Abstract
The ability of Streptococcus mutans to catabolize cellobiose, a β-linked glucoside generated during the hydrolysis of cellulose, is shown to be regulated by a transcriptional regulator, CelR, which is encoded by an operon with a phospho-β-glucosidase (CelA) and a cellobiose-specific sugar phosphotransferase system (PTS) permease (EIICel). The roles of CelR, EIICel components, and certain fructose/mannose-PTS permeases in the transcriptional regulation of the cel locus were analyzed. The results revealed that (i) the celA and celB (EIIBCel) gene promoters require CelR for transcriptional activation in response to cellobiose, but read-through from the celA promoter contributes to expression of the EIICel genes; (ii) the EIICel subunits were required for growth on cellobiose and for transcriptional activation of the cel genes; (iii) CcpA plays little direct role in catabolite repression of the cel regulon, but loss of specific PTS permeases alleviated repression of cel genes in the presence of preferred carbohydrates; and (iv) glucose could induce transcription of the cel regulon when transported by EIICel. CelR derivatives containing amino acid substitutions for five conserved histidine residues in two PTS regulatory domains and an EIIA-like domain also provided important insights regarding the function of this regulator. Based on these data, a model for the involvement of PTS permeases and the general PTS proteins enzyme I and HPr was developed that reveals a critical role for the PTS in CcpA-independent catabolite repression and induction of cel gene expression in S. mutans.
The pathogen primarily responsible for human tooth decay, Streptococcus mutans, can utilize a wide variety of carbohydrates found in the diet and in host-derived macromolecules (6, 16). The S. mutans genome contains more than a dozen phosphoenolpyruvate (PEP)-dependent sugar phosphotransferase system (PTS) permeases dedicated to the internalization and phosphorylation of simple sugars (22, 26, 27). S. mutans depends on the PTS for the acquisition of energy sources and for acid production through glycolysis, an integral part of its pathogenic mechanisms. A recent study probing the expression of various PTS permeases in S. mutans cells grown in a variety of carbohydrates supports that most of these permeases are subject to transcriptional regulation (5).
S. mutans can assimilate a number of β-glucosides, including cellobiose, esculin, arbutin, and salicin (8, 21). Cellobiose utilization pathways have been of particular interest because of their potential for the development of alternative energy sources (9, 23). A previous study identified a genetic locus in S. mutans UA159 that is required for the catabolism of cellobiose, including genes for phospho-β-glucosidase (celA), a putative transcriptional regulator (celR), and the B, A, and C domains of the cellobiose PTS enzyme EIICel (celB, celC, and celD), respectively (21). Deletion of celA or celD resulted in loss of growth on cellobiose. Hence, utilization of cellobiose by S. mutans consists of concurrent uptake and phosphorylation of the disaccharide through the cellobiose PTS, followed by hydrolysis of cellobiose-6-phosphate to free glucose and glucose-6-phosphate by the phospho-β-glucosidase enzyme CelA (21).
The primary effector of carbohydrate catabolite repression (CCR) in low-G+C-content gram-positive bacteria is CcpA, a DNA binding protein that modulates transcription of a variety of genes in response to carbohydrate flow through the glycolytic pathway (3, 10, 11, 15). Although an apparent CcpA homologue in S. mutans plays a role in the control of genes involved in many processes, particularly energy metabolism and virulence (3), CcpA-independent CCR appears to be a dominant control point for the coordination of carbohydrate catabolism in this organism. A primary effector of CcpA-independent CCR in S. mutans is the glucose/mannose PTS permease EIIMan (1, 2), but we have recently shown that at least two other PTS permeases in S. mutans exert dominant control over catabolic operons in response to preferred carbohydrate sources, often without direct involvement by CcpA (32).
As part of our ongoing efforts to dissect the molecular mechanisms of catabolite modification of gene expression in S. mutans, we employed a series of genetic analyses to dissect the transcriptional regulation of the cellobiose operon in strain UA159. Results detailed in this report demonstrated the complex nature of the control of cellobiose utilization by S. mutans and provided valuable insights into the signal transduction and catabolite control pathways governing carbohydrate utilization.
MATERIALS AND METHODS
Bacterial strains, growth conditions, assays, and reagents.
Escherichia coli strains were grown in Luria-Bertani medium supplemented, when needed, with antibiotics at the following concentrations: ampicillin (100 μg ml−1), kanamycin (Km; 40 μg ml−1), erythromycin (Em; 500 μg ml−1), and spectinomycin (Sp; 50 μg ml−1). S. mutans UA159 and its derivatives were cultured in brain heart infusion broth (Difco Laboratories, Detroit, MI) at 37°C with 5% CO2 and 95% air, with antibiotics added at the following concentrations when necessary: Km (700 μg ml−1), Em (5 μg ml−1), Sp (500 μg ml−1), and tetracycline (10 μg ml−1). For chloramphenicol acetyltransferase (CAT) assays, PTS assays and growth rate comparisons for S. mutans strains were grown in tryptone-vitamin (TV) base medium (7) with the specified concentrations of glucose, fructose, mannose, or cellobiose. All chemical reagents and antibiotics were obtained from Sigma Chemical Co. Growth curves for S. mutans were generated using a Bioscreen C reader (Oy Growth Curves Ab Ltd., Helsinki, Finland), with readings taken every 30 min. CAT (24) and PEP-dependent sugar PTS (18) assays were performed as previously described (30, 34).
DNA manipulation.
Standard recombinant DNA techniques were performed to engineer plasmids. All restriction and modifying enzymes were purchased from New England Biolabs (Beverly, MA) and used as recommended by the supplier. DNA purification was carried out using the QIAprep spin miniprep kit and QIAquick DNA purification kits purchased from Qiagen, Inc. (Valencia, CA). All primers were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA).
Inactivation of the genes carried by the cel locus by allelic exchange was performed using the PCR-ligation-transformation technique (17). All the antibiotic markers used in this study, including the Km, Sp, and Em cassettes, have been shown to have no polar effects on the expression of genes downstream of the insertion site (32). All mutants were confirmed by PCR, followed by DNA sequencing, including confirmation of the sequence for the chromosomal content of the arms used for recombination. In some cases, strains carrying multiple mutations were generated by transformation with chromosomal DNA isolated from other mutant strains of S. mutans, followed by selection for the appropriate antibiotic resistance and PCR confirmation.
