Abstract
We determined the prevalence of rickettsiae in Dermacentor adults at 15 localities in Canada. Rickettsia rickettsii was not detected in any tick, whereas Rickettsia peacockii was present in 76% of Dermacentor andersoni adults and Rickettsia montanensis in 8% of Dermacentor variabilis adults. This host specificity was maintained in localities where both tick species occurred in sympatry.
Dermacentor andersoni and Dermacentor variabilis are vectors of Rickettsia rickettsii (6), the etiological agent of Rocky Mountain spotted fever (RMSF) in humans. RMSF has been a notifiable disease in the United States since the 1920s, with over 3,600 cases reported between 1997 and 2002 (9). Nonpathogenic rickettsiae have also been reported for both tick species (3, 12, 13). The detection and identification of Rickettsia in ticks have greatly improved in accuracy and sensitivity since the advent of PCR-based techniques. The rickettsial citrate synthase (gltA) and the 190-kDa surface protein (ompA) genes have been used to distinguish among species of Rickettsia and to determine the prevalence of different rickettsiae in D. andersoni or D. variabilis adults within the United States, primarily at localities where these two tick species do not coexist (1, 13, 19, 27). Serological studies of the prevalence of Rickettsia in the United States are also based on an examination of ticks from allopatric populations (2, 20). Comparisons of the prevalence of rickettsiae in sympatric and allopatric populations of D. andersoni and D. variabilis would provide insight into the host specificity and transmission of Rickettsia species.
RMSF is not a reportable disease in Canada. As a consequence, little is known of the frequency of RMSF, except for a few published cases in Alberta between 1923 and 1943 (5, 11, 15). There is no detailed information of the distribution and prevalence of rickettsial species in Canada, even though D. andersoni and D. variabilis are relatively common (28). The geographic ranges of these tick species in Canada are largely allopatric, except for a zone of sympatry in central Saskatchewan (28). The aim of the present study was to determine the species of Rickettsia present and their relative prevalence in adult ticks from allopatric and sympatric populations of D. andersoni and D. variabilis in Canada.
Total genomic DNA (gDNA) was extracted and column purified (10) from 1,326 adult ticks collected in 2005 (May through July) and 2007 (April through June) from 15 localities (Table 1). The presence of rickettsiae in ticks was determined by amplification of a 381-bp fragment of gltA by PCR from the tick gDNA using primers RpCS.877p and RpCS.1258n (22) and the following conditions: 95°C for 5 min, followed by 25 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s and a final extension at 74°C for 5 min. The results of the PCR analyses revealed that a large proportion (76%) of D. andersoni adults were infected with Rickettsia, while relatively few (8%) D. variabilis adults tested positive for Rickettsia (Table 1). There was no significant difference in prevalence of Rickettsia in D. andersoni males (73%; n = 205) and females (79%; n = 303; P = 0.128), or in the prevalence of Rickettsia in D. variabilis males (9%; n = 382) and females (7%; n = 436; P = 0.420). The prevalence of infection in D. andersoni varied among localities (36 to 96%); the lowest prevalence was recorded within Danielson Provincial Park (Table 1). The prevalence of Rickettsia in D. variabilis was very low (0 to 8%) at most localities, except within Blackstrap Provincial Park, where 33% of ticks were Rickettsia positive (Table 1). There was heterogeneity in the prevalence of Rickettsia within Blackstrap Provincial Park with a significantly greater (P < 0.001) proportion of Rickettsia-infected D. variabilis individuals on the western side of Blackstrap Lake (39%; n = 115) than on the eastern side (4%; n = 26).
TABLE 1.
