Abstract
Group B Streptococcus (GBS) is major cause of invasive disease in newborn infants and the leading cause of neonatal meningitis. To gain access to the central nervous system (CNS), GBS must not only subvert host defenses in the bloodstream but also invade and survive within brain microvascular endothelial cells (BMEC), the principal cell layer composing the blood-brain barrier (BBB). While several GBS determinants that contribute to the invasion of BMEC have been identified, little is known about the GBS factors that are required for intracellular survival and ultimate disease progression. In this study we sought to identify these factors by screening a random GBS mutant library in an in vitro survival assay. One mutant was identified which contained a disruption in a two-component regulatory system homologous to CiaR/CiaH, which is present in other streptococcal pathogens. Deletion of the putative response regulator, ciaR, in GBS resulted in a significant decrease in intracellular survival within neutrophils, murine macrophages, and human BMEC, which was linked to increased susceptibility to killing by antimicrobial peptides, lysozyme, and reactive oxygen species. Furthermore, competition experiments with mice showed that wild-type GBS had a significant survival advantage over the GBS ΔciaR mutant in the bloodstream and brain. Microarray analysis comparing gene expression between wild-type and ΔciaR mutant GBS bacteria revealed several CiaR-regulated genes that may contribute to stress tolerance and the subversion of host defenses by GBS. Our results identify the GBS CiaR response regulator as a crucial factor in GBS intracellular survival and invasive disease pathogenesis.
Streptococcus agalactiae, also known as group B Streptococcus (GBS), is a gram-positive encapsulated bacterium that asymptomatically colonizes the vaginal and gastrointestinal tracts of healthy human adults (8). However, in susceptible hosts, particularly the newborn and the elderly, it is one of the main causes of fatal invasive disease (37). Moreover, GBS is currently the leading cause of neonatal meningitis in the United States. Mortality remains high in GBS meningitis despite antibiotic therapy, and 25 to 50% of surviving infants are left with permanent neurological sequelae, including cognitive deficits, cerebral palsy, blindness, deafness, or seizures (9). In order to cause meningitis, blood-borne bacteria must be able to survive and multiply in the bloodstream, interact with cerebral endothelial cells, and finally breach the blood-brain barrier (BBB) to gain access to the central nervous system (CNS).
The BBB maintains the homeostasis of the CNS microenvironment by restricting the access of macromolecules, cells, and pathogens. The majority of the BBB is anatomically represented by the cerebral microvascular endothelium. At the level of the microvasculature, brain microvascular endothelial cells (BMEC) are joined by tight junctions and display a paucity of pinocytosis (3, 4), thereby effectively limiting CNS access. Yet despite the highly restrictive nature of the BBB, certain bacterial pathogens, such as GBS, are able to penetrate it and gain entry into the CNS. It has been shown previously that GBS adheres to, invades, and survives inside human BMEC (hBMEC) (34). Several GBS components have been identified that promote interaction with hBMEC in vitro and penetration of the BBB in vivo, including FsbA (53), PilB (29), Lmb (54), beta-hemolysin/cytolysin (βh/c) (7), and lipoteichoic acid (LTA) (6); however, the bacterial factors that mediate survival within brain endothelial cells are unknown.
Studies with animals and humans have shown that the risk of GBS meningitis is correlated with the magnitude and duration of high-level bacteremia (10). Thus, prior to CNS dissemination, GBS must first reach and replicate in the bloodstream, subverting the host innate immune response, including phagocytic cells. Consequently, bacterial resistance to phagocyte clearance in the bloodstream is a key aspect of GBS disease pathogenesis. In addition, the BBB itself plays a sentinel role in bacterial surveillance by secreting chemokines that promote neutrophil recruitment and activation in response to infection (7). Thus, professional phagocytic cells such as neutrophils and macrophages play a leading role in the control of GBS disease. While GBS is typically considered an extracellular pathogen, it can persist in both phagocytic (5) and nonphagocytic cells (34). The GBS βh/c and carotenoid pigment have been shown to contribute to survival in phagocytes and resistance to oxidative damage (28). In addition, we have also recently demonstrated that a GBS major pilus protein, PilB, promotes resistance to host phagocytic killing; survival was linked to the ability of PilB to mediate GBS resistance to cathelicidin antimicrobial peptides (AMPs) (30).
Cathelicidins are naturally occurring cationic AMP antibiotics that are expressed in epithelial cells, neutrophils, and macrophages and constitute an important component of the innate host defense against invasive bacterial infections (33, 35, 45). Recently, murine cathelicidin (cathelicidin-related AMP, or mCRAMP) was shown to be induced in the endothelial cells of the BBB and in cells of the meninges after meningococcal infection (2). Thus, we speculated that GBS factors that confer resistance to AMPs would also promote survival in host cells, an essential step in survival in the bloodstream, BBB penetration, and the pathogenesis of neonatal meningitis. Here we used random transposon mutagenesis to discover a GBS two-component regulatory system (TCRS) that confers resistance to mCRAMP. We provide evidence that the response regulator, CiaR, plays a role in controlling the intracellular survival and virulence potential of GBS through protection against multiple host defense effectors.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The GBS wild-type (WT) strain COH1, a highly virulent serotype III clinical isolate (57), was used in this study. GBS was grown in Todd-Hewitt broth (THB; Hardy Diagnostics) as standing cultures or on THB agar (THA) plates at 37°C. For antibiotic selection, 5 μg/ml erythromycin or 2 μg/ml chloramphenicol (Cm) was used. Escherichia coli strains were grown in Luria-Bertani broth (LB; Hardy Diagnostics), and 500 μg/ml erythromycin or 10 μg/ml Cm was used for antibiotic selection. Functional assays, unless otherwise noted, were performed with bacteria grown to early-exponential phase in THB. Next, bacteria were washed with pyrogen-free phosphate-buffered saline (PBS; Invitrogen, Carlsbad, CA) and adjusted to the desired concentration.
