Abstract
Streptococcus pneumoniae is the causative agent of multiple diseases, including otitis media, pneumonia, bacteremia, and meningitis. Pneumolysin (Ply), a member of the cholesterol-dependent cytolytic pore-forming toxins, is produced by virtually all clinical isolates of S. pneumoniae, and strains in which the Ply gene has been deleted are severely attenuated in mouse models of infection. In contrast to all other members of the cholesterol-dependent cytolysin family, Ply lacks a signal peptide for export. Instead, Ply has been hypothesized to be released upon autolysis or, alternatively, via a nonautolytic mechanism that remains ill defined. We determined by use of cell fractionation and Western blotting that, during in vitro growth, exported Ply is localized primarily to the cell wall compartment in 18 different serotypes in the absence of detectable cell lysis. Hemolytic assays revealed that this cell wall-localized Ply is active. Additionally, cell wall-localized Ply is accessible to extracellular protease and is detergent releasable.
Streptococcus pneumoniae, a gram-positive pathogen and colonizer of the human nasopharynx, is one of the leading causes of otitis media, sinusitis, meningitis, pneumonia, and bacteremia (12). In developed countries, S. pneumoniae places a large burden on the medical community and a huge economic burden on parents and caregivers of children. For example, in the United States, ear infections represent the number one cause for a child under five years of age to visit a pediatrician, and these infections result in an estimated cost of $3 billion to $4 billion per year (24). In developing countries, particularly those in sub-Saharan Africa and southeastern Asia, consequences of S. pneumoniae infections are much more severe. Every year, millions of children die as a result of pneumonia, bacteremia, and meningitis caused by S. pneumoniae (26).
S. pneumoniae has many virulence factors that contribute to its ability to cause disease. One of these is the 53-kDa pore-forming toxin pneumolysin (Ply). Ply has multiple independent activities (12, 23). Ply activates complement (21), stimulates host cell apoptosis (8), and acts as a cholesterol-dependent cytolysin (CDC) by binding to cholesterol in the host cell plasma membrane, oligomerizing, inserting into the eukaryotic membrane, and forming a pore 350 to 450 Å in diameter (2, 9). Virtually all clinical isolates express Ply, and strains in which ply has been deleted are attenuated in animal models of colonization and infection (19). A toxoid (nonlytic) version of Ply has been shown to constitute a protective antigen against subsequent S. pneumoniae challenge in mice (1, 20).
Ply functions extracellularly, yet unlike all other members of the CDC family, Ply has no N-terminal signal peptide (25). Early studies of Ply reported that it has a cytoplasmic localization in S. pneumoniae (13). This report, along with the observation that Ply is not found in culture supernatants until cells have undergone autolysis, suggests a cell lysis-dependent release of Ply. Autolysis is a well-studied stationary-phase phenomenon that occurs in vitro (16). LytA, a cell wall-bound murein hydrolase, is required for autolysis; two other minor lysins, LytC and CbpD, also contribute to this process (10). It is unclear whether autolysis is a laboratory artifact or not; however, strains deficient in LytA are attenuated in mouse models of infection, and this is suggestive of a role for autolysis in vivo (6). The release of Ply into the host milieu upon autolysis has been a working hypothesis in the field for the past 20 years, and as such, subsequent studies that have analyzed Ply's activity or presence have considered only a cytoplasmic or lysis-mediated extracellular location.
Recently, evidence contradicting this model has emerged. Certain strains have been reported to release Ply into the culture supernatant in early stationary phase, when autolysis of the culture has not yet occurred (4). Additionally, Ply has been reported to be released in the absence of LytA, indicating an autolysis-independent release of Ply (3).
In this report, we build on these studies to show lysis-independent export of Ply, specifically to the cell wall compartment. This cell wall localization is surprising, given that Ply lacks not only a signal peptide but also any of the known gram-positive cell wall anchor motifs. Additionally, we show that this cell wall-localized Ply has hemolytic activity, is accessible to protease, and is detergent releasable.
MATERIALS AND METHODS
Strains and growth conditions.
