Abstract
When salt-tolerant Myxococcus cells are moved to a seawater environment, they change their growth, morphology, and developmental behavior. Outer membrane proteins and signal transduction pathways may play important roles in this shift. Chip hybridization targeting the genes predicted to encode 226 two-component signal transduction pathways and 74 outer membrane proteins of M. xanthus DK1622 revealed that the expression of 55 corresponding genes in the salt-tolerant strain M. fulvus HW-1 was significantly modified (most were downregulated) by the presence of seawater. Sequencing revealed that these seawater-regulated genes are highly homologous in both strains, suggesting that they have similar roles in the lifestyle of Myxococcus. Seven of the genes that had been reported in M. xanthus DK1622 are involved in different cellular processes, such as fruiting body development, sporulation, or motility. The outer membrane (Om) gene Om031 had the most significant change in expression (downregulated) in response to seawater, while the two-component system (Tc) gene Tc105 had the greatest increase in expression. Their homologues MXAN3106 and MXAN4042 were knocked out in DK1622 to analyze their functions in response to changes in salinity. In addition to having increased salt tolerance, sporulation of the MXAN3106 mutant was enhanced compared to that of DK1622, whereas mutating gene MXAN4042 produced contrary results. The results indicated that the genes that are involved in the cellular processes that are significantly changed in response to salinity may also be involved the salt tolerance of Myxococcus cells. Regulating the expression levels of these multifunctional genes may allow cells to quickly and efficiently respond to changing conditions in coastal environments.
Isolation techniques based on the formation of fruiting body structures reveal that myxobacteria primarily inhabit various terrestrial environments (5, 20). Salt-tolerant fruiting myxobacterial strains from coastal environments can also be found if the enriching medium is prepared with diluted seawater (16). The salt-tolerant myxobacterial strains exhibit significant shifts in growth, development, and cellular behavior in response to the presence or absence of seawater. For example, when grown in medium with yeast cells as the only nutrient source, the salt-tolerant strain Myxococcus fulvus HW-1 requires a high cell concentration to initiate growth if the cells were collected from seawater-free cultures; the threshold number of cells required for growth decreases if the cells were from seawater-containing cultures (31). On nutrient-deficient plates, salt-tolerant Myxococcus strains are able to develop typical fruiting bodies only when the seawater concentration is low or absent (the optimal seawater concentration for the development of fruiting bodies varies from 0% to 60% for different salt-tolerant Myxococcus strains) (27). Although the development process is inhibited by high concentrations of seawater, the myxospores can be developed directly from vegetative cells (31). Phylogeny, morphology, and cellular behavior suggest that the salt-tolerant strains are probably soil myxobacterial strains that have adapted to seawater conditions (31). The underlying mechanism of how the salt-tolerant myxobacteria adapted to seawater is important for understanding the evolution of the myxobacterial multicellular lifestyle in natural environments.
Two-component systems are widely used in bacteria. They regulate gene expression in various cellular metabolic processes (24), including the adjustment to osmotic pressure, via extracellular or intracellular signal transduction pathways. For example, the two-component system EnvZ-OmpR in Escherichia coli cells regulates the cellular osmotic balance by controlling the expression of membrane proteins OmpC and OmpF (9). Besides two-component systems, outer membrane proteins, such as channel proteins or transporters, may also play predominant roles in maintaining the intracellular osmotic balance by changing their function or expression when cells live in seawater environments. In this study, a low-density chip that targeted the predicted open reading frames (ORFs) of two-component systems (Tc) and outer membrane proteins (Om) of M. xanthus DK1622 was constructed to evaluate the expression of the corresponding genes in the salt-tolerant Myxococcus strain M. fulvus HW-1. The chip hybridization survey revealed that the expression levels of 55 genes in HW-1 were significantly different in the presence or absence of seawater. The genes that had increased or decreased expression in HW-1 were sequenced for comparison. Further, two gene loci of the homologues to one significantly upregulated and one significantly downregulated gene of M. fulvus HW-1 were knocked out in the DK1622 strain to analyze their functions in response to changes in salinity.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. M. xanthus DK1622 and M. fulvus HW-1 were cultured in CTT broth (10) or CTT containing different concentrations of seawater or NaCl (HCTT) (31). Development experiments were performed on TPM (13) or TPM containing different concentrations of seawater or NaCl (HTPM). E. coli cells were cultivated in liquid Luria-Bertani medium (LB) or on solid LB containing 1.5% agar. Myxococcus cells were incubated at 30°C, while E. coli cells were grown at 37°C. When necessary, kanamycin was added to the media at a final concentration of 40 μg/ml.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype or description | Source or reference |
|---|---|---|
| Strains | ||
| M. fulvus HW-1 (ATCC BAA-855) | Wild type | 31 |
| M. xanthus | ||
| DK1622 | Wild type | 12; Z. Yang, Virginia Tech |
| YL0401 | MXAN3106 in-frame deletion in DK1622 | This study |
| YL0402 | MXAN4042 in-frame deletion in DK1622 | This study |
| E. coli XLI-Blue MR | Δ(mcrA)183 Δ(mcrCB-hsdSMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac | Stratagene Co. |
| Plasmids | ||
| pBJ113 | Gene replacement vector with KG cassette; Kmr | 11; Z. Yang, Virginia Tech |
| pBJ-MXAN3106d | MXAN3106 in-frame deletion in pBJ113 | This study |
| pBJ-MXAN4042d | MXAN4042 in-frame deletion in pBJ113 | This study |
Microarray design and construction.
The genomic sequence of M. xanthus DK1622 (GenBank accession number CP000113) was retrieved from the GenBank database. The genes predicted to encode outer membrane proteins and two-component systems were selected to construct a low-density microarray (the detailed procedure is described in supplemental material S1). In total, 300 ORFs were arrayed, targeting 226 two-component proteins and 74 outer membrane proteins. The efficacy of the microarray was tested by hybridizing the chip to genomic DNA from M. xanthus DK1622 (labeled with fluorescent dye Cy5) and M. fulvus HW-1 (labeled with fluorescent dye Cy3).
Extraction of total RNA.
M. fulvus HW-1 cells were grown in CTT or HCTT (containing 20% seawater) liquid broth with shaking at 30°C. Cells at the mid-log phase of growth were harvested, washed, and resuspended in an RNase-free solution containing 25% phenol-ethanol (5% acid phenol and 95% ethanol, pH 4.5). After 10 min of incubation on ice, total RNA was isolated using the Promega SV total RNA purification kit according to the manufacturer's instructions. The RNA was further purified by DNase I (Takara) digestion and phenol-chloroform extraction. The RNA concentration, purity, and integrity were estimated by the absorbance at 260 nm, absorbance at 260/280 nm, and agarose gel electrophoresis, respectively.
Synthesis of cDNA, labeling, hybridization, and data analysis.
