Abstract
The gram-positive bacterial pathogen Listeria monocytogenes has evolved mechanisms to rapidly replicate in the host cytosol, implying efficient utilization of host-derived nutrients. However, the contribution of host nutrient scavenging versus that of bacterial biosynthesis toward rapid intracellular growth remains unclear. Nutrients that contribute to growth of L. monocytogenes include branched-chain fatty acids (BCFAs), amino acids, and other metabolic intermediates generated from acyl-coenzyme A, which is synthesized using lipoylated metabolic enzyme complexes. To characterize which biosynthetic pathways support replication of L. monocytogenes inside the host cytosol, we impaired lipoate-dependent metabolism by disrupting two lipoate ligase genes that are responsible for bacterial protein lipoylation. Interrupting lipoate-dependent metabolism modestly impaired replication in rich broth medium but strongly inhibited growth in defined medium and host cells and impaired the generation of BCFAs. Addition of short BCFAs and amino acids restored growth of the A1A2-deficient (A1A2−) mutant in minimal medium, implying that lipoate-dependent metabolism generates amino acids and BCFAs. BCFAs alone rescued intracellular growth and spread in L2 fibroblasts of the A1A2− mutant. Lipoate-dependent metabolism was also required in vivo, as a wild-type strain robustly outcompeted the lipoylation-deficient mutant in a murine model of listeriosis. The results of this study suggest that lipoate-dependent metabolism contributes to both amino acid and BCFA biosynthesis and that BCFA biosynthesis is preferentially required for intracellular growth of L. monocytogenes.
The gram-positive food-borne pathogen Listeria monocytogenes replicates in diverse settings, ranging from soil to the intracellular environment of mammalian host cells (41, 43, 44). The ability of L. monocytogenes to thrive in soil and food indicates that the bacterium has the capacity to adapt to different nutritional environments. When carbon source availability changes, L. monocytogenes alters the activity of PrfA, the global virulence regulator (11, 18, 29). While the nutritional milieu surrounding L. monocytogenes affects virulence gene expression by altering PrfA function, deletion of prfA also appears to decrease the metabolite pool of branched-chain amino acids (BCAAs) (8, 23, 42). It is not fully understood why PrfA, a regulator that enables virulence, would influence biosynthesis. Genes involved in aromatic amino acid biosynthesis are important for L. monocytogenes virulence, but it is of interest that some single-amino-acid auxotrophs are not attenuated for growth in the cytosol, implying that the bacterium acquires host-derived amino acids to support intracellular growth (26, 40). Recent studies tracking carbon flux during intracellular growth of L. monocytogenes demonstrated that >50% of bacterial amino acids were provided by the host, despite the capability of the bacterium to synthesize many of those amino acids (9). Thus, the presence of biosynthetic pathways in bacterial genomes may not be a clear indicator of the requirement for these pathways during growth inside a host organism, and other approaches must be used to determine which metabolic precursors are synthesized by the bacterium during intracellular infection and which are scavenged from the host environment.
Strategies for increasing biosynthesis or obtaining key nutrients from the host are essential aspects of pathogenesis (4). Intracellular pathogens such as Salmonella enterica serovar Typhimurium and Mycobacterium tuberculosis can adapt to the mammalian host environment by differentially regulating nutrient transporters and metabolic biosynthesis pathways (14, 36). L. monocytogenes may share these adaptive strategies when transitioning from the extracellular environment to the host cytosol, as it upregulates genes that enable the biosynthesis of BCAAs, as well as genes encoding glycerol-3-phosphate dehydrogenase and enzymes of the pentose phosphate pathway (6, 8, 19). L. monocytogenes also exploits the cytosolic nutrient environment by expressing a hexose phosphate transporter and induces sugar transporter gene expression when replication is shifted from the vacuole to the cytosol (6, 7, 18). Thus, L. monocytogenes can respond to nutritional changes by synthesizing or transporting metabolic intermediates to support virulence.
One nutrient that L. monocytogenes cannot synthesize is lipoate, an essential cofactor for metabolic enzyme complexes such as pyruvate dehydrogenase (PDH) and branched-chain keto-acid dehydrogenase (BCKD) (35). We previously demonstrated that during growth under nutrient-limiting conditions, L. monocytogenes requires the lipoate ligase LplA1 for utilization of physiological concentrations of host-derived lipoyl peptides (22). Despite the presence of another functional lipoate ligase, LplA2, LplA1 is essential for bacterial pathogenesis of L. monocytogenes (22). It is not yet fully understood why lipoate-dependent metabolism is important for bacterial virulence. Studies of microbial physiology by the use of model organisms such as the gram-negative bacterium Escherichia coli and the gram-positive bacterium Bacillus subtilis have demonstrated that lipoylated PDH generates acetyl-coenzyme A (acetyl-CoA) from pyruvate, while lipoylated BCKD makes branched acyl-CoAs from BCAA catabolism (20). In contrast to the results seen with E. coli, generation of tricarboxylic acids in L. monocytogenes is noncyclical and is unlikely to generate energy in the form of ATP, as it is missing α-ketoglutarate dehydrogenase activity (8). Thus, the main function of the lipoylated metabolic enzyme complexes of Listeria bacteria may be to enable biosynthesis of amino acids and branched-chain fatty acids (BCFAs), key components of the bacterial membrane of L. monocytogenes (47). BCFAs enhance survival of L. monocytogenes under conditions of adverse pH and cold stress, although it is unknown what role they play during pathogenesis (12, 47). While recent studies demonstrated that the majority of amino acids that L. monocytogenes utilizes during intracellular growth are derived from the host, the bioavailability of other key metabolites, such as BCFAs, has not been determined (9).
Neither PDH nor BCKD is predicted to be functional in the absence of lipoate, and mutations that render E. coli unable to ligate lipoate or lipoate precursors to metabolic enzymes are lethal in the absence of nutritional supplements that allow the organism to bypass the requirement for the tricarboxylic acid cycle (30). To assess the overall requirement for lipoate-dependent metabolism in biosynthesis and intracellular growth, we measured the ability of an L. monocytogenes mutant deficient in lipoylation of the bacterial target proteins represented by lplA1::Tn917ΔlplA2 (A1A2 deficient [A1A2−]) to replicate in vitro and in vivo. In contrast to the previously characterized ΔlplA1 mutant, which exhibits lipoylation of bacterial proteins when L. monocytogenes is in the extracellular environment, the A1A2− mutant did not detectably lipoylate any bacterial target proteins. In this report, we demonstrate that lipoate-dependent metabolism in L. monocytogenes controls amino acid and anteiso-BCFA biosynthesis. Lipoate-dependent metabolism was not essential for L. monocytogenes growth in rich medium but was required for optimal cytosolic replication and growth under nutrient-limiting conditions. Growth of the A1A2− mutant in defined medium could be rescued by enrichment with amino acids and BCFA precursors, but only the BCFA precursors stimulated intracellular growth. These data indicate that intracellular growth of L. monocytogenes requires bacterial biosynthesis of BCFAs and suggest that the presence of BCFAs may be growth limiting in the cytosolic environment.
MATERIALS AND METHODS
Bacterial strains and growth.
Strains used in this study are described in Table 1. Prior to infection of cells in tissue culture, L. monocytogenes was grown overnight in brain heart infusion (BHI; Difco) medium at 37°C. For extracellular growth curves, bacteria were grown in either BHI medium or improved minimal medium (IMM) in a Bioscreen growth curve analyzer (Growth Curves). IMM was prepared as previously described except that, where indicated, filter-sterilized supplements were added (33).
TABLE 1.
Bacterial strains used in this study
| Name | Strain | Genotype/description | Reference |
|---|---|---|---|
| WT | 10403S | L. monocytogenes serotype 1/2 a, parental strain used as the WT | 10 |
| AlA2− | MOR125 | 10403S lplA1::Tn917ΔlplA2 | 22 |
| ΔlplA1 | DP-L4263 | 10403S ΔlplA1 | 31 |
| ΔlplA2 | MOR129 | 10403S ΔlplA2 | 22 |
| LLO− | DP-L2161 | 10403S Δhly | 16 |
| WTr | DP-L3903 | 10403S::Tn917; unknown site of insertion, WT phenotype | 2 |
Protein analysis.
