Abstract
The Sac10b protein family, also known as Alba, is widely distributed in Archaea. Sac10b homologs in thermophilic Sulfolobus species are very abundant. They bind both DNA and RNA with high affinity and without sequence specificity, and their physiological functions are still not fully understood. Mma10b from the euryarchaeote Methanococcus maripaludis is a mesophilic member of the Sac10b family. Mma10b is not abundant and constitutes only ∼0.01% of the total cellular protein. Disruption of mma10b resulted in poor growth of the mutant in minimal medium at near the optimal growth temperature but had no detectable effect on growth in rich medium. Quantitative proteomics, real time reverse transcription-PCR, and enzyme assays revealed that the expression levels of some genes involved in CO2 assimilation and other activities were changed in the Δmma10b mutant. Chromatin immunoprecipitation suggested a direct association of Mma10b with an 18-bp DNA binding motif in vivo. Electrophoretic mobility shift assays and DNase I footprinting confirmed that Mma10b preferentially binds specific sequences of DNA with an apparent Kd in the 100 nM range. These results suggested that the physiological role of Mma10b in the mesophilic methanococci is greatly diverged from that of homologs in thermophiles.
Proteins of the Sac10b protein family (also called Alba) are proposed to be a major type of chromosome-associated proteins in Archaea (reviewed in reference 33). Sac10b homologs have been identified in all archaea whose genomes have been sequenced except for the mesophilic euryarchaeotes belonging to Halobacteria and Methanosarcinales (10). The proteins from the hyperthermophilic archaea of the genus Sulfolobus have been extensively studied, but their physiological functions are not well established.
Sac10b homologs show high affinities for DNA and bind cooperatively to DNA without apparent sequence specificity (25, 44). The proteins are able to constrain DNA into negative supercoils, and this ability is dramatically enhanced at temperatures higher than 45°C (50). Therefore, it is possible that the proteins affect DNA compaction at temperatures optimal for the growth of the hyperthermophiles. Given their DNA binding properties and abundance in thermophiles, these proteins have been proposed to play a role in DNA protection and stability at high temperature (19, 50). Native Sso10b from Sulfolobus solfataricus is acetylated on Lys16, and acetylation decreases the affinity of the protein for DNA (4). Thus, the Sac10b protein family is also referred to as Alba, which stands for “acetylation lowers binding affinity.” The deacetylase Sir2 associates with Sso10b in vivo (4), and the transacetylase Pat acetylates Sso10b specifically on Lys16 in vitro (27). The presence of a reversible Sso10b acetylation system suggests a histone-like reversible modification of Sac10b homologs, which could be important for controlling DNA availability for replication, transcription, and recombination. A role of Sac10b homologs in transcriptional regulation is also supported by the observation that deacetylation of Sso10b by Sir2 mediated transcriptional repression in an in vitro transcription system (4). The Sir2/Pat system is absent in many euryarchaeotes, including methanococci. However, other acetyltransferases, e.g., Elp3 homologs, could be functional in the modification of Sac10b homologs and the regulation of DNA compaction (45).
Although originally identified as DNA binding proteins, the Sac10b family proteins also bind RNA. The overall structure of Sso10b resembles the RNA binding IF3-C fold (44). Moreover, phylogenetic analysis indicates that the Sac10b family is related to two eukaryotic protein families involved in RNA binding (2). Ssh10b from Sulfolobus shibatae binds both DNA and RNA in vitro, and RNAs are bound in vivo (12). In addition, both DNase and RNase release Sso10b from cellular nucleic acid-protein complexes, suggesting that this protein is associated with both DNA and RNA in vivo (27).
Although genes encoding Sac10b homologs have been identified in mesophiles, none of these proteins has been biochemically characterized. Therefore, it is of interest to compare these homologs to their counterparts from the hyperthermophiles, especially in view of the suggestion that the latter may be involved in thermal adaptation. Methanococci are hydrogenotrophic methanogens belonging to the phylum Euryarchaeota and are classified into genera depending in part upon their growth temperatures. The genera Methanococcus, Methanothermococcus, and Methanocaldococcus have optimal growth temperatures of 35 to 40°C, 60 to 65°C, and 68 to 85°C, respectively (46). The presence of Sac10b homologs in all these organisms permits a comparison of properties of the Sac10b homologs at different growth temperatures. In addition, the readily available genetic systems in mesophilic methanococci are of value for the investigation of the in vivo role of the Sac10b family. Heinicke et al. (14) constructed a mutant of the mesophilic, heterotrophic Methanococcus voltae with a deletion of the gene encoding a Sac10b homolog. While the mutation did not affect growth in complex medium, two-dimensional gel electrophoresis of the cellular proteins identified changes in the expression of genes encoding a helix-turn-helix DNA binding protein, a cystathione β-synthase domain-containing protein, and pyruvate oxidoreductase (Por) subunit C (14).
In this study, Mma10b from Methanococcus maripaludis was characterized to elucidate the physiological properties of Sac10b homologs in mesophiles. The expression level of Mma10b, the Sac10b homolog from M. maripaludis, was the lowest among the Sac10b homologs from the extreme thermophile Methanothermococcus thermolithotrophicus and the hyperthermophile Methanocaldococcus jannaschii. Disruption of the gene encoding Mma10b in M. maripaludis limited autotrophic growth and resulted in changes of the expression of several genes. A chromatin immunoprecipitation (ChIP) assay detected specific associations of Mma10b with the coding regions of genes in vivo. The association of Mma10b with DNA was sequence dependent in vitro. These results suggested that the role of Mma10b differs greatly from that of homologs in thermophiles.
MATERIALS AND METHODS
Strains, media and culture conditions.
The strains and plasmids used in this study are listed in Table 1. M. maripaludis was grown in 28-ml aluminum seal tubes with 275 kPa of H2-CO2 (80:20 [vol/vol]) at 37°C in 5 ml of McN (minimal medium), McNA (McN plus 10 mM sodium acetate), McNAla (McN plus 1 mM alanine), McNY (McN plus 0.2% [wt/vol] yeast extract), or McC (McNA plus 0.2% [wt/vol] Casamino Acids plus 0.2% [wt/vol] yeast extract) as described previously (47). Antibiotics were not included when comparing the growth of the wild type and mutants. The inocula were 0.1 ml of cultures grown in the same medium. The inocula for cultures of the mutant S590 and the strain S591 were started with frozen stocks for all experiments to ensure that a revertant at second loci had not been selected. Puromycin (2.5 μg/ml) was added when needed. The growth was determined by measuring the increase in absorbance at 600 nm. In general, the exponential growth of methanococci was observed only at low cell densities or below an absorbance of 0.4, and cells entered a linear growth phase at higher cell densities due to the low solubility of H2.
TABLE 1.
Archaeal strains and plasmids
| Strain or plasmid | Genotype or description | Source or reference |
|---|---|---|
| Methanococcus maripaludis strains | ||
| S2 | Wild type | 47 |
| S590 | Δmma10b::pac | This work |
| S591 | Δmma10b::pac/pMEV2-mma10b | This work |
| Methanocaldococcus jannaschii JAL-1 | Wild type | 20 |
| Methanothermococcus thermolithotrophicus SN1 | Wild type | 18 |
| Methanococcus voltae PS | Wild type | 3 |
| Plasmids | ||
| pIJA03 | Purr methanogen integration vector | 37 |
| pIJA03-mma10b | pIJA03 with the upstream and downstream regions of the mma10b gene (MMP1613) | This work |
| pMEV2 | Neomycin shuttle vector | 22 |
| pMEV2-mma10b | pMEV2 with mma10b gene | This work |
| pET11a-mja10b | M. jannaschii MJ0212 cloned into pET11a | This work |
| pET15b-mth10b | M. thermolithotrophicus EF565170 cloned into pET11b | This work |
| pET15b-mvo10b | M. voltae CAF74922 cloned into pET11b | This work |
| pET15b-mma10b | M. maripaludis MMP1613 cloned into pET11b | This work |
| pB9 | M. maripaludis genome fragment (position 1307469-1307986) cloned into pCR2.1-TOPO | This work |
Protein expression and purification.
The full-length genes for Sac10b homologs from M. jannaschii, M. thermolithotrophicus, M. voltae, and M. maripaludis were PCR amplified from genomic DNA (primer sequences are available upon request).
For the expression of Mja10b, the PCR product was digested with NdeI and BamHI and ligated into pET11a (Novagen), yielding the expression vector pET11a-mja10b. Escherichia coli BL21(DE3) cells were transformed with pET11a-mja10b and grown at 37°C in LB medium supplemented with 0.1 mg/ml ampicillin until the absorbance at 600 nm reached 0.8 to 1.0. The expression of Mja10b was induced for 3 to 4 h by adding isopropyl-1-thio-β-d-galactopyranoside (IPTG) to a final concentration of 1 mM. Cells were harvested by centrifugation, resuspended in buffer A (30 mM potassium phosphate [pH 6.6], 0.1 mM EDTA, and 1 mM dithiothreitol [DTT]), and lysed by sonication. The lysate was centrifuged at 150,000 × g for 30 min, and the supernatant was heated for 15 min at 75°C. After centrifugation, the supernatant was applied to a 1-ml Mono S column (Pharmacia). Proteins bound to the column were eluted with a KCl gradient from 0 to 1 M in buffer A. Fractions containing Mja10b were pooled and dialyzed against 10 mM potassium phosphate buffer (pH 7.0) and 10% (wt/vol) glycerol.
For the expression of Mth10b, Mma10b, and Mvo10b, the PCR products were digested with restriction enzymes and ligated into pET15b containing a His tag sequence (Novagen). Transformation and protein expression were carried out as described above for Mja10b. After harvesting, cells were resuspended in buffer B (20 mM potassium phosphate buffer [pH 7.4], 0.5 M NaCl, 1 mM DTT, and 10% [wt/vol] glycerol) and lysed by sonication. The lysates were centrifuged at 150,000 × g for 30 min, and the supernatants were applied to a 1-ml HisTrap affinity column (Pharmacia). Proteins bound to the column were eluted with an imidazole gradient from 0.05 to 0.5 M. Protein fractions containing the Sac10b homolog were pooled and dialyzed against 10 mM potassium phosphate buffer (pH 7.0) and 10% glycerol (wt/vol). After purification, the N-terminal His tag of the protein was cleaved using the Thrombin CleanCleave kit (Sigma).
