Abstract
AspT, the aspartate:alanine antiporter of Tetragenococcus halophilus, a membrane protein of 543 amino acids with 10 putative transmembrane (TM) helices, is the prototype of the aspartate:alanine exchanger (AAE) family of transporters. Because TM3 (isoleucine 64 to methionine 85) has many amino acid residues that are conserved among members of the AAE family and because TM3 contains two charged residues and four polar residues, it is thought to be located near (or to form part of) the substrate translocation pathway that includes the binding site for the substrates. To elucidate the role of TM3 in the transport process, we carried out cysteine-scanning mutagenesis. The substitutions of tyrosine 75 and serine 84 had the strongest inhibitory effects on transport (initial rates of l-aspartate transport were below 15% of the rate for cysteine-less AspT). Considerable but less-marked effects were observed upon the replacement of methionine 70, phenylalanine 71, glycine 74, arginine 76, serine 83, and methionine 85 (initial rates between 15% and 30% of the rate for cysteine-less AspT). Introduced cysteine residues at the cytoplasmic half of TM3 could be labeled with Oregon green maleimide (OGM), whereas cysteines close to the periplasmic half (residues 64 to 75) were not labeled. These results suggest that TM3 has a hydrophobic core on the periplasmic half and that hydrophilic residues on the cytoplasmic half of TM3 participate in the formation of an aqueous cavity in membranes. Furthermore, the presence of l-aspartate protected the cysteine introduced at glycine 62 against a reaction with OGM. In contrast, l-aspartate stimulated the reactivity of the cysteine introduced at proline 79 with OGM. These results demonstrate that TM3 undergoes l-aspartate-induced conformational alterations. In addition, nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis analyses and a glutaraldehyde cross-linking assay suggest that functional AspT forms homo-oligomers as a functional unit.
In some strains of the lactic acid bacterium Tetragenococcus halophilus, a proton motive force (PMF) is generated by the combined action of an intracellular l-aspartate decarboxylation reaction catalyzed by an l-aspartate-4-decarboxylase (AspD [EC 4.1.1.12]) and an electrogenic aspartate1−:alanine0 exchange reaction catalyzed by an aspartate:alanine antiporter (AspT [TC# 2.A.81.1.1]): l-aspartate (out) + l-alanine (in) → l-aspartate (in) + l-alanine (out). The PMF generated is sufficiently high to drive ATP synthesis via the bacterial FoF1 ATPase. This combination of PMF and ATP synthesis has been proposed as a proton motive metabolic cycle, and the prototype model is found in Oxalobacter formigenes (3, 9, 30). Such decarboxylation reactions are thought to be advantageous for cells because the reactions generate metabolic energy and regulate the intracellular pH. In previous works using proteoliposomes, we found that the aspartate:alanine exchange catalyzed by AspT is electrogenic (1, 2). The biochemical features of substrate transport by AspT indicate that the protein can be classified as a conventional secondary transport protein and that it is an electrogenic antiporter similar to the prototype precursor:product exchanger OxlT, an oxalate:formate antiporter that is a member of the major facilitator superfamily, from O. formigenes (3, 9, 30, 39). AspT belongs to the newly classified aspartate:alanine exchanger (AAE) family (TC# 2.A.81) of transporters in the transporter classification system developed by Saier et al. (http://www.tcdb.org/index.php). Recently, the results of a BLAST (http://www.ncbi.nlm.nih.gov/BLAST/; 7) search of the nucleotide sequence of the aspT gene and the amino acid sequence of the AspT protein against current nucleotide and protein databases have suggested that AAE family transporters are conserved in many bacterial species (10, 19). The putative broad distribution of AspT orthologues and paralogues in bacteria suggests that an additional biochemical study of AspT can be a valuable part of the ongoing investigation of membrane transport.
AspT is a membrane protein containing 543 amino acids (57.2 kDa). The membrane topology of AspT has been studied by means of alkaline phosphatase and β-lactamase fusion methods (33); it has also been studied by the substituted-cysteine accessibility method (SCAM) (6), which uses the impermeant, fluorescent thiol-specific probe OGM and the impermeant, nonfluorescent thiol-specific probe [2-(trimethylammonium)ethyl]methanethiosulfonate bromide (34). These analyses revealed that AspT has a unique topology: the protein has 10 transmembrane helices (TMs), a large hydrophilic cytoplasmic loop (about 180 amino acids) between TM5 and TM6, and N and C termini located at the periplasm. TM3 contains amino acid residues that are well conserved among members of the AAE family. Moreover, our topological analysis indicated that there are isolated charged residues (aspartate 67 and arginine 76) in the middle of TM3. For the transporters of polar molecules such as aspartate, the substrate transport pathway can be expected to be enriched with residues that are more hydrophilic than residues found elsewhere in the TMs.
In the present study, we applied SCAM to the TM3 of AspT to further elucidate the role of TM3 in the transport process. An analysis of the effects of the amino acid substitutions on transporter function identified amino acids that are crucial for transport. Furthermore, our SCAM results suggest that TM3 participates in the formation of a hydrophilic cleft in the membrane and implicate the TM in ligand-induced conformational alterations. In addition, nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analyses of purified AspT showed that some single-cysteine variants form S-S-linked homodimers in membranes. We speculated that AspT forms oligomers in the membrane and that the TM3s are located close to each other, as shown in the similar case of the multidrug exporter AcrB, in which the extramembrane central pore α-helices of each AcrB protomer are close to each other in the trimer formation (32). To confirm the homo-oligomer formation of AspT, we developed a solubilization and purification scheme using n-dodecyl-β-d-maltoside (DDM), and we studied the oligomerization of AspT by means of a glutaraldehyde cross-linking assay.
MATERIALS AND METHODS
Chemicals, cells, and expression plasmids.
l-[2,3-3H]aspartic acid (1.07 GBq/mmol) was purchased from Amersham-Pharmacia Biotech (Piscataway, NJ). 1-O-n-Octyl-β-d-glucopyranoside (OG) and DDM were obtained from Nacalai Tesque (Kyoto, Japan). Escherichia coli phospholipid was provided by Avanti Polar Lipids (Alabaster, AL) (8). OGM was purchased from Invitrogen Co. (Carlsbad, CA). Escherichia coli strain XL1-Blue harboring pMS421 (Spcr LacIq)—referred to as strain XL3 (3)—was used for the expression of the asp operon with pTrc99A (Amersham-Pharmacia Biotech).
Site-directed mutagenesis.
Single-cysteine variants were constructed by the oligodeoxyribonucleotide-directed dual amber mutagenesis method (Takara Bio, Tokyo, Japan) (21). The pKF 19k/18k vector (Takara Bio) harboring the gene for cysteine-less AspT-(His)6 was used as a DNA template for the mutagenesis method. DNA fragments encoding AspT in which a single cysteine had been introduced were ligated back into the corresponding site of the pBlue cysteine-less AspHis. DNA fragments encoding single-cysteine AspT-(His)6 were cloned into pTrcAsp instead of wild-type AspT. The DNA sequences of all the mutagenized AspT proteins were verified by DNA sequence analysis, as described previously (2).
Production of single-cysteine AspT-(His)6.
A preculture of E. coli XL3 carrying pTrc single-cysteine AspT-His or pTrc99A was diluted 100-fold in fresh Luria-Bertani (LB) medium containing 30 mM d-glucose, 30 μg/ml carbenicillin, and 30 μg/ml spectinomycin. These cells were grown for 2.5 h at 37°C with shaking to an optical density at 650 nm of 0.5 and were then diluted twofold in fresh LB broth containing 30 mM d-glucose, 60 mM l-aspartate, and 1 mM pyridoxal 5′-phosphate. The cell suspensions were incubated statically for 1 h at 37°C. Afterward, 200 μM (final concentration) isopropyl-β-d-thiogalactoside was added to each culture, and the static inductions of the AspT variants were allowed to proceed for 12 h at 37°C.
Solubilization, reconstitution, and transport assay of AspT variants.