Bacteria were prepared and analyzed by immunoblotting with an anti-lipoic acid antibody (Calbiochem) as previously described (31), except that washed exponential-phase cultures (at an optical density at 600 nanometers [OD600] of 0.5) or stationary-phase cultures (OD600 of 1.2) were used after growth in BHI medium at 37°C. Bacteria were pelleted and then lysed with FastProtein Blue lysing matrix (MP Biomedicals) by processing in a FastPrep machine for 40 s at an intensity setting of 6.0.
Lactate, fatty acid analysis, and amino acid analysis.
Lactate was quantified using a lactate assay kit (BioVision; catalog no. K607-100), which was quantified by a colorimetric change at 570 nm. The amount of lactate in the supernatant was quantified by comparison with a standard curve. For fatty acid analysis, bacterial pellets were examined by gas chromatographic analysis of cellular fatty acid methyl esters by use of a Sherlock microbial identification system (Microbial ID). Bacterial pellets were lysed using an MP Fast Protein Blue matrix and a Bio101 FastPrep machine (40 s; setting, 6.0). One milligram of bacterial protein was submitted for amino acid analysis to the Molecular Structure Facility of the University of California at Davis, where lysate was hydrolyzed with 6N HCl for 24 h at 110°C and dissolved in a sodium citrate buffer containing 40 nmol of norleucine/ml, and 50.0 μl was injected into an L-8800 Hitachi analyzer. Subsequent peaks and amino acid concentrations were identified using Beckman System Gold software.
Acetoin quantification.
Acetoin concentrations in selected culture samples were determined using a previously validated derivatization method (38). A total of 200 μl of reagent-grade acetonitrile (ACN) and 50 μl of a 2,4-dinitrophenylhydrazine (DNPH) stock solution in acidified methanol (0.5% [wt/vol] DNPH, 4% [vol/vol] H2SO4) was added to 200 μl of filtered culture supernatant in 2-ml plastic microcentrifuge tubes. Samples were then vortexed briefly and allowed to react for 6 min at room temperature in the dark. An Agilent 1100 series high-pressure liquid chromatograph (HPLC) equipped with a UV diode array detector set to 345 nm and a Phenomenex C18 (reverse-phase) column (250 mm by 2.0 mm; 5-μm particle size) was used to detect the acetoin-DNP derivatization product, by the use of injection volumes of 50 μl. The mobile phase was a mixture of ACN and distilled deionized water (DDI) pumped at 0.6 ml/min according to the following gradient: 0 to 13 min, 0:100 to 75:25 ACN:DDI; 13 to 17 min, 75:25 to 65:35 ACN:DDI; 17 to 17.5 min, 65:35 to 0:100 ACN:DDI; and 17.5 to 21 min, 0:100 ACN:DDI. Under these conditions, acetoin-DNP exhibited a characteristic retention time (tR) of 10.8 min. Peak identity was confirmed via derivatization and HPLC separation of acetoin standards prepared in fresh BHI media. Similarly, derivatized acetoin standards in BHI were used to generate a calibration curve for acetoin concentrations as a function of acetoin-DNP peak areas at tR = 10.8 min over a range of 0 to 5 mM acetoin.
BMDM isolation.
Primary bone marrow-derived macrophages (BMDMs) were isolated from 6- to 8-week-old C57BL/6 female mice (Jackson Laboratory) and cultured as described previously (34).
Bacterial infections.
Mammalian cells were infected as previously described (31). Bacterial cultures were grown at 37°C in BHI medium to stationary phase and washed. Per 24-well dish, 1.2 × 106 RAW and 4 × 106 BMDM or J774 macrophages were infected for 30 min at a multiplicity of infection (MOI) of 0.6 for the wild type (WT) and 12 for the AlA2− mutant in J774 macrophages and BMDMs and an MOI of 20 for the WT and of 133 for the AlA2− mutant in RAW264.7 cells. Per 24-well dish, 5 × 106 L2 cells were infected for 60 min at an MOI of 1 for the WT and 20 for the AlA2− mutant in L2 cells. Following infection, host cells were washed twice with Dulbecco's phosphate-buffered saline (D-PBS) with Ca2+ Mg2+ and incubated with medium containing 10 μg of gentamicin ml−1 for the duration of the experiment. These conditions resulted in ∼10% host cell infection at 1 h. Infected host cells were lysed in 5 ml of sterile water and plated on Luria-Bertani (LB) agar, where CFUs were counted after 72 h of incubation at 37°C to permit growth of the AlA2− mutant.
Intracellular escape assays.
A total of 4 × 106 BMDMs per six-well plate were grown on coverslips and infected as described above. At 2 h postinfection, coverslips were removed and fixed in 3.7% paraformaldehyde in D-PBS. After being washed three times with 0.1% Tween 20 in D-PBS and blocked for 10 min with TBS-T (25 mM Tris HCl, 150 mM NaCl, 0.1% Tween 20, 1% bovine serum albumin), anti-L. monocytogenes antibody (Difco) was added to the coverslips for 1 h and washed, and then secondary antibody was added for 30 min with rhodamine phalloidin. Intracellular bacteria were scored for the presence or absence of actin clouds. A minimum of 50 macrophages and a total of at least 150 bacteria were randomly scored per experimental replicate.
L2 plaque assays.
Plaque assays were performed as previously described (31). In L2 plaque assays, BCFA precursors and soy protein hydrolysate supplements were added to 3 ml of top agar per well after an initial 1-h infection and developed for 5 days prior to addition of Neutral Red stain to visualize necrotic foci. Plaque diameters were measured (n = 20) and expressed as percentages of the mean diameters of WT plaques by the use of Canvas software (Deneba).
Mouse infections.
Competitive index (CI) infections were performed as previously described (2). Exponential-phase bacterial cultures were diluted in Ca2+- and Mg2+-free D-PBS, and 5 × 105 WT bacteria and 5 × 107 AlA2− mutant bacteria in 200 μl were injected intraperitoneally into 4- to 6-week-old C57BL/6 J mice (Jackson Laboratories). At 24 h and 72 h postinfection, the animals were sacrificed, and livers and spleens were homogenized in 0.1% NP-40 containing 0.1 μg of erythromycin (Erm) ml−1, serially diluted, and plated on drug-free LB agar or LB agar containing 1 μg of Erm ml−1. The CI was calculated by dividing the number of WT CFUs (Erm sensitive [Erms]) by the number of mutant CFUs (Erm resistant [Ermr]) after normalizing for the input ratio.
Statistical analysis.
Samples were analyzed with a Student's t test by assuming unequal variance (Microsoft Excel).