GBS mutant screen using an in vitro survival assay.
Random transposon mutagenesis of GBS COH1 was performed (6), and mutants were screened in an in vitro AMP survival assay. Individual mutants were grown in 96-well plates in the presence of 8 μM polymyxin B, a cationic AMP derived from the soil bacterium Bacillus polymyxa (Sigma-Aldrich), overnight at 37°C. Putative mutants that displayed inhibited growth compared to that of the WT strain were retested and confirmed in further assays. The site of Tn917 insertion in hypersensitive mutants was determined using single-primer PCR (23) with the Tn917 primer (5′-CTAAACACTTAAGAGAATTG-3′) as described previously (6). The BLAST algorithm was used to compare the sequences obtained with the published genome of GBS COH1 and all entries in the GenBank database.
Targeted mutagenesis and complementation analysis.
Plasmid insertional mutagenesis and precise, in-frame allelic exchange of GBS ciaR with the chloramphenicol acetyltransferase (cat) gene were performed according to our established methods (6). Briefly, primers ciaR-upF (5′-ACGTGGATCCGAACATGCTATTGAAGAAGCTC-3′), ciaR-upR (5′-CAACGGTGGTATATCCAGTGATTTTTTTCTCCATAGTATATCCTTTCGATGTAC-3′), ciaR-downF (5′-CTGCGATGAGTGGCAGGGCGGGGCGTAAGATAAGTAAATTCAAAAA-3′), and ciaR-downR (5′-GCGTTCTAGACTTGAAGCGATATTTTCACTATT-3′) were used to amplify a 750-bp fragment directly upstream and downstream, respectively, of the ciaR gene. The ciaR-upR and ciaR-downF primers were constructed with 25-bp extensions corresponding to the 5′ and 3′ ends of the cat gene, respectively. In a subsequent PCR, upstream and downstream PCR fragments were combined with a 660-bp amplicon of the complete cat gene using primers ciaR-upF and ciaR-downR. The resultant PCR amplicon, containing an in-frame replacement of the ciaR gene with cat, was subcloned into the temperature-sensitive vector pHY304. Allelic-exchange mutagenesis was performed as described previously (19), and the COH1 ΔciaR mutant was confirmed by PCR analysis and restriction digestion. For complementation studies, ciaR plus 300 bp of flanking DNA was PCR amplified from the COH1 chromosome using ciaR-complF (5′-TAGCGGATCCGCGATATGGCGATTAGCCG-3′) and ciaR-complR (5′-AGGCCTCGAGTTAATCGGTTTCGACATGC-3′) and was cloned into shuttle expression vector pDCerm (19), yielding plasmid pciaR.
Cell culture.
The hBMEC line was obtained from Kwang Sik Kim (Johns Hopkins University, Baltimore, MD) and has been described previously (51). hBMEC were cultured using RPMI 1640 (Gibco) supplemented with 10% fetal bovine serum (FBS; Gibco), 10% NuSerum (BD Biosciences, San Jose, CA), and modified Eagle's medium nonessential amino acids (Gibco). Tissue culture flasks and 24-well plates were precoated with 1% rat tail collagen to support the hBMEC monolayers. The murine macrophage-like cell line J774 was maintained in RPMI 1640 plus 10% FBS.
Adherence, invasion, and intracellular survival assays.
WT and ΔciaR GBS strains were analyzed for their capacities to adhere to, invade, and survive inside hBMEC as described previously (6, 34). Briefly, early-log-phase WT and ΔciaR mutant GBS bacteria (1 × 105 CFU) were added to a monolayer of hBMEC in 24-well plates in a final volume of 500 μl. The plates were centrifuged at 800 × g for 5 min to synchronize the infection and were then incubated at 37°C under 5% CO2. After 2 h, the monolayers were washed three times with PBS before the addition of 1 ml of RPMI-10% FBS containing gentamicin (100 μg/ml) and penicillin G (5 μg/ml) for two additional hours to kill extracellular and surface-adherent bacteria. Monolayers were disrupted with PBS containing 0.025% Triton X-100, and the number of intracellular bacteria was quantified by plating serial dilutions on THA plates. To assess intracellular survival in hBMEC and J774 macrophages over time, intracellular CFU were enumerated at the indicated time points after the addition of antibiotics. To assess the number of surface-adherent (total cell-associated) bacteria, bacteria were quantified from hBMEC monolayers after 30 min of incubation as described above except that they were washed six times with PBS before lysing and plating. All assays were performed in triplicate and repeated at least three times.
Neutrophil survival assays.
Human neutrophils were purified from healthy human volunteers by Histopaque-Ficoll-Paque gradient centrifugation according to the manufacturers' instructions (Histopaque-1119 [Sigma]; Ficoll-Paque Plus [GE Healthcare]). Contaminating erythrocytes were removed by hypotonic lysis. Final neutrophil purity and viability were >95%. Experiments were performed as previously described (30) with minor modifications. Briefly, WT and ΔciaR mutant GBS strains were grown to early-exponential phase, washed, and resuspended in PBS (1 × 106 CFU/ml). Bacteria (100 μl = 1 × 105 CFU) were incubated at 37°C for 15 min with 400 μl of autologous serum. Next, 1 × 104 opsonized bacteria were added to the purified neutrophils at a multiplicity of infection of 1:100 in RPMI 1640-10% FBS and were incubated at 37°C under rotating conditions. At the indicated time points, neutrophils were lysed by the addition of distilled water, and the numbers of surviving bacteria were quantified by serial dilution plating on THA plates. The percentage of recovered bacteria was calculated as the ratio of CFU recovered to CFU in the initial inoculum. Assays were performed in triplicate and repeated at least three times.
AMP killing assays.
MICs for liquid growth inhibition assays were determined, and bacterial killing kinetics were compared, as described previously (30, 35). The cathelicidin mCRAMP was synthesized and purified (>99%) by the Louisiana State University Protein Facility. WT and ΔciaR mutant GBS strains were incubated with the indicated amount of AMP at 37°C. At the indicated time points, bacteria were plated on THA for CFU enumeration. Assays were performed in triplicate and repeated at least three times.