The preparation of the AspT variant proteoliposomes was carried out as previously described (34). In brief, membrane ghosts were prepared by an osmotic shock procedure (8). The membrane ghosts were solubilized with 1.25% (wt/vol) OG in the presence of 0.4% (wt/vol) E. coli phospholipid, 100 mM KH2PO4 (pH 7), and 20% glycerol (8). Control extracts were prepared in the same way but without added protein. These solubilized membrane proteins were reconstituted in a final volume of 1 ml with 800 μl of detergent extracts (1.2 mg of protein) (or control lipid extract), 130 μl of bath-sonicated liposomes (5.9 mg of E. coli phospholipid), and 18 μl of 15% OG, with the balance made up by 100 mM KH2PO4 (pH 7). After incubation of the mixture for 20 min on ice, proteoliposomes (or control liposomes) were formed at room temperature (R.T.) by rapid injection into 20 ml of a loading buffer containing 100 mM KH2PO4 (pH 7) and 100 mM l-aspartate as the potassium salt. The substrate-loaded proteoliposomes (or liposomes) were kept at R.T. for 20 min.
Unless otherwise noted, the initial rates of l-[2,3-3H]aspartate incorporation were measured in duplicate at 25°C by means of a filtration assay (34, 45, 51). Proteoliposomes were applied directly to the center of a 0.22-μm-pore-size GSTF Millipore filter (Millipore Co., Billerica, MA) and washed twice with 5 ml of chilled assay buffer (100 mM KH2PO4 [pH 7], 100 mM K2SO4). Upon release of the vacuum, proteoliposomes were covered with preincubated assay buffer containing 0.1 mM l-[2,3-3H]aspartate, and the reaction was terminated after 1 min by filtration and washing. AspT function is usually reported as relative specific activity by the normalization of observed rates to levels of AspT production as determined by immunoblot analysis (described below).
Immunoblot analysis.
The detergent extracts of the membrane ghosts of each of the single-cysteine variants and the cysteine-less parent were analyzed by reducing SDS-PAGE. For the SDS-PAGE, 45 μg of proteins was used, and after electrophoresis, the proteins were transferred to a polyvinylidene difluoride membrane (Nippon Genetics, Tokyo, Japan) by semidry electrophoretic blotting (Bio-Rad Laboratories, Hercules, CA) and probed with an anti-AspT rabbit polyclonal antibody (Operon Biotechnologies, Tokyo, Japan). Rabbits were injected with two different synthetic polypeptides (SKLPISDHLKTLYSNQ and NDVSERVGSDASPF) as antigens with adjuvant to elicit an immune response to AspT peptides. AspT production was detected by chemiluminescence with a LAS-3000 imaging system (Fujifilm, Tokyo, Japan), and signals were quantified with NIH Image software (v. 1.63). Antisera were used at the following dilutions: anti-AspT rabbit polyclonal antibody, 1:2,000, and anti-rabbit immunoglobulin G goat polyclonal antibody-horseradish peroxidase conjugate (StressGen Bioreagents Co., Victoria, Canada), 1:2,000. The production of each AspT variant was normalized to that of the cysteine-less parental control found on the same gel.
Site-directed fluorescence labeling.
The exposure of TM3 positions to the aqueous environment (the extracellular medium or the cytosol) was assessed in the single-cysteine variants. The membrane ghosts (prepared as described above) were resuspended in 20 mM potassium phosphate (pH 8) or 20 mM potassium phosphate (pH 8) containing 100 mM l-aspartate and incubated for 20 min at 25°C with 40 μM OGM, an impermeable thiol-active agent (50). The reaction was quenched by the addition of 6 mM β-mercaptoethanol and three cycles of washing with distilled water. The protein was solubilized by resuspending the membrane vesicles in 1 ml of solubilization buffer (50 mM Tris-HCl [pH 7], 0.75 mM phenylmethylsulfonyl fluoride, 1% Triton X-100, 0.1% SDS). After the mixture was incubated at 4°C for 2 h on a rotary platform shaker, the insoluble debris was removed by centrifugation at 4°C (17,500 × g for 30 min), and AspT was purified in a one-step affinity procedure (17, 34). In brief, six-histidine-tagged AspT was precipitated with 50 μl of Ni-nitrilotriacetic acid (NTA) Superflow resin (Qiagen, Valencia, CA) by overnight batch incubation at 4°C on a rotary platform shaker. The resin, with bound AspT, was washed on ice with a total of 3 ml of wash buffer (solubilization buffer supplemented with 200 mM sodium fluoride and 25 mM imidazole). AspT was eluted by a brief centrifugation at 4°C with 60 μl of elution buffer (50 mM Tris-HCl [pH 7], 2% SDS, and 0.5 M imidazole).
To label cysteines exposed at the extracellular surface, intact cells were harvested by centrifugation (6,000 × g for 30 min) and suspended in buffer A (100 mM K2SO4, 50 mM KH2PO4 [pH 8]), and the absorbance at 530 nm was adjusted to 8.5. OGM (40 μM final concentration) was added to 5 ml of the cell suspension, and the mixture was incubated for 20 min at 25°C. The labeling reaction was quenched by the addition of 6 mM (final concentration) β-mercaptoethanol. The cells were collected immediately and washed three times with buffer B [100 mM K2SO4, 50 mM 3-(N-morpholino)propanesulfonic acid (pH 7) as the potassium salt]. The membrane ghosts were prepared from labeled cells, and these membranes served as the source of AspT for solubilization and purification as described above.
Detection of OGM-labeled protein.
The protein was subjected to SDS-PAGE with a 10% polyacrylamide gel matrix. For the SDS-PAGE, 10 μl of elution sample was used, and after electrophoresis, the gel was rinsed briefly with a destaining solution (10% glacial acetic acid, 15% methanol). Fluorescence profiles were recorded with a LAS-3000 imaging system. After the fluorescence profiles were recorded, the protein content of each lane was evaluated by staining the same gel with Coomassie brilliant blue (CBB).
Expression, solubilization, and purification of wild-type AspT-His [AspT(WT)-His] for analysis of oligomerization.
A preculture of E. coli XL3 carrying pTrc AspT(WT)-His was diluted 100-fold in fresh LB medium containing 30 mM d-glucose, 30 μg/ml carbenicillin, and 30 μg/ml spectinomycin. These cells were grown for 2.5 h at 37°C with shaking to an optical density at 650 nm of 0.5 and were then diluted twofold in fresh LB broth containing 30 mM d-glucose, 60 mM l-aspartate, and 1 mM pyridoxal 5′-phosphate. Afterward, 200 μM (final concentration) isopropyl-β-d-thiogalactoside was added to the cultures, and the induction of AspT(WT)-His by static culture was allowed to proceed for 12 h at 37°C. The membrane ghosts were prepared by an osmotic shock procedure (8). The cells harvested from each liter of culture solution were suspended in 7.5 ml of a lysis solution (500 μg/ml lysozyme [Seikagaku Co., Tokyo, Japan], 40 μg/ml DNase I [Sigma, St. Louis, MO], 10 mM Tris-HCl [pH 7.5], 1 mM phenylmethylsulfonyl fluoride) and incubated at 37°C for 30 min. The cells were disrupted by a 10-fold dilution into 45 ml of iced distilled water. After the cells were disrupted, the cytoplasmic proteins released were removed by two cycles of washing with iced distilled water and centrifugation (44). The membrane ghosts were solubilized (8) at 4°C for 6 h with 1.5% (wt/vol) DDM in the presence of 200 mM l-aspartate, 100 mM KH2PO4 (pH 7), and 20% glycerol. After centrifugation at 12,000 × g for 60 min, the supernatant was incubated with a Ni-NTA affinity column (375-μl bed volume for a 1-liter culture) at 4°C for 6 h. The column was washed on ice with a total of 7.5 ml/1-liter culture of wash buffer (200 mM l-aspartate and 20 mM KH2PO4 [pH 7], 20% glycerol, 0.05% DDM, and 25 mM imidazole). Then, AspT(WT)-His was eluted by a brief centrifugation in the cold with elution buffer (200 mM l-aspartate and 20 mM KH2PO4 [pH 7], 20% glycerol, 0.05% DDM, and 0.5 M imidazole) at 375 μl/1 liter of culture.
Reconstitution and transport assay of purified AspT(WT)-His.