The S. pneumoniae strains used in this study are listed in Table 1. S. pneumoniae was grown to an optical density at 600 nm (OD600) of 0.3 in Todd-Hewitt broth supplemented with 0.5% yeast extract plus 5 μl/ml Oxyrase (Oxyrase, Inc., Mansfield, OH). These cultures were mixed with glycerol to a final concentration of 20% glycerol, aliquoted into 300-μl starter cultures, and stored at −80°C. Starter cultures were thawed and diluted into Todd-Hewitt broth supplemented with 0.5% yeast extract plus Oxyrase and grown to midexponential phase, at an OD600 of 0.6, except where indicated.
TABLE 1.
Strains used in this study
| Strain | Relevant genotype or description | Serotype | Reference or source |
|---|---|---|---|
| AC353 | TIGR4, Smr derivative | 4 | 11 |
| STM 226 | AC353 ply::magellan2 | 4 | 11 |
| AC1770 | D39 | 2 | 25 |
| AC409 | D39 ply::Em | 2 | C. Rosenow |
| AC324 | WU2 | 3 | J. Yother |
| AC1356 | Clinical isolate | 1 | J. Weiser |
| AC1357 | Clinical isolate | 6A | J. Weiser |
| AC1359 | Clinical isolate | 6B | J. Weiser |
| AC1362 | Clinical isolate | 23F | J. Weiser |
| AC1363 | Clinical isolate | 8 | J. Weiser |
| AC1365 | Clinical isolate | 9V | J. Weiser |
| P1807 | lytA | 4 | 22 |
Fractionation of S. pneumoniae.
Cells were grown to an OD600 of 0.6 and centrifuged at 14,100 × g for 3 min. The culture supernatants were saved, and proteins were precipitated with 6% (vol/vol) trichloroacetic acid (TCA) and resuspended in 70 μl 50 mM Tris, pH 7.5. The cell pellets were washed once with phosphate-buffered saline (PBS) and resuspended in 70 μl cell wall digestion buffer (1× protease inhibitor cocktail [Roche], 300 U/μl mutanolysin, 1 mg/ml lysozyme in a 30% sucrose-10 mM Tris [pH 7.5] buffer) and incubated at 37°C for 3 h with rocking. Protoplasts were separated from the cell wall by centrifugation at 14,100 × g for 10 min. Protoplasts were resuspended in 70 μl 50 mM Tris buffer, pH 7.5.
Hemolytic assays.
Hemolytic assays were performed as described in reference 3, with some modifications. Samples of culture supernatants (not TCA precipitated), cell wall, and protoplasts were pooled, and 100 μl of each fraction was serially twofold diluted in assay buffer (10 mM dithiothreitol [DTT] and 0.1% bovine serum albumin in PBS) in a 96-well U-bottom plate. Fifty microliters of triple-washed 2% sheep red blood cells was added to the dilutions and incubated for 1 h at 37°C. Plates were spun at 233 × g for 10 min, and hemolytic units were determined by eye. Hemolytic units are equal to the reciprocal of the highest dilution at which there was 100% lysis. Cell wall and protoplast hemolytic units were divided by 10 to normalize them to the same volume as the culture supernatant.
Western blotting analysis.
Equal volumes of samples were boiled in sodium dodecyl sulfate (SDS) sample buffer (50 mM Tris-HCl, pH 6.8, 2% SDS, 0.5% bromophenol blue, 10% glycerol, 100 mM β-mercaptoethanol), cooled, and loaded onto an SDS-polyacrylamide gel electrophoresis (PAGE) gel and run for 25 min at 75 V and an additional 90 min at 125 V. Proteins were transferred to a nitrocellulose membrane at 25 V for 1 h. Membranes were cut and blocked with 5% skim milk in PBS for 1 h. Primary antibody to Ply (Statens Serum Institut) at 1:1,000, CodY (a gift of A.L. Sonenshein) at 1:10,000, or RrgB (14) at 1:2,500 diluted in milk was applied to membrane for 1 h at room temperature. Membranes were washed three times for 10 min each with PBS-0.1% Tween. Horseradish peroxidase-conjugated antibody (Roche) was applied to membrane at a 1:2,500 dilution in milk. Membranes were washed as described above and developed with the ECL-Plus horseradish peroxidase Western blotting detection kit (Amersham).
Proteinase K treatment.