Mouse infection model.
In vivo competition experiments to assess the virulence potential and relative fitness of the bacteria were performed as described previously (30). Male CD-1 mice (6 to 8 weeks old; Charles River Laboratories, Wilmington, MA) were injected intravenously with 2 × 108 early-logarithmic-phase bacteria at a 1:1 ratio of WT and ΔciaR strains. At 24 h, blood was collected for enumeration of bacteria on THA alone and on THA containing Cm (2 μg/ml) in order to distinguish between the survival frequencies of WT and mutant strains. At 72 h, the blood, spleen, and brain were collected, and bacteria were enumerated as described above. Experiments were repeated twice (8 to 10 mice/group). Additional analysis by PCR was performed to confirm the presence or absence of the ciaR gene on recovered CFU.
RNA isolation.
GBS strains were cultured in THB to a final optical density at 600 nm of 0.3, and 50 ml of bacteria was harvested by centrifugation. For RNA extraction, a 950-ml suspension of 0.7 g QBio lysing matrix B (MP Biomedicals, Solon, OH) in 50 mM sodium acetate (pH 4.0)-0.5% sodium dodecyl sulfate-25% acidic phenol (Amresco, Solon, OH) was added to the cell pellet. Cells were mechanically disrupted using a FastPrep 120 device (MP Biomedicals, Solon, OH) with a speed setting of 5 and a pulse length of 22 s. The mixture was separated by centrifugation, and the aqueous phase was recovered. Two volumes of 100% ethanol were added, and sodium acetate (pH 5.2) was added to a final concentration of 0.3 M. After incubation for 1 h at −20°C and recovery by centrifugation, the RNA pellet was air dried and resuspended in a final volume of 100 ml diethyl pyrocarbonate-treated H2O. One milliliter of Trizol reagent (Sigma-Aldrich) was added, mixed by inversion, and incubated at room temperature for 5 min. Next, 200 ml of chloroform was added, and the mixture was shaken vigorously and allowed to remain at room temperature for 2 min. After centrifugation for 10 min, the aqueous phase was recovered, and RNA was precipitated with an equal volume of 100% isopropanol. The pellet was air dried and resuspended in 50 ml diethyl pyrocarbonate-treated water. The remaining DNA was removed by treatment with RNase-free DNase I (Promega, Madison, WI), and RNA was further purified using an RNeasy (Qiagen, Valencia, CA) column according to the manufacturer's instructions. Purified RNA was stored at −80°C.
DNA microarray design and analysis.
A custom DNA oligonucleotide microarray (designated DWS530259N) was designed by Affymetrix (Santa Clara, CA) and manufactured by Nimblegen (Madison, WI) to function on the Affymetrix GeneChip platform. The NimbleExpress 49 format with a feature size of 17 μm was utilized. Oligonucleotides (25-mers) were selected using the genomic sequence of GBS strain COH1 (55). Each potential coding region was represented by 24 perfectly matched and 24 mismatched oligonucleotides when possible. The oligonucleotide sets were tiled twice on the chip, giving two technical replicates per chip. RNA integrity was assessed with an Agilent 2100 bioanalyzer using an RNA 6000 Nano LabChip kit (Agilent, Palo Alto, CA). GeneChip targets were prepared according to the manufacturer's protocols (prokaryotic expression manual; Affymetrix). cDNA was synthesized from 10 mg of total RNA using Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA). Carryover RNA was degraded with 1 N NaOH followed by 1 N HCl. cDNA was purified and concentrated using a MinElute PCR purification kit (Qiagen). Samples were fragmented with DNase I (Promega, Madison, WI) and biotin end labeled with GeneChip DNA labeling reagent (Affymetrix). A hybridization cocktail containing 3.9 mg labeled cDNA was hybridized to the arrays. Hybridization was performed for 16 h with a rotation of 60 rpm at a temperature of 45°C in a GeneChip hybridization oven, model 640 (Affymetrix). Arrays were washed and stained with a streptavidin-phycoerythrin conjugate using predefined protocols with a GeneChip fluidics station, model 450 (protocol ProkGE-WS2_450). Arrays were scanned with a GeneChip 3000 7G scanner (Affymetrix). Scanned microarray images were analyzed using GeneChip Operating Software, version 1.4 (Affymetrix). Arrays were globally scaled to a target intensity value of 500 in order to compare individual experiments. The absolute call (present, marginal, or absent) for the expression of each gene in each sample, as well as the direction and level of change in gene expression between samples, was identified using the software mentioned above.
Quantitative RT-PCR analysis.
The Beacon Design program (Premier Biosoft International, Palo Alto, CA) was used to design appropriate primers for quantitative real-time PCR (RT-PCR). Purified RNA was used as a template with the Superscript III first-strand synthesis kit (Invitrogen, Carlsbad, CA). The resulting cDNA was then used as a template in a PCR with SYBR Green and iTaq (Bio-Rad, Hercules, CA). Fluorescence from SYBR Green was measured by a Bio-Rad iCycler thermocycler. To ensure that each primer pair produced only a single amplicon, melting curve analysis and electrophoresis of PCR products on agarose gels were performed. The ΔΔCT method was used to estimate changes in gene expression between WT COH1 and ΔciaR strains, with rpsL (a housekeeping gene) as a reference. To verify that the ΔΔCT method was appropriate for estimating relative transcript levels, primer validation was performed, demonstrating that the efficiencies of the target and the reference were approximately equal and that the absolute value of the slope of the log input RNA amount versus ΔCT was <0.1.
Statistical analysis.
Differences in the enumeration of CFU in the blood, brain, and spleen for the murine competition model were analyzed using the paired t test. For functional assays, differences in CFU enumeration were analyzed using Student's t test. Significance was accepted at a P value of <0.05.