The homogeneity of purified proteins was confirmed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (see Fig. S1 in the supplemental material).
Quantitative immunoblotting.
Mja10b, Mth10b, and Mma10b, which were purified to homogeneity, were injected into rabbits to generate polyclonal antiserum. For the Western blot of Mma10b, crude extracts (containing ∼400 μg protein) from M. maripaludis strains S2, S590, and S591 and recombinant Mma10b (25 to 200 ng) were loaded onto a 12% SDS-polyacrylamide gel. After electrophoresis, proteins were electrophoretically transferred onto a polyvinylidene difluoride (PVDF) membrane (Bio-Rad). The membrane was then incubated sequentially with rabbit anti-Mma10b antiserum and goat anti-rabbit immunoglobulin G (IgG) conjugated to horseradish peroxidase (Bio-Rad). The immunoblots were developed with SuperSignal West Femto Maximum Sensitivity substrate (Pierce) and exposed to CL-XPosure film (Pierce). For the Western blots of Mja10b and Mth10b, crude extract (containing 60 and 200 μg of protein) from M. jannaschii and M. thermolithotrophicus, respectively, was loaded onto a 12% SDS-polyacrylamide gel, electrophoretically transferred onto a PVDF membrane (Bio-Rad), incubated sequentially with rabbit antiserum and goat anti-rabbit IgG conjugated to alkaline phosphatase (Bio-Rad), and visualized with nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate (Promega).
Measurement of protein aggregation.
Aggregation of Sac10b homologs following heat treatment was monitored by 90° light scattering at 488 nm on a Shimadzu RF5301PC spectrofluorimeter. The proteins (0.1 mg/ml) were incubated at the indicated temperatures for various lengths of time in 10 mM potassium phosphate buffer (pH 7.0) and 10% (wt/vol) glycerol. The effect of salt on protein aggregation was determined by comparing incubations with no addition of salt and with 400 mM KCl.
Construction of the Δmma10b::pac mutant.
The Δmma10b::pac mutant was made by transformation of the wild-type M. maripaludis strain S2 with pIJA03-mma10b, which was constructed from the integration vector pIJA03. The plasmid pIJA03 lacks the origin of replication for methanococci and contains a pac cassette, which encodes puromycin resistance (11, 22). To construct pMma10b, a 937-bp region upstream and a 969-bp region downstream of the mma10b gene was PCR amplified and cloned into MCS1 and MCS2 of pIJA03, respectively. The orientation of each insert was confirmed by restriction mapping.
pIJA03-mma10b was transformed into M. maripaludis strain S2 by the polyethylene glycol method (42). After transformation, cultures were plated on McC medium plus puromycin. Puromycin-resistant isolates were restreaked on the same medium, and isolated colonies were then transferred to broth cultures containing 5 ml McC medium plus puromycin. After growth, 2 ml of the culture was used for determination of the genotype and phenotype. The remaining culture was used for preparation of frozen stocks (41).
The genotype of the Δmma10b::pac mutant (S590) was confirmed by Southern hybridization. Southern hybridizations were performed using the DIG High Prime DNA Labeling and Detection Starter Kit I (Roche, Mannheim, Germany). The DNA template of the probe was made by digestion of pIJA03-mma10b with KpnI and NheI. These sites were in the multiple-cloning sites that flanked the downstream PCR products. The 972-bp fragment was purified by agarose gel electrophoresis and gel extraction using the QIAquick gel extraction kit (Qiagen). The genomic DNA was digested with BglII and then hybridized with the probe. The hybridizations with S2 and S590 genomic DNAs generated expected products of ∼2,300 bp and ∼3,400 bp, respectively.
For complementation, the mma10b gene with the 100-bp upstream region was PCR amplified. The PCR products and the shuttle vector pMEV2 (22) were digested with XhoI and BglII and purified from the gel. The cloning of the PCR products into the digested pMEV2 replaced the strong promoter PhmvA with the native promoter of mma10b. The resulting plasmid, pMEV2-mma10b, was transformed into S590 and screened on McC plates containing neomycin as previously described (22). The complementation strain was named S591.
Enzyme assays.
The Por and CO dehydrogenase/acetyl coenzyme A (acetyl-CoA) synthase (CODH/ACS) activities were determined as described previously (23). M. maripaludis strains S2, S590, and S591 were grown in McN medium to an absorbance at 600 nm of about 0.4. The cells from 100-ml cultures were harvested anaerobically by centrifugation and resuspended in assay buffer containing 100 mM potassium Tricine (pH 8.6), 5 mM MgCl2, and 1 mM thiamine pyrophosphate. The cells were lysed by freezing at −20°C. Unbroken cells were removed by centrifugation at 10,000 × g for 10 min at room temperature. The protein concentrations were determined by Bradford protein assays (Bio-Rad). One unit of enzyme activity was defined as 1 μmol of methyl viologen reduced per minute.
Differential 14N-15N labeling of proteins.
To prepare differentially 14N-15N-labeled proteins, the wild-type strain S2 and the Δmma10b mutant S590 were grown in McNA medium containing either 4.6 mM unlabeled [14N]ammonium sulfate or 4.6 mM ≥99% [15N]ammonium sulfate in sidearm bottles. These bottles were manufactured from 160-ml serum bottles by fusing a 28-ml aluminum seal tube to the side. They could be pressurized to 138 kPa and contained 10 ml of culture. To prevent contamination with other nitrogen compounds, glassware was immersed in 1 M HCl overnight, rinsed with deionized water, and dried before use. Rubber stoppers were autoclaved for 20 min in 0.5 M NaOH and rinsed thoroughly with deionized water. Cultures were inoculated a day after addition of sulfide and ammonium sulfate to the medium. The inocula were 0.1 ml of cultures grown in the same medium. After inoculation, cultures were initially pressurized to 138 kPa with H2-CO2 gas (80:20 [vol/vol]) and repressurized twice daily. When S2 and S590 cultures reached an average absorbance at 600 nm of 0.54, four cultures of the unlabeled S2 and four cultures of 15N-labeled S590 were mixed together to generate sample A. The reverse-labeled cultures were mixed to generate sample B. Cells were harvested by centrifugation at 10,000 × g for 30 min at 4°C. The pellets were suspended in 0.5 ml of 100 mM ammonium bicarbonate buffer (pH 7.8), and 5 μl of 0.5 M phenylmethylsulfonyl fluoride was added to inhibit protease activity. The cells were lysed by freezing at −20°C. Upon thawing, 10 U of DNase I was added to the cell lysate, and the crude extracts were incubated at room temperature for 1 h. After DNA digestion, the crude extracts were centrifuged at 16,000 × g for 30 min to remove unbroken cells. Because of the carryover of 14NH4Cl from the inocula, the proteins from cultures grown with [15N]ammonium sulfate were ∼98% enriched with [15N].
Quantitative proteomics.
Protein digestion, fractionation, and mass spectrometry were performed as described previously (48). The accurate mass of each peptide was obtained from the m/z values of monoisotopic peaks. The number of nitrogen atoms in each peptide was determined from the mass separation between the monoisotopic peaks of the peptide and its 15N-enriched counterpart. The identification of peptides was automated using the software described previously (48). A measured mass peak was considered identified when it was within 10 ppm of the mass of a predicted tryptic peptide that had the same nitrogen content. A protein (open reading frame [ORF]) was considered identified when at least one unique peptide, i.e., predicted to be encoded in only one ORF, was identified.
Multiple peptide pairs from the same ORF were usually detected because more than one unique peptide was detected for many proteins, and most peptides were detected in multiple matrix-assisted laser desorption/ionization spots. The number of peptide pairs collected per ORF was designated n. The relative signal intensity (peak height) of 14N and 15N peptide peaks in each peak pair was used as a measure of relative peptide level. Multiple measurements of one protein were averaged to obtain the relative protein levels in S2 and S590. The average relative protein levels for all proteins were normalized to 1. Differential protein levels were regarded as significant if N was ≥2, the normalized relative protein levels were not between 0.7 and 1.5 (equivalent to >1.5-fold changes), and the 95% confidence interval for the normalized ratio did not include the value 1.00.
Real time RT-PCR.
To compare the levels of gene expression in the wild-type and mutant strains of M. maripaludis, total RNA was purified from 100 ml of cells, which had grown in McNA to an absorbance at 600 nm of about 0.4, using the Trizol LS reagent (Invitrogen). The purified RNA (20 μg) was incubated with RNase-free DNase (10 units) for 30 min at 37°C to remove residual DNA. Reverse transcription-PCR (RT-PCR) was performed with a mixture of 1 μg of template RNA, 200 U of Moloney murine leukemia virus reverse transcriptase (Promega), 1 μM of the reverse primer for a tested gene or the 16S rRNA gene, and 30 U of RNase inhibitor (Promega) in a total volume of 25 μl. After 1 h at 42°C, RNA was removed by incubation with 4 U of RNase H (Takara) for 30 min at 37°C. Real-time PCR was performed in a ABI Prism 7000 sequence detection system (Applied Biosystems) with a 25-μl mixture containing 12.5 μl of 2× Sybr green PCR master mix (Applied Biosystems), 200 μM each of the primers for the tested gene, and a dilution of the reverse transcription products. Cycling conditions included incubation at 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 30 s and 60°C for 1 min. The standard curves for quantification of the expression of the tested genes and the 16S rRNA gene were constructed with dilutions of PCR-amplified DNA fragments containing corresponding genes under the same conditions. Levels of expression of the tested genes were normalized against those of the 16S rRNA gene.
ChIP.