Localities and coordinates of the collection sites of D. andersoni and D. variabilis adults within Canada and the number of ticks that were positive for infection with Rickettsia using PCR analyses of the gltA gene
| Locality in Canada | Coordinates | No. of D. andersoni adults:
|
No. of D. variabilis adults:
|
||
|---|---|---|---|---|---|
| Tested | Positive for Rickettsia (%) | Tested | Positive for Rickettsia (%) | ||
| Lethbridge, AB | 49°44′N, 112°50′W | 100 | 72 (72) | ||
| Cypress Hills, AB | 49°25′N, 110°15′W | 61 | 37 (61) | ||
| Saskatchewan Landing Provincial Park, SK | 50°38′N, 107°57′W | 101 | 97 (96) | 100 | 0 (0) |
| Grasslands National Park, SK | 49°13′N, 107°42′W | 17 | 15 (88) | 1 | 0 (0) |
| Buffalo Pound Provincial Park, SK | 50°36′N, 105°25′W | 35 | 30 (86) | 100 | 2 (2) |
| Douglas Provincial Park, SK | 51°02′N, 106°28′W | 14 | 13 (93) | 40 | 0 (0) |
| Danielson Provincial Park, SK | 51°15′N, 106°49′W | 61 | 22 (36) | 100 | 0 (0) |
| Outlook, SK | 51°28′N, 107°04′W | 18 | 17 (94) | 12 | 0 (0) |
| Harris, SK | 51°42′N, 107°37′W | 101 | 84 (83) | 12 | 0 (0) |
| Saskatoon, SK | 52°10′N, 106°36′W | 38 | 0 (0) | ||
| Blackstrap Provincial Park, SK | 51°47′N, 106°25′W | 141 | 46 (33) | ||
| Bradwell, SK | 51°54′N, 106°13′W | 100 | 7 (7) | ||
| Wakaw, SK | 52°36′N, 105°51′W | 44 | 0 (0) | ||
| Minnedosa, MB | 50°14′N, 99°50′W | 100 | 8 (8) | ||
| Kenora, ON | 49°45′N, 94°29′W | 30 | 2 (7) | ||
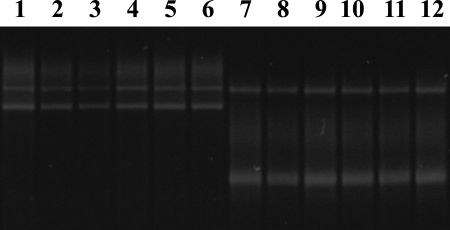
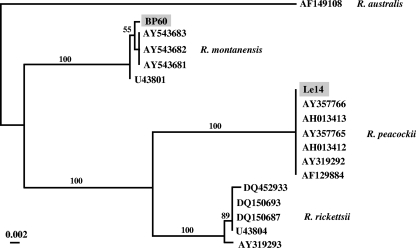
Genetic variation among the 452 gltA amplicons derived from Rickettsia-positive ticks was examined using single-strand conformation polymorphism (SSCP) analyses (10, 14). Two different SSCP banding patterns (i.e., profiles) were detected among samples: one profile (type I) was displayed by all D. andersoni individuals positive for Rickettsia, and the second (type II) was displayed only by D. variabilis individuals positive for Rickettsia (Fig. 1). The gltA sequences derived from 11 column-purified amplicons of type I were identical to each other and to the sequence for Rickettsia peacockii (GenBank accession number AF129885) (25). The eight type II gltA amplicons derived from Rickettsia-infected D. variabilis individuals were identical in nucleotide sequence to one another and to a sequence for Rickettsia montanensis (accession number U74756) (23). The presence of R. peacockii in D. andersoni adults and R. montanensis in D. variabilis adults was confirmed by the amplification and sequencing of a 532-bp fragment of ompA (22) from a single individual of each tick species that contained rickettsiae using primers Rr190.70p and Rr190.602n (22) and the same conditions used for gltA except that 30 amplification cycles were used. The ompA amplicon from D. andersoni was identical in sequence to that reported previously for R. peacockii (accession number U55821) (19). The ompA amplicon from D. variabilis most closely matched the sequence for R. montanensis (accession number AY543682) (1), but it differed at a single nucleotide position. The results of a phylogenetic analysis showed that there was strong statistical support for the inclusion of the Rickettsia species from D. variabilis within the clade of R. montanensis (Fig. 2).
FIG. 1.
SSCP analysis of gltA amplicons from total gDNA from D. andersoni (SSCP profile I) and D. variabilis (SSCP profile II). Lanes 1 to 6 and 7 to 12 contain gltA amplicons derived from single D. andersoni and D. variabilis individuals, respectively.
FIG. 2.
A neighbor-joining tree depicting the relationships of the ompA sequences of Rickettsia from D. andersoni (Le14) and D. variabilis (BP60) obtained in the present study with those of R. peacockii (accession numbers AF129884, AH013412, AH013413, AY357765, and AY357766), R. montanensis (AY543681, AY543682, AY543683, and U43801), Rickettsia australis (AF149108), and R. rickettsii (AY319293, DQ452933, DQ150693, DQ150687, and U43804) derived from GenBank. The numbers above the branches in the tree indicate the statistical support following bootstrap analyses (1,000 iterations) for each clade. R. australis was used to root the tree (26).
