Abstract
Haemophilus ducreyi is an obligate human pathogen and the causative agent of the sexually transmitted, genital ulcerative disease chancroid. The genome of strain 35000HP contains two known porin proteins, OmpP2A and OmpP2B. Loss of OmpP2A and OmpP2B expression in the mutant 35000HP::P2AB resulted in no obvious growth defect or phenotype. Comparison of outer membrane profiles indicated increased expression of the 58.5-kDa chaperone, GroEL, in the porin-deficient mutant. A proteomics-based comparison resulted in the identification of 231 proteins present in membrane-associated protein samples, of which a subset of 56 proteins was differentially expressed at a level of 1.5-fold or greater in the porin-deficient strain 35000HP::P2AB relative to that in 35000HP. Twenty of the differentially expressed proteins were selected for real-time PCR, resulting in the validation of 90% of the selected subgroup. Proteins identified in these studies suggested a decreased membrane stability phenotype, which was verified by disk diffusion assay. Loss of OmpP2A and OmpP2B resulted in global protein expression changes which appear to compensate for the absence of porin expression in 35000HP::P2AB.
Genital ulcers can result from infections with a number of sexually transmitted pathogens, including Haemophilus ducreyi (22). Infection with H. ducreyi is uncommon in the United States but has been identified as a cofactor in the transmission of human immunodeficiency virus in developing nations, where both diseases are endemic (14, 50). As with all gram-negative bacteria, the outer membrane (OM) is the primary permeability barrier for H. ducreyi (34, 35). Porin proteins are important components of the OM, comprising a significant portion of the OM protein content of and functioning as the primary means for hydrophilic solutes, wastes, and antimicrobial agents to cross the OM (34, 35). The genomes of enteric, gram-negative bacteria commonly possess several porin encoding genes (4, 19, 27, 37). However, the genome of 35000HP contains only two known porin genes, ompP2A and ompP2B. Interestingly, unlike 35000HP, most clinical isolates of H. ducreyi express OmpP2A exclusively (40). OmpP2A and OmpP2B share 27% to 33% homology with the OmpP2 porin of Haemophilus influenzae Rd (40, 45, 49). Deletion of ompP2 in H. influenzae type b resulted in a construct with a pronounced growth defect that was avirulent in vivo (9). In contrast to results of these previous studies, the deletion of both ompP2A and ompP2B in 35000HP::P2AB had no statistically significant effect on pustule formation in the human challenge model (20).
In the present study, we performed a proteomics-based, comparative analysis of 35000HP::P2AB to 35000HP in order to identify protein expression differences that may correlate with phenotypic differences caused by (or resulting from) the absence of OmpP2A and OmpP2B. We have detected the expression of 231 proteins, a subset of which is differentially expressed at both the protein and transcript level. These results suggest that a global change in protein expression occurs in 35000HP::P2AB which functionally compensates for the loss of OmpP2A and OmpP2B.
MATERIALS AND METHODS
Bacterial strains, culture media, and growth conditions.
H. ducreyi strains 35000HP and 35000HP::P2AB have been described previously (20, 47). These strains were routinely cultured at 35.5°C on supplemented chocolate agar or in H. ducreyi broth as described previously (8).
Membrane-associated protein isolation and analysis.
Total membrane preparations (MP) for sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-PAGE) were isolated as previously described (46). MP samples for the Protein Biomarker Discovery Service (ProtTech Inc., Norristown, PA) were isolated as described previously (46), with the following modification: protein samples were resuspended in a modified buffer Z containing 50 mM HEPES (final concentration) substituted for 50 mM Tris, pH 8.0 (final concentration), to prevent interference with the lysine residue acylation reaction. SDS-PAGE and Western immunoblot analysis were performed as described previously (25). All lanes contained 10 μg/ml of protein as determined by the Lowry protein assay (Sigma-Aldrich, Springfield, MO).
Antibody development and characterization.
We previously developed antisera specific to either OmpP2A or OmpP2B, and the development of monoclonal antibody (MAb) 2C7 has been described elsewhere (47). MAb 1B2-1B7 was purchased from the American Type Culture Collection and has been previously demonstrated to bind the lipooligosaccharide (LOS) of H. ducreyi (12, 13, 32, 54). The GroEL-specific MAb 2G3 was generated following whole-cell immunization with H. ducreyi strains 35000, CIP542, and 33921 utilizing a previously described protocol (7, 17).
RNA isolation.
Broth cultures inoculated with either 35000HP or 35000HP::P2AB were grown to an optical density at 600 nm of 0.950. Ten-milliliter aliquots were immediately treated with RNAlater (Ambion, Austin, TX) to prevent RNA degradation. RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. RNA samples were treated with Baseline-ZERO DNase (Epicentre, Madison, WI) to remove contaminating genomic DNA, and RNA clean up was performed using the RNeasy mini kit (Qiagen, Valencia, CA) RNA clean-up protocol as per the manufacturer's instructions. RNA was converted to cDNA using the high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA).
RT-PCR analysis.
All primers used in this study are listed in Table 1 and were designed using PrimerQuest Software (http://www.idtdna.com/Scitools/Applications/Primerquest/). Primer specificity and amplification efficiency were validated as described previously (29). Quantitative, real-time PCR (qRT-PCR) was performed in a Rotor-Gene 3000 (Corbett Research, Sydney, Australia) RT instrument using the QuantiTect SYBR green PCR kit (Qiagen) using the following thermocycling parameters: 95°C for 15 min, followed by 40 cycles of 94°C for 15 s, 60°C for 30 s, and 72°C for 30 s. RT data were collected by the Rotor-Gene 6 analysis program and transformed using the 2−ΔΔCT method in Microsoft Excel 2007 prior to statistical analysis in GraphPad Prism 4.0 (La Jolla, CA) (29).
TABLE 1.
Nucleotide sequences of qRT-PCR primers used in this study
| Primer | Sequence 5′ → 3′ |
|---|---|
| aceE-F | ACCGAGCCTTCTTTCGCTAGTTGT |
| aceE-R | TACGGATGGCTTTGGTCGTTCTGA |
| argH-F | GCACAAATTAGTGGCAACGGCTGA |
| argH-R | GCCAATGCACCACAAAATGGA |
| cafA-F | AAACCGCCGSGTTCATCACAATGG |
| cafA-R | GAACCAAAGGCGCTCGCTTAACAA |
| frdA-F | TTAATGACGGAAGGGTGTCGTGGT |
| frdA-R | TTTGTCACGTGGGCCTAACTCCAT |
| groEL-F | AACTTTAGTGGTTAATACTATGCGTGGT |
| groEL-R | ACGGTCGCCGAAGCC |
| HD1190-F | AGTGCAACGGACGGTGGTAATACT |
| HD1190-R | GCTCAAGAAGAAGCAGGCGGTTTA |
| HD1337-F | GGTGCTTGTTTATGGGCTGCCATT |
| HD1337-R | CGAAGAAATTCGCGCGATTATTGCACA |
| HD1400-F | GCACTCTGGCAACAACAGCCATTA |
| HD1400-R | CACGCTCTTTGCTTAACGCTGTGA |
| HD1654-F | GCCATTTAATGTAACCGCAGGGCA |
| HD1654-R | TAAATGGCGCCAATCGGCATTACC |
| imp-F | ACTTTGCGGGCGAAGAAATTA |
| imp-R | TGTGGTGCCTGTTGTTCAATTCGG |
| mukB-F | GTTGTCTTGCTGTTGGCGGTGTA |
| mukB-R | CGGAATTTAATCAGCAAGCGGCGA |
| nudH-F | ATGACGCAAGTCAGCCGGTATGTA |
| nudH-R | AACCCAACGCCAACCATCAAACTC |
| nusG-F | TTACGATGGCGAGGTTTATCCGCA |
| nusG-R | TGCCACGTGTAATGGGCTTCATTG |
| putP-F | AGGCGGTCGTCGTTTAGGTAGTTT |
| putP-R | GCCCGCAACCAGTTTCCAGTTAAA |
| recB-F | GACTTTCAACGCGCAGCAGAACAT |
| recB-R | TTGCTTGGAATGGCTGAATAGCGG |
| rluB-F | ACACTTACTTTAGCCGGTCGGGTT |
| rluB-R | AATTCAGCACCGTTTAGCACACCC |
| rpoD-F | AGTACAGTGTGCGGTGGCTGAATA |
| rpoD-R | TCTGCGACCACATCTGATGCTTCT |
| secB-F | TAGAAGACAGTGGCGATACGGCAT |
| secB-R | AGTACGCTAGGGCATTGTGATGCT |
| suhB-F | TTTACTGCGGTACGTGGTGAAGGT |
| suhB-R | TAGCACCGGTTAAATCACGACGCT |
| uup-F | TTGCCGAACCTTGTAATTCACGCC |
| uup-R | AAGGTATTAAAGCACGCCGAACGC |
Disk diffusion assays.