The ChIP assays were performed with a ChIP assay kit (USB Corporation) as described by the manufacturer with the following modifications. Cells of M. maripaludis strains S2 and S590 were grown in 100 ml of McNA medium to an optical density of 0.6 to 0.8. Cross-linking was performed by adding 2.7 ml of formaldehyde directly to the culture (1% final concentration) and incubating for 20 min at room temperature with gentle agitation. The cross-linking reaction was then quenched by adding glycine to a final concentration of 0.125 M. Cells were harvested and washed twice with chilled 1× phosphate-buffered saline. Cells were then resuspended in 3 ml of lysis buffer with addition of 1× protease inhibitor cocktail and passed twice through a French pressure cell at 100 MPa to break the cells and shear DNA into short fragments. The cell extracts were centrifuged at 20,000 × g for 10 min to remove debris, and then 700 μl of supernatant was incubated with 70 μl of preblocked protein A-agarose beads for 1 h at 4°C with gentle agitation. The mixture was centrifuged at 3,000 × g for 5 min, and the supernatant was incubated with 7 μl of antiserum overnight at 4°C with gentle agitation. Antibody was not added for the negative control reaction. The immune complexes were captured with 70 μl of preblocked protein A-agarose beads, washed, and eluted as described by the manufacturer. The immunoprecipitated DNA was purified with a DNA Clean & Concentrator kit (Zymo Research).
The purified DNA fragments were blunted with T4 DNA polymerase (New England Biolabs), ligated to linkers for PCR by blunt-end ligation, and PCR amplified as described previously (13). The amplified DNA fragments were cloned into pCR2.1-TOPO using a TOPO TA cloning kit (Invitrogen). A 96-clone library was generated for each sample. The sequencing of the insert DNA fragments was performed by the Sequencing and Synthesis Facility at the University of Georgia.
Prediction of protein binding sites.
The Mma10b binding site for DNA was predicted by the Gibbs Motif Sampler program (http://bayesweb.wadsworth.org/gibbs/gibbs.html) using DNA sequences of the fragments coprecipitated with Mma10b in the ChIP analysis. A diagram of the binding motif was created with the WebLogo program (http://weblogo.berkeley.edu/). The presence of a binding motif in a DNA fragment or the whole genome was predicted using the Motif Locator program with a score cutoff of 9.0 (http://www.cmbl.uga.edu/software/motloc.html).
Electrophoretic mobility shift assays (EMSA).
For gel shift assays in agarose gel, about 100 ng DNA was incubated with 0 to 15 μM of Mma10b for 30 min at 37°C in 20 mM Tris-HCl (pH 7.6), 1 mM DTT, 100 μg/ml bovine serum albumin (BSA), and 10% (wt/vol) glycerol in a total volume of 20 μl. DNA-protein complexes were resolved by electrophoresis in 0.7% or 1.2% agarose gels in 1× TAE buffer, which was comprised of 40 mM Tris HCl, 20 mM acetic acid, and 1 mM disodium EDTA. After electrophoresis, the gels were stained with ethidium bromide and visualized by UV.
EMSA in polyacrylamide gel was performed with a 58- or 60-bp double-stranded DNA (dsDNA) or a 64-nucleotide (nt) single-stranded RNA fragment. The 58-mer DNA corresponded to a sequence fragment in the wild-type M. maripaludis genome (positions 1307685 to 1307742) and contained the predicted Mma10b binding site. The sequence of one strand of the 60-mer DNA was 5′-GATCCCCCAATGCTTCGTTTCGTATCACACACCCCAAAGCCTTCTGCTTTGAATGCTGCC. The predicted Mma10b binding site was absent in this fragment. The sequence of the RNA fragment was 5′-GAAUACUCAAGCUUAUGCAUGCGGCCGCAUCUAGAGGGCCCGGAUCCCUCGAGGUCGACGAAUU. The DNA and RNA fragments were labeled at the 5′ end with [γ-32P]ATP using T4 polynucleotide kinase (Promega). The labeled 60-bp and 58-bp DNA fragments (20 to 30 nM) were incubated with Sac10b homologs (0 to 10 μM) at 22°C and 37°C, respectively, in 20 mM Tris-HCl (pH 7.6), 1 mM DTT, 100 μg/ml BSA, and 8% (wt/vol) glycerol in a total volume of 20 μl. The labeled RNA fragment was heated at 90°C for 1 min and chilled on ice, and then 20 to 30 nM of the RNA fragment was incubated with Sac10b homologs (0 to 10 μM) for 10 min at 22°C in 20 mM Tris-HCl (pH 7.6), 1 mM DTT, 100 μg/ml BSA, and 8% (wt/vol) glycerol in a total volume of 20 μl. DNA-protein or RNA-protein complexes were resolved by electrophoresis in 8% nondenaturing polyacrylamide gels. When KCl was added to the incubation mixtures, the electrophoresis was performed with 1× TBE buffer, which was comprised of 89 mM Tris, 89 mM boric acid, and 2 mM disodium EDTA. For incubations without added salts, the electrophoresis was performed in 0.1× TBE. After electrophoresis, the gels were visualized by exposure to X-ray films.
DNase I footprinting.
The DNase I footprinting method was modified from that described by Yindeeyoungyeon and Schell (51). DNA fragments were prepared by PCR using pB9 as the template and primer pair *B9F and B9R. The *B9F primer was labeled with 6-carboxyfluorescein (6-FAM) at the 5′ end. The PCR amplicon was an approximately 400-bp 6-FAM-labeled fragment and contained two predicted Mma10b binding sites. The PCR products were gel purified with a QuickClean DNA gel extraction kit (GenScript Corporation).
Approximately 450 ng of 6-FAM-labeled DNA (15 nM final concentration) and 9 μg of purified Mma10b (15 μM final concentration) were incubated in 20 mM Tris-HCl pH 7.6, 1 mM DTT, 100 μg/ml BSA, and 10% (wt/vol) glycerol in a total volume of 60 μl. The samples without addition of Mma10b were supplemented with BSA to yield the same total protein concentration. After incubation at 37°C for 1 h, samples were digested with 15 μl DNase I (0.01 U/μl final concentration), which was freshly prepared by diluting the 10 U/μl stock of RQ1 RNase-free DNase (Promega). The digestion was performed at room temperature for 4 min, and then the reaction was stopped by adding 15 μl stop solution (20 mM EGTA, pH 8.0). The digested DNA was purified with a DNA Clean & Concentrator kit (Zymo Research, Orange, CA, USA). The DNA fragments were analyzed on the ABI 3730 genetic analyzer (Applied Biosystems) at the Sequencing and Synthesis Facility at the University of Georgia.
RESULTS
Mma10b is expressed at low levels.
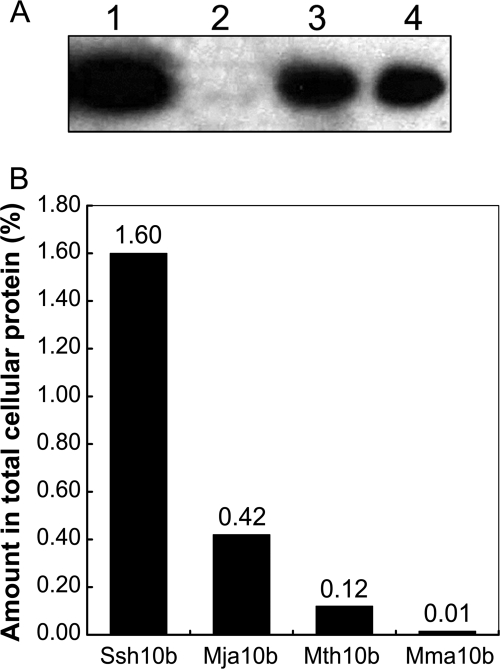
The protein levels of Sac10b homologs from Sulfolobus shibatae (Ssh10b), Methanocaldococcus jannaschii (Mja10b), Methanothermococcus thermolithotrophicus (Mth10b), and Methanococcus maripaludis (Mma10b) were compared by quantitative Western blotting. The protein level of Mma10b was very low. In two independent measurements, Mma10b constituted 0.009 to 0.014% of the total cellular protein (Fig. 1A). In a separate experiment, the cellular concentration of Mvo10b was found to be below the level of detection, or <0.01% of the total cellular protein (data not shown). In contrast, the Sac10b homologs were more abundant in thermophiles. Ssh10b, Mja10b, and Mth10b constituted 1.6%, 0.42%, and 0.12% of the total cellular protein, respectively (Fig. 1B). Thus, the protein levels of Sac10b homologs in methanococci were lower than that in Sulfolobus and negatively correlated with the methanococcal optimum growth temperatures.
FIG. 1.
Expression levels of Sac10b homologs determined by Western blotting. (A) Western blotting of Mma10b. Proteins were separated with a 12% SDS-polyacrylamide gel and then transferred onto a PVDF membrane. The membrane was incubated sequentially with rabbit anti-Mma10b antiserum and anti-rabbit IgG-horseradish peroxidase conjugate. The proteins were detected with enhanced chemiluminescent substrates and exposed to CL-XPosure film for 5 s. Lane 1, 2, and 3, loaded with 400 μg of protein crude extracts of S2 (wild type), S590 (the Δmma10b mutant), and S591 (the complemented Δmma10b mutant), respectively. Lane 4, loaded with 25 ng of recombinant Mma10b. (B) Amounts of Sac10b homologs in wild-type cells as determined by Western blotting. Percentages of Sac10b homologs in total cellular protein were the averages of two independent measurements.
Aggregation of Sac10b homologs with heat treatment.
