Abstract
Motility is an essential characteristic for mesophilic Aeromonas strains. We identified a new polar flagellum region (region 6) in the A. hydrophila AH-3 (serotype O34) chromosome that contained two additional polar stator genes, named pomA2 and pomB2. A. hydrophila PomA2 and PomB2 are highly homologous to other sodium-conducting polar flagellum stator motors as well as to the previously described A. hydrophila AH-3 PomA and PomB. pomAB and pomA2B2 were present in all the mesophilic Aeromonas strains tested and were independent of the strains' ability to produce lateral flagella. Unlike MotX, which is a stator protein that is essential for polar flagellum rotation, here we demonstrate that PomAB and PomA2B2 are redundant sets of proteins, as neither set on its own is essential for polar flagellum motility in either aqueous or high-viscosity environments. Both PomAB and PomA2B2 are sodium-coupled stator complexes, although PomA2B2 is more sensitive to low concentrations of sodium than PomAB. Furthermore, the level of transcription in aqueous and high-viscosity environments of pomA2B2 is reduced compared to that of pomAB. The A. hydrophila AH-3 polar flagellum is the first case described in which two redundant sodium-driven stator motor proteins (PomAB and PomA2B2) are found.
The bacterial flagellum usually is powered by a reversible rotator motor at the base of the flagellum structure, which uses energy from either the proton or sodium ion gradient to drive the rotation of the flagellum filament. The flagellum motor is divided into two substructures: the stator and the rotor. The rotor is composed of the FliM and FliN proteins, which form the C ring structure, and the FliG protein. These three proteins act as a switch that controls the direction of flagellum rotation, clockwise or counterclockwise. The stator is the stationary component of the motor and consists of different proteins surrounding the rotor, which constitute proton or sodium ion channels that couple the flow of ions to flagellum rotation (8, 10). In the proton-driven motor of Escherichia coli and Salmonella enterica serovar Typhimurium, the stator is composed of two integral membrane proteins, MotA and MotB (9, 31, 45). MotA has four transmembrane domains, whereas MotB has one transmembrane domain as well as a peptidoglycan-binding motif at its C terminus (17, 51). These two proteins form a complex, in a ratio of four MotA to two MotB, that conducts protons across the inner membrane (29). However, the stator of the Vibrio parahaemolyticus proton-driven lateral flagellum motor (MotAL [LafT] and MotBL [LafU]) requires an additional protein, which has a peptidoglycan-binding domain, for motor function called MotY (LafY) (44). Torque generation in the proton-driven motors is obtained through the electrostatic interaction between conserved charges on the cytoplasmic domains of the FliG and MotA proteins (28).
In the sodium-driven motor of alkaliphilic Bacillus species, the stators require two proteins, MotsP and MotS (24), whereas the polar flagellum stators of Vibrio species, such as V. alginolyticus and V. parahaemolyticus, require four proteins: PomA, PomB, MotX, and MotY (3, 34, 48). MotP/PomA and MotS/PomB proteins are homologous to the proton-driven MotA and MotB, respectively. MotX and MotY do not have paralogous proteins in E. coli and are components of the T ring (46), which is located beneath the P ring of the polar flagellum basal body in Vibrio species. In V. alginolyticus, MotX and MotY are required for the assembly of the PomAB complex in the polar flagellum motor (30). MotY has an N-terminal region that is essential for the association of the stator unit around the rotor (30) and, like MotB and PomB, has a peptidoglycan-binding motif in its C-terminal region (33). It is believed that torque generation in sodium-driven motors is obtained in a manner similar to that of proton-driven motors (4, 21), although charged residues that are critical for flagellum rotation appear to be different or additional charged residues may be required (19, 49).
Mesophilic Aeromonas species are ubiquitous waterborne bacteria and pathogens of reptiles, amphibians, and fish (6). In humans, Aeromonas hydrophila, belonging to hybridization groups 1 and 3 (HG1 and HG3), A. veronii biovar sobria (HG8/HG10), and A. caviae (HG4) have been associated with gastrointestinal and extraintestinal diseases such as wound infections of healthy humans and, less commonly, with septicemias of immunocompromised patients (25). The swimming motility of all mesophilic aeromonads has been linked to a single polar unsheathed flagellum, expressed constitutively, that is required for the adherence and invasion of human and fish cell lines (13, 36, 40). Certain strains also are able to express many unsheathed peritrichous lateral flagellum when grown in viscous environments or on surfaces that increase bacterial adherence and are required for swarming motility and biofilm formation (14, 20). The expression of two distinct flagellum systems is relatively uncommon, although it has been observed in V. parahaemolyticus (32), Azospirillum brasilense (38), Rhodospirillum centenum (26), and Plesiomonas shigelloides (23).
Recently, we reported the genes involved in A. hydrophila lateral and polar flagellum formation, and surprisingly we found that PomB mutations do not affect swimming (13, 14), in contrast to the similar mutants described for V. cholerae, V. alginolyticus, and V. parahaemolyticus (11, 21). In this work, we describe a new A. hydrophila AH-3 pomAB-like locus (pomCD) and its implication in polar flagellum motility. Furthermore, we investigated the distribution of these genes among mesophilic Aeromonas species.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. E. coli strains were grown on Luria-Bertani (LB) Miller broth and LB Miller agar at 37°C, and Aeromonas strains were grown in tryptic soy broth (TSB) or agar (TSA) at 30°C. When required, ampicillin (50 μg/ml), kanamycin (50 μg/ml), chloramphenicol (25 μg/ml), rifampin (rifampicin; 100 μg/ml), spectinomycin (50 μg/ml), and tetracycline (20 μg/ml) were added to the different media.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Reference or source |
|---|---|---|
| Strains | ||
| A. hydrophila | ||
| AH-3 | A. hydrophila wild type, serogroup O:34 | 35 |
| AH-405 | AH-3, spontaneously Rifr | 1 |
| AH-4442 | AH-405, flhA::Kmr | 13 |
| AH-4444 | AH-405, pomB::Kmr | 13 |
| AH-4448 | In-frame AH-405ΔpomAB | This work |
| AH-4449 | AH-405ΔpomAB, pomB2::miniTn5Km-1, Kmr | This work |
| AH-4450 | AH-405ΔpomAB, ΔlafTU::Kmr | This work |
| AH-4452 | AH-405ΔpomAB, pomB2::Cmr | This work |
| AH-4461 | AH-405, motX::Kmr | 13 |
| AH-4470 | AH-405, pomB2::Cmr | This work |
| AH-4471 | AH-405ΔpomA2B2 | This work |
| AH-4472 | AH-405, pomB::Kmr, pomB2::Cmr | This work |
| AH-4473 | AH-405ΔpomAB, ΔpomA2B2 | This work |
| AH-5503 | AH-405, lafK::Kmr | 14 |
| AH-5510 | AH-405ΔlafTU::Kmr | This work |
| AH-5511 | AH-405, lafK::Kmr, ΔpomAB | This work |
| AH-5512 | AH-405, lafK::Kmr, Δ pomA2B2 | This work |
| E. coli | ||
| DH5α | F−endA hdsR17(rK− mK+) supE44 thi-1 recA1 gyr-A96 φ80lacZ | 22 |
| XL1-Blue | endA1 recA1 hsdR17 supE44 thi-1 gyrA96 relA1 lac (F′ proAB lacIZΔM15 Tn10) | Stratagene |
| S17-1(λpir) | thi thr1 leu tonA lacY supE recA::RP4-2 (Tc::Mu) Kmr λpir with miniTn5Km1 | 16 |
| MC1061(λpir) | thi thr1 leu6 proA2 his4 argE2 lacY1 galK2 ara14 xyl5 supE44 λpir | 41 |
| Plasmids | ||
| pGEMT | Cloning vector, Apr | Promega |
| pBCSK | Cloning vector with lacZ gene, Cmr | Stratagene |
| pRK2073 | Helper plasmid, Spr | 41 |
| pCM100 | pGP704 suicide plasmid, pir dependent, Cmr | 50 |
| pUC4-KIXX | Plasmid carrying 1.6-kb aph cassette, Apr, Kmr | Pharmacia |
| pDM4 | Suicide plasmid, pir dependent with sacAB genes, oriR6K, Cmr | 37 |
| pACYC184 | Plasmid vector, Cmr, Tcr | 42 |
| pACYC-POMAB | pACYC184 with pomAB genes, Tcr | This work |
| pACYC-POMA2B2 | pACYC184 with pomA2B2 genes, Tcr | This work |
| pCM-POMB2 | pCM100 with a internal fragment of AH-3 pomB2 gene, Cmr | This work |
| pDM-LAFTU | pDM4 with AH-3 lafTU::Km, Cmr, Kmr | This work |
| pDM-POMA2B2 | pDM4 with AH-3Δ pomA2B2, Cmr | This work |
| pDM-POMAB | pDM4 with AH-3ΔpomAB, Cmr | This work |
Tcr, tetracycline resistant; Kmr, kanamycin resistant; Apr, ampicillin resistant; Rifr, rifampin resistant; Cmr, chloramphenicol resistant; Spr, spectinomycin resistant.