Our molecular analyses of 508 D. andersoni and 818 D. variabilis adults from 15 localities revealed the presence of R. peacockii in D. andersoni and R. montanensis in D. variabilis. This host-specificity was maintained at the seven localities where both tick species occurred in sympatry. These findings are consistent with the results of studies conducted in the United States, where R. peacockii has been reported only for D. andersoni (7, 19) and R. montanensis only for D. variabilis (1, 2, 12, 21). Philip and Casper (20) reported R. montanensis for D. andersoni from the western side of Bitterroot Valley (Montana), based on serotyping of rickettsiae from ticks. However, this probably represents a case of an incorrect identification of the rickettsiae. Philip and Casper (20) demonstrated that there were four serotypes within 106 rickettsial isolates from D. andersoni and attributed these to be R. rickettsii (9%), Rickettsia rhipicephali (44%), Rickettsia bellii (i.e., 369-C; 39%) and R. montanensis (i.e., “Rickettsia montana”; 8%). In contrast, Burgdorfer et al. (7) showed that R. peacockii occurs on the western side of Bitterroot Valley at a prevalence of 8 to 16%. It is, therefore, likely that the fourth rickettsial species detected by Philip and Casper (20) was not R. montanensis but R. peacockii, especially if the antibodies used in their assay were cross-reactive with both species. If this were the case, then R. montanensis would also represent a rickettsial species that is host specific for D. variabilis.
We only detected single-species rickettsial infections in both tick species. This is typical for Dermacentor spp. (1, 13, 27), except for the reports of a single D. variabilis adult from Ohio infected with R. bellii, R. montanensis, and R. rickettsii (8) and of a single Dermacentor occidentalis adult infected with R. bellii and R. rhipicephali (27). The prevalence of R. peacockii in D. andersoni at different localities (36 to 96%) was significantly greater than that for R. montanensis in D. variabilis (0 to 33%). This is likely due to the mode of transmission of R. peacockii, which is thought to be exclusively transovarial (i.e., from female ticks to their offspring) (7, 19). The prevalence of R. montanensis in D. variabilis at 12 of the 15 sites in the present study (0 to 8%) was similar to that for D. variabilis populations in Ohio (<0.1%) (21), Massachusetts (1%) (12), and Maryland (4%) (1). The relatively low prevalence of R. montanensis in ticks compared to that for R. peacockii suggests that horizontal transmission is required for the maintenance of this species in populations of D. variabilis. R. montanensis has been detected in mice (Peromyscus spp.) and voles (Microtus spp.) (18), hosts used by D. variabilis (4, 16), suggesting that small mammals may act as reservoirs for this species of Rickettsia.
The results of the present study also showed that the other rickettsial species recorded in D. andersoni and/or D. variabilis in the United States (i.e., the pathogenic R. rickettsii [6] and the nonpathogenic R. bellii and R. rhipicephali [13]) were not detected in any of the 1,326 ticks tested. The prevalence of R. rickettsii in D. andersoni adults in the Bitterroot Valley of Montana varies from 1.5 to 5% (6), while infections of R. rickettsii in D. variabilis range from 0.1% in Ohio (21) to 8.6% in Maryland (24). The lack of detection of R. rickettsii in D. andersoni from the nine localities in Canada may be associated with the relatively high proportion of ticks infected with R. peacockii. Although R. peacockii is closely related to R. rickettsii (19), it appears to be nonpathogenic to D. andersoni and has no effect on the fecundity of infected females (18). The greater incidence of RMSF on the western side of Bitterroot Valley compared to the eastern side of the valley has been shown to be associated with a significantly lower prevalence of R. peacockii (7, 19). Only 8 to 16% of D. andersoni on the western side of the Bitterroot Valley are infected with R. peacockii (7), whereas the prevalence is 70 to 80% for ticks on the eastern side (7, 19), which is equivalent to the average prevalence of R. peacockii in D. andersoni in the present study (76%). It has also been shown that establishment of R. rickettsii in the ovarial tissues of D. andersoni is prevented by an “interference phenomenon” when ticks are already infected with R. peacockii (7). D. variabilis adults infected with R. montanensis are also known to prevent the establishment of R. rickettsii (17). Thus, R. peacockii and R. montanensis have epidemiological significance with respect to R. rickettsii because of a negative effect on its enzootic maintenance. However, the relatively low prevalence of D. variabilis adults infected with R. montanensis in 13 of the Canadian localities we examined would not account for the apparent absence of R. rickettsii. Therefore, other factors must be responsible for this.