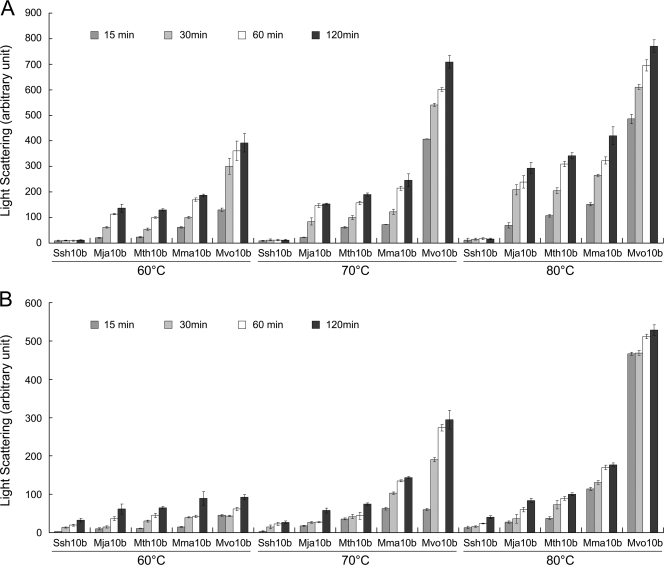
The heat-induced aggregation of Sac10b homologs was monitored by light scattering to indicate the irreversible denaturation of proteins at high temperature. Some proteins from the mesophilic methanococci are stable at high temperature, which may be evidence for a thermophilic origin of archaea (38). For instance, the temperature optimum of Por from M. maripaludis is 60°C (23). Thus, it was of interest to determine whether the Sac10b homologs from the mesophiles were also resistant to heat. As shown in Fig. 2A, Mma10b and Mvo10b denatured faster than Mth10b and Mja10b at temperatures higher than 60°C, suggesting that mesophilic Sac10b homologs were not as heat stable as thermophilic homologs.
FIG. 2.
Heat-induced aggregation of Sac10b homologs. Recombinant proteins Ssh10b, Mja10b, Mth10b, Mma10b, and Mvo10b were suspended at a concentration of 0.1 mg/ml in buffer containing 10 mM potassium phosphate (pH 7.0) and 10% (vol/vol) glycerol. Protein aggregation was monitored by 90° light scattering at 488 nm on a Shimadzu RF5301PC spectrofluorimeter. (A) Incubation without additions. (B) Incubation with 0.4 M KCl. Error bars indicate standard deviations of 10 measurements from one experiment.
The methanococci are marine organisms, and the intracellular concentration of K+ in methanococci is 0.4 to 1.3 M (32, 36). Moreover, high ionic strengths are important for the full folding of archaeal histones and their function in negative supercoiling of DNA (35). Therefore, high ionic strengths may influence the stability of Sac10b homologs. As shown in Fig. 2B, protein aggregation with 0.4 M of KCl was reduced compared to that in an incubation without salt, but the rates of aggregation followed the same trend under both conditions, i.e., Mvo10b > Mma10b > Mth10b > Mja10b. The rapid denaturation of Mvo10b and Mma10b indicated that they were not functional at high temperatures, and Sac10b homologs were adapted to the organism's optimum growth temperature.
Large differences in the heat-induced aggregation suggested that Mma10b and Mvo10b were not closely related. Extensive phylogenetic analyses of the Sac10b homologs in the euryarchaeotes supported this conclusion (data not shown). In contrast to most proteins of the two mesophilic methanococci, the sequence of Mma10b was only 57% identical to that of Mvo10b, and it was more similar to that of Mth10b (79% identical) from the thermophile M. thermolithotrophicus. Sulfolobus and several other archaeal species encode two copies of the Sac10b homologs, Alba1 and Alba2, which are 30% to 40% identical and very likely are paralogs with different functions (19). The sequences of Mma10b, Mth10b, and Mja10b were more closely related to that of Alba1 (48% to 58% identical), while Mvo10b was equidistant to Alba1 and Alba2 (40% identical). Because of their low sequence similarity, Mma10b and Mvo10b may have been derived from paralogous lineages, which might explain their different responses to high temperatures.
Comparisons of affinities of Mma10b for DNA and RNA with other Sac10b homologs.
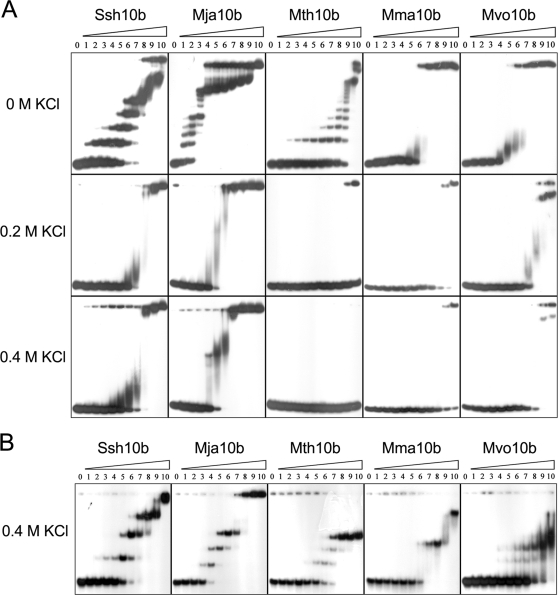
Previous studies reported that the association of Sac10b homologs from Sulfolobales with DNA was sequence independent (27). The affinity of Mma10b for oligonucleotide DNA was compared with those of other Sac10b homologs by EMSA. A 32P-labeled 60-bp dsDNA fragment was titrated with Sac10b homologs (Fig. 3A). The affinities of Mja10b and Mth10b for DNA were similar to that of Ssh10b. At low protein concentrations, they formed a series of low-molecular-weight protein-DNA complexes that were well resolved. At higher protein concentrations, they formed slowly migrating aggregates which were unable to enter the 8% gels. The aggregates presumably resulted from uncharacterized protein-protein and/or protein-DNA interactions. Unlike the thermophilic proteins, Mma10b and Mvo10b formed only high-molecular-weight complexes with DNA, suggesting different patterns of protein-DNA interactions. The amount of Mma10b required to retard half of the input DNA (Kd) was about 600 nM.
FIG. 3.
Analysis of binding of Sac10b homologs to DNA and RNA by EMSA. (A) Binding of Sac10b homologs to a 32P-labeled 60-bp dsDNA fragment. The proteins were incubated with the DNA (20 to 30 nM) at 22°C for 10 min in the presence of 0, 0.2 or 0.4 M KCl. Protein-DNA complexes were resolved by electrophoresis on an 8% nondenaturing polyacrylamide gel. The gel was exposed to X-ray film. From lane 0 to 10, the protein concentrations were 0, 0.02, 0.04, 0.08, 0.16, 0.31, 0.63, 1.25, 2.5, 5, and 10 μM, respectively. (B) Binding of Sac10b homologs to a 32P-labeled 64-nt single-stranded RNA fragment. The proteins were incubated with RNA (20 to 30 nM) at 22°C for 10 min in the presence of 0.4 M KCl. Protein-RNA complexes were resolved by electrophoresis on an 8% nondenaturing polyacrylamide gel. The gel was exposed to X-ray film. From lane 0 to 10, the protein concentrations were 0, 4 × 10−5, 1.6 × 10−4, 6.3 × 10−4, 2.5 × 10−3, 0.01, 0.04, 0.16, 0.63, 2.5, and 10 μM, respectively.
The presence of salt affected the protein-DNA interaction. At 0.2 M of KCl, none of the Sac10b homologs was able to generate stable DNA shifts, but all formed aggregates at high protein concentrations (Fig. 3A). At 0.4 M of KCl, the apparent Kd of Mma10b increased by about 10-fold to 7 μM, and Mth10b was unable to form aggregates (Fig. 3A).
In contrast, the RNA binding patterns of these proteins were independent of the salt concentration, and the affinities for a 64-nt RNA fragment were only slightly lower with 0.4 M KCl than with no salt (data not shown). All Sac10b homologs were able to form well-resolved complexes with the RNA in the presence of 0.4 M KCl (Fig. 3B). Ssh10b and Mja10b also formed high-molecular-weight aggregates at higher protein concentrations. In contrast, the other three proteins did not form aggregates even at protein concentrations of as high as 10 μM. The protein concentrations that were required to retard half of the input RNA were about 10 nM for Ssh10b, 2.5 nM for Mja10b, 150 nM for Mth10b and Mma10b, and 600 nM for Mvo10b. Therefore, the mesophilic methanococcal Sac10b homologs possessed higher affinities for oligonucleotide RNA than DNA in vitro, especially in the presence of high salt concentrations.
Disruption of mma10b impairs autotrophic growth.
Since mesophilic Sab10b homologs were expressed at low levels, they may perform a very different and possibly dispensable function compared to homologs in thermophiles. M. maripaludis was used here as a model to elucidate the physiological role of Sac10b homologs in mesophilic methanococci. The gene encoding Mma10b (Mmp1613) was deleted by gene replacement with the pac cassette, which encodes puromycin resistance in methanococci (see Fig. S2 in the supplemental material). The deletion of mma10b was confirmed by Southern hybridization (data not shown) and Western blotting (Fig. 1A). Since the gene was transcribed on the opposite strand from its neighbors, this mutation was not expected to affect transcription of downstream genes.
When grown in minimal medium, the Δmma10b mutant, strain S590, experienced a longer lag phase, lower growth rate during exponential phase, and lower maximum growth yield than the wild type (Fig. 4). The differences in the growth of S590 and the wild-type S2 were consistent in three independent experiments. To confirm that these differences were due to the mutation, the complementation strain S591 was constructed by transforming the plasmid pMEV2-mma10b into S590. Because Mma10b was expressed at only low levels in the wild type, the native promoter was used for the complementation. This resulted in expression of Mma10b that was about 40% lower than that of the wild type (Fig. 1A). The complementation partially restored growth. In a typical experiment, the durations of the lag phase of S2, S590, and S591 were about 3, 10, and 15 h, respectively. The doubling times (means ± standard deviations for five cultures) of S2, S590, and S591 were 5.8 ± 0.7, 10.2 ± 0.6, and 6.7 ± 1.1 h, respectively. The maximum growth yields (means ± standard deviations for five cultures) of S2, S590, and S591 were 0.48 ± 0.00, 0.31 ± 0.01, and 0.45 ± 0.02 mg dry weight/ml of culture, respectively. Thus, at the lower levels of expression in the complemented strain, only the lag phase was severely affected. The longer lag phase may have been caused by the lower efficiency of mma10b expression from the shuttle vector and a requirement for more time to accumulate Mma10b sufficient for optimal growth.
FIG. 4.
Growth of the Δmma10b::pac mutant S590 in minimal medium at 37°C. (▪), wild-type strain S2; (□), Δmma10b mutant S590; (▴), strain S591 (complementation of S590 with mma10b expressed from pMEV2-mma10b). Error bars are the standard deviations from 12 measurements in three independent experiments.
