Abstract
We performed chromatin immunoprecipitation-microarray analysis to investigate differences in function between StpA and H-NS in Escherichia coli cells. StpA binding regions essentially overlap those of H-NS in wild-type cells, while they are reduced to one-third in the hns mutant. The H-NS binding profile was unaffected by stpA inactivation.
In prokaryotic cells, a group of highly expressed small proteins (e.g., FIS, IHF, HU, H-NS, and StpA) called nucleoid-associated proteins (NAPs) is involved in genomic compaction and regulation of gene expression, although the detailed architecture of nucleoid is not yet fully understood (1). H-NS is a NAP that has been extensively studied in Escherichia coli and related bacteria (3). Subsequent to the binding of H-NS dimers to phased A tracts, oligomerization is triggered between contiguous dimers. Genome-wide chromatin immunoprecipitation-microarray (ChIP-chip) analyses of binding sites of H-NS in E. coli and Salmonella cells have revealed that H-NS takes part, directly or indirectly, as a transcriptional silencer of many unlinked genes, most of which are horizontally acquired (3a, 8a, 10). H-NS assembles into patches of oligomers bridging the segments of DNA in vitro, and the bridging of DNA segments by H-NS is proposed to play a role in nucleoid organization in vivo by maintaining negatively supercoiled microdomains (2, 4, 13).
StpA, an H-NS paralog, is another NAP and shows 58% sequence identity with H-NS at the amino acid level. Biochemical analysis revealed that the DNA binding preferences of H-NS and StpA are similar and that StpA can also constrain DNA supercoils (15, 17). Furthermore, both H-NS and StpA can repress the expression of the galU promoter (17). Together, these observations indicate that the H-NS and StpA proteins have similar properties, although inactivation of stpA in E. coli has no apparent effect on transcriptional repression and growth rate, which are both affected by the hns mutation (14, 17). These findings have led to the proposal that StpA plays a role supplementary to that of H-NS.
To investigate the difference in function between StpA and H-NS, we compared the in vivo distributions of StpA and H-NS across the E. coli genome by using ChIP-chip technology. (For whole-genome profiles of the FLAG-tagged H-NS and StpA binding sites, together with information on the materials and methods used in this work, see the supplemental material.) Western blot analysis revealed that antibodies against H-NS and StpA cross-react with the antigens of each other (Fig. 1A and B), making these antibodies unsuitable for use in ChIP-chip experiments with the wild-type strain. Therefore, we used strains expressing either StpA or H-NS tagged with 3×FLAG epitopes at the C-terminal end in this work (Table 1 and Fig. 1C). Results shown in Fig. 1 indicate that comparable levels of StpA and H-NS are expressed during the exponential growth phase of the strains used here. The addition of a FLAG tag to H-NS and StpA had no effect on the protein expression levels of H-NS or StpA (Fig. 1A and B), and no growth rate impairment was observed in cells expressing either FLAG-tagged StpA or H-NS in otherwise wild-type backgrounds (data not shown).
FIG. 1.
Comparison of StpA and H-NS protein expression levels in the strains used for ChIP-chip analysis. Total cellular proteins of each strain were prepared by trichloroacetic acid precipitation and separated by Tris-Tricine sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Expression levels of native StpA in wild-type (wt) (lane 1) and Δhns mutant (lane 3) cells, FLAG-tagged StpA in wild-type (lane 2) and Δhns mutant (lane 4) cells, and FLAG-tagged StpA(F21C) in Δhns mutant (lane 5) and wild-type (lane 6) cells were detected by the StpA antipeptide antibody (A). Expression levels of native H-NS in wild-type (lane 1) and ΔstpA mutant (lane 3) cells, FLAG-tagged H-NS in wild-type (lane 2) and ΔstpA mutant (lane 4) cells were detected by the H-NS antibody (B). Expression levels of FLAG-tagged H-NS in wild-type (lane 1) and ΔstpA mutant (lane 3) cells, FLAG-tagged StpA in wild-type (lane 2) and Δhns mutant (lane 4) cells, and FLAG-tagged StpA(F21C) in Δhns mutant cells were detected by anti-FLAG antibody (C). σ70 was used as an internal control and detected by anti-σ70 antibody.
TABLE 1.