Disk diffusion assays were performed as described previously (30), with the following modifications. Chocolate agar plates, inoculated with 200 μl of 35000HP or 35000HP::P2AB suspended in brain heart infusion to an optical density at 600 nm of 0.2, were incubated for 30 min at 35.5°C, 5% CO2 prior to application of paper disks saturated with the appropriate detergent or hydrophobic antibiotic. Each detergent or hydrophobic antibiotic was assayed in quadruplicate during three independent experiments at the following concentrations: cetyltrimethylammonium bromide (CTAB; 100 mg/ml), N-lauroyl sarcosine (100 mg/ml), SDS (100 mg/ml), Triton X-100 (10%, vol/vol), Tween 20 (10%, vol/vol), novobiocin (10 mg/ml), polymyxin B (10 mg/ml), and deoxycholate (100 mg/ml). All chemicals were purchased from Sigma-Aldrich (Springfield, MO). Statistical significance was determined by a paired, two-tailed Student's t test in GraphPad Prism 4.0.
Protein differential expression analysis.
Differential expression of membrane-associated proteins was determined by the Protein Biomarker Discovery Service offered by ProtTech, Inc. This service utilizes 1-D gel electrophoresis coupled with subsequent light chromatography-tandem mass spectrometry (LC-MS-MS) analysis of isotope-coded affinity-tagged membrane-associated protein samples (28). This isotope-coded affinity-tagged technique, known as lysine-residue isotope tagging, is a proprietary extension of previously established N-terminal protein labeling techniques (33, 55, 56). Data analysis was performed as described previously (21).
MS analysis.
Matrix-assisted laser desorption ionization-MS services were performed at the Department of Biochemistry Proteomics Core Facility, University at Buffalo, SUNY.
RESULTS
Characterization of the 35000HP::P2AB mutant.
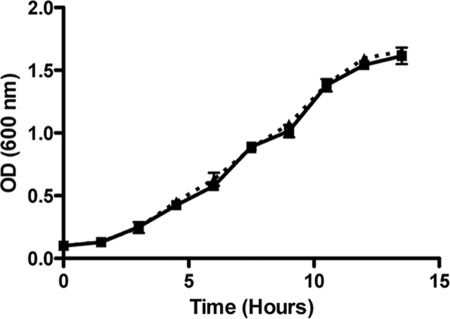
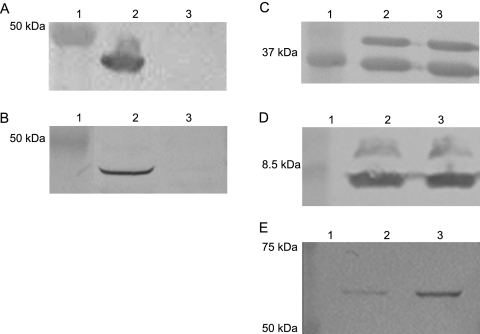
We have previously described the construction of a 35000HP mutant defective in expression of both OmpP2A and OmpP2B (20). Comparative growth analysis of 35000HP and 35000HP::P2AB demonstrated that the loss of both porins had no effect on growth in standard culture medium (Fig. 1). These data are in striking contrast to previous studies describing a severe growth defect in an H. influenzae type b OmpP2 mutant (9). In addition, deletion of classical porins in other gram-negative bacteria often results in either a growth defect or a lethal phenotype (1, 6, 8-10, 41). To explore possible explanations for this unexpected result, MPs were isolated from 35000HP and 35000HP::P2AB and analyzed for any differences in the protein profiles by SDS-PAGE (data not shown) and Western blot analysis (Fig. 2). Western blots probed with OmpP2A-specific (Fig. 2A) and OmpP2B-specific (Fig. 2B) antisera confirmed the proper phenotypes of 35000HP and 35000::P2AB. Whereas Western blots probed with the OmpA homolog-specific MAb 2C7 (Fig. 2C) and LOS-specific MAb 1B2-1B7 (Fig. 2D) demonstrate that 35000HP and 35000::P2AB expressed equivalent levels of these membrane components, a Western blot probed with MAb 2G3 demonstrated increased reactivity to 35000HP::P2AB relative to 35000HP (Fig. 2E). MAb 2G3 reacts to a 58.5-kDa protein with an apparent molecular weight consistent with the H. ducreyi heat shock and chaperonin protein GroEL (unpublished results). To confirm that MAb 2G3 was specific to GroEL, the 58.5-kDa band was excised and subjected to matrix-assisted laser desorption ionization-MS and peptide mass fingerprint analysis. The NCBI database was queried using the Mascot search engine (http://www.matrixscience.com), which returned a single, high-probability hit (Mowse score, 160) to the H. ducreyi GroEL.
FIG. 1.
Growth comparison of H. ducreyi 35000HP to the porin-deficient 35000HP::P2AB. The growth of 35000HP (squares) was compared to that of 35000HP::P2AB (triangles) in H. ducreyi broth. No discernible, statistically significant difference was identified in the doubling time of 35000HP compared to that of 35000HP::P2AB (P = 0.482; n = 3).
FIG. 2.
Observation of the increased expression of a 58.5-kDa protein in the porin-deficient mutant. Western blot analysis of 35000HP (lane 2) and 35000HP::P2AB (lane 3) total MPs using antibodies specific to OmpP2A (A), OmpP2B (B), OmpA2/MOMP (C), LOS (D), and GroEL (E). Molecular size standards (lane 1) are shown in kilodaltons.
Comparative proteomic analysis.
To determine if the increase in GroEL expression represented a singular response or was indicative of one or more previously unrecognized phenotypes, MP preparations from 35000HP and 35000HP::P2AB were compared for differential protein expression by ProtTech, Inc. (Norristown, PA). This 1-D gel electrophoresis coupled with a subsequent LC-MS-MS-based technique provides enhanced identification of membrane-associated and hydrophobic proteins compared to 2-D electrophoresis-based approaches (16, 52). A total of 231 proteins were identified between both strains, of which 170 (74%) have not been previously detected in prior H. ducreyi proteomics studies (Table 2), thus demonstrating the value of this method as a complement to standard 2-D electrophoresis-based techniques (39, 43). In total, 56 proteins were identified as being differentially expressed in the porin-deficient mutant relative to 35000HP (1.5-fold or greater), with the average differentially expressed protein being identified by 3.7 ± 0.6 peptides. Thirty-six proteins were increased in expression in 35000HP::P2AB relative to that in 35000HP, including the cytoplasmic chaperone SecB, the proline permease PutP, and the stress-associated dinucleoside polyphosphate hydrolase NudH. Twenty proteins were decreased in expression in 35000HP::P2AB relative to that in 35000HP, including the LOS/lipopolysaccharide (LPS) export protein Imp, the serum resistance protein DsrA, and the antiphagocytic proteins LspA1 and LspA2. A complete list of the differentially expressed proteins, exhibiting 1.5- to 33.3-fold changes in expression, is shown in Table 3. When the differentially expressed proteins were organized by the cluster of orthologous groups (COG) entry present for each protein in the 35000HP genome, a number of functional categories was identified (Fig. 3). Closer analysis of each functional category yielded interesting results (Fig. 3B). In particular, the number of differentially expressed metabolism-associated proteins indicated that a wide variety of metabolic processes had been affected. This change in metabolism is also mirrored by changes in proteins involved in transcription and translation. These data suggest that OmpP2A and OmpP2B may function as general diffusion pores, as has been described for OmpP2 of H. influenzae (51). Further analysis of the differentially expressed proteins identified a subset involved in membrane biogenesis. The number of affected chaperone, secretory, peptidoglycan, and membrane-associated proteins also suggests that OmpP2A and OmpP2B function in a structural capacity in the OM of 35000HP and that the loss of these proteins could result in a membrane biogenesis and/or stability defect. Finally, the loss of OmpP2A and OmpP2B resulted in the differential expression of several proteins with no defined function.