The solubilized membrane proteins were reconstituted in a final volume of 1 ml with 800 μl of detergent extracts (10 μg of protein) (or control lipid extract), 130 μl of bath-sonicated liposomes (5.9 mg of E. coli phospholipid), and 18 μl of 15% OG, with the balance made up by 100 mM KH2PO4 (pH 7). After incubation for 20 min on ice, proteoliposomes (or control liposomes) were formed at R.T. by rapid injection of the mixtures into 20 ml of a loading buffer containing 100 mM KH2PO4 (pH 7) and 100 mM l-aspartate as the potassium salt. The substrate-loaded proteoliposomes (or liposomes) were kept at R.T. for 20 min. To assay for l-aspartate transport by l-[2,3-3H]aspartate-loaded particles, proteoliposomes were diluted 20-fold from the concentrated stock preparation into an appropriate volume of assay buffer (100 mM KH2PO4 [pH 7] and 100 mM K2SO4). After 1 to 3 min of preincubation at 25°C, l-[2,3-3H]aspartate was added to a final concentration of 100 μM; at various times, 50- to 100-μl aliquots were removed for membrane filtration (0.22-μm-pore-size GSTF Millipore filters). The membrane filters were followed by two washes with 5 ml of assay buffer (8).
Glutaraldehyde cross-linking.
Aliquots (0.4 μg) of the purified AspT(WT)-His (0.01 mg/ml in wash buffer containing either 0.05% DDM or 1% SDS, for cross-linking in native or denaturing conditions, respectively) were subjected to in vitro cross-linking by incubation with 1 to 100 mM glutaraldehyde at 25°C for 30 min. The reaction was terminated by the addition of gel sample buffer containing 4% SDS, 125 mM Tris-HCl (pH 6.8), and 10% β-mercaptoethanol, and the samples were analyzed by SDS-PAGE (7.5% polyacrylamide gel matrix). The gels were silver stained and optically scanned using ImageJ (National Institutes of Health [http://rsb.info.nih.gov/ij/]).
RESULTS
Transport activities of single-cysteine variants of TM3 in a functional AspT.
We performed a multiple alignment of 10 AAE family transporters to identify conserved amino acids in the AspT proteins. The alignment showed that TM3 has many amino acid residues that are conserved among members of the AAE family (Fig. 1). To clarify the role of TM3 in l-aspartate transport, we performed a complete cysteine-scanning mutagenesis of TM3. In the first step, each amino acid from threonine 59 to phenylalanine 88, including TM3 (isoleucine 64 to methionine 85), was individually replaced with cysteine in a functional AspT molecule devoid of all three native cysteine residues (AspT cysteine-less). The level of active transport was measured by using l-aspartate-loaded proteoliposomes in which each variant of AspT was reconstituted. Because Abe et al. (2) demonstrated that E. coli crude membranes reconstituted in proteoliposomes possess background aspartate:aspartate self-exchange activities, we measured the background l-aspartate self-exchange activity of membranes prepared from E. coli harboring the empty vector pTrc99A. We found that the resultant background l-aspartate self-exchange activity was 11.7% of that of the AspT cysteine-less variant (Table 1). Given the background activity of E. coli membranes, the substitutions of tyrosine 75 and serine 84 had the strongest inhibitory effects on transport; the initial rates of l-aspartate transport were below 15% of the rate for cysteine-less AspT (Table 1). Considerable but less-marked effects were observed upon the replacement of methionine 70, phenylalanine 71, glycine 74, arginine 76, serine 83, and methionine 85 (initial rates between 15% and 30% of the rate for cysteine-less AspT). In contrast, the replacement of amino acids in the periplasmic half of TM3 (isoleucine 64 to phenylalanine 69) had little or no effect on AspT function.
FIG. 1.
Multiple sequence alignment of members of the AAE family of transporters. A multiple alignment was generated with ClustalW2 (version 1.83) with sequences obtained from a BLASTP search (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi) against AspT of T. halophilus (accession number Q8L3K8) at the European Bioinformatics Institute server (http://www.ebi.ac.uk/Tools/clustalw2/index.html) (35, 36). The chosen matrix was BLOSUM; penalties were 10 (open gap), 0.05 (extending gap), and 10 (gap distance). The resulting alignment was optically improved with the Boxshade 3.31 program (http://bioweb.pasteur.fr/seqanal/interfaces/boxshade.html); conserved residues are highlighted in black (identical to consensus) or gray (similar to consensus). The term AspT refers to the homology with AspT of T. halophilus (Thal). The bacterial host strains and accession numbers for the AspT homologs are as follows: Lactobacillus reuteri (Lreu), ABQ82551; Lactobacillus acidophilus (Laci), AAV43508; Parabacteroides distasonis (Pdis), ABR42837; Ralstonia metallidurans (Rmet), ABF08864; Bordetella bronchiseptica (Bbro), CAE32035; Bordetella parapertussis (Bpar), CAE37441; Francisella tularensis (Ftul), ABK89250; Bradyrhizobium japonicum (Bjap), BAC48740; and Comamonas testosteroni (Ctes), BAC65228. Experimental evidence for aspartate:alanine antiporter activity exists only for T. halophilus and C. testosteroni. Numbers indicate the position in the primary sequence and refer to the first amino acid of the alignment.
TABLE 1.
Production levels and specific activities of single-cysteine variants relative to those of cysteine-less AspT
| Variant | Production (%)a | Sp act (%)b |
|---|---|---|
| —c | — | 11.7 ± 1.24 |
| Cysteine-less | 100.0 | 100.0 |
| T59C | 111.0 ± 1.0 | 110.0 ± 21.3 |
| L60C | 139.2 ± 1.29 | 49.1 ± 2.3 |
| L61C | 154.2 + 0.35 | 121.6 ± 18.3 |
| G62C | 79.2 + 0.76 | 259.6 ± 25.4 |
| D63C | 136.0 ± 0.92 | 75.2 ± 4.04 |
| I64C | 191.5 + 0.91 | 101.1 ± 2.66 |
| F65C | 56.9 + 2.04 | 50.1 ± 2.91 |
| F66C | 54.5 + 2.68 | 38.3 ± 0.29 |
| D67C | 89.3 ± 1.8 | 45.5 ± 3.46 |
| F68C | 52.3 + 0.81 | 101.2 ± 12.3 |
| F69C | 48.4 + 2.04 | 59.0 ± 2.99 |
| M70C | 72.2 ± 1.61 | 26.1 ± 1.26 |
| F71C | 51.7 ± 0.53 | 27.8 ± 0.71 |
| A72C | 55.2 ± 0.67 | 54.3 ± 7.67 |
| I73C | 63.2 ± 1.51 | 37.3 ± 7.55 |
| G74C | 30.6 ± 1.17 | 17.2 ± 3.69 |
| Y75C | 53.1 ± 0.3 | 11.3 ± 8.6 |
| R76C | 24.9 + 0.95 | 22.9 ± 6.3 |
| V77C | 45.9 ± 2.37 | 71.4 ± 10.0 |
| G78C | 27.8 ± 4.38 | 28.1 ± 1.72 |
| P79C | 36.5 + 3.88 | 34.1 ± 3.9 |
| S80C | 64.6 ± 2.45 | 70.2 ± 3.76 |
| F81C | 31.0 ± 4.96 | 33.7 ± 0.71 |
| I82C | 42.2 + 4.4 | 65.6 + 5.58 |
| S83C | 107.9 ± 2.97 | 18.7 ± 1.79 |
| S84C | 101.1 ± 2.42 | 8.7 ± 1.51 |
| M85C | 63.3 + 2.5 | 28.1 ± 10.2 |
| K86C | 75.8 ± 1.6 | 40.6 ± 6.53 |
| K87C | 79.2 ± 0.44 | 35.6 ± 2.07 |
| F88C | 144.3 ± 5.82 | 14.8 ± 1.41 |
Production is given relative to that of the cysteine-less parent (mean ± standard deviation).
Initial rates of l-[2,3-3H]aspartate transport, normalized for the levels of AspT production, are given relative to the rate for the cysteine-less parent, whose activity was 4.24 nmol min−1 mg−1 of protein.
Membrane ghosts from control cells (without AspT expression) were used for control reconstitution and the transport assay. Data reflect mean values ± standard deviations found in three independent trials. For each single-cysteine variant, an equal amount of protein was used for reconstitution in l-aspartate-loaded proteoliposomes.
Definition of the hydrophobic core region and water-filled cavity of TM3.