Cells were grown to an OD600 of 0.6, pelleted, washed once, and resuspended in PBS. Cells were treated with various concentrations (0.05, 0.1, and 0.2 μg/ml) of proteinase K for 1 min at room temperature. The cells were washed once with protease inhibitor cocktail (Roche) and were then fractionated into cell wall and protoplasts as described above. Ten microliters of each sample was run on an SDS-PAGE gel, and Western blotting was performed to detect Ply and CodY.
Released-protein assay.
Cells were grown to an OD600 of 0.6, pelleted, and washed once in PBS. Cells were then treated with 500 μl of various compounds (0.1, 0.2, 0.5, and 1 M NaCl or MgCl2; 10 mM DTT; 0.1 M Na2CO3; 8 M urea; 0.1% or 0.05% SDS or H2O) for 15 min at room temperature. Cells were pelleted and supernatant proteins were concentrated with TCA as described above. Protein pellets were resuspended in 100 μl 50 mM Tris, pH 7.5. Cell pellets were resuspended with 100 μl 50 mM Tris, pH 7.5. Ten microliters of each sample was run on an SDS-PAGE gel, and Western blotting was performed to detect Ply and CodY.
RESULTS
Hemolytic activity is contained in the cell wall fraction.
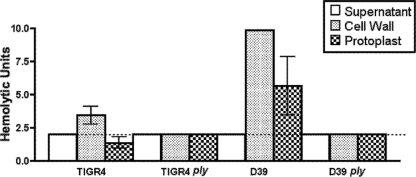
While assaying hemolytic activity by using the standard method, we noted that washed S. pneumoniae cells had much higher levels of hemolytic activity than the supernatants despite the fact that the cells had not been lysed (data not shown), suggesting a surface-localized pool of Ply. To investigate this hypothesis, we fractionated midexponential-phase cultures of a serotype 4 strain (TIGR4) and a serotype 2 strain (D39) into culture supernatant and cell wall and protoplast fractions and assayed each fraction for hemolytic activity. The cell wall was isolated by enzymatic digestion with mutanolysin and lysozyme in an osmotic stabilizing buffer, followed by collection of the protoplasts by centrifugation. Upon hemolytic assay of each fraction, the cell wall fraction was shown to contain the majority of the activity (Fig. 1). ply mutants with either background had no hemolytic activity (Fig. 1). Acapsular strains showed the same partitioning of hemolytic activity as their encapsulated parent strains, indicating that the hemolytic activity in the cell wall was not reliant upon encapsulation (data not shown).
FIG. 1.
Hemolytic assay of culture supernatant and cell wall and protoplast fractions of TIGR4 (serotype 4), D39 (serotype 2), TIGR4 ply, and D39 ply strains. The cell wall fraction has the most hemolytic activity of all fractions in both wt strains, whereas no hemolytic activity was detected in the ply mutant derivatives. The dotted line represents the limit of detection. Bars show the means of three biological replicates, and error bars indicate the standard errors of the means.
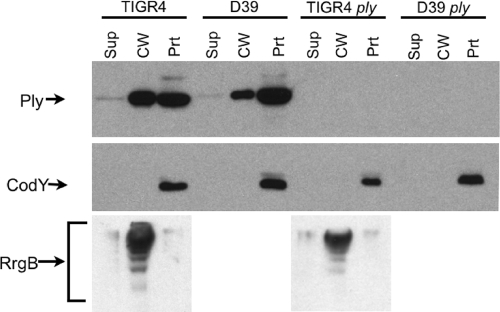
Consistent with the hemolytic assays, Western blotting revealed the presence of a large portion of Ply in the cell wall fraction in the strains of two different serotypes grown to midexponential phase and very little Ply in the culture supernatants (Fig. 2). Antibodies to a known cytoplasmic protein, CodY, detected protein only in the protoplast fraction, indicating that no detectable lysis had occurred (Fig. 2). Antibodies to a known TIGR4 cell wall protein, RrgB, detected protein primarily in the cell wall fraction for both wild-type (wt) TIGR4 and TIGR4 ply strains, indicating that the cell fractionation method successfully separated the cell wall from the protoplasts (Fig. 2). Growth of bacteria without the addition of Oxyrase did not affect the cell wall localization of Ply (data not shown). Although the amounts of Ply detected by Western blotting in the protoplast fraction and the cell wall fraction were roughly equal, we observed a low hemolytic activity in the protoplast fraction compared to that in the cell wall fraction (compare Fig. 1 and 2). The reason for this reduced activity is unknown, but we have ruled out the possibility of an inhibitory compound in the protoplast by showing that mixing the cell wall and protoplast fractions 1:1 yielded a precisely additive hemolytic activity (data not shown).