Microarray data accession number.
The microarray data set has been deposited in the NCBI Gene Expression Omnibus (GEO) repository under accession no. GSE14259.
RESULTS
Identification of a GBS gene locus associated with AMP resistance.
To identify GBS factors involved in intracellular survival, we screened a random Tn917 transposon library of the virulent serotype III GBS strain COH1 (6) in an in vitro AMP survival assay (see Materials and Methods). Approximately 3,000 Tn917 mutants were screened for AMP sensitivity, and two independent mutants that displayed greater susceptibility to AMP killing than the WT parent strain were identified. Subsequent genomic sequencing demonstrated that both mutants contained a transposon insertion in the GBS SAN_1100 gene, which encodes a putative sensor histidine kinase (CiaH) and is present in a locus with the SAN_1101 gene, encoding a putative DNA-binding response regulator (CiaR) (Fig. 1A). This locus is homologous to the CiaR/CiaH (CiaR/H) TCRS present in other streptococcal pathogens (41, 44, 58). TCRSs, composed of a membrane-bound sensor and a cytoplasmic response regulator, are used by bacteria to sense and respond to changes in environmental stimuli. BLAST analysis of the GBS COH1 CiaR/H locus revealed that this locus is present in all published GBS strains, including serotypes Ia, Ib, II, III, and V (100% identity; 100% similarity).
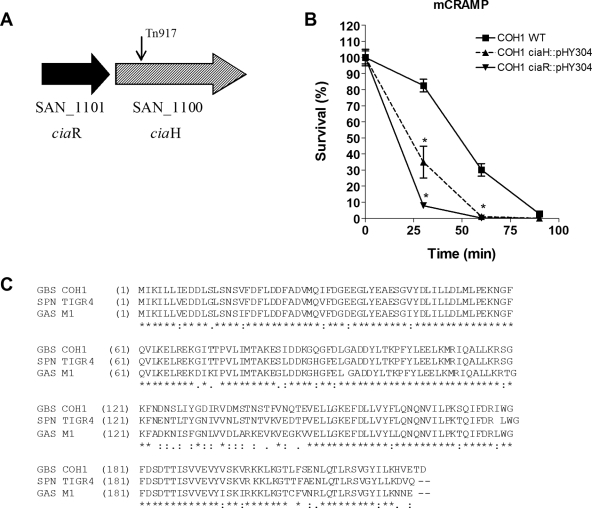
FIG. 1.
The GBS CiaR/H system contributes to AMP resistance. (A) Schematic representation of the GBS ciaR/ciaH region. The position of the Tn917 insertion in original mutants is indicated by arrows. (B) Killing kinetics of WT COH1 and the COH1ΔciaR::pHY304 and COH1ΔciaH::pHY304 mutants after exposure to 18 μM mCRAMP. (C) Alignment of the amino acid sequence of the ciaR gene product in GBS COH1 with those of its homologues in Streptococcus pneumoniae (SPN) TIGR4 and Streptococcus pyogenes (group A Streptococcus [GAS]) M1. Stars indicate residues that are identical in all sequences; colons indicate conserved substitutions; and periods indicate semiconserved substitutions.
To confirm our results and demonstrate the requirement of individual cia locus genes for GBS resistance to AMP killing, we performed targeted plasmid insertional mutagenesis, as described previously (40), on the ciaH and ciaR genes, generating the isogenic mutants COH1ΔciaH::pHY304 and COH1ΔciaR::pHY304, respectively. We then examined the susceptibilities of WT and cia mutant GBS strains to killing by mCRAMP. Both isogenic GBS ΔciaH::pHY304 and ΔciaR::pHY304 mutants exhibited enhanced susceptibility to mCRAMP over time relative to that of the WT strain (Fig. 1B). Since the CiaR-deficient mutant appeared to have a more pronounced phenotype than the ΔciaH mutant, we continued our studies focusing on the role of CiaR. At the amino acid level, GBS CiaR demonstrated high similarity to CiaR homologues in Streptococcus pneumoniae (89% identity; 95% similarity) and Streptococcus pyogenes (80% identity; 89% similarity) (Fig. 1C).
GBS CiaR promotes survival in phagocytic cells.
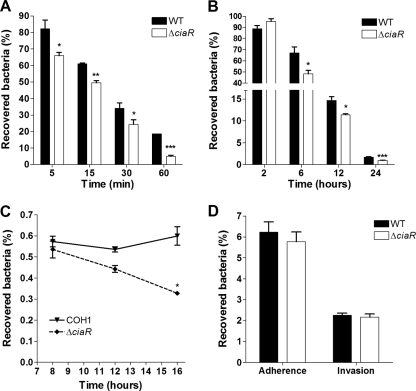
Studies with animals and humans have shown that high-level bacteremia is required for the development of meningitis (10). Thus, prior to CNS dissemination, blood-borne GBS must resist the host's innate defense mechanisms, including killing by neutrophils and macrophages. Since the ΔciaR::pHY304 mutant was more susceptible to killing by AMPs than the ΔciaH mutant, we hypothesized that CiaR would contribute to the survival of GBS during its encounter with phagocytic cells. To further examine the role of CiaR, we performed precise, in-frame allelic-exchange replacement of ciaR in WT GBS COH1 to generate the isogenic mutant COH1ΔciaR by using our standard methodologies (6). We next evaluated the total survival and intracellular survival of WT and ΔciaR mutant bacteria in the presence of purified human neutrophils and the murine macrophage cell line J774, respectively. As shown in Fig. 2A, ciaR-deficient GBS bacteria were killed by human neutrophils more rapidly than the WT GBS strain. We observed no differences in growth/survival between the WT and the ΔciaR mutant in autologous serum alone (data not shown). Similarly, the survival kinetics of intracellular bacteria demonstrated that significantly fewer ΔciaR than WT GBS bacteria were recovered over time after phagocytosis by J774 macrophages (Fig. 2B). Both strains were initially phagocytosed to similar degrees (data not shown). The surface-associated GBS β-h/c is a major virulence factor that contributes to GBS phagocyte survival and virulence potential in animal disease models (7, 28); however, no differences in β-h/c activity between the WT and ΔciaR mutant strains were observed (data not shown).