Promoter-reporter gene fusions were constructed by inserting a promoter-containing sequence, including the cognate ribosome-binding site, into the integration vector plasmid pJL84 in front of a cat gene from Staphylococcus aureus that lacks a promoter and ribosome-binding site (34). DNA sequences flanking the promoter-cat fusion were derived from the mtlA-phnA locus, which serves as the integration site after transformation into S. mutans. All gene fusions were confirmed by sequencing before being used to transform S. mutans, and the correct conformation of the integration cassette in the chromosome was verified by PCR.
Construction of pBGEA and pBGE integration vectors.
After being digested with BamHI, two small DNA fragments were released from pBGK (31), and the largest fragment was circularized, yielding pBGK-Bm. Deletion of the two BamHI fragments led to the removal of the promoter region of the gtfA gene and loss of the tetracycline resistance gene. A HincII site, residing in the middle of the remaining gtfA fragment, was then used to linearize the plasmid through partial HincII digestion. A DNA fragment carrying a nonpolar Em cassette was released from a previously constructed plasmid, NpEm1, by restriction digestion with EcoRI and SalI, followed by treatment with the Klenow fragment of DNA polymerase I. Ligation of these two DNA fragments created a new integration vector, pBGE-alpha, with the Em marker oriented in the same direction as for the transcription of gtfA. pBGE-alpha was used to generate pBGEA by removing the NruI/BamHI fragment. Subsequently, pBGE was made by deleting the EcoRI/PstI fragment that harbors the ampicillin resistance gene from pBGEA. In all three vectors, there are two unique restriction enzyme sites, XbaI and BsrGI, available for DNA cloning (see Fig. S1 in the supplemental material). In this study, pBGE was used to deliver a promoterless copy of celR and its derivatives onto the chromosome of S. mutans for genetic analysis (see below). This vector is particularly useful for genetic studies of S. mutans when the gene of interest is not tolerated well by E. coli.
PTS regulation domain (PRD) truncation and histidine replacement mutants of celR were engineered through recombinant PCRs (33). All celR derivatives were cloned into the integration vector pBGE via the XbaI/BsrGI sites, confirmed by sequencing, and then inserted into the gtfA site of a celR mutant carrying a PcelA-cat fusion. Subcloning was performed to generate some of the celR mutants containing multiple histidine replacements.
RNA isolation, reverse transcription-PCR (RT-PCR), and quantitative real-time RT-PCR.
Total RNA was extracted from S. mutans cultures using the RNeasy minikit (Qiagen) according to a protocol adapted for small-scale purification (4). cDNA templates were then generated from 2 μg of total RNA with random hexamers using the SuperScript III first-strand synthesis system (Invitrogen), according to the instructions from the supplier.
For RT-PCR, the following primers were designed to amplify DNA fragments overlapping the celA and celB structural genes: 5′-CAAATGGAGCTCACATTTAAGATTAAT-3′ (forward) and 5′-CATTGGGATTGGAACGTAGGC-3′ (reverse). Real-time PCRs were carried out using an iCycler iQ real-time PCR detection system (Bio-Rad, Hercules, CA) and iQ SYBR green supermix (Bio-Rad), according to the protocols provided by the supplier. Three individual cultures from each strain were used to prepare total RNA and cDNA, and triplicates were included in subsequent reactions for each cDNA sample, along with appropriate controls, as detailed elsewhere (4). Transcript levels of three genes were probed using gene-specific primers as follows: for celA, 5′-AAT GGG CAA CAA ATG TTT TCG-3′ (forward) and 5′-ATT CGG TAA CAA GGT GAT AAG G-3′ (reverse); for celB, 5′-CGC ATT GAA GCA GAC AAC TAT G-3′ (forward) and 5′-CAT TGG GAT TGG AAC GTA GGC-3′ (reverse); and for celR, 5′-TTC TTG ATG AGT GCC GTG AAG G-3′ (forward) and 5′-TTC GGG CAT ATT GCT CAA CTC C-3′ (reverse).
RESULTS
Genetic organization of the cel locus.
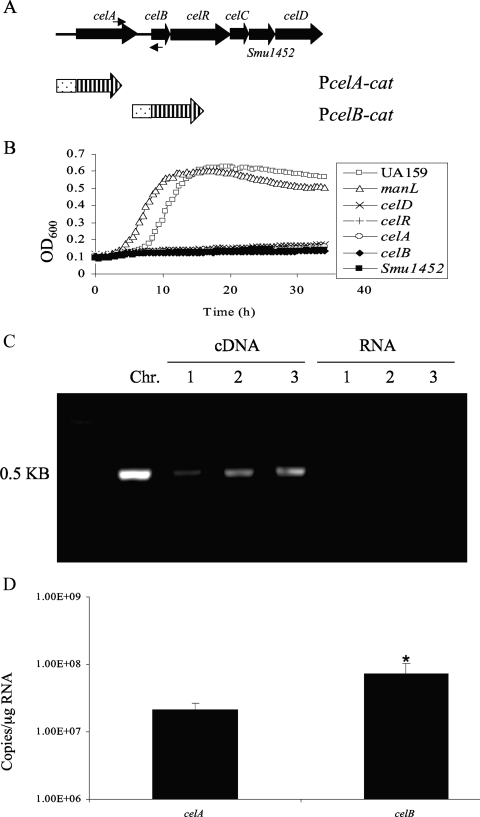
A six-gene locus in the genome of S. mutans strain UA159 (Fig. 1A), including the 6-phospho-β-glucosidase gene celA; genes for a predicted cellobiose-specific PTS enzyme II, celB, celC, and celD; a hypothetical gene, Smu1452; and a regulatory gene, celR, appears to be organized into two transcriptional units arising from promoters that are proximal to celA and celB. It has been previously reported that defects in celA or celD in S. mutans resulted in loss of growth on cellobiose as the sole carbohydrate source (21). Our results indicated that celA, celB, celC, celD, Smu1452, and celR are all required for the growth of S. mutans on cellobiose (Fig. 1B and some data not shown).
FIG. 1.
Genetic organization of the cellobiose utilization locus in S. mutans. (A) Schematic representation of the cel locus, with six putative open reading frames depicted as large, filled arrows. celA encodes a 6-phospho-β-glucosidase; celB, celC, and celD encode the IIB, IIA, and IIC components, respectively, of a putative cellobiose-specific PTS enzyme II; Smu1452 encodes a conserved hypothetical protein; and celR encodes a putative regulatory protein. A pair of small, filled arrows represents primers used in the RT-PCRs (see panel C). CAT fusions are shown as dotted bars (promoter) and barred arrows (cat). (B) Growth curves of strain UA159, a manL mutant (JAM1), and celA, celB, celR, celD, and Smu1452 mutants. Cells were cultured overnight in TV with 0.5% glucose and then diluted 100-fold into TV with 10 mM cellobiose. Growth was monitored with a Bioscreen C reader. OD600, optical density at 600 nm. (C) The result of the RT-PCRs using a pair of primers (A) targeting the region overlapping the coding sequences of celA and celB. Templates of the reactions include chromosomal DNA (Chr.), cDNA generated using random hexamers, and total RNA from wild-type strain UA159. All reactions were performed in triplicate. (D) Transcript levels of celA and celB measured using quantitative real-time RT-PCR. Total RNA samples were extracted from three independent cultures of UA159 grown to mid-exponential phase in TV supplemented with 0.5% cellobiose before being subjected to RT using random hexamers. Primers specific for celA and celB were then used in subsequent quantitative amplification against the same pool of cDNA. All reactions were performed in triplicate. An asterisk represents a P value of less than 0.05.