FIG. 2.
Western blot analysis of fractionated S. pneumoniae TIGR4, D39, TIGR4 ply, and D39 ply strains. Ply is found in the cell wall of both wt strains, whereas the cytoplasmic control protein CodY is found exclusively in the protoplast fraction. RrgB, a multimeric cell wall protein specific to TIGR4, is found primarily in the cell wall lane for the two TIGR4 strains. Ply is absent in the ply mutant strain. Sup, culture supernatant; CW, cell wall; Prt, protoplast.
Ply is surface exposed.
Accessibility to proteinase K was used to further assess the surface localization of Ply on intact cells. wt TIGR4 cells were incubated for 1 min with increasing concentrations of proteinase K and fractionated, and Ply was assayed by Western blotting. Ply was rapidly depleted from the cell wall fraction but not from the protoplast fraction (Fig. 3). Cytoplasmic CodY was not affected by any concentration of proteinase K used. This result shows that Ply in the cell wall compartment is accessible to exogenously added protease and thus is surface exposed.
FIG. 3.
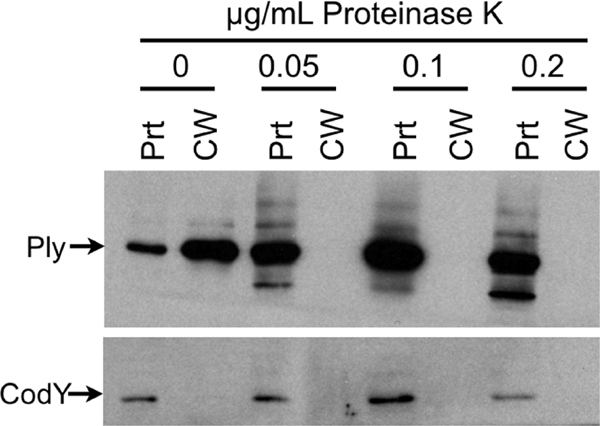
Ply is surface exposed. Cells were treated with increasing amounts of proteinase K for 1 min at room temperature, treated with protease inhibitor, fractionated into cell wall and protoplast compartments, and Western blotted for Ply and CodY. Ply is hydrolyzed in the cell wall by proteinase K treatment without any cell lysis. CW, cell wall; Prt, protoplast.
Cell wall-localized Ply is a general phenomenon.
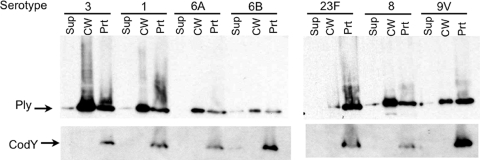
The genome contents of S. pneumoniae bacteria are known to be up to 10% different from strain to strain, and although ply is highly conserved (15), it is possible that other gene products specific to TIGR4 and D39 are contributing to the cell wall localization of Ply. To determine whether or not cell wall-localized Ply is a general phenomenon, we fractionated and Western blotted a bank of clinical isolates composed of 16 additional serotypes. Although there are differences in the ratios of cell wall-localized Ply to protoplast-localized Ply, all 16 contained a substantial amount of Ply in the cell wall and little in the culture supernatant. A representative blot from this survey is shown in Fig. 4. The serotype 23F strain was unique among the 18 strains analyzed in this study in that it had much more Ply in the protoplast fraction than in the cell wall fraction (Fig. 4). However, Ply was nevertheless detectable within the cell wall, with no detectable Ply in the culture supernatant. Again, the CodY cytoplasmic control revealed no detectable cell lysis in any of the cultures.
FIG. 4.