Synthesis of cDNA, labeling, and hybridization were performed as previously described (7). Briefly, 20 μg of sample RNA and 2 μl of quality control RNA were reverse transcribed to cDNA using random hexamer primers. After polymerization, the RNA was hydrolyzed with alkali, and the cDNA was purified using the QiaQuick PCR purification kit (Qiagen). The purified cDNAs from CTT and HCTT cultures were labeled with Cy3-dCTP and Cy5-dCTP, respectively, using random primers. The labeled cDNAs were combined for hybridization. After hybridization and washing, the slides were scanned with a GenePix 4100A scanner (Axon Instruments Inc.). Fluorescent spots and local background intensities were quantified using GenePix Pro 6.0 software. The data were normalized using the internal standard and “housekeeping gene” methods (28). Differentially expressed genes were filtered by the “fold change” method (2). Twofold changes in the level of expression were considered significant.
Quantitative real-time PCR.
The purified cDNA served as the template, with the 16S rRNA gene as an internal control. Primers were designed using Beacon Designer (Premier Biosoft International) to produce fragments of 80 to 150 bp. The primer pair (forward/reverse) for the amplification of the 16S rRNA gene was 5′-CGGCGTGACAAGTCGGGTGTGAAAG-3′/5′-CGTCTCAGCGTCAGTTACCGTCCAG-3′. For the Tc105 gene the primer pair was 5′-TGTCCATCAGCGGCGAGTCC-3′/5′-GGCGATGAAGGGCTTGTCA CG-3′, and for the Om031 gene it was 5′-TCCTCGTCGTCGGCATCA-3′/5′-GTAGCGGGTCTCGGCTGTT-3′. The PCRs were in a total volume of 25 μl containing 12.5 μl Sybr green PCR master mix (Takara), 0.5 μl of each primer (10 μM), 0.5 μl cDNA, and 11 μl double-distilled water. PCR was performed in a Bio-Rad Chromo4 real-time PCR detection system using standard conditions. Quadruplicate samples were examined for each gene, and the data were analyzed using the 2−ΔΔCT method as described previously (17).
In-frame deletion of genes in M. xanthus DK1622.
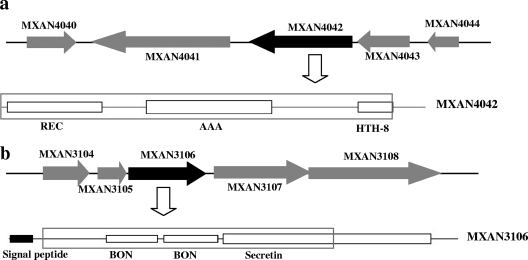
In-frame deletions of MXAN3106 and MXAN4042 in M. xanthus DK1622 were performed using the positive-negative KG cassettes described by Ueki et al. (26). The in-frame deletion of MXAN4042 includes a 1,524-bp fragment containing the predicted coding regions of the REC domain at amino acids (aa) 4 to 115, the AAA domain at aa 163 to 307 of MXAN4042, and also a 171-bp noncoding segment in front of the gene (Fig. 1a). The MXAN3106 protein has three predicted transmembrane regions, of which two (aa 249 to 276 and aa 368 to 385) were knocked out; the signal peptide (aa 5 to 24) remained (in total 951 bp [317 aa] were deleted [Fig. 1b]). Upstream and downstream homologous arms were amplified from the DK1622 genome using PrimeSTAR HS DNA polymerase (Takara). The primer sets MXAN3106dUF/UR (5′-GCGGCAAGCACTGCGACTTCG-3′/5′-GCGAATTCCTGACGGTGGTGCCATCCT-3′; EcoRI site is indicated by underlining and italics; protection bases are indicated by underlining) (product = 986 bp) and MXAN3106dDF/DR (5′-CGGAATTCGGACCGCAACGGCAACATC-3′/5′-TCCTCGTAGGGCTCGTCTTCTTC-3′) (product = 978 bp) were designed for the amplification of MXAN3106, and MXAN4042dUF/UR (5′-AGCCGTCCTTGTCCTCGAT-3′/5′-GCGAATTCTGTTCCACGCCTCGGGCAACT-3′) (product = 875 bp) and MXAN4042dDF/DR (5′-GCGAATTCAATGCGATCTCGACCTTGTG-3′/5′-AATCCCGAGGAGTTCCAGTTGGTTA-3′) (product = 879 bp) were designed for MXAN4042. After amplification, the two homologous arms were treated with EcoRI and then ligated to form an internal in-frame deletion fragment. The fragment was then PCR amplified, purified, and ligated into the SmaI site of pBJ113, producing the deletion plasmid pBJ-MXAN3106d or pBJ-MXAN4042. The deletion plasmids were transferred separately into M. xanthus DK1622 by electroporation. Kmr transformants were selected and then inoculated onto CTT agar supplemented with 1% galactose. Deletion mutants were identified by galactose resistance, Km sensitivity, and PCR amplification.
FIG. 1.
Physical organization of Tc105 (MXAN4042) (a) and Om031 (MXAN3106) (b) in M. xanthus DK1622, showing the putative functional domains. The deletions are boxed.
Effects of salts or seawater on growth and development.
The effects of salts or seawater on cell growth and development were measured using previously described methods (30,31). Cells in the mid-log growth phase were inoculated into CTT containing different concentrations of salts or seawater. The absorbance at 600 nm was measured every 4 h to evaluate the growth. The generation time was determined by counting the cell numbers during the early exponential growth phase with a Helber bacterial counting chamber under a phase-contrast microscope. The generation time (G) was calculated as follows: G = 0.301 × Δt/(log10 X2 − log10 X1), where Δt is the interval between two collection times (in hours) and X1 and X2 are the cell concentrations (cells per milliliter) at the collection times.
To assay cell development, cells were grown to mid-log phase, harvested, and resuspended in TPM buffer at a density of 5 × 109 cells/ml. Aliquots of 10 μl were spotted on TPM agar plates containing different concentrations of salts or seawater. The plates were incubated at 30°C for 5 days. Cell development was examined under a dissection microscope. Five-day TPM cultures were assayed for sporulation. The cells were resuspended in 100 μl TPM buffer and slightly sonicated for homogenization. After 2 h of incubation at 50°C, the cells were serially diluted, mixed with CTT (containing 0.3% agar), and poured onto CTT plates. After 5 days of incubation at 30°C, the colonies were counted.
Viability staining.
The autolysis of cells during development was measured using the Live/Dead BacLight bacterial viability kit (Invitrogen; L7012) as described by Nariya and Inouye (19) with minor modifications. The cells were collected from cultures developing on TPM containing 0% or 50% seawater. The collected cells were washed twice with TM buffer, adjusted to 5 × 108 cells per milliliter, and stained according to the manufacturer's protocol. The cells were observed and counted under a fluorescence microscope (Nikon TE2000s). The viability of cells was also measured by culturing serially diluted cells on CTT plates without heat treatment.