FIG. 2.
GBS ciaR enhances intracellular survival in hBMEC and J774 macrophages, as well as resistance to killing by human neutrophils. (A) Killing kinetics of WT and ΔciaR mutant GBS upon exposure to human neutrophils. (B) Survival in J774 macrophages. (C) Survival in hBMEC. (D) Adherence to and invasion of hBMEC by WT and ΔciaR GBS (multiplicity of infection, 1). *, P < 0.05; **, P < 0.01. All experiments were performed at least three times with similar results. Results of representative experiments are shown.
CiaR contributes to survival in hBMEC.
Recently, it has been shown that mCRAMP is expressed in the endothelial cells of the BBB during bacterial infection (2). Thus, we hypothesized, as an extension of our findings with phagocytic cells, that CiaR would contribute to enhanced survival in endothelial cells. To test this hypothesis, we performed quantitative hBMEC intracellular survival assays as described previously (6, 34) (see also Materials and Methods). The numbers of surviving intracellular organisms were quantified at different time points after the initial 2-h infection and 2 h of incubation with extracellular antibiotics. Data are expressed as the percentages of intracellular CFU after a total of 8, 12, and 16 h. Consistent with previously published studies (34), WT GBS was capable of surviving in hBMEC for as long as 16 h with no observable differences in monolayer integrity (data not shown). The COH1ΔciaR mutant, however, exhibited a dramatic decrease in survival in hBMEC over time compared to that of the WT strain (Fig. 2C).
To rule out the possibility that this difference reflected a deficiency in the initial adherence to or invasion of hBMEC, we performed additional experiments to assess the level of total cell-associated (surface-adherent plus intracellular) bacteria and the invasive capability of the COH1ΔciaR mutant strain. As shown in Fig. 2D, COH1 WT and COH1ΔciaR mutant bacteria exhibited similar abilities to bind and invade hBMEC. Also, there was no difference in growth kinetics between WT GBS COH1 and COH1ΔciaR mutant bacteria in THB or in the RPMI-based culture medium used in our in vitro hBMEC assays (data not shown). Other studies of the meningeal pathogen E. coli K1 have demonstrated that the exopolysaccharide capsule mediates intracellular survival and trafficking in the brain endothelium (24). However, our analysis of GBS capsular sialic acids by 1,2-diamino-4,5-methylenedioxybenzene (DMB) derivatization and reverse-phase high-performance liquid chromatography (27) revealed no difference in capsular sialic acid content between the WT and the ΔciaR mutant strain (data not shown). Additionally, in previous studies, β-h/c has been implicated in promoting apoptosis in different cell types (22, 28). Thus, it is possible that β-h/c-mediated apoptosis of hBMEC may impact GBS survival and thus the differences in intracellular survival observed between the WT and the ciaR mutant. However, the GBS strain used in these studies, COH1, is very weakly hemolytic (34), and additionally, we observed no differences in β-h/c activity between WT COH1 and the COH1ΔciaR mutant (see above). Thus, our results indicate that the ciaR gene plays a specific role in facilitating the survival of GBS inside hBMEC, without significantly altering the growth rate, capsule production, β-h/c production, or adherence to and invasion of hBMEC.
CiaR confers resistance to killing by AMPs, lysozyme, and oxidants.
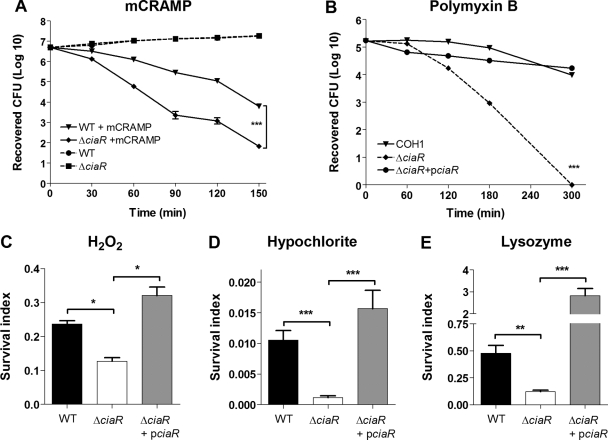
Host cells employ multiple mechanisms to kill intracellular pathogens, including the release of pore-forming cationic AMPs, cell wall-destabilizing enzymes, and reactive oxygen species generated during the oxidative burst. We hypothesized that CiaR promotes intracellular survival by conferring resistance to one or more of these antimicrobial mechanisms. We first confirmed our previous results by comparing the sensitivities of the allelic replacement mutant COH1ΔciaR and WT COH1 to mCRAMP (MICs, 2 μM versus 4 μM) and polymyxin B (MICs, 18 μg/ml versus 32 μg/ml), a cationic AMP derived from the bacterium Bacillus polymyxa. In a more sensitive killing kinetics assay, the isogenic ΔciaR mutant exhibited enhanced susceptibility to mCRAMP (Fig. 3A) and polymyxin B (Fig. 3B), whereas both strains grew equally well under these conditions in the absence of AMP. Furthermore, complementation of the COH1ΔciaR mutant with an expression plasmid bearing a copy of the WT ciaR gene (pciaR) restored resistance to polymyxin B back to WT levels (Fig. 3B). The presence of the vector-only control in the WT and ΔciaR mutant strains did not affect the recovery of surviving bacteria (data not shown). We next examined the susceptibilities of WT GBS and the ΔciaR mutant strain to hydrogen peroxide (H2O2) and hypochlorite (HOCl), two of the principal oxidants of phagolysosome killing, as well as the granular enzyme lysozyme. We observed a significant decrease in the ability of the COH1ΔciaR mutant to survive upon exposure to these effectors (Fig. 3C to E); again, single-gene complementation of the ΔciaR mutant with ciaR restored survival to WT levels (Fig. 3C to E). Correspondingly, complementation of the ΔciaR mutant also restored intracellular survival in J774 macrophages (data not shown). Thus, our results establish unambiguously that the GBS ciaR gene contributes to intracellular survival by mediating resistance to multiple antimicrobial effectors.