Motility assays (swarming and swimming).
Freshly grown bacterial colonies were transferred with a sterile toothpick into the center of swarm agar (1% tryptone, 0.5% NaCl, 0.5% agar) or swim agar (1% tryptone, 0.5% NaCl, 0.25% agar). The plates were incubated face up for 24 to 48 h at 25°C, and motility was assessed by examining the migration of bacteria through the agar from the center toward the periphery of the plate. Moreover, swimming motility was assessed by light microscopy observations in liquid media. When required, the specific inhibitor for the sodium-driven flagellar motors, amiloride (Sigma), was added at 0.5 to 2 mM to the motility assay media, or motility assay media with different sodium chloride concentrations (0 to 100 mM) were used.
TEM.
For transmission electron microscopy (TEM), bacterial suspensions were placed on Formvar-coated grids and negatively stained with a 2% solution of uranyl acetate, pH 4.1. Preparations were observed on a Hitachi 600 transmission electron microscope.
MiniTn5Km-1 mutagenesis.
The conjugal transfer of transposition element miniTn5Km-1 from E. coli S17-1λpirKm-1 (16) to A. hydrophila AH-4448 (AH405ΔpomAB) was carried out in a conjugal drop incubated for 6 h at 30°C with the ratio 1:5:1, corresponding to E. coli S17-1λpirKm-1, A. hydrophila AH-4448, and A. hydrophila HB101/pRK2073 (helper plasmid), respectively. Serial dilutions of the mating mix were plated on TSA supplemented with rifampin and kanamycin in order to select mutants.
DNA techniques.
DNA manipulations were carried out essentially as previously described (42). DNA restriction endonucleases, T4 DNA ligase, E. coli DNA polymerase Klenow fragment, and alkaline phosphatase were used as recommended by the suppliers. PCR was performed using Taq DNA polymerase (Invitrogen) in a gene amplifier PCR system 2400 thermal cycler (Perkin Elmer). Colony hybridizations were carried out by colony transfer onto positive nylon membranes (Roche) and then lysed according to the manufacturer's instructions. Probe labeling with digoxigenin, hybridization, and detection (GE Healthcare) were carried out as recommended by the suppliers.
RT-PCR.
For reverse transcription-PCR (RT-PCR), total RNA was isolated from A. hydrophila AH-3 grown at 25°C in liquid media (TSB) by RNA Protect bacterial reagent (Qiagen) and an RNeasy mini kit (Qiagen). To ensure that RNA was devoid of contaminating DNA, the preparation was treated with RNase-free TurboDNase I (Ambion). First-strand cDNA synthesis was carried out using the Thermoscript RT-PCR system (Invitrogen) and random primers on 3 μg of total DNase-digested RNA according to the manufacturer's instructions. PCR without reverse transcriptase was performed to confirm the absence of contaminating DNA in the RNA sample. Semiquantitative PCR is an image estimation of the size of the amplified bands before they reach the plateau. For semiquantitative PCR, second-strand synthesis and subsequent DNA amplification were carried out using Accuprime TaqDNA polymerase (Invitrogene) and one pair of oligonucleotides, 5′-ATCCAGGCCATGTTCCATC-3′ and 5′-CAACCGCCGTTCAACCTG-3′, to amplify the pomA DNA fragment of 227 bp, and another pair of oligonucleotides, 5′-CGCGGATCCGTATGATAA-3′ and 5′-CAAGAGCGAAGACAAGCTG-3′, to amplify the pomB2 DNA fragment of 170 bp; the oligonucleotides were designed using the Prime program from the Genetics Computer Group package (Madison, WI). To analyze the amount of cDNA template, 15-μl aliquots were removed for each PCR sample every five cycles, starting at cycle 15 and ending at cycle 35. Amplicons at each time point were analyzed by agarose gel electrophoresis with ethidium bromide staining. A. hydrophila ribosomal 16S primers were used as a control for the amount of cDNA template.
Cloning of DNA flanking miniTn5Km-1 insertions.
Chromosomal DNA of miniTn5Km-1 mutants was digested with EcoRI, PstI, and EcoRV, purified, ligated into the vector pBCSK (Stratagene), and introduced into E. coli XL1-Blue. Recombinant plasmids containing the transposon with flanking insertions were selected on LB plates supplemented with kanamycin and chloramphenicol. The miniTn5Km-1 flanking sequences were obtained by using specific primers to the I and O end of miniTn5Km-1 (5′-AGATCTGATCAAGAGACAG-3′ and 5′-ACTTGTGTATAAGAGTCAG-3′, respectively) as well as M13for and T3 primers.
Nucleotide sequencing and computer sequence analysis.
Plasmid DNA for sequencing was isolated by a Qiagen plasmid purification kit (Qiagen, Inc., Ltd.) as recommended by the supplier. In some cases, inverse PCR was used to amplify a chromosomal DNA fragment for sequencing, as described previously (14). Double-stranded DNA sequencing was performed by using the Sanger dideoxy chain termination method (43) with an ABI Prism dye terminator cycle-sequencing kit (Perkin-Elmer). Custom-designed primers used for DNA sequencing were purchased from Isogen Life Sciences.
The DNA sequence was translated in all six frames, and their deduced amino acid sequences were inspected in the GenBank, EMBL, and SwissProt databases by using the BLASTX, BLASTP, or PSI-BLAST network service at the NCBI (2). The protein family profiling was performed using the protein family database Pfam at the Sanger Center (7). The determination of possible terminator sequences was done by using the Terminator program from the Genetics Computer Group package (Madison, WI). Other online sequence analysis services also were used.
Construction of defined mutants.
The chromosomal in-frame pomAB strain and pomA2B2 deletion mutant, A. hydrophila AH-4448 and AH-4471, respectively, were constructed by allelic exchange as described by Milton et al. (37). Briefly, DNA regions upstream (fragment AB) and downstream (fragment CD) of pomAB were amplified using two sets of asymmetric PCRs. DNA fragment AB contains 750 bp upstream of pomA and the first eight codons of pomA. DNA fragment CD contains the first base in codon 276 of pomB to 577 bp downstream of pomB. DNA fragments AB and CD were annealed at the overlapping regions provided by the primers B and C and amplified as a single fragment using primers A and D (Table 2). For the pomA2B2 mutant construction, a 2,318-bp DNA fragment containing pomA2B2 was amplified by PCR using the primer pair POMA2-F and POMB2-R (Table 2). The amplified fragment was digested with SacI to make a pomA2B2 deletion. Two DNA fragments containing 682 bp upstream of pomA2 and 601 bp downstream of pomB2, respectively, were recovered and ligated. The fusion product was amplified as a single fragment of 1,283 bp using POMA2-F and POMB2-R primers. The pomAB and pomA2B2 fusion products were purified and sequenced, BamHI digested (the BamHI site is present in primers), ligated into BglII-digested and phosphatase-treated pDM4 vector (37), electroporated into E. coli MC1061, and plated on chloramphenicol plates at 30°C to obtain pDM-POMAB and pDM-POMA2B2 plasmids, respectively. The introduction of the plasmids into rifampin-resistant (Rifr) A. hydrophila AH-405 was performed as previously described (13). Transconjugants were selected on plates containing chloramphenicol and rifampin. PCR analysis confirmed that the vector had integrated correctly into the chromosomal DNA. After sucrose treatment, transformants that were rifampin resistant (Rifr) and chloramphenicol sensitive (Cms) were chosen and confirmed by PCR.