TABLE 2.
Complete list of membrane-associated proteins identified by Protein Biomarker Discovery Service
| NCBI RefSeq accession no. | Protein characteristic(s)a |
|---|---|
| NP_872672.1 | Major OM protein homolog, OmpA2 |
| NP_873123.1 | Lipoprotein Hlp |
| NP_872671.1 | Major OM protein |
| NP_872802.1 | Periplasmic zinc transporter |
| NP_873651.1 | Hypothetical OM protein |
| NP_873192.1 | 30S ribosomal protein S7b |
| NP_872695.1 | Periplasmic nitrate reductaseb |
| NP_873124.1 | Lipoprotein HlpBb |
| NP_874240.1 | FKBP-type peptidyl-prolyl cis-trans isomerase FkpA |
| NP_874283.1 | 30S ribosomal protein S4b |
| NP_872858.1 | 15-kDa OM lipoproteinb |
| NP_872909.1 | Manganese superoxide dismutase |
| NP_874147.1 | 60-kDa chaperonin |
| NP_873267.1 | Endo-1,4-beta-xylanase Ab |
| NP_873991.1 | 30S ribosomal protein S2 |
| NP_873158.1 | Heme binding protein |
| NP_873136.1 | Hypothetical protein HD0595b |
| NP_872850.1 | Hypothetical protein HD0256 |
| NP_874290.1 | 30S ribosomal protein S5b |
| NP_873316.1 | Hypothetical protein HD0805b |
| NP_873652.1 | OM protein D-15 |
| NP_874221.1 | 50S ribosomal protein L7/L12 |
| NP_874308.1 | 50S ribosomal protein L2b |
| NP_873552.1 | Spermidine/putrescine-binding periplasmic protein |
| NP_874222.1 | 50S ribosomal protein L10 |
| NP_873987.1 | Ribosome releasing factor |
| NP_874254.1 | Collagen adhesin NcaA |
| NP_874343.1 | Hemoglobin-binding protein HgbA |
| NP_873635.1 | OM protein P4 |
| NP_872793.1 | Chaperone protein DnaK |
| NP_874312.1 | 30S ribosomal protein S10b |
| NP_873494.1 | DNA-binding protein HUb |
| NP_873733.1 | Tight adherence protein Db |
| NP_874137.1 | 18,000-molecular-weight peptidoglycan-associated OM lipoprotein PAL |
| NP_873827.1 | Hypothetical protein HD1409b |
| NP_874224.1 | 50S ribosomal protein L11b |
| NP_873538.1 | Nucleoside diphosphate kinase |
| NP_874163.1 | Translation initiation factor IF3b |
| NP_873902.1 | 30S ribosomal protein S9 |
| NP_873019.1 | Pyruvate kinase II |
| NP_873133.1 | Hypothetical protein HD0591b |
| NP_873623.1 | Large supernatant protein 2b |
| NP_873911.1 | Large supernatant protein 1b |
| NP_874292.1 | 50S ribosomal protein L6b |
| NP_872899.1 | Transaldolase |
| NP_874286.1 | 50S ribosomal protein L36b |
| NP_873852.1 | OM protein P2 homolog |
| NP_874288.1 | 50S ribosomal protein L15b |
| NP_872690.1 | Fine tangled pili major subunit |
| NP_872796.1 | Hypothetical protein HD0192b |
| NP_874256.1 | DNA-directed RNA polymerase omega subunitb |
| NP_873235.1 | 3-Oxoacyl-(acyl-carrier-protein) reductase, truncated |
| NP_872807.1 | 50S ribosomal protein L27b |
| NP_873584.1 | Dihydrodipicolinate synthaseb |
| NP_874160.1 | Hypothetical protein HD1798b |
| NP_873288.1 | 50S ribosomal protein L32b |
| NP_873671.1 | Hypothetical protein HD1218b |
| NP_872936.1 | Heat shock protein HtpXb |
| NP_873637.1 | RNA polymerase sigma-70 factorb |
| NP_873668.1 | Small protein Ab |
| NP_873029.1 | Hypothetical protein HD0457b |
| NP_873262.1 | RNA-binding protein Hfqb |
| NP_874092.1 | 50S ribosomal protein L31b |
| NP_874225.1 | Transcription antitermination protein NusG |
| NP_874223.1 | 50S ribosomal protein L1 |
| NP_873210.1 | Hypothetical protein HD0680 |
| NP_874284.1 | 30S ribosomal protein S11b |
| NP_872663.1 | Coproporphyrinogen III oxidaseb |
| NP_872680.1 | Elongation factor Tu |
| NP_873194.1 | Elongation factor Tu |
| NP_874042.1 | Phosphoglyceromutase |
| NP_874291.1 | 50S ribosomal protein L18b |
| NP_874280.1 | 30S ribosomal protein S16b |
| NP_872641.1 | ATP synthase subunit Bb |
| NP_873551.1 | HSP-70 cofactor |
| NP_873350.1 | Superoxide dismutase [Cu-Zn]b |
| NP_873801.1 | Hypothetical protein HD1377b |
| NP_872827.1 | Phosphotransferase system phosphocarrier protein HPrb |
| NP_873040.1 | Conserved putative lipoproteinb |
| NP_873191.1 | 30S ribosomal protein S12b |
| NP_873285.1 | Serum resistance protein DsrA |
| NP_873532.1 | 30S ribosomal protein S18b |
| NP_873221.1 | RNA polymerase sigma factorb |
| NP_874075.1 | Organic solvent tolerance protein precursorb |
| NP_873197.1 | Export protein SecB |
| NP_873277.1 | Lipoproteinb |
| NP_873102.1 | Aminopeptidase A/Ib |
| NP_873292.1 | Hypothetical protein HD0776b |
| NP_874344.1 | Large-conductance mechanosensitive channelb |
| NP_872854.1 | Periplasmic serine protease do |
| NP_874177.1 | Iron (chelated) ABC transporter, periplasmic-binding protein |
| NP_873279.1 | Acid phosphatase stationary-phase survival proteinb |
| NP_874108.1 | Putative uroporphyrinogen III C-methyltransferaseb |
| NP_872940.1 | Hypothetical protein HD0358b |
| NP_874148.1 | Co-chaperonin GroES |
| NP_874289.1 | 50S ribosomal protein L30b |
| NP_874140.1 | Colicin transport protein, TolQb |
| NP_874120.1 | Ferritin-like protein 1 |
| NP_874012.1 | Dihydrolipoamide acetyltransferase |
| NP_873182.1 | Hypothetical protein HD0646b |
| NP_873496.1 | Hypothetical protein HD1006b |
| NP_873530.1 | 30S ribosomal protein S6 |
| NP_874117.1 | Preprotein translocase subunit YajCb |
| NP_874285.1 | 30S ribosomal protein S13b |
| NP_872698.1 | Possible cytochrome c-type proteinb |
| NP_872785.1 | 3-Hydroxydecanoyl-acyl carrier protein dehydrataseb |
| NP_873254.1 | 50S ribosomal protein L28b |
| NP_873904.1 | Hypothetical protein HD1495b |
| NP_874263.1 | Acyl carrier protein |
| NP_873249.1 | Hypothetical protein HD0725b |
| NP_874191.1 | Xanthine phosphoribosyltransferaseb |
| NP_873669.1 | Hypothetical protein HD1215b |
| NP_873895.1 | Integration host factor, alpha chainb |
| NP_874296.1 | 50S ribosomal protein L24b |
| NP_873876.1 | Translation initiation factor IF2b |
| NP_872806.1 | 50S ribosomal protein L21b |
| NP_873499.1 | Putative solute/DNA competence effectorb |
| NP_874282.1 | DNA-directed RNA polymerase alpha subunit |
