Abstract
ISAba1 is an insertion sequence that is widely distributed in Acinetobacter baumannii. We demonstrated here that ISAba1 and the composite transposon Tn2006 are capable of transposition, generating 9-bp target site duplications. The expression of the ISAba1 transposase-encoding gene was downregulated by translational frameshifting.
Insertion sequences (IS) are the smallest and the most abundant transposable elements (<2.5 kb) capable of independent transposition in microbial genomes (20). They cause insertion mutations and genome rearrangements and enhance the spread of resistance and virulence determinants within species (2, 12, 16, 18, 23). Based on previous in silico analyses, ISAba1 is bracketed by 15-bp short inverted repeat sequences. ISAba1 is bound by 9-bp short direct repeats that correspond to target site duplications likely generated upon transposition and possesses the acidic amino acid triad DDE (5, 10, 13).
ISAba1 has been identified in Acinetobacter baumannii (10), which is a gram-negative bacterium causing nosocomial outbreaks and showing a multidrug resistance phenotype (28, 34). ISAba1 has been identified in association with several antibiotic resistance genes in A. baumannii (17, 26, 30, 32). The role of ISAba1 in the expression of the antibiotic resistance gene of A. baumannii has been demonstrated for blaampC, encoding the naturally occurring cephalosporinase, and for the blaOXA-23 gene, encoding a carbapenem-hydrolyzing oxacillinase, but it might act similarly with other resistance genes (6, 7, 10). ISAba1 might also be responsible for the mobility of blaOXA-23, with two copies bracketing this ß-lactamase gene and forming a composite transposon (defined as Tn2006) (7). The main objective of this study was to determine the functionality of ISAba1 as a mobile element and to analyze its impact on the plasticity of the A. baumannii genome.
In order to follow the transposition of ISAba1, this element was tagged. An ScaI restriction site was inserted upstream of the transposase coding sequence and downstream of the right inverted repeat in order to not impair the transposase-encoding gene, using primers pre-ISAba1-5′ext and ISAba1-3′extScaI (Table 1). ISAba1-ScaI was cloned in pCR-BluntII-TOPO vector (kanamycin resistant). A PCR product corresponding to the entire blaTEM-1 gene sequence, encoding an ampicillin resistance marker (25), was inserted in the ScaI restriction site, giving rise to recombinant plasmid pISAba1-TEM-1. The recombinant plasmid, introduced in the Escherichia coli TOP10 recipient strain by electrotransformation as previously described (21), was selected on Trypticase soy agar plates containing kanamycin (30 μg/ml) and amoxicillin (50 μg/ml). The recombinant plasmid pISAba1-TEM-1 was electroporated into E. coli RZ211 (pOX38-Gen; Genr, a transfer-proficient F plasmid derivative) for transposition experiments (8). Selection was performed on gentamicin (8 μg/ml)-, kanamycin (30 μg/ml)-, and amoxicillin (50 μg/ml)-containing plates. E. coli RZ211 was then used as a donor for mating-out assays with E. coli J53 Azider, with selection on gentamicin (8 μg/ml) and azide (100 μg/ml) with or without amoxicillin (50 μg/ml)-containing plates, as described previously (14, 22). The transposition frequency was calculated by dividing the number of Genr Amxr Azider transconjugants by the number of Genr Azider transconjugants. All of the Genr Amxr Azider colonies were screened for kanamycin susceptibility to exclude those that may have resulted from nontransposition events. The transposition frequency (mean plus or minus standard deviation) determined in E. coli (ISAba1-TEM-1) was (2.1 ± 0.7) × 10−7. (For the measurement of transposition frequencies, standard deviation was calculated from three independent cultures. Statistical analysis was performed using the Student t test; a P value of ≤0.05 was considered significant.)
TABLE 1.