We hypothesized that CelR activates transcription from the celA and celB promoters by binding to the promoter regions of these genes. Using two cat fusions, PcelA-cat and PcelB-cat, constructed by using promoter regions upstream of the coding sequences for celA and celB, respectively (Fig. 1A), expression of both PcelA-cat and PcelB-cat was found to be highly induced by cellobiose (Table 1). Induction of celA was inhibited by the presence of readily metabolizable carbohydrates, including glucose, fructose, or mannose. Galactose, which is not effective at eliciting CCR in S. mutans, had no repressive effect on cel gene expression. CAT activity from both promoter fusions was drastically reduced when assayed in a CelR-deficient mutant grown in a combination of galactose and cellobiose. When a wild-type copy of the celR gene was introduced into the celR mutant via integration behind the gtfA promoter on the chromosome, expression of celA and growth on cellobiose for this mutant were partially restored (see below).
TABLE 1.
CAT-specific activities of promoter fusions PcelA-cat and PcelB-cat in the backgrounds of UA159 and the celR mutanta
| Sugar(s) | PcelA-cat
|
PcelB-cat
|
||
|---|---|---|---|---|
| celR+ | celR | celR+ | celR | |
| Glucose | 0.97 (0.07) | NT | 4.38 (1.14) | 0.51 (0.15) |
| Fructose | 0.68 (0.10) | NT | NT | NT |
| Mannose | 0.78 (0.52) | NT | NT | NT |
| Galactose | 5.93 (0.63) | 0.79 (0.20) | NT | NT |
| Cellobiose | 615.35 (58.10) | No growth | 1,905.40 (107.10) | No growth |
| Cellobiose/glucose | 1.78 (0.17) | NT | NT | NT |
| Cellobiose/fructose | 0.50 (0.32) | NT | NT | NT |
| Cellobiose/mannose | 0 (0) | NT | NT | NT |
| Cellobiose/galactose | 531.11 (39.9) | 0 (0) | 1,157.30 (41.2) | 2.41 (0.18) |
Cells were grown to mid-exponential phase (optical density at 600 nm, ∼0.4) in TV-based media before being harvested for CAT assays. Sugars were each used at a concentration of 0.5%, including the mixed sugars. Data represent the average and standard deviation (in parentheses) of three independent assays. NT, not tested.
The possibility of read-through for transcripts arising from the celA promoter into celB was investigated using RT-PCR and real-time quantitative RT-PCR. RNA samples extracted from S. mutans UA159 grown in TV-cellobiose were subjected to RT using random hexamers, and the reaction products were then amplified using a specific set of primers targeting a DNA fragment encompassing the 3′ end of celA and the 5′ region of celB. The RT-PCR results (Fig. 1C) indicated that read-through can occur between these two genes. Quantitative real-time RT-PCR using primers specific to celA and celB, amplifying them from the same cDNA products, revealed about 3-fold-higher levels of the celB transcript than those of celA (Fig. 1D). The expression levels from these two promoters were also compared using cat fusions. Consistent with the measurements of mRNA, the celB promoter was shown to produce nearly 3-fold-higher CAT activity than that of the celA promoter in cells grown on cellobiose (Table 1).
cel-PTS components are required for transcription of the cel regulon.
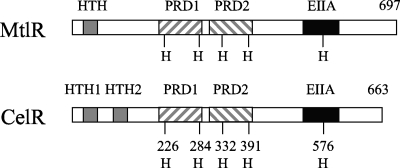
The putative transcriptional regulator CelR is 27% identical to the MtlR transcriptional regulator of Geobacillus stearothermophilus, which has been suggested to regulate a mannitol transport and utilization pathway (12-14). Despite limited sequence homology, CelR and MtlR of G. stearothermophilus (Fig. 2) have predicted DNA binding motifs (two in CelR and one in MtlR) near the N terminus, tandem PRDs in the central portion of the proteins, and a PTS IIA-like domain near the C terminus. Five conserved histidine residues are present in the protein sequences of MtlR and CelR, with two in each PRD and one in the EIIA-like domain (Fig. 2). It was suggested that transport of mannitol by the PTS resulted in the dephosphorylation of the conserved histidine residues in the IIA-like domain and the first PRD of MtlR, which allows for the activation of transcription of the mtl operon (12, 14).
FIG. 2.
Computer predictions of the organization of MtlR from G. stearothermophilus and CelR from S. mutans. The helix-turn-helix motifs (HTH), PRDs, and the EIIA-like domains (EIIA) are represented as gray, diagonally striped, and filled bars, respectively. Also shown are the locations of five conserved histidine residues (H) for each protein.
Given the similarities in the CelR and MtlR transcriptional regulators, we explored the effects of mutations in the cellobiose PTS permease genes celB, celC, and celD on the ability of CelR to activate cel operon expression. Mutants carrying a PcelA-cat fusion were grown to mid-exponential phase in TV base medium supplemented with the noncatabolite-repressing sugar galactose and cellobiose. Deletion of CelD (IIC) resulted in nearly complete loss of PcelA promoter activity, and loss of CelB (IIB) led to diminished celA expression, but loss of CelC (IIA) resulted in high levels of CAT expressed from the celA promoter (Table 2). Assuming that deletion of CelD (EIIC) resulted in constitutive phosphorylation of CelC (IIA) or CelB (IIB), and that loss of CelB led to constitutive phosphorylation of CelC, these data suggest that phosphorylation of CelR by CelC or CelB may repress transcription from the celA and celB promoters.
TABLE 2.