Cell wall localization of Ply is a general phenomenon. Fractionation and Western blot analysis of seven representative serotype strains from a bank of clinical isolates. All isolates contain Ply in the cell wall fraction. Sup, culture supernatant; CW, cell wall; Prt, protoplast.
Ply is localized to the cell wall across exponential and early stationary growth phases.
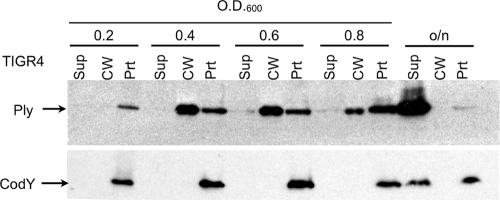
To determine if Ply's localization changed at particular stages of growth, cells were grown to stationary phase and samples were taken throughout growth. The samples were separated into culture supernatants and cell wall and protoplast fractions, and Ply was detected by Western blotting as described above. The fractions were normalized to the same amount of cells by using equivalent OD600 units. Ply was found initially only in the protoplast fraction at early exponential phase but appeared in the cell wall fraction at all later stages of growth. Upon overnight growth, autolysis occurred, leaving most of the Ply and approximately half of the CodY in the culture supernatant (Fig. 5).
FIG. 5.
Ply is localized to the cell wall fraction throughout exponential phase and into stationary phase. Fractionation and Western blot analysis of cells collected and normalized to OD at various points throughout growth. Sup, culture supernatant; CW, cell wall; Prt, protoplast; o/n, overnight.
Ply is noncovalently attached to the cell wall.
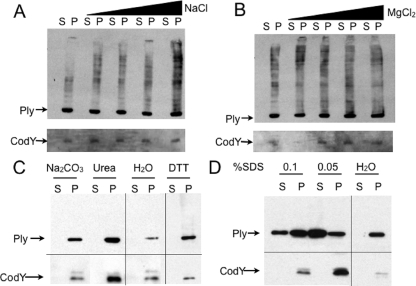
Midexponential-growth-phase cells were treated with various compounds in order to probe the nature of the attachment of Ply to the cell wall. High-salt washes with both monovalent and divalent cations, NaCl and MgCl2, respectively, were unable to release Ply, indicating that Ply is not attached via an ionic interaction (Fig. 6A and B). Ply was not released by DTT or Na2CO3, indicating that the single Cys in Ply is not involved in disulfide bond formation in the case of DTT or thioester bond formation in the case of Na2CO3 (Fig. 6C). Treatment with 8 M urea, a denaturant, did not release Ply from the cell surface, suggesting that Ply may be attached to the cell wall via an interaction that does not require properly folded proteins (Fig. 6C). In contrast to the treatments described above, SDS was able to release Ply from the cells (Fig. 6D). This result suggests a hydrophobic, noncovalent interaction with a cell wall component.
FIG. 6.
Ply release assays. Cells were treated with increasing concentrations (0 to 1 M) of either monovalent cation (NaCl) (A) or divalent cation (MgCl2) (B), 8 M urea, 10 mM DTT, or 0.1 M Na2CO3 (C), or 0.1% or 0.05% SDS (D) and then separated into released proteins (S) and attached proteins (P) and Western blotted. (A and B) High-salt washes do not remove Ply from the cell surface. (C) Urea cannot release Ply from the cell surface. Neither Na2CO3 nor DTT can release Ply from the cell surface. (D) SDS washes do remove Ply from the cell surface.
Ply localization is not dependent upon LytA.
A lytA mutant strain was grown to midexponential phase; fractionated into culture supernatant, cell wall, and protoplast components; and Western blotted to determine the localization of Ply (Fig. 7). Ply was found in the cell wall in the lytA mutant strain, indicating that Ply's cell wall localization in vitro is not dependent upon LytA.
FIG. 7.