Sequences of primers used in this study
| Primera | Sequence (5′→3′) | Reference or source |
|---|---|---|
| ISAba1a (+) | ATGCAGCGCTTCTTTGCAGG | 9 |
| ISAba1b (−) | AATGATTGGTGACAATGAAG | 9 |
| Pre-ISAba15′ext (+) | GCATGATTAGCTCCTCTG | This work |
| ISAba13′extScaI (−) | ATCCTCTGTACACGACAAATATACTTCACAGAACCC | This work |
| ISAba1-GSP1 (−) | TGAAAACATATTGAAAATCA | This work |
| ISAba1-GSP2 (−) | GAAGCGCTGCATACGTCGAT | This work |
| ISAba1-GSP3 (−) | GTGGTAAGCACTTGATGGGC | This work |
| ISAba1mut1 (+) | AACTTCTGCAATCGTGTTAAAAAGAACTTCATTGTCACC | This work |
| ISAba1mut2 (−) | GGTGACAATGAAGTTCTTTTTAACACGAATGC | This work |
| Pre-TEM1 (+) | GTATCCGCTCATGAGACAATA | 23 |
| Pre-TEM2 (−) | TCTAAAGTATATATGAGTAAACTTGGTCTG | 23 |
| ISAba15′ext (+) | CTCTACACATATCATAAGGCAGC | This work |
| Pre-TEM3′ext (−) | CCTACTTGCTTTATCCTGTCTAGCCG | This work |
| T3 | AATTAACCCTCACTAAAGGG | Stratagene |
| T7 | CGGGATATCACTCAGCATAATG | Stratagene |
| UAP | CTACTACTACTACTAGGCAGGCGTCGACTAGTAC | Invitrogen |
+, forward; −, reverse.
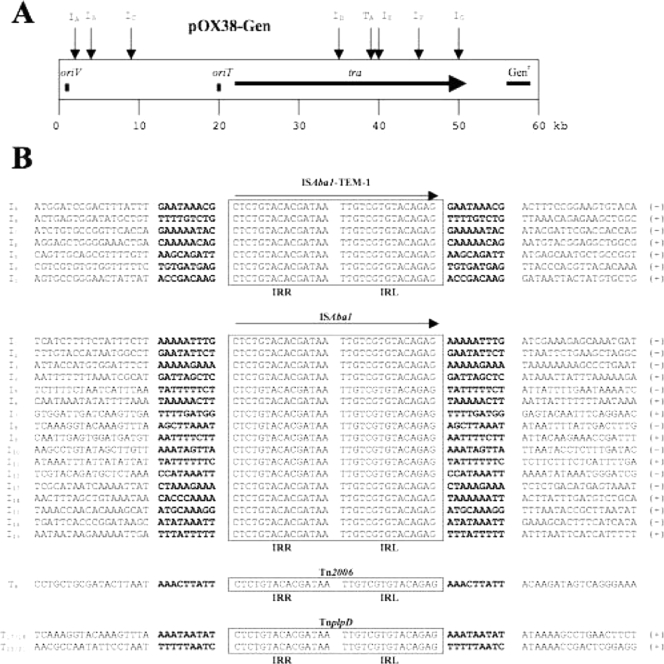
The insertion sites of ISAba1-TEM-1 were determined with seven randomly chosen Genr Amxr Azider Kans transconjugants by DNA sequencing (29) the external neighboring regions of the inverted repeats using primers ISAba1-5′ext and ISAba1-3′ext (Applied Biosystems 3100 sequencer) (Table 1). A 9-bp target site duplication, consistent with a transposition event, was observed. Insertions had occurred on seven different sites, and alignment of those insertion site sequences together with those identified in the genome of A. baumannii AYE, a multidrug-resistant clinical isolate (24, 33), revealed a consensus motif (AAATAAATT) (see Fig. 2) corresponding to an AT-rich target site sequence.
FIG. 2.