CAT activities representing expression of celA in wild-type strain UA159 and celD-, celB-, and celC-deficient mutantsa
| Strain | PcelA-cat CAT activity (nmol [mg protein]−1 min−1) with indicated sugar(s)
|
||
|---|---|---|---|
| Glucose | Galactose | Cellobiose/galactose | |
| celD+ celB+ cel+ | 0.97 (0.07) | 5.93 (0.63) | 531.11 (39.9) |
| celD (IICCel) | 0.21 (0.29) | 1.10 (0.40) | 1.03 (1.79) |
| celB (IIBCel) | 0.12 (0.20) | 42.10 (6.33) | 78.26 (11.41) |
| celC (IIACel) | 33.34 (0.78) | 2,647.0 (164.4) | 2,441.13 (167.10) |
Cells were grown to mid-exponential phase in TV-based media supplemented with 0.5% sugar, before being harvested for CAT assays. Data represent the average and standard deviation (in parentheses) of three independent assays.
If activation of the cel operon by CelR occurs when phosphate from PEP is preferentially transferred to the incoming cellobiose instead of CelR, then activation of celA expression should take place in the celC (IIA) mutant background in the absence of cellobiose. Indeed, when CAT activity was measured in a celC mutant carrying a PcelA-cat gene fusion and growing under nonrepressing conditions in TV-galactose medium, the expression level of celA was similar to that of TV-galactose-cellobiose cultures (Table 2). Activation of celA expression in the celC mutant was also apparent in TV containing the repressing sugar glucose (Table 2), although expression levels were about 80-fold lower than when the cells were grown with the nonrepressing carbohydrate galactose. It was also noted that loss of CelB caused increased expression from the celA promoter in cells growing in galactose alone. Consistent with the prediction that dephosphorylated CelR led to constitutive expression of celA in the celC and celB mutants, a combination of celC and celR mutations resulted in no celA expression when tested in TV-galactose (data not shown). Furthermore, similar effects of mutations in celC, celB, and celD on the expression of celB were observed when these experiments were repeated using the PcelB-cat fusion (data not shown). The simplest interpretation, again, is that CelC (IIA) and CelB (IIB) are capable of phosphorylating CelR.
Role of EIIABMan in the regulation of the cel operon.
In a previous microarray study, loss of EIIABMan (manL) led to enhanced expression of celA, celC, and celD (1). Subsequently, we confirmed that a manL mutant of S. mutans expressed higher levels of celC mRNA than the parental strain when grown in TV medium containing glucose and cellobiose (32). To further our understanding of the regulatory role played by EIIABMan in the uptake and utilization of carbohydrates by S. mutans, a strain carrying a manL deletion was used to host the PcelA-cat fusion. Loss of EIIABMan resulted in alleviation of the expression of PcelA-cat in cells grown with glucose and cellobiose (Table 3). The same strain showed a small, albeit significant, derepression of celA expression when grown on mannose and cellobiose, but little difference was observed in the wild-type and manL mutant strains growing on fructose and cellobiose. Similar results were obtained when the expression of celB was monitored in a manL mutant strain (data not shown). These data are consistent with our previous results (32) which demonstrate that EIIABMan is required for CCR of various catabolic operons in the presence of glucose or mannose but not fructose.
TABLE 3.
CAT activities representing expression of celA in wild-type strain UA159 and manL- and ccpA-deficient mutantsa
| Sugar(s) | PcelA-cat CAT activity (nmol [mg protein]−1 min−1) for indicated strains
|
||
|---|---|---|---|
| manL+ ccpA+ | manL | ccpA | |
| Cellobiose/glucose | 1.78 (0.17) | 795.61 (39.1) | 2.32 (0.13) |
| Cellobiose/fructose | 0.50 (0.32) | 1.15 (0.19) | 0.42 (0.07) |
| Cellobiose/mannose | 0 (0) | 1.27 (0.37) | 1.77 (0.56) |
| Glucose | 0.97 (0.07) | 1,371.95 (92.8) | 1.97 (0.74) |
| Glucose/fructose | NT | 2.04 (0.72) | NT |
| Glucose/mannose | NT | 2.61 (1.72) | NT |
| Glucose/galactose | NT | 1,273.40 (150.90) | NT |
Each sugar was added at a concentration of 0.5%, including the mixed sugars. Data represent the average and standard deviation (in parentheses) of three independent assays. NT, not tested.
Interestingly, the manL mutant also showed a maximal level of derepression for celA expression when cultured in TV base medium supplemented only with glucose. Similar results were obtained when testing a manL mutant carrying the PcelB-cat fusion (data not shown). Although the cel locus has been shown to be inducible by cellobiose and repressed by glucose, the fact that activation occurs in the manL mutant grown in glucose indicates that glucose may act as an inducer, perhaps by serving as a substrate for transport by EIICel. Alternatively, loss of EIIABMan may be sufficient for the activation of cel expression via alleviation of CCR. We therefore measured CAT levels expressed from PcelA-cat in the manL mutant background with cells grown in combination with glucose and fructose, mannose, or galactose. As shown in Table 3, the manL PcelA-cat strain produced high levels of CAT activity in glucose-containing medium only when the second carbohydrate was the nonrepressing sugar galactose but not when the repressing sugar fructose or mannose was present. Similar results were obtained when the PcelB-cat gene fusion was tested in the manL mutant background (data not shown). Since induction of PcelA-cat by cellobiose in the wild-type background was also repressible by fructose or mannose but not by galactose, these data provide further support for the notion that glucose can serve as an inducer of the cel operon.
EIICel is able to transport glucose.
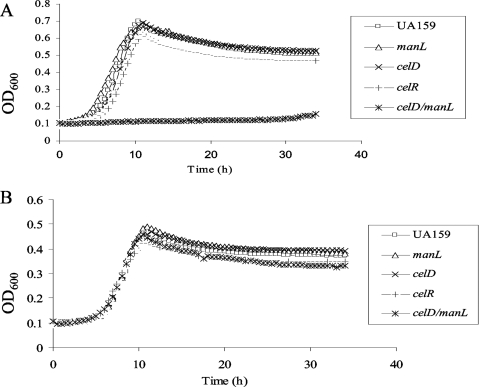
The simplest explanation for how glucose could enhance cel gene expression is that glucose can be transported through the cellobiose PTS. To test this hypothesis, we compared the growth rates of the manL (JAM1), celR, and celD mutants with that of the wild-type strain in TV medium supplemented with 10 mM cellobiose, glucose, or fructose. Loss of CelR or CelD resulted in loss of growth in medium containing cellobiose as the only carbohydrate (Fig. 1B). S. mutans JAM1 (manL) displayed slightly slower growth in glucose medium, as previously reported (2). However, JAM1 also displayed a shorter lag phase when the strain was diluted into cellobiose medium from an overnight TV-glucose culture (Fig. 1B), probably due to the elevated expression of cel genes in glucose medium in the absence of EIIABMan, as noted above. However, no reduction in the growth rate was evident for the celR or celD mutant during growth on 10 mM glucose (Fig. 3A). In order to rule out the possibility that increases in manL expression or ManL activity could compensate for diminished glucose uptake in the celD or celR mutants, we tested the growth of a manL celD double mutant in TV-glucose. As shown in Fig. 3A, the double mutant manL celD, as well as manL celR (data not shown), produced little growth in TV base medium containing 10 mM glucose after more than 30 h of incubation, clearly indicating that EIICel contributes significantly to the internalization of glucose. In contrast, all mutants exhibited growth similar to that of the wild-type strain in TV-fructose medium (Fig. 3B), further supporting that the growth defect observed in glucose was due to the loss of glucose uptake systems.