When acetate, alanine, Casamino Acids, or yeast extract was added to the medium, the growth of S590 was indistinguishable from that of the wild-type strain (see below). Thus, the growth defect appeared to be restricted to minimal medium. A Δehb mutant of M. maripaludis has a similar phenotype. It is impaired in autotrophic growth and possesses high specific activities of the key autotrophic enzymes, CODH/ACS and Por (30). The specific activities of both enzymes from cells grown in minimal medium in exponential growth phase increased in S590 (Table 2). The differences between S2 and S590 were significant, with P values of <0.05. The complementation strain S591 had activities close to the wild-type levels.
TABLE 2.
Por and CODH/ACS activities of M. maripaludis strains S2, S590, and S591a
| Strain | Sp act (U/mg)b
|
|
|---|---|---|
| POR | CODH-ACS | |
| S2 | 304 ± 25 | 216 ± 21 |
| S590 | 467 ± 59 | 305 ± 26 |
| S591 | 273 ± 22 | 195 ± 16 |
The cultures were grown in McN medium.
Data are the averages and standard deviations for nine assays from three independent cultures. One unit of enzyme activity was defined as 1 μmol of methyl viologen reduced per minute.
To determine if Mma10b was involved in thermal adaptation, the growth response of the Δmma10b mutant to temperature was characterized (Fig. 5). In minimal medium, the growth rate of S590 was less than that of the wild-type strain at near 37°C, which was close to the temperature optimum (Fig. 5). Growth of S2 and growth of S590 were similar at both lower and higher temperatures. In contrast, growth in media with abundant sources of reduced carbon was the same for S590 and S2 at all tested temperatures. Presumably, the process(es) affected in the mutant was growth limiting only at high growth rates under conditions where autotrophic CO2 fixation was required. In any case, the similar growth of the mutant and wild type at high temperatures did not support a role for Mma10b in thermoadaptation.
FIG. 5.
Effect of temperature on the growth of the Δmma10b::pac mutant S590. (▪), wild-type strain S2; (□), mutant S590. The specific growth rates (μ) during the exponential phase were calculated from the slopes of the semilogarithmic plots. Representative error bars are the standard deviations from eight measurements in two independent experiments. Growth rates in McN medium with the indicated additions are shown.
Quantitative proteomic analysis of protein expression patterns.
To elucidate the influence of mma10b disruption on gene expression, the proteomes of the Δmma10b mutant S590 and the wild-type S2 were compared. Both S590 and S2 were cultured in McNA medium and harvested during the exponential growth phase, conditions where their growth was indistinguishable. Proteins encoded by 442 of the 1,722 ORFs were detected at least once, and 327 proteins were detected by multiple measurements (see Table S1 in the supplemental material). By the criteria described in Materials and Methods, the levels of 8 of the 327 proteins increased significantly in S590 relative to the wild type, and the levels of 6 proteins decreased significantly (Table 3).
TABLE 3.
Changes in expression of certain proteins in the mutant S590 determined by quantitative proteomic analysisa
| Regulation and ORFb | nc | Protein ratio
|
Annotatione | |
|---|---|---|---|---|
| Meand | SE | |||
| Upregulation | ||||
| MMP0003 | 4 | 1.38 | 0.11 | 2-Oxoglutarate oxidoreductase alpha subunit, korA |
| MMP0092 | 2 | 1.66 | 0.10 | DNA-directed RNA polymerase subunit F |
| MMP0148 | 3 | 1.36 | 0.12 | Acetyl-CoA synthetase, AMP forming, acsA |
| MMP0276 | 2 | 1.57 | 0.06 | Conserved hypothetical protein |
| MMP0341 | 4 | 1.26 | 0.07 | Pyruvate carboxylase subunit A |
| MMP0414 | 6 | 1.51 | 0.03 | Threonyl-tRNA synthetase |
| MMP0594 | 2 | 1.53 | 0.11 | Conserved hypothetical archaeal protein |
| MMP0684 | 3 | 2.05 | 0.06 | Heat shock protein Hsp20 |
| MMP0872 | 4 | 5.84 | 0.04 | Porphobilinogen deaminase |
| MMP0971 | 6 | 1.52 | 0.06 | Adenylosuccinate lyase |
| MMP1157 | 2 | 1.79 | 0.09 | Desulfoferrodoxin, ferrous iron binding site |
| MMP1504 | 2 | 1.32 | 0.05 | Pyruvate oxidoreductase subunit beta |
| Downregulation | ||||
| MMP0196 | 5 | 0.76 | 0.11 | ABC-type iron(III) transport system, periplasmic binding protein |
| MMP0197 | 4 | 0.77 | 0.08 | ABC-type iron(III) transport system, permease component |
| MMP0944 | 2 | 0.60 | 0.02 | Hypothetical protein |
| MMP0956 | 7 | 0.66 | 0.18 | DNA topoisomerase I |
| MMP1363 | 6 | 0.74 | 0.15 | RNA polymerase, subunit A′ |
| MMP1364 | 5 | 0.74 | 0.10 | RNA polymerase, subunit A" |
| MMP1666 | 2 | 0.42 | 0.23 | Flagellin B1 precursor |
| MMP1667 | 3 | 0.36 | 0.38 | Flagellin B2 |
| MMP1670 | 5 | 0.68 | 0.15 | Flagellum accessory protein D |
| MMP1671 | 3 | 0.59 | 0.29 | Flagellum accessory protein E |
The cultures for proteomic analysis were grown in McNA medium.
Expression of the ORFs in bold changed only 1.25- to 1.5-fold, but the accuracy of these changes were supported by changes of neighboring ORFs or other members of the same pathway.
Number of proteome measurements for the ORF.
Mean of the S590/S2 ratio of protein levels.
From reference 15.
The levels of several enzymes involved in carbon assimilation increased moderately in S590, including the Por β subunit (PorB, Mmp1504), the 2-ketoglutarate oxidoreductase α subunit (Mmp0003), pyruvate carboxylase subunit A (Mmp0341), and acetyl-CoA synthetase (Mmp0148). While the differences in protein levels were less than 1.5-fold, they were statistically significant. Por expression also increased in the Δmvo10b mutant of M. voltae (14).
The changes in gene expression revealed by quantitative proteomics were confirmed by real time RT-PCR. Six potentially regulated ORFs were selected (Table 4). The direction of change in the steady-state levels of mRNA in all cases agreed with proteomics. For all of the six ORFs, the extent of changes in mRNA levels was greater than the proteome, a trend that was consistent with previous observations with M. maripaludis (49).
TABLE 4.
Relative transcript levels of genes of the mutant S590 determined by quantitative RT-PCRa
| ORF | Transcript ratiob | Annotationc |
|---|---|---|
| MMP0684 | 6.82 ± 1.38 | Heat shock protein Hsp20 |
| MMP0872 | 5.58 ± 1.15 | Porphobilinogen deaminase |
| MMP0092 | 3.56 ± 0.74 | DNA-directed RNA polymerase subunit F |
| MMP1504 | 3.23 ± 0.64 | Pyruvate oxidoreductase subunit beta |
| MMP0944 | 0.54 ± 0.11 | Hypothetical protein |
| MMP0956 | 0.18 ± 0.05 | DNA topoisomerase I |
The cultures for quantitative RT-PCR analysis were grown in McNA medium.
S590/S2 mRNA ratio determined by quantitative RT-PCR. Data are the averages and standard deviations of three measurements.
From reference 15.
Mma10b is associated with DNA in vivo.
Mma10b bound DNA with a low affinity in vitro. The ChIP assay was employed to determine if Mma10b was associated with chromosomal DNA in vivo. To confirm the specificity of the immunoprecipitation, Δmma10b mutant (S590) cells were used as a control. PCR amplification of coprecipitated DNA from wild-type cells generated multiple DNA fragments ranging from 100 to 1,000 bp, while the immunoprecipitation with S590 cells did not yield enough DNA to be visible with ethidium bromide on agarose gels after PCR amplification (see Fig. S3 in the supplemental material). Therefore, DNA purified from ChIP samples did not result from nonspecific immunoprecipitation.
The sequences of 46 clones with inserts of DNA fragments that coprecipitated with Mma10b were determined (see Table S2 in the supplemental material). Some of these fragments were part of the operons which encoded proteins that were differentially expressed in the Δmma10b mutant. These included genes for DNA-directed RNA polymerase subunits and genes involved in flagella biosynthesis. These results indicated that associations of Mma10b with coding regions of the genes might affect gene expression. Mma10b was also associated with some genes that were not differentially expressed, such as MMP0383, which encoded the surface layer protein (see Table S1 in the supplemental material), indicating that the binding of Mma10b did not necessarily affect gene expression, at least under the conditions tested.
Mma10b binds DNA with sequence specificity.
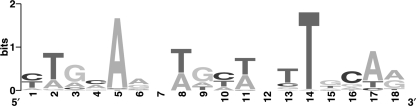
To identify potential Mma10b binding sites, the DNA sequences identified in the ChIP experiment were analyzed with the Gibbs Sampler program (40). This program predicted an 18-bp binding motif derived from 46 potential binding sites, in which the highly conserved A (5th position) and T (14th position) were separated by 8 bp (Fig. 6). The 2nd and the 17th positions were also preferably T and A/T, respectively. Based upon this degenerate sequence, the presence or absence of a binding site in a DNA fragment was analyzed by the Motif Locator program at a cutoff score of 9.0 (28). The higher the cutoff score, the more stringent the binding site identification. The cutoff value was validated by EMSA in agarose gel with five different DNA fragments with lengths of 500 to 1,000 bp. For three fragments in which the predicted binding site was present at a cutoff of 9.0, the apparent Kd on agarose gels was <1 μM (Fig. 7A and data not shown). For the two other fragments in which the predicted binding site was absent at a cutoff of 9.0, the apparent Kd was >10 μM (Fig. 7A and data not shown). Because both fragments also contained one predicted binding site at a cutoff of 8.0, this cutoff value was not stringent enough to predict the presence of binding sites. Using the same criterion, the whole genome of M. maripaludis contained about 4,000 Mma10b binding sites. Among the 46 coprecipitated DNA fragments, 32 sequences contained at least one predicted binding site. All of the predicted binding sites were located in the protein-coding regions (see Table S2 in the supplemental material). Other binding sites not recognized by the sequence analyses may also exist, since some of the coprecipitated DNA fragments did not contain the degenerate motif.