Target sites of ISAba1 and Tn2006 insertions. (A) Positions of the ISAba1-TEM-1 and Tn2006 insertions in plasmid pOX38-Gen. Insertions of the tagged IS (IA to IG) and of the composite transposons (TA) are indicated by vertical arrows. The origin of replication (oriV), the origin of transfer (oriT), the tra genes required for plasmid transfer, and the gentamicin resistance gene (Genr) are indicated. (B) Nucleotide sequence alignments of the seven pOX38-Gen::ISAba1-TEM-1 transconjugants and the 17 copies of ISAba1 in the A. baumannii AYE genome, pOX38-Gen::Tn2006 transconjugants, and TnplpD made by the 17 to 18 and 20 to 21 ISAba1 copy numbers and environment are shown. Nucleotide sequences of the end regions of ISAba1-TEM-1, ISAba1, and Tn2006 are boxed. Boldfaced letters indicate target site sequences duplicated upon transposition. Gray boxes indicate conserved nucleotides in the environment of the ISAba1-TEM-1 and Tn2006 insertions. Orientation of the IS of ISAba1-TEM-1, ISAba1, and Tn2006 is indicated by (+) and (−).
Since changes in growth conditions may affect the transposition efficiency of several mobile elements (19), the transposition of ISAba1 was determined after addition of several antibiotics as described previously (14). The antibiotic concentrations studied were 1/2, 1/4, and 1/10 of the MICs. The ciprofloxacin effect was studied since fluoroquinolones have been shown to induce antibiotic resistance in Vibrio cholerae through an SOS-mediated response (3). Transposition assays were performed as described above. No statistical difference was observed for ISAba1 transposition frequency by adding different antibiotics at the studied concentrations (Table 2).
TABLE 2.
ISAba1 transposition frequency in E. coli without and with antibioticsa
| Antibiotic (μg/ml) | Transposition frequency [(mean ± SD) × 10−7] |
|---|---|
| None | 2.1 ± 1.0 |
| Ceftazidime (0.12) | 1.8 ± 0.8 |
| Ceftazidime (0.06) | 2.4 ± 0.5 |
| Ceftazidime (0.02) | 7.5 ± 5.8 |
| Cefotaxime (0.02) | 5.2 ± 1.7 |
| Cefotaxime (0.01) | 3.2 ± 2.1 |
| Cefotaxime (0.005) | 4.0 ± 3.2 |
| Ciprofloxacin (0.001) | 3.3 ± 2.6 |
| Ciprofloxacin (0.0005) | 3.6 ± 0.6 |
| Ciprofloxacin (0.0002) | 2.6 ± 1.0 |
| Imipenem (0.06) | 1.4 ± 1.1 |
| Imipenem (0.03) | 6.6 ± 6.1 |
| Imipenem (0.01) | 3.4 ± 2.0 |
| Nalidixic acid (0.5) | 4.5 ± 3.1 |
| Nalidixic acid (0.25) | 1.2 ± 0.7 |
| Nalidixic acid (0.1) | 1.6 ± 1.0 |
| Piperacillin (6) | 2.9 ± 0.2 |
| Piperacillin (3) | 1.8 ± 0.2 |
| Piperacillin (1) | 3.1 ± 3.8 |
In each case, three independent experiments were performed, and the mean and standard deviations were calculated. Statistical analysis was performed using the Student t test, and a P value of ≤0.05 was considered significant. MICs for the E. coli RZ211 strain (pISAba1-TEM-1) were as follows: ceftazidime, 0.25 μg/ml; cefotaxime, 0.047 μg/ml; ciprofloxacin, 0.002 μg/ml; imipenem, 0.12 μg/ml; nalidixic acid, 1 μg/ml; and piperacillin, 12 μg/ml.