RESULTS
L. monocytogenes growth in the absence of lipoate ligases in rich medium.
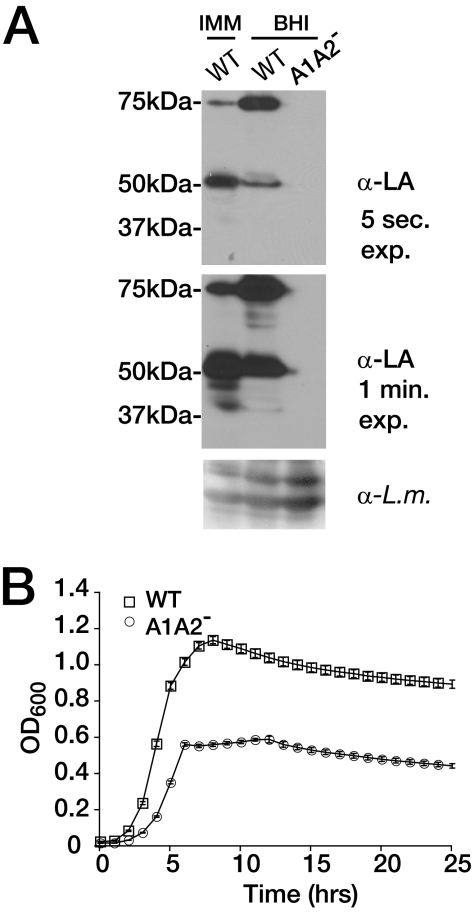
To determine the role of lipoate-dependent metabolism in L. monocytogenes extracellular growth, we characterized a previously constructed bacterial mutant deficient in both LplA1 and LplA2 (lplA1::Tn917ΔlplA2, designated A1A2−) and lacking all detectable bacterial lipoylation (22). In the absence of these two ligases, we were unable to detect lipoylated bacterial target proteins, as observed by immunoblot analysis with an antibody against lipoic acid (Fig. 1A) (22). The 75-kDa lipoylated protein species in the WT was previously identified as the E2 subunit of PDH, and the migration of the 50-kDa protein species is consistent with the predicted size for the lipoylated E2 subunit of BCKD (31). L. monocytogenes encodes a functional BCKD, as when the genes encoding subunits of BCKD are disrupted, BCFA biosynthesis is perturbed (47). Thus, the two dominant lipoylated bands present in WT L. monocytogenes, but absent in the A1A2− mutant, likely correspond to bacterial PDH and BCKD. We therefore conclude that the lipoate ligases LplA1 and LplA2 are responsible for all detectable protein lipoylation in L. monocytogenes (22). Notably, PDH represented 22% of total lipoylated proteins in IMM-grown cultures, while PDH represented 61% of total lipoylated proteins after growth in the rich (BHI) medium. Conversely, BCKD represented 78% of total lipoylated proteins in IMM-grown cultures, while it represented only 39% of total lipoylated proteins in BHI-grown cultures. These data suggest that the growth environment of L. monocytogenes influences the levels of lipoylated BCKD and PDH, with a limiting nutrient environment resulting in increased levels of lipoylated BCKD compared to PDH.
FIG. 1.
In the absence of lipoate ligases, L. monocytogenes can grow in nutrient-rich conditions. (A) The WT and the AlA2− mutant were grown in BHI medium or IMM to stationary phase (OD600 of 0.6 for the A1A2− mutant in BHI medium or 1.2 for the WT in BHI medium and IMM). Lysates containing equivalent bacterial numbers were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with an anti-lipoic acid (α-LA) antibody. The blot was exposed for 5 s (5 sec. exp.) or 1 min (1 min. exp.). The results of anti-LA immunoblotting after 1 min of exposure are shown to illustrate the lack of detectable lipoylation in the A1A2− mutant. The E2 subunit of bacterial PDH corresponds to the 75-kDa band. The blot was stripped and reprobed with polyclonal anti-L. monocytogenes (α-L.m.) antibody to confirm equivalent levels of protein loading. (B) Bacterial growth was determined by measuring OD600 values over time for the WT and the AlA2− mutant inoculated in BHI medium and grown at 37°C with aeration in a Bioscreen growth curve analyzer. Error bars represent standard deviations.
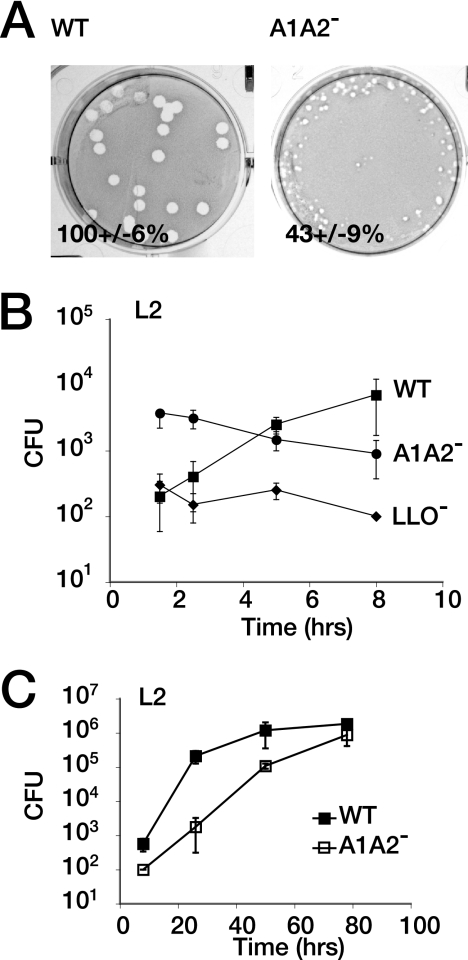
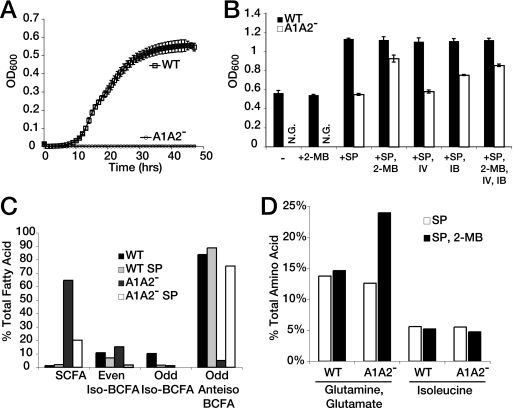
Without lipoylation, neither PDH nor BCKD can function to generate biosynthetic intermediates (32). We considered the possibility that complete loss of lipoylation would result in a substantial replication defect. Analysis of bacterial growth in BHI medium showed that the doubling times of the WT and the AlA2− mutant during exponential growth were similar (63 to 66 min), although the AlA2− mutant entered stationary phase earlier than the WT strain (Fig. 1B). This trend was confirmed by measuring the number of CFUs (data not shown). We had previously demonstrated that the replication defect of an L. monocytogenes LplA1-deficient strain could be complemented by a plasmid containing the lplA1 gene; this plasmid was used to transform the A1A2− mutant and the WT and ΔlplA1 control strains (22, 31). However, the A1A2− mutant was not conducive to transformation; thus, complementation was not achieved (data not shown). Taken together, these data demonstrate that L. monocytogenes is able to grow in rich medium even in the absence of lipoyl ligase function.
Metabolic alterations in the absence of lipoylation.
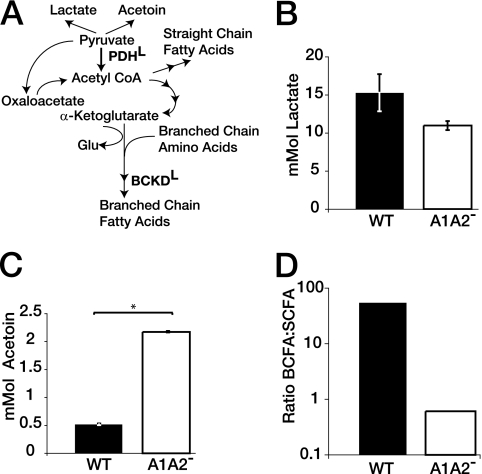
In the absence of lipoylated PDH, pyruvate generated from glycolysis would likely be converted into alternate metabolites such as lactate and acetoin through the lactate dehydrogenase and the 2-3-butanediol pathways (Fig. 2). To test whether we were altering known lipoate-dependent metabolic pathways, we grew the WT and the AlA2− mutant in BHI medium and quantified the lactate, acetoin, and fatty acid content (Fig. 2). While lactate levels were relatively similar, the amount of secreted acetoin was fourfold higher in the AlA2− mutant compared to WT results (Fig. 2B and C; also see Fig. S1 in the supplemental material). Quantification of total amino acid content showed an 8% drop in the levels of glutamate and glutamine in the AlA2− mutant, but there were no other striking differences in total amino acid levels between the WT and the A1A2− mutant (Table 2). These results were not surprising, as L. monocytogenes can scavenge amino acids from its growth environment and BHI medium is rich in amino acids (26). In contrast to the amino acid results, we expected the fatty acid content to be altered when lipoylation of BCKD was disrupted, as BCKD-deficient mutants have a decreased capacity to generate odd-numbered-carbon-length BCFAs with the methyl branch in the anteiso orientation (47). To measure fatty acid composition, we performed fatty acid analysis of bacteria grown in BHI medium to the stationary phase (Fig. 2D; also see Fig. S2 in the supplemental material). Straight-chain fatty acids predominated in the AlA2− mutant, while BCFAs were the predominant fatty acid species in the WT, demonstrating that lipoylation is essential for L. monocytogenes BCFA biosynthesis even in rich medium. The differences in total fatty acid composition between the WT and the A1A2− mutant were also reflected in the membrane fatty acid composition (see Fig. S3 in the supplemental material). We therefore conclude that despite the ability of L. monocytogenes to grow in BHI medium without lipoylation, fundamental metabolic pathways that generate acetoin and BCFAs were altered in the absence of lipoate ligase activity.
