Abstract
Iron oxide sheaths and filaments are commonly found in hydrothermal environments and have been shown to have a biogenic origin. These structures were seen in the flocculent material associated with two submarine volcanoes along the Kermadec Arc north of New Zealand. Molecular characterization of the bacterial communities associated with the flocculent samples indicated that no known Fe-oxidizing bacteria dominated the recovered clone libraries. However, clones related to the recently described Fe-oxidizing bacterium Mariprofundus ferrooxydans were obtained from both the iron-containing flocculent (Fe-floc) and sediment samples, and peaks corresponding to Mariprofundus ferrooxydans, as well as the related clones, were observed in several of our terminal restriction fragment length polymorphism profiles. A large group of epsilonproteobacterial sequences, for which there is no cultured representative, dominated clones from the Fe-floc libraries and were less prevalent in the sediment sample. Phylogenetic analyses indicated that several operational taxonomic units appeared to be site specific, and statistical analyses of the clone libraries found that all samples were significantly different from each other. Thus, the bacterial communities in the Fe-floc samples were not more closely related to each other than to the sediment communities.
Biogenic iron oxide sheaths and filaments occur in a variety of hydrothermal environments, and the bacteria involved in their genesis could have significant impacts on local elemental cycles (9). These filaments are found as thin layers on seafloor rocks (16) and lava crusts (29), on sulfides and devices incubated at venting sites (10, 23), and as thick flocculent mats covering vast areas near hydrothermal environments (1, 20, 22, 34). The sheaths and filaments are often visually similar to those produced by the neutrophilic Fe-oxidizing betaproteobacteria Leptothrix spp. and Gallionella spp. In some cases it is clear either that the structures are directly produced by Fe-oxidizing bacteria (FeOB) (12) or that bacteria are serving as nucleation sites for the iron precipitation (16, 36). In other instances only the sheaths or filaments remain, leading to speculation of a bacterial origin (9, 18, 29).
An increasing number of novel FeOB associated with iron precipitate formation have been isolated from marine hydrothermal environments. Edwards et al. (11) isolated nine alpha- and gammaproteobacterial strains of FeOB from sulfide rubble and fine-grained sulfide sediments collected from Juan de Fuca Ridge in the northeast Pacific Ocean. Bacteria, mostly gammaproteobacteria within the genus Pseudoalteromonas, capable of Mn and Fe oxidation were cultivated from iron-containing flocculent (Fe-floc) mats occurring at Vailulu'u Seamount, Samoa (34). Fe-oxidizing gammaproteobacteria related to the Xanthomonas group appear to be a predominant phylotype at Loihi Seamount, Hawaii (9, 14). In a recent report, Emerson et al. (12) characterized two additional strains of FeOB, PV-1 and JV-1, isolated from iron-containing flocculent mats at Loihi Seamount. These strains, designated Mariprofundus ferrooxydans, were shown to be members of the proposed zeta class of proteobacteria capable of producing filamentous structures consisting of Fe-oxyhydroxide (12).
Flocculent mat material located near hydrothermal environments has been shown to contain hollow tubes of Fe-oxides. Of the sites studied, the mats present at Loihi Seamount, Hawaii, have been the most extensively characterized (21, 22). The presence of dense flocculent mats has also been documented at Juan de Fuca Ridge in the northeast Pacific Ocean (20), Franklin Seamount in the Western Woodlark Basin, Papua New Guinea (1), Vailulu'u Seamount off the coast of Samoa (34), and the Mariana Island arc/back arc system stretching from Iwo Jima to Guam (5). At both the Vailulu'u and Juan de Fuca Ridge sites, the presence of Fe-floc material was reported shortly after volcanic activity, within 4 years and 1 month, respectively (20, 34).
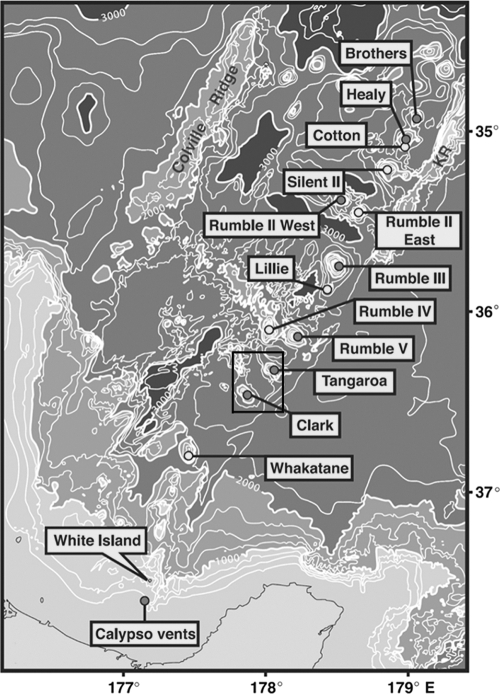
The Kermadec Arc is part of the larger Lau-Havre-Taupo arc/back arc system associated with subduction of the Pacific plate beneath the Australian plate (Fig. 1). The entire system is approximately 2,500 km in length and is thought to consist of at least 90 submarine volcanoes, although not all demonstrate hydrothermal activity (7). A 1999 survey of 13 submarine volcanoes occurring in the southern 260 km of the Kermadec Arc found that at least 7 were hydrothermally active (6). Analysis of plume water showed variation in the chemical composition between plumes, with unique signatures being detected between vent sites as well as within the same seamount (6). On a recent research cruise, dense Fe-containing flocculent mats were observed in the vicinity of several hydrothermally active submarine volcanoes (personal observations).
FIG. 1.
Map of Kermadec Arc, showing the locations of the seamounts in relation to New Zealand. Samples were collected from Clark and Tangaroa Seamounts (indicated by the box). (Reprinted from reference 6 with permission.)