Translational control of transposition by frameshifting has been demonstrated for several IS (9, 15). ISAba1 contains two consecutive and overlapping open reading frames (ORFs), with the second ORF (orfB) at phase −1 relative to the upstream ORF (orfA) at phase 0 (Fig. 1). It was therefore likely that a frameshift might be necessary to give rise to a unique and likely functional transposase (5, 27). Analysis of the ISAba1 sequence showed a frameshift motif, made of seven adenosines at position 458. The AAA AAA A motif was changed to AAA AAG AA in order to generate a coding sequence that does not require any frameshifting and therefore to generate a constitutive expression of the transposase gene (the change is underlined). Site-directed mutagenesis was performed to add a guanine residue on the pISAba1-TEM-1 plasmid, yielding the pISAba1-mut-TEM-1 plasmid, according to the manufacturer's recommendations (QuikChange site-directed mutagenesis kit; Stratagene). Transposition experiments were performed as described above. Transposition efficiency (transposition frequency mean plus or minus standard deviation) was (1.8 ± 5.0) × 10−4 using the modified ISAba1 (ISAba1-mut-TEM-1), corresponding to a 1,000-fold increase compared to that of the native ISAba1 (Tn2006; see below). This increased transposition efficiency might result from a constitutive expression of the transposase. ISAba1 exhibits an A7 motif that we showed to be responsible for a negative regulation of tnpA expression.
FIG. 1.
Sequence of ISAba1. The deduced amino acid sequence is designated by a single-letter code below the nucleotide sequence, and left and right inverted repeats (IRL and IRR, respectively) are boxed. The asterisks indicate stop codons. The −35 and −10 promoter boxes are indicated. Pin corresponds to the promoter of the orfAB transposase gene, and Pout to the promoter provided by ISAba1 for expression of adjacent genes. The +1 of transcription for tnpA is indicated in boldface type. The A7 motif is underlined, and the DDE motif is boldfaced.
The genetics of acquisition of the blaOXA-23 gene had been previously investigated, and the composite transposon Tn2006 was identified (7). The entire Tn2006 sequence was cloned in pBK-CMV vector (kanamycin resistant), and transposition events were analyzed as described previously (14). The blaOXA-23 gene conferring resistance to amoxicillin provided a marker to follow transposition events. The transposition efficiency of Tn2006 was found to be (1.6 ± 2.5) × 10−8, being 10-fold lower than that observed with a single ISAba1 element, suggesting that ISAba1 transposition frequency might decrease with the length of the mobilized DNA fragment.
To demonstrate that the ISAba1 element possesses promoter sequences for tnpA gene expression, the site of transcription initiation for the tnpA gene was mapped from RNA of A. baumannii AYE (33), using the 5′ RACE (rapid amplification of cDNA ends) PCR technique (version 2.0; Invitrogen, Life Technologies, Cergy-Pontoise, France). The +1 transcription start was found to be 73 bp upstream of the start codon. The promoter Pin was subsequently defined to be made of the −10 (TACTAT) and −35 (TAATAA) boxes separated by 18 bp (Fig. 1). We showed here that the tnpA gene possesses promoter sequences, conferring on ISAba1 the property to be an autonomous mobile element.
In order to expand the knowledge related to ISAba1 distribution among the Acinetobacter genus, 14 different species of Acinetobacter were screened by PCR. ISAba1 was found in wild-type and in carbapenem-resistant A. baumannii isolates obtained from worldwide sources but also in five other Acinetobacter species (Table 3).
TABLE 3.
Distribution of ISAba1 in Acinetobacter spp.
| Species | Place(s) of origin | No. of isolate(s) | No. of positive isolate(s)b |
|---|---|---|---|
| Acinetobacter baumannii | Variousa | 50 | 40 |
| Acinetobacter haemolyticus | Belgium | 2 | 2 |
| Acinetobacter johnsonii | France | 3 | 1 |
| Acinetobacter junii | France | 9 | 7 |
| Acinetobacter lwoffii | France | 3 | 2 |
| Germany | 1 | 1 | |
| Acinetobacter radioresistens | France | 3 | 0 |
| Acinetobacter schindleri | France | 1 | 0 |
| Acinetobacter ursingii | France | 1 | 0 |
| Acinetobacter genomospecies 3 | France | 1 | 0 |
| Acinetobacter genomospecies 9 | France | 3 | 3 |
| Germany | 1 | 1 | |
| Acinetobacter genomospecies 10 | France | 1 | 0 |
| Acinetobacter genomospecies 13 | France | 1 | 0 |
| Acinetobacter genomospecies 15 | France | 1 | 0 |
| Acinetobacter genomospecies 16 | France | 1 | 0 |
| Acinetobacter genomospecies 17 | France | 1 | 0 |
Place of origin and number of isolates for A. baumannii strains: France (23), Romania (3), Belgium (1), Turkey (1), Sweden (3), Tunisia (1), Tahiti (2), Benin (1), Libya (1), Vietnam (1), New Caledonia (2), South Africa (2), Egypt (1), Monaco (1), Réunion (1), United States (2), Switzerland (1), China (1), Russia (1), Spain (1).
