Peptidoglycan (PG) is the major component of the bacterial cell wall and is recognized by animals as a signaling molecule indicating the presence of bacteria. PG recycling during cell growth and division is well regulated, but a few gram-negative bacteria also release monomeric forms of PG (2). Until recently, the interaction between these PG monomers and animal host cells was thought to be restricted to pathogenic associations. In this issue, Adin et al. describe a mechanism by which the beneficial bacterium Vibrio fischeri releases PG monomers, lending insight into the role that these microbe-associated molecular pattern (MAMP) molecules play in host morphogenesis of the Hawaiian bobtail squid Euprymna scolopes (1).
PG monomers.
In gram-negative bacteria, PG consists of subunits of N-acetylglucosamine and N-acetylmuramic acid connected to a short pentapeptide side chain of l-alanyl-d-γ-glutamyl-meso-diaminopimelyl-d-alanyl-d-alanine (2). During normal recycling of PG, lytic transglycosylases cleave the N-acetylmuramic acid-β-1,4-N-acetylglucosamine linkage, generating PG monomers (2, 7). In Escherichia coli, a permease, AmpG, then aids these PG monomers in entering the cytoplasm, where they are recycled and incorporated into the remodeled cell wall (2, 9). Some gram-negative bacteria, however, release PG monomers that can have a dramatic effect on eukaryotic host epithelial cells. This phenomenon was first described for the pathogens Bordetella pertussis and Neisseria gonorrhoeae, where release of PG monomers (identical in structure but named tracheal cytotoxin in B. pertussis and PG cytotoxin in N. gonorrhoeae) causes sloughing of ciliated epithelial cells and contributes to the etiology of whooping cough and gonorrhea, respectively (2, 10, 12).
The squid-vibrio association and PG.
The symbiosis between the benthic Hawaiian bobtail squid, Euprymna scolopes, and the bioluminescent bacterium Vibrio fischeri is used as a model system to study the effects of beneficial bacteria on the development of animal host tissues (6, 8, 13). Each generation, the host is colonized by V. fischeri cells from the environment. Upon entry, these symbionts are housed in a structure called a light organ, the light of which is used by the host to camouflage itself during its nocturnal feeding behavior. Because the potential symbionts represent only a small fraction of the ambient microbial assemblage found in the surrounding seawater, E. scolopes and V. fischeri have evolved mechanisms that increase the probability of successful colonization, while discouraging infection of host tissues by nonspecific microorganisms. One such mechanism is a process by which the host harvests V. fischeri from seawater by using mucus secretions originating from superficial ciliated epithelia that aggregate environmental bacteria (reviewed in reference 8). The induction of these mucus secretions is initiated by bacterial PG. V. fischeri is able to outcompete other environmental bacteria in this mucus and migrate to and colonize epithelium-lined crypt spaces located in the center of the light organ, which is positioned below the superficial ciliated epithelium that is responsible for the mucus secretions (Fig. 1) (8). Following successful colonization, V. fischeri induces apoptosis and regression of these superficial ciliated fields via the synergistic action of PG monomers (identical to those released by B. pertussis and N. gonorrhoeae) and lipopolysaccharide (4). V. fischeri PG monomers have also been shown to cause the trafficking of host phagocytic hemocytes into the ciliated fields, presumably a process that aids in the symbiont-induced host cell morphogenesis (5).
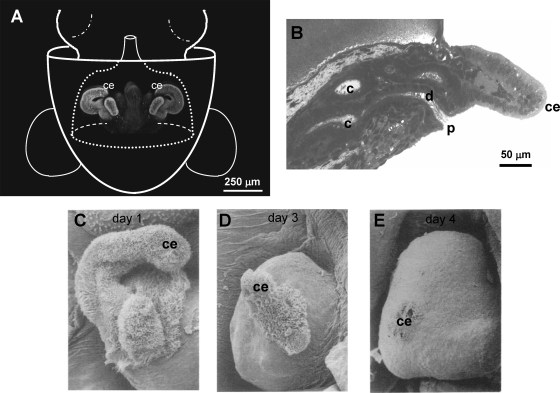
FIG. 1.
Effect of V. fischeri monomers on host light-organ morphogenesis. (A) At hatching, the juvenile host's light organ is positioned in the center of the mantle cavity, where it is exposed to seawater and a background of environmental bacteria. A ciliated epithelium (ce) located on the surface of the light organ aids V. fischeri cells in colonizing the host (7a). (B) During this process, the symbionts migrate through pores (p) on the surface, down a ciliated duct (d), and into epithelium-lined crypt spaces (c), where colonization takes place. (C to E) V. fischeri PG monomers along with lipopolysaccharide lead to cell death and regression of the superficial ciliated epithelium (ce) over a 4-day period. (Adapted from reference 2a with the permission of the publisher.)