| NP_874327.1 | Hypothetical protein HD2003 |
| NP_874111.1 | ABC-type transport protein Uupb |
| NP_873974.1 | Condensin subunit B |
| NP_874219.1 | DNA-directed RNA polymerase beta subunitb |
| NP_873097.1 | 5"-Phosphoribosylglycinamide transformylaseb |
| NP_873358.1 | 2-Dehydro-3-deoxyphosphooctonate aldolaseb |
| NP_873559.1 | Cysteine desulfurase |
| NP_873624.1 | Anaerobic glycerol-3-phosphate dehydrogenase, subunit Ab |
| NP_873795.1 | Hypothetical protein HD1371b |
| NP_873981.1 | ATP-dependent RNA helicaseb |
| NP_874039.1 | Hypothetical protein HD1654b |
| NP_874114.1 | DNA gyrase subunit Ab |
| NP_872657.1 | Fumarate reductase |
| NP_872776.1 | Dinucleoside polyphosphate hydrolaseb |
| NP_872798.1 | RNase Eb |
| NP_873314.1 | “Haemophilus somnus” lipoprotein C homologb |
| NP_873489.1 | Sodium/proline symporter, proline permeaseb |
| NP_873554.1 | Exodeoxyribonuclease V, beta subunitb |
| NP_873994.1 | GTP-binding protein Erab |
| NP_874019.1 | Cytoplasmic axial filament proteinb |
| NP_874161.1 | 50S ribosomal protein L20b |
| NP_874167.1 | Thiamine biosynthesis protein ThiIb |
| NP_874218.1 | DNA-directed RNA polymerase beta" subunitb |
| NP_872924.1 | Probable pseudouridylate synthaseb |
| NP_873015.1 | Inositol-1-monophosphataseb |
| NP_873352.1 | DNA polymerase III subunit betab |
| NP_873522.1 | Argininosuccinate lyaseb |
| NP_873683.1 | DNA polymerase Ib |
| NP_873822.1 | Putative soluble lytic murein transglycosylaseb |
| NP_873909.1 | Inositol-5-monophosphate dehydrogenase |
| NP_873978.1 | DNA topoisomerase IV subunit Ab |
| NP_874013.1 | 2-Oxoglutarate dehydrogenase |
| NP_874067.1 | Type III restriction enzymeb |
| NP_874255.1 | ATP-dependent DNA helicase RecGb |
| NP_874330.1 | ATP-dependent protease ATP-binding subunitb |
| NP_873678.1 | Transcriptional regulatory proteinb |
| NP_872770.1 | RNase activity regulator protein RraAb |
| NP_873187.1 | Opacity associated protein Ab |
| NP_873533.1 | 50S ribosomal protein L9 |
| NP_874341.1 | Hypothetical protein HD2023b |
| NP_872931.1 | Nitrate reductase, cytochrome c-type proteinb |
| NP_873712.1 | Protein-export membrane proteinb |
| NP_873322.1 | Arginine ABC transporter, periplasmic-binding proteinb |
| NP_874304.1 | 50S ribosomal protein L16b |
| NP_874305.1 | 30S ribosomal protein S3b |
| NP_873175.1 | Possible cell division proteinb |
| NP_873241.1 | Twin arginine translocase protein Ab |
| NP_873417.1 | Hypothetical protein HD0923b |
| NP_873558.1 | Hypothetical protein HD1080b |
| NP_873615.1 | Serine transporterb |
| NP_874095.1 | Na+/H+ antiporter proteinb |
| NP_874189.1 | Probable OM proteinb |
| NP_873253.1 | 50S ribosomal protein L33b |
| NP_874227.1 | Putative oligopeptide transporterb |
| NP_874302.1 | 30S ribosomal protein S17b |
| NP_872773.1 | Inorganic pyrophosphatase |
| NP_873718.1 | Single-strand DNA-binding protein |
| NP_873875.1 | Ribosome-binding factor Ab |
| NP_873990.1 | Elongation factor Ts |
| NP_874138.1 | Colicin tolerance proteinb |
| NP_874162.1 | 50S ribosomal protein L35b |
| NP_872836.1 | S-Adenosyl-methyltransferaseb |
| NP_872825.1 | Glucose-specific phosphotransferase system enzyme IIA componentb |
| NP_872882.1 | Hypothetical protein HD0292b |
| NP_873347.1 | Putative secreted proteaseb |
| NP_873491.1 | Hypothetical protein HD1001b |
| NP_873542.1 | Hypothetical protein HD1060b |
| NP_873812.1 | Molybdopterin converting factor subunit 2b |
| NP_874081.1 | Hypothetical protein HD1710b |
| NP_874171.1 | Thioredoxin |
| NP_872949.1 | Ferric uptake regulation proteinb |
| NP_873141.1 | Hypothetical protein HD0600 |
| NP_873397.1 | Cytolethal distending toxin protein Ab |
| NP_874145.1 | Cytochrome d ubiquinol oxidase, subunit Ib |
| NP_874229.1 | Peptide deformylaseb |
| NP_873284.1 | Mannose-specific phosphotransferase IIAB component |
| NP_873537.1 | Probable pseudouridine synthaseb |
| NP_873649.1 | (3R)-Hydroxymyristoyl-acyl carrier protein dehydrataseb |
| NP_873684.1 | Outer-membrane lipoprotein carrier protein precursorb |
| NP_873695.1 | 50S ribosomal protein L25b |
| NP_873769.1 | Thiol/disulfide interchange protein |
| NP_873885.1 | Hypothetical protein HD1472b |
| NP_874309.1 | 50S ribosomal protein L23b |
| NP_874277.1 | 50S ribosomal protein L19b |
| NP_872711.1 | Hypothetical protein HD0097b |
| NP_872838.1 | Penicillin-binding protein 3b |
| NP_872902.1 | Dipeptide transport system permease proteinb |
| NP_873167.1 | 2,3,4,5-Tetrahydropyridine-2-carboxylate N-succinyltransferase |
| NP_873199.1 | Trigger factor |
| NP_873226.1 | Possible TetR family transcriptional regulatorb |
| NP_873335.1 | UDP-glucose-4-epimeraseb |
| NP_874007.1 | Hypothetical protein HD1618b |
| NP_874027.1 | Possible integral membrane protein of DedA familyb |
| NP_874105.1 | 30S ribosomal protein S15b |
| NP_874192.1 | Aminoacyl-histidine dipeptidaseb |
| NP_874297.1 | 50S ribosomal protein L14b |
| NP_872715.1 | Hypothetical protein HD0101b |
| NP_872816.1 | Heme-binding protein Ab |
| NP_872842.1 | UDP-N-acetylmuramoyl-l-alanyl-d-glutamate synthetaseb |
| NP_872976.1 | Electron transport complex protein RnfC |
| NP_873011.1 | Transcription antitermination protein NusBb |
| NP_873283.1 | Mannose-specific phosphotransferase system IIC componentb |
| NP_873507.1 | Probable macrolide-specific efflux proteinb |
| NP_873672.1 | Hypothetical protein HD1219b |
| NP_873685.1 | Ribosomal protein S6 modification proteinb |
| NP_873703.1 | Exoribonuclease IIb |
| NP_873728.1 | Recombination factor protein RarAb |
| NP_873764.1 | Hypothetical protein HD1332b |
| NP_873846.1 | Alanyl-tRNA synthetaseb |
| NP_873849.1 | Hypothetical protein HD1432b |
| NP_873984.1 | Pyridoxine biosynthesis proteinb |
| NP_874196.1 | TDP-4-keto-6-deoxy-d-glucose transaminaseb |
| NP_874310.1 | 50S ribosomal protein L4b |
TABLE 3.