Number of isolates in which ISAba1-like copies were detected.
In silico analysis of the A. baumannii AYE genome (24, 33) identified 21 copies of ISAba1, differing only by a single-base-pair substitution (guanine to adenine at position 65) located upstream of orfA, and therefore, modifying neither the coding sequence nor the promoter sequences of the transposase gene. ISAba1-related gene disruption was found for different genes. Seven configurations in which ISAba1 was located close to and upstream of genes were identified, likely providing promoter sequences enhancing their expression. Six copies of ISAba1 were in such configuration that they were forming composite transposons. Two copies (both bracketed by target site duplications) surrounded the plpD gene encoding a putative phospholipase D, and the same ISAba1-plpD-ISAba1 structure was identified twice as a composite transposon named TnplpD (Fig. 2). Two copies of TnplpD had thus been very likely generated upon ISAba1-mediated transposition, giving rise to a plpD multicopy. By analyzing in silico the recently available A. baumannii genome sequences, nine copies of ISAba1 were identified from strain AB0057 (1) and only a single copy was identified in strain ATCC 17978 (31), whereas ISAba1 was absent from strains AB307-0294, AB900 (1), SDF (33), and ACICU (11).
Here, we demonstrated that ISAba1 and the composite transposon Tn2006 were capable of transposition in E. coli strains as well as the ability of ISAba1 to mobilize an antibiotic resistance gene. The ISAba1 element contains two ORFs, encoding a functional transposase, regulated by a mechanism named programmed translational frameshifting that has already been identified at least for IS3 family members (4). This study reports on the very first functional properties of an IS element in A. baumannii.
Acknowledgments
This work was financed by a grant from the Ministère de l'Éducation Nationale et de la Recherche, Université Paris XI, France, and mostly by a grant from the European Community (DRESP2; grant LSHM-CT-2005-018705) and the INSERM, France.
Footnotes
Published ahead of print on 9 January 2009.
REFERENCES
- 1.Adams, M. D., K. Goglin, N. Molyneaux, K. M. Hujer, H. Lavender, J. J. Jamison, I. J. MacDonald, K. M. Martin, T. Russo, A. A. Campagnari, A. M. Hujer, R. A. Bonomo, and S. R. Gill. 2008. Comparative genome sequence analysis of multidrug-resistant Acinetobacter baumannii. J. Bacteriol. 1908053-8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aubert, D., T. Naas, C. Héritier, L. Poirel, and P. Nordmann. 2006. Functional characterization of IS1999, an IS4 family element involved in mobilization and expression of β-lactam resistance genes. J. Bacteriol. 1886506-6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beaber, J. W., B. Hochhut, and M. K. Waldor. 2004. SOS response promotes horizontal dissemination of antibiotic resistance genes. Nature 172-74. [DOI] [PubMed] [Google Scholar]
- 4.Chandler, M., and O. Fayet. 1993. Translational frameshifting in the control of transposition in bacteria. Mol. Microbiol. 7497-503. [DOI] [PubMed] [Google Scholar]
- 5.Chandler, M., and J. Mahillon. 2002. Insertion sequences revisited, p. 305-366. In N. L. Craig, R. Craigie, M. Gellert, and A. M. Lambowitz (ed.), Mobile DNA II. ASM Press, Washington, DC.