Swarming assay.
To measure the swarming capacity of the mutants, the method described by Shi and Zusman (22) was employed with minor modifications. Aliquots (2 μl, 5 × 109 cells/ml) were inoculated onto CTT medium containing 1.5% or 0.3% agar. Different concentrations of NaCl or seawater were added to the medium to measure their effects on motility (27). The diameters of the initial colonies were 0.4 ± 0.1 cm. After 3 or 5 days of incubation at 30°C, the sizes of the swarming colonies were measured, and the types of cellular motility at edges of the colonies were observed under a phase-contrast microscope (Nikon Eclipse TE2000-S).
Agglutination assay.
Cellular agglutination was assayed using the method previously described by Shimkets (23) with minor modifications. The cells were grown in CTT medium overnight and harvested by centrifugation at 10,000 × g for 2 min at 4°C. The cells were then washed once with MOPS (morpholinepropanesulfonic acid) buffer (pH 6.8), resuspended, and adjusted to an absorbance of 0.6 at 600 nm. Agglutination and cellular cohesion were determined in buffer containing 10 mM MOPS (pH 6.8), 1 mM MgCl2, and 1 mM CaCl2. The absorbance at 600 nm was measured every 10 min. This experiment was repeated twice.
Liquid colorimetric assay.
To measure the uptake and binding of dyes, a liquid colorimetric assay was performed with Congo red and trypan blue according to the method described previously by Black and Yang (3).
Nucleotide sequence accession numbers.
The sequences of the genes from M. fulvus HW-1 that were upregulated or downregulated in response to the presence of seawater have been deposited in the GenBank databases with accession numbers EU744193 to EU744246.
RESULTS
Changes in gene expression profiles of two-component systems and outer membrane proteins in salt-tolerant M. fulvus HW-1 in response to the presence of seawater.
Bioinformatic analysis of the complete genomic sequence of M. xanthus DK1622 revealed more than 200 ORFs involved in two-component signal transduction pathways (8), forming an extensive regulatory network for its complex lifestyle (32). Signal transduction pathways, together with outer membrane proteins, are thought to play predominant roles in the development of salt-tolerant myxobacteria adapting to oceanic conditions. A low-density microarray was constructed from the genomic sequence of M. xanthus DK1622 to survey the expression profile changes in salt-tolerant strain M. fulvus HW-1 in response to the presence of seawater. In total, 300 selected ORFs were used to construct a hybridization chip, including 226 ORFs predicted to encode proteins of two-component systems and 74 ORFs predicted to encode outer membrane proteins (the detailed procedure for the preparation of the microarray is described in supplemental material S1). Chip hybridization confirmed that more than 90% of the DK1622 probes produced positive signals on HW-1 genomic DNA (26 unhybridized ORFs are listed in Table S2 in the supplemental material). The chip was then hybridized with total RNA extracted from HW-1 cells grown in the presence or absence of seawater. The survey revealed 55 genes that had significantly changed expression levels. Most of these 55 genes were downregulated by the presence of seawater, while only a few were upregulated by seawater (48 versus 7) (Table 2).
TABLE 2.
Hybridization signals from the HW-1 genomic microarray extracted from fresh cultures labeled with Cy3 and seawater cultures labeled with Cy5
| ID | Locus | Description | Hybridization signal [log2 (Cy5/Cy3)] | GenBank accession no. | Lengths (bp) of corresponding genes in HW-1/DK1622 | % Nucleotide/amino acid identity |
|---|---|---|---|---|---|---|
| Om001 | MXAN5970 | Peptidase, S8 (subtilisin) family | −1.354 | EU744193 | 2,307/2,310 | 91/89 |
| Om003 | MXAN4440 | Hypothetical protein | −1.544 | EU744194 | 3,597/3,597 | 88/91 |
| Om005 | MXAN4407 | Conserved domain protein | 1.322 | EU744195 | 2,058/1,821 | 87/85 |
| Om008 | MXAN4254 | OmpA domain protein | −1.676 | EU744196 | 1,863/1,863 | 90/88 |
| Om009 | MXAN4092 | Hypothetical protein | −1.727 | EU744197 | 1,077/1,185 | 75/74 |
| Om019 | MXAN0411 | Hypothetical protein | −1.535 | EU744198 | 2,361/2,406 | 85/82 |
| Om021 | MXAN0562 | Phosphate-selective porins O and P | −1.244 | EU744199 | 1,374/1,416 | 88/87 |
| Om031 | MXAN3106 | Protein transporter, outer bacterial membrane secretin (secretin) family | −3.265 | EU744200 | 1,449/1,449 | 95/97 |
| Om034 | MXAN3553 | Hypothetical protein | −2.04 | EU744201 | 1,296/1,284 | 90/90 |
| Om035 | MXAN2482 | Hypothetical protein | −2.135 | EU744202 | 1,503/1,497 | 89/84 |
| Om044 | MXAN2426 | Hypothetical protein | −1.305 | EU744203 | 1,638/1,626 | 89/88 |
| Om050 | MXAN2883 | Lipoprotein, putative | −2.878 | EU744204 | 1,296/1,326 | 89/88 |
| Om051 | MXAN0990 | Cation efflux system protein CusC | −1.988 | EU744205 | 1,371/1,371 | 90/90 |
| Om055 | MXAN7340 | Hypothetical protein | −2.33 | EU744206 | 525/444 | 88/94 |
| Om061 | MXAN6134 | Ig-like domain/kelch domain protein | −1.708 | Not amplified | Not amplified | Not amplified |
| Om065 | MXAN2928 | Hypothetical protein | −1.41 | EU744207 | 1,194/1,200 | 81/77 |
| Om069 | MXAN4799 | OmpA domain protein | −1.586 | EU744208 | 1,848/1,848 | 91/94 |
| Om070 | MXAN4728 | Outer membrane protein, bacterial surface antigen | −1.653 | EU744209 | 2,487/2,466 | 93/95 |
| Om073 | MXAN2708 | Organic solvent tolerance protein, putative | −2.381 | EU744210 | 2,637/2,637 | 90/90 |
| Om078 | MXAN5490 | Conserved hypothetical protein | −1.905 | EU744211 | 1,251/1,251 | 88/86 |