Differential expression of membrane-associated proteins in 35000HP::P2AB relative to that in 35000HP
| NCBI RefSeq accession no. | Protein | Function | COG assignment | No. of peptidesa | Fold change in expressionb |
|---|---|---|---|---|---|
| NP_872657.1 | FrdA | Fumarate reductase (flavoprotein subunit) | C | 2 | 4.2 |
| NP_872698.1 | NapB | Nitrate reductase (cytochrome c subunit) | C | 4 | 1.7 |
| NP_872776.1 | NudH | Dinucleoside polyphosphate hydrolase | LR | 2 | 2.2 |
| NP_872819.1 | ClpX | Periplasmic protease | O | 4 | ND |
| NP_872924.1 | RluB | Probable pseudouridylate synthase | J | 2 | 33.3 |
| NP_872976.1 | RnfC | Iron-sulfur binding NADH dehydrogenase | C | 5 | ND |
| NP_873015.1 | SuhB | Inositol-1-monophosphatase | G | 2 | 5.9 |
| NP_873097.1 | PurT | Phosphoribosylglycinamide formyltransferase | F | 4 | ND |
| NP_873171.1 | AccC | Acetyl coenzyme A carboxylase (biotin carboxylase subunit) | I | 3 | ND |
| NP_873182.1 | HD0646 | Ligand-gated channel (CirA superfamily) | S | 1 | ND |
| NP_873191.1 | RpsL | 30S ribosomal protein S12 | J | 4 | 2.0 |
| NP_873197.1 | SecB | Chaperone/export protein | U | 2 | ND |
| NP_873221.1 | RpoD | RNA polymerase general sigma factor | K | 3 | 2.6 |
| NP_873288.1 | RpmF | 50S ribosomal protein L32 | J | 2 | ND |
| NP_873292.1 | HD0776 | Protein of unknown function | S | 3 | ND |
| NP_873489.1 | PutP | Proline permease | ER | 2 | 6.7 |
| NP_873522.1 | ArgH | Argininosuccinate lyase | E | 2 | 3.9 |
| NP_873554.1 | RecB | Exodeoxyribonuclease V (beta subunit) | L | 2 | 25.0 |
| NP_873651.1 | HD1190 | Predicted OM protein (OmpH-like) | M | 2 | 2.0 |
| NP_873672.1 | HD1219 | Protein of unknown function | S | 3 | ND |
| NP_873728.1 | RarA | DNA recombination factor | L | 8 | ND |
| NP_873766.1 | Kgd | Alpha-ketoglutarate decarboxylase | C | 4 | ND |
| NP_873801.1 | HD1377 | Protein of unknown function | S | 3 | 6.3 |
| NP_873812.1 | MoaE | Molybdopterin converting factor (subunit 2) | H | 1 | ND |
| NP_873822.1 | HD1400 | Putative soluble lytic murein transglycosylase | M | 2 | 2.1 |
| NP_873873.1 | Pta | Phosphate acetyltransferase | CR | 3 | ND |
| NP_873912.1 | ManB | Probable phosphomannomutase | G | 4 | ND |
| NP_873974.1 | MukB | Chromatin remodeling | D | 2 | 2.1 |
| NP_873995.1 | Rnc | RNase III | K | 3 | ND |
| NP_874012.1 | AceF | Dihydrolipoamide acetyltransferase | C | 11 | ND |
| NP_874147.1 | GroEL | 60-kDa chaperonin | O | 12 | 3.3 |
| NP_874148.1 | GroES | Co-chaperonin with GroEL | O | 4 | 2.0 |
| NP_874162.1 | RpmI | 50S ribosomal protein L35 | J | 3 | 3.3 |
| NP_874219.1 | RpoB | DNA-directed RNA polymerase (beta subunit) | K | 2 | ND |
| NP_874225.1 | NusG | Transcription antitermination protein | K | 2 | 3.6 |
| NP_874227.1 | HD1887 | Putative oligopeptide transporter | S | 1 | ND |
| NP_873852.1 | OmpP2B | Porin | M | 1 | ND* |
| NP_873623.1 | LspA2 | Hemagglutinin-like macrophage phagocytic inhibitor | U | 30 | −2.0 |
| NP_874013.1 | AceE | Pyruvate dehydrogenase | C | 2 | −1.9 |
| NP_874019.1 | CafA | RNase G | J | 2 | −9.4 |
| NP_874039.1 | HD1654 | Protein of unknown function | S | 2 | −4.0 |
| NP_874075.1 | Imp | LPS/LOS export and organic solvent tolerance protein | M | 3 | −1.6 |
| NP_874111.1 | Uup | ABC-type transport protein | R | 2 | −1.5 |
| NP_874280.1 | RpsP | 30S ribosomal protein S16 | J | 3 | −1.5 |
| NP_874286.1 | RpmJ | 50S ribosomal protein L36 | J | 8 | −2.0 |
| NP_873133.1 | HD0591 | Putative two-component sensor kinase of LemA family | S | 3 | −1.5 |
| NP_873235.1 | FabG | 3-Oxoacyl-(acyl carrier protein) reductase | IQR | 1 | ND* |
| NP_873285.1 | DsrA | Serum resistance protein | UW | 1 | ND* |
| NP_873911.1 | LspA1 | Hemagglutinin-like macrophage phagocytic inhibitor | U | 10 | −2.0 |
| NP_874095.1 | NhaB | Na+/H+ antiporter protein | P | 1 | ND* |
| NP_874191.1 | Gpt | Xanthine-guanosine phosphoribosyltransferase | F | 1 | ND* |
| NP_874224.1 | RplK | 50S ribosomal protein L11 | J | 1 | ND* |
| NP_874256.1 | RpoZ | DNA-directed RNA polymerase omega subunit | K | 4 | −2.0 |
| NP_874282.1 | RpoA | DNA-directed RNA polymerase alpha subunit | K | 4 | −2.0 |
| NP_874285.1 | RpsM | 30S ribosomal protein S13 | J | 2 | −2.0 |
| NP_874312.1 | RpsJ | 30S ribosomal protein S10 | J | 5 | ND* |
Number of peptides used to identify the protein by LC-MS-MS analysis. The average differentially expressed protein was identified by 3.7 ± 0.6 peptides.
Several proteins were present in detectable quantities in one but not both samples, preventing quantitative analysis of protein expression. ND, not detected in 35000HP; ND*, not detected in 35000HP::P2AB.
FIG. 3.
COG classification of differentially expressed proteins in 35000HP::P2AB relative to those in 35000HP. (A) Proteins whose expression increased or decreased in 35000HP::P2AB relative to those in 35000HP were grouped by similar functional category: I, information storage and processing; II, cellular processing and signaling; III, metabolism; and IV, poorly categorized. (B) Differential expression of proteins within similar functional categories was further delineated by individual COG assignment. Proteins demonstrating increased expression in 35000HP::P2AB are denoted by solid bars, while proteins demonstrating decreased expression are denoted by hatched bars. Several proteins have multiple COG assignments; COG assignments for individual proteins are listed in Table 3.
Verification of proteomics data by qRT-PCR.
We validated our proteomics data by qRT-PCR analysis to correlate gene expression with protein expression. Twenty of the 56 proteins were selected for verification, representing slightly more than a third of the differentially expressed protein data set. These data correlated with the protein expression results, serving to validate 18 of the 20 selected proteins and representing a 90% accuracy rate for the Protein Biomarker Discovery Service (Table 4). The change in gene expression for cafA and mukB failed to meet the 1.5-fold cutoff value, indicating that either their cognate proteins are not increased in expression in the porin-deficient mutant or that posttranscriptional regulatory mechanisms are responsible for the increase in expression detected by the Protein Biomarker Discovery Service.