Nucleotide sequence accession numbers.
The sequences of the gltA and ompA genes for representative samples have been deposited in GenBank under accession numbers FM883668 to FM883671.
Acknowledgments
Funding for this work was provided to N.B.C. from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Canadian Foundation for Innovation. S.J.D. is a recipient of a NSERC Graduate Scholarship.
We thank John Allen, Alvin Gajadhar, Murray Lankester, Brad Scandrett, and Travis Quirk, who provided some ticks, and Lorilee Flavelle and Chantel Krakowetz for technical assistance.
Footnotes
Published ahead of print on 16 January 2009.
REFERENCES
- 1.Ammerman, N. C., K. I. Swanson, J. M. Anderson, T. R. Schwartz, E. C. Seaberg, G. E. Glass, and D. E. Norris. 2004. Spotted-fever group Rickettsia in Dermacentor variabilis, Maryland. Emerg. Infect. Dis. 10:1478-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, J. F., L. A. Magnarelli, R. N. Philip, and W. Burgdorfer. 1986. Rickettsia rickettsii and Rickettsia montana from ixodid ticks in Connecticut. Am. J. Trop. Med. Hyg. 35:187-191. [DOI] [PubMed] [Google Scholar]
- 3.Bell, E. J., D. B. Lackman, H. G. Stoenner, and G. M. Kohls. 1963. Nonpathogenic rickettsias related to spotted fever group isolated from ticks, Dermacentor variabilis and Dermacentor andersoni from eastern Montana. J. Immunol. 90:770-781. [PubMed] [Google Scholar]
- 4.Bishopp, F. C., and H. L. Trembley. 1945. Distribution and hosts of certain North American ticks. J. Parasitol. 31:1-54. [Google Scholar]
- 5.Bow, M. R., and J. H. Brown. 1945. Tick-borne diseases of man in Alberta. Can. Med. Assoc. J. 53:459-465. [PMC free article] [PubMed] [Google Scholar]
- 6.Burgdorfer, W. 1975. A review of Rocky Mountain spotted fever (tick-borne typhus), its agent, and its tick vectors in the United States. J. Med. Entomol. 12:269-278. [DOI] [PubMed] [Google Scholar]
- 7.Burgdorfer, W., S. F. Hayes, and A. J. Mavros. 1981. Nonpathogenic rickettsiae in Dermacentor andersoni: a limiting factor for the distribution of Rickettsia rickettsii, p. 585-594. In W. Burgdorfer and R. L. Anacker (ed.), Rickettsiae and rickettsial diseases. Academic Press, New York, NY.
- 8.Carmichael, J. R., and P. A. Fuerst. 2006. A rickettsial mixed infection in a Dermacentor variabilis tick from Ohio. Ann. N. Y. Acad. Sci. 1078:334-337. [DOI] [PubMed] [Google Scholar]
- 9.Chapman, A. S., S. M. Murphy, L. J. Demma, R. C. Holman, A. T. Curns, J. H. McQuiston, J. W. Krebs, and D. L. Swerdlow. 2006. Rocky Mountain spotted fever in the United States, 1997-2002. Vector-Borne Zoonotic Dis. 6:170-178. [DOI] [PubMed] [Google Scholar]
- 10.Dergousoff, S. J., and N. B. Chilton. 2007. Differentiation of three species of ixodid tick, Dermacentor andersoni, D. variabilis and D. albipictus, by PCR-based approaches using markers in ribosomal DNA. Mol. Cell. Probes 21:343-348. [DOI] [PubMed] [Google Scholar]
- 11.Duncan, J. H. 1937. Rocky Mountain spotted fever in Canada. Can. Med. Assoc. J. 37:575-577. [PMC free article] [PubMed] [Google Scholar]
- 12.Feng, W. C., E. S. Murray, W. Burgdorfer, J. M. Spielman, G. Rosenberg, K. Dang, C. Smith, C. Spickert, and J. L. Waner. 1980. Spotted fever group rickettsiae in Dermacentor variabilis from Cape Cod, Massachusetts. Am. J. Trop. Med. Hyg. 29:691-694. [DOI] [PubMed] [Google Scholar]
- 13.Gage, K. L., M. E. Schrumpf, W. Burgdorfer, and T. G. Schwan. 1994. DNA typing of rickettsiae in naturally infected ticks using a polymerase chain reaction/restriction fragment length polymorphism system. Am. J. Trop. Med. Hyg. 50:247-260. [DOI] [PubMed] [Google Scholar]
- 14.Gasser, R. B., M. Hu, N. B. Chilton, B. E. Campbell, A. J. Jex, D. Otranto, C. Cafarchia, I. Beveridge, and X. Zhu. 2006. Single-strand conformation polymorphism (SSCP) for the analysis of genetic variation. Nat. Protoc. 1:3121-3128. [DOI] [PubMed] [Google Scholar]
- 15.Gibbons, R. J. 1939. Survey of Rocky Mountain spotted fever and sylvatic plague in western Canada during 1938. Can. J. Public Health 30:184-187. [Google Scholar]
- 16.Gregson, J. D. 1956. The Ixodoidea of Canada, p. 92. Canada Department of Agriculture, Ottawa, Ontario, Canada.