Based on the observation of iron-containing flocculent mats at these sites, the goal of this project was to compare the composition of bacterial communities within the mats from two seamounts along the Kermadec Arc and a sediment sample. The results of this study will address a number of questions, including the following: (i) is the known Fe-oxidizing bacterium Mariprofundus ferrooxydans as common in the Fe-floc samples from these seamounts as from those at Loihi Seamount, Hawaii?; (ii) are Fe-floc bacterial communities more closely related to each other than to the communities in the surrounding sediment?; (iii) how similar are the bacterial communities inhabiting two Fe-floc samples obtained from different seamounts? To address these questions, clone libraries were constructed for each sample and the resulting sequence data were used for phylogenetic analyses. In order to examine additional samples, terminal restriction fragment length polymorphism (T-RFLP) analyses were employed. Rarefaction analysis and the diversity estimators chao 1 and ACE were used to determine clone library coverage and predict the diversity present at each site. Multidimensional scaling (MDS), analysis of similarity (ANOSIM), and LIBSHUFF (J. R. Henriksen; http://libshuff.mib.uga.edu) were then used to statistically compare the bacterial community compositions between sites (4, 32).
MATERIALS AND METHODS
Site details.
The Tangaroa Fe-flocculent mat sample was obtained from a depth of 669 m at 36°19.367′S 178°01.823′ E (Fig. 1). Temperature was recorded within the mat as 14.2°C. A sediment sample was collected from the vicinity of the Tangaroa Seamount at a depth of 653 m at 36°19.399′S 178°01.823′E, with a temperature of 12°C. The Clark Fe-flocculent mat sample was recovered at 881 m depth with the closest recorded position at 36°26.843′S 177°50.310′E The temperature of the overlying water was 6.1°C. Additional sediment, Fe-floc, and ambient water samples were collected from both the Tangaroa and Clark Seamounts and used for phylogenetic studies.
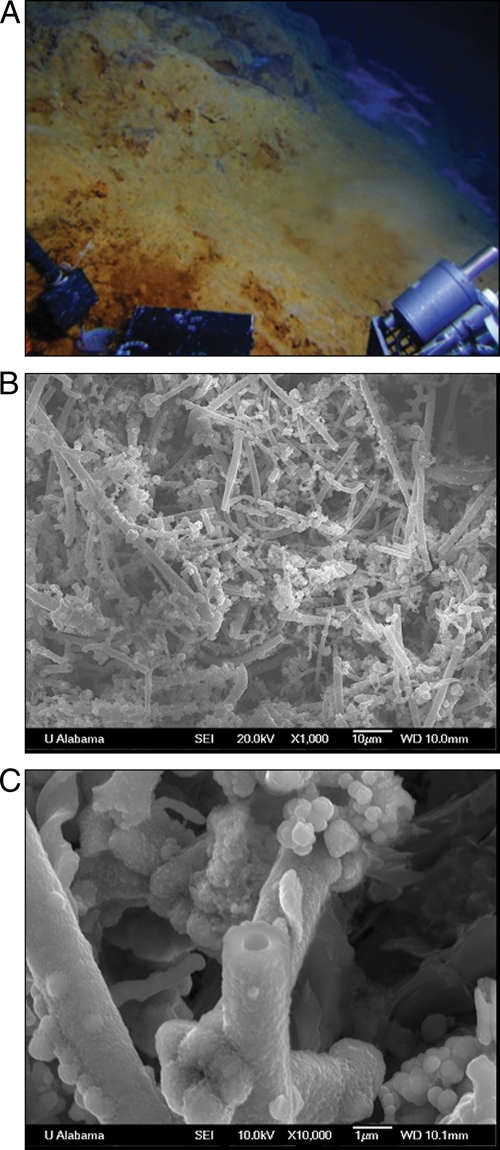
The Tangaroa Fe-floc material covered a vast area on the order of hundreds of square meters and had a thin, hazy, white layer over portions. The Clark Fe-floc material covered a smaller area of approximately 10 square meters (Fig. 2A). The sediment sample was collected near a field of long-necked barnacles and consisted of fine, gray sediment with small pieces of elemental sulfur. Distances between sites are 40.16 km for Tangaroa Fe-floc and Clark Fe-floc, 0.9884 km for Tangaroa Fe-floc and Tangaroa sediment, and 39.49 km for Clark Fe-floc and Tangaroa sediment.
FIG. 2.
(A) The Fe-floc mat as it appeared from the Pisces V submersible. (B and C) High-resolution scanning electron microscope images of Fe-floc samples indicated the presence of sheaths and filaments. Bar, 10 μm (B) or 1 μm (C).
Sampling.
Samples were acquired using the manned submersible Pisces V. Mat and sediment materials were suctioned into acrylic collection containers mounted on the submersible's carousel work platform using the vacuum suction arm of the submersible. Water samples were collected in Niskin bottles deployed by the sub's manipulator arm. Aboard the research vessel, samples were removed aseptically and portions of the sediment and floc samples were preserved in 70% ethanol or stored in sterile 10% glycerol in artificial seawater at −80°C. Water samples (300 to 500 ml) were filtered through 0.2-μm carbonate filters and the filters were preserved in 2 ml of RNAlater (Ambion).
Microscopy.
A Nikon E600 phase-contrast microscope with a 100× oil immersion objective was used for phase-contrast microscopy. A JEOL 7000 field emission scanning electron microscope with energy dispersive X-ray (EDX) spectroscopy was used for additional images and EDX analysis. Small samples of the mat and sediment material were rinsed three times in distilled H2O to remove residual sodium chloride. Samples were dropped directly onto either an aluminum or a carbon mount and dried in a laminar flow hood prior to microscopic examination.
Clone library construction.