TABLE 2.
Primers used in the construction of chromosomal in-frame pomAB and pomA2B22 deletion mutants
| Primer | Sequencea | Amplified fragment |
|---|---|---|
| pomAB mutant | ||
| A primer | 5′-CGCGGATCCACAGCGGGTCAAGGAAATA-3′ | AB fragment |
| B primer | 5′-CCCATCCACTAAAC TTAAACACAGGATCAGGCCAAACAT-3′ | |
| C primer | 5′-TGTTTAAGTTTAGTGGATGGGGTGCAACAGGCGGTAGAG-3′ | CD fragment |
| D primer | 5′-CGCGGATCCACGCTTGT CAAACATGGTG-3′ | |
| pomA2B22 mutant | ||
| POMA2-F | 5′-CGCGGATCCATGGTTTCCAGCTCTTCCA-3′ | pomA2B22 fragment |
| POMB2-R | 5′-CGCGGATCCTGGTGACATCGATCACCTG-3′ |
Underlined letters show overlapping regions; double-underlined letters show BamHI restriction site.
The defined insertion pomB2 mutants were obtained using a method based on suicide plasmid pCM100 (50). Briefly, an internal fragment of the selected gene was amplified by PCR, ligated into pGEM-Teasy (Promega), and transformed into E. coli XL1-Blue. The DNA insert was sequenced, recovered by EcoRI restriction digestion, blunt ended, and ligated into EcoRV-digested and phosphatase-treated pCM100. Recombinant pCM-POMB2 plasmid was transformed into E. coli MC1061(λpir) and selected for chloramphenicol resistance (Cmr). Triparental mating with the mobilizing strain HB101/pRK2073 was used to transfer the recombinant plasmid into the A. hydrophila AH-405 rifampin-resistant strain to obtain the defined insertion mutant AH-4470, selecting for Rifr and Cmr. The correct construction was verified by Southern blot hybridization.
The defined A. hydrophila lafTU mutant AH-5510 was constructed by the PCR amplification of lafTU internal gene fragments. The PCR product was sequenced and ligated into the vector pGEMTeasy (Promega) and transformed into E. coli XL1-Blue. A recombinant plasmid containing lafTU genes was EcoRV digested to make a lafTU deletion, and fragment deletion was performed instead of using the SmaI-digested kanamycin-resistant cassette from pUC4-KIXX. The inserted cassette contains an outward-reading promoter that ensures the expression of downstream genes when inserted in the correct orientation (12); however, such an insertion will alter the regulation of such genes. The presence of a single HindIII restriction site in the SmaI-digested cassette allowed its orientation to be determined. The DNA fragments containing lafTU with the kanamycin-resistant cassette were recovered and ligated into suicide vector pDM4 (37) to construct the pDM-LAFTU plasmid. The recombinant plasmid was electroporated into E. coli MC1061(λpir) and plated on chloramphenicol and kanamycin plates at 30°C. The plasmids with mutated genes were transferred into rifampin-resistant A. hydrophila AH-405 by triparental mating using E. coli MC1061(λpir) (containing the insertion construct) and the mobilizing strain HB101/pRK2073. Transconjugants were selected on plates containing chloramphenicol, kanamycin, and rifampin. PCR analysis confirmed that the vector had integrated correctly into the chromosomal DNA. To complete the allelic exchange, the integrated suicide plasmid was forced to recombine out of the chromosome by adding 5% sucrose to the agar plates. The pDM4 vector contains sacB, which produces an enzyme that converts sucrose into a product that is toxic to gram-negative bacteria. Transconjugants surviving on plates with 5% sucrose that were rifampin resistant, kanamycin resistant, and chloramphenicol sensitive were chosen and confirmed by PCR. The A. hydrophila pomAB-lafTU double mutant AH-4450 was obtained by the introduction of the suicide plasmid pDM-LAFTU into the A. hydrophila pomAB mutant AH-4448 using the method previously described.
Plasmid construction for complementation studies.
Plasmid pACYC-POMAB, containing the complete pomAB genes, and the plasmid pACYC-POMA2B2, containing pomA2B2 genes from A. hydrophila AH-3, were obtained by the PCR amplification of genomic DNA using oligonucleotides 5′-GCATCGCCACTGAGTCAC-3′ and 5′-ATACCGGCTAACGAGACCA-3′, to generate a band of 1,800 bp, and 5′-TGGCCGATAATAAGCCATC-3′ and 5′-AGCTCTTGACGCAGCTTTT-3′, to generate a band of 1,976 bp. The amplified band was sequenced, ligated into pGEM-Teasy (Promega), and transformed into E. coli XL1-Blue. The DNA insert was recovered by EcoRI restriction digestion, ligated into EcoRI phosphatase-treated pACYC184 vector (42), and introduced into E. coli DH5α. Both inserted fragment orientations were determined by restriction analysis and sequencing.
Nucleotide sequence accession number.
The nucleotide sequences reported in this paper have been submitted to the GenBank/EMBL database under accession number FJ409648.
RESULTS
LafTU and PomAB function in A. hydrophila AH-3 polar flagellum motility.
Previous work demonstrated that the A. hydrophila pomB defined insertion mutant, AH-4444, is able to swim and swarm as well as wild-type A. hydrophila AH-3. In contrast, the A. hydrophila motX mutant, AH-4461, is unable to swim but still is able to swarm (13). In this work, we constructed an in-frame pomAB mutant, AH-4448, and analyzed its ability to swim and swarm. As previously described for AH-4444 (pomB), the A. hydrophila AH-4448 (pomAB) mutant was able to swarm and swim as well as the wild-type strain (Table 3). In order to explore whether the lateral flagellum stator proteins LafTU were involved in polar flagellum motility, we constructed A. hydrophila AH-5510, with a defined mutant in the lafTU genes, and analyzed its abilities to swim and swarm. The AH-5510 (lafTU) mutant strain showed an 80% decrease in radial expansion on swarm agar plates, which is similar to that observed for A. hydrophila AH-3 structural lateral flagellum mutants previously (14). The swimming motility of the AH-5510 (lafTU) mutant was identical to that of wild-type A. hydrophila AH-3 (Table 3), and TEM showed both lateral and polar flagella (data not shown).
TABLE 3.
Motility phenotypes of A. hydrophila AH-3 in-frame and defined insertion mutants and in trans-complemented mutants
| Strain and type of mutant | Gene defect interval or plasmid used | Motility
|
Flagellation typec
|
||
|---|---|---|---|---|---|
| Swarminga | Swimmingb | Lateral | Polar | ||
| In frame and defined insertion | |||||
| AH-3 | Wild type | 3.7 ± 0.2 | + | + | + |
| AH-5503 | lafK | 0.6 ± 0.1 | + | − | + |
| AH-5510 | lafTU | 0.6 ± 0.1 | + | + | + |
| AH-4444 | pomB | 3.4 ± 0.1 | + | + | + |
| AH-4448 | pomAB | 3.5 ± 0.2 | + | + | + |
| AH-4470 | pomB2 | 3.4 ± 0.3 | + | + | + |
| AH-4471 | pomA2B22 | 3.4 ± 0.1 | + | + | + |
| AH-4472 | pomB-B2 | 1.1 ± 0.1 | − | + | + |
| AH-4473 | pomAB-A2B22 | 1.2 ± 0.2 | − | + | + |
| AH-4452 | pomAB-B2 | 1.1 ± 0.1 | − | + | + |
| AH-4449 | |||||
| AH-4450 | lafTU-pomAB | 0.5 ± 0.1 | + | + | + |
| AH-5511 | lafK-pomAB | 0.6 ± 0.1 | + | − | + |
| AH-5512 | lafK- pomA2B22 | 0.5 ± 0.1 | + | − | + |
| In trans complemented | |||||
| AH-4472 | pACYC-POMAB | 3.3 ± 0.2 | + | + | + |
| pACYC-POMA2B2 | 3.4 ± 0.2 | ||||
| AH-4473 | pACYC-POMAB | 3.2 ± 0.1 | + | + | + |
| pACYC- POMA2B2 | 3.4 ± 0.2 | ||||
| AH-4452 | pACYC-POMAB | 3.2 ± 0.2 | + | + | + |
| pACYC- POMA2B2 | 3.1 ± 0.1 | ||||
Migration (in centimeters) of bacteria through the swarm agar plates from the center toward the periphery of the plate.