TABLE 4.
qRT-PCR analysis of gene expression for differentially expressed proteins
| NCBI accession no. | Gene | Mean fold change ± SEa |
|---|---|---|
| gi33149018 | groEL | 4.0 ± 2.5 |
| gi33151304 | frdA | 5.5 ± 1.0 |
| gi33151423 | nudH | 2.0 ± 0.4 |
| gi33151571 | rluB | 1.7 ± 0.06 |
| gi33151662 | suhB | 2.2 ± 0.3 |
| gi33151844 | secB | 2.1 ± 0.7 |
| gi33151868 | rpoD | 1.9 ± 0.3 |
| gi33152136 | putP | 2.1 ± 0.04 |
| gi33152169 | argH | 2.3 ± 0.6 |
| gi33152201 | recB | 3.4 ± 1.1 |
| gi33152298 | HD1190 | 4.1 ± 2.3 |
| gi33152448 | HD1377 | 2.6 ± 0.6 |
| gi33152469 | HD1400 | 1.9 ± 0.2 |
| gi33152621 | mukB | 1.2 ± 0.09 |
| gi33152660 | aceE | −3.5 ± 0.2 |
| gi33152666 | cafA | −1.4 ± 0.1 |
| gi33152686 | HD1654 | −2.3 ± 0.1 |
| gi33152722 | imp | −3.4 ± 0.8 |
| gi33152758 | uup | −5.5 ± 1.0 |
| gi33152872 | nusG | 2.3 ± 0.5 |
Mean fold change in gene expression in 35000HP::P2AB relative to 35000HP, normalized to gyrA expression as previously described (32).
35000HP::P2AB exhibits increased membrane permeability to hydrophobic agents.
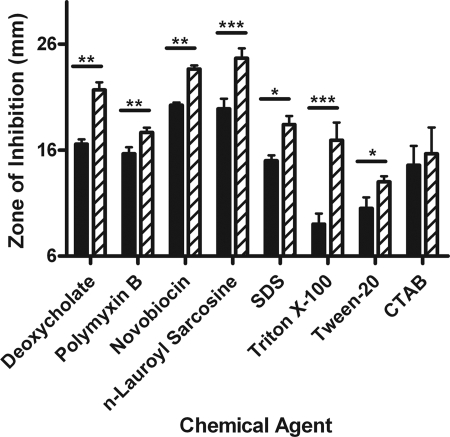
Changes in proteins associated with LPS/LOS export (Imp), peptidoglycan biosynthesis (HD1400), the OM (LspA1/2 and DsrA), and stress-associated chaperone function (GroEL/ES) in 35000HP::P2AB suggested that the loss of OmpP2A and OmpP2B may play a role in maintaining the structural integrity of the OM. We initially performed antibiotic sensitivity studies which showed that 35000HP::P2AB was more susceptible to erythromycin, a porin-independent antibiotic, but more resistant to the porin-dependent antibiotics tetracycline, ciprofloxacin, and tigecycline compared to the wild type (data not shown). We performed subsequent disk diffusion assays assessing the stability of the membrane to challenge from detergents and hydrophobic antibiotics. These data demonstrated that the porin-deficient mutant was more sensitive than the wild type to all detergents tested with the exception of the cationic detergent CTAB (Fig. 4). 35000HP::P2AB also exhibited increased sensitivity to hydrophobic antibiotics that do not enter the cell through porin proteins (Fig. 4 and data not shown). Taken together, these data indicate that the loss of OmpP2A and OmpP2B renders the OM more permeable to hydrophobic solutes.
FIG. 4.
Disk diffusion analysis of membrane stability. Disk diffusion assays were performed to evaluate the stability of 35000HP (solid bars) and 35000HP::P2AB (hatched bars). Asterisks denote values that differ in a statistically significant manner at a P value of 0.05 (*), 0.005 (**), or 0.0005 (***).
DISCUSSION
The loss of classical porin expression in gram-negative pathogens often results in decreased fitness in both in vitro and in vivo environments (1, 6, 8-10, 41). However, 35000HP::P2AB did not exhibit any loss of viability or demonstrate any growth defect in vitro or in vivo in the human challenge model (20). Proteomic comparison of 35000HP::P2AB to 35000HP identified 231 proteins, 56 of which were determined to be differentially expressed. The differentially expressed proteins represented 18 separate COG classifications whose functions were predominantly associated with metabolism, protein trafficking, and membrane biogenesis. Differential expression was verified by qRT-PCR for 18 out of 20 selected proteins, representing a 90% success rate among the tested subset. Taken together, we suggest that the loss of OmpP2A and OmpP2B expression in 35000HP::P2AB results in global changes in protein expression and affects a wide range of cellular processes, the alteration of which appears to compensate for the loss of porin function in standard growth conditions.
Analysis of the COG assignments of the differentially expressed proteins indicates several interesting deviations in global protein expression in 35000HP::P2AB relative to that in 35000HP. Alterations in the metabolism-associated proteins are more numerous than any other COG category. Increased expression of the putative oligopeptide permease HD1887 and the proline permease PutP is anticipated to facilitate increased proline uptake in the porin-deficient mutant, an activity that has been demonstrated to help Escherichia coli survive a multitude of environmental stresses (23, 44, 53). Likewise, expression of argininosuccinate lyase (ArgH) was similarly increased. ArgH catalyzes the conversion of argininosuccinate into fumarate and arginine. Arginine stockpiling has also been demonstrated to occur in E. coli under a number of stressful growth conditions, including low pH and phosphate, nitrogen, and carbon deprivation (11, 18, 34). Such changes suggest that OmpP2A and OmpP2B function as general diffusion pores and/or facilitate the specific uptake of one or more cofactors involved in multiple metabolic pathways (2, 51).
The decreased expression of LspA2 and DsrA in the porin-deficient mutant is another interesting observation. Although the mechanisms that transport LspA2 and DsrA to the OM and surface are different, the energy expended in synthesizing and exporting these proteins is fairly significant. Thus, it is possible that the decreased expression of these two proteins may represent an attempt to minimize the metabolic burden on the porin-deficient mutant consistent with the alterations in metabolic activity mentioned above. In contrast, expression of MOMP and OmpA2, two major OM protein components, was unchanged. These data suggest that these last two proteins are more important for the survival and growth of the porin mutant in vitro than LspA2 and DsrA. However, far more mechanistic studies are needed to more accurately address these observations.
GroEL involvement in stress responses is well studied for many bacteria, including H. ducreyi (18, 26, 36, 38, 48). 35000HP::P2AB exhibits increased expression of NudH, a dinucleoside polyphosphate hydrolase involved in the breakdown of toxic compounds, maintenance of normal metabolite pools, and the degradation of intercellular signaling molecules, including the alarmone diadenosine tetraphosphate (AP4A) (3, 31). As diadenosine oligophosphates such as AP4A have been demonstrated to link chaperone expression and stress responses in other organisms, it is possible a similar mechanism is at work in 35000HP::P2AB (3, 31). Similarly, NudH may function to integrate membrane and metabolic stress responses within the porin-deficient mutant (3, 31).
SecB is a cytoplasmic chaperone responsible for the primary binding of nascently synthesized polypeptides bound for the OM (11). Increased SecB expression in 35000HP::P2AB is matched by a similar increase in the expression of the conserved hypothetical protein, HD1190. In silico analysis of HD1190 demonstrated the presence of an OmpH-like sequence motif by CDART (conserved domain architecture tool) analysis and that it is highly similar to OmpH-like proteins of other Pasteurellaceae by BLAST (15). OmpH, also known as Skp, is one of three periplasmic chaperones that bind immature OMPs and target them to the OM for transport (24, 42). Several prominent secreted and OM proteins, notably LspA1/2 and DsrA, are decreased in expression in the porin-deficient mutant. The changes in translation-associated, chaperone, and OM protein expression in 35000HP::P2AB suggest alterations in protein export and/or secretion in the absence of OmpP2A and OmpP2B.