Total DNA was extracted from Fe-floc and sediment samples using the MoBio UltraClean soil DNA isolation kit. Small subunit rRNA genes were amplified by PCR using the universal bacterial primers 8F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1392R (5′-ACGGGCGGTGTGTACA-3′). Each 50-μl PCR mixture contained 1 μl template DNA, 2 U Taq DNA polymerase (Eppendorf), 1× Taq buffer, 2.75 μM Mg(O-acetate)2, 1× Taq Master PCR Enhancer, each deoxynucleoside triphosphate at a concentration of 20 μM, and each primer at a concentration of 0.4 μM. The PCR conditions were 85°C for 5 min, 30 cycles of 94°C for 45 s, 55°C for 1 min, and 72°C for 90 s, and a final 7-min extension at 72°C. PCR-amplified products were purified by agarose gel electrophoresis with a QIAquick gel extraction kit (Qiagen), cloned into the pCR 2.1 vector with a TOPO TA cloning kit, and transformed into TOP10 chemically competent cells (Invitrogen). Plasmids containing the appropriately sized insert were purified using the QIAprep Spin miniprep kit (Qiagen) and sequenced by Macrogen Inc., South Korea.
DNA sequence analysis.
DNA sequence data were compiled and edited in Sequencher (version 4.7). Clone sequences were compared to sequences in the GenBank database using BLASTn searches. Potentially chimeric sequences were determined using the CHECK_CHIMERA program of the Ribosomal Database Project. Sequence alignments were performed using Bioedit (version 7.0.9); PAUP* (35) and GARLI (37) were used for maximum likelihood phylogenetic calculations.
T-RFLP analysis.
Using the DNA extracted previously, samples were PCR amplified as described for clone library construction with the modification of a fluorescent S-hexachlorofluorescein (HEX) label on the 8F primer. Triplicate 100-μl PCR products were pooled, cleaned, and concentrated using the Montage PCR centrifugal filter device (Millipore). Digests were conducted individually using HaeIII and HinfI restriction endonucleases. Reactions were carried out using 400 ng cleaned PCR products, 1× enzyme buffer, 2 U of restriction endonuclease, and sterile distilled H2O to a total volume of 50 μl. Reaction mixtures were incubated at 37°C for 8 h prior to denaturing the enzyme and stored at 4°C. DNA was ethanol precipitated overnight and centrifuged, and the pellet was dried using a Centrivac. Pellets were resuspended in 10 μl of deionized formamide and 0.5 μl 6-carboxytetramethylrhodamine size standard prior to being analyzed with an ABI 3100 genetic analyzer with a 50-cm capillary array (Applied Biosystems). Fragment lengths were determined using the Local Southern size-calling algorithm of the Genemapper analysis software version 3.7 (Applied Biosystems).
Data matrices were constructed using peaks above a threshold of 50 fluorescence units, which was considered to be the background level. Peaks smaller than 100 bp and greater than 500 bp were removed from the data set to avoid uncertainties associated with fragment size determination. The variable threshold method proposed by Osbourne et al. (28) was used to determine which peaks were analyzed. These data were normalized to the total fluorescence units per sample, and the resulting profiles were analyzed using T-Align (33). Data matrices were analyzed using PRIMER 5.0 as described below.
Statistical analyses.
DOTUR was used to perform rarefaction analyses, and the nonparametric abundance estimators chao 1 and ACE were used to estimate the diversity present in the samples (30). Regression curves and R values were generated using Sigma Plot from data obtained in DOTUR. Bray-Curtis similarity matrices were constructed using square root transformations of distance matrices. Nonmetric, multidimensional scaling (MDS) plots were used to visualize differences in the clone libraries and T-RFLP profiles and one-way ANOSIM methods were used for statistical comparisons between the clone libraries and T-RFLP profiles (PRIMER 5.0). LIBSHUFF statistical comparisons were conducted using webLIBSHUFF.
Nucleotide sequence accession numbers.
The sequences of 102 rRNA gene clones have been deposited in GenBank and are available as accession numbers FJ535252 to FJ535353.
RESULTS
Flocculent mat composition.
Phase-contrast microscopy revealed the presence of both sheaths and filaments (as described in reference 13) within the flocculent material. Bacterial cells were shown to be associated with the sheaths (hollow tubes) and filaments (thinner and not typically straight) for only a small number of the structures (∼1 to 5%) (data not shown). Because the samples were collected via suction, it is not possible to determine if many of the cells were dislodged during collection or were no longer associated with the filaments prior to collection. Scanning electron microscope/EDX analysis revealed that the flocculent material from both seamounts consisted of long, hollow sheaths and irregular filamentous aggregates (Fig. 2B and C) that were composed mainly of Fe-oxides and silica. The hollow sheaths were 1 to 2 μm in diameter and 50 to 200 μm in length; however, actual lengths could not be determined due to potential breakage during sampling and/or processing. The average of four EDX spectra from each Fe-floc sample indicated that the Clark flocculent material had a higher percentage of silica and other elements than the Tangaroa flocculent material. The Clark Fe-floc was composed of 71% Fe, 17.5% Si, 4.1% Mg, 5.3% P, and 2.2% Ca, while the Tangaroa Fe-floc consisted of 88.2% Fe, 7.1% Si, 2.4% Mg, 1.5% P, and 0.73% Ca. These percentages are in general agreement with EDX analyses of the Fe-oxide microtubes produced by the betaproteobacterium Leptothrix ochracea (Fe/Si/P ratio of 80:15:5) (17).
Molecular analysis.