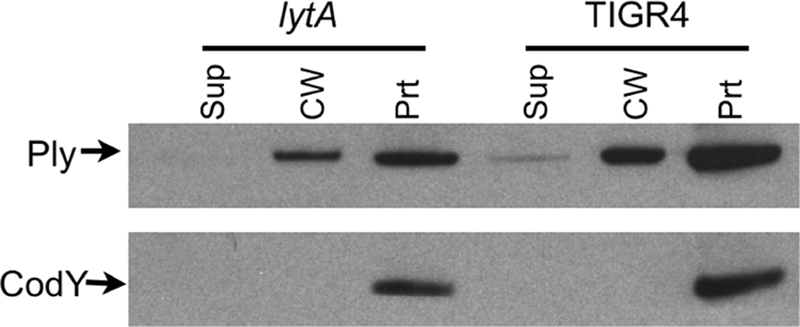
Ply localization in a lytA background. Fractionation and Western blot analysis of a lytA mutant strain. Ply is found in the cell wall fraction. Sup, culture supernatant; CW, cell wall; Prt, protoplast.
DISCUSSION
Ply is unique among the members of the CDC family in that it contains no recognizable export sequence, yet it is known to function outside of the bacterial cell (25). For the past 20 years, the model in the field has been that Ply is released upon autolysis. This model is supported by the fact that Ply is not found in large amounts in the culture supernatant until stationary phase is reached and cells begin autolysing through LytA murein hydrolysis activity (19). Additionally, lytA and ply null mutants are both attenuated in animal models of infection (6, 7), and this outcome has been interpreted to indicate that without autolysis, Ply cannot be released and, therefore, cannot function (19). Contradicting that model, Briles and colleagues reported that Ply is released into culture supernatants in vitro in a lytA-null background (3). Our data support this new model of autolysis-independent release of Ply, as we show that Ply is localized to the cell wall in multiple serotypes at stages of growth where autolysis is not occurring. Further supporting the autolysis-independent model of Ply release is the fact that Ply is found at the cell wall in a lytA mutant strain.
Initially we detected hemolytic activity on washed S. pneumoniae cells, and since these cells were intact, we hypothesized that there might be a surface-exposed pool of Ply. To test this hypothesis, we performed two experiments: one was to test whole cells in an acapsular background to see if the hemolytic activity was lost in the absence of capsule, and the other was to fractionate the cells into cell wall and protoplast components and measure each fraction's hemolytic titer. The acapsular strain yielded the same surface hemolytic activity as its isogenic encapsulated strain, which indicated that the putative surface-exposed Ply was not located within or trapped beneath the capsule. When we performed hemolytic assays on cell wall and protoplast fractions, we discovered that the cell wall contained most of the cellular hemolytic activity, indicating a cell wall-localized pool of Ply.
Western blotting supported the notion that there is a cell wall-localized pool of Ply. Cytoplasmic contamination of the cell wall fraction was ruled out by probing the same blot with antibody to a cytoplasmic protein, CodY. CodY was found only in the protoplast lanes and never in the cell wall fraction, indicating that no cytoplasmic spillover occurred in the fractionation process. We confirmed Ply's cell surface localization by an independent assay for accessibility to extracellular proteolysis. Proteinase K treatment resulted in the rapid and complete digestion of cell wall-localized Ply while not affecting intracellular pools of Ply or CodY. Thus, we conclude that a pool of surface-accessible Ply is present within the cell wall compartment of S. pneumoniae.
Although the serotype designation of S. pneumoniae refers to the makeup of the capsule, different strains can be up to 10% different in genome content from each other. It is therefore important to determine whether or not any given phenotype is general or strain specific. We analyzed clinical isolates representing 16 additional serotypes for Ply subcellular localization. In all 16 additional isolates, we found Ply in the cell wall, indicating that cell wall-localized Ply is a general phenomenon.
In addition to lacking a secretion signal, Ply lacks all known cell wall anchoring motifs. We probed the chemical nature of the attachment of Ply to the cell wall by trying to release Ply with various compounds that should disrupt ionic interactions, thioester bonds, disulfide bonds, or hydrophobic interactions or that denature proteins. Of the types of attachments considered, only a hydrophobic interaction with a cell wall component was supported, since the detergent SDS could release Ply into the supernatant. Thus, we hypothesize that Ply is noncovalently linked to the cell wall. This hypothesis is supported by our observation that after cell wall digestion, Ply resolves at its expected molecular mass of 53 kDa. If Ply were covalently linked to another protein which was itself attached to the cell wall or to a large unit of cell wall produced by mutanolysin and lysozyme cleavage, we would expect Ply to run at a molecular mass higher than what its sequence predicts.