| Tc001 | MXAN0314 | Sensory box histidine kinase/response regulator | −1.727 | EU744212 | 2,076/2,067 | 89/88 |
| Tc012 | MXAN0524 | Response regulator | 1.110 | EU744213 | 510/510 | 93/92 |
| Tc022 | MXAN2962 | Response regulator | −1.273 | EU744214 | 378/408 | 95/96 |
| Tc026 | MXAN0612 | Sensory box histidine kinase | −1.857 | EU744215 | 2,172/2,172 | 93/92 |
| Tc031a | MXAN7440 | Sigma-54-dependent DNA-binding response regulator Nla24 | −1.409 | EU744216 | 1,356/1,356 | 95/96 |
| Tc037a | MXAN5364 | DNA-binding response regulator HsfA | −1.37 | EU744217 | 1,458/1,458 | 97/98 |
| Tc049 | MXAN4261 | Sigma-54-dependent DNA-binding response regulator (I) | −1.685 | EU744218 | 1,359/1,365 | 91/95 |
| Tc050 | MXAN4675 | Response regulator | −1.747 | EU744219 | 1,116/1,116 | 93/96 |
| Tc053a | MXAN6996 | Response regulator/sensor histidine kinase AsgD | −1.023 | EU744220 | 2,322/2,325 | 90/90 |
| Tc070 | MXAN6029 | Chemotaxis sensor histidine kinase/response regulator | 1.130 | EU744221 | 2,145/2,160 | 94/95 |
| Tc073 | MXAN6224 | DNA-binding response regulator, Fis family | −1.423 | EU744222 | 552/525 | 93/95 |
| Tc079 | MXAN3738 | Response regulator | −1.732 | EU744223 | 675/675 | 82/75 |
| Tc080 | MXAN4232 | Response regulator | −1.498 | EU744224 | 1,236/1,236 | 94/96 |
| Tc094 | MXAN6966 | Response regulator/sensory box histidine kinase | −1.639 | EU744225 | 1,971/1,971 | 92/93 |
| Tc099 | MXAN6051 | Chaperone protein HtpG | −1.381 | EU744226 | 1,965/1,959 | 95/96 |
| Tc101 | MXAN7206 | Sensor histidine kinase/response regulator | −1.400 | EU744227 | 4,140/4,077 | 83/83 |
| Tc105a | MXAN4042 | Sigma-54-dependent DNA-binding response regulator Nla6 | 1.211 | EU744228 | 1,455/1,455 | 95/98 |
| Tc107 | MXAN4240 | Sigma-54-dependent DNA-binding response regulator | −1.706 | EU744229 | 1,416/1,416 | 95/99 |
| Tc111 | MXAN6735 | Sensor histidine kinase/response regulator | 1.020 | EU744230 | 7,437/7,431 | 94/96 |
| Tc118 | MXAN6032 | Response regulator/chemotaxis protein CheW, putative | −1.899 | EU744231 | 867/867 | 96/95 |
| Tc127 | MXAN0935 | Response regulator | −1.651 | EU744232 | 687/618 | 91/91 |
| Tc137 | MXAN6015 | Sensor histidine kinase | −1.345 | EU744233 | 1,647/1,629 | 89/88 |
| Tc142 | MXAN0459 | Sensor histidine kinase | −1.011 | EU744234 | 1,410/1,401 | 93/92 |
| Tc150 | MXAN0937 | Sigma-54-dependent DNA-binding response regulator | −1.563 | EU744235 | 1,368/1,365 | 95/98 |
| Tc153 | MXAN7001 | Response regulator | −1.379 | EU744236 | 369/369 | 93/97 |
| Tc154 | MXAN7123 | Sensor histidine kinase | 1.032 | EU744237 | 1,602/1,542 | 89/86 |
| Tc176a | MXAN0264 | DNA gyrase, B subunit (GyrB) | −1.690 | EU744238 | 2,448/2,448 | 96/97 |
| Tc177 | MXAN1093 | DNA-binding response regulator | −1.454 | EU744239 | 606/606 | 97/100 |
| Tc178a | MXAN4149 | Response regulator FrzS | −1.572 | EU744240 | 1,689/1,693 | 91/90 |
| Tc180 | MXAN0712 | Sensor histidine kinase/response regulator | −1.334 | EU744241 | 5,904/6,456 | 93/92 |
| Tc182a | MXAN5123 | Sensor histidine kinase MrpA | 1.050 | EU744242 | 1,026/1,026 | 95/95 |
| Tc184 | MXAN0089 | Glutathione S-transferase domain protein | −1.369 | EU744243 | 645/645 | 89/89 |
| Tc185 | MXAN3290 | Sensor histidine kinase/response regulator | −1.074 | EU744244 | 1,593/1,701 | 93/96 |
| Tc186 | MXAN3555 | Sigma-54-dependent DNA-binding response regulator | −2.170 | EU744245 | 1,425/1,425 | 96/98 |
| Tc225 | MXAN0460 | Response regulator | −2.599 | EU744246 | 768/768 | 96/98 |
Reported gene in DK1622.
Seawater-regulated genes in HW-1.
Among the 55 seawater-regulated genes, seven of the 35 Tc genes have reported functions, but none of the 20 Om genes have been described in DK1622 or other Myxococcus strains. The 55 seawater-regulated genes of M. fulvus HW-1 were further sequenced for comparison. Except for Om061 (the corresponding gene in DK1622 is MXAN6134 and is predicted to encode an immunoglobulin-like domain/kelch domain protein), all of the other 54 genes were obtained from HW-1 (Table 2). The 19 sequenced Om genes from HW-1 showed 74% to 97% identity with their DK1622 homologues at the amino acid level (most had 85% to 95% identity). Among the seawater-regulated Om genes, the only one that was upregulated was Om005, which had 85% identity to its homologue MXAN4407 in DK1622 at the amino acid level. Compared to outer membrane proteins, the 35 two-component system-related proteins had higher homology between DK1622 and HW-1 (Table 2). Of the seven reported Tc genes from DK1622, two were upregulated, while the other five were downregulated in HW-1 in the presence of seawater. These genes in DK1622 are involved in different cellular processes. For instance, 8 of the 28 ntrC-like activator (Nla) genes in DK1622 are required for development (4), of which the homologues of nla6 and nla24 in HW-1 were upregulated and downregulated in the presence of seawater (Table 2). Similar to nla6, the histidine kinase MrpA gene (MXAN5123), the other gene in HW-1 that is upregulated in response to the presence of seawater, was reported to be necessary for the development of myxospores but not for fruiting body morphogenesis (25). AsgD (MXAN6996) participates in the production of the A-signal, a mixture of amino acids or small peptides (14, 15), and the asgD mutation resulted in arrested development at an early stage. The homologue of DK1622 asgD (90% amino acid sequence identity) was downregulated in HW-1 in the presence of seawater. High homologies of the sequences suggested similar roles of these seawater-regulated genes in both strains DK1622 and HW-1.