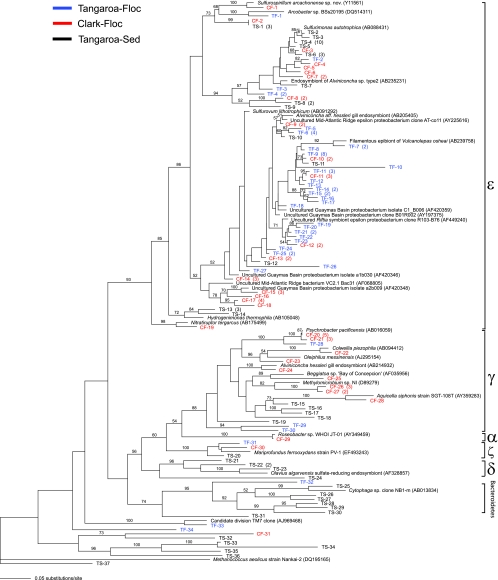
A total of 161 near-complete 16S rRNA gene sequences were analyzed from the three clone libraries (52 clones from the Tangaroa Fe-floc, 55 clones from the Clark Fe-floc, and 54 clones from the Tangaroa sediment). Epsilonproteobacterial clones dominated the libraries, with 45 clones from the Tangaroa Fe-floc, 34 from the Clark Fe-floc, and 30 from the Tangaroa sediment. These epsilonproteobacteria accounted for 67.3% of the total library (Fig. 3). The gammaproteobacteria were the next largest group represented in the clone libraries. The Clark Fe-floc contained 18 gammaproteobacterial clones, the Tangaroa Fe-floc contained 1 clone, and the Tangaroa sediment contained 5 clones (Fig. 3). Three clones fell into the proposed zetaproteobacteria, sharing 92 to 94% sequence similarity with M. ferrooxydans. One clone from each of the floc and sediment samples was recovered. The majority of the Bacteriodetes recovered were from the Tangaroa sediment sample (six clones), with only one clone detected from the Tangaroa Fe-floc and none from the Clark Fe-floc. Four Tangaroa sediment clones were affiliated with the deltaproteobacteria and one Clark Fe-floc clone was related to the alphaproteobacteria (Fig. 3). The remaining clones did not cluster into readily identifiable categories or were most closely related to candidate divisions, such as TM7, OP1, and OP11. No betaproteobacterial clones were found, although this group has been historically associated with the capacity for iron oxidation and the formation of similar Fe-oxide-rich, sheath-like structures.
FIG. 3.
Maximum likelihood phylogenetic tree of Fe-floc and sediment clone libraries. The numbers in parentheses for clones indicate the total number of clones with >99% sequence similarity. Bar, 5 nucleotide substitutions per 100 bp. Bootstrap values greater than 50% are shown above the appropriate branches.
Although significant overlap was present in the clone libraries, several lineages appeared to be site specific. For example, clones related to Sulfurimonas autotrophica and Cytophaga spp. were only recovered from the sediment samples, while clones related to Psychrobacter pacificensis were only detected in the Clark Fe-floc clone library. A large cluster of epsilonproteobacterial clones from the Fe-floc samples had no related cultured representatives. Unsurprisingly, a number of the clones were related to sequences from hydrothermal systems, deep sea sediments, and symbionts of vent-associated macrofauna (Fig. 3).
Richness estimates.
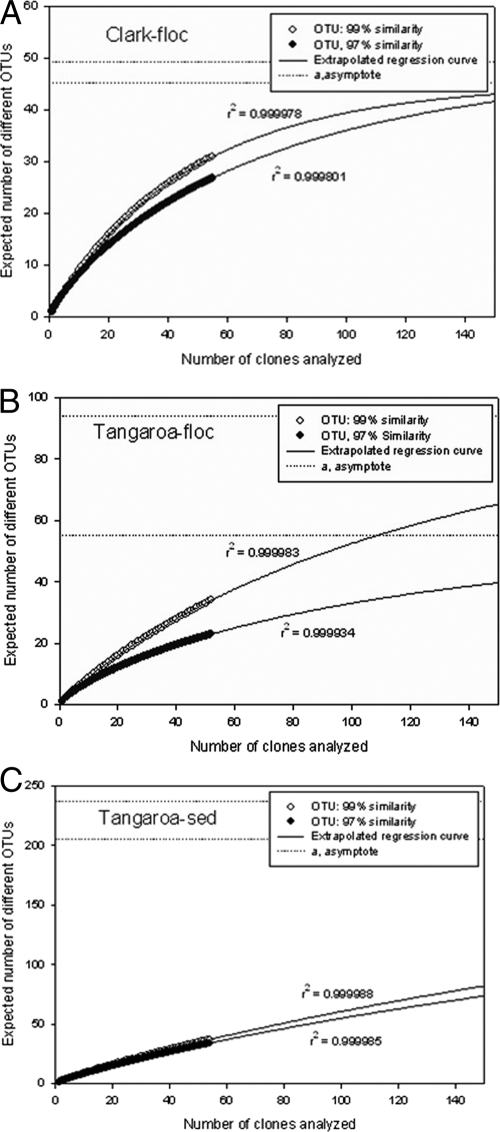
For this study, operational taxonomic units (OTUs) were defined as sequences with either 99% or 97% similarity. Using these definitions, the total number of OTUs represented in the clone libraries was determined. The three libraries contained 31 to 37 OTUs using the 99% similarity definition and dropped to 23 to 34 OTUs when the 97% similarity definition was employed (Table 1). Rarefaction analyses (Fig. 4) were used to evaluate the adequacy of sampling for each library, and the nonparametric abundance estimators chao 1 and ACE were used to predict total richness. Estimates of total richness varied depending upon the method of analysis and the definition of OTU used, although the trends were consistent between methods (Table 2). In all cases, the sediment sample showed the highest richness and the least sampling coverage.
TABLE 1.
Bacterial diversity estimates and clone library coverage of Fe-floc and sediment clone libraries
| Site (no. of clones) | % OTU definition | No. of OTUs | Rarefaction regression (% coverage)a
|
||
|---|---|---|---|---|---|
| Asymptote | chao 1 | ACE | |||
| Tangaroa-Floc | >99 | 34 | 94 (36) | 77 (44) | 120 (28) |
| (52) | >97 | 23 | 55 (42) | 49 (47) | 54 (43) |
| Clark-Floc (55) | >99 | 31 | 45 (69) | 48 (65) | 48 (65) |
| >97 | 27 | 49 (55) | 61 (44) | 59 (46) | |
| Tangaroa-Sed (54) | >99 | 37 | 237 (16) | 192 (19) | 260 (14) |
| >97 | 34 | 206 (17) | 160 (21) | 151 (23) | |
The asymptote (of the regression equation), chao 1, and ACE columns indicate the projected diversity as the number of OTUs expected.
FIG. 4.