−, no motility in liquid media; +, motility in liquid media.
−, absence of flagella; +, flagella equivalent to those of the wild type.
Since neither lafTU nor pomAB mutations affect A. hydrophila AH-3 swimming motility, we constructed an A. hydrophila strain mutant in both lafTU and pomAB genes (AH-4450). Mutant strain AH-4450 showed reduced swarming motility, similar to that of the AH-5510 (lafTU) mutant; however, its swimming motility was not affected, being similar to that observed for the A. hydrophila AH-4448 (pomAB) single mutant and AH-3 wild-type strain (Table 3). These results suggested that lafTU-encoded proteins are involved only in lateral flagellum motility and do not participate in polar flagellum rotation.
Identification of a new locus of polar flagellum motor in A. hydrophila AH-3.
We performed a miniTn5Km-1 mutagenesis using the A. hydrophila AH-4448 mutant (AH-405ΔpomAB) as a recipient strain to find a second polar stator locus of A. hydrophila AH-3 that is involved in polar flagellum motility. Transconjugants were screened for greatly reduced or null swimming motility in swim agar and the inability to move in liquid media, as determined by light microscopy. Fifteen transposon insertion mutants unable to swim were analyzed by TEM after growth in liquid media. Only the A. hydrophila AH-4449 transposon insertion mutant was unable to swim (Table 3), but it did produce polar flagella. As no EcoRV restriction sites were present in the transposon, the altered-motility mutant was analyzed for the presence of the transposon by the Southern hybridization of EcoRV-digested chromosomal DNA. A single band was detected in the mutant, indicating that the mutant had a single copy of the minitransposon in its genome.
The DNA flanking the transposon in the A. hydrophila AH-4449 insertion mutant was cloned into pBCSK as described in Materials and Methods. The nucleotide sequencing of the cloned fragment revealed an open reading frame (ORF) whose predicted amino acid sequence shared homology with different sodium-driven flagellum motor proteins, such as the V. parahaemolyticus PomB-homologous protein, MotB, and PomB of V. alginolyticus, V. cholerae, and Shewanella oneidensis. Since a PomB protein already was described as a polar flagellum motor protein in A. hydrophila AH-3 polar flagellum region 3 (13), we named this new flagellum motor protein PomB2.
Organization of A. hydrophila AH-3 polar flagellum region 6 (pomA2B2 loci).
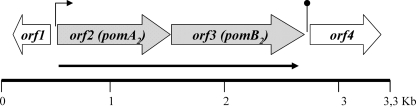
To amplify and sequence the A. hydrophila AH-3 genomic region that contains the pomB2 gene, progressive inverse PCR with specific oligonucleotides was performed as described in Materials and Methods. The sequence analysis of 3,399 bp obtained from the amplified fragments revealed four complete ORFs. ORF2 to ORF4 are transcribed in the same direction, and ORF1 is transcribed in the opposite direction (Fig. 1). The start codon of ORF2 was located 221 bp upstream of ORF1, the ORF3 start was 5 bp downstream of ORF2, and ORF4 was separated from ORF3 by 145 bp. Sequence analysis in silico showed putative ribosome-binding sites upstream of each of the ORF start codons, a putative σ28 promoter sequence (TAAA-N14-GCCGATAA) upstream of the ORF2 start codon, and a transcriptional terminator rho-independent sequence downstream of ORF3 (Fig. 1).
FIG. 1.
Genetic organization of A. hydrophila AH-3 polar flagellum region 6. ORFs and their transcriptional directions are indicated by block arrows. The black arrow indicates the transcriptional unit. Small horizontal arrows indicate the location of putative promoter sequences. Lollipop structures depict the approximate position of the putative transcriptional rho-independent terminators.
The characteristics of the individual proteins and their protein homologies were analyzed using the BLASTP program (2) of the NCBI database and are shown in Table 4. ORF1 was homologous to the exonuclease VII small subunit of different bacteria, such as Vibrio spp. and Shewanella spp. (50 to 64% identity). ORF2 and ORF3 are related to polar flagellum stator proteins that are involved in the formation of a sodium-conducting channel to generate rotational motion in the sodium-type flagellum motor (25). The ORF2-derived amino acid sequence was homologous to the flagellum motor protein ASA-0993 of A. salmonicida and protein AHA-3318 of A. hydrophila ATCC7966T (96 to 94% identity, respectively), as well as to the sodium-driven flagellum motor proteins MotA (a PomA homologue) of V. parahaemolyticus and PomA of Vibrio and Shewanella species (62 to 63% identity). ORF3 was homologous to the flagellum motor protein AHA-3317 of A. hydrophila ATCC7966 (94% identity), MotB of V. parahaemolyticus (a PomB homologue), and PomB of different Vibrio and Shewanella species (60 to 62% identity). The ORF4-derived amino acid sequence was homologous to the transcription elongation factor GreA from different bacteria, such as Salmonella spp. and Yersinia spp.
TABLE 4.
Characteristics of the A. hydrophila AH-3 polar flagella region 6
| ORF no. | Nucleotide position | Protein size (bp) | Molecular weight | pI | Predicted function | Homologous gene | %Identity/ %similarity |
|---|---|---|---|---|---|---|---|
| 1 | 684-433 | 83 | 9.3 | 4.5 | Exonuclease VII | SamaDRAFT_0389 of Shewanella amazonensis | 64/83 |
| xseB of Vibrio fischeri | 59/78 | ||||||
| xseB of Haemophilus influenzae | 57/72 | ||||||
| 2 | 905-1663 | 252 | 27.0 | 5.1 | Flagella motor protein | AHA-3318 of A. hydrophila ATCC7966T | 94/98 |
| pomA of Vibrio cholerae | 66/81 | ||||||
| motA of Vibrio parahaemolyticus | 64/80 | ||||||
| 3 | 1669-2562 | 297 | 32.5 | 5.0 | Flagella motor protein | AHA-3317 of A. hydrophila ATCC7966T | 94/96 |
| motB of Vibrio parahaemolyticus | 59/74 | ||||||
| pomB of Vibrio cholerae | 57/72 | ||||||
| 4 | 2707-3183 | 158 | 17.5 | 4.7 | Transcription elongation factor | greA of Salmonella enterica serovar Typhi | 77/87 |
| greA of Yersinia pestis | 75/85 |
Since A. hydrophila AH-3 PomA and PomB already have been described as two polar flagellum motor proteins encoded by genes located in the A. hydrophila AH-3 polar flagellum region 3 (13), we named these newly discovered polar flagellum stator proteins PomA2 and PomB2, respectively, and designated these polar flagellar loci region 6.
Distribution of the pomA2B2 and pomAB genes in mesophilic Aeromonas strains.
The distribution of pomA2B2 and pomAB genes was analyzed in mesophilic Aeromonas strains (n = 50) by dot blot hybridization experiments against total genomic DNA using independent PCR probes. The distribution of these two sets of polar stator genes were performed using two PCR probes for pomAB and pomA2B2. These two probes hybridized to the chromosomal DNA of all mesophilic Aeromonas strains tested, whether the strains were able to produce lateral flagella or not.
Analysis of A. hydrophila pomA2B2 loci defined mutants and complementation studies.
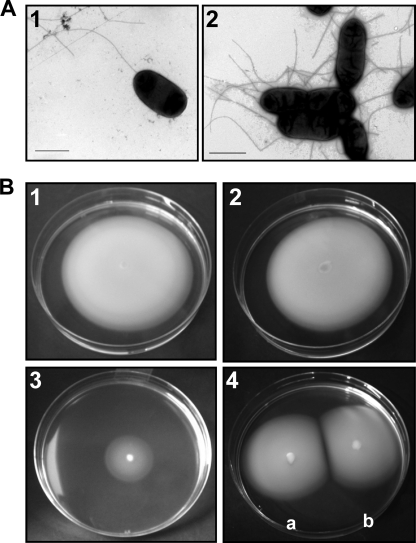
We constructed A. hydrophila AH-3 defined mutants in pomB2 (AH-4470) and pomA2B2 (AH-4471), and we analyzed their ability to form polar and lateral flagella as well as their motility phenotype. TEM analyses showed that AH-4470 (pomB2) and AH-4471 (pomA2B2) mutants are able to form both flagellum types (polar and lateral) (Fig. 2A). Motility assays showed that both mutants are able to swim in liquid media (examined by light microscopy) and swarm on swarming motility agar as well as wild-type A. hydrophila AH-3 (Table 3, Fig. 2B). These results are identical to the ones obtained for the A. hydrophila pomB (AH-4444) and pomAB (AH-4448) mutants.