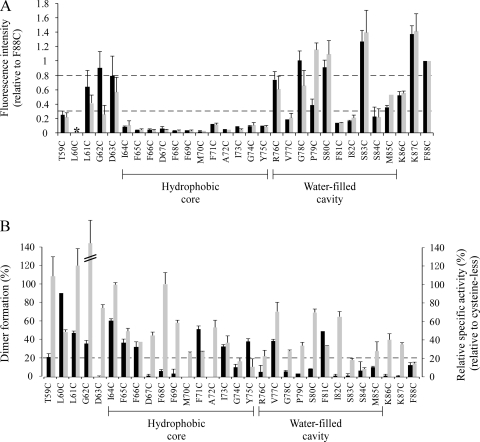
To obtain a comprehensive picture of the accessibility of the cysteines at the various positions of TM3, we employed a labeling approach specific for each artificially introduced cysteine. Randomly oriented membrane ghosts containing the corresponding single-cysteine AspT variants were labeled with the hydrophilic fluorescent probe OGM. Studies of OxlT have shown that cysteine residues exposed to the aqueous phase can be identified by their accessibility to OGM (18, 50, 51), which is known to be membrane impermeant under the conditions used here (34, 50). After membrane ghosts labeling, the AspT variants were solubilized from the membranes, purified by Ni-NTA affinity chromatography, and subjected to SDS-PAGE. The fluorescence of each AspT variant labeled with OGM was detected by the UV irradiation of the gels (Fig. 2A, bottom panel). The intensities of the fluorescence and CBB were normalized to the corresponding intensities of the F88C variant, whose mutation is located in the intracellular hydrophilic loop. The labeling efficiency of the cysteine in each variant was assessed as the ratio of the intensities of the fluorescent and CBB bands (Fig. 3A, black bars).
FIG. 2.
OGM binding to single-cysteine variants of TM3. The hydrophobic core of TM3 was defined experimentally by OGM labeling of the indicated single-cysteine variants spanning the entire transmembrane segment. (A and B) Membrane ghosts of each variant were exposed to OGM in the absence (A) or presence (B) of l-aspartate prior to protein solubilization, purification under denaturing conditions using solubilization with 1% Triton X-100 and 0.1% SDS and elution with 2% SDS, and analysis using nonreducing SDS-PAGE (10% polyacrylamide gel matrix). (C) Whole-cell OGM labeling (34) was applied to six single-cysteine variants that showed high accessibility to OGM in membrane ghost labeling. E. coli cells that expressed each variant were exposed to OGM in the absence of l-aspartate. Purified proteins from OGM-exposed E. coli cells were analyzed using nonreducing SDS-PAGE as described above. A single gel containing all samples was used to record the fluorescence signal with a LAS-3000 imaging system (bottom panel) before staining with CBB to visualize protein (top panel).
FIG. 3.
Conformational alteration induced by l-aspartate and disulfide dimer formation of single-cysteine variants in the cell membrane. (A) The levels of fluorescence intensity and CBB staining in nonreducing SDS-PAGE (Fig. 2A and B) were normalized to the corresponding levels of the F88C variant, whose mutation is located in an intracellular hydrophilic loop. The labeling efficiency of cysteine in each variant is shown as the ratio of the normalized levels of fluorescence intensity and CBB staining with black bars (absence of l-aspartate) and gray bars (presence of l-aspartate). The asterisk indicates that L60C showed strong disulfide dimer formation; the amount of monomer was too small to normalize the strength of the fluorescence. (B) The bands representing monomeric and dimeric forms were quantified by nonreducing SDS-PAGE analysis (Fig. 2A, top panel). The efficiency of the disulfide bond formation of each single-cysteine variant is shown as the ratio of dimer to total protein (black bars). The specific activity of each variant is shown by the gray bars.
Despite the fact that amino acids isoleucine 64 to methionine 85 are predicted to form a membrane-spanning domain (TM3), cysteines placed at several positions within this region were modified by OGM (Fig. 2A, bottom panel). Introduced cysteine residues corresponding to the positions of glycine 78, serine 80, and serine 83 were particularly accessible (≥0.8 relative to phenylalanine 88) to OGM (Fig. 2A, bottom panel, and Fig. 3A, black bars). This region of the protein must therefore be exposed to the aqueous environment. The intermediate accessibility (<0.8 but ≥0.3 relative to phenylalanine 88) was observed for the cysteine residues introduced in place of arginine 76, proline 79, and methionine 85. A negative response (<0.3 relative to phenylalanine 88) was found at the 12 cysteines close to the periplasmic half of TM3 (from isoleucine 64 to tyrosine 75), valine 77, phenylalanine 81, isoleucine 82, and serine 84 (Fig. 2A, bottom panel, and Fig. 3A, black bars). On the whole, positions in the C-terminal (cytoplasmic) half of TM3 showed higher accessibility than positions in the N-terminal (periplasmic) half of the TM. To study the accessibility of introduced cysteine residues at the C-terminal (cytoplasmic) half of TM3 from the periplasmic side, we used whole-cell OGM labeling for six variants (G62C, D63C, G78C, S80C, S83C, and K87C) that showed high accessibility to OGM in membrane ghost labeling (Fig. 2A, bottom panel, and Fig. 3A, black bars). G62C and D63C, which are predicted to be located at the periplasmic side, maintained their good accessibility to OGM in whole-cell labeling, whereas G78C, S80C, S83C, and K87C showed remarkable defects in their accessibility to OGM in whole-cell labeling (Fig. 2C).
Influence of ligand binding on cysteine accessibility.
To further explore the role of TM3 in AspT function, we examined the influence of a native ligand (l-aspartate) on the accessibility of introduced cysteine residues in single-cysteine variants to OGM. Membrane ghosts containing the single-cysteine AspT variants were labeled after incubation with 100 mM l-aspartate. Subsequently, the AspT variants were isolated, and fluorescence was detected as described above (Fig. 2B, bottom panel). The analysis revealed four groups of single-cysteine AspT variants with the following characteristics (Fig. 2A and B, bottom panels, and Fig. 3A). (i) Cysteines in the positions of leucine 61, aspartic acid 63, arginine 76, glycine 78, serine 80, serine 83, methionine 85, lysine 86, lysine 87, and phenylalanine 88 were accessible to OGM (≥0.3 relative to phenylalanine 88), and the labeling reaction was not markedly influenced by the presence of l-aspartate. (ii) Cysteines introduced at threonine 59, from isoleucine 64 to tyrosine 75, or at valine 77, phenylalanine 81, isoleucine 82, or serine 84 showed only low reactivity toward OGM (<0.3 relative to phenylalanine 88), and the addition of l-aspartate did not stimulate cysteine reactivity. (iii) The cysteine residue introduced at glycine 62 was labeled by OGM, and the labeling reaction was inhibited by l-aspartate. (iv) l-Aspartate stimulated the reactivity of cysteine at proline 79. The observed substrate protection can be explained by direct steric hindrance; by ligand-induced conformational alterations, which either bury the corresponding residue within the protein or move it from an aqueous environment into an apolar environment; or by both steric hindrance and conformational alterations.
Oligomerization of purified AspT.
Nonreducing SDS-PAGE analysis indicated that 12 of the single-cysteine variants formed S-S-linked homodimers (≥20% of purified single-cysteine AspT) in the native E. coli membrane (Fig. 2A, top panel, and Fig. 3B, black bars). This result suggests that AspT may form homo-oligomers in the membrane and that the TM3s of the individual protomers are close to each other. To confirm the homo-oligomer formation of AspT by means of a glutaraldehyde cross-linking assay, we developed a solubilization and purification scheme under DDM-solubilized conditions.
An AspT variant with a six-histidine tag inserted into the large hydrophilic loop, AspT(WT)-His (34), was expressed in E. coli, solubilized in detergent (DDM), and purified by a conventional one-step procedure employing Ni-NTA chromatography (Fig. 4A). We observed a single band on an SDS-PAGE gel at a position lower than that expected from the polypeptide sequence (57.2 kDa) of the AspT variant; similar results have been observed for other membrane proteins (3, 13). Then we reconstituted the purified AspT variant into proteoliposomes and confirmed that it catalyzed the l-aspartate self-exchange reaction. The proteoliposomes containing the purified AspT variant were loaded with 100 mM potassium l-aspartate. Then the proteoliposomes were resuspended in 0.1 M potassium phosphate buffer (pH 7), and l-aspartate exchange reactions were started by the addition of 100 μM (final concentration) external l-[3H]aspartate. l-[3H]aspartate was incorporated into proteoliposomes by l-aspartate self-exchange. The steady-state incorporation of l-[3H]aspartate was approximately 3,300 nmol/mg of protein (Fig. 4B). Moreover, accumulated l-[3H]aspartate was released by the addition of excess unlabeled l-aspartate, suggesting that we succeeded in purifying the AspT, while completely maintaining its transport activity.
FIG. 4.