Rarefaction curves showing total diversity predicted from clone libraries. Open circles represent an OTU definition of 99% sequence similarity. Black circles represent an OTU definition of 97% sequence similarity. Dotted lines indicate the asymptotes of extrapolated regression curves (solid lines) and therefore the level of predicted total diversity. (A) Clark Fe-floc sample; (B) Tangaroa Fe-floc sample; (C) Tangaroa sediment sample.
TABLE 2.
Statistical analyses of Fe-floc and sediment clone libraries
| Comparison | ANOSIM
|
LIBSHUFF
|
||
|---|---|---|---|---|
| R statistic | P value | ΔCXY, ΔCYX | P value | |
| Tangaroa-floc vs Clark-floc | 0.172 | 0.001 | 0.311, 1.938 | 0.001 |
| Tangaroa-floc vs Tangaroa-sediment | 0.34 | 0.001 | 0.723, 2.097 | 0.001 |
| Clark-floc vs Tangaroa-sediment | 0.079 | 0.006 | 2.418, 1.038 | 0.001 |
OTU distribution.
Using a 99% sequence similarity definition for OTUs, the dominant groups were examined for each sample. The most populous OTU in the Tangaroa Fe-floc sample contained eight clones which had no related cultured representative but were most closely related to uncultured proteobacterial clone B01R002 from a Guaymas Basin sediment sample (95%) (8). Additional OTUs contained four clones (1 OTU), three clones (1 OTU), and two clones (6 OTUs), with all remaining OTUs containing single sequences (25 OTUs). The dominant OTU in the Clark Fe-floc sample contained five clones that were most closely related to Psychrobacter pacificensis (99%) (26). Other Clark Fe-floc OTUs contained four clones (1 OTU), three clones (5 OTUs), and two clones (7 OTUs), with all remaining OTUs containing singlets (17 OTUs). Ten clones were in the dominant OTU from the Tangaroa sediment clone library. This group was most closely related to Sulfurimonas autotrophica (98%) (19). Three OTUs contained three clones, 3 OTUs contained two clones, and all remaining OTUs were composed of singlet sequences (30 OTUs).
Statistical analysis.
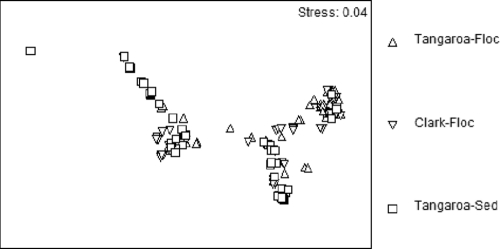
Nonmetric MDS analysis of clone library similarity matrices formed two distinct clusters. The clusters were independent of sample site, with representatives from each sample present in each cluster (Fig. 5). ANOSIM indicated that all clone libraries were significantly different (P < 0.05 for all sites) with the lowest R value found between the Clark Fe-floc and the Tangaroa sediment clone libraries (Table 2). R values closer to 1 indicate a greater difference within the samples, while R values closer to 0 indicate more similarity (3). LIBSHUFF comparison of clone libraries also indicated significant differences between each library (P < 0.05 for all sites) (Table 2).
FIG. 5.
Nonmetric MDS plot of clones. This plot was generated using a Bray-Curtis similarity matrix with a square root transformation of the clone library distance matrix.
T-RFLP analysis.
A total of 133 and 172 terminal restriction fragments (TRFs) were identified using HinfI and HaeIII, respectively, from the five Fe-floc samples and two sediment samples analyzed. An average of 19 HinfI and 25 HaeIII TRFs were detected per sample. Using HinfI, the Clark sediment sample demonstrated the lowest number of peaks (12), while the greatest number of peaks (29) was observed in one of the Clark floc samples. For HaeIII digests, one of the three Tangaroa floc samples had the lowest number of peaks (11), while the greatest number of peaks (37) was observed in the second Clark floc sample.
An artificial digest of a portion of the Mariprofundus ferrooxydans small subunit rRNA gene using HinfI yielded a 218-bp fragment. Corresponding peaks were observed in two of the three Tangaroa Fe-floc samples, one of the two Clark Fe-floc samples, and neither of the sediment samples. Artificial digests of the three clones closely related to Mariprofundus ferrooxydans with HinfI yielded fragments of ∼192 bp. Corresponding peaks for these presumed zetaproteobacterial clones were identified in all but two of the samples. Artificial digests with HaeIII yielded peaks corresponding to Mariprofundus ferrooxydans (255 bp) in four of the seven samples. Peaks corresponding to the zetaproteobacteria detected in our clone libraries (221 bp) were also present in four of the seven samples.
Several peaks were found in all of the samples (HaeIII peaks at 192 and 403 bp; a HinfI peak at 300 bp). Conversely, a number of peaks were only detected in Fe-floc samples. For the HinfI digest, peaks at 218, 221, 223, and 298 bp were present in all but two of the Fe-floc samples and neither of the sediment samples. The HaeIII digest showed peaks at 105 and 417 bp that were present in all but one of the Fe-floc samples and neither sediment sample. The HaeIII peak at 202 bp was only present in Clark Fe-floc samples, while the peak at 323 bp was only found in the three Clark samples (two floc samples and one sediment sample).
MDS analysis of similarity matrices based on either binary (presence or absence of peaks) or normalized peak area showed clustering of four out of the five Fe-floc samples relative to that of the sediment samples. One of the Tangaroa floc samples grouped more closely with the sediment samples than with the other Fe-floc samples. Both water samples clustered separately from all other samples. ANOSIM comparison of sediment versus Fe-floc yielded an R value of 0.073, while comparison of the Fe-floc samples from the two seamounts was 0.083; however, neither was supported by statistically significant P values (0.38 and 0.4, respectively). Analysis of the water samples showed that they were distinct from both sediment and Fe-floc samples (floc-water, R = 0.95 and P = 0.05; sediment water, R = 1 and P = 0.33).