FIG. 3.
Growth curves for strain UA159 and manL, celD, celR, and celD manL mutants in TV medium supplemented with 10 mM glucose (A) or fructose (B). OD600, optical density at 600 nm.
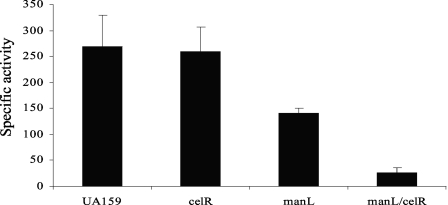
To more directly evaluate the role of EIICel in the transport of glucose, glucose PTS activity was measured in strain UA159 and the manL, celR, and manL celR mutants grown in TV base medium supplemented with 0.5% (27.5 mM) glucose medium. With 0.5% glucose in the growth medium, compared to 10 mM glucose, the manL celR double mutant grows very slowly but yielded a sufficient amount of cells for biochemical assays. As shown in Fig. 4, deletion of celR alone had little impact on glucose PTS activity. Deletion of both manL and celR resulted in glucose PTS activity at a minimal level. Therefore, substantial evidence supports that the cellobiose PTS enzyme II complex is capable of transporting both cellobiose and glucose.
FIG. 4.
Results from PTS assays measuring the PEP-dependent glucose-PTS activity in strain UA159 and celR, manL, and manL celR mutants in cells that were grown to mid-exponential phase in TV supplemented with 0.5% of glucose. Error bars represent the standard deviations of three independent samples.
Role of other PTS permeases in catabolite repression of the cel regulon.
We previously demonstrated that strains carrying deletions of levD, encoding a fructose/mannose porter, or of two fructose porters (FruI, FruCD) showed decreased sensitivity to CCR when grown in the cognate sugars for these permeases (32). To more thoroughly investigate the mechanisms of inhibition for expression of celA by fructose and mannose (Table 1), expression from the PcelA-cat fusion in cultures prepared using fructose or mannose and cellobiose was monitored in mutants lacking the genes for fructose and mannose PTS permeases (30, 34). Deletion of individual fructose PTS (fruI, fruCD, and levD) or mannose PTS porters (levD and manL) (fruI; L. Zeng and R. Burne, unpublished data) caused only low levels of derepression for celA expression when cells were cultured in fructose or mannose and cellobiose. However, when mutants carrying deletions of multiple fructose or mannose porters were used in these assays, alleviation of celA expression became more obvious (Table 4). These data indicate that negative regulation over the utilization of β-glucosides can be exerted by multiple preferred carbohydrates, in an additive fashion, via their cognate PTS porters. At the same time, as shown in Table 3, loss of CcpA had little effect on the repression of celA (and celB) (data not shown) by these preferred sugars.
TABLE 4.
Alleviation of catabolite repression by fructose or mannose through deletion of PTS permeases
| Strain | PcelA-cat CAT activity (nmol [mg protein]−1 min−1) with indicated sugarsa
|
|
|---|---|---|
| Fructose/cellobiose | Mannose/cellobiose | |
| levD+ manL+ fruI+ fruCD+ | 0.50 (0.32) | 0 (0) |
| levD | 1.98 (0.51) | 0.57 (0.07) |
| manL | 1.15 (0.19) | 1.27 (0.37) |
| fruI fruCD | 0.60 (0.02) | 1.00 (0.25) |
| fruI fruCD levD | 49.41 (5.12) | 0.87 (0.03) |
| manL levD fruI | 159.90 (4.55) | 614.70 (34.00) |
Data represent the average and standard deviation (in parentheses) of three independent assays.
Mutational analysis of CelR.
Based on the similarities between CelR and MtlR and on the characteristics of the celC and celB mutants, we postulated that the five conserved histidine residues in the two PRDs and the EIIA domain of CelR played important roles in the regulation of cel operon expression. Attempts to clone celR in E. coli on some of the commonly used plasmid vectors for complementation analysis in streptococci caused cytotoxicity against the host, prohibiting us from delivering celR into S. mutans on a plasmid. To circumvent this technical problem, an integration vector was engineered (pBGE; see Materials and Methods) based on pBGK (31) that allowed for stable cloning of a promoterless copy of celR in E. coli. Integration into the gtfA locus following transformation of S. mutans allowed for expression of celR from sequences upstream of the integration arms. The expression levels of the native and complementing celR genes were compared using quantitative RT-PCR (see Fig. S2 in the supplemental material). The transcript level of the integrated copy of celR was about 20% as abundant as the native copy in the wild-type organism (data not shown), but constitutive levels of expression for celR were achieved from the gtfA site under the conditions tested. Importantly, the wild-type copy of celR could partially restore the expression of the cel locus, allowing for growth on cellobiose (data not shown) and expression of a PcelA-cat fusion (Table 5). Although the levels of expression for the celA promoter from the complemented celR were about 6-fold lower than those in the wild-type background (Table 1), the degree of induction by cellobiose in the complemented strains was sufficient to assess the role of the putative phosphorylation sites in CelR for regulation of cel gene expression.
TABLE 5.