- 17.Macaluso, K. R., D. E. Sonenshine, S. M. Ceraul, and A. F. Azad. 2002. Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second Rickettsia. J. Med. Entomol. 39:809-813. [DOI] [PubMed] [Google Scholar]
- 18.Niebylski, M. L., M. G. Peacock, and T. G. Schwan. 1999. Lethal effect of Rickettsia rickettsii on its tick vector (Dermacentor andersoni). Appl. Environ. Microbiol. 65:773-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niebylski, M. L., M. E. Schrumpf, W. Burgdorfer, E. R. Fischer, K. L. Gage, and T. G. Schwan. 1997. Rickettsia peacockii sp. nov., a new species infecting wood ticks, Dermacentor andersoni, in western Montana. Int. J. Syst. Bacteriol. 47:446-452. [DOI] [PubMed] [Google Scholar]
- 20.Philip, R. N., and E. A. Casper. 1981. Serotypes of spotted fever group rickettsiae isolated from Dermacentor andersoni (Stiles) ticks in western Montana. Am. J. Trop. Med. Hyg. 30:230-238. [DOI] [PubMed] [Google Scholar]
- 21.Pretzman, C., N. Daugherty, K. Poetter, and D. Ralph. 1990. The distribution and dynamics of Rickettsia in the tick population of Ohio. Ann. N. Y. Acad. Sci. 590:227-236. [DOI] [PubMed] [Google Scholar]
- 22.Regnery, R. L., C. L. Spruill, and B. D. Plikaytis. 1991. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J. Bacteriol. 173:1576-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roux, V., E. Rydkina, M. Eremeeva, and D. Raoult. 1997. Citrate synthase gene comparison, a new tool for phylogenetic analysis, and its application for the rickettsiae. Int. J. Syst. Bacteriol. 47:252-261. [DOI] [PubMed] [Google Scholar]
- 24.Schriefer, M. E., and A. F. Azad. 1994. Changing ecology of Rocky Mountain spotted fever, p. 314-326. In D. E. Sonenshine and T. N. Mather (ed.), Ecological dynamics of tick-borne zoonoses. Oxford University Press, New York, NY.
- 25.Simser, J. A., A. T. Palmer, U. G. Munderloh, and T. J. Kurtti. 2001. Isolation of a spotted fever group Rickettsia, Rickettsia peacockii, in a Rocky Mountain wood tick, Dermacentor andersoni, cell line. Appl. Environ. Microbiol. 67:546-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stenos, J., and D. Walker. 2000. The rickettsial outer-membrane protein A and B genes of Rickettsia australis, the most divergent rickettsia of the spotted fever group. Int. J. Syst. Evol. Microbiol. 50:1775-1779. [DOI] [PubMed] [Google Scholar]
- 27.Wikswo, M. E., R. Hu, G. A. Dasch, L. Krueger, A. Arugay, K. Jones, B. Hess, S. Bennett, V. Kramer, and M. E. Eremeeva. 2008. Detection and identification of spotted fever group rickettsiae in Dermacentor species from southern California. J. Med. Entomol. 45:509-516. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson, P. R. 1967. The distribution of Dermacentor ticks in Canada in relation to bioclimatic zones. Can. J. Zool. 45:517-537. [DOI] [PubMed] [Google Scholar]