DISCUSSION
The hollow Fe-oxide tubes and filaments that were visualized in this study are consistent with the findings of other research groups that have examined similar flocculent material. Samples of flocculent mat material with Fe-oxide filaments have been obtained from Loihi Seamount, Hawaii; Franklin Seamount, Papua New Guinea; Juan de Fuca Ridge, northeast Pacific Ocean; Vailulu'u Seamount, Samoa; and recently from cold seeps located at the Chefren Mud Volcano (Nile Deep Sea Fan, eastern Mediterranean) (1, 20, 22, 27, 34). This study adds evidence for the occurrence of these biogenic Fe-oxides at locations with hydrothermal venting. Although the chemical composition of the sheaths and filaments was similar to what was reported for the microtubes produced by the betaproteobacterium Leptothrix ochracea (17), no betaproteobacteria were recovered in the clone libraries. This suggests that other FeOB may produce Fe-oxide structures with similar chemical composition.
Phylogenetic analysis revealed that the Fe-flocculent mats occurring at Tangaroa and Clark Seamounts were not dominated by known FeOB. Mariprofundus ferrooxydans was the only characterized FeOB that was related to our clones. This organism has been reported to be common within Fe-floc communities associated with Loihi Seamount, Hawaii (12), and the Mariana Island arc/back arc (5). Under laboratory conditions, M. ferrooxydans forms helical stalks upon oxidation of iron (12); however, sheaths and filaments within the mat material obtained from Loihi Seamount shared similar morphologies to those obtained in our studies (13), suggesting the presence of species of FeOB other than M. ferrooxydans. Each of the three clone libraries in the present study contained one clone that was most closely related to M. ferrooxydans. The Tangaroa-floc, Clark-floc, and Tangaroa sediment clones shared 94%, 93%, and 92% sequence similarity to M. ferrooxydans, respectively. Using at least one of the restriction enzymes, TRFs corresponding to those generated by artificial digests of M. ferrooxydans or related zetaproteobacteria were consistently found in both the Fe-oxide mat and sediment samples. The presence of these peaks suggests that the zetaproteobacteria may be common inhabitants of hydrothermal environments around the world. When a suitable source of iron is present, they may flourish, forming extensive Fe-floc mats. Once the iron source has been utilized or has dissipated, the iron filaments could remain while the majority of FeOB die off. Alternatively, M. ferrooxydans-related strains may be minor inhabitants at all the sites but are not responsible for formation of the Fe-floc material. The large group of epsilonproteobacteria without a cultured representative could harbor the organisms responsible for the iron sheath and filament formation. Supporting this theory, this group was largely dominated by OTUs from the Tangaroa and Clark Fe-floc samples. Cultivation experiments would be needed to determine if there are novel FeOB present in this group of epsilonproteobacteria.
The greater species richness predicted to be in the sediment sample, as indicated by the rarefaction curves and richness estimators, suggests that the Fe-flocculent mats may create a specialized niche that is less able to support diverse bacterial populations. This could be due to several factors, including the range of available energy sources present in the sediment versus the flocculent mat samples or the suitability of the materials as a substrate for colonization. Alternately, FeOB may outcompete other bacteria during the formation of the Fe-floc material, and further colonization by other bacteria after sheath and filament formation may be hindered by their presence. Additional studies are necessary to determine nutrient availability and colonization patterns at these sites. Interestingly, the TRF pattern for the samples showed the Clark floc material to be the most diverse (based on total number of peaks), rather than the sediment samples. However, it is difficult to draw definitive conclusions from these data, because related organisms will often provide a single peak rather than multiple peaks and the peaks for rare species may be below the arbitrarily determined level of detection.
The application of statistical methods to phylogenetic data has enhanced ecological evaluation of microbial communities. ANOSIM and LIBSHUFF analyses are valuable methods for determining statistically significant differences between sets of 16S rRNA gene sequence data (4, 24, 32). Although phylogenetic analyses of the clone libraries showed that distinct groups were present in the Fe-floc samples, both methods of statistical analysis found the two floc samples to be different. The finding that the Fe-floc clone libraries were not only statistically different from the sediment but also from each other suggests that the community composition within the mats varies. This was supported by the detection of site-specific lineages in the clone libraries as well as the observation of several site-specific TRFs in the T-RFLP profiles. Other peaks in the T-RFLP profiles appeared to be specific to the floc samples. Although MDS analysis of the TRFLP profiles demonstrated clustering of four out of the five Fe-floc samples analyzed, ANOSIM analyses of the samples were not statistically significant. This is most likely due to the low sample number available for statistical analyses but should not be entirely discounted.
There are a number of factors that could affect the bacterial community composition within the Fe-floc samples. The age of the Fe-floc material must be considered, as it has been shown that these mats can form rapidly and remain indefinitely (13, 20). Alternately, the composition of bacterial communities on iron precipitates has been shown to change with depth of the mat material (15). Venting characteristics and underlying substrate could also influence the composition of the bacterial communities present within the Fe-flocs (9, 10, 25). Although fluid samples were not taken in the immediate vicinity of the Fe-floc material, general vent fluid chemistry for the two arc volcanoes varies. Venting at Tangaroa averaged 30°C, while some vents at Clark discharged fluids near 200°C. The Tangaroa vents had higher hydrogen sulfide levels (∼1,000 μmol/kg) compared to the Clark vents (∼250 μmol/kg), while the pH of vents at both seamounts was around 5, lower than the optimal pH growth range of 6.2 to 6.5 for M. ferrooxydans (12). Interestingly, neither seamount demonstrated high iron concentrations, with Tangaroa vents averaging 0.006 mM/kg and Clark vents averaging 0.015 mM/kg (G. Massoth and D. Butterfield, personal communication). Surprisingly, these iron concentrations are approximately 2 orders of magnitude lower than levels reported at Loihi Seamount (∼1 mmol/kg) (31), where similar Fe-floc formations are common and are characterized by the presence of M. ferrooxydans (13, 22). The low concentration of iron in the vent fluids from Tangaroa and Clark Seamounts suggests our observations of widespread iron-rich filamentous material at these sites may be a remnant of earlier, more robust venting or the result of biogeochemical inputs from fluids moving through the underlying sediment (as postulated in reference 27).