Mutational analyses of CelR regarding the roles of PRD and five conserved histidine residues
| Strain | PcelA-cat CAT activity (nmol [mg protein]−1 min−1) with indicated sugar(s)a
|
||
|---|---|---|---|
| Glucose | Galactose | Galactose/cellobiose | |
| celR/pBGE | 1.13 (0.54) | 0.60 (0.24) | 0.94 (0.65) |
| celR/celR+ | 3.40 (0.82) | 3.97 (0.76) | 88.86 (2.88) |
| celR/celRDPRD | 0.68 (0.09) | 0.38 (0.19) | 0.80 (0.31) |
| celR/celRH226A | 0.20 (0.20) | 0.07 (0.12) | 0.46 (0.22) |
| celR/celRH226P | 0.54 (0.21) | 0.56 (0.18) | 0.94 (0.15) |
| celR/celRH284A | 0.34 (0.28) | 5.59 (0.62) | 21.35 (4.21) |
| celR/celRH332A | 0.16 (0.28) | 0.18 (0.19) | 0.31 (0.12) |
| celR/celRH391A | 0 (0) | 0.88 (0.26) | 3.17 (0.78) |
| celR/celRH391P | 3.10 (0.65) | 5.36 (0.61) | 144.73 (16.02) |
| celR/celRH576A | 0.59 (0.14) | 1.05 (0.61) | 1.60 (0.50) |
| celR/celRH576P | 0.11 (0.10) | 0.54 (0.48) | 0.50 (0.13) |
| celR/celRH226A H284A | 1.56 (0.82) | 0.13 (0.07) | 1.56 (0.51) |
| celR/celRH332A H391P | 1.13 (0.27) | 0.34 (0.59) | 0.14 (0.24) |
| celR/celRH284A H576A | 0.63 (0.28) | 0.72 (0.15) | 0.78 (0.25) |
| celR/celRH284A H391P | 1.16 (0.06) | 5.36 (1.00) | 14.35 (1.74) |
Data represent the average and standard deviation (in parentheses) of three independent assays.
Site-directed mutagenesis was used to engineer a variety of celR derivatives that carried single mutations, or combinations of mutations, of the conserved histidine residues in the predicted PRDs and EIIA-like domain. A mutated form of celR was also constructed that lacked both PRDs but had the rest of the sequence intact (celRDPRD). The ability of the strains expressing the various forms of CelR to express the cel genes was evaluated, and a number of conclusions were derived from these experiments (Table 5). First, PRDs are required for the activity of CelR. The CelR mutant with both PRDs deleted completely lost its ability to activate expression of celA in response to cellobiose. Second, the loss of two conserved histidine residues, H284 and H391 (Fig. 2), caused constitutive expression of the celA, possibly due to the loss of sites that could be targeted by EIIABCel. The other three histidine residues, H226, H332, and H576, were required for transcriptional activation by CelR, as replacement of these residues by alanine created proteins incapable of activating the expression of celA (Table 5). Furthermore, mutations at positions 226, 332, and 576 were dominant to mutations at position 284 or 391, since the H226A H284A, H332A H391P, and H284A H576A double mutations failed to activate celA expression. In contrast, the combined H284A H391P mutations led to constitutive expression of celA. These data demonstrate that phosphorylation of CelR at His226, His332, and His576 is required for activation, and phosphorylation at His284 and His391 inhibits CelR-dependent transcriptional activation. It is also of interest that an H391P mutant of CelR resulted in constitutive activation of celA expression, whereas an H391A mutant of CelR failed to do so (Table 5). As controls, two additional histidine-to-proline replacements in CelR, H226P and H576P, were engineered, but neither of them caused constitutive activation of celA (Table 5).
DISCUSSION
Carbon catabolite repression in low-G+C-content gram-positive bacteria often requires the binding of CcpA to the promoter regions of CCR-susceptible genes (10). Recent studies have proven that CcpA can directly affect CCR of particular genes in S. mutans and can exert primary control over expression of genes for energy metabolism and virulence (3). Notwithstanding, studies with the fruA and levD operons of S. mutans and microarray analysis have shown that CcpA-independent CCR is primarily controlled by a minimum of three sugar-specific PTS permeases that sense glucose, mannose, or fructose and repress genes for the uptake and catabolism of nonpreferred sugars (1, 32). While a comprehensive picture of the mechanisms underlying PTS-dependent expression of genes in S. mutans has yet to develop, the cellobiose utilization operon appears to be a useful model for the further exploration of mechanisms by which PTS components govern carbohydrate utilization in this pathogen.
CelR of S. mutans has considerable similarity to MtlR of G. stearothermophilus, but there are a number of important distinctions between cel gene regulation in S. mutans and mtl gene expression in G. stearothermophilus and between the S. mutans cel gene cluster and other operons regulated by PRD-containing proteins. First, no catabolite response element for CcpA binding has been detected within the cel regulon, whereas catabolite response elements overlap with the mannitol promoter (12) in G. stearothermophilus and are common in catabolic operons of other gram-positive bacteria (29). Consistent with this finding, deletion of ccpA in S. mutans did not markedly affect cel expression. Instead, alleviation of CCR was observed when the genes for the enzyme II PTS permeases encoded by manL, fruI, or levD were mutated. Second, genetic analysis showed that deletion of celC (IIA), and to a lesser extent, celB (IIB), resulted in increased celA expression, but loss of celD (IIC) resulted in constitutively repressed cel expression. Therefore, EIIACel may be the primary regulator involved in phosphorylation of CelR, though EIIBCel is likely involved. In contrast, data obtained from in vitro studies revealed that phosphorylation of purified MtlR depended on EIICBMtl (in the presence of EIIAMtl) but not on EIIAMtl alone (9). In fact, most PRD-containing regulators are phosphorylated by the IIB component of the cognate enzyme II permease (25, 28). Third, whereas deletion of the PRD in the Bacillus subtilis LevR transcriptional activator led to constitutive expression of the lev operon (13), loss of PRDs in CelR completely eliminated cel activation. This result indicates that the role of the PRDs in modulation of CelR activity may be more complex than in some other PRD-containing regulatory proteins. Finally, the five conserved histidine residues found in MtlR appear to have different roles than those in CelR. When the histidine residues in PRD-I of MtlR are phosphorylated by enzyme I (EI)/HPr and that of the IIA-like domain by EIICBMtl, repression is elicited. In contrast, phosphorylation of the histidine residues in PRD-II (by HPr) promotes DNA binding (12). A similar pattern was noted in the LicT transcriptional antiterminator in B. subtilis (19). However, three histidine residues (H226, H332, and H576) in CelR are required for activation and two (H284 and H391) for repression, and the histidine residues found within a single PRD do not appear to act in concert.
PRDs, when phosphorylated, have been suggested to foster the dimerization of the regulatory proteins and binding to their cognate elements in target genes (28). Based on the conservation in the primary sequence, secondary structures, and functions of CelR observed thus far, we believe that H391 of CelR is one of the sites phosphorylated by the cel-PTS components and that dephosphorylation of histidine 391 is required for the protein to achieve a secondary structure that is necessary for the activity of CelR. We are presently working to reconstitute the phosphotransferase system in vitro to demonstrate conclusively that the histidines serve as the sites for modification, although this work has been temporarily hampered by difficulties expressing a full-length CelR protein in E. coli. Given the complexity of this system as described thus far, we predict that the mechanisms will differ somewhat from established paradigms.