FIG. 2.
L. monocytogenes metabolism is altered in the absence of lipoylation. (A) Model of major lipoate-dependent pathways in L. monocytogenes. Both PDHL and BCKDL require lipoylation (L) for function. Glu, glutamate. (B and C) The WT and the AlA2− mutant were grown to the stationary phase in BHI medium and pelleted, and the amounts of secreted lactate (B) and acetoin (C) were quantified in the remaining supernatant (n = 3). Error bars represent standard deviations. For panel B, the amount of lactate in the supernatant was quantified by comparison with a standard curve. For panel C, filtered culture supernatant was derivatized and products were detected by HPLC. Acetoin peak identities and concentrations were confirmed by HPLC separation of derivatized acetoin standards prepared in filtered BHI medium. Acetoin levels of the WT and the AlA2− mutant were significantly different (Student's t test [P < 0.05, as indicated by the asterisk]). (D) Pellets of stationary-phase WT and the AlA2− mutant grown in BHI medium (n = 2) were analyzed by gas chromatographic analysis of cellular fatty acid methyl esters by use of a Sherlock microbial identification system (Microbial ID), and the ratios of short-chain fatty acids (SCFA) to BCFAs are shown.
TABLE 2.
Total amino acid content of bacterial lysatea
| Amino acid | % Amino acid contentb
|
|||||
|---|---|---|---|---|---|---|
| WT
|
A1A2−
|
|||||
| BHI | SP | SP + MB | BHI | SP | SP + MB | |
| Aspartic acid | 9.05 | 8.19 | 9.10 | 9.23 | 9.08 | 7.72 |
| Threonine | 4.99 | 5.10 | 3.94 | 5.23 | 5.73 | 4.75 |
| Serine | 3.08 | 3.21 | 3.77 | 3.59 | 3.84 | 3.23 |
| Glxc | 21.92 | 22.62 | 13.75 | 14.60 | 12.60 | 23.94 |
| Proline | 3.79 | 3.16 | 3.39 | 3.60 | 3.47 | 3.10 |
| Glycine | 8.40 | 9.94 | 10.82 | 10.82 | 10.47 | 9.54 |
| Alanine | 7.27 | 8.61 | 8.42 | 8.53 | 10.77 | 9.17 |
| Valine | 5.85 | 6.31 | 7.63 | 6.87 | 7.50 | 6.45 |
| Cystine | 0.08 | 0.03 | 0.30 | 0.06 | 0.17 | 0.06 |
| Methionine | 0.64 | 0.33 | 0.59 | 0.60 | 0.33 | 0.48 |
| Isoleucine | 4.42 | 4.66 | 5.61 | 5.20 | 5.53 | 4.72 |
| Leucine | 6.16 | 6.40 | 7.40 | 7.18 | 7.47 | 6.37 |
| Tyrosine | 2.11 | 2.04 | 2.31 | 2.29 | 2.43 | 2.17 |
| Phenylalanine | 2.79 | 2.82 | 3.27 | 3.07 | 3.38 | 2.99 |
| β-Alanine | 0.24 | 0.14 | 0.68 | 0.54 | 0.23 | 0.19 |
| Homocystine | 1.31 | 0.37 | 0.19 | 0.24 | 0.21 | 0.52 |
| Hydroxylysine | 0.06 | 0.04 | 0.10 | 0.00 | 0.06 | 0.03 |
| Ornithine | 0.37 | 0.17 | 0.10 | 0.09 | 0.09 | 0.07 |
| Tryptophan | 0.13 | 0.06 | 0.07 | 0.17 | 0.00 | 0.08 |
| Lysine | 7.31 | 7.32 | 7.84 | 7.52 | 7.42 | 6.09 |
| l-Methylhistidine | 1.07 | 0.43 | 1.12 | 1.11 | 0.24 | 0.68 |
| Histidine | 1.70 | 1.51 | 1.67 | 1.58 | 1.66 | 1.40 |
| Arginine | 3.44 | 3.37 | 4.50 | 4.26 | 3.86 | 3.16 |
| Hydroxyproline | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Asparagine | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| Proline | 3.79 | 3.16 | 3.41 | 3.61 | 3.47 | 3.10 |
The inoculum was grown in BHI medium or IMM containing 6% (wt/vol) soy protein hydrolysate (SP) with or without 5 mM 2-methyl butyrate (2-MB).
Individual amino acid percentages were calculated after quantification of total amino acid content in 1 mg of bacterial protein lysate by amino acid analysis.
Glx represents both glutamine and glutamate.
L. monocytogenes pathogenesis requires lipoate-dependent metabolism.
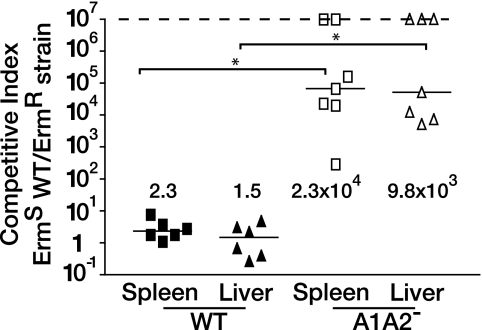
To determine the absolute requirement for lipoate-dependent metabolism in virulence, we tested the ability of the AlA2− mutant to grow in a murine model of L. monocytogenes infection. Erms WT L. monocytogenes was mixed with the Ermr AlA2− mutant and coinjected into female 6- to 8-week-old C57BL/6 mice. After 24 h, livers and spleens were harvested, and CFUs were enumerated. The CI of infection was calculated by dividing the number of recovered WT bacteria by the number of AlA2− mutant bacteria. We previously demonstrated that the CIs for the ΔlplA1 mutant are 31.6 and 603.0 for the liver and spleen of C57BL/6 mice, respectively, indicating that the WT significantly outcompetes a partial-lipoylation mutant in vivo (22). After 24 h, the WT outcompeted the AlA2− mutant by 9,872- and 23,391-fold in the liver and spleen of C57BL/6 mice; for some animals, no AlA2− mutant bacteria were recovered (Fig. 3). Therefore, L. monocytogenes deficient in lipoyl ligase function is substantially less fit in a murine model of infection than bacteria with a partial lipoylation defect or WT bacteria.
FIG. 3.
L. monocytogenes pathogenesis requires lipoate-dependent metabolism. Exponentially growing bacteria were mixed and injected intraperitoneally into 4- to 6-week-old female C57BL/6 mice. After 24 h, spleens and livers were harvested and plated onto LB agar with and without Erm. The CI was calculated by dividing the number of drug-sensitive (WT) CFUs by the number of drug-resistant (AlA2− mutant) CFUs. The horizontal solid lines represent the median values, and the dotted upper line represents mice for which no AlA2− CFUs were detected. The values from mice for which CFUs could not be detected were not used when CI values were calculated. Statistically significant differences between two groups were determined by Student's t test (P < 0.05, as indicated by the asterisks).
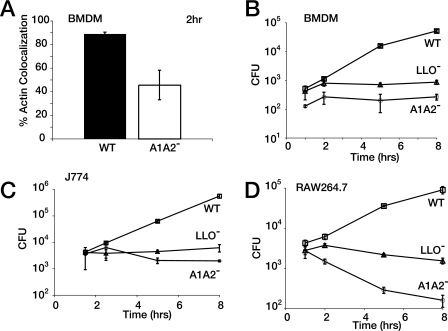
Replication of L. monocytogenes in phagocytic cells requires lipoate-dependent metabolism.