Increased expression of HD1400, a putative lytic murein transglycosylase may permit increased protein secretion into the periplasm or may increase cell wall permeability. The loss of OmpP2A and OmpP2B also resulted in the decreased expression of the Imp protein. In E. coli, Imp is an essential OM protein required for the export of LPS (5). Phenotypic analyses of Imp mutants demonstrated increased sensitivity to detergents and hydrophobic antibiotics, as well as increased membrane permeability to maltodextrins (5, 41). As inferred from the decreased expression of Imp in 35000HP::P2AB, the porin-deficient mutant exhibits increased sensitivity to both hydrophobic antibiotics and detergents (Fig. 4). These observations suggest that 35000HP::P2AB may be subject to increased cell envelope permeability, at both the OM and the cell wall, and that this property may partially offset the loss of OmpP2A and OmpP2B in a nonspecific manner. The decreased membrane stability of the porin-deficient mutant is interesting because this strain remains virulent in the human challenge model (20). It is possible that clearance of H. ducreyi in this model does not involve membrane stability and it is also possible that the porin-deficient mutant may be less virulent in the later stages of infection, a parameter that cannot be measured in this human system. However, more studies are needed to address these hypotheses.
To our knowledge, this report constitutes the first comparative proteomic analysis of a bacterium deficient in the expression of both known porin proteins. While the loss of OmpP2A and OmpP2B expression in 35000HP::P2AB has multiple effects on bacterial physiology, this mutant has no obvious growth defect and remains fully virulent in vivo (20). The survival of the porin-deficient mutant is an important observation as porin mutants in Haemophilus spp. exhibit severe phenotypic defects, including loss of viability (9). Additionally, we cannot rule out the possibility that other presently undetected proteins may also demonstrate altered expression or function to compensate for the loss of OmpP2A and OmpP2B in 35000HP::P2AB. We are currently extending our analyses of the proteins identified in this study with particular emphasis on membrane stability and permeability, metabolic profiling, and nutrient uptake. These data will be instrumental in characterizing the general and specific functions of OmpP2A and OmpP2B for H. ducreyi biology and pathogenesis.
Acknowledgments
This work was supported by a grant to AAC from the John R. Oishei Foundation.
We thank N. R. Luke and S. R. Gill for critical review of the manuscript and D. Zhang (ProtTech, Inc.) for assistance and advice in the interpretation of the proteomics data.
Footnotes
Published ahead of print on 19 December 2008.
REFERENCES
- 1.Afkar, E., G. Reguera, M. Schiffer, and D. R. Lovley. 2005. A novel Geobacteraceae-specific outer membrane protein J (OmpJ) is essential for electron transport to Fe(III) and Mn(IV) oxides in Geobacter sulfurreducens. BMC Microbiol. 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen, C., E. Maier, G. Kemmer, J. Blass, A. K. Hilpert, R. Benz, and J. Reidl. 2003. Porin OmpP2 of Haemophilus influenzae shows specificity for nicotinamide-derived nucleotide substrates. J. Biol. Chem. 27824269-24276. [DOI] [PubMed] [Google Scholar]
- 3.Bessman, M. J., D. N. Frick, and S. F. O'Handley. 1996. The MutT proteins or “Nudix” hydrolases, a family of versatile, widely distributed, “housecleaning” enzymes. J. Biol. Chem. 27125059-25062. [DOI] [PubMed] [Google Scholar]
- 4.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 2771453-1474. [DOI] [PubMed] [Google Scholar]
- 5.Braun, M., and T. J. Silhavy. 2002. Imp/OstA is required for cell envelope biogenesis in Escherichia coli. Mol. Microbiol. 451289-1302. [DOI] [PubMed] [Google Scholar]
- 6.Bucarey, S. A., N. A. Villagra, M. P. Martinic, A. N. Trombert, C. A. Santiviago, N. P. Maulen, P. Youderian, and G. C. Mora. 2005. The Salmonella enterica serovar Typhi tsx gene, encoding a nucleoside-specific porin, is essential for prototrophic growth in the absence of nucleosides. Infect. Immun. 736210-6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campagnari, A. A., T. F. Ducey, and C. A. Rebmann. 1996. Outer membrane protein B1, an iron-repressible protein conserved in the outer membrane of Moraxella (Branhamella) catarrhalis, binds human transferrin. Infect. Immun. 643920-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carbonetti, N. H., V. I. Simnad, H. S. Seifert, M. So, and P. F. Sparling. 1988. Genetics of protein I of Neisseria gonorrhoeae: construction of hybrid porins. Proc. Natl. Acad. Sci. USA 856841-6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cope, L. D., S. E. Pelzel, J. L. Latimer, and E. J. Hansen. 1990. Characterization of a mutant of Haemophilus influenzae type b lacking the P2 major outer membrane protein. Infect. Immun. 583312-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darcan, C., R. Ozkanca, and K. P. Flint. 2003. Survival of nonspecific porin-deficient mutants of Escherichia coli in black sea water. Lett. Appl. Microbiol. 37380-385. [DOI] [PubMed] [Google Scholar]
- 11.Driessen, A. J. 2001. SecB, a molecular chaperone with two faces. Trends Microbiol. 9193-196. [DOI] [PubMed] [Google Scholar]
- 12.Edwards, K. J., S. Allen, B. W. Gibson, and A. A. Campagnari. 2005. Characterization of a cluster of three glycosyltransferase enzymes essential for Moraxella catarrhalis lipooligosaccharide assembly. J. Bacteriol. 1872939-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filiatrault, M. J., B. W. Gibson, B. Schilling, S. Sun, R. S. Munson, Jr., and A. A. Campagnari. 2000. Construction and characterization of Haemophilus ducreyi lipooligosaccharide (LOS) mutants defective in expression of heptosyltransferase III and β1,4-glucosyltransferase: identification of LOS glycoforms containing lactosamine repeats. Infect. Immun. 683352-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleming, D. T., and J. N. Wasserheit. 1999. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex. Transm. Infect. 753-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geer, L. Y., M. Domrachev, D. J. Lipman, and S. H. Bryant. 2002. CDART: protein homology by domain architecture. Genome Res. 121619-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Görg, A., W. Weiss, and M. J. Dunn. 2004. Current two-dimensional electrophoresis technology for proteomics. Proteomics 43665-3685. [DOI] [PubMed] [Google Scholar]
- 17.Haase, E. M., A. A. Campagnari, J. Sarwar, M. Shero, M. Wirth, C. U. Cumming, and T. F. Murphy. 1991. Strain-specific and immunodominant surface epitopes of the P2 porin protein of nontypeable Haemophilus influenzae. Infect. Immun. 591278-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hennequin, C., A. Collignon, and T. Karjalainen. 2001. Analysis of expression of GroEL (Hsp60) of Clostridium difficile in response to stress. Microb. Pathog. 31255-260. [DOI] [PubMed] [Google Scholar]
- 19.Iacono, M., L. Villa, D. Fortini, R. Bordoni, F. Imperi, R. Bonnal, T. Sicheritz-Ponten, G. De Bellis, P. Visca, A. Cassone, and A. Carattoli. 2008. Whole-genome pyrosequencing of an epidemic multidrug-resistant Acinetobacter baumannii strain belonging to the European clone II group. Antimicrob. Agents Chemother. 522616-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janowicz, D., N. R. Luke, K. R. Fortney, B. P. Katz, A. A. Campagnari, and S. M. Spinola. 2006. Expression of OmpP2A and OmpP2B is not required for pustule formation by Haemophilus ducreyi in human volunteers. Microb. Pathog. 40110-115. [DOI] [PubMed] [Google Scholar]
- 21.Jin, S., D. S. Daly, D. L. Springer, and J. H. Miller. 2008. The effects of shared peptides on protein quantitation in label-free proteomics by LC/MS/MS. J. Proteome Res. 7164-169. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, L. F., D. J. Coetzee, and R. E. Dorrington. 2005. Sentinel surveillance of sexually transmitted infections in South Africa: a review. Sex. Transm. Infect. 81287-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kempf, B., and E. Bremer. 1998. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol. 170319-330. [DOI] [PubMed] [Google Scholar]