- 6.Corvec, S., N. Caroff, E. Espaze, C. Giraudeau, H. Drugeon, and A. Reynaud. 2003. AmpC cephalosporinase hyperproduction in Acinetobacter baumannii clinical strains. J. Antimicrob. Chemother. 52629-635. [DOI] [PubMed] [Google Scholar]
- 7.Corvec, S., L. Poirel, T. Naas, H. Drugeon, and P. Nordmann. 2007. Genetics and expression of the carbapenem-hydrolyzing oxacillinase gene blaOXA-23 in Acinetobacter baumannii. Antimicrob. Agents Chemother. 511530-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derbyshire, K. M., L. Hwang, and N. D. Grindley. 1987. Genetic analysis of the interaction of the sequence insertion IS903 transposase with its terminal inverted repeats. Proc. Natl. Acad. Sci. USA 848048-8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escoubas, J. M., D. Lane, and M. Chandler. 1994. Is the IS1 transposase, InsAB′, the only IS1-encoded protein for efficient transposition? J. Bacteriol. 1765864-5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Héritier, C., L. Poirel, and P. Nordmann. 2006. Cephalosporinase over-expresssion resulting from insertion of ISAba1 in Acinetobacter baumannii. Clin. Microbiol. Infect. 12123-130. [DOI] [PubMed] [Google Scholar]
- 11.Iacono, M., L. Villa, D. Fortini, R. Bordoni, F. Imperi, R. J. Bonnal, T. Sicheritz-Ponten, G. De Bellis, P. Visca, A. Cassone, and A. Carattoli. 2008. Whole-genome pyrosequencing of an epidemic multidrug-resistant Acinetobacter baumannii strain belonging to the European clone II group. Antimicrob. Agents Chemother. 522616-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato, N., K. Yamazoe, C. G. Han, and E. Ohtsubo. 2003. New insertion sequence elements in the upstream region of cfiA in imipenem-resistant Bacteroides fragilis strains. Antimicrob. Agents Chemother. 47979-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kulkosky, J., K. S. Jones, R. A. Katz, J. P. Mack, and A. M. Skalka. 1992. Residues critical for retroviral integrative recombination in a region that is highly conserved among retroviral/retrotransposon integrases and bacterial insertion sequence transposases. Mol. Cell. Biol. 122331-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lartigue, M.-F., L. Poirel, D. Aubert, and P. Nordmann. 2006. In vitro analysis of ISEcp1B-mediated mobilization of naturally occurring β-lactamase gene blaCTX-M of Kluyvera ascorbata. Antimicrob. Agents Chemother. 501282-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Licznar, P., N. Melhede, M.-F. Prère, N. Wills, R. F. Gesteland, J. F. Atkins, and O. Fayet. 2003. Programmed translational-1 frameshifting on hexanucleotide motifs and the whobble properties of tRNAs. EMBO J. 224770-4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin, H., T-Y. Li, M.-H. Xie, and Y. Zhang. 2007. Characterization of the variants, flanking genes, and promoter activity of the Leifsonia xyli subsp. cynodontis insertion sequence IS1237. J. Bacteriol. 1893217-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naas, T., F. Namdari, H. Réglier-Poupet, C. Poyart, and P. Nordmann. 2007. Panresistant extended-spectrum ß-lactamase SHV-5-producing Acinetobacter baumannii from New York City. J. Antimicrob. Chemother. 601174-1176. [DOI] [PubMed] [Google Scholar]
- 18.Nagai, T., L. S. Phan Tran, Y. Inatsu, and Y. Itoh. 2000. A new IS4 family insertion sequence, IS4Bsu1, responsible for genetic instability of poly-γ-glutamic acid production in Bacillus subtilis. J. Bacteriol. 1822387-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagy, Z., and M. Chandler. 2004. Regulation of transposition in bacteria. Res. Microbiol. 155387-398. [DOI] [PubMed] [Google Scholar]