Among the seawater-regulated genes, Om031 had the most significant change in expression (downregulated) in response to the presence of seawater, while Tc105 was the most significantly upregulated Tc gene. To confirm the chip hybridization results, expression of the Om031 and Tc105 genes was also assayed by quantitative real-time PCR. The expression of Om031 was decreased 8.13-fold, while Tc105 expression was increased 3.3-fold in the presence of seawater. These results were consistent with the chip hybridization results. DK1622 MXAN4042 (nla6) is the gene that corresponds to HW-1 Tc105, and they have 98% amino acid sequence identity. Nla6 (ntrC-like activators) contains REC and AAA (ATPase associated with a variety of cellular activities) functional domains (Fig. 1a). The outer membrane protein gene Om031 from HW-1 also has high similarity to the MXAN3106 gene from DK1622 (97% amino acid sequence identity). The MXAN3106 gene was predicted to encode a protein transporter that contains one secretin and two BON (bacterial OsmY and nodulation) domains (Fig. 1b). The changes in expression and bioinformatic analysis results strongly suggested that the products of these two genes played important roles in the adaptation of Myxococcus cells to oceanic conditions. Because a genetic system for deletion is not available for HW-1 (30), the homologues of Om031 and Tc105 were separately knocked out in DK1622 (producing mutants YL0401 and YL0402) to study the functions of these seawater-regulated genes in the presence of seawater.
Growth and salt tolerance of YL0401 and YL0402 mutants.
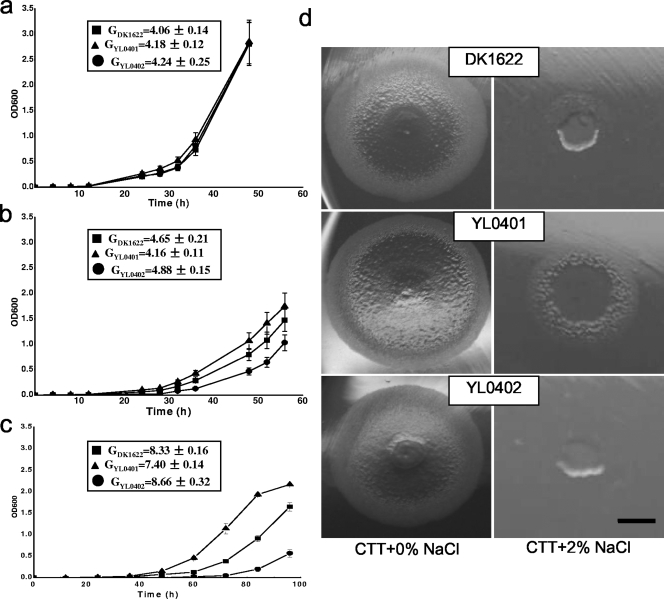
The two mutants had growth curves and generation times similar to those of DK1622 in CTT broth (Fig. 2a). When 0.5% (g/100 ml) NaCl was added, the cellular growth of both the mutants and the wild-type strain was delayed, but mutant YL0401 grew slightly better than wild-type DK1622, while the growth of YL0402 was slower than that of the wild type (Fig. 2b). Higher concentrations of NaCl led to more significant differences in the growth of the three strains (Fig. 2c). The generation times of the three strains also changed significantly. At 2% NaCl, mutant YL0401 was still able to grow weakly, but the wild-type strain and mutant YL0402 had almost no growth (Fig. 2d). The results indicated that the expression of MXAN4042 was beneficial for cellular growth under high-osmolarity conditions, while mutating MXAN3106 allowed the cells to be able to grow with higher salinity. This is consistent with the changes in expression of their homologues in the salt-tolerant strain HW-1 in response to the presence of seawater.
FIG. 2.
Effect of NaCl on growth of DK1622, YL0401, and YL0402. (a) CTT broth without addition of NaCl; (b) CTT broth with addition of 0.5% NaCl; (c) CTT broth with addition of 1.25% NaCl; (d) colonies on CTT medium containing 0% and 2.0% NaCl. The generation times of the three strains in panels a, b, and c were calculated in the same period in the beginning of the exponential growth stage. Error bars indicate standard deviations. The bar in panel d is equal to 0.5 cm for each panel.
Development and sporulation of the YL0401 and YL0402 mutants.
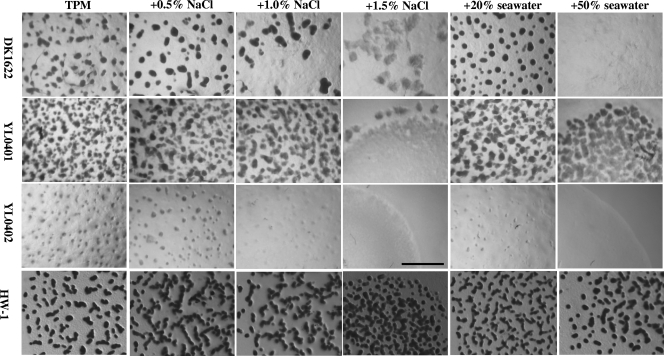
On the TPM developmental medium with no added NaCl or seawater, the salt-tolerant M. fulvus strain HW-1 was able to form fruiting bodies, but with lower density than that with the addition of NaCl or seawater. Deleting the MXAN4042 gene in DK1622 caused the mutant (YL0402) to aggregate weakly, to form rather rudimentary fruiting bodies (Fig. 3), and to have significantly decreased sporulation (about 4.5 × 10−3 times of that of DK1622). This was similar to effect of the insertion mutation of MXAN4042 (nla6), reported by Caberoy et al. (4). However, the developmental behavior of the MXAN3106 mutant (YL0401) was strange. Its fruiting body structures formed on TPM were rather small and atypical, but the mounds were densely distributed in the swarm (Fig. 3). The sporulation of YL0401 was also increased to about 4.3 times the population in DK1622 (Table 3).
FIG. 3.
Fruiting body development of mutants DK0401 and YL0402 and the wild-type strain YL1622 of M. xanthus and of the salt-tolerant M. fulvus strain HW-1 on TPM containing different concentrations of NaCl or seawater. The bar is equal to 1.0 mm for each panel.
TABLE 3.
Sporulation of mutants and the wild-type strain DK1622 on TPM containing different concentrations of NaCl or seawater
| Strain | % Sporulation (yield)a on TPM with:
|
|||||
|---|---|---|---|---|---|---|
| No addition | 0.5% NaCl | 1.0% NaCl | 1.5% NaCl | 20% seawater | 50% seawater | |
| DK1622 | 100.0 ± 5.1 | 116.9 ± 8.8 | 29.0 ± 4.2 | 2.71 ± 0.25 | 156.6 ± 0.0 | 0.197 ± 0.07 |
| YL0401 | 429.7 ± 6.9 (4.3) | 551.0 ± 64.7 (4.7) | 143.0 ± 25.5 (4.9) | 19.0 ± 0.0 (7.0) | 469.9 ± 5.1 (3.0) | 55.4 ± 6.8 (281.2) |
| YL0402 | 0.45 ± 0.06 (4.5 × 10−3) | 0.29 ± 0.10 (2.5 × 10−3) | 2.93 × 10−4 (1.0 × 10−5) | 1.5 × 10−4 (5.5 × 10−5) | 0.76 ± 1.5 (4.9 × 10−3) | 3.5 × 10−5 (1.8 × 10−4) |
DK1622 cells formed 8.07×106 myxospores per colony on TPM, which was taken as 100% sporulation. Accordingly, the sporulation of DK1622 on other media and of the two mutants on each media were determined. The mean values and standard deviations for the spore assays are shown as percentages of the value for DK1622 (wild type). The yield relative to DK1622 is also shown.