FIG. 2.
(A) TEM of an A. hydrophila AH-3 defined mutant in pomB2 (AH-4470) grown at 25°C on liquid medium (1) and grown at 25°C on swarm agar (2). Bacteria were gently placed onto Fonvard-coated copper grids and negatively stained using 2% uranyl acetate. Bar, 1 μm. (B) Swarming motility observed for A. hydrophila AH-3 (1), pomB2 mutant AH-4470 (2), pomB-pomB2 double mutant AH-4472 (3), and double mutant AH-4472 complemented with plasmid pACYC-POMAB (4a) or pACYC-POMA2B2 (4b) independently. The pomA2B2 double mutant AH-4471 shows the same TEM result and swarming phenotype as pomB2 mutant AH-4470. The double mutants AH-4473 (pomAB-A2B2) and AH-4452 (pomAB-B2) show the same swarming phenotype as the AH-4472 mutant (pomB-B2).
Since pomAB or pomA2B2 disruptions do not abolish A. hydrophila AH-3 polar flagellum motility, we constructed mutants in both loci and analyzed their swimming and swarming phenotype. In order to disrupt the pomB2 gene in either the pomAB or pomB mutant background (AH-4448 and AH-4444, respectively), we introduced the suicide plasmid pCM-POMB2 into each one of these mutants. We also disrupted the pomA2B2 genes in the pomAB mutant strain (AH-4448) background by the introduction of the suicide plasmid pDM-POMA2B2 into this mutant strain. These three double mutants were constructed as described in Materials and Methods. Motility assays showed that AH-4473 (pomAB-A2B2), AH-4452 (pomAB-B2), and AH-4472 (pomB-B2) mutants were unable to swim (examined by light microscopy) and showed a decreased radial expansion (74% reduction) in swarm agar similar to that of the AH-4449 (pomAB-B2) transposon insertion mutant rather than to that of wild-type A. hydrophila AH-3 (Table 3, Fig. 2B), since polar flagella contribute to the swarming motility.
Complementation studies were undertaken to determine if wild-type polar flagellum motility could be restored to the mutants by providing pomAB or pomA2B2 genes in trans. Plasmids pACYC-POMAB and pACYC-POMA2B2, containing the A. hydrophila AH-3 pomAB and pomA2B2 genes, respectively, were introduced independently by triparental mating into the AH-4473 (pomAB-A2B2), AH-4452 (pomAB-B2), and AH-4472 (pomB-B2) mutant strains. These three mutants were able to swim and swarm as well as the A. hydrophila AH-3 wild type when one of the recombinant plasmids was introduced into them (Fig. 2B).
Transcription of pomAB and pomA2B2.
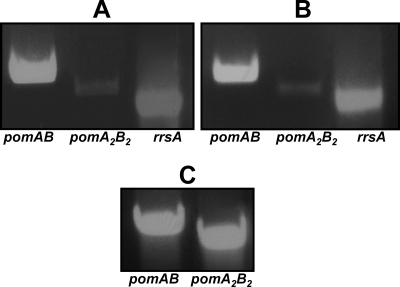
Previous studies demonstrated that A. hydrophila AH-3 pomAB, in polar flagellum region 3, constitute an independent transcriptional unit (13). To assure the cotranscription of genes in polar flagellum region 6, primer pairs that overlapped pomA2B2 and pomB2-orf4 were designed near the 3′ end of the upstream gene and near the 5′ end of the downstream gene. An RT-PCR product of the expected size was detected only for pomA2B2 amplification (Fig. 1). To analyze the level of transcription of A. hydrophila AH-3 pomAB and pomA2B2 in liquid and solid media at 25°C, semiquantitative RT-PCR assays were carried out as described in Materials and Methods. These assays show significantly higher levels of pomAB transcription than those of pomA2B2 genes in both media (Fig. 3), suggesting that pomAB is the prevalent polar flagellum stator.
FIG. 3.
RT-PCR cDNA fragments obtained from the pomAB (pomA internal fragment), pomA2B2 (pomB2 internal fragment), and rrsA (ribosomal 16S internal fragment) genes of A. hydrophila AH-3 total RNA isolated when the strain was grown at 25°C in liquid (A) or solid medium (B). (C) PCR was performed with the pomAB and pomA2B2 genes using 100 μg of A. hydrophila AH-3 genomic DNA as the control primer.
A. hydrophila polar flagellum energy source.
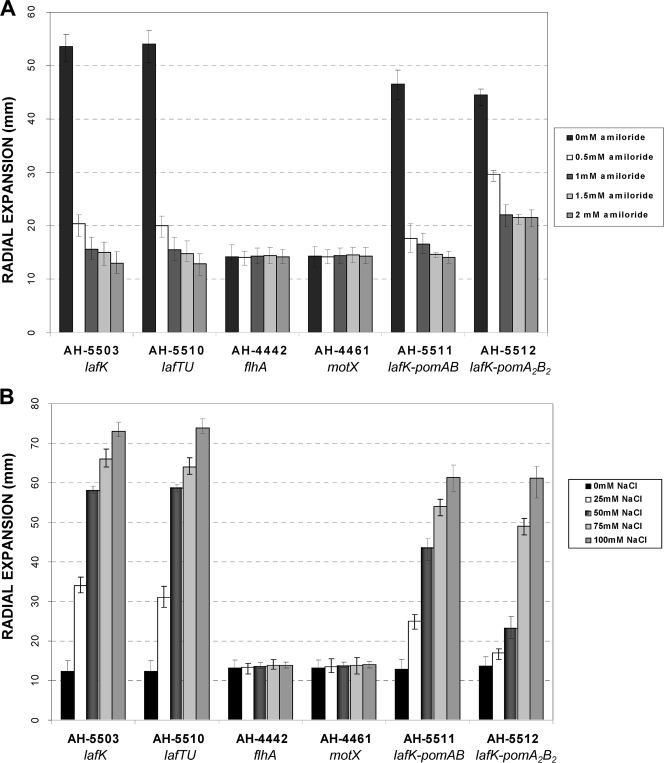
The structural lateral flagellum AH-5503 mutant (lafK), which was previously described (14), as well as the lateral flagellar stator motor mutant AH-5510 (lafTU) were used to determine if the A. hydrophila polar flagellum stator motor employed sodium ions as an energy source to rotate. We measured the radial expansion of these mutants after growth at 25°C in swim agar plates containing 0 to 2 mM amiloride dissolved in dimethyl sulfoxide, a potent inhibitor of Na+ channels in different organisms (6), and plates containing 0 to 100 mM NaCl. In both mutants, the radial expansion decreased 63, 71, 73, and 76% in swim agar plates with 0.5, 1, 1.5, and 2 mM of amiloride, respectively (Fig. 4A). Both mutants showed a radial expansion of around 2.7, 4.7, 5.3, and 5.9 times higher in swim agar plates with 25, 50, 75, and 100 mM NaCl, respectively, than on the same kind of plates without NaCl (Fig. 4B). Swimming motility was completely inhibited in liquid media with 2 mM amiloride, and the amount of motile fraction increased when the NaCl concentration increased (Table 5). The motile fraction does not increase with different KCl concentrations (data not show). Furthermore, we measured the radial expansion of the structural polar flagellum mutant AH-4442 (flhA) as well as the polar stator A. hydrophila motX mutant (AH-4461), which was previously described (13), in swim and swarm agar plates containing 0 to 2 mM amiloride and plates containing 0 to 100 mM NaCl. These two mutants, which possess only functional lateral flagella, showed no difference in their radial expansion in the presence of amiloride or different concentrations of sodium ions (Fig. 4). These results suggest that only the polar flagellum motor is inhibited by the sodium channel inhibitor amiloride, and its rotation is sodium ion dependent.
FIG. 4.
