity of Tetr clones to nickel salts (15). Furthermore, the efficiency might be improved by the use of other positive selection strategies, such as the introduction of meganuclease restriction sites and selection by induction of double-strand breaks in the chromosome (2, 16). An approach described by Olson et al. (13) may also be of interest, since a circular targeting construct is generated by PCR and integrated into the chromosome without Red-mediated recombination.
Our novel approach should accelerate functional analyses of bacterial genes and enable new approaches for manipulation of bacterial genomes for research in bacterial pathogenesis, as well as for strain construction for vaccine development and industrial microbiology.
Supplementary Material
Acknowledgments
This work was supported by grant HE1964 from the Deutsche Forschungsgemeinschaft and by a grant from Elitenetzwerk Bayern.
We thank Joyce Karlinsey for providing crucial support for the Bochner-Maloy selection procedure.
Footnotes
Published ahead of print on 16 January 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Baba, T., T. Ara, M. Hasegawa, Y. Takai, Y. Okumura, M. Baba, K. A. Datsenko, M. Tomita, B. L. Wanner, and H. Mori. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cox, M. M., S. L. Layton, T. Jiang, K. Cole, B. M. Hargis, L. R. Berghman, W. G. Bottje, and Y. M. Kwon. 2007. Scarless and site-directed mutagenesis in Salmonella enteritidis chromosome. BMC Biotechnol. 7:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellis, H. M., D. Yu, T. DiTizio, and D. L. Court. 2001. High efficiency mutagenesis, repair, and engineering of chromosomal DNA using single-stranded oligonucleotides. Proc. Natl. Acad. Sci. USA 98:6742-6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerlach, R. G., N. Claudio, M. Rohde, D. Jäckel, C. Wagner, and M. Hensel. 2008. Cooperation of Salmonella pathogenicity islands 1 and 4 is required to breach epithelial barriers. Cell. Microbiol. 10:2364-2376. [DOI] [PubMed] [Google Scholar]
- 6.Gerlach, R. G., S. U. Hölzer, D. Jäckel, and M. Hensel. 2007. Rapid engineering of bacterial reporter gene fusions by using Red recombination. Appl. Environ. Microbiol. 73:4234-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerlach, R. G., D. Jäckel, N. Geymeier, and M. Hensel. 2007. Salmonella pathogenicity island 4-mediated adhesion is coregulated with invasion genes in Salmonella enterica. Infect. Immun. 75:4697-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerlach, R. G., D. Jäckel, B. Stecher, C. Wagner, L. Lupas, W. D. Hardt, and M. Hensel. 2007. Salmonella pathogenicity island 4 encodes a giant non-fimbrial adhesin and the cognate type 1 secretion system. Cell. Microbiol. 9:1834-1850. [DOI] [PubMed] [Google Scholar]
- 9.Karlinsey, J. E., and K. T. Hughes. 2006. Genetic transplantation: Salmonella enterica serovar Typhimurium as a host to study sigma factor and anti-sigma factor interactions in genetically intractable systems. J. Bacteriol. 188:103-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koronakis, E., C. Hughes, I. Milisav, and V. Koronakis. 1995. Protein exporter function and in vitro ATPase activity are correlated in ABC-domain mutants of HlyB. Mol. Microbiol. 16:87-96. [DOI] [PubMed] [Google Scholar]
- 11.Maloy, S. R., V. L. Stewart, and R. K. Taylor. 1996. Genetic analysis of pathogenic bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 12.Morgan, E., A. J. Bowen, S. C. Carnell, T. S. Wallis, and M. P. Stevens. 2007. SiiE is secreted by the Salmonella enterica serovar Typhimurium pathogenicity island 4-encoded secretion system and contributes to intestinal colonization in cattle. Infect. Immun. 75:1524-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olson, M. M., L. J. Templeton, W. Suh, P. Youderian, F. S. Sariaslani, A. A. Gatenby, and T. K. Van Dyk. 2007. Production of tyrosine from sucrose or glucose achieved by rapid genetic changes to phenylalanine-producing Escherichia coli strains. Appl. Microbiol. Biotechnol. 74:1031-1040. [DOI] [PubMed] [Google Scholar]
- 14.Oppenheim, A. B., A. J. Rattray, M. Bubunenko, L. C. Thomason, and D. L. Court. 2004. In vivo recombineering of bacteriophage lambda by PCR fragments and single-strand oligonucleotides. Virology 319:185-189. [DOI] [PubMed] [Google Scholar]
- 15.Podolsky, T., S. T. Fong, and B. T. Lee. 1996. Direct selection of tetracycline-sensitive Escherichia coli cells using nickel salts. Plasmid 36:112-115. [DOI] [PubMed] [Google Scholar]
- 16.Posfai, G., V. Kolisnychenko, Z. Bereczki, and F. R. Blattner. 1999. Markerless gene replacement in Escherichia coli stimulated by a double-strand break in the chromosome. Nucleic Acids Res. 27:4409-4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang, R. F., and S. R. Kushner. 1991. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100:195-199. [PubMed] [Google Scholar]
- 18.Warming, S., N. Costantino, D. L. Court, N. A. Jenkins, and N. G. Copeland. 2005. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 33:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu, D., H. M. Ellis, E. C. Lee, N. A. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.