FIG. 6.
Consensus sequence of the Mma10b binding site. The Mma10b binding site for DNA was predicted by the Gibbs Motif Sampler program using DNA sequences of the fragments associated with Mma10b in the ChIP analysis (see Table S2 in the supplemental material). The diagram was created with the WebLogo program.
FIG. 7.
Analysis of sequence-dependent association of Mma10b with DNA by agarose (A and B) and polyacrylamide gel (C) EMSA. (A) Binding of Mma10b to linear DNA fragments with and without the binding motif. The DNA concentration was 15 nM. Protein-DNA complexes were resolved by electrophoresis on 1.2% agarose gels. From lane 0 to 5, the protein concentrations were 0, 1, 2, 3.75, 7.5, and 15 μM, respectively. The presence or absence of Mma10b binding sites was predicted by the Motif Locator program with a score cutoff of 9.0. (B) Binding of Mma10b to closed, circular supercoiled and linear forms of the plasmid pB9. The linear plasmid was generated after digestion with HindIII. Based upon the Motif Locator program with a score cutoff of 9.0, the DNA sequences contained four potential Mma10b binding sites. The DNA concentration was 2 nM. Protein-DNA complexes were resolved by electrophoresis on 0.7% agarose gels. From lane 0 to 5, the protein concentrations were 0, 1, 2, 3.75, 7.5, and 15 μM, respectively. (C) Binding of Mma10b to 32P-labeled 58-bp dsDNA fragments. The DNA concentration was 20 nM. Protein-DNA complexes were resolved by electrophoresis on an 8% nondenaturing polyacrylamide gel. The gel was exposed to X-ray film. From lane 0 to 10, the protein concentrations were 0, 0.02, 0.04, 0.08, 0.16, 0.31, 0.63, 1.25, 2.5, 5, and 10 μM, respectively. The upper panel used a DNA fragment with the wild-type sequence (positions 1307685 to 1307742 in the chromosome, which included “binding site a” in Fig. 8). The lower panel used a fragment where key bases in the motif were changed as indicated.
Furthermore, the sequence-dependent association was not influenced by the DNA structure. For a plasmid containing four predicted binding sites, Mma10b bound equally well to the supercoiled plasmid and linear DNAs (Fig. 7B). At a protein concentration of 0.8 μM, migration of both forms of the input DNAs was retarded. Moreover, at higher protein concentrations, multiple protein-DNA bands formed, suggesting that more than one binding site may be occupied and that Mma10b may bind cooperatively as oligomers. At the highest protein concentration of 15 μM, the complexes formed with both supercoiled DNA and linear DNA migrated faster than the protein-free DNA, suggesting that DNA may be condensed. The presence of a high salt concentration reduced the association of Mma10b with binding motif-containing DNA fragments. With 0.4 M of KCl, the plasmid DNA was not retarded by 15 μM of Mma10b in agarose gel (data not shown).
The sequence-dependent binding of Mma10b to short dsDNA was further examined by EMSA in polyacrylamide gels. This method may be more accurate for Sac10b homolog binding to nucleic acids than the use of agarose gels because smaller changes in mobility can be detected due to the small pore size of polyacrylamide gels and the lower ionic strength used during electrophoresis may stabilize binding. The affinities of Mma10b for two 58-bp dsDNA fragments were compared (Fig. 7C). For the DNA fragment possessing the binding motif sequence, the apparent Kd was about 120 nM by this method. For the DNA fragment with A and Ts at the 2nd, 5th, 14th, and 17th positions changed to G, the Kd was about 600 nM, which was close to that of a 60-bp dsDNA fragment that lacked a predicted binding site (Fig. 3A). Therefore, Mma10b associated with motif-containing DNA at least fivefold more strongly than with bulk DNA. Moreover, two discrete shifts, which may represent protein binding in different cooperative fashions, were observed (Fig. 7C). The cooperative association of Ssh10b with DNA was described previously (7). In Fig. 7C, the first shift, which may have lower protein oligomerization state than the second shift, appeared at lower Mma10b concentrations in the presence of the binding site than in its absence.
The predicted DNA binding sites of Mma10b were further validated by DNase I footprinting analysis. A 389-bp DNA fragment with two predicted binding sites was used in this assay (Fig. 8A). Binding site “a” had been previously shown to associate with Mma10b by EMSA (Fig. 7C). The electropherograms of the 389-bp DNA fragment after DNase I digestion clearly revealed that two regions were protected by incubation with Mma10b, and these two regions corresponded to the positions within the predicted binding sites (Fig. 8). A third region (around nt 130) was also protected, even though a binding motif was not identified in this region. These results suggested that Mma10b did not coat DNA but bound to specific loci.
FIG. 8.
Identification of DNA regions protected by Mma10b in DNase I footprinting analyses. (A) Schematic diagram of the 389-bp DNA fragment (positions 1307598 to 1307986 in the chromosome) used in the footprinting reactions. One DNA strand was fluorescently labeled via PCRs with the primer B9F, which was labeled with 6-FAM at the 5′ end. The DNA binding sites (a and b), which were predicted by the program Motif Locator with the consensus binding sequence, are indicated by shaded boxes. (B) Electropherograms of fragments generated by footprinting reactions in the presence (15 μM) and absence of Mma10b. The patterns were viewed with the Peak Scanner software v1.0 (Applied Biosystems, Foster City, CA). The regions that were protected by Mma10b during the digestion with DNase I are indicated as regions I and II. (C) Expanded view of region I from panel B. (D) Expanded view of region II from panel B. Red and blue lines represent reactions in the presence (15 μM) and absence of Mma10b, respectively. Specific sites that were protected by Mma10b are marked (•). The horizontal bars indicate the positions of the predicted binding sites a and b. The DNA sequences of sites a and b are shown below the bars.
DISCUSSION
Proteins of the Sac10b family are abundant in the thermophilic archaea and have been proposed to play important roles in thermoadaption. Assuming a thermophilic origin for the archaea (38), these proteins could be molecular fossils in mesophiles like M. maripaludis. However, two lines of evidence suggest that this is not the case and that this protein has retained a function in the mesophiles. First, the mesophilic methanococcal proteins are labile to denaturation at high temperature. This result suggests that their amino acid composition has evolved under selection for a functional protein. Second, disruption of mma10b results in impaired autotrophic growth and changes in gene expression. Thus, the protein plays some role in cellular processes.
Comparisons between thermophilic and mesophilic Sac10b homologs.
The physiological properties of thermophilic and mesophilic methanococcal Sac10b homologs are remarkably different, indicating that the function of these proteins has diverged greatly. First, the expression levels of Sac10b homologs are much lower in mesophilic methanococci than in thermophiles. In the mesophile M. maripaludis, Mma10b accounts for only ∼0.01% of the total protein. Second, in vitro the affinity of Mma10b for DNA is sequence dependent, suggesting that Mma10b binds preferentially at specific loci in vivo. Third, the disruption of mma10b affects growth only in minimal medium, showing that this protein is not essential. Attempts to delete ssh10b in S. shibatae were not successful, indicating that disruption of ssh10b may lead to a lethal mutation. These differences indicate that the protein family has evolved from a more crucial role in thermophiles to a nonessential role in mesophiles. This is supported by the absence of Sac10b homologs in mesophilic Methanosarcina and Halobacteria species.
Mma10b binds to DNA with sequence specificity and has a lower affinity for DNA without a specific binding site. When a single sequence-specific binding site is available, the apparent Kd for short dsDNA is ∼120 nM as determined by polyacrylamide gel EMSA, which is about two times higher than that of recombinant Sso10b and similar to that of acetylated Sso10b (19). When the binding site is absent, the apparent Kd increases to ∼600 nM. Two features of mesophilic Sac10b homologs (Mma10b and Mvo10b) probably contribute to their low nonspecific affinities for DNA. First, Lys16 (Sso10b numbering) is replaced with Asn and Glu in Mma10b and Mvo10b, respectively. Lys16 plays a key role in DNA binding (4). In Sso10b, replacement of Lys16 with Ala reduces the affinity by 7- to 14-fold, and replacement with Glu reduces the affinity by ∼40-fold. Therefore, the substitutions observed in the mesophilic methanococcal proteins are expected to lower their affinities for DNA. Second, the mesophilic Sac10b homologs are less highly charged than those from the thermophiles. For instance, the pIs calculated from the amino acid contents are as follows: Ssh10b (pI = 10.5) > Mja10b (pI = 9.7) > Mth10b (pI = 8.0) > Mvo10b (pI = 7.9) > Mma10b (pI = 5.8). The intracytoplasmic pH of mesophilic methanococci is around 6.5 to 8 (9). Thus, Mma10b and Mvo10b are expected to be neutral or negatively charged in vivo. In contrast, Sso10b and most other chromatin proteins are highly basic and possess a positively charged DNA binding surface. Presumably, the reduced positive charges on the mesophilic Sac10b homologs limit their nonspecific binding to nucleic acids.
Comparison of Mma10b with other DNA binding proteins.
Mma10b is very different from typical transcription regulators. For instance, NrpR in M. maripaludis binds its operator sequences with a Kd of 0.3 nM (21). On the other hand, the low abundance of Mma10b makes it unlike common chromatin architectural proteins such as archaeal histones and MC1, which make up ∼5% of total cellular protein. For instance, based upon a protein content of ∼ 4.3 × 10−13 g of protein per cell and a molecular mass of Mma10b of 11.8 kDa, M. maripaludis contains ∼2,200 copies of Mma10b per cell. If the cell contains only one copy of the chromosome and one dimer of Mma10b binds 18 bp of DNA, Mma10b could bind no more than 1% of the genome. M. jannaschii cells contain multiple copies of the chromosome (26). If M. maripaludis cells also contain multiple copies, Mma10b could only bind an even smaller proportion of the genome.