In summary, results from analyses of clone libraries and T-RFLP profiles indicate that while bacterial communities vary both between Fe-floc mats and sediment samples, there do appear to be OTUs that are specific to the Fe-floc mats. Our results also indicate that presumptive zetaproteobacteria, although not dominant members of the community, do occur at these sites and may be globally important constituents of hydrothermal environments. The presence of the large group of epsilonproteobacteria within our clone libraries that have no cultured representative suggests that these microbes may be important inhabitants of this Fe-floc niche, either as novel FeOB or as inhabitants of the environment after filament formation by FeOB. To date, all characterized hydrothermal vent-associated epsilonproteobacteria either oxidize reduced sulfur or reduce sulfur while oxidizing hydrogen or formate (2). As iron is the fourth most abundant element in the Earth's crust, gaining an understanding of its transformations and role in supporting lithotrophic bacterial communities is important for advancing our knowledge of deep sea benthic biodiversity.
Acknowledgments
We thank the crews of the KOK and Pisces V for collection assistance, the New Zealand government for permits, and Marc Slattery and Deborah Gochfeld as well as our colleagues from New Zealand for field assistance. We also thank Gary Massoth and Dave Butterfield (UW/NOAA PMEL) for sharing their vent chemistry data and Michael Bersch for assistance with scanning electron microscope/EDX analysis.
This work was supported by funding from the National Institute for Undersea Science and Technology, grant number NA16RU1496.
Footnotes
Published ahead of print on 29 December 2008.
REFERENCES
- 1.Boyd, T., and S. Scott. 2001. Microbial and hydrothermal aspects of ferric oxyhydroxides and ferrosic hydroxides: the example of Franklin Seamount, Western Woodlark Basin, Papua New Guinea. Geochem. Trans. 2:45-56. [Google Scholar]
- 2.Campbell, B. J., A. S. Engel, M. L. Porter, and K. Takai. 2006. The versatile ɛ-proteobacteria: key players in sulphidic habitats. Nat. Rev. Microbiol. 4:458-468. [DOI] [PubMed] [Google Scholar]
- 3.Clarke, K. R., and R. M. Warwick. 2001. Change in marine communities: an approach to statistical analysis and interpretation, 2nd ed., p. 6-5. PRIMER-E, Plymouth Marine Laboratory, United Kingdom.
- 4.Crump, B. C., and J. E. Hobbie. 2005. Synchrony and seasonality in bacterioplankton communities of two temperate rivers. Limnol. Oceanogr. 50:1718-1729. [Google Scholar]
- 5.Davis, R. E., and C. L. Moyer. 2008. Extreme spatial and temporal variability of hydrothermal microbial mat communities along the Mariana Island Arc and southern Mariana back-arc system. J. Geophys. Res. doi: 10.1029/2007JB005413. [DOI]
- 6.de Ronde, C. E. J., E. T. Baker, G. J. Massoth, J. E. Lupton, I. C. Wright, R. A. Feely, and R. R. Greene. 2001. Intra-oceanic subduction-related hydrothermal venting, Kermadec volcanic arc, New Zealand. Earth Planet. Sci. Lett. 193:359-369. [Google Scholar]
- 7.de Ronde, C. E. J., K. Faure, C. J. Bray, D. A. Chappell, and I. C. Wright. 2003. Hydrothermal fluids associated with seafloor mineralization at two southern Kermadec arc volcanoes, offshore New Zealand. Miner. Deposita 38:217-233. [Google Scholar]
- 8.Dhillon, A., A. Teske, J. Dillon, D. A. Stahl, and M. L. Sogin. 2003. Molecular characterization of sulfate-reducing bacteria in the Guaymas Basin. Appl. Environ. Microbiol. 69:2765-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards, K. J., W. Bach, T. M. McCollom, and D. R. Rogers. 2004. Neutrophilic iron-oxidizing bacteria in the ocean: their habitats, diversity, and roles in mineral deposition, rock alteration, and biomass production in the deep-sea. Geomicrobiol. J. 21:393-404. [Google Scholar]
- 10.Edwards, K. J., T. M. McCollom, H. Konishi, and P. R. Buseck. 2003. Seafloor bioalteration of sulfide minerals: results from in situ incubation studies. Geochim. Cosmochim. Acta 67:2843-2856. [Google Scholar]
- 11.Edwards, K. J., D. R. Rogers, C. O. Wirsen, and T. M. McCollom. 2003. Isolation and characterization of novel psychrophilic, neutrophilic, Fe-oxidizing, chemolithoautotrophic alpha- and gammaproteobacteria from the deep sea. Appl. Environ. Microbiol. 69:2906-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emerson, D., J. A. Rentz, T. G. Lilburn, R. E. Davis, H. Aldrich, C. Chan, and C. L. Moyer. 2007. A novel lineage of proteobacteria involved in formation of marine Fe-oxidizing microbial mat communities. PLoS ONE 2:e667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emerson, D., and C. L. Moyer. 2002. Neutrophilic Fe-oxidizing bacteria are abundant at the Loihi Seamount hydrothermal vents and play a major role in Fe oxide deposition. Appl. Environ. Microbiol. 68:3085-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emerson, D., and C. Moyer. 1997. Isolation and characterization of novel iron-oxidizing bacteria that grow at circumneutral pH. Appl. Environ. Microbiol. 63:4784-4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emerson, D., and N. P. Revsbech. 1994. Investigation of an iron-oxidizing microbial mat community located near Aarhus, Denmark: field studies. Appl. Environ. Microbiol. 60:4022-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fortin, D., F. G. Ferris, and S. D. Scott. 1998. Formation of Fe-silicates and Fe-oxides on bacterial surfaces in samples collected near hydrothermal vents on the Southern Explorer Ridge in the Northeast Pacific Ocean. Am. Mineral. 83:1399-1408. [Google Scholar]