The wild-type strain DK1622 was able to form complete fruiting body structures at low concentrations of NaCl (0.5%) or seawater (20%), and the sporulation was slightly increased (Fig. 3 and Table 3). In the presence of 1.0% NaCl, the fruiting body structure was still complete, but the number of fruiting bodies was decreased, and consequently fewer myxospores were produced. Increasing the concentration of NaCl to 1.5% or that of seawater to 50% caused DK1622 cells to form rudimentary fruiting body mounds, or even prevented aggregation, and sporulation was greatly decreased. On the other hand, YL0401 formed small, atypical fruiting bodies that were densely distributed on colonies on TPM containing 0.5% and 1.0% NaCl or 20% seawater. Even on media with 50% seawater, the mutant was still able to form rudimentary fruiting bodies, while the aggregation and morphogenesis of DK1622 was completely inhibited (Fig. 3). Sporulation in mutant YL0401 was also stronger than that in DK1622 at different concentrations of salinity, especially in the presence of 50% seawater, where the sporulation of YL0401 was about 300 times that of DK1622 (Table 3). This was similar to the behavior of salt-tolerant strain HW-1 in response to the presence of different concentrations of seawater (31). In contrast, YL0402 cells were unable to form any fruiting body structures or even cellular mounds on TPM with added NaCl or seawater. The sporulation ability of YL0402 was also consequently decreased, especially in the presence of high salinity (Table 3).
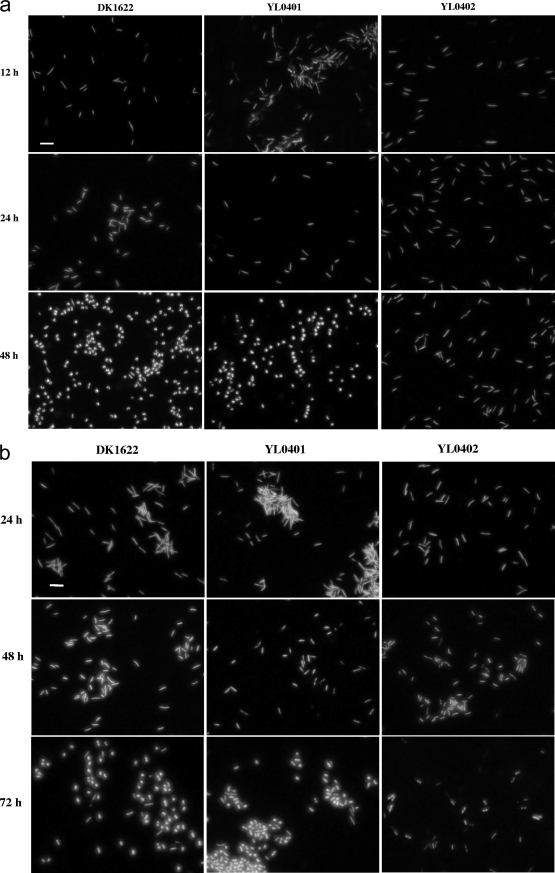
The MXAN4042 (nla6) and MXAN3106 genes may thus have positive and negative roles in the survival of Myxococcus cells in the ocean. To determine the effects of mutation and the presence of seawater on the survival of cells, the TPM cultures were further analyzed using the live/dead staining method (19) and the culturing method without heat treatment (thus, the formed colonies were from all the living cells, including myxospores and vegetative cells). After 12 h of incubation on TPM plates, there was almost no phenotypic difference between the three strains. The cells were in bacilliform (Fig. 4), and the number of living DK1622 cells was 40.67% ± 2.42% of the inoculation, while it was 39.90% ± 2.54% for YL0401 cells and 51.25% ± 4.19% for YL0402 cells. After 24 h of incubation, the cells were still mostly in bacilliform, while myxospores started to form in DK1622 and YL0401 but not in YL0402. The remaining living cells decreased to 21.6% ± 4.47% for DK1622, 28.1% ± 4.33% for YL0401, and 30.8% ± 4.08% for YL0402. After 48 h on TPM, there were about 1 to 2% bacilliform cells mixed with the spherical myxospores in DK1622, but almost no bacilliform cells could be seen in YL0401. However, the remaining living YL0402 cells were still in bacilliform (Fig. 4a). The morphogenesis of myxospores thus started during the first 24 h of incubation but proceeded mainly during the 24- to 48 h period. After 48 h of incubation, the remaining living cells of the three strains decreased to 5.3% ± 0.40% for DK1622, 4.25% ± 0.20% for YL0401, and 0.47% ± 0.04% for YL0402. The similar survival rates for DK1622 and YL0401 suggested that the MXAN3106 gene did not contribute to the developmental autolysis, while mutating the MXAN4042 gene caused a significant increase in the survival rates of cells during the early times of development, but later the cells quickly decreased.
FIG. 4.
Live/dead staining of YL0401 and YL0402 mutants and the wild-type strain DK1622 of M. xanthus on TPM (a) and TPM plus 50% seawater (b). The bar is equal to 5.0 μm for each panel.
Compared to survival on TPM without seawater, the survival rates of all three of the strains, especially the YL0401 mutant, were significantly increased by the presence of seawater. After 24 h of incubation on TPM containing 50% seawater, the remaining living DK1622 cells were 57.7% ± 1.47%, YL0401 cells were 86.5 ± 3.47%, and YL0402 cells were 52.0% ± 2.94% of the inoculation. A further 24 h of incubation led to the remaining living cells decreasing to 3.27% ± 0.20% for DK1622, 2.88% ± 0.25% for YL0401, and 5.29% ± 0.44% of the inoculation for YL0402. However, all three strains were still mostly in bacilliform (Fig. 4b). DK1622 and YL0401 cells were transmuted into myxospores during the 48- to 72-h period on TPM containing 50% seawater, whereas YL0402 cells were still in bacilliform and were weakly stained (Fig. 4b). The remaining living YL0402 cells were greatly fewer than the remaining living DK1622 and YL0401 cells after 5 days of incubation on TPM containing 50% seawater (Table 3). These results indicated that the autolysis of Myxococcus cells in the presence of seawater proceeded in a pattern similar to that in the absence of seawater, while the development of myxospores was delayed. Deletion of the MXAN3106 gene increased the sporulation ability of mutant YL0401 in the presence of high concentrations of seawater, where rudimentary fruiting body structures were able to be formed (Fig. 3). Because YL0401 and DK1622 cells had similar survival rates on TPM containing 50% seawater, the significant difference in sporulation between YL0401 and DK1622 in the presence of 50% seawater (Table 3) was the result of mutation of the MXAN3106 gene.