Bacterial strains used in this study
| E. coli strain | Relevant characteristic(s) | Source or reference |
|---|---|---|
| W3110 | Prototroph | Laboratory stock |
| TON1816 | W3110 hns-3×FLAG Kmr | 12 |
| ZEU01 | W3110 stpA-3×FLAG Kmr | This work |
| ZEU02 | W3110 ΔstpA::cat | This work |
| ZEU03 | W3110 ΔstpA::cat hns-3×FLAG Kmr | This work |
| RM539 | W3110 Δhns::Kmr | 5 |
| ZEU04 | W3110 Δhns::KmrstpA-3×FLAG | This work |
| ZEU05 | W3110 Δhns::KmrstpA(F21C)-3×FLAG cat | This work |
| ZEU06 | W3110 Δhns::Kmr ΔstpA::cat | This work |
Using the FLAG-tagged strains, we first compared the genome-wide distribution of StpA and H-NS in otherwise wild-type cells (ZEU01 and TON1816 cells, respectively). Exponential-phase cultures at an optical density at 600 nm of 0.4 growing aerobically in LB medium were treated with formaldehyde to cross-link proteins to DNA, and DNA-protein complexes containing StpA or H-NS were immunoprecipitated with the anti-FLAG antibody. After de-cross-linking by heat treatment, coprecipitated DNA was amplified by PCR and terminally labeled for hybridization with a high-density oligonucleotide tiling chip. DNA fragments enriched by immunoprecipitation were quantitatively estimated by dividing the signal intensities of DNA in the immunoprecipitated fraction by those of DNA isolated from the whole-cell extract fraction before purification (enrichment factor). Visualization of enrichment factors for each probe along the genome coordinates revealed no significant differences between the distribution profiles of StpA and H-NS across the E. coli genome (Fig. 2, lanes 1 and 2; see also Fig. S4 in the supplemental material).
FIG. 2.
Typical binding profiles of H-NS and StpA in exponential-phase E. coli cells with different genetic backgrounds. H-NS and StpA binding signals (enrichment factors) in the region between bp 1,751,000 and 1,825,000 (A) and in the region between bp 2,049,000 and 2,137,000 (B) are demonstrated. Overlapping profiles of H-NS and StpA binding signals in wild-type cells are shown in lane 1 and lane 2. Unaffected H-NS binding signals in the stpA mutant (lane 3), reduction of both FLAG-tagged and native StpA binding sites (lane 4 and lane 5) in the hns mutant, and StpA(F21C) binding sites in the hns mutant (lane 6) are also represented. The enrichment factors for each 25-mer probe, calculated by dividing the signal intensities of the DNA in the immunoprecipitated fraction by those of the DNA in the supernatant, are shown by vertical bars at the corresponding positions on the genome. The enrichment factor is shown to the right of the distribution map. The positions and directions of the open reading frames are indicated at the bottom.
Next, we mapped the localization of H-NS and StpA in stpA and hns single mutants (ZEU03 and ZEU04 cells), respectively. We found that the inactivation of stpA had no effect on the distribution profile of H-NS (Fig. 2, lane 3), implying that H-NS has an ability to bind to the regions observed in wild-type cells without StpA. This feature of H-NS explains the lack of a discriminative phenotype for the stpA mutant; it has a normal growth rate (14, 17), and its transcriptome retains a profile similar to that of wild-type cells (9).
In contrast, there was a marked reduction in the number of StpA binding regions in hns mutant cells (Fig. 2, lane 4). For the method used to quantitatively estimate the dependency of StpA binding on the presence of H-NS, see the supplemental material. We counted 375 H-NS binding sites in wild-type cells, and 85% of these were also recognized as H-NS binding sites in the stpA mutant. By comparison, only 34% of the 474 binding sites for StpA in wild-type cells were recognized in the hns mutant. In these experiments, we observed impairment of growth of the hns mutant when the FLAG tag was fused to StpA (see Fig. S2 in the supplemental material). Therefore, we repeated the ChIP-chip experiment with anti-StpA antibody and found a similar reduction in the number of StpA binding sites (Fig. 2, lane 5; see Fig. S5 in the supplemental material).