Significance of this work.
The discovery that a beneficial symbiont employs PG monomers to induce eukaryotic cell morphogenesis as part of the normal developmental program of an animal host broadens the role of MAMPs in microbe-animal interactions beyond that of recognition and removal of pathogens by the innate immune system (4). Adin et al. continue this story by describing a potential mechanism by which V. fischeri accumulates extracellular PG monomers. The authors identified and targeted V. fischeri homologues to ampG, which transports PG into the cytoplasm, and lytic transglycosylase genes (ltgA, ltgD, and ltgY) for mutagenesis. These genes were chosen because B. pertussis and N. gonorrhoeae employ different mechanisms to make their PG monomers. Whereas in B. pertussis the disruption of ampG leads to the release of PG monomer into the extracellular environment, N. gonorrhoeae uses lytic transglycosylase activity to generate and release PG monomer (reviewed in reference 2). Based on a number of elegant experiments, the major findings of this study include the following: (i) ampG mutants have a 100-fold increase in PG monomer release; (ii) mutations of transglycosylase genes in V. fischeri led to a decrease in PG monomer release; and (iii) a triple mutant lacking ltgA, ltgD, and ltgY colonized the host but left these squid open to secondary infection.
Because the V. fischeri ampG mutant led to a significant increase in PG monomer release while the inactivation of three transglycosylase genes led to the opposite result, the authors conclude that PG monomer release in V. fischeri more resembles that of N. gonorrhoeae. Interestingly, knocking out all three lytic transglycosylase genes led to susceptibility to a superinfection of host light organs by another strain of V. fischeri, suggesting that the inability to produce PG monomers leads to less regression of the superficial ciliated epithelium and presumably continued colonization of the host light organ. One of the amazing aspects of the squid-vibrio association is the high level of specificity (i.e., only V. fischeri has been demonstrated to colonize the light organ, even though the host crypt spaces are physically open to the surrounding seawater, containing on average 106 cells/ml of nonsymbiotic bacteria) (8). As the authors so aptly state, PG monomers may help establish this specificity by “closing the door” and preventing future colonization by potential pathogens or interlopers.
Future questions.
Besides the results outlined above, this study also generated a number of very useful future tools, V. fischeri strains that result in significant increased and decreased PG monomer activity. V. fischeri PG monomer and PG derivatives have been implicated in a number of developmental events in the squid host (8, 13). These mutants will no doubt prove to be crucial resources for elucidating the role(s) that PG monomers play in establishing, and perhaps also in maintaining, the squid-vibrio association. Besides the induction of host mucus secretions preceding bacterial aggregation, hemocyte trafficking, and regression of the superficial ciliated epithelium, a number of other developmental events in this association may be mediated by V. fischeri PG monomer and/or PG derivatives. For example, by 48 h after colonization, host mucus accumulates in the crypt spaces while mucus secretion from the cells of the superficial epithelium ceases (8). Furthermore, the presence of V. fischeri in the crypt spaces is required to prevent continued mucus secretion, as removal of the symbionts from the light organ with the use of antibiotics restores the ability of the host to secrete mucus (8). Does PG or its derivatives play a role in regulating these changes in host mucosecretory behavior?
The host light organ continues to develop after colonization as the hatchling animal grows into an adult. The crypt spaces must accommodate a 1,000-fold increase in V. fischeri cells during this period, and a number of changes occur in the overall architecture of the tissues that house the symbionts. Are PG monomers involved with initiating any of these later developmental events, as they are during establishment of the association? The host also has a unique behavior in that every day at dawn, greater than 95% of the symbionts are expelled from the light organ along with a component of host cells comprised of sloughed epithelial cells, epithelial cell fragments, and phagocytic blood cells or hemocytes, the main cellular component of the host's innate immune system (7). This behavior serves two purposes, to seed the environment with symbionts for future host generations and to presumably regulate V. fischeri growth in the crypt spaces. The role of the sloughed epithelial cells in the association is unknown, but this cellular debris may serve as a nutritional source for the symbionts. PG monomers from B. pertussis and N. gonorrhoeae have been implicated in ciliated cell sloughing in humans (2). The mutants generated in this study may help researchers to understand if V. fischeri PG monomers are involved with this daily restructuring of the host epithelium as well as mediation of the host's immune response, leading to the establishment and maintenance of homeostasis in the crypt spaces.
How might V. fischeri MAMPs such as PG monomers interact with the host at the molecular level? A number of pattern recognition receptors such as Toll-like receptor and PG recognition proteins along with downstream signaling pathways such as the NF-κB signaling pathway have been implicated in recognizing MAMPs and mediating host responses to microorganisms (2, 11). In E. scolopes, homologues to all of these have been found, and now host gene expression of these various receptors and pathways may be studied in response to varying V. fischeri PG monomer release in vivo (3).