Radial expansion at 25°C in swim agar plates containing no amiloride or 0.5, 1, 1.5, and 2 mM of amiloride (A) and containing no NaCl or 25, 50, 75, and 100 mM of NaCl (B) of A. hydrophila AH-3 structural flagellum mutants AH-5503 and AH-4442 (lateral and polar mutants, respectively), lateral stator mutant AH-5510 (lafTU), polar stator mutant AH-4461 (motX), and double mutants AH-5511 (lafK-pomAB) and AH-5512 (lafK-pomA2B2). Motility was determined by measuring the diameter (in millimeters) of the zone of expansion from the point of inoculation after 48 h of growth. The standard deviations were determined from five different experiments.
TABLE 5.
Effect of amiloride and NaCl in liquid media
| Substance and concn. (mM) | Motile fraction (motile cells/total no. of cells) of mutant:
|
|||
|---|---|---|---|---|
| AH-5503 (lafK) | AH-5510 (lafTU) | AH-5511 (lafK-pomAB) | AH-5512 (lafK-pomA2B22) | |
| Amiloride | ||||
| 0 | 0.66 | 0.65 | 0.67 | 0.64 |
| 0.5 | 0.58 | 0.56 | 0.58 | 0.6 |
| 1 | 0.42 | 0.44 | 0.39 | 0.47 |
| 1.5 | 0.29 | 0.30 | 0.28 | 0.35 |
| 2 | 0.03 | 0.05 | 0.03 | 0.05 |
| NaCl | ||||
| 0 | 0.06 | 0.03 | 0.05 | 0.08 |
| 25 | 0.42 | 0.46 | 0.44 | 0.35 |
| 50 | 0.65 | 0.67 | 0.62 | 0.51 |
| 75 | 0.69 | 0.70 | 0.68 | 0.61 |
| 100 | 0.78 | 0.77 | 0.76 | 0.75 |
aThe motile fraction was determined by counting approximately the number of motile cells out of the total number of cells according to Fukuoka et al. (19).
Since both flagellum types contribute to motility in viscous media and in order to independently analyze the pomAB and pomA2B2 energy source, we constructed double mutants that were mutated in lateral flagellum system genes (lafK) and the polar flagellum stator motor genes pomAB and pomA2B2, as described above. Double mutant strains AH-5511 (lafK-pomAB) and AH-5512 (lafK-pomA2B2) were confirmed by PCR. Motility assays of the A. hydrophila AH-5511 (lafK-pomAB) mutant showed 63, 65, 69, and 70% radial expansion reduction, and the AH-5512 (lafK-pomA2B2) mutant showed 34, 51, 52, and 52% radial expansion reduction in swimming agar containing 0.5, 1, 1.5, and 2 mM amiloride, respectively (Fig. 4A). Both mutants also showed an increase in radial expansion corresponding to an increase in sodium ion concentrations. The radial expansion of mutant AH-5511 (lafK-pomAB) was 1.9, 3.4, 4.2, and 4.8 times higher and that of mutant AH-5512 (lafK-pomA2B2) was 1.2, 1.7, 3.6, and 4.5 times higher in swim agar with 25, 50, 75, and 100 mM NaCl, respectively, than those for the mutants in swim agar without NaCl (Fig. 4B). Furthermore, we analyzed the ability of these two mutants (AH-5511 and AH-5512) as well as the lateral flagellum mutant AH-5503 to move in swarm agar plates, and all of them showed around 0.5 to 0.6 cm radial expansion (Table 3).
DISCUSSION
Mesophilic Aeromonas species have a constitutive single polar flagellum that propels the bacterium in aqueous environments and contributes to adhesion and biofilm formation (13, 27). Recently, five A. hydrophila AH-3 chromosomal regions involved in the formation and function of the polar flagellum have been described; two of them (regions 3 and 4) contain genes whose encoded proteins show high homology to different polar flagellum stator motor proteins (13). The A. hydrophila polar flagellar region 3 contains the pomA and pomB genes, which encode proteins that are homologous to Pseudomonas MotA and MotB, respectively (15), and region 4 contains motX, which encodes a protein homologous to the sodium-driven motor MotX of V. parahaemolyticus (33). MotX is involved in A. hydrophila polar flagellum rotation, because its mutation abolishes polar flagellum motility. Nevertheless, a PomB (13) or PomAB mutation does not abolish polar flagellum motility in liquid media, which is similar to the result observed in Pseudomonas aeruginosa MotAB mutants (18, 47) and in contrast to those for V. cholerae, V. alginolyticus, and V. parahaemolyticus PomAB mutants (11, 21).
These results suggested two possibilities about A. hydrophila AH-3 polar flagellum motility: the lateral flagellum stator LafTU can supply PomAB function, and A. hydrophila AH-3 has a second polar stator that is involved in polar flagellum motility. To explore the first possibility, we constructed a defined A. hydrophila AH-3 mutant in lafTU and a double mutant in both lafTU and pomAB. Analyses of these mutants showed that both have lateral and polar flagella, are able to swim in liquid media, and show an 80% decrease in radial expansion in swarm agar plates compared to results for the wild type at 25°C (Table 3). The fact that this double mutant does not abolish polar flagellum motility suggests the second possibility.
To investigate this hypothesis, we performed transposon insertion mutagenesis using the A. hydrophila AH-405ΔpomAB mutant as the recipient strain and isolated the mutant AH-4449, which is unable to swim in liquid media but still is able to produce polar flagella. The mutant allowed us to find pomA2 and pomB2. In contrast to the previously described A. hydrophila AH-3 pomAB loci, pomA2B2 loci are located in a separate chromosomal region, as described for the V. parahaemolyticus sodium-type flagellum stator genes motAB (34). However, P. aeruginosa also has shown redundant polar flagellar stator genes, motAB and motCD (18), whose chromosomal distribution is similar to those of A. hydrophila pomAB and pomA2B2, respectively. Also, A. hydrophila ATCC7966T has pomA2B2 genes homologous to AHA-3318 and AH-3317 in a chromosomal location identical to that for A. hydrophila AH-3. Sequence alignments showed that A. hydrophila PomA2 and PomB2 display higher homology with sodium-conducting polar flagellum stator motors than do A. hydrophila AH-3 PomA and PomB (Fig. 5). Analyses of charged residues showed that E. coli MotA-charged residues R90 and E98, involved in torque generation, are conserved in A. hydrophila PomA2 and PomA, as well as in Vibrio spp. PomA (MotA) as residues R88 and E96. The C-terminal region of A. hydrophila PomA2 and of Vibrio spp. PomA show three important charged residues (K203, R215, and D220) that are essential for Vibrio sodium-dependent motor function (39) that are not present in either E. coli MotA or in A. hydrophila PomA. The D32 E. coli MotB residue and D24 Vibrio spp. PomB (MotB) residue, which play critical roles in ion flux and energy conversion, are conserved in A. hydrophila PomB2 (D20) and PomB (D24). Furthermore, the analysis of different mutants with single and multiple lesions in pomAB and/or pomA2B2 suggest that A. hydrophila PomAB and PomA2B2 are able to compensate for the loss of each other and are a redundant set of proteins that do not have differential motility roles, and the single loss of PomAB or PomA2B2 is not essential in polar flagellum motility in aqueous or in viscous environments. In contrast, in Pseudomonas aeruginosa either stator (MotAB or MotCD) is sufficient for swimming, but both are necessary for swarming motility (47). Semiquantitative RT-PCR assays of A. hydrophila pomAB and pomA2B2 after growth in liquid media and on solid plates showed a transcription level of pomAB genes at least 10 times higher than that of pomA2B2 genes in both media. These results suggest that although both sets of proteins are involved in polar flagellum motility, pomAB is prevalent in liquid and viscous media.