The cellobiose utilization pathway is proving to be an excellent model system to evaluate the role in CCR of general and sugar-specific components of the PTS. Results obtained during our genetic analyses clearly suggest critical functions in the regulation of the cel regulon played by EIICel components and the general PTS enzymes EI and HPr. Previous studies with MtlR and the mannitol PTS suggested that while EIIMtl components, in the absence of inducing substrate, are required for repressing the expression of the mtl operon through phosphorylation of MtlR, CCR is exerted when phosphoryl groups were “siphoned” away from EI/HPr while rapidly metabolizable sugars (e.g., glucose) are being transported. It is likely that similar roles are played by EIIABCel and EI/HPr in the regulation of cel gene expression; i.e., phosphorylation of CelR (at H284 and H391) by EIIABCel represses CelR activity, while phosphorylation of all three other histidine residues by EI/HPr is required for activation. The fact that glucose is able to both induce (via EIICel) and repress (via EIIMan) the cel operon, with the latter being apparently dominant over the former, seems to imply a direct role of ManL in catabolite repression of this pathway. However, we also showed that two fructose/mannose PTS porters (LevD and FruI) also contribute to CCR of the cel operon, doing so in an additive fashion (Table 4). In fact, in order to observe the most-profound alleviation of the repression of cel genes by mannose, both levD and fruI had to be mutated (Table 4). Collectively, these observations favor that EI/HPr participate in CCR through phosphorylation of H226, H332, and H576 of CelR, whereas ManL, FruI, or LevD could impose sugar-specific CCR on cel regulon indirectly by affecting the phosphorylation state of EI/HPr (Fig. 5). Specifically, in the presence of their cognate sugar, ManL, FruI, or LevD would “siphon” phosphate from HPr to the incoming carbohydrate. Notably, since glucose is another substrate for EIICel, this hypothesis assumes that EIIMan possesses a significantly higher affinity for glucose than does EIICel. In fact, when the rate of PEP-dependent phosphorylation of glucose by EIICel or EIIMan was measured against a range of glucose concentrations, using bacterial cultures from manL or celR mutants, the results indicated that EIIMan requires a 100- to 200-fold-lower concentration of glucose than did EIICel for optimal activity.
FIG. 5.
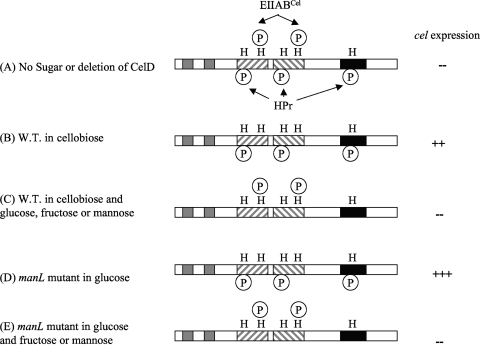
Schematic model depicting the regulation of the cel operon via differential phosphorylation of the CelR protein by components of the PTS. The model predicts that full activity of the CelR protein requires phosphorylation by EI and HPr at histidine residues 226, 332, and 576, with concomitant existence of histidine 284 and 391 in a dephosphorylated state due to the transfer of phosphate groups from EIICel to incoming cellobiose. (A) When no carbohydrate is present, all five histidines are phosphorylated, resulting in repression (-) of cel expression. (B) Transport of cellobiose results in dephosphorylation of H284 and H391 and activation (++) of the cel gene. W.T., wild type. (C) The presence of preferred carbohydrates (glucose, fructose, or mannose) inhibits CelR activation by directing phosphate from HPr to incoming sugars, resulting in dephosphorylation of His226, 332, and 576. (D) A manL mutant relies on EIICel for glucose transport (at a lower rate than EIIMan), which leads to dephosphorylation at H284 and H391, while allowing EI/HPr to maintain phosphorylation at H226, H332, and H576; this combined effect produces full activation (+++) of CelR activity. (E) Activation of the cel regulon in the manL mutant by glucose is repressed by the presence of fructose or mannose, due to flow of the phosphoryl group from HPr to the incoming fructose or mannose.
It was also of interest that a nonpolar mutant in the Smu1452 gene was incapable of growing on cellobiose. Smu1452 encodes a putative 173-amino-acid protein with four predicted transmembrane domains. A previous study had indicated that transcription of Smu1452 is induced along with other members of the cel regulon in response to cellobiose (5). Apparent homologues of Smu1452 have been found in Streptococcus pneumoniae R6 and Streptococcus gordonii, and in both cases, they are part of a well-conserved cellobiose operon (http://www.oralgen.lanl.gov/). Consistent with our finding, the homologue of Smu1452 in S. pneumoniae appears necessary for cellobiose utilization (20). We are currently exploring the potential role of this hypothetical protein in carbohydrate transport and catabolism by S. mutans.
Supplementary Material
Acknowledgments
This work was supported by grants DE12236 and T32 DE007200 from the National Institute of Dental and Craniofacial Research.