To determine where in the L. monocytogenes intracellular life cycle lipoate-dependent metabolism was required, we measured escape and proliferation of the AlA2− mutant in phagocytes. We infected macrophages with the WT or the AlA2− mutant and quantified the colocalization of L. monocytogenes with polymerized host actin, as colocalization occurs only in the cytosol (37). The AlA2− mutant was able to escape from the vacuole into the cytosol, albeit with lower efficiency than the WT, as it colocalized with host actin at 2 h postinfection in BMDMs (Fig. 4A). However, the AlA2− mutant did not replicate in the nutrient-limiting environment of the J774 or BMDM cytosol in the course of an 8-h infection (Fig. 4B and C). When we examined the ability of the AlA2− mutant to survive and grow in the RAW264.7 peritoneal macrophage cell line, the AlA2− mutant was more susceptible to killing, demonstrating that lipoate-dependent metabolism may be important for survival as well as for intracellular growth (Fig. 4D). These data indicate that lipoate-dependent metabolism is vital for replication of L. monocytogenes inside macrophages.
FIG. 4.
Lipoate-dependent metabolism is essential for growth during infection of phagocytes. After overnight growth in BHI medium, stationary-phase cultures of the WT, the AlA2− mutant, and an LLO− mutant of L. monocytogenes were washed and used to infect BMDMs (A and B), J774 macrophages (C), and RAW264.7 macrophages (D) for 30 min. (A) Intracellular bacteria were visualized at 2 h postinfection in BMDMs by colocalization of L. monocytogenes with actin clouds by the use of immunofluorescence with an anti-Listeria antibody and rhodamine-phalloidin. For panels B, C, and D, after extracellular bacteria were washed away, the extracellular medium was treated with 10 μg of gentamicin/ml. At the indicated times postinfection, cells were osmotically lysed and the lysate was plated on LB agar to determine colony-forming-unit values. Error bars represent standard deviations.
Growth and spread of lipoate-dependent metabolism mutants in L2 cells.
As different cell types may provide distinct nutrient environments for L. monocytogenes, we also measured bacterial growth in nonphagocytic cells. Murine L2 fibroblasts were infected with the WT or the AlA2− mutant and were incubated for several days to allow necrotic foci to form. We visualized plaques by Neutral Red staining and measured plaque diameter as an indicator of bacterial replication and cell-cell spread. Notably, the AlA2− mutant was able to form plaques, although the plaques were substantially reduced in size compared with the WT results and formed only around the edge of the well, where the agar overlay was thickest (Fig. 5A). When the thickness of the overlay was decreased, the A1A2− mutant was unable to form plaques (data not shown), possibly due to exposure to environmental oxygen, as lipoate and lipoylated enzymes can contribute to resistance to oxidative stress (3, 5, 39). We also examined the intracellular growth of the WT alone, the AlA2− mutant, and a vacuole-restricted listeriolysin-deficient (LLO−) mutant in L2 cells by enumerating CFUs over both a short and a long period of infection (Fig. 5B and C). After an initial lag, the AlA2− mutant could replicate in L2 cells, although its optimal doubling time of 4 h never approached the optimal doubling time of the WT at 57 min (Fig. 5B and C). The ability of the L. monocytogenes lipoylation mutant to grow in L2 cells suggests that the intracellular environment of some cell types may provide nutrients that bypass the requirement for lipoate-dependent metabolism.
FIG. 5.
Optimal growth in L2 fibroblasts requires lipoate-dependent metabolism. The WT, the AlA2− mutant, and the LLO− mutant of L. monocytogenes were grown to the stationary phase in BHI medium and used to infect L2 cells for 1 h. Infected monolayers were washed and treated with 50 μg of gentamicin/ml. (A) A 3-ml agar overlay was placed over infected cells, and plaques allowed to develop for 5 days, at which time they were visualized by the addition of Neutral Red. Plaque diameters (n = 20) were measured and are expressed as percentages of the mean diameters of WT plaques. (B and C) Infected L2 cells were osmotically lysed and plated on LB agar to enumerate CFU. Error bars represent standard deviations.
Excess environmental amino and fatty acids bypass the requirement for lipoate-dependent metabolism.
Critical nutrients for bacterial replication may be sequestered or absent in the host cytosol (4). To determine whether growth of L. monocytogenes requires lipoate-dependent metabolism to generate critical growth intermediates when the bacterium is in a nutrient-limiting environment, we grew the A1A2− mutant and the WT in IMM, a defined medium developed for the growth of WT L. monocytogenes (Fig. 6A) (33). While WT L. monocytogenes efficiently replicated in IMM, the AlA2− mutant did not, indicating that lipoate-dependent metabolism was necessary for bacterial growth under conditions of nutrient limitation. As the AlA2− mutant could grow in BHI medium but not in IMM, we hypothesized that one important function of lipoate-dependent metabolism is biosynthesis of key metabolites, which would be more critical in nutrient-poor environments. We therefore reasoned that we could identify which biosynthetic pathways might be supported by lipoate-dependent metabolism by taking a nutrient supplementation approach. WT or AlA2− mutant bacteria were inoculated into IMM supplemented with additional amino acids and fatty acids, and bacterial growth was measured over time. When amino acids were added to IMM in the form of soy protein hydrolysate, the AlA2− mutant exhibited a dramatic increase in growth (Fig. 6B). The presence of soy protein hydrolysate somewhat enhanced the growth of WT bacteria as well. When an alternate source of amino acids, vitamin-free casein hydrolysate, was used to supplement IMM, it also supported an increase in the growth of the AlA2− mutant similar to that observed with soy protein hydrolysate (data not shown). When amino acids were added individually, growth did not occur, implying that lipoate-dependent metabolism controls multiple pathways of amino acid biosynthesis. As the BCFA profile was altered in the AlA2− mutant after growth in BHI medium, we also added the BCFA precursors 2-methylbutyrate, isovalerate, and isobutyrate. Addition of BCFA precursors alone to IMM did not enable growth of the AlA2− mutant in the absence of soy protein hydrolysate. When isovalerate, a precursor for even-numbered-carbon-length iso-BCFAs, was added with soy protein hydrolysate, replication of the AlA2− mutant was not affected (Fig. 6B). However, addition of 2-methylbutyrate and isobutyrate, precursors for odd-numbered-carbon-chain-length BCFAs, did partially enhance replication of the AlA2− mutant while not affecting growth of WT (Fig. 6B). Of the two odd-numbered-carbon-chain-length BCFA precursors, 2-methylbutyrate, a precursor for anteiso-BCFAs, supported replication of the AlA2− mutant better than isobutyrate, an iso-BCFA precursor (Fig. 6B). These data suggest that under non-nutrient-limiting conditions such as the use of BHI medium, L. monocytogenes uses lipoate-dependent metabolism for production of BCFAs. However, when the growth environment becomes limiting with respect to amino acid and BCFA precursors in the manner seen with IMM medium, lipoate-dependent metabolism becomes essential for growth.
FIG. 6.
L. monocytogenes growth in the absence of excess environmental amino and fatty acids requires lipoate-dependent metabolism. (A) Individual colonies picked from fresh BHI plates containing the WT and the AlA2− mutant were inoculated into IMM, and the OD600 was recorded over time at 37°C using a Bioscreen growth curve analyzer. (B) Bacteria were grown as described for panel A to the stationary phase with or without (−) additional supplementation with 6% (wt/vol) soy protein hydrolysate (SP), 5 mM 2-methylbutyrate (2-MB), 5 mM isovalerate (IV), and/or 5 mM isobutyrate (IB). Error bars in panels A and B represent standard deviations. N.G., no growth. (C) Fatty acid content of bacterial pellets after growth in BHI medium or in IMM supplemented with 6% (wt/vol) soy protein hydrolysate (SP). Data are reported as percentages of total quantified fatty acids and clustered based on the even or odd numbers of carbons as well as on whether the fatty acid branch was anteiso or iso in orientation. (D) Amino acid content of bacterial lysate after growth in IMM supplemented with 6% (wt/vol) SP or 5 mM 2-MB. Data are reported as the percentage of total quantified amino acids in each individual sample.