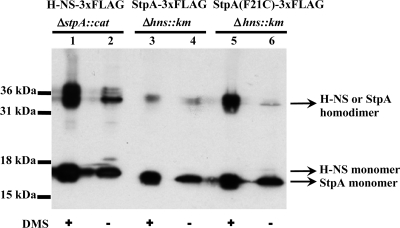
Dimers are the basic DNA binding blocks of H-NS and StpA, and the efficiency of dimer formation of StpA is known to be lower than that of H-NS (7, 8). Indeed, in vitro cross-linking experiments demonstrated that although the total cellular amounts of H-NS and StpA were comparable, the amount of StpA dimer was markedly lower than that of H-NS in the strains used here (Fig. 3). Thus, we replaced Phe 21 with Cys (F21C mutation) in StpA, which has been reported to improve the efficiency of dimerization (7), and the F21C mutation increased the dimerization ability of StpA to a level comparable to that of H-NS (Fig. 3). However, ChIP-chip analysis of StpA(F21C) with ZEU05 cells indicated that stabilization of the StpA dimer does not dramatically change the StpA binding profile of the hns mutant, increasing the relative ratio of StpA binding sites from 34 to 50% of the level in wild-type cells (Fig. 2, lane 6). These results suggest that the reduction in the number of StpA binding sites in the hns mutant is not due to the limited amount of the StpA dimer but resulted from differences between the intrinsic DNA binding properties of the StpA dimer and the H-NS dimer. It is also possible that the inactivation of hns induces a DNA structure unfavorable for StpA binding.
FIG. 3.
Dimerization proficiency of H-NS and StpA. Crude extracts of cells expressing either H-NS-3×FLAG or StpA/StpA(F21C)-3×FLAG were subjected to in vitro cross-linking and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Lanes 1 and 2, hns-3×FLAG ΔstpA::cat; lanes 3 and 4, stpA-3×FLAG Δhns::Kmr; lanes 5 and 6, stpA(F21C)-3×FLAG Δhns::Kmr. The protein bands were detected by immunoblotting with anti-FLAG antibodies. Sizes of protein standards are indicated on the left, and the presumed positions of H-NS and StpA dimers are indicated on the right. DMS, dimethyl suberimidate.
Wolf et al. reported that StpA has the ability to repress its own expression, even in the hns mutant, but does not repress the bgl operon in the hns mutant (16; see Fig. S3 in the supplemental material). Our findings are consistent with these observations and revealed that about two-thirds of the StpA binding sites in wild-type cells are dependent on H-NS, while the remaining one-third are recognized by the StpA dimer in the absence of H-NS. Our transcriptome analysis (12) indicated that the reduction of the StpA binding sites in hns mutant cells results in the desilencing of many genes that are covered by H-NS and StpA in wild-type cells, and this would lead to impairment of the growth rate of the hns mutant.
StpA binding spreads to regions covered by H-NS in wild-type cells. This spreading may occur through H-NS/StpA heterodimer formation and/or interaction between H-NS and StpA homodimers and indicates that StpA has the ability to function as a molecular backup for H-NS. Recently, Noom et al. proposed that most of the H-NS in the cell is bound to DNA, based on the calculation of the molecular number of H-NS necessary to cover the binding sites identified by ChIP-chip experiments (11). During the exponential phase, rapid DNA replication in bacterial cells may cause a temporal shortage of H-NS, where the supplemental function of StpA to maintain gene silencing and negatively supercoiled microdomains will be crucial, although growth conditions under which the stpA mutation shows severe effects await further study.
Inactivation of stpA in the hns mutant resulted in further impairment of cell growth, suggesting the importance of the remaining StpA binding in the hns mutant. Interestingly, a stringent response has been observed in the hns stpA double mutant, even under nonstringent conditions, probably because of the reduction of negative supercoiling of genomic DNA (6). The remaining level of StpA binding in the hns mutant might ensure minimal activity to maintain the genome DNA topology to ensure continued cell viability, although it is also possible that desilencing of StpA-bound genes has deleterious effects on cell growth.
Microarray data accession number.
Raw data (.CEL format) from the ChIP-chip experiments described here have been deposited in the ArrayExpress database (http://www.ebi.ac.uk/microarray-as/ae/) under accession no. E-MEXP-1880.
Supplementary Material
Acknowledgments
We are grateful to Chiharu Ueguchi and Koichi Ito for bacterial strains. We also thank Hirofumi Aiba for the H-NS antibody.