Footnotes
Published ahead of print on 23 January 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Abranches, J., M. M. Candella, Z. T. Wen, H. V. Baker, and R. A. Burne. 2006. Different roles of EIIABMan and EIIGlc in regulation of energy metabolism, biofilm development, and competence in Streptococcus mutans. J. Bacteriol. 1883748-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abranches, J., Y. Y. Chen, and R. A. Burne. 2003. Characterization of Streptococcus mutans strains deficient in EIIABMan of the sugar phosphotransferase system. Appl. Environ. Microbiol. 694760-4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abranches, J., M. M. Nascimento, L. Zeng, C. M. Browngardt, Z. T. Wen, M. F. Rivera, and R. A. Burne. 2008. CcpA regulates central metabolism and virulence gene expression in Streptococcus mutans. J. Bacteriol. 1902340-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn, S. J., J. A. Lemos, and R. A. Burne. 2005. Role of HtrA in growth and competence of Streptococcus mutans UA159. J. Bacteriol. 1873028-3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ajdic, D., and V. T. Pham. 2007. Global transcriptional analysis of Streptococcus mutans sugar transporters using microarrays. J. Bacteriol. 1895049-5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banas, J. A. 2004. Virulence properties of Streptococcus mutans. Front. Biosci. 91267-1277. [DOI] [PubMed] [Google Scholar]
- 7.Burne, R. A., Z. T. Wen, Y. Y. Chen, and J. E. Penders. 1999. Regulation of expression of the fructan hydrolase gene of Streptococcus mutans GS-5 by induction and carbon catabolite repression. J. Bacteriol. 1812863-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuffini, A., T. A. Kral, and L. Daneo-Moore. 1982. Regulation of the growth rate of Streptococcus mutans. J. Dent. Res. 61502-505. [DOI] [PubMed] [Google Scholar]
- 9.Desvaux, M. 2006. Unravelling carbon metabolism in anaerobic cellulolytic bacteria. Biotechnol. Prog. 221229-1238. [DOI] [PubMed] [Google Scholar]
- 10.Deutscher, J. 2008. The mechanisms of carbon catabolite repression in bacteria. Curr. Opin. Microbiol. 1187-93. [DOI] [PubMed] [Google Scholar]
- 11.Giammarinaro, P., and J. C. Paton. 2002. Role of RegM, a homologue of the catabolite repressor protein CcpA, in the virulence of Streptococcus pneumoniae. Infect. Immun. 705454-5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henstra, S. A., R. H. Duurkens, and G. T. Robillard. 2000. Multiple phosphorylation events regulate the activity of the mannitol transcriptional regulator MtlR of the Bacillus stearothermophilus phosphoenolpyruvate-dependent mannitol phosphotransferase system. J. Biol. Chem. 2757037-7044. [DOI] [PubMed] [Google Scholar]
- 13.Henstra, S. A., B. Tolner, R. H. ten Hoeve Duurkens, W. N. Konings, and G. T. Robillard. 1996. Cloning, expression, and isolation of the mannitol transport protein from the thermophilic bacterium Bacillus stearothermophilus. J. Bacteriol. 1785586-5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henstra, S. A., M. Tuinhof, R. H. Duurkens, and G. T. Robillard. 1999. The Bacillus stearothermophilus mannitol regulator, MtlR, of the phosphotransferase system. A DNA-binding protein, regulated by HPr and IICBmtl-dependent phosphorylation. J. Biol. Chem. 2744754-4763. [DOI] [PubMed] [Google Scholar]
- 15.Iyer, R., N. S. Baliga, and A. Camilli. 2005. Catabolite control protein A (CcpA) contributes to virulence and regulation of sugar metabolism in Streptococcus pneumoniae. J. Bacteriol. 1878340-8349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuramitsu, H. K. 2003. Molecular genetic analysis of the virulence of oral bacterial pathogens: an historical perspective. Crit. Rev. Oral Biol. Med. 14331-344. [DOI] [PubMed] [Google Scholar]
- 17.Lau, P. C., C. K. Sung, J. H. Lee, D. A. Morrison, and D. G. Cvitkovitch. 2002. PCR ligation mutagenesis in transformable streptococci: application and efficiency. J. Microbiol. Methods 49193-205. [DOI] [PubMed] [Google Scholar]
- 18.LeBlanc, D. J., V. L. Crow, L. N. Lee, and C. F. Garon. 1979. Influence of the lactose plasmid on the metabolism of galactose by Streptococcus lactis. J. Bacteriol. 137878-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindner, C., A. Galinier, M. Hecker, and J. Deutscher. 1999. Regulation of the activity of the Bacillus subtilis antiterminator LicT by multiple PEP-dependent, enzyme I- and HPr-catalysed phosphorylation. Mol. Microbiol. 31995-1006. [DOI] [PubMed] [Google Scholar]
- 20.McKessar, S. J., and R. Hakenbeck. 2007. The two-component regulatory system TCS08 is involved in cellobiose metabolism of Streptococcus pneumoniae R6. J. Bacteriol. 1891342-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Old, L. A., S. Lowes, and R. R. Russell. 2006. Genomic variation in Streptococcus mutans: deletions affecting the multiple pathways of beta-glucoside metabolism. Oral Microbiol. Immunol. 2121-27. [DOI] [PubMed] [Google Scholar]
- 22.Postma, P. W., J. W. Lengeler, and G. R. Jacobson. 1993. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 57543-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwarz, W. H. 2001. The cellulosome and cellulose degradation by anaerobic bacteria. Appl. Microbiol. Biotechnol. 56634-649. [DOI] [PubMed] [Google Scholar]
- 24.Shaw, W. V. 1975. Chloramphenicol acetyltransferase activity from chloramphenicol-resistant bacteria. Methods Enzymol. 43737-755. [DOI] [PubMed] [Google Scholar]
- 25.Stulke, J., M. Arnaud, G. Rapoport, and I. Martin-Verstraete. 1998. PRD—a protein domain involved in PTS-dependent induction and carbon catabolite repression of catabolic operons in bacteria. Mol. Microbiol. 28865-874. [DOI] [PubMed] [Google Scholar]
- 26.Vadeboncoeur, C., M. Frenette, and L. A. Lortie. 2000. Regulation of the pts operon in low G+C Gram-positive bacteria. J. Mol. Microbiol. Biotechnol. 2483-490. [PubMed] [Google Scholar]
- 27.Vadeboncoeur, C., and M. Pelletier. 1997. The phosphoenolpyruvate:sugar phosphotransferase system of oral streptococci and its role in the control of sugar metabolism. FEMS Microbiol. Rev. 19187-207. [DOI] [PubMed] [Google Scholar]
- 28.van Tilbeurgh, H., and N. Declerck. 2001. Structural insights into the regulation of bacterial signalling proteins containing PRDs. Curr. Opin. Struct. Biol. 11685-693. [DOI] [PubMed] [Google Scholar]
- 29.Warner, J. B., and J. S. Lolkema. 2003. CcpA-dependent carbon catabolite repression in bacteria. Microbiol. Mol. Biol. Rev. 67475-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen, Z. T., C. Browngardt, and R. A. Burne. 2001. Characterization of two operons that encode components of fructose-specific enzyme II of the sugar:phosphotransferase system of Streptococcus mutans. FEMS Microbiol. Lett. 205337-342. [DOI] [PubMed] [Google Scholar]
- 31.Wen, Z. T., and R. A. Burne. 2001. Construction of a new integration vector for use in Streptococcus mutans. Plasmid 4531-36. [DOI] [PubMed] [Google Scholar]
- 32.Zeng, L., and R. A. Burne. 2008. Multiple sugar:phosphotransferase system permeases participate in catabolite modification of gene expression in Streptococcus mutans. Mol. Microbiol. 70197-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng, L., Y. Dong, and R. A. Burne. 2006. Characterization of cis-acting sites controlling arginine deiminase gene expression in Streptococcus gordonii. J. Bacteriol. 188941-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng, L., Z. T. Wen, and R. A. Burne. 2006. A novel signal transduction system and feedback loop regulate fructan hydrolase gene expression in Streptococcus mutans. Mol. Microbiol. 62187-200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.