FIG. 3.
CiaR contributes to GBS resistance to killing by antimicrobial effectors. (A and B) Killing kinetics of WT GBS, the ΔciaR mutant, and the ΔciaR mutant complemented with pciaR upon exposure to 16 μM mCRAMP (A) or 64 μM polymyxin B (B). (C to E) Survival of WT GBS, the ΔciaR mutant, and the complemented strain after exposure to 0.03% H2O2 (C), 0.01% hypochlorite (D), or 100 ng/ml of lysozyme (E). The survival index was calculated as total CFU recovered/CFU in the starting inoculum. *, P < 0.05; **, P < 0.01; ***, P < 0.001. All experiments were performed at least three times with similar results. Results of representative experiments are shown.
CiaR promotes virulence in a mouse model of infection.
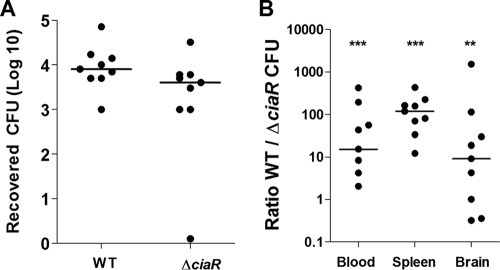
Thus far, our data indicate that CiaR expression promotes survival in brain endothelium and host phagocytic cells such as neutrophils and macrophages. To assess whether GBS CiaR contributes to bacterial survival and virulence potential in vivo, we studied bacterial competition in a murine model (30). Mice were challenged intravenously with equal amounts (2 × 108 CFU) of WT COH1 and COH1ΔciaR, and bacteremia was assessed 24 h postinjection by using antibiotic selection and PCR-based screening to distinguish between the WT and the mutant strain (30). At 24 h, we did not observe a significant difference in the survival of ΔciaR bacteria versus the WT strain (Fig. 4A). At the experimental end point (72 h), blood, spleens, and brains were collected for the enumeration of surviving bacteria. Despite the initial similarities of the WT and mutant strains in establishing acute high-grade bacteremia at 24 h, by 72 h consistently more WT than ΔciaR mutant GBS bacteria were recovered from the blood, brain, and spleen (Fig. 4B). This finding demonstrates a crucial role for CiaR in bacterial fitness and further corroborates the contribution of CiaR to GBS survival in vivo.
FIG. 4.
GBS CiaR is essential for bacterial fitness and overall virulence. Shown are analyses of bacterial counts in blood 24 h after intravenous injection with equal amounts of WT and ΔciaR mutant GBS strains (A) and in blood, spleen, and brain tissues at the experimental end point of 72 h (B). CFU were enumerated on THA plates or on THA plates supplemented with Cm to distinguish between WT and mutant bacteria. An overall ratio of 1 indicates equal numbers of WT and ΔciaR GBS CFU. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Identification of genes differentially regulated in the ΔciaR mutant.
Since CiaR displays homology to other known streptococcal response regulators, we predicted that it affects the transcriptional regulation of GBS genes relevant to intracellular survival and pathogenesis. Microarray analysis using COH1 Affymetrix GeneChips was performed to compare the transcriptional profiles of the WT COH1 strain and the isogenic COH1ΔciaR mutant cultured under identical conditions. The two strains grew with identical kinetics, and global gene expression was examined at early-log phase (optical density at 600 nm, 0.3). GCOS 1.4 software (Affymetrix) was used to identify differentially regulated genes according to the following criteria: presence in at least one of the two strains, the same direction of change in both experiments, and at least a twofold increase or decrease in expression in both of the two experiments (Table 1). The microarray data showed the absence of the ciaR transcript in the ΔciaR mutant, confirming the disruption of the ciaR gene. The expression of the predicted sensor gene ciaH, located 3′ to ciaR, was unaffected by the ΔciaR mutation (data not shown). Only one gene (purQ), with a predicted function of purine and pyrimidine biosythesis, was upregulated more than twofold. More dramatic was the downregulation of seven genes in the ΔciaR mutant. Four of the seven downregulated genes encoded hypothetical proteins or proteins of unknown function. Strikingly, three genes that are predicted to be involved in proteolysis stood out, because their expression was downregulated at least 2.5-fold in both experiments. Gene ontology (GO) analysis of molecular function and biological processes predicted that these genes encode products with metalloendopeptidase (GO:0004222) and proteolysis (GO:0006508) activities. These genes, SAN_0039, SAN_0058, and SAN_0059, belong to the M23/M37 family of metallopeptidases, which catalyze the hydrolysis of nonterminal peptide linkages in oligopeptides or polypeptides (43).
TABLE 1.