Oligomerization of AspT. (A) Purification of AspT(WT)-His. SDS-PAGE analysis with a 10% polyacrylamide gel matrix of purified AspT(WT)-His under nondenaturing conditions using solubilization with 1.5% DDM and elution with 0.05% DDM. (B) l-Aspartate transport assay with proteoliposomes containing purified AspT(WT)-His. Proteoliposomes loaded with 100 mM l-aspartate and 100 mM potassium phosphate (pH 7.0) were placed in assay buffer (100 mM potassium phosphate [pH 7], 100 mM potassium sulfate) at 10 μg of protein/ml along with 100 μM l-[3H]aspartate. The uptake of l-[3H]aspartate by the proteoliposomes containing purified AspT(WT)-His is shown (⋄). Proteoliposomes were allowed to take up l-[3H]aspartate for 7.5 min. At the time indicated by the arrow, l-aspartate was added to achieve a final concentration of 15 mM (▪). Samples were removed, filtered, and washed at the times indicated. (C) Glutaraldehyde cross-linking of the purified AspT(WT)-His. Image of silver-stained SDS-PAGE gel (7.5% polyacrylamide gel matrix) of AspT(WT)-His after 1 to 100 mM glutaraldehyde (GA) cross-linking in 0.05% DDM or 1% SDS (left panel) and densitometric scans of silver-stained SDS-PAGE gels (right panel) of AspT after 100 mM glutaraldehyde cross-linking in 0.05% DDM.
To confirm the oligomer formation of the purified protein, we carried out a glutaraldehyde cross-linking assay. Directed at surface-exposed amino groups, glutaraldehyde has been used to analyze the quaternary structures of purified multisubunit membrane proteins (13, 26, 28, 38, 40, 52). The treatment of purified AspT protein with glutaraldehyde showed shifts of the monomer band (at ∼50 kDa on a denaturing SDS gel) to the position (100 kDa) expected for homo-dimers (Fig. 4C). Moreover, cross-linker treatment in a denaturing detergent (SDS) failed to shift the monomer band. This result supports the idea that the band shifts were caused by intermolecular cross-linking. The glutaraldehyde-treated samples, whether cross-linked or not, ran as fuzzy bands, as expected from the adduct heterogeneity derived from the different extents of glutaraldehyde modification in the individual monomers and dimers by this reaction. The results of the glutaraldehyde cross-linking assay suggest that functional AspT formed homo-oligomers under DDM-solubilized conditions.
DISCUSSION
Identification of sites involved in both substrate binding and translocation is a central goal of ongoing studies of AAE family transporters. Solute transporters such as AspT and OxlT have a network of key amino acid residues that facilitate substrate movement into and out of an appropriate binding site, thereby defining a substrate translocation pathway through the protein (48, 49). For transporters of polar molecules like aspartate, this pathway is assumed to be enriched in residues of a more hydrophilic character than found elsewhere in the transmembrane helices, as is the case for the substrate translocation pathways found in other transporters (12, 20, 25, 29, 49). For these reasons, because TM3 is enriched with hydrophilic amino acid residues, including aspartate 67 and arginine 76, and because these are the only charged residues in the TMs of AspT, TM3 deserves attention. Therefore, residues on the same helical face as aspartate 67 or arginine 76 might also be expected to be involved in the substrate translocation pathway. To investigate the role of TM3, we performed a complete cysteine-scanning mutagenesis study of TM3 in the presence and absence of l-aspartate to learn more about the membrane topology, possible ligand-binding sites, and the functional dynamics of AspT.
Impacts of cysteine substitution.
Cysteine scanning of TM3 revealed that the replacements of tyrosine 75 and serine 84 had the strongest inhibitory effects on transport (initial rates of l-aspartate transport were below 15% of the rate for cysteine-less AspT), and the alteration of 9 of the 22 amino acid (isoleucine 64 to methionine 85) positions tested reduced AspT function (initial rates of l-aspartate transport were below 30% of the rate for cysteine-less AspT; Table 1). The results indicated that three groups of amino acid residues played important roles in l-aspartate transport processes: (i) amino acids located at the hydrophilic face in the hydrophobic core (methionine 70 and phenylalanine 71), (ii) the amino acids of the GxxxG motif (glycine 74, tyrosine 75, arginine 76, valine 77, and glycine 78), and (iii) amino acids located at the C-terminal end of the helix (serine 83, serine 84, and methionine 85). In the first group, phenylalanine 71 is well conserved as an aromatic amino acid (tyrosine or phenylalanine) within the AAE family (Fig. 1). Methionine 70 is not conserved within the family, but the substitution of methionine 70 with cysteine may cause local structural perturbations affecting the adjacent functionally important region around phenylalanine 71, thereby explaining the reduced transport activity of the corresponding variant. Therefore, we speculated that methionine 70 and phenylalanine 71 are located on the hydrophilic face of the hydrophobic core of TM3 (Fig. 5B, top panel). In the second group, glycine 74 and glycine 78 are completely conserved within the family. Tyrosine 75 is well conserved as an aromatic amino acid (tyrosine or phenylalanine). Arginine 76 of TM3 is not conserved within the AAE family, but a charged or polar amino acid is found at this position in other members of the family (Fig. 1). Therefore, we speculated that arginine 76 is located in the hydrophilic water-filled cavity, and this speculation is supported by the fact that this residue was accessible to sulfhydryl reagents (Fig. 2A and B and 3A; see also Discussion below). Glycine 74 and glycine 78 may be important for the structure or conformational flexibility of TM3. A similar role has been reported for specific glycine residues in the lactose permease LacY of E. coli (4, 24, 47). In fact, our results indicate that TM3 of AspT is involved in ligand-induced conformational alterations, as described below. Because cysteine substitution variants at phenylalanine 71 and tyrosine 75 (groups 1 and 2, respectively) showed high frequencies of disulfide bond formation on nonreducing SDS-PAGE, disulfide bond formation may decrease the flexibility of TM3, which may in turn decrease the transport activities. In group 3, serine 84 is well conserved (but not completely) as a small amino acid (serine, glycine, or alanine). The substitution of serine 83 or methionine 85 with cysteine may cause local structural perturbations affecting the adjacent functionally important region around serine 84, thereby explaining the reduced transport activities of the corresponding variants. Taken together, the results of the activity measurements confirmed the particular functional significance of TM3 in the transport process.
FIG. 5.
Integrated view of AspT TM3. This figure summarizes the cysteine accessibility data shown in Table 1 and Fig. 2 and 3. (A) Side view of TM3. The residues in black circles are highly accessible to OGM (≥0.8 relative to F88C), the residues in gray circles show an intermediate accessibility (<0.8 but ≥0.3 relative to F88C), and the residues in unshaded circles are not accessible or only slightly accessible (<0.3 relative to F88C). The reactivity of the cysteine at the amino acid position shown by the black square was stimulated by l-aspartate; the amino acid at the position indicated by the black triangle was protected by l-aspartate. The shaded area demarcates the water-filled cavity of TM3, which is accessible to OGM (Fig. 2A and B). (B) Helical-wheel projection of the hydrophobic core (isoleucine 64 to tyrosine 75; top panel) and the water-filled cavity (arginine 76 to methionine 85; bottom panel) with specific activities; symbols are the same as described for panel A. Specific activities are shown in squares: black squares, ≥50% of that of cysteine-less AspT; dark gray squares, <50%, ≥30%; light gray squares, <30%, ≥15%; and unshaded squares, <15%) (Table 1). Asterisks within the helical wheel indicate cysteine substitutions that show clear disulfide dimer formation in nonreducing SDS-PAGE (Fig. 2 and 3).
Assignment of the hydrophobic core and water-filled cavity.