As the WT was able to generate substantially more BCFAs than the A1A2− mutant after growth in BHI medium (Fig. 2D) and as BCAAs can be precursors for BCFAs, we sought to determine whether addition of amino acid sources and short BCFA precursors would enhance the ability of the A1A2− mutant to generate BCFAs. Without supplementation, over 80% of the total fatty acids in the WT were anteiso-BCFAs, whereas addition of soy protein hydrolysate and 2-methylbutyrate increased WT anteiso-BCFA content to only 90% of the total fatty acid content (Fig. 6C and Table 3). In contrast, addition of soy protein hydrolysate to BHI medium substantially altered the BCFA profile of the A1A2− mutant to nearly the levels found with the WT, increasing the odd-numbered-carbon-length anteiso-BCFAs from 5% to 75% of the total fatty acid content (Fig. 6C, Table 3). Supplementation with 2-methylbutyrate in addition to amino acids increased the odd-numbered-carbon-length anteiso-BCFAs from 75% to 95% of total fatty acid content in the AlA2− mutant, while the percentage remained constant at 90% in the WT (Table 3). The total amino acid content of the AlA2− mutant after supplementation with soy protein and 2-methylbutyrate remained largely unaltered from the amino acid content of the WT, with the exception of the level of glutamine and glutamate, which was increased from 13% to 24% of the total recovered amino acids in the A1A2− mutant (Table 2). Together, soy protein hydrolysate and 2-methylbutyrate restored the growth of the AlA2− mutant to nearly WT levels (Fig. 6B), while soy protein hydrolysate supplementation alone restored the BCFA profile to nearly WT levels (Fig. 6C) but only partially restored growth. These data indicate that the requirement for lipoate-dependent metabolism-mediated biosynthesis during L. monocytogenes replication in nutrient-restricted medium can be bypassed by supplementation with amino acids, which not only provide building blocks for protein synthesis but also provide primers for anteiso-BCFA synthesis.
TABLE 3.
Total fatty acid content of bacterial pelleta
| Fatty acid typeb | % Fatty acid contentc
|
|||||
|---|---|---|---|---|---|---|
| WT growth environment
|
A1A2− mutant growth environment
|
|||||
| BHI | SP | SP + MB | BHI | SP | SP + MB | |
| Even-numbered-carbon-chain-length SCFAd | 1.24 | 0.94 | 0.67 | 57.6 | 3.49 | 1.23 |
| Odd-numbered-carbon-chain-length SCFAd | 0 | 1.08 | 0.62 | 7.06 | 16.66 | 3.02 |
| Even-numbered-carbon-chain-length iso-BCFAe | 2.42 | 2.63 | 2.49 | 26.83 | 4.21 | 0.46 |
| Odd-numbered-carbon-chain-length iso-BCFAe | 11.58 | 6.45 | 6.06 | 1.22 | 0.26 | 0 |
| Odd-numbered-carbon-chain-length anteiso-BCFAf | 83.83 | 88.70 | 89.95 | 5.10 | 75.25 | 95.22 |
The inoculum was grown in BHI medium or IMM containing 6% (wt/vol) soy protein hydrolysate (SP) with or without 5 mM 2-methyl butyrate (2-MB).
Omega allicyclic minor fatty acid components are not included in this table.
Values are representative of the results of two independent experiments.
Short-chain fatty acid (SCFA) values represent cumulative percentages of straight-chain fatty acids with either an odd- or an even-numbered-carbon-chain length.
Iso-BCFA values represent cumulative percentages of iso-BCFAs with either an odd- or an even-numbered-carbon-chain length.
Anteiso-BCFA values represent cumulative percentages of anteiso-BCFAs with an odd-numbered-carbon-chain length.
BCFA precursor supplementation restores intracellular growth and spread of the L. monocytogenes A1A2− mutant.
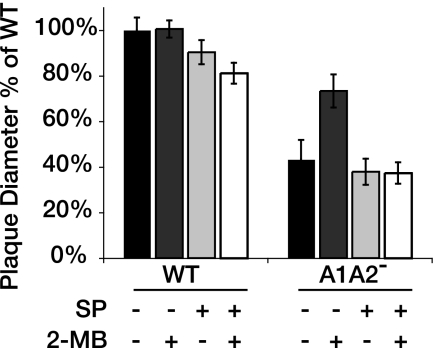
L. monocytogenes scavenges 50% to 100% of its amino acids from the host environment, but scavenging of BCFA precursors from the cytosol has not been investigated (9, 26). Biosynthesis of BCFA in L. monocytogenes is largely dependent upon the presence of BCKD, which requires lipoylation for activity (Fig. 2A). If the A1A2− mutant cannot grow in the cytosol of host cells because it does not scavenge BCFAs from the host and has a reduced ability to generate BCFAs, we hypothesized that we would be able to bypass the requirement for lipoate-dependent metabolism in the A1A2− mutant by exogenous supplementation with BCFA precursors. We supplemented L2 cells with and without soy protein hydrolysate and 2-methylbutyrate and assessed the ability of the AlA2− mutant to grow and spread (Fig. 7). Addition of soy protein hydrolysate had an inhibitory effect on the growth of L2 cells (data not shown) and also had a slight inhibitory effect on the intracellular growth and spread of WT L. monocytogenes. We observed no enhancement of AlA2− mutant plaque formation in the presence of soy protein hydrolysate compared to untreated L2 cell results. We also tested whether 5 mM 2-methylbutyrate, which had no apparent effect on L2 cells at a concentration of up to 500 mM, affected growth of the AlA2− mutant. In contrast to the behavior of the AlA2− mutant in IMM, where amino acid supplementation was more stimulatory than 2-methylbutyrate, addition of 5 mM 2-methylbutyrate alone significantly enhanced the growth and spread of the lipoylation-deficient mutant in L2 cells (Fig. 7). We infer from these data that in the host cell cytosol, L. monocytogenes can scavenge sufficient amino acids for growth but requires lipoate-dependent metabolism for biosynthesis of anteiso-BCFAs, a major component of the listerial membrane.
FIG. 7.
BCFA precursors bypass the requirement for lipoate-dependent metabolism in intracellular growth and spread. The WT and the AlA2− mutant of L. monocytogenes were grown to the stationary phase and used to infect L2 cells for 1 h. Extracellular bacteria grown in BHI medium were washed and treated with 50 μg of gentamicin/ml. A 3-ml overlay was placed on infected cells, and plaques were allowed to develop for 5 days before visualization by the addition of Neutral Red. Plaque diameters (n = 20) were measured and are expressed as percentages of the mean diameters of WT plaques. The overlay was supplemented with 0.75% soy protein hydrolysate (SP) and/or 5 mM 2-methylbutyrate (2-MB) or was left unsupplemented (−). Error bars represent standard deviations.
DISCUSSION
In this study, we investigated the mechanism by which lipoate ligases in L. monocytogenes contribute to bacterial metabolism during intracellular growth and virulence. Disrupting both lipoate ligase genes resulted in a complete loss of detectable lipoylated proteins in bacterial cell extract. In the absence of lipoylation, the AlA2− mutant was able to grow in rich medium but exhibited changes in acetoin and BCFA metabolism. Replication of the AlA2− mutant in nutrient-limiting conditions and in the host cytosol was substantially impaired. Supplementation with nonessential amino acids and anteiso-BCFA precursors to the AlA2− mutant restored growth in minimal medium and returned the profile of anteiso-BCFAs to a profile similar to that seen with the WT. Notably, in the nonphagocytic L2 cell line, the metabolic block in growth was partially complemented by the addition of anteiso-BCFA precursors but not by that of amino acids. These data imply that the cytosolic environment dictates bacterial biosynthesis of BCFAs and suggest that lipoate-dependent metabolism in L. monocytogenes enables this process to facilitate replication and virulence.