DISCUSSION
Salt-tolerant myxobacteria isolated from coastal areas were thought to be soil myxobacterial strains that had undergone degenerative adaptation to oceanic conditions. Alternatively, the salt-tolerant myxobacterial cells may passively migrate between seawater and nonseawater conditions and thus have developed different acclimation strategies, showing significant differences in growth, motility, development of fruiting bodies, and sporulation (31). Because terrestrial myxobacteria are salt sensitive and are normally unable to grow in the presence of high salinity (reference 21 and our unpublished data), developing salt tolerance is essential for myxobacterial strains living in coastal regions. Outer membrane proteins and signal transduction pathways are thought to play important roles in the shift of cellular behaviors. Our chip hybridization survey revealed that 35 of 226 two-component systems and 20 of 74 outer membrane proteins were significantly upregulated or downregulated in salt-tolerant Myxococcus strain HW-1 in the presence of seawater. These genes were mostly downregulated, possibly reflecting a simpler lifestyle for salt-tolerant myxobacterial cells in oceanic environments (31). Sequencing the seawater-regulated genes in HW-1 revealed that they are highly homologous to their homologues in DK1622, suggesting they have similar roles in both strains. Of the seawater-regulated genes, only seven Tc genes had been reported in Myxococcus. The genes reported in M. xanthus DK1622 are involved in different cellular processes, such as motility (nla24 and frzS), development of fruiting bodies (asgD), and sporulation (nla6 and mrpA). The reported functions of the corresponding genes in DK1622 suggested that one of the acclimation mechanisms of the salt-tolerant Myxococcus cells is probably to increase their sporulation when the process of fruiting body development is decreased by the presence of seawater (31). For example, Caberoy et al. made an insertion mutation of nla6 in DK1622, which led to a short delay in aggregation and seriously reduced sporulation efficiency (4). Our results indicated that MXAN4042 (nla6) not only was involved in the development of fruiting bodies and myxospores but also increased the initiation of programmed cell death during the developmental process. The homologue of MXAN4042 was also the most significantly upregulated Tc gene in salt-tolerant strain HW-1 in response to the presence of seawater, which probably reflected the need of a greater sporulation ability in seawater conditions, where the fruiting body development was seriously inhibited (31). On the other hand, Om031 (MXAN3106) was the gene most dramatically downregulated by seawater. Deleting MXAN3106 in DK1622 caused the cells to be more tolerant to high salinity, as well as stronger sporulation. Deleting the two genes did not affect the swarming ability of cells on either soft (0.3%) or hard (1.5%) agar containing different concentrations of NaCl or seawater. Similar to that of DK1622, the colony expansions of the mutants were slightly affected by the presence of 0.5% NaCl or 20% seawater but were seriously repressed by increased salinity (data not shown). The cellular dye-binding ability and intercellular clumping ability of the two mutants were also little changed from those of the wild-type strain (data not shown). The results suggested that the changes in the fruiting and sporulation abilities of the mutants were seemingly not produced by changes in extracellular matrix. However, in our previous study, we determined that mutation of the S-motility-related gene locus mts in M. fulvus HW-1 resulted in deficiencies in S motility, development of fruiting bodies and myxospores, and also salt tolerance (30). Thus, the genes that are involved in the cellular processes that were significantly changed in response to salinity may influence the salt tolerance of Myxococcus cells. Increasing or decreasing the expression levels of these multifunctional genes allows cells to quickly respond and efficiently acclimate to changing conditions in different environments.
The multifunctionality of genes helps cells to efficiently use cellular resources, which may be an important mechanism that myxobacteria use to acclimate to various environmental conditions. In seawater conditions, the homologue of MXAN4042 (nla6) was the most significantly upregulated Tc gene in salt-tolerant strain HW-1 in response to the presence of seawater. LacZ fusion expression indicated that MXAN4042 in DK1622 was highly expressed in both growth and development stages (the colonies of mutants were bright blue on CTT and TPM media containing 40 μg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside [X-Gal] [unpublished data]). MXAN4042 was predicted to encode a sigma-54-dependent DNA-binding response regulator (4). As an enhancer binding protein (8), the protein not only regulates developmental autolysis, fruiting body formation, and sporulation but also is involved in salt tolerance of Myxococcus cells. Perhaps Nla6 is a global positive regulator for the survival of Myxococcus cells not only under nutrient-deficient conditions but also under other adverse conditions such as high salinity. The MXAN3106 protein was predicted to containing one secretin and two BON (bacterial OsmY and nodulation) domains. The secretin domain is thought to help localize the protein to the outer membrane, while the BON domains are involved in protecting the cell from osmotic shock as well as other cell membrane-localized processes, such as secretion of a pilus, by interacting with the phospholipid membranes (1, 29). Results from bioinformatics and experiments clearly indicated that the MXAN3106 protein is involved in the osmotic regulation of the cellular membrane. Deleting the MXAN3106 gene increased not only sporulation but also the salt tolerance of cells and thus increased survival rates under high-salinity conditions. In salt-tolerant cells, expression of the Om031 (MXAN3106) gene was downregulated, which allowed the cells to adapt to high-salt conditions. Our unpublished data on LacZ fusion expression also indicated that MXAN3106 and its downstream gene MXAN3107 were both highly expressed during the growth and development stages.
Besides the studied MXAN4042 (Tc105) and Om031 (MXAN3106) genes, bioinformatic analysis of other seawater-regulated genes also suggested that they were probably involved in the salt tolerance or the shift in response to the presence of seawater. For example, the product of the Om005 (MXAN4407) gene had low homology to the AsmA protein from E. coli. This protein participates in inhibition of OmpF assembly. OmpF, in contrast to OmpC, is more necessary under low-osmolarity conditions, and its expression is repressed by high osmotic pressure (6, 18). Om005 in HW-1 may play a role similar to that of AsmA. The homologue of DK1622 asgD (90% amino acid sequence identity) was downregulated in HW-1 in the presence of seawater, probably reflecting the inhibiting effects of seawater on the development of fruiting bodies. Further related experiments are being undertaken in our laboratory.
Supplementary Material
Acknowledgments
This work was financially supported by grants from the National Nature Science Foundation of China (30600007 and 30671192), the Chinese 863 programs (2006AA02Z171 and 2006AA02Z225), and the National Nature Science Foundation of Shandong Province, China (Q2006D01).
We thank Roberta Greenwood for her help in editing the manuscript.