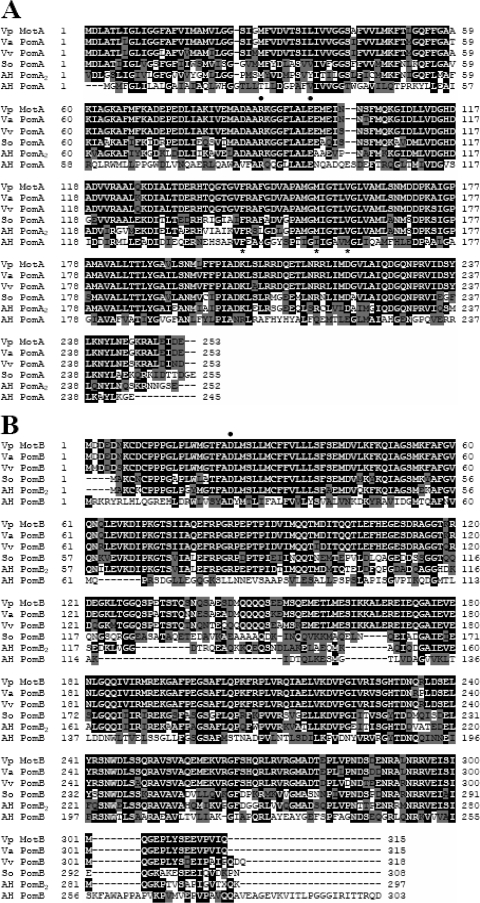
FIG. 5.
(A) Sequence alignment of a Vibrio parahaemolyticus PomA homologue (Vp MotA), Vibrio alginolyticus PomA (Va PomA), Vibrio vulnificus PomA (Vv PomA), Shewanella oneidensis PomA (So PomA), A. hydrophila AH-3 PomA2 (AH PomA2), and PomA (AH PomA). (B) Sequence alignment of a V. parahaemolyticus PomB homologue (Vp MotB), V. alginolyticus PomB (Va PomB), V. vulnificus PomB (Vv PomB), S. oneidensis PomB (So PomB), and A. hydrophila AH-3 PomB2 (AH PomB2) and PomB (AH PomB). The deduced amino acid sequences were aligned using Clustal W. White letters with a black background indicate identical amino acid residues, and black letters with a gray background indicate similar amino acid residues. The black circles over the residues show conserved charged residues in proton and sodium motors, and the asterisks show charged residues essential in Vibrio sodium-dependent motor function.
Swimming motility assays in the presence of different amiloride concentrations, as well as in different NaCl concentrations, indicated that only the A. hydrophila polar flagellum stator is sodium dependent, whereas the lateral flagellar stator is not. To analyze the pomAB and pomA2B2 energy source independently, we performed amiloride swimming inhibition assays with AH-5511 (lafK-pomAB) and AH-5512 (lafK-pomA2B2), as well as swimming assays with different NaCl concentrations. These assays showed that the radial expansion of the lateral flagellum mutant (lafK) without amiloride or with 25 to 100 mM NaCl is higher than the radial expansion of the lafK-pomAB or lafK-pomA2B2 polar flagellum stator mutant, suggesting that both polar flagellum stators contribute to motility in viscous media, as is the case with the swim agar plates. Furthermore, both polar flagellum stator mutants showed a reduction in radial expansion when grown in the presence of amiloride, as well as increased radial expansion when the sodium ion concentration was increased in the medium. However, the AH-5512 (lafK-pomA2B2) mutant responds to sodium at a higher concentration than the AH-5511 (lafK-pomAB) mutant. These results lead us to the conclusion that PomAB and PomA2B2 constitute redundant polar flagellum stators that are sodium ion dependent and are used for swimming motility only, but PomA2B2 is more sensitive to low-level sodium ion variations than PomAB, and PomAB seems to be specialized to function at higher sodium levels.
Both A. hydrophila PomB and PomB2 possess one transmembrane domain at their N termini and an OmpA domain at their C-terminal sequences that probably are involved in peptidoglycan interaction (17) and may be responsible for anchoring the force generator. A. hydrophila PomA and PomA2 possess four transmembrane domains and the flagellar motor protein MotA family signature A-[LMF]-x-[GAT]-T-[LIVMF]-x-G-x-[LIVMF]-x(7)-P. A similar situation is described for P. aeruginosa, because there are two sets of motAB-like genes, motAB and motCD, as well as another gene, motY, which contributes to proton-driven flagellum motility. The loss of either motAB-like gene still resulted in motile bacteria in aqueous environments, motCD disruption abolished polar flagellum motility in viscous environments (15% Ficoll), and only mutations of both sets of genes abrogated polar flagellum motility in aqueous solutions (47). In Pseudomonas, MotCD is essential for the polar flagellum proton motor rotation in viscous media, but in Aeromonas, for which polar flagellum stator motor is sodium dependent, neither PomAB nor PomA2B2 are essential for polar flagellum rotation in viscous media. Most aeromonads have an entirely distinct lateral flagellum system for motility in highly viscous media or over surfaces.
The A. hydrophila polar flagellum is the first case in which two redundant sodium-driven stator motor proteins (PomAB and PomA2B2) are found. The redundancy is based on the fact that neither set on its own is essential for polar flagellum motility in either aqueous or high-viscosity environments.
Acknowledgments
This work was supported by Plan Nacional de I + D and FIS grants (Ministerio de Educación, Ciencia y Deporte and Ministerio de Sanidad, Spain) and from Generalitat de Catalunya (Centre de Referència en Biotecnologia). M.W. received a predoctoral fellowship from Ministerio de Educación, Ciencia y Deporte. J.G.S. was supported by grants from the Wellcome Trust and the Bardhan Research and Education Trust.
We thank Maite Polo for her technical assistance.
Footnotes
Published ahead of print on 30 January 2009.
REFERENCES
- 1.Altarriba, A., S. Merino, R. Gavín, R. Canals, A. Rabaan, J. G. Shaw, and J. M. Tomas. 2003. A polar flagella operon (flg) of Aeromonas hydrophila contains genes required for lateral flagella expression. Microb. Pathog. 34249-259. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, F. S., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zang, W. Miller, and J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acid Res. 253389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asai, Y., S. Kojima, H. Kato, N. Nishioka, I. Kawagishi, and M. Homma. 1997. Putative channel component for the fast-rotating sodium-driven flagella motor of a marine bacterium. J. Bacteriol. 1795104-5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asai, Y., T. Yakushi, I. Kawagishi, and M. Homma. 2003. Ion-coupling determinants of Na+-driven and H+-driven flagellar motors. J. Mol. Biol. 327453-463. [DOI] [PubMed] [Google Scholar]
- 5.Atsumi, T., Y. Maekawa, H. Tokuda, and Y. Imae. 1992. Amiloride at pH 7.0 inhibits the Na+-driven flagellar motors of Vibrio alginolyticus but allows cell growth. FEBS Lett. 314114-116. [DOI] [PubMed] [Google Scholar]
- 6.Austin, B., and C. Adams. 1996. Fish pathogens, p. 197-243. In B. Austin, M. Altwegg, P. J. Gosling, and S. W. Joseph (ed.), The genus Aeromonas. John Wiley and Sons, New York, NY.