- 20.Nevers, P., and H. Sadler. 1977. Transposable genetic elements as agents of gene instability and chromosomal rearrangements. Nature 268109-115. [DOI] [PubMed] [Google Scholar]
- 21.Philippon, L. N., T. Naas, A.-T. Bouthors, V. Barakett, and P. Nordmann. 1997. OXA-18, a class D clavulanic acid-inhibited extended-spectrum β-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 412188-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poirel, L., M. Guibert, S. Bellais, T. Naas, and P. Nordmann. 1999. Integron and carbenicillinase-mediated reduced susceptibility to amoxicillin-clavulanic acid in isolates of multidrug-resistant Salmonella enterica serotype Typhimurium DT104 from French patients. Antimicrob. Agents Chemother. 431098-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poirel, L., J.-W. Decousser, and P. Nordmann. 2003. Insertion sequence ISEcp1B is involved in expression and mobilization of a blaCTX-M β-lactamase gene. Antimicrob. Agents Chemother. 472938-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poirel, L., O. Menuteau, N. Agoli, C. Cattoen, and P. Nordmann. 2003. Outbreak of extended-spectrum β-lactamase VEB-1 producing isolates of Acinetobacter baumannii in a French hospital. J. Clin. Microbiol. 413542-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poirel, L., H. Mammeri, and P. Nordmann. 2004. TEM-121, a novel complex mutant of TEM-type ß-lactamase from Enterobacter aerogenes. Antimicrob. Agents Chemother. 484528-4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poirel, L., and P. Nordmann. 2006. Genetics structures at the origin of acquisition and expression of the carbapenem-hydrolyzing oxacillinase gene blaOXA-58 in Acinetobacter baumannii. Antimicrob. Agents Chemother. 501442-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prère, M.-F., M. Chandler, and O. Fayet. 1990. Transposition in Shigella dysenteriae: isolation and analysis of IS911, a new member of the IS3 group of insertion sequences. J. Bacteriol. 1724090-4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruiz, M., S. Marti, F. Fernandez-Cuenca, A. Pascual, and J. Vila. 2007. Prevalence of ISAba1 in epidemiologically unrelated Acinetobacter baumannii clinical isolates. FEMS Microbiol. Lett. 27463-66. [DOI] [PubMed] [Google Scholar]
- 29.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 745463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segal, H., S. Garny, and B. G. Elisha. 2005. Is ISAba1 customized for Acinetobacter? FEMS Microbiol. Lett. 243425-429. [DOI] [PubMed] [Google Scholar]
- 31.Smith, M. G., T. A. Gianoulis, S. Pukatzki, J. J. Mekalanos, L. N. Ornston, M. Gerstein, and M. Snyder. 2007. New insights into Acinetobacter baumannii pathogenesis revealed by high-density pyrosequencing and transposon mutagenesis. Genes Dev. 21601-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turton, J. F., M. E. Ward, N. Woodford, M. E. Kaufmann, R. Pike, D. M. Livermore, and T. L. Pitt. 2006. The role of ISAba1 in expression of OXA carbapenemase genes in Acinetobacter baumannii. FEMS Microbiol. Lett. 25872-77. [DOI] [PubMed] [Google Scholar]
- 33.Vallenet, D., P. Nordmann, V. Barbe, L. Poirel, S. Mangenot, E. Bataille, C. Dossat, S. Gas, A. Kreimeyer, P. Lenoble, S. Oztas, J. Poulain, B. Segurens, C. Robert, C. Abergel, J. M. Claverie, D. Raoult, C. Médigue, J. Weissenach, and S. Cruveiller. 2008. Comparative analysis of acinetobacters: three genomes for three lifestyles. PLoS ONE 19e1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou, H., Q. Yang, Y. S. Yu, Z. Q. Wei, and L. J. Li. 2007. Clonal spread of imipenem-resistant Acinetobacter baumannii among different cities of China. J. Clin. Microbiol. 454054-4057. [DOI] [PMC free article] [PubMed] [Google Scholar]