Even though Mma10b is not abundant enough to coat the entire genome, it could still modulate nucleoid architecture. The nucleoid in enteric bacteria is organized in loops of ∼10 kb, which are connected and closed by DNA binding proteins (8, 31). If the M. maripaludis chromosome is organized in the same manner, Mma10b is abundant enough to play a role in loop maintenance. Likewise, the cellular level of Mma10b is similar to that of Lrp in E. coli, which contains ∼1,000 dimers per cell (1). The DNA binding affinity of Mma10b is also close to that of LrpC in Bacillus subtilis, which binds intrinsically curved DNA with higher affinity (Kd of ∼80 nM) than noncurved DNA (Kd of ∼700 nM) (39). Therefore, the physiological role of Mma10b could resemble that of Lrp in some bacteria, which contributes to nucleoid organization by wrapping and bridging DNA (5, 8, 24, 39).
Binding of DNA by Mma10b in vivo.
The ChIP experiment identified association of Mma10b with some DNA regions in vivo. Sequence analysis with these regions identified an 18-bp binding motif and indicated that the whole genome contains about 4,000 Mma10b binding sites. Thus, there is about 0.3 molecule of the physiologically active dimer per binding site, and Mma10b dimer would have to act at below stoichiometric levels. About 99% of the predicted binding sites are located inside protein-coding regions. Therefore, the associations of Mma10b with DNA need to be reversible during transcription.
The Kd of the Mma10b association with a motif-containing 58-bp dsDNA fragment was about 120 nM at low salt concentrations. While the presence of salt near physiological concentrations reduced its affinity, it would not necessarily eliminate the association of Mma10b with DNA. At intracellular concentrations of Mma10b (∼2 μM) and DNA binding sites (∼4 μM, assuming one genome per cell), 70% of the total amount of Mma10b would be associated with specific DNA binding sites with Kds of 1 μM. Moreover, the calculated ratio of Mma10b-DNA complexes to free Mma10b molecules might be underestimated, as the nucleoid is highly compacted in the living cells, and the local concentrations of Mma10b and DNA binding sites could be much higher.
The oligomeric state of Mma10b when binding to DNA is not clear. The footprinting experiment suggested that Mma10b protected DNA regions of about 10 to 20 bp in length. A model of DNA binding by Sac10b homologs proposes that the binding stoichiometry varies between 5 and 20 bp DNA per dimer, depending on the binding density (43, 44). This model suggests that within 10- to 20-bp DNA regions, Mma10b could bind as either a dimer, tetramer, or octamer.
Binding of RNA by Mma10b.
Although no evidence was obtained for RNA binding by Mma10b in vivo, this possibility is not excluded. First, Mma10b and Mvo10b possess high affinities for RNA, even in the presence of physiological concentrations of salt. Second, the low abundance of Mma10b cannot eliminate a role in mRNA or ribosome binding. Based upon the total amount of RNA in methanococci of 6 × 10−8 to 10 × 10−8 μg cell−1 (17) and the fact that 4% of the total RNA is mRNA in growing cells (29), the mRNA content in methanococci is expected to be 1,400 to 2,400 molecules per cell. With 2,200 Mma10b monomers per cell, there is about 0.5 molecule of the physiologically active dimer per mRNA molecule. Similarly, M. maripaludis contains 1.0 × 104 to 2.3 × 104 ribosomes cell−1, or about twice the value reported for E. coli (16). Thus, there is about 0.1 to 0.2 molecule of Mma10b dimer per ribosome. This level is comparable to the levels of bacterial translation initiation factors (IF1, -2, and -3), which in E. coli are present at a level of 0.2 to 0.3 molecule per ribosome (6). Thus, the levels of Mma10b are consistent with a role in mRNA or rRNA binding, but only at nonstoichiometric levels.
Influence of Mma10b on gene expression.
The influence of Mma10b on carbon assimilation is suggested by the poor growth in minimal medium and increased protein levels of carbon assimilation enzymes in the Δmma10b mutant. In minimal medium, M. maripaludis reduces CO2 to acetyl-CoA by use of CODH/ACS. Acetyl-CoA is then reductively carboxylated to pyruvate by Por (23, 34). Exogenous acetate and alanine are alternative sources for acetyl-CoA and pyruvate, respectively. Because the addition of acetate or alanine restores the growth of the Δmma10b mutant to the wild-type level, the disruption of mma10b appears to impair an early step(s) of CO2 fixation, such as converting CO2 to acetyl-CoA or pyruvate. Compared with a Δehb mutant which is also defective in CO2 assimilation, the expression of a similar group of proteins is affected but with smaller changes in the Δmma10b mutant (30, 49). First, enzymes involved in autotrophy, such as Por and CODH/ACS, are upregulated in both mutants. Second, proteins of the fla operon are downregulated in both mutants. The regulation of flagellar genes in M. maripaludis is complex and is affected by levels of H2 and phosphate as well as growth rate (16). The striking similarities between gene regulation in the Δmma10b and the Δehb mutants suggest that the disruption of mma10b limits autotrophic CO2 flux. However, there is no reason to propose a specific regulatory role for Mma10b. CO2 fixation imposes large bioenergetic demands upon methanococci, and any mutation that lowers the growth efficiency could negatively affect this process.
Conclusions.
The Sac10b homologs of the mesophilic methanococci are clearly functional. The proteins are expressed at low levels, suggesting that their function is very different from that of the hyperthermophilic proteins. The association of Mma10b with DNA in vitro is sequence dependent, indicating that it binds to the chromosome at specific loci. However, the affinity is much lower than that of transcriptional regulators. Mutations that disrupt the gene alter the growth properties of the cells and change the expression levels of several genes. While the association of Mma10b at specific sites could change DNA structure, whether it acts in concert with other DNA binding proteins and directly affects gene expression remains an area for future investigations.
Supplementary Material
Acknowledgments
We thank Magdalena Sieprawska-Lupa for preparing protein samples for proteomic study. We thank Iris Porat for designing plasmid pIJA03-mma10b. We thank Timothy R. Hoover for help with the ChIP experiment. We thank Kenneth L. Jones and Travis C. Glenn for help with the DNase I footprinting experiment. We thank Jan Mrazek and Xiangxue Guo for help with sequence analysis for identification of binding motifs.
This work was supported in part by grant DE-FG02-97ER20269 from DOE Energy Biosciences to W. B. Whitman; grant IR01RR019767-04 from NIH to J. I. Amster; grant 2004CB719603 from the National Basic Research Program of China to L. Huang; grants 30730003, 30621005, and 39925001 from the National Natural Science Foundation of China (NSFC) to L. Huang; and grant KSCX2-YW-G-023 from the Chinese Academy of Sciences to L. Huang.
Footnotes
Published ahead of print on 23 January 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Ali Azam, T., A. Iwata, A. Nishimura, S. Ueda, and A. Ishihama. 1999. Growth phase-dependent variation in protein composition of the Escherichia coli nucleoid. J. Bacteriol. 1816361-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aravind, L., L. M. Iyer, and V. Anantharaman. 2003. The two faces of Alba: the evolutionary connection between proteins participating in chromatin structure and RNA metabolism. Genome Biol. 4R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balch, W. E., G. E. Fox, L. J. Magrum, C. R. Woese, and R. S. Wolfe. 1979. Methanogens: reevaluation of a unique biological group. Microbiol. Mol. Biol. Rev. 43260-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell, S. D., C. H. Botting, B. N. Wardleworth, S. P. Jackson, and M. F. White. 2002. The interaction of Alba, a conserved archaeal chromatin protein, with Sir2 and its regulation by acetylation. Science 296148-151. [DOI] [PubMed] [Google Scholar]
- 5.Beloin, C., J. Jeusset, B. Revet, G. Mirambeau, F. Le Hegarat, and E. Le Cam. 2003. Contribution of DNA conformation and topology in right-handed DNA wrapping by the Bacillus subtilis LrpC Protein. J. Biol. Chem. 2785333-5342. [DOI] [PubMed] [Google Scholar]
- 6.Bremer, H., and P. P. Dennis. 1996. Modulation of chemical composition and other parameters of the cell by growth, p. 1553-1569. In F. C. Neidhardt, R. Curtiss, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella, 2nd ed., vol. 2. ASM Press, Washington, DC. [Google Scholar]
- 7.Cui, Q., Y. Tong, H. Xue, L. Huang, Y. Feng, and J. Wang. 2003. Two conformations of archaeal Ssh10b: the origin of its temperature-dependent interaction with DNA. J. Biol. Chem. 27851015-51022. [DOI] [PubMed] [Google Scholar]
- 8.Dame, R. T. 2005. The role of nucleoid-associated proteins in the organization and compaction of bacterial chromatin. Mol. Microbiol. 56858-870. [DOI] [PubMed] [Google Scholar]
- 9.Dybas, M., and J. Konisky. 1992. Energy transduction in the methanogen Methanococcus voltae is based on a sodium current. J. Bacteriol. 1745575-5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forterre, P., F. Confalonieri, and S. Knapp. 1999. Identification of the gene encoding archeal-specific DNA-binding proteins of the Sac10b family. Mol. Microbiol. 32669-670. [DOI] [PubMed] [Google Scholar]