In L. monocytogenes, when genes encoding BCKD are disrupted, anteiso-BCFAs are synthesized at significantly reduced levels (12, 47). The inability to produce anteiso-BCFAs through BCKD can be bypassed by supplementation with 2-methylbutyrate, a precursor for anteiso-BCFAs. BCKD mutants exhibit defects in the cold stress response, possibly because anteiso-BCFAs contribute substantially to the single-membrane bilayer found in gram-positive bacteria (45). Here, we found that in the absence of lipoylated metabolic enzymes, including BCKD, the A1A2− mutant produces skewed ratios of anteiso-BCFAs. Amino acid supplementation of the AlA2− mutant in defined medium could substantially restore the anteiso-BCFA profile, even in the absence of functional BCKD. Thus, L. monocytogenes BCFAs must also be generated by a BCKD-independent mechanism. Although L. monocytogenes can scavenge amino acids from the cytosolic environment, replication of the A1A2− mutant was still significantly impaired (26). Since only 2-methylbutyrate, an anteiso-BCFA precursor, could supplement growth of the A1A2− mutant during intracellular growth, the scavenged cytosolic amino acids were presumably sufficient to replete bacterial amino acid pools but not in sufficient excess to drive BCFA biosynthesis. Together, these data suggest that the intracellular environment does not provide enough amino acids or enough BCFA precursors to bypass the requirement for lipoate-dependent fatty acid biosynthesis. The cytosols of different host cell types may offer distinct nutrient profiles, some of which could minimize the requirement for lipoate-dependent metabolism-mediated biosynthesis in L. monocytogenes infection. In support of the idea that the host may provide various nutrients to bypass metabolic pathway requirements in different cell types, L. monocytogenes requires a gene encoding glycerol kinase 1 in some cell types but not in others (18). It will be of interest to determine whether L. monocytogenes and other bacteria that require lipoate-dependent metabolism for growth exhibit tropism toward tissues rich in nutrients, as such tissues could lessen the energetic burden of biosynthesis.
The different mechanisms by which L. monocytogenes generates anteiso-BCFAs from BCAAs have not been well characterized, although studies of other bacterial species suggest that the requisite methyl branch essential for BCFA biosynthesis is primarily derived from degradation of BCAAs into branched carboxylic acids (20). Alternatively, BCFA can be directly generated from branched short-chain carboxylic acids such as 2-methylbutyrate or from β-oxidation of existing endogenous or exogenous BCFAs (20, 24). These branched carboxylic acids can then be activated with respect to their CoA esters with BCKD or an acyl-CoA synthetase, or they can be activated by a decarboxylase. In bacteria such as E. coli and B. subtilis, the branched primers then condense with malonyl-acyl carrier protein derived from malonyl-CoA and eventually form elongated BCFAs (20). In B. subtilis lysate, when the large BCKD complex is removed by ultracentrifugation, the remaining lysate can still generate BCFAs by the use of a branched-chain α-keto acid decarboxylase (KDCA) (21). KDCA decarboxylates α-keto acid derivatives of BCAAs, and in B. subtilis, this primer source is thought to then condense with a malonyl-acyl carrier protein derivative to ultimately generate BCFAs (20). By homology to a characterized KDCA of Lactococcus lactis, L. monocytogenes EGD-e encodes a candidate KDCA (lmo1984, ilvB) that shares with it 21% identity and 66% similarity. Future experiments will determine whether L. monocytogenes can generate BCFAs through KDCA as an alternative mechanism to BCKD and whether this pathway contributes to virulence.
The influence of nutrients on virulence is widespread among pathogens (4). In the Vibrio cholerae El Tor strain, the production of 2,3-butanediol enables survival under acidic growth conditions and enhances murine intestinal colonization (46). In S. Typhimurium, formate induces invasiveness, and in Streptococcus pneumoniae, sucrose transport and metabolism contributes to colonization and disease (15, 17). Finally, in Plasmodium falciparum, lipoate scavenging contributes to mitochondrial lipoylation and enables growth in erythrocytes (1, 13). We have demonstrated that lipoate-dependent metabolism is needed for the production of anteiso-BCFAs, which play a key role in L. monocytogenes intracellular survival and replication. High percentages of anteiso-BCFA are thought to increase L. monocytogenes resistance to the lantibiotic nisin, a peptide antibiotic naturally produced by nonpathogenic bacteria (27). Since BCFAs also contribute to the ability of L. monocytogenes to survive cold and alkaline stress, we speculate that they additionally allow the bacteria to survive the unfriendly environment of the host cytosol (25, 40). Other studies have also emphasized a key role for fatty acids in virulence. In S. aureus, insertional inactivation of BCKD disrupts BCFA composition and the ability of the bacterium to resist oxidative stress and alkaline pH (39). In M. tuberculosis, microarray studies have revealed an upregulation of genes encoding fatty acid catabolic pathways during growth in the macrophage vacuole (36). Finally, deletion of acyl carrier protein in Toxoplasma gondii leads to loss of PDH lipoylation and abrogation of apicoplast fatty acid synthesis, resulting in attenuation of virulence (28). Collectively, these findings suggest that lipoate-dependent metabolism plays a critical role in the virulence of pathogens by enabling de novo biosynthesis of critical metabolic intermediates that may not be provided in the nutrient-restrictive environment of the host.
Supplementary Material
Acknowledgments
We gratefully acknowledge members of the O'Riordan laboratory and John Cronan for helpful discussions. We also thank C. Alteri and M. Fonseca for critical review of the manuscript.
This work was funded by National Institutes of Health grant R01A1064540 (to M.O.). K.K. was a trainee of the Molecular Mechanisms of Pathogenesis Training Grant (T32A1007528) and a recipient of a departmental Willison fellowship.
Footnotes
Published ahead of print on 30 January 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Allary, M., J. Z. Lu, L. Zhu, and S. T. Prigge. 2007. Scavenging of the cofactor lipoate is essential for the survival of the malaria parasite Plasmodium falciparum. Mol. Microbiol. 631331-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auerbuch, V., L. L. Lenz, and D. A. Portnoy. 2001. Development of a assay to evaluate the virulence of Listeria monocytogenes actA mutants during primary and secondary infection of mice. Infect. Immun. 695953-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biewenga, G. P., G. R. Haenen, and A. Bast. 1997. The pharmacology of the antioxidant lipoic acid. Gen. Pharmacol. 29315-331. [DOI] [PubMed] [Google Scholar]
- 4.Brown, S. A., K. L. Palmer, and M. Whiteley. 2008. Revisiting the host as a growth medium. Nat. Rev. Microbiol. 6657-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryk, R., C. D. Lima, H. Erdjument-Bromage, P. Tempst, and C. Nathan. 2002. Metabolic enzymes of mycobacteria linked to antioxidant defense by a thioredoxin-like protein. Science 2951073-1077. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee, S. S., H. Hossain, S. Otten, C. Kuenne, K. Kuchmina, S. Machata, E. Domann, T. Chakraborty, and T. Hain. 2006. Intracellular gene expression profile of Listeria monocytogenes. Infect. Immun. 741323-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chico-Calero, I., M. Suárez, B. González-Zorn, M. Scortti, J. Slaghuis, W. Goebel, J. A. Vázquez-Boland, and the E. L. G. Consortium. 2002. Hpt, a bacterial homolog of the microsomal glucose-6-phosphate translocase, mediates rapid intracellular proliferation in Listeria. Proc. Natl. Acad. Sci. USA 99431-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisenreich, W., J. Slaghuis, R. Laupitz, J. Bussemer, J. Stritzker, C. Schwarz, R. Schwarz, T. Dandekar, W. Goebel, and A. Bacher. 2006. 13C isotopologue perturbation studies of Listeria monocytogenes carbon metabolism and its modulation by the virulence regulator PrfA. Proc. Natl. Acad. Sci. USA 1032040-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eylert, E., J. Schär, S. Mertins, R. Stoll, A. Bacher, W. Goebel, and W. Eisenreich. 2008. Carbon metabolism of Listeria monocytogenes growing inside macrophages. Mol. Microbiol. 691008-1017. [DOI] [PubMed] [Google Scholar]