Footnotes
Published ahead of print on 16 January 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Bitter, W. 2003. Secretins of Pseudomonas aeruginosa: large holes in the outer membrane. Arch. Microbiol. 179307-314. [DOI] [PubMed] [Google Scholar]
- 2.Black, M. A., and R. W. Doerge. 2002. Calculation of the minimum number of replicate spots required for detection of significant gene expression fold change in microarray experiments. Bioinformatics 181609-1616. [DOI] [PubMed] [Google Scholar]
- 3.Black, W. P., and Z. Yang. 2004. Myxococcus xanthus chemotaxis homologs DifD and DifG negatively regulate fibril polysaccharide production. J. Bacteriol. 1861001-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caberoy, N. B., R. D. Welch, J. S. Jakobsen, S. C. Slater, and A. G. Garza. 2003. Global mutational analysis of NtrC-like activators in Myxococcus xanthus: identifying activator mutants defective for motility and fruiting body development. J. Bacteriol. 1856083-6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawid, W. 2000. Biology and global distribution of myxobacteria in soils. FEMS. Microbiol. Rev. 24403-427. [DOI] [PubMed] [Google Scholar]
- 6.Deng, M., and R. Misra. 1996. Examination of AsmA and its effect on the assembly of Escherichia coli outer membrane proteins. Mol. Microbiol. 21605-612. [DOI] [PubMed] [Google Scholar]
- 7.Eriksson, S., S. Lucchini, A. Thompson, M. Rhen, and J. C. Hinton. 2003. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol. Microbiol. 47103-118. [DOI] [PubMed] [Google Scholar]
- 8.Goldman, B. S., W. C. Nierman, D. Kaiser, S. C. Slater, A. S. Durkin, J. Eisen, C. M. Ronning, W. B. Barbazuk, M. Blanchard, C. Field, C. Halling, G. Hinkle, O. Iartchuk, H. S. Kim, C. Mackenzie, R. Madupu, N. Miller, A. Shvartsbeyn, S. A. Sullivan, M. Vaudin, R. Wiegand, and H. B. Kaplan. 2006. Evolution of sensory complexity recorded in a myxobacterial genome. Proc. Natl. Acad. Sci. USA 10315200-15205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hess, J. F., R. B. Bourret, and M. I. Simon. 1991. Phosphorylation assays for proteins of the two-component regulatory system controlling chemotaxis in Escherichia coli. Methods Enzymol. 200188-204. [DOI] [PubMed] [Google Scholar]
- 10.Hodgkin, J., and D. Kaiser. 1977. Cell-to-cell stimulation of movement in nonmotile mutants of Myxococcus. Proc. Natl. Acad. Sci. USA 742938-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Julien, B., D. Kaiser, and A. Garza. 2000. Spatial control of cell differentiation in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 979098-9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaiser, D. 1979. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 765952-5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kearns, D. B., B. D. Campbell, and L. J. Shimkets. 2000. Myxococcus xanthus fibril appendages are essential for excitation by a phospholipid attractant. Proc. Natl. Acad. Sci. USA 9711505-11510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuspa, A., L. Plamann, and D. Kaiser. 1992. Identification of heat-stable A-factor from Myxococcus xanthus. J. Bacteriol. 1743319-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuspa, A., L. Plamann, and D. Kaiser. 1992. A-Signalling and the cell density requirement for Myxococcus xanthus development. J. Bacteriol. 1747360-7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li, Y. Z., W. Hu, Y. Q. Zhang, Z. J. Qiu, Y. Zhang, and B. H. Wu. 2002. A simple method to isolate salt-tolerant myxobacteria from marine samples. J. Microbiol. Methods 50205-209. [DOI] [PubMed] [Google Scholar]
- 17.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 18.Misra, R., and Y. Miao. 1995. Molecular analysis of asmA, a locus identified as the suppressor of OmpF assembly mutants of Escherichia coli K-12. Mol. Microbiol. 16779-788. [DOI] [PubMed] [Google Scholar]
- 19.Nariya, H., and M. Inouye. 2008. MazF, an mRNA interferase, mediates programmed cell death during multicellular Myxococcus development. Cell 13255-66. [DOI] [PubMed] [Google Scholar]
- 20.Reichenbach, H. 1999. The ecology of myxobacteria. Environ. Microbiol. 115-21. [DOI] [PubMed] [Google Scholar]
- 21.Reichenbach, H., and M. Dworkin. 1992. The myxobacteria, p. 3416-3487. In H. G. Trüper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes, 2nd ed. Springer-Verlag, Berlin, Germany.
- 22.Shi, W., and D. R. Zusman. 1993. The two motility systems of Myxococcus xanthus show different selective advantages on various surfaces. Proc. Natl. Acad. Sci. USA 903378-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimkets, L. J. 1986. Correlation of energy-dependent cell cohesion with social motility in Myxococcus xanthus. J. Bacteriol. 166837-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stock, A. M., V. L. Robinson, and P. N. Goudreau. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69183-215. [DOI] [PubMed] [Google Scholar]
- 25.Sun, H., and W. Shi. 2001. Genetic studies of mrp, a locus essential for cellular aggregation and sporulation of Myxococcus xanthus. J. Bacteriol. 1834786-4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ueki, T., S. Inouye, and M. Inouye. 1996. Positive-negative KG cassettes for construction of multi-gene deletions using a single drug marker. Gene 183153-157. [DOI] [PubMed] [Google Scholar]
- 27.Wang, B., W. Hu, C. Y. Zhang, J. Y. Zhao, D. M. Jiang, Z. H. Wu, and Y. Li. 2007. Adaptation of salt-tolerant Myxococcus strains and their motility systems to the ocean conditions. Microb. Ecol. 5443-51. [DOI] [PubMed] [Google Scholar]
- 28.Wilson, D. L., M. J. Buckley, C. A. Helliwell, and I. W. Wilson. 2003. New normalization methods for cDNA microarray data. Bioinformatics 191325-1332. [DOI] [PubMed] [Google Scholar]
- 29.Yeats, C., and A. Bateman. 2003. The BON domain: a putative membrane-binding domain. Trends Biochem. Sci. 28352-355. [DOI] [PubMed] [Google Scholar]
- 30.Zhang, C. Y., K. Cai, H. Liu, Y. Zhang, H. W. Pan, B. Wang, Z. H. Wu, W. Hu, and Y. Z. Li. 2007. A new locus important for Myxococcus social motility and development. J. Bacteriol. 1897937-7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang, Y. Q., Y. Z. Li, B. Wang, Z. H. Wu, C. Y. Zhang, X. Gong, Z. J. Qiu, and Y. Zhang. 2005. Characteristics and living patterns of marine myxobacterial isolates. Appl. Environ. Microbiol. 713331-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zusman, D. R., A. E. Scott, Z. M. Yang, and J. R. Kirby. 2007. Chemosensory pathways, motility and development in Myxococcus xanthus. Nat. Rev. Microbiol. 5862-872. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.