Genes affected in CiaR mutant vs WT
| TIGR locus | Gene/putative identification | TIGR cellular role category | Fold change
|
SD | ||
|---|---|---|---|---|---|---|
| Gene array
|
Quantitative RT-PCR | |||||
| Expt 1 | Expt 2 | |||||
| Downregulated genes | ||||||
| SAN_1101 | DNA-binding response regulator CiaR | Regulatory functions: DNA interactions, two-component systems | −1,489.28 | −394.96 | −576 | N/A |
| SAN_2180 | Conserved hypothetical protein | Hypothetical proteins: conserved | −7.39 | −3.84 | −5.11 | 2.75 |
| SAN_0058 | Peptidase, M23/M37 family | Protein fate: degradation of proteins, peptides, and glycopeptides | −6.46 | −3.89 | −5.36 | 0.93 |
| SAN_0059 | Peptidase, M23/M37 family | Protein fate: degradation of proteins, peptides, and glycopeptides | −4.64 | −3.14 | −6.03 | 0.77 |
| SAN_0039 | Peptidase, M23/M37 family | Protein fate: degradation of proteins, peptides, and glycopeptides | −3.95 | −2.92 | −4.56 | 0.32 |
| SAN_2168 | PAP2 family protein | Unknown function: general | −3.72 | −3.54 | −1.14 | 0.16 |
| SAN_2294 | Hypothetical protein | Unknown function | −2.98 | −1.23 | −1.45 | 0.65 |
| SAN_0238 | Oxidoreductase, putative | Unknown function: enzymes of unknown specificity | −2.09 | −3.23 | −1.79 | 0.1 |
| Upregulated gene | ||||||
| SAN_2409 | Phosphoribosylformylglycinamidine synthase (PurQ) | Purine ribonucleotide biosynthesis | 2.1 | 2.73 | 1.09 | 0.31 |
To continue our analysis and confirm our microarray expression data by an independent method, we assessed the relative abundances of the most highly downregulated mRNAs in the ΔciaR mutant by quantitative RT-PCR (Table 1). As observed in our microarray studies, the transcript levels of the genes encoding the hypothetical protein (SAN_2180) and the peptidases (SAN_0039, SAN_0058, and SAN_0059) were significantly downregulated in the CiaR-deficient strain. However, in contrast to the microarray results, we detected no change in transcript levels for the SAN_2168, SAN_2294, and SAN_0238 genes, encoding hypothetical or putative proteins of unknown function, and no change for purQ (SAN_2409). This independent analysis further confirms several targets positively regulated by CiaR that may play a role in bacterial survival and the subversion of host defense mechanisms.
DISCUSSION
High-level bacteremia and bloodstream survival are essential steps in the pathogenesis of GBS neonatal meningitis and ultimate BBB penetration (9). This requires bacteria to develop various strategies to resist the host cell defense system, including resistance to phagolysosome killing. Using a transposon library screen, targeted mutagenesis, and genetic complementation, our studies demonstrate a requirement for the CiaR response regulator in GBS survival in host cells. Decreased intracellular survival by the GBS ΔciaR mutant in vitro was correlated with diminished virulence potential in vivo. Deletion of the ciaR gene did not affect hBMEC adherence and invasion or phagocyte uptake mechanisms; thus, GBS CiaR appears to contribute to a general mechanism of survival in both endothelial and professional phagocytic cells.
Cathelicidins are cationic AMPs that constitute an ancient component of the innate immune system and are efficient at killing a broad range of organisms, including bacteria, fungi, and enveloped viruses (26). They display a wide tissue distribution, including mucosal surfaces, neutrophils, and macrophages, and have recently been shown to be present in brain endothelium (2). Yet bacterial pathogens have evolved various mechanisms to defend themselves against mammalian AMPs, including cell surface modulation to prevent AMP binding, active efflux, and proteolytic degradation (32, 36, 46). We hypothesized that specific GBS factors that promote AMP resistance would likely play a role in bacterial survival and virulence. In a genetic screen, the GBS CiaR/H locus was identified as conferring resistance to the antimicrobial action of AMPs. Both CiaH and CiaR contributed to bacterial survival, as evidenced by the fact that isogenic ciaH and ciaR mutants exhibited enhanced susceptibility to mCRAMP. While our data suggest that this locus plays a role in GBS intracellular and AMP survival, it is likely not the only GBS factor that contributes to resistance. To date, several GBS factors have been shown to contribute to AMP resistance: the d-alanylation of cell wall LTA (39), the cell wall-associated penicillin-binding protein 1a (15), the pilus structural subunit PilB (30), and now the recently identified CiaR/H TCRS.
Bacterial TCRSs are widespread, with hundreds of members identified in pathogenic and nonpathogenic bacteria. Signaling by a TCRS is the most prevalent strategy by which bacteria monitor and respond to environmental signals (17, 31, 52). A membrane-bound sensor histidine kinase senses environmental changes and subsequently activates its cognate cytoplasmic response regulator, which acts to modulate transcription (31). Analysis of sequenced GBS genomes has revealed the presence of genes predicted to encode as many as 20 TCRSs (12, 55), compared to only 13 in S. pyogenes (13), S. pneumoniae (56), and Streptococcus mutans (1). Thus, it has been suggested that TCRSs play a particularly important role in GBS niche establishment and adaptation to various environments within the human host (20). Only a few TCRSs in GBS have been described to date, including DltR/S, which functions to control the d-alanylation of GBS LTA (38); RgfA/C, which appears to regulate the expression of C5a peptidase (50); and the CovR/S (CsrR/S) global regulatory system, which controls multiple GBS virulence determinants (20, 25). From our study, the GBS CiaR/H TCRS likely senses changes encountered in the intracellular host cell environment, since CiaR enhanced GBS survival in neutrophils, macrophages, and nonphagocytic brain endothelial cells. Increased survival was likely attributable to increased resistance to host killing mechanisms, including killing by AMPs, lysozyme, and oxidative stress. Our results are in agreement with observations for the role of the CiaR/H system in other streptococcal species (41, 44, 58), including S. pneumoniae. The CiaR/H system in S. pneumoniae has been shown to contribute to genetic competence, susceptibility to antibiotics, autolysis, oxidative-stress tolerance, thermotolerance, and pneumococcal virulence in vivo in mouse infection models (58). Unlike S. pneumoniae, GBS is not naturally genetically competent or prone to autolysis; thus, we speculate that while there may be some functional overlap between the two systems, the role of GBS CiaR/H may be more specifically defined. In support of this idea, we observed that the most highly downregulated genes in the GBS CiaR-deficient mutant, genes predicted to encode metallopeptidases, are not present in the genomes of the other streptococcal species that utilize the CiaR/H system (S. pyogenes, S. pneumoniae, and S. mutans).