- 7.Bateman, A., E. Birney, L. Cerruti, R. Durbin, L. Etwiller, S. R. Eddy, S. Griffiths-Jones, K. L. Howe, M. Marshall, and E. L. L. Sonnhammer. 2002. The pfam protein families database. Nucleic Acids Res. 30276-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berg, H. C. 2003. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 7219-54. [DOI] [PubMed] [Google Scholar]
- 9.Blair, D. F., and H. C. Berg. 1990. The MotA protein of E. coli is a proton conducting component of the flagellar motor. Cell 60439-449. [DOI] [PubMed] [Google Scholar]
- 10.Blair, D. F. 2003. Flagellar movement driven by proton translocation. FEBS Lett. 54586-95. [DOI] [PubMed] [Google Scholar]
- 11.Boles, B. R., and L. L. McCarter. 2000. Insertional inactivation of genes encoding components of the sodium-type flagellar motor and switch of Vibrio parahaemolyticus. J. Bacteriol. 1821035-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bott, M., M. Meyer, and P. Dimroth. 1995. Regulation of anaerobic citrate metabolism in Klebsiella pneumoniae. Mol. Microbiol. 18533-546. [DOI] [PubMed] [Google Scholar]
- 13.Canals, R., S. Ramirez, S. Vilches, G. Horsburgh, J. G. Shaw, J. M. Tomás, and S. Merino. 2006. Polar flagellum biogenesis in Aeromonas hydrophila. J. Bacteriol. 188542-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canals, R., M. Altarriba, S. Vilches, G. Horsburgh, J. G. Shaw, J. M. Tomás, and S. Merino. 2006. Analysis of the lateral flagellar gene system of Aeromonas hydrophila AH-3. J. Bacteriol. 188852-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dasgupta, N., M. C. Wolfgang, A. Goodman, S. K. Arora, J. Jyot, S. Lory, and R. Ramphal. 2003. A four-tiered transcriptional regulatory circuit controls flagellar biogenesis in Pseudomonas aeruginosa. Mol. Microbiol. 50809-824. [DOI] [PubMed] [Google Scholar]
- 16.De Lorenzo, V., M. Herrero, U. Jakubzik, and K. N. Timmis. 1990. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J. Bacteriol. 1726568-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Mot, R., and J. Vanderleyden. 1994. The C-terminal sequence conservation between OmpA-related outer membrane proteins and MotB suggests a common function in both gram-positive and gram-negative bacteria, possibly in the interaction of these domains with peptidoglycan. Mol. Microbiol. 12333-334. [DOI] [PubMed] [Google Scholar]
- 18.Doyle, T. B., A. C. Hawkins, and L. L. McCarter. 2004. The complex flagellar torque generator of Pseudomonas aeruginosa. J. Bacteriol. 1866341-6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukuoka, H., T. Yakushi, and M. Homma. 2004. Concerted effects of amino acid substitutions in conserved charged residues and other residues in the cytoplasmic domain of PomA, a stator component of Na+-driven flagella. J. Bacteriol. 1866749-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gavín, R., A. A. Rabaan, S. Merino, J. M. Tomás, I. Gryllos, and J. G. Shaw. 2002. Lateral flagella of Aeromonas species are essential for epithelial cell adherence and biofilm formation. Mol. Microbiol. 43383-397. [DOI] [PubMed] [Google Scholar]
- 21.Gosink, H. K., and C. C. Häse. 2000. Requirements for conversion of the Na+-drive flagellar motor of Vibrio cholerae to the H+-drive motor of Escherichia coli. J. Bacteriol. 1824234-4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166557-580. [DOI] [PubMed] [Google Scholar]
- 23.Inoue, K., Y. Kosako, K. Suzuky, and T. Shimada. 1991. Peritrichous flagellation in Plesiomonas shigelloides strains. Jpn. J. Med. Sci. Biol. 44141-146. [DOI] [PubMed] [Google Scholar]
- 24.Ito, M., D. B. Hicks, T. M. Henkin, A. A. Guffanti, B. D. Powers, L. Zvi, K. Uematsu, and T. A. Krulwich. 2004. MotPS is the stator-force generator for motility of alkaliphilic Bacillus, and its homologue is a second functional Mot in Bacillus subtilis. Mol. Microbiol. 531365-2958. [DOI] [PubMed] [Google Scholar]
- 25.Jaques, S., Y. K. Kim, and L. L. McCarter. 1999. Mutations conferring resistance to phenamil and amiloride, inhibitors of sodium-drive motility of Vibrio parahaemolyticus. Proc. Natl. Acad. Sci. USA 965740-5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang, Z. Y., and C. E. Bauer. 1997. Analysis of a chemotaxis operon from Rhodospirillum centenum. J. Bacteriol. 1795712-5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirov, S. M., M. Castrisios, and J. G. Shaw. 2004. Aeromonas flagella (polar and lateral) are enterocyte adhesins that contribute to biofilm formation on surfaces. Infect. Immun. 721939-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kojima, S., and D. F. Blair. 2001. Conformational change in the stator of the bacterial flagella motor. Biochemistry 4013041-13050. [DOI] [PubMed] [Google Scholar]
- 29.Kojima, S., and D. F. Blair. 2004. Solubilization and purification of the MotA/MotB complex of Escherichia coli. Biochemistry 4326-34. [DOI] [PubMed] [Google Scholar]
- 30.Kojima, S., A. Shinohara, H. Terashima, T. Yakushi, M. Sakuma, M. Homma, K. Namba, and K. Imada. 2008. Insights into the stator assembly of the Vibrio flagellar motor from the crystal structure of MotY. Proc. Natl. Acad. Sci. USA 1057696-7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macnab, R. 1996. Flagella and motility, p. 123-145. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, DC.
- 32.McCarter, L. L., and M. Silverman. 1990. Surface-induced swarmer cell differentiation of Vibrio parahaemolyticus. Mol. Microbiol. 41057-1062. [DOI] [PubMed] [Google Scholar]
- 33.McCarter, L. L. 1994. MotY, a component of the sodium-type flagellar motor. J. Bacteriol. 1764219-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCarter, L. L. 2001. Polar flagella motility of the Vibrionaceae. Microbiol. Mol. Biol. Rev. 65445-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merino, S., S. Camprubi, and J. M. Tomas. 1991. The role of lipopolysaccharide in complement-killing of Aeromonas hydrophila strains of serotype O:34. J. Gen. Microbiol. 1371583-1590. [DOI] [PubMed] [Google Scholar]
- 36.Merino, S., X. Rubires, A. Aguilar, and J. M. Tomás. 1997. The role of flagella and motility in the adherence and invasion to fish cell lines by Aeromonas hydrophila serogroup O:34 strains. FEMS Microbiol. Lett. 151213-217. [DOI] [PubMed] [Google Scholar]
- 37.Milton, D. L., R. O'Toole, P. Horstedt, and H. Wolf-Watz. 1996. Flagellin A is essential for the virulence of Vibrio anguillarum. J. Bacteriol. 1781310-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moens, S., K. Michiels, V. Keijer, F. Van Leuven, and J. Vanderleyden. 1995. Cloning, sequencing, and phenotypic analysis of laf1, encoding the flagellin of the lateral flagella of Azospirillum brasilense Sp7. J. Bacteriol. 1775419-5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Obara, M., T. Yakushi, S. Kojima, and M. Homma. 2008. Roles of charged residues in the C-terminal region of PomA, a stator component of the Na+-driven flagellar motor. J. Bacteriol. 1903565-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabaan, A. A., I. Gryllos, J. M. Tomas, and J. G. Shaw. 2001. Motility and the polar flagellum are required for Aeromonas caviae adherence to Hep-2 cells. Infect. Immun. 694257-4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubirés, X., F. Saigi, N. Piqué, N. Climent, S. Merino, S. Albertí, J. M. Tomás, and M. Requé. 1997. A gene (wbbL) from Serratia marcescens N28b (O4) complements the rfb-50 mutation of Escherichia coli K-12 derivatives. J. Bacteriol. 1797581-7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 43.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 745463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stewart, B. J., and L. L. McCarter. 2003. Lateral flagellar gene system of Vibrio parahaemolyticus. J. Bacteriol. 1854508-4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stolz, B., and H. C. Berg. 1991. Evidence for interactions between MotA and MotB, torque-generating elements of the flagellar motor of Escherichia coli. J. Bacteriol. 1737033-7037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terashima, H., H. Fukuoka, T. Yakushi, S. Kojima, and M. Homma. 2006. The Vibrio motor proteins, MotX and MotY, are associated with the basal body of Na+-driven flagella and required for stator formation. Mol. Microbiol. 621170-1180. [DOI] [PubMed] [Google Scholar]
- 47.Toutain, Ch M., M. E. Zegans, and G. A. O'Toole. 2005. Evidence for two flagellar stators and their toll in the motility of Pseudomonas aeruginosa. J. Bacteriol. 187771-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yorimitsu, T., and M. Homma. 2001. Na+-driven flagellar motor of Vibrio. Biochim. Biophys. Acta 150582-93. [DOI] [PubMed] [Google Scholar]
- 49.Yorimitsu, T., Y. Sowa, A. Ishijima, T. Yakushi, and M. Homma. 2002. The systematic substitutions around the conserved charged residues of the cytoplasmic loop of Na+-driven flagellar motor component PomA. J. Mol. Biol. 320403-413. [DOI] [PubMed] [Google Scholar]
- 50.Yu, H. B., P. S. Srinivasa Rao, H. C. Lee, S. Vilches, S. Merino, J. M. Tomas, and K. Y. Leung. 2004. A type III secretion system is required for Aeromonas hydrophila AH-1 pathogenesis. Infect. Immun. 721248-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou, J. D., R. T. Fazzio, and D. F. Blair. 1995. Membrane topology of the MotA protein of Escherichia coli. J. Mol. Biol. 251237-242. [DOI] [PubMed] [Google Scholar]