To obtain a comprehensive picture of the accessibility of cysteines at the various positions of TM3, randomly oriented membrane ghosts containing single-cysteine AspT variants were labeled with the hydrophilic fluorescent probe OGM. The labeling experiments (Fig. 2A) revealed a striking discontinuity in OGM accessibility along TM3. Thus, when AspT was labeled in situ, OGM failed to react with cysteines within the N-terminal 12-residue segment (residues 64 to 75), whereas inserted cysteines at the C-terminal segment yielded fluorescent products (Fig. 2A). These results indicate that residues 64 to 75 form the hydrophobic core of TM3. The existence of a hydrophobic core within the membrane has recently also been reported for OxlT (18, 51). There are two possible general explanations for such findings. The first possibility is that the region of low OGM reactivity (positions 64 to 75) is within the lipid bilayer and hence inaccessible to the impermeant probe. Once OGM has modified its target, the OGM carboxylate lies about 8 to 10 Å from the target cysteinyl sulfur; therefore, cysteines that react with OGM can be expected to be within 8 to 10 Å of the external or internal aqueous phase. If TM3 forms a transmembrane α-helix with 20 to 25 residues that span the 30- to 40-Å bilayer, a central core of some 10 residues (e.g., residues 64 to 75) can be expected to be inaccessible to OGM for purely geometric reasons. The second possible explanation is that cysteines in this core are unreactive for some reasons having to do with their chemical environment. Cysteines in a nonpolar (i.e., lipid) environment are generally thought to have high pKa values (pKa = 14) characteristic of the side chain of cysteine (18). In the second explanation, the reactive sulfide (−S1−) would not be present at a sufficiently high concentration to permit its chemical modification with OGM. We caution, however, that these two explanations are not mutually exclusive. A transmembrane helix might have positions that face other helices or the hydrophilic translocation pathway (e.g., see the work of Goswitz and Brooker [20]); these positions might be inaccessible but chemically reactive. In contrast, there might be positions that face the membrane lipid, and these positions might be accessible but chemically unreactive.
In addition, it is noteworthy that most positions (6 of 10 residues [arginine 76 to methionine 85]) located in the cytoplasmic half of TM3 showed high reactivities toward OGM (Fig. 2A and B). The data indicate that the C-terminal half of the putative α-helix is exposed to an aqueous cavity that is open to the cytoplasmic side of the membrane. This cavity most likely extends from the cytoplasmic end of TM3 to the middle of the domain (residues 76 to 85). To study the accessibility of the water-filled cavity at the C-terminal (cytoplasmic) half of TM3 from the periplasmic side, we applied whole-cell OGM labeling for six variants (G62C, D63C, G78C, S80C, S83C, and K87C) that showed high accessibility to OGM in membrane ghost labeling (Fig. 2A). G62C and D63C, which are predicted to be located on the periplasmic side of the membrane, maintained their good accessibility to OGM in whole-cell labeling. In contrast, G78C, S80C, S83C, and K87C showed remarkable defects in their accessibility to OGM in whole-cell labeling (Fig. 2C). This result suggests that amino acid residues located in the C-terminal half of TM3 are inaccessible from the periplasmic side and that the water-filled cavity in the C-terminal half of TM3 is open to the cytoplasmic side.
The probable side view of TM3 (helical cylinder) predicted from the results of the OGM labeling assay is shown in Fig. 5A. The N-terminal half of TM3 (isoleucine 64 to tyrosine 75) is enriched with hydrophobic amino acids (such as phenylalanine, isoleucine, methionine, and alanine), and cysteine substitution of the hydrophobic sector was less inhibitory to the transport activities of the variants. Moreover, many single-cysteine variants in the hydrophobic sector were labeled with OGM only to a small extent. In contrast, the C-terminal half (arginine 76 to methionine 85) of TM3 includes many hydrophilic amino acid residues (such as arginine and serine), and cysteine substitution caused a loss of transport function. Many of the cysteine-substituted variants in the hydrophilic sector were well labeled with OGM. Taken together, the results indicate that the local environment around the N-terminal half of TM3 differs from that around the C-terminal half.
The center of TM3 contains a proline residue (position 79) and glycine residues (positions 74 and 78) that may be involved in the formation of kinks and bends, thereby distorting the regular α-helical structure. This possibility is implied by the crystal structure of LacY, in which only 3 of 12 helices appear to be straight; all the others are arched, kinked, or S-shaped (4). Therefore, we used helical-wheel analysis to separately examine the N-terminal half forming a hydrophobic core (isoleucine 64 to tyrosine 75) and the C-terminal half forming a water-filled cavity (arginine 76 to tyrosine 75) (Fig. 5B).
A display of the hydrophobic core (isoleucine 64 to tyrosine 75) as a helical wheel (Fig. 5B, top panel) clearly showed that the attributed TM3 residues are asymmetrical. Thus, one helical face was enriched in residues whose substitution by cysteine was usually without marked function loss (except for Y75C). In contrast, at the other helical face, cysteine-scanning mutagenesis resulted in larger function loss; note that this latter face includes the charged residue aspartate 67, which is the only charged residue located in the hydrophobic core. The substitution of aspartate 67 with cysteine resulted in a moderate loss of transport activity (45.5% of the initial transport ratio of cysteine-less AspT). In addition, aspartate 67 is not conserved within the AAE family (Fig. 1). These results suggest that aspartate 67 does not play crucial roles in AspT function, but the location of aspartate 67 supports the idea that the latter helical face is exposed to hydrophilic environments. Despite the fact that tyrosine 75 seems to be located in the hydrophobic sector, the significant loss of function observed in the Y75C variant might be attributable to the GxxxG motif; this motif includes tyrosine 75, and its function was affected by the substitution, as described below. A display of the cytoplasmic half of TM3 (arginine 76 to methionine 85) as a helical wheel (Fig. 5B, bottom panel) showed that many of the cysteine-substituted variants in the segment were OGM labeled and showed function loss. In particular, on the face that includes arginine 76, the three residues proline 79, serine 80, and serine 83 were highly accessible to OGM. We therefore propose that the cytoplasmic half of TM3 is located in (or forms a part of) the hydrophilic water-filled cavity and plays an important role in the substrate transport of AspT.
l-Aspartate-induced conformational alteration.
In TM3, some positions showed variable accessibility to OGM dependent on whether or not l-aspartate was present (Fig. 2A and B, bottom panels, and Fig. 3A). This phenomenon has been explained by an l-aspartate-induced conformational alteration that makes these positions accessible or inaccessible to the aqueous phase and leads to l-aspartate binding at, or close to, these residues.
In the case of the cysteine introduced at proline 79, which is in a cytoplasmic cavity, the reactivity of cysteine was markedly stimulated in the presence of l-aspartate. Although the presence of l-aspartate inhibited the reactivity of the cysteine introduced at glycine 62, which is in a periplasmic loop, position 62 exhibited high accessibility to OGM only in the absence of l-aspartate (Fig. 2A and B, lanes G62C and P79C). The l-aspartate-dependent protection of G62C against OGM labeling may be achieved by direct steric hindrance derived from l-aspartate binding to the positions close to glycine 62, but this reason does not explain the stimulatory effect of l-aspartate on the OGM labeling of P79C. A second possibility is that there may be an l-aspartate-induced conformational alteration that buries or exposes the residues within the protein. In brief, in the absence of substrate, glycine 62 is exposed to the periplasmic surface, and proline 79 is located at the cytoplasmic end of the hydrophobic core. Substrate binding induces a structural conformation change, and those residues (glycine 62 and proline 79) move to the periplasmic end of the hydrophobic core and the cytoplasmic water-filled cavity, respectively. This hypothesis clearly explains the reasons for the protective effect and stimulatory effect of OGM labeling by substrate binding. Similar substrate-induced conformational alterations have been shown by means of the site-directed chemical labeling of OxlT, LacY, and the Na+/proline transporter PutP of E. coli (16, 37, 46).
Role of TM3 in AspT function.
Nonreducing SDS-PAGE analyses of purified single-cysteine AspT showed that some single-cysteine variants formed S-S-linked homodimers in membranes (Fig. 2A, top panel, and Fig. 3B, black bars). Moreover, a glutaraldehyde cross-linking assay suggested that functional AspT formed homo-oligomers under DDM-solubilized conditions (Fig. 4C). AspT solubilized only with DDM showed transport activity after reconstitution in proteoliposomes (Fig. 4B); this result suggests that DDM-solubilized AspT molecules probably kept active oligomer forms. Because these experiments do not show functional subunit stoichiometry within a protein complex in the native membrane, it is difficult to determine the oligomeric stoichiometry of the AspT complex from these experiments. However, these results do suggest that AspT formed homo-oligomers in the membrane and that the TM3s of the individual protomers are close to each other. In many membrane proteins, the GxxxG motif (two glycine residues separated by any three residues) is often found to be important for mediating the interaction of TM helices (11, 42). Early studies (including mutagenesis, computational modeling, and thermodynamic characterization) on glycophorin, a TM dimer, showed the central role of GxxxG (5, 15, 27) for TM interaction. For secondary transporters, the human serotonin transporter and the osmosensor and osmoprotectant transporter of E. coli have recently been reported, and GxxxG motifs in their TMs play an important role in their oligomerization and helix packing (14, 23). Interestingly, in the AAE family transporters, we found highly conserved GxxxG motifs in the hinge region (GYxxGP) that connects the N- and C-terminal halves of TM3 (Fig. 1). As a predictable role of the GxxxG motif in TM3 of an AspT protomer, the motif might contribute to the oligomerization of AspT by means of helix packing through the proximity of the individual TM3 in each AspT protomer.