- 24.Kleinschmidt, J. H. 2003. Membrane protein folding on the example of outer membrane protein A of Escherichia coli. Cell. Mol. Life Sci. 601547-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klesney-Tait, J., T. J. Hiltke, I. Maciver, S. M. Spinola, J. D. Radolf, and E. J. Hansen. 1997. The major outer membrane protein of Haemophilus ducreyi consists of two OmpA homologs. J. Bacteriol. 1791764-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Layton, J. C., and P. L. Foster. 2005. Error-prone DNA polymerase IV is regulated by the heat shock chaperone GroE in Escherichia coli. J. Bacteriol. 187449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, D. G., J. M. Urbach, G. Wu, N. T. Liberati, R. L. Feinbaum, S. Miyata, L. T. Diggins, J. He, M. Saucier, E. Deziel, L. Friedman, L. Li, G. Grills, K. Montgomery, R. Kucherlapati, L. G. Rahme, and F. M. Ausubel. 2006. Genomic analysis reveals that Pseudomonas aeruginosa virulence is combinatorial. Genome Biol. 7R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, J., H. Steen, and S. P. Gygi. 2003. Protein profiling with cleavable isotope-coded affinity tag (cICAT) reagents: the yeast salinity stress response. Mol. Cell. Proteomics 21198-1204. [DOI] [PubMed] [Google Scholar]
- 29.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 30.Luke, N. R., S. Allen, B. W. Gibson, and A. A. Campagnari. 2003. Identification of a 3-deoxy-d-manno-octulosonic acid biosynthetic operon in Moraxella catarrhalis and analysis of a KdsA-deficient isogenic mutant. Infect. Immun. 716426-6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLennan, A. G. 2006. The Nudix hydrolase superfamily. Cell. Mol. Life Sci. 63123-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melaugh, W., N. J. Phillips, A. A. Campagnari, R. Karalus, and B. W. Gibson. 1992. Partial characterization of the major lipooligosaccharide from a strain of Haemophilus ducreyi, the causative agent of chancroid, a genital ulcer disease. J. Biol. Chem. 26713434-13439. [PubMed] [Google Scholar]
- 33.Nam, H. W., R. Simpson, and Y. S. Kim. 2005. N-terminal isotope tagging with propionic anhydride: proteomic analysis of myogenic differentiation of C2C12 cells. J. Chromatogr. B 82691-107. [DOI] [PubMed] [Google Scholar]
- 34.Nikaido, H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67593-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nikaido, H., and M. Vaara. 1985. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 491-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park, S. H., K. H. Oh, and C. K. Kim. 2001. Adaptive and cross-protective responses of Pseudomonas sp. DJ-12 to several aromatics and other stress shocks. Curr. Microbiol. 43176-181. [DOI] [PubMed] [Google Scholar]
- 37.Parkhill, J., G. Dougan, K. D. James, N. R. Thomson, D. Pickard, J. Wain, C. Churcher, K. L. Mungall, S. D. Bentley, M. T. Holden, M. Sebaihia, S. Baker, D. Basham, K. Brooks, T. Chillingworth, P. Connerton, A. Cronin, P. Davis, R. M. Davies, L. Dowd, N. White, J. Farrar, T. Feltwell, N. Hamlin, A. Haque, T. T. Hien, S. Holroyd, K. Jagels, A. Krogh, T. S. Larsen, S. Leather, S. Moule, P. O'Gaora, C. Parry, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413848-852. [DOI] [PubMed] [Google Scholar]
- 38.Parsons, L. M., R. J. Limberger, and M. Shayegani. 1997. Alterations in levels of DnaK and GroEL result in diminished survival and adherence of stressed Haemophilus ducreyi. Infect. Immun. 652413-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Post, D. M., and B. W. Gibson. 2007. Proposed second class of Haemophilus ducreyi strains show altered protein and lipooligosaccharide profiles. Proteomics 73131-3142. [DOI] [PubMed] [Google Scholar]
- 40.Prather, D. T., M. Bains, R. E. Hancock, M. J. Filiatrault, and A. A. Campagnari. 2004. Differential expression of porins OmpP2A and OmpP2B of Haemophilus ducreyi. Infect. Immun. 726271-6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sampson, B. A., R. Misra, and S. A. Benson. 1989. Identification and characterization of a new gene of Escherichia coli K-12 involved in outer membrane permeability. Genetics 122491-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schäfer, U., K. Beck, and M. Muller. 1999. Skp, a molecular chaperone of gram-negative bacteria, is required for the formation of soluble periplasmic intermediates of outer membrane proteins. J. Biol. Chem. 27424567-24574. [DOI] [PubMed] [Google Scholar]
- 43.Scheffler, N. K., A. M. Falick, S. C. Hall, W. C. Ray, D. M. Post, R. S. Munson, Jr., and B. W. Gibson. 2003. Proteome of Haemophilus ducreyi by 2-D SDS-PAGE and mass spectrometry: strain variation, virulence, and carbohydrate expression. J. Proteome Res. 2523-533. [DOI] [PubMed] [Google Scholar]
- 44.Shahjee, H. M., K. Banerjee, and F. Ahmad. 2002. Comparative analysis of naturally occurring l-amino acid osmolytes and their d-isomers on protection of Escherichia coli against environmental stresses. J. Biosci. 27515-520. [DOI] [PubMed] [Google Scholar]
- 45.Sikkema, D. J., and T. F. Murphy. 1992. Molecular analysis of the P2 porin protein of nontypeable Haemophilus influenzae. Infect. Immun. 605204-5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spinola, S. M., G. E. Griffiths, K. L. Shanks, and M. S. Blake. 1993. The major outer membrane protein of Haemophilus ducreyi is a member of the OmpA family of proteins. Infect. Immun. 611346-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spinola, S. M., L. M. Wild, M. A. Apicella, A. A. Gaspari, and A. A. Campagnari. 1994. Experimental human infection with Haemophilus ducreyi. J. Infect. Dis. 1691146-1150. [DOI] [PubMed] [Google Scholar]
- 48.Spory, A., A. Bosserhoff, C. von Rhein, W. Goebel, and A. Ludwig. 2002. Differential regulation of multiple proteins of Escherichia coli and Salmonella enterica serovar Typhimurium by the transcriptional regulator SlyA. J. Bacteriol. 1843549-3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Srikumar, R., D. Dahan, M. F. Gras, M. J. Ratcliffe, L. van Alphen, and J. W. Coulton. 1992. Antigenic sites on porin of Haemophilus influenzae type b: mapping with synthetic peptides and evaluation of structure predictions. J. Bacteriol. 1744007-4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steen, R. 2001. Eradicating chancroid. Bull. W. H. O. 79818-826. [PMC free article] [PubMed] [Google Scholar]
- 51.Vachon, V., D. J. Lyew, and J. W. Coulton. 1985. Transmembrane permeability channels across the outer membrane of Haemophilus influenzae type b. J. Bacteriol. 162918-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilkins, M. R., E. Gasteiger, J. C. Sanchez, A. Bairoch, and D. F. Hochstrasser. 1998. Two-dimensional gel electrophoresis for proteome projects: the effects of protein hydrophobicity and copy number. Electrophoresis 191501-1505. [DOI] [PubMed] [Google Scholar]
- 53.Wood, J. M., E. Bremer, L. N. Csonka, R. Kraemer, B. Poolman, T. van der Heide, and L. T. Smith. 2001. Osmosensing and osmoregulatory compatible solute accumulation by bacteria. Comp. Biochem. Physiol. A 130437-460. [DOI] [PubMed] [Google Scholar]
- 54.Young, W. W., Jr., J. Portoukalian, and S. Hakomori. 1981. Two monoclonal anticarbohydrate antibodies directed to glycosphingolipids with a lacto-N-glycosyl type II chain. J. Biol. Chem. 25610967-10972. [PubMed] [Google Scholar]
- 55.Zappacosta, F., and R. S. Annan. 2004. N-terminal isotope tagging strategy for quantitative proteomics: results-driven analysis of protein abundance changes. Anal. Chem. 766618-6627. [DOI] [PubMed] [Google Scholar]
- 56.Zhang, X., Q. K. Jin, S. A. Carr, and R. S. Annan. 2002. N-terminal peptide labeling strategy for incorporation of isotopic tags: a method for the determination of site-specific absolute phosphorylation stoichiometry. Rapid Commun. Mass Spectrometry 162325-2332. [DOI] [PubMed] [Google Scholar]