Research on host-microbe interactions continues to reveal an intricate and conserved repertoire of signals used to mediate molecular cross talk in both pathogenic and beneficial associations. The use of MAMPs such as PG monomers in the initiation and establishment of both types of relationships demonstrates that cell-cell communication between bacteria and eukaryotes is ubiquitous in nature, often serving critical functions in these associations.
Acknowledgments
Thanks go to Joerg Graf (University of Connecticut) and Karen Visick (Loyola University, Chicago, IL) for critical reading of the manuscript.
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
Footnotes
Published ahead of print on 16 January 2009.
REFERENCES
- 1.Adin, D. M., J. T. Engle, W. E. Goldman, M. J. McFall-Ngai, and E. V. Stabb. 2009. Mutations in ampG and lytic transglycosylase genes affect the net release of peptidoglycan monomers from Vibrio fischeri. J. Bacteriol. 1912012-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cloud-Hansen, K. A., S. B. Peterson, E. V. Stabb, W. E. Goldman, M. J. McFall-Ngai, and J. Handelsman. 2006. Breaching the great wall: peptidoglycan and microbial interactions. Nat. Rev. Microbiol. 4710-716. [DOI] [PubMed] [Google Scholar]
- 2a.Doino, J. A., and M. J. McFall-Ngai. 1995. A transient exposure to symbiosis-competent bacteria induces light organ morphogenesis in the host squid. Biol. Bull. 189347-355. [DOI] [PubMed] [Google Scholar]
- 3.Goodson, M. S., M. Kojadinovic, J. V. Troll, T. E. Scheetz, T. L. Casavant, M. B. Soares, and M. J. McFall-Ngai. 2005. Identifying components of the NF-κB pathway in the beneficial Euprymna scolopes-Vibrio fischeri light organ symbiosis. Appl. Environ. Microbiol. 716934-6946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koropatnick, T. A., J. T. Engle, M. A. Apicella, E. V. Stabb, W. E. Goldman, and M. J. McFall-Ngai. 2004. Microbial factor-mediated development in a host-bacterial mutualism. Science 3061186-1188. [DOI] [PubMed] [Google Scholar]
- 5.Koropatnick, T. A., J. R. Kimbell, and M. J. McFall-Ngai. 2007. Responses of host hemocytes during the initiation of the squid-Vibrio symbiosis. Biol. Bull. 21229-39. [DOI] [PubMed] [Google Scholar]
- 6.McFall-Ngai, M. J., and E. G. Ruby. 1991. Symbiont recognition and subsequent morphogenesis as early events in an animal-bacterial mutualism. Science 2541491-1494. [DOI] [PubMed] [Google Scholar]
- 7.Nyholm, S. V., and M. J. McFall-Ngai. 1998. Sampling the light-organ microenvironment of Euprymna scolopes: description of a population of host cells in association with the bacterial symbiont Vibrio fischeri. Biol. Bull. 19589-97. [DOI] [PubMed] [Google Scholar]
- 7a.Nyholm, S. V., E. V. Stabb, E. G. Ruby, and M. J. McFall-Ngai. 2000. Establishment of an animal-bacterial association: recruiting symbiotic vibrios from the environment. Proc. Natl. Acad. Sci. USA 9710231-10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nyholm, S. V., and M. J. McFall-Ngai. 2004. The winnowing: establishing the squid-vibrio symbiosis. Nat. Rev. Microbiol. 2632-642. [DOI] [PubMed] [Google Scholar]
- 9.Park, J. T. 1995. Why does Escherichia coli recycle its cell wall peptides? Mol. Microbiol. 17421-426. [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal, R. S., W. Nogami, B. T. Cookson, W. E. Goldman, and W. J. Folkening. 1987. Major fragment of soluble peptidoglycan released from growing Bordetella pertussis is tracheal cytotoxin. Infect. Immun. 552117-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Royet, J., and R. Dziarski. 2007. Peptidoglycan recognition proteins: pleiotropic sensors and effectors of antimicrobial defences. Nat. Rev. Microbiol. 5264-277. [DOI] [PubMed] [Google Scholar]
- 12.Sinha, R. K., and R. S. Rosenthal. 1980. Release of soluble peptidoglycan from growing gonococci: demonstration of anhydro-muramyl-containing fragments. Infect. Immun. 29914-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Visick, K. V., and E. G. Ruby. 2006. Vibrio fischeri and its host: it takes two to tango. Curr. Opin. Microbiol. 9632-638. [DOI] [PubMed] [Google Scholar]