This work was supported by KAKENHI (Grant-in-Aid for Scientific Research) on Priority Areas Systems Genomics from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Footnotes
Published ahead of print on 16 January 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Azam, T. A., and A. Ishihama. 1999. Twelve species of the nucleoid-associated protein from Escherichia coli: sequence recognition specificity and DNA binding affinity. J. Biol. Chem. 27433105-33113. [DOI] [PubMed] [Google Scholar]
- 2.Dorman, C. J. 2004. H-NS: a universal regulator for a dynamic genome. Nat. Rev. Microbiol. 2391-400. [DOI] [PubMed] [Google Scholar]
- 3.Fang, F. C., and S. Rimsky. 2008. New insights into transcriptional regulation by H-NS. Curr. Opin. Microbiol. 11113-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Grainger, D. C., D. Hurd, M. D. Goldberg, and S. J. Busby. 2006. Association of nucleoid proteins with coding and non-coding segments of the Escherichia coli genome. Nucleic Acids Res. 344642-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardy, C. D., and N. R. Cozzarelli. 2005. A genetic selection for supercoiling mutants of Escherichia coli reveals proteins implicated in chromosome structure. Mol. Microbiol. 571636-1652. [DOI] [PubMed] [Google Scholar]
- 5.Ito, K., T. Oshima, T. Mizuno, and Y. Nakamura. 1994. Regulation of lysyl-tRNA synthetase expression by histone-like protein H-NS of Escherichia coli. J. Bacteriol. 1767383-7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansson, J., C. Balsalobre, S. Y. Wang, J. Urbonaviciene, D. J. Jin, B. Sonden, and B. E. Uhlin. 2000. Nucleoid proteins stimulate stringently controlled bacterial promoters: a link between the cAMP-CRP and the (p)ppGpp regulons in Escherichia coli. Cell 102475-485. [DOI] [PubMed] [Google Scholar]
- 7.Johansson, J., S. Eriksson, B. Sonden, S. N. Wai, and B. E. Uhlin. 2001. Heteromeric interactions among nucleoid-associated bacterial proteins: localization of StpA-stabilizing regions in H-NS of Escherichia coli. J. Bacteriol. 1832343-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansson, J., and B. E. Uhlin. 1999. Differential protease-mediated turnover of H-NS and StpA revealed by a mutation altering protein stability and stationary-phase survival of Escherichia coli. Proc. Natl. Acad. Sci. USA 9610776-10781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Lucchini, S., G. Rowley, M. D. Goldberg, M. Harrison, and J. C. Hinton. 2006. H-NS mediates the silencing of laterally acquired genes in bacteria. PLoS Pathog. 2e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Müller, C. M., U. Dobrindt, G. Nagy, L. Emody, B. E. Uhlin, and J. Hacker. 2006. Role of histone-like proteins H-NS and StpA in expression of virulence determinants of uropathogenic Escherichia coli. J. Bacteriol. 1885428-5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navarre, W. W., S. Porwollik, Y. Wang, M. McClelland, H. Rosen, S. J. Libby, and F. C. Fang. 2006. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science 313236-238. [DOI] [PubMed] [Google Scholar]
- 11.Noom, M. C., W. W. Navarre, T. Oshima, G. J. Wuite, and R. T. Dame. 2007. H-NS promotes looped domain formation in the bacterial chromosome. Curr. Biol. 17R913-R914. [DOI] [PubMed] [Google Scholar]
- 12.Reference deleted.
- 13.Rimsky, S. 2004. Structure of the histone-like protein H-NS and its role in regulation and genome superstructure. Curr. Opin. Microbiol. 7109-114. [DOI] [PubMed] [Google Scholar]
- 14.Sonden, B., and B. E. Uhlin. 1996. Coordinated and differential expression of histone-like proteins in Escherichia coli: regulation and function of the H-NS analog StpA. EMBO J. 154970-4980. [PMC free article] [PubMed] [Google Scholar]
- 15.Tupper, A. E., T. A. Owen-Hughes, D. W. Ussery, D. S. Santos, D. J. Ferguson, J. M. Sidebotham, J. C. Hinton, and C. F. Higgins. 1994. The chromatin-associated protein H-NS alters DNA topology in vitro. EMBO J. 13258-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf, T., W. Janzen, C. Blum, and K. Schnetz. 2006. Differential dependence of StpA on H-NS in autoregulation of stpA and in regulation of bgl. J. Bacteriol. 1886728-6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang, A., S. Rimsky, M. E. Reaban, H. Buc, and M. Belfort. 1996. Escherichia coli protein analogs StpA and H-NS: regulatory loops, similar and disparate effects on nucleic acid dynamics. EMBO J. 151340-1349. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.