- 11.Gernhardt, P., O. Possot, M. Foglino, L. Sibold, and A. Klein. 1990. Construction of an integration vector for use in the archaebacterium Methanococcus voltae and expression of a eubacterial resistance gene. Mol. Gen. Genet. 221273-279. [DOI] [PubMed] [Google Scholar]
- 12.Guo, R., H. Xue, and L. Huang. 2003. Ssh10b, a conserved thermophilic archaeal protein, binds RNA in vivo. Mol. Microbiol. 501605-1615. [DOI] [PubMed] [Google Scholar]
- 13.Hecht, A., S. Strahl-Bolsinger, and M. Grunstein. 1996. Spreading of transcriptional represser SIR3 from telomeric heterochromatin. Nature 38392-96. [DOI] [PubMed] [Google Scholar]
- 14.Heinicke, I., J. Muller, M. Pittelkow, and A. Klein. 2004. Mutational analysis of genes encoding chromatin proteins in the archaeon Methanococcus voltae indicates their involvement in the regulation of gene expression. Mol. Genet. Genomics 27276-87. [DOI] [PubMed] [Google Scholar]
- 15.Hendrickson, E. L., R. Kaul, Y. Zhou, D. Bovee, P. Chapman, J. Chung, E. Conway de Macario, J. A. Dodsworth, W. Gillett, D. E. Graham, M. Hackett, A. K. Haydock, A. Kang, M. L. Land, R. Levy, T. J. Lie, T. A. Major, B. C. Moore, I. Porat, A. Palmeiri, G. Rouse, C. Saenphimmachak, D. Soll, S. Van Dien, T. Wang, W. B. Whitman, Q. Xia, Y. Zhang, F. W. Larimer, M. V. Olson, and J. A. Leigh. 2004. Complete genome sequence of the genetically tractable hydrogenotrophic methanogen Methanococcus maripaludis. J. Bacteriol. 1866956-6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendrickson, E. L., Y. Liu, G. Rosas-Sandoval, I. Porat, D. Soll, W. B. Whitman, and J. A. Leigh. 2008. Global responses of Methanococcus maripaludis to specific nutrient limitations and growth rate. J. Bacteriol. 1902198-2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hennigan, A. N., and J. N. Reeve. 1994. mRNAs in the methanogenic archaeon Methanococcus vannielii: numbers, half-lives and processing. Mol. Microbiol. 11655-670. [DOI] [PubMed] [Google Scholar]
- 18.Huber, H., M. Thomm, H. König, G. Thies, and K. O. Stetter. 1982. Methanococcus thermolithotrophicus, a novel thermophilic lithotrophic methanogen. Arch. Microbiol. 13247-50. [Google Scholar]
- 19.Jelinska, C., M. J. Conroy, C. J. Craven, A. M. Hounslow, P. A. Bullough, J. P. Waltho, G. L. Taylor, and M. F. White. 2005. Obligate heterodimerization of the archaeal Alba2 protein with Alba1 provides a mechanism for control of DNA packaging. Structure 13963-971. [DOI] [PubMed] [Google Scholar]
- 20.Jones, W. J., J. A. Leigh, F. Mayer, C. R. Woese, and R. S. Wolfe. 1983. Methanococcus jannaschii sp. nov., an extremely thermophilic methanogen from a submarine hydrothermal vent. Arch. Microbiol. 136254-261. [Google Scholar]
- 21.Lie, T. J., G. E. Wood, and J. A. Leigh. 2005. Regulation of nif expression in Methanococcus maripaludis: roles of the euryarchaeal repressor NrpR, 2-oxoglutarate, and two operators. J. Biol. Chem. 2805236-5241. [DOI] [PubMed] [Google Scholar]
- 22.Lin, W., and W. Whitman. 2004. The importance of porE and porF in the anabolic pyruvate oxidoreductase of Methanococcus maripaludis. Arch. Microbiol. 18168-73. [DOI] [PubMed] [Google Scholar]
- 23.Lin, W. C., Y.-L. Yang, and W. B. Whitman. 2003. The anabolic pyruvate oxidoreductase from Methanococcus maripaludis. Arch. Microbiol. 179444-456. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Torrejon, G., M. I. Martinez-Jimenez, and S. Ayora. 2006. Role of LrpC from Bacillus subtilis in DNA transactions during DNA repair and recombination. Nucleic Acids Res. 34120-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lurz, R., M. Grote, J. Dijk, R. Reinhardt, and B. Dobrinski. 1986. Electron microscopic study of DNA complexes with proteins from the Archaebacterium Sulfolobus acidocaldarius. EMBO J. 53715-3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malandrin, L., H. Huber, and R. Bernander. 1999. Nucleoid structure and partition in Methanococcus jannaschii: an archaeon with multiple copies of the chromosome. Genetics 1521315-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marsh, V. L., S. Y. Peak-Chew, and S. D. Bell. 2005. Sir2 and the acetyltransferase, Pat, regulate the archaeal chromatin protein, Alba. J. Biol. Chem. 28021122-21128. [DOI] [PubMed] [Google Scholar]
- 28.Mrazek, J., S. Xie, X. Guo, and A. Srivastava. 2008. AIMIE: a web-based environment for detection and interpretation of significant sequence motifs in prokaryotic genomes. Bioinformatics 241041-1048. [DOI] [PubMed] [Google Scholar]
- 29.Neidhardt, F. C., and H. E. Umbarger. 1996. Chemical composition of Escherichia coli, p. 143-170. In F. C. Neidhardt, R. Curtiss, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella, 2nd ed., vol. 1. ASM Press, Washington, DC. [Google Scholar]
- 30.Porat, I., W. Kim, E. L. Hendrickson, Q. Xia, Y. Zhang, T. Wang, F. Taub, B. C. Moore, I. J. Anderson, M. Hackett, J. A. Leigh, and W. B. Whitman. 2006. Disruption of the operon encoding Ehb hydrogenase limits anabolic CO2 assimilation in the archaeon Methanococcus maripaludis. J. Bacteriol. 1881373-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Postow, L., C. D. Hardy, J. Arsuaga, and N. R. Cozzarelli. 2004. Topological domain structure of the Escherichia coli chromosome. Genes Dev. 181766-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robertson, D. E., D. Noll, and M. F. Roberts. 1992. Free amino acid dynamics in marine methanogens. Beta-amino acids as compatible solutes. J. Biol. Chem. 26714893-14901. [PubMed] [Google Scholar]
- 33.Sandman, K., and J. N. Reeve. 2006. Archaeal histones and the origin of the histone fold. Curr. Opin. Microbiol. 9520-525. [DOI] [PubMed] [Google Scholar]
- 34.Shieh, J., and W. B. Whitman. 1988. Autotrophic acetyl coenzyme A biosynthesis in Methanococcus maripaludis. J. Bacteriol. 1703072-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soares, D., I. Dahlke, W. T. Li, K. Sandman, C. Hethke, M. Thomm, and J. N. Reeve. 1998. Archaeal histone stability, DNA binding, and transcription inhibition above 90 degrees C. Extremophiles 275-81. [DOI] [PubMed] [Google Scholar]
- 36.Sprott, G. D., and K. F. Jarrell. 1981. K+, Na+, and Mg2+ content and permeability of Methanospirillum hungatei and Methanobacterium thermoautotrophicum. Can. J. Microbiol. 27444-451. [DOI] [PubMed] [Google Scholar]
- 37.Stathopoulos, C., W. Kim, T. Li, I. Anderson, B. Deutsch, S. Palioura, W. Whitman, and D. Soll. 2001. Cysteinyl-tRNA synthetase is not essential for viability of the archaeon Methanococcus maripaludis. Proc. Natl. Acad. Sci. USA 9814292-14297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stetter, K. O. 1994. The lesson of Archaeabacteria, p. 143-151. In S. Boengtson (ed.), Early life on earth. Columbia University Press, New York, NY.
- 39.Tapias, A., G. Lopez, and S. Ayora. 2000. Bacillus subtilis LrpC is a sequence-independent DNA-binding and DNA-bending protein which bridges DNA. Nucleic Acids Res. 28552-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson, W., E. C. Rouchka, and C. E. Lawrence. 2003. Gibbs Recursive Sampler: finding transcription factor binding sites. Nucleic Acids Res. 313580-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tumbula, D. L., J. Keswani, J. Shieh, and W. B. Whitman. 1995. Maintenance of methanogen stock cultures in glycerol at −70°C, p. 85-87. In F. T. Robb, A. R. Place, K. R. Sowers, H. J. Schreir, S. DasSarma, E. M. Fleischmann (ed.), Archaea—a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 42.Tumbula, D. L., R. A. Makula, and W. B. Whitman. 1994. Transformation of Methanococcus maripaludis and identification of a PstI-like restriction system. FEMS Microbiol. Lett. 121309-314. [Google Scholar]
- 43.Wang, G., R. Guo, M. Bartlam, H. Yang, H. Xue, Y. Liu, L. Huang, and Z. Rao. 2003. Crystal structure of a DNA binding protein from the hyperthermophilic euryarchaeon Methanococcus jannaschii. Protein Sci. 122815-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wardleworth, B. N., R. J. M. Russell, S. D. Bell, G. L. Taylor, and M. F. White. 2002. Structure of Alba: an archaeal chromatin protein modulated by acetylation. EMBO J. 214654-4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White, M. F., and S. D. Bell. 2002. Holding it together: chromatin in the Archaea. Trends Genet. 18621-626. [DOI] [PubMed] [Google Scholar]
- 46.Whitman, W. B. 2001. Genus I. Methanococcus, p. 236-240. In D. R. Boone, R. W. Castenholz, and G. M. Garrity (ed.), Bergey's mannual of systematic bacteriology, vol. 1. Springer, New York, NY. [Google Scholar]
- 47.Whitman, W. B., J. Shieh, S. Sohn, D. S. Caras, and U. Premachandran. 1986. Isolation and characterization of 22 mesophilic methanococci. Syst. Appl. Microbiol. 7235-240. [Google Scholar]
- 48.Wong, R. L., and I. J. Amster. 2006. Combining low and high mass ion accumulation for enhancing shotgun proteome analysis by accurate mass measurement. J. Am. Soc. Mass Spectrom. 17205-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xia, Q., E. L. Hendrickson, Y. Zhang, T. Wang, F. Taub, B. C. Moore, I. Porat, W. B. Whitman, M. Hackett, and J. A. Leigh. 2006. Quantitative proteomics of the archaeon Methanococcus maripaludis validated by microarray analysis and real time PCR. Mol. Cell Proteomics 5868-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xue, H., R. Guo, Y. Wen, D. Liu, and L. Huang. 2000. An abundant DNA binding protein from the hyperthermophilic archaeon Sulfolobus shibatae affects DNA supercoiling in a temperature-dependent fashion. J. Bacteriol. 1823929-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yindeeyoungyeon, W., and M. A. Schell. 2000. Footprinting with an automated capillary DNA sequencer. BioTechniques 291034-1041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.