The 10 single-cysteine variants of sodium:proton antiporter NhaA TM9 are known to form dimers without cross-linking reagents, and the positions forming dimers are located at the N-terminal half of NhaA TM9 (43). Pulsed electron paramagnetic resonance distance measurements of NhaA demonstrated that the TM9s of each protomer of NhaA are close to each other (22). Such spontaneous disulfide bond formation found in single-cysteine variants of a TM is generally thought to reflect the proximity of the TM in one protomer to that in the other protomer in its three-dimensional structure. Because six single-cysteine variants with substitutions located in the N-terminal half of AspT TM3 spontaneously formed a dimer by disulfide bonding under nonreducing conditions, we predicted that the N-terminal half of TM3 in one protomer is close to that of TM3 in the other protomer. Notably, five phenylalanine residues and one tyrosine residue are predicted to exist in the hydrophobic core (Fig. 5A). Aromatic amino acid residues have been shown to mediate the self-assembly of several soluble proteins through π-π interaction between aromatic rings (31, 41). Very recently, aromatic amino acid residues in the TMs of membrane proteins have been shown to promote oligomerization (41). It is also plausible that the aromatic amino acid residues in the hydrophobic core of AspT TM3 are involved in oligomerization. The single-cysteine variants (F66C, I73C, I64C, F71C, and Y75C) that formed an S-S-linked dimer in the hydrophobic core are clustered at the hydrophobic face (Fig. 5B, top panel). However, the single-cysteine variants in the probable substrate translocation pathway showed lower transport activities than the wild type, and these variants also showed low frequencies of disulfide bond formation; this result suggests that the positions of TM3 in one protomer are not close to the corresponding positions of the TM3s in other protomers. Therefore, we presume that the TM3 hydrophobic core consists of one face that is involved in oligomerization and another face that forms the substrate translocation pathway.
Recently, structural studies of the potassium channel and Ca2+-ATPase revealed that the small side chains of the amino acid residues found in the GxxxG motifs of these proteins play an important role in the stability and conformational changes of the proteins during substrate transport (11). For AspT, cysteine substitutions for highly conserved GxxxG motifs decreased l-aspartate transport activities (Table 1). Moreover, we found l-aspartate-induced conformational alterations in TM3, and the most drastic alterations of the reactivity of substituted cysteine residues toward OGM were observed at proline 79, which is located next to the GxxxG motif (Fig. 2A and B). Therefore, contribution to the flexibility of TM3 during substrate transport might be another potential role for the GxxxG motif in TM3 of AspT. To determine the substrate transport mechanism of AspT, ongoing studies are focusing on the GxxxG motif of TM3 and other TMs that form putative substrate translocation pathways.
Acknowledgments
This work was supported by a research fellowship for young scientists from the Japan Society for the Promotion of Science. K.N. is a research fellow of the Japan Society for the Promotion of Science.
We thank Di (Cody) Kang (Department of Physiology, the Johns Hopkins School of Medicine Institute) for helpful comments concerning protein purifications. We thank the Kikkoman Corporation for their gift of the T. halophilus asp operon.
Footnotes
Published ahead of print on 30 January 2009.
REFERENCES
- 1.Abe, K., H. Hayashi, and P. C. Maloney. 1996. Exchange of aspartate and alanine. Mechanism for development of a proton-motive force in bacteria. J. Biol. Chem. 2713079-3084. [DOI] [PubMed] [Google Scholar]
- 2.Abe, K., F. Ohnishi, K. Yagi, T. Nakajima, T. Higuchi, M. Sano, M. Machida, R. I. Sarker, and P. C. Maloney. 2002. Plasmid-encoded asp operon confers a proton motive metabolic cycle catalyzed by an aspartate-alanine exchange reaction. J. Bacteriol. 1842906-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abe, K., Z. S. Ruan, and P. C. Maloney. 1996. Cloning, sequencing, and expression in Escherichia coli of OxlT, the oxalate:formate exchange protein of Oxalobacter formigenes. J. Biol. Chem. 2716789-6793. [DOI] [PubMed] [Google Scholar]
- 4.Abramson, J., I. Smirnova, V. Kasho, G. Verner, H. R. Kaback, and S. Iwata. 2003. Structure and mechanism of the lactose permease of Escherichia coli. Science 301610-615. [DOI] [PubMed] [Google Scholar]
- 5.Adams, P. D., D. M. Engelman, and A. T. Brunger. 1996. Improved prediction for the structure of the dimeric transmembrane domain of glycophorin A obtained through global searching. Proteins 26257-261. [DOI] [PubMed] [Google Scholar]
- 6.Akabas, M. H., D. A. Stauffer, M. Xu, and A. Karlin. 1992. Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science 258307-310. [DOI] [PubMed] [Google Scholar]
- 7.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215403-410. [DOI] [PubMed] [Google Scholar]
- 8.Ambudkar, S. V., and P. C. Maloney. 1986. Bacterial anion exchange. Use of osmolytes during solubilization and reconstitution of phosphate-linked antiport from Streptococcus lactis. J. Biol. Chem. 26110079-10086. [PubMed] [Google Scholar]
- 9.Anantharam, V., M. J. Allison, and P. C. Maloney. 1989. Oxalate:formate exchange. The basis for energy coupling in Oxalobacter. J. Biol. Chem. 2647244-7250. [PubMed] [Google Scholar]
- 10.Barabote, R. D., D. G. Tamang, S. N. Abeywardena, N. S. Fallah, J. Y. Fu, J. K. Lio, P. Mirhosseini, R. Pezeshk, S. Podell, M. L. Salampessy, M. D. Thever, and M. H. Saier, Jr. 2006. Extra domains in secondary transport carriers and channel proteins. Biochim. Biophys. Acta 17581557-1579. [DOI] [PubMed] [Google Scholar]
- 11.Curran, A. R., and D. M. Engelman. 2003. Sequence motifs, polar interactions and conformational changes in helical membrane proteins. Curr. Opin. Struct. Biol. 13412-417. [DOI] [PubMed] [Google Scholar]
- 12.Doyle, D. A., J. Morais Cabral, R. A. Pfuetzner, A. Kuo, J. M. Gulbis, S. L. Cohen, B. T. Chait, and R. MacKinnon. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 28069-77. [DOI] [PubMed] [Google Scholar]
- 13.Fang, Y., L. Kolmakova-Partensky, and C. Miller. 2007. A bacterial arginine-agmatine exchange transporter involved in extreme acid resistance. J. Biol. Chem. 282176-182. [DOI] [PubMed] [Google Scholar]
- 14.Feng, J., and Z. Chun-Cheng. 2007. Thermodynamics of nucleosomal core particles. Biochemistry 462594-2598. [DOI] [PubMed] [Google Scholar]
- 15.Fleming, K. G., A. L. Ackerman, and D. M. Engelman. 1997. The effect of point mutations on the free energy of transmembrane alpha-helix dimerization. J. Mol. Biol. 272266-275. [DOI] [PubMed] [Google Scholar]
- 16.Frillingos, S., and H. R. Kaback. 1996. Probing the conformation of the lactose permease of Escherichia coli by in situ site-directed sulfhydryl modification. Biochemistry 353950-3956. [DOI] [PubMed] [Google Scholar]
- 17.Fu, D., and P. C. Maloney. 1997. Evaluation of secondary structure of OxlT, the oxalate transporter of Oxalobacter formigenes, by circular dichroism spectroscopy. J. Biol. Chem. 2722129-2135. [DOI] [PubMed] [Google Scholar]
- 18.Fu, D., and P. C. Maloney. 1998. Structure-function relationships in OxlT, the oxalate/formate transporter of Oxalobacter formigenes. Topological features of transmembrane helix 11 as visualized by site-directed fluorescent labeling. J. Biol. Chem. 27317962-17967. [DOI] [PubMed] [Google Scholar]