Although we have shown the stable attachment of Ply to the cell wall in vitro, Ply presumably must be released from the bacterial cell in order to function in vivo. The mechanism by which Ply is released from the cell wall in the host has yet to be determined. It is possible that there is a host-derived signal that serves as a trigger to release Ply or that host contact triggers Ply release. Alternatively, the cell wall composition of S. pneumoniae may be different in vivo such that Ply is not sequestered in the cell wall but is instead released from it.
Virulence studies with lytA mutant strains having the same attenuated virulence as ply mutant strains in animal models of infection have been interpreted as Ply release and autolysis being synonymous, adding support to the autolysin-dependent release of Ply model (19). However, in certain mouse strains that are highly susceptible to S. pneumoniae infection, the phenotypes of lytA and ply mutants can be separated (3). We show here that Ply localizes to the cell wall in vitro in a lytA mutant strain, challenging the model of autolysis-dependent release of Ply and supporting the model of autolysis-independent release of Ply. However, it is possible that lytA mutant strains fail to either properly express or localize Ply in vivo.
This study does not address the mechanism by which Ply is transported across the cytoplasmic membrane of S. pneumoniae. There are several examples of streptococcal proteins that are exported and localized to the cell wall yet do not contain a signal peptide or a cell wall anchor motif. Among them are enolase (5, 18) and streptococcal surface GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (17). The mechanism for the export of these proteins is not known. Thus, Ply may fall into a potentially large class of signal peptide-lacking proteins that are exported across the cell membrane and attached to the cell wall through novel mechanisms.
Acknowledgments
This work was funded by the Howard Hughes Medical Institute and the Center for Gastroenterology Research on Absorptive and Secretory Processes, NEMC (grant P30 DK34928).
We thank the Camilli laboratory for much critical feedback on this study.
Footnotes
Published ahead of print on 23 January 2009.
REFERENCES
- 1.Alexander, J. E., R. A. Lock, C. C. Peeters, J. T. Poolman, P. W. Andrew, T. J. Mitchell, D. Hansman, and J. C. Paton. 1994. Immunization of mice with pneumolysin toxoid confers a significant degree of protection against at least nine serotypes of Streptococcus pneumoniae. Infect. Immun. 625683-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alouf, J. E. 2000. Cholesterol-binding cytolytic protein toxins. Int. J. Med. Microbiol. 290351-356. [DOI] [PubMed] [Google Scholar]
- 3.Balachandran, P., S. K. Hollingshead, J. C. Paton, and D. E. Briles. 2001. The autolytic enzyme LytA of Streptococcus pneumoniae is not responsible for releasing pneumolysin. J. Bacteriol. 1833108-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benton, K. A., J. C. Paton, and D. E. Briles. 1997. Differences in virulence for mice among Streptococcus pneumoniae strains of capsular types 2, 3, 4, 5, and 6 are not attributable to differences in pneumolysin production. Infect. Immun. 651237-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergmann, S., M. Rohde, G. S. Chhatwal, and S. Hammerschmidt. 2001. Alpha-enolase of Streptococcus pneumoniae is a plasmin(ogen)-binding protein displayed on the bacterial cell surface. Mol. Microbiol. 401273-1287. [DOI] [PubMed] [Google Scholar]
- 6.Berry, A. M., R. A. Lock, D. Hansman, and J. C. Paton. 1989. Contribution of autolysin to virulence of Streptococcus pneumoniae. Infect. Immun. 572324-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry, A. M., J. Yother, D. E. Briles, D. Hansman, and J. C. Paton. 1989. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect. Immun. 572037-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun, J. S., J. E. Sublett, D. Freyer, T. J. Mitchell, J. L. Cleveland, E. I. Tuomanen, and J. R. Weber. 2002. Pneumococcal pneumolysin and H(2)O(2) mediate brain cell apoptosis during meningitis. J. Clin. Investig. 10919-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert, R. J., J. L. Jimenez, S. Chen, I. J. Tickle, J. Rossjohn, M. Parker, P. W. Andrew, and H. R. Saibil. 1999. Two structural transitions in membrane pore formation by pneumolysin, the pore-forming toxin of Streptococcus pneumoniae. Cell 97647-655. [DOI] [PubMed] [Google Scholar]