- 17.Hashimoto, H., S. Yokoyama, H. Asaoka, Y. Kusano, Y. Ikeda, M. Seno, J. Takada, T. Fujii, M. Nakanishi, and R. Murakami. 2007. Characteristics of hollow microtubes consisting of amorphous iron oxide nanoparticles produced by iron oxidizing bacteria, Leptothrix ochracea. J. Magn. Magn. Mater. 310:2405-2407. [Google Scholar]
- 18.Hofmann, B. A., and J. D. Farmer. 2000. Filamentous fabrics in low-temperature mineral assemblages: are they fossil biomarkers? Implications for the search for a subsurface fossil record on the early Earth and Mars. Planet. Space Sci. 48:1077-1086. [Google Scholar]
- 19.Inagaki, F., K. Takai, H. Kobayashi, K. H. Nealson, and K. Horikoshi. 2003. Sulfurimonas autotrophica gen. nov., sp. nov., a novel sulfur-oxidizing epsilon-proteobacterium isolated from hydrothermal sediments in the Mid-Okinawa Trough. Int. J. Syst. Evol. Microbiol. 53:1801-1805. [DOI] [PubMed] [Google Scholar]
- 20.Juniper, S. K., P. Martineu, J. Sarrazin, and Y. Gelinas. 1995. Microbial-mineral floc associated with nascent hydrothermal activity on Co-Axial Segment, Juan de Fuca Ridge. Geophys. Res. Lett. 22:179-182. [Google Scholar]
- 21.Karl, D. M., A. M. Brittain, and B. D. Tilbrook. 1989. Hydrothermal and microbial processes at Loihi Seamount, a mid-plate hot-spot volcano. Deep-Sea Res. 36:1655-1673. [Google Scholar]
- 22.Karl, D. M., G. M. McMurtry, A. Malahoff, and M. O. Garcia. 1988. Loihi Seamount, Hawaii: a mid-plate volcano with a distinctive hydrothermal system. Nature 335:532-535. [Google Scholar]
- 23.Kennedy, C. B., S. D. Scott, and F. G. Ferris. 2003. Ultrastructure and potential sub-seafloor evidence of bacteriogenic iron oxides from axial volcano, Juan de Fuca Ridge, North-east Pacific Ocean. FEMS Microbiol. Ecol. 43:247-254. [DOI] [PubMed] [Google Scholar]
- 24.Klaus, J. S., I. Janse, J. M. Heikoop, R. A. Sanford, and B. W. Fouke. 2007. Coral microbial communities, zooxanthellae and mucus along gradients of seawater depth and coastal pollution. Environ. Microbiol. 9:1291-1305. [DOI] [PubMed] [Google Scholar]
- 25.Luther, G. W., III, T. F. Rozan, M. Taillefert, D. B. Nuzzio, C. Di Meo, T. M. Shank, R. A. Lutz, and S. C. Cary. 2001. Chemical speciation drives hydrothermal vent ecology. Nature 410:813-816. [DOI] [PubMed] [Google Scholar]
- 26.Maruyama, A., D. Honda, H. Yamamoto, K. Kitamura, and T. Higashihara. 2000. Phylogenetic analysis of psychrophilic bacteria isolated from the Japan Trench, including a description of the deep-sea species Psychrobacter pacificensis sp. nov. Int. J. Syst. Evol. Microbiol. 50:835-846. [DOI] [PubMed] [Google Scholar]
- 27.Omoregie, E. O., V. Mastalerz, G. de Lange, K. L. Straub, A. Kappler, H. Roy, A. Stadnitskaia, J.-P. Foucher, and A. Boetius. 2008. Biogeochemistry and community composition of iron- and sulfur-precipitating microbial mats at the Chefren Mud Volcano (Nile Deep Sea Fan, eastern Mediterranean). Appl. Environ. Microbiol. 74:3198-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osborne, C. A., G. N. Rees, Y. Bernstein, and P. H. Janssen. 2006. New threshold and confidence estimates for terminal restriction fragment length polymorphism analysis of complex bacterial communities. Appl. Environ. Microbiol. 72:1270-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perfit, M. R., J. R. Cann, D. J. Fornari, J. Engels, D. K. Smith, W. I. Ridley, and M. H. Edwards. 2003. Interaction of sea water and lava during submarine eruptions at mid-ocean ridges. Nature 426:62-65. [DOI] [PubMed] [Google Scholar]
- 30.Schloss, P. D., and J. Handelsman. 2005. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl. Environ. Microbiol. 71:1501-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sedwick, P. N., G. M. McMurtry, and J. D. Macdougall. 1992. Chemistry of hydrothermal solutions from Pele's Vents, Loihi Seamount, Hawaii. Geochim. Cosmochim. Acta 56:3643-3667. [Google Scholar]
- 32.Singleton, D. R., M. A. Furlong, S. L. Rathbun, and W. B. Whitman. 2001. Quantitative comparisons of 16S rRNA gene sequence libraries from environmental samples. Appl. Environ. Microbiol. 67:4374-4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith, C. J., B. S. Danilowicz, A. K. Clear, F. J. Costello, B. Wilson, and W. G. Meijer. 2005. T-Align, a web-based tool for comparison of multiple terminal restriction fragment length polymorphism profiles. FEMS Microbiol. Ecol. 54:375-380. [DOI] [PubMed] [Google Scholar]
- 34.Staudigel, H., S. R. Hart, A. Pile, B. E. Bailey, E. T. Baker, S. Brooke, D. P. Connelly, L. Haucke, C. R. German, I. Hudson, D. Jones, A. A. Koppers, J. Konter, R. Lee, T. W. Pietsch, B. M. Tebo, A. S. Templeton, R. Zierenberg, and C. M. Young. 2006. Vailulu'u Seamount, Samoa: life and death on an active submarine volcano. Proc. Natl. Acad. Sci. USA 103:6448-6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swofford, D. L. 2003. PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4. Sinauer Associates, Sunderland, MA.
- 36.Ueshima, M., and K. Tazaki. 2001. Possible role of microbial polysaccharides in nontronite formation. Clays Clay Miner. 49:292-299. [Google Scholar]
- 37.Zwickl, D. J. 2006. Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. Ph.D. dissertation. The University of Texas at Austin.