- 19.Fujiki, T., K. Nanatani, K. Nishitani, K. Yagi, F. Ohnishi, H. Yoneyama, T. Uchida, T. Nakajima, and K. Abe. 2007. Membrane topology of aspartate:alanine antiporter AspT from Comamonas testosteroni. J. Biochem. (Tokyo) 14185-91. [DOI] [PubMed] [Google Scholar]
- 20.Goswitz, V. C., and R. J. Brooker. 1995. Structural features of the uniporter/symporter/antiporter superfamily. Protein Sci. 4534-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hashimoto-Gotoh, T., K. Yasojima, and A. Tsujimura. 1995. Plasmids with a kanamycin-resistance gene for site-directed mutagenesis using the oligodeoxyribonucleotide-directed dual amber method. Gene 167333-334. [DOI] [PubMed] [Google Scholar]
- 22.Hilger, D., Y. Polyhach, E. Padan, H. Jung, and G. Jeschke. 2007. High-resolution structure of a Na+/H+ antiporter dimer obtained by pulsed electron paramagnetic resonance distance measurements. Biophys. J. 933675-3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horschitz, S., T. Lau, and P. Schloss. 2007. Glycine residues G338 and G342 are important determinants for serotonin transporter dimerisation and cell surface expression. Neurochem. Int. doi: 10.1016/j.neuint.2007.09.005. [DOI] [PubMed]
- 24.Jung, K., H. Jung, P. Colacurcio, and H. R. Kaback. 1995. Role of glycine residues in the structure and function of lactose permease, an Escherichia coli membrane transport protein. Biochemistry 341030-1039. [DOI] [PubMed] [Google Scholar]
- 25.Kaback, H. R. 1998. Structure/function studies on the lactose permease of Escherichia coli. Acta Physiol. Scand. Suppl. 64321-33. [PubMed] [Google Scholar]
- 26.Lai, F. A., M. Misra, L. Xu, H. A. Smith, and G. Meissner. 1989. The ryanodine receptor-Ca2+ release channel complex of skeletal muscle sarcoplasmic reticulum. Evidence for a cooperatively coupled, negatively charged homotetramer. J. Biol. Chem. 26416776-16785. [PubMed] [Google Scholar]
- 27.Lemmon, M. A., J. M. Flanagan, J. F. Hunt, B. D. Adair, B. J. Bormann, C. E. Dempsey, and D. M. Engelman. 1992. Glycophorin A dimerization is driven by specific interactions between transmembrane alpha-helices. J. Biol. Chem. 2677683-7689. [PubMed] [Google Scholar]
- 28.Maduke, M., D. J. Pheasant, and C. Miller. 1999. High-level expression, functional reconstitution, and quaternary structure of a prokaryotic ClC-type chloride channel. J. Gen. Physiol. 114713-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maloney, P. C. 1990. Resolution and reconstitution of anion exchange reactions. Philos. Trans. R. Soc. Lond. B 326437-454. [DOI] [PubMed] [Google Scholar]
- 30.Maloney, P. C., R. T. Yan, and K. Abe. 1994. Bacterial anion exchange: reductionist and integrative approaches to membrane biology. J. Exp. Biol. 196471-482. [DOI] [PubMed] [Google Scholar]
- 31.McGaughey, G. B., M. Gagné, and A. K. Rappé. 1998. π-Stacking interactions. Alive and well in proteins. J. Biol. Chem. 27315458-15463. [DOI] [PubMed] [Google Scholar]
- 32.Murakami, S., N. Tamura, A. Saito, T. Hirata, and A. Yamaguchi. 2004. Extramembrane central pore of multidrug exporter AcrB in Escherichia coli plays an important role in drug transport. J. Biol. Chem. 2793743-3748. [DOI] [PubMed] [Google Scholar]
- 33.Nanatani, K., F. Ohonishi, H. Yoneyama, T. Nakajima, and K. Abe. 2005. Membrane topology of the electrogenic aspartate-alanine antiporter AspT of Tetragenococcus halophilus. Biochem. Biophys. Res. Commun. 32820-26. [DOI] [PubMed] [Google Scholar]
- 34.Nanatani, K., T. Fujiki, K. Kanou, M. Takeda-Shitaka, H. Umeyama, L. Ye, X. Wang, T. Nakajima, T. Uchida, P. C. Maloney, and K. Abe. 2007. Topology of AspT, the aspartate:alanine antiporter of Tetragenococcus halophilus, determined by site-directed fluorescence labeling. J. Bacteriol. 1897089-7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearson, W. R. 1990. Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol. 18363-98. [DOI] [PubMed] [Google Scholar]
- 36.Pearson, W. R., and D. J. Lipman. 1988. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 852444-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pirch, T., S. Landmeier, and H. Jung. 2003. Transmembrane domain II of the Na+/proline transporter PutP of Escherichia coli forms part of a conformationally flexible, cytoplasmic exposed aqueous cavity within the membrane. J. Biol. Chem. 27842942-42949. [DOI] [PubMed] [Google Scholar]
- 38.Roth, G. J., C. J. Siok, and J. Ozols. 1980. Structural characteristics of prostaglandin synthetase from sheep vesicular gland. J. Biol. Chem. 2551301-1304. [PubMed] [Google Scholar]
- 39.Ruan, Z. S., V. Anantharam, I. T. Crawford, S. V. Ambudkar, S. Y. Rhee, M. J. Allison, and P. C. Maloney. 1992. Identification, purification, and reconstitution of OxlT, the oxalate:formate antiport protein of Oxalobacter formigenes. J. Biol. Chem. 26710537-10543. [PubMed] [Google Scholar]
- 40.Sakaguchi, T., Q. Tu, L. H. Pinto, and R. A. Lamb. 1997. The active oligomeric state of the minimalistic influenza virus M2 ion channel is a tetramer. Proc. Natl. Acad. Sci. USA 945000-5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sal-Man, N., D. Gerber, I. Bloch, and Y. Shai. 2007. Specificity in transmembrane helix-helix interactions mediated by aromatic residues. J. Biol. Chem. 28219753-19761. [DOI] [PubMed] [Google Scholar]
- 42.Senes, A., D. E. Engel, and W. F. DeGrado. 2004. Folding of helical membrane proteins: the role of polar, GxxxG-like and proline motifs. Curr. Opin. Struct. Biol. 14465-479. [DOI] [PubMed] [Google Scholar]
- 43.Tzubery, T., A. Rimon, and E. Padan. 2008. Structure-based functional study reveals multiple roles of transmembrane segment IX and loop VIII-IX in NhaA Na+/H+ antiporter of Escherichia coli at physiological pH. J. Biol. Chem. 28315975-15987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varadhachary, A., and P. C. Maloney. 1990. A rapid method for reconstitution of bacterial membrane proteins. Mol. Microbiol. 41407-1411. [DOI] [PubMed] [Google Scholar]
- 45.Wang, X., R. I. Sarker, and P. C. Maloney. 2006. Analysis of substrate-binding elements in OxlT, the oxalate:formate antiporter of Oxalobacter formigenes. Biochemistry 4510344-10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang, X., L. Ye, C. C. McKinney, M. Feng, and P. C. Maloney. 2008. Cysteine scanning mutagenesis of TM5 reveals conformational changes in OxlT, the oxalate transporter of Oxalobacter formigenes. Biochemistry 475709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weinglass, A. B., and H. R. Kaback. 1999. Conformational flexibility at the substrate binding site in the lactose permease of Escherichia coli. Proc. Natl. Acad. Sci. USA 9611178-11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan, R. T., and P. C. Maloney. 1993. Identification of a residue in the translocation pathway of a membrane carrier. Cell 7537-44. [PubMed] [Google Scholar]
- 49.Yan, R. T., and P. C. Maloney. 1995. Residues in the pathway through a membrane transporter. Proc. Natl. Acad. Sci. USA 925973-5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ye, L., Z. Jia, T. Jung, and P. C. Maloney. 2001. Topology of OxlT, the oxalate transporter of Oxalobacter formigenes, determined by site-directed fluorescence labeling. J. Bacteriol. 1832490-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ye, L., and P. C. Maloney. 2002. Structure/function relationships in OxlT, the oxalate/formate antiporter of Oxalobacter formigenes: assignment of transmembrane helix 2 to the translocation pathway. J. Biol. Chem. 27720372-20378. [DOI] [PubMed] [Google Scholar]
- 52.Yernool, D., O. Boudker, E. Folta-Stogniew, and E. Gouaux. 2003. Trimeric subunit stoichiometry of the glutamate transporters from Bacillus caldotenax and Bacillus stearothermophilus. Biochemistry 4212981-12988. [DOI] [PubMed] [Google Scholar]