- 10.Guiral, S., T. J. Mitchell, B. Martin, and J. P. Claverys. 2005. Competence-programmed predation of noncompetent cells in the human pathogen Streptococcus pneumoniae: genetic requirements. Proc. Natl. Acad. Sci. USA 1028710-8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hava, D. L., and A. Camilli. 2002. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol. Microbiol. 451389-1406. [PMC free article] [PubMed] [Google Scholar]
- 12.Jedrzejas, M. 2001. Pneumococcal virulence factors: structure and function. Microbiol. Mol. Biol. Rev. 65187-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson, M. 1977. Cellular location of pneumolysin. FEMS Microbiol. Lett. 2243-245. [Google Scholar]
- 14.LeMieux, J., D. L. Hava, A. Basset, and A. Camilli. 2006. RrgA and RrgB are components of a multisubunit pilus encoded by the Streptococcus pneumoniae rlrA pathogenicity islet. Infect. Immun. 742453-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lock, R. A., Q. Y. Zhang, A. M. Berry, and J. C. Paton. 1996. Sequence variation in the Streptococcus pneumoniae pneumolysin gene affecting haemolytic activity and electrophoretic mobility of the toxin. Microb. Pathog. 2171-83. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell, T. J., J. E. Alexander, P. J. Morgan, and P. W. Andrew. 1997. Molecular analysis of virulence factors of Streptococcus pneumoniae. Soc. Appl. Bacteriol. Symp. Ser. 2662S-71S. [PubMed] [Google Scholar]
- 17.Pancholi, V., and V. A. Fischetti. 1992. A major surface protein on group A streptococci is a glyceraldehyde-3-phosphate-dehydrogenase with multiple binding activity. J. Exp. Med. 176415-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pancholi, V., and V. A. Fischetti. 1998. Alpha-enolase, a novel strong plasmin(ogen) binding protein on the surface of pathogenic streptococci. J. Biol. Chem. 27314503-14515. [DOI] [PubMed] [Google Scholar]
- 19.Paton, J. C., P. W. Andrew, G. J. Boulnois, and T. J. Mitchell. 1993. Molecular analysis of the pathogenicity of Streptococcus pneumoniae: the role of pneumococcal proteins. Annu. Rev. Microbiol. 4789-115. [DOI] [PubMed] [Google Scholar]
- 20.Paton, J. C., R. A. Lock, C. J. Lee, J. P. Li, A. M. Berry, T. J. Mitchell, P. W. Andrew, D. Hansman, and G. J. Boulnois. 1991. Purification and immunogenicity of genetically obtained pneumolysin toxoids and their conjugation to Streptococcus pneumoniae type 19F polysaccharide. Infect. Immun. 592297-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paton, J. C., B. Rowan-Kelly, and A. Ferrante. 1984. Activation of human complement by the pneumococcal toxin pneumolysin. Infect. Immun. 431085-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pericone, C. D., D. Bae, M. Shchepetov, T. McCool, and J. N. Weiser. 2002. Short-sequence tandem and nontandem DNA repeats and endogenous hydrogen peroxide production contribute to genetic instability of Streptococcus pneumoniae. J. Bacteriol. 1844392-4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubins, J. B., and E. N. Janoff. 1998. Pneumolysin: a multifunctional pneumococcal virulence factor. J. Lab. Clin. Med. 13121-27. [DOI] [PubMed] [Google Scholar]
- 24.Stool, S. E., and M. J. Field. 1989. The impact of otitis media. Pediatr. Infect. Dis. J. 8S11-S14. [PubMed] [Google Scholar]
- 25.Walker, J. A., R. L. Allen, P. Falmagne, M. K. Johnson, and G. J. Boulnois. 1987. Molecular cloning, characterization, and complete nucleotide sequence of the gene for pneumolysin, the sulfhydryl-activated toxin of Streptococcus pneumoniae. Infect. Immun. 551184-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams, B. G., E. Gouws, C. Boschi-Pinto, J. Bryce, and C. Dye. 2002. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect. Dis. 225-32. [DOI] [PubMed] [Google Scholar]