- 10.Freitag, N. E., L. Rong, and D. A. Portnoy. 1993. Regulation of the prfA transcriptional activator of Listeria monocytogenes: multiple promoter elements contribute to intracellular growth and cell-to-cell spread. Infect. Immun. 612537-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbreth, S. E., A. K. Benson, and R. W. Hutkins. 2004. Catabolite repression and virulence gene expression in Listeria monocytogenes. Curr. Microbiol. 4995-98. [DOI] [PubMed] [Google Scholar]
- 12.Giotis, E. S., D. A. McDowell, I. S. Blair, and B. J. Wilkinson. 2007. Role of branched-chain fatty acids in pH stress tolerance in Listeria monocytogenes. Appl. Environ. Microbiol. 73997-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Günther, S., P. J. McMillan, L. J. Wallace, and S. Müller. 2005. Plasmodium falciparum possesses organelle-specific alpha-keto acid dehydrogenase complexes and lipoylation pathways. Biochem. Soc. Trans. 33977-980. [DOI] [PubMed] [Google Scholar]
- 14.Heithoff, D. M., C. P. Conner, P. C. Hanna, S. M. Julio, U. Hentschel, and M. J. Mahan. 1997. Bacterial infection as assessed by in vivo gene expression. Proc. Natl. Acad. Sci. USA 94934-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang, Y., M. Suyemoto, C. D. Garner, K. M. Cicconi, and C. Altier. 2008. Formate acts as a diffusible signal to induce Salmonella invasion. J. Bacteriol. 1904233-4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikonomidis, G., Y. Paterson, F. J. Kos, and D. A. Portnoy. 1994. Delivery of a viral antigen to the class I processing and presentation pathway by Listeria monocytogenes. J. Exp. Med. 1802209-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iyer, R., and A. Camilli. 2007. Sucrose metabolism contributes to in vivo fitness of Streptococcus pneumoniae. Mol. Microbiol. 661-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joseph, B., S. Mertins, R. Stoll, J. Schär, K. R. Umesha, Q. Luo, S. Müller-Altrock, and W. Goebel. 2008. Glycerol-metabolism and PrfA activity in Listeria monocytogenes. J. Bacteriol. 1905412-5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joseph, B., K. Przybilla, C. Stühler, K. Schauer, J. Slaghuis, T. M. Fuchs, and W. Goebel. 2006. Identification of Listeria monocytogenes genes contributing to intracellular replication by expression profiling and mutant screening. J. Bacteriol. 188556-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaneda, T. 1991. Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol. Rev. 55288-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaneda, T. 1988. Stereoselectivity in the 2-methylbutyrate incorporation into anteiso fatty acids in Bacillus subtilis mutants. Biochim. Biophys. Acta 96010-18. [DOI] [PubMed] [Google Scholar]
- 22.Keeney, K. M., J. A. Stuckey, and M. X. O'Riordan. 2007. LplA1-dependent utilization of host lipoyl peptides enables Listeria cytosolic growth and virulence. Mol. Microbiol. 66758-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim, H.-J., M. Mittal, and A. L. Sonenshein. 2006. CcpC-dependent regulation of citB and lmo0847 in Listeria monocytogenes. J. Bacteriol. 188179-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunau, W. H., V. Dommes, and H. Schulz. 1995. β-Oxidation of fatty acids in mitochondria, peroxisomes, and bacteria: a century of continued progress. Prog. Lipid Res. 34267-342. [DOI] [PubMed] [Google Scholar]
- 25.Liu, S., J. E. Graham, L. Bigelow, P. D. Morse, and B. J. Wilkinson. 2002. Identification of Listeria monocytogenes genes expressed in response to growth at low temperature. Appl. Environ. Microbiol. 681697-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marquis, H., H. G. Bouwer, D. J. Hinrichs, and D. A. Portnoy. 1993. Intracytoplasmic growth and virulence of Listeria monocytogenes auxotrophic mutants. Infect. Immun. 613756-3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martínez, B., and A. Rodríguez. 2005. Antimicrobial susceptibility of nisin resistant Listeria monocytogenes of dairy origin. FEMS Microbiol. Lett. 25267-72. [DOI] [PubMed] [Google Scholar]
- 28.Mazumdar, J., E. H Wilson, K. Masek, C. A Hunter, and B. Striepen. 2006. Apicoplast fatty acid synthesis is essential for organelle biogenesis and parasite survival in Toxoplasma gondii. Proc. Natl. Acad. Sci. USA 10313192-13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milenbachs, A. A., D. P. Brown, M. Moors, and P. Youngman. 1997. Carbon-source regulation of virulence gene expression in Listeria monocytogenes. Mol. Microbiol. 231075-1085. [DOI] [PubMed] [Google Scholar]
- 30.Morris, T. W., K. E. Reed, and J. E. Cronan, Jr. 1995. Lipoic acid metabolism in Escherichia coli: the lplA and lipB genes define redundant pathways for ligation of lipoyl groups to apoprotein. J. Bacteriol. 1771-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Riordan, M., M. A. Moors, and D. A. Portnoy. 2003. Listeria intracellular growth and virulence require host-derived lipoic acid. Science 302462-464. [DOI] [PubMed] [Google Scholar]
- 32.Perham, R. N. 2000. Swinging arms and swinging domains in multifunctional enzymes: catalytic machines for multistep reactions. Annu. Rev. Biochem. 69961-1004. [DOI] [PubMed] [Google Scholar]
- 33.Phan-Thanh, L., and T. Gormon. 1997. A chemically defined minimal medium for the optimal culture of Listeria. Int. J. Food Microbiol. 3591-95. [DOI] [PubMed] [Google Scholar]
- 34.Portnoy, D. A., P. S. Jacks, and D. J. Hinrichs. 1988. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 1671459-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Premaratne, R. J., W. J. Lin, and E. A. Johnson. 1991. Development of an improved chemically defined minimal medium for Listeria monocytogenes. Appl. Environ. Microbiol. 573046-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schnappinger, D., S. Ehrt, M. I. Voskuil, Y. Liu, J. A. Mangan, I. M. Monahan, G. Dolganov, B. Efron, P. D. Butcher, C. Nathan, and G. K. Schoolnik. 2003. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J. Exp. Med. 198693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schnupf, P., and D. A. Portnoy. 2007. Listeriolysin O: a phagosome-specific lysin. Microbes Infect. 91176-1187. [DOI] [PubMed] [Google Scholar]
- 38.Shirk, M. C., W. P. Wagner, and R. Fall. 2002. Isoprene formation in Bacillus subtilis: a barometer of central carbon assimilation in a bioreactor? Biotechnol. Prog. 181109-1115. [DOI] [PubMed] [Google Scholar]
- 39.Singh, V. K., D. S. Hattangady, E. S. Giotis, A. K. Singh, N. R. Chamberlain, M. K. Stuart, and B. J. Wilkinson. 2008. Insertional inactivation of branched-chain α-keto acid dehydrogenase in Staphylococcus aureus leads to decreased branched-chain membrane fatty acid content and increased susceptibility to certain stresses. Appl. Environ. Microbiol. 745882-5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stritzker, J., J. Janda, C. Schoen, M. Taupp, S. Pilgrim, I. Gentschev, P. Schreier, G. Geginat, and W. Goebel. 2004. Growth, virulence, and immunogenicity of Listeria monocytogenes aro mutants. Infect. Immun. 725622-5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swaminathan, B., and P. Gerner-Smidt. 2007. The epidemiology of human listeriosis. Microbes Infect. 91236-1243. [DOI] [PubMed] [Google Scholar]
- 42.Trivett, T. L., and E. A. Meyer. 1971. Citrate cycle and related metabolism of Listeria monocytogenes. J. Bacteriol. 107770-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welshimer, H. J. 1968. Isolation of Listeria monocytogenes from vegetation. J. Bacteriol. 95300-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welshimer, H. J. 1960. Survival of Listeria monocytogenes in soil. J. Bacteriol. 80316-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willecke, K., and A. B. Pardee. 1971. Fatty acid-requiring mutant of Bacillus subtilis defective in branched chain α-keto acid dehydrogenase. J. Biol. Chem. 2465264-5272. [PubMed] [Google Scholar]
- 46.Yoon, S. S., and J. J. Mekalanos. 2006. 2,3-Butanediol synthesis and the emergence of the Vibrio cholerae El Tor biotype. Infect. Immun. 746547-6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu, K., D. O. Bayles, A. Xiong, R. K. Jayaswal, and B. J. Wilkinson. 2005. Precursor and temperature modulation of fatty acid composition and growth of Listeria monocytogenes cold-sensitive mutants with transposon-interrupted branched-chain α-keto acid dehydrogenase. Microbiology (Reading) 151615-623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.