We identify the GBS CiaR response regulator as a key player in survival in both endothelial cells and phagocytes; however, the exact mechanism(s) by which GBS CiaR controls gene expression to promote resistance to host defense mechanisms and intracellular survival remains unclear. To further understand the mechanism by which the CiaR/H system contributes to increased GBS intracellular survival, we used microarray and quantitative RT-PCR analyses to compare the transcriptional profiles of the WT and ΔciaR mutant GBS strains. In S. pneumoniae, CiaR/H controls genes involved in teichoic acid modification, sugar metabolism, stress tolerance, chromosome segregation, protease maturation, and other factors of unknown function (14). In particular, a positively controlled stress-induced serine protease, HtrA (high-temperature requirement A), has been shown to be an important mediator of virulence and resistance to oxidative stress (18, 48). However, our analysis of GBS CiaR-controlled genes revealed only slight downregulation of the GBS htrA homologue (−1.27-fold in experiment 1 and −0.17-fold in experiment 2 [data not shown]). The most highly downregulated gene (SAN_2180) was unknown but shared homology (42% identity; 60% similarity) to genes in Lactococcus lactis involved in acid tolerance and multistress tolerance (42). Like lactococci, GBS is well adapted to acidic environments, since its own growth is accompanied by lactic acid production; thus, it would be expected to have efficient acid stress resistance and inducible responses to an acidic pH. The other three downregulated genes that were confirmed by quantitative RT-PCR—SAN_0039, SAN_0058, and SAN_0059—have predicted protease activity. Bacterial proteases play significant roles in resistance to host defense mechanisms and disease pathogenesis. In general, they have been reported to promote the intracellular survival of pathogens by various mechanisms, a few of which include disruption of phagosome maturation (11), evasion of the autophagic pathway (21), vacuole escape (47), and cleavage of host antimicrobial effectors such as AMPs (36). The metallopeptidases regulated by GBS CiaR all exhibit a high degree of homology (70% similarity; 56% identity) to zooA, a streptococcal gene encoding zoocin A, a bacteriocin-like inhibitory substance (49). Zoocin A is produced by Streptococcus equi subsp. zooepidemicus 4881 and has been shown to be a penicillin binding protein acting as a d-alanyl endopeptidase (16). Thus, we speculate that a general mechanism related to cell wall stabilization or restructuring might contribute to the observed multiple-stress tolerance mediated by CiaR. Continued studies of how GBS senses and responds to the host intracellular environment will increase our understanding of the ways in which this and other bacterial pathogens subvert the host immune system to cause disease.
Acknowledgments
We are grateful to Monique Stins and Kwang Sik Kim for providing hBMEC, to Mandy Lewis for capsular sialic acid analysis, and to James Hoch for helpful discussions. Microarray and real-time PCR analysis were performed by the Research Core Facility, Louisiana State University Health Sciences Center—Shreveport (Shreveport, LA). We also thank Paula Polk for conducting the microarray analysis and Rona Scott for helpful discussions.
This work was supported by grant R21AI073818 from NIAID/NIH to D.W.S. and by a Burroughs Wellcome Fund Career Award and grant R01NS051247 from NINDS/NIH to K.S.D.
Footnotes
Published ahead of print on 29 December 2008.
REFERENCES
- 1.Ajdić, D., W. M. McShan, R. E. McLaughlin, G. Savic, J. Chang, M. B. Carson, C. Primeaux, R. Tian, S. Kenton, H. Jia, S. Lin, Y. Qian, S. Li, H. Zhu, F. Najar, H. Lai, J. White, B. A. Roe, and J. J. Ferretti. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 9914434-14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergman, P., L. Johansson, H. Wan, A. Jones, R. L. Gallo, G. H. Gudmundsson, T. Hokfelt, A. B. Jonsson, and B. Agerberth. 2006. Induction of the antimicrobial peptide CRAMP in the blood-brain barrier and meninges after meningococcal infection. Infect. Immun. 746982-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betz, A. L. 1992. An overview of the multiple functions of the blood-brain barrier. NIDA Res. Monogr. 12054-72. [PubMed] [Google Scholar]
- 4.Betz, A. L. 1985. Epithelial properties of brain capillary endothelium. Fed. Proc. 442614-2615. [PubMed] [Google Scholar]
- 5.Cornacchione, P., L. Scaringi, K. Fettucciari, E. Rosati, R. Sabatini, G. Orefici, C. von Hunolstein, A. Modesti, A. Modica, F. Minelli, and P. Marconi. 1998. Group B streptococci persist inside macrophages. Immunology 9386-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doran, K. S., E. J. Engelson, A. Khosravi, H. C. Maisey, I. Fedtke, O. Equils, K. S. Michelsen, M. Arditi, A. Peschel, and V. Nizet. 2005. Blood-brain barrier invasion by group B Streptococcus depends upon proper cell-surface anchoring of lipoteichoic acid. J. Clin. Investig. 1152499-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doran, K. S., G. Y. Liu, and V. Nizet. 2003. Group B streptococcal beta-hemolysin/cytolysin activates neutrophil signaling pathways in brain endothelium and contributes to development of meningitis. J. Clin. Investig. 112736-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doran, K. S., and V. Nizet. 2004. Molecular pathogenesis of neonatal group B streptococcal infection: no longer in its infancy. Mol. Microbiol. 5423-31. [DOI] [PubMed] [Google Scholar]
- 9.Edwards, M. S., M. A. Rench, A. A. Haffar, M. A. Murphy, M. M. Desmond, and C. J. Baker. 1985. Long-term sequelae of group B streptococcal meningitis in infants. J. Pediatr. 106717-722. [DOI] [PubMed] [Google Scholar]
- 10.Ferrieri, P., B. Burke, and J. Nelson. 1980. Production of bacteremia and meningitis in infant rats with group B streptococcal serotypes. Infect. Immun. 271023-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fratti, R. A., J. Chua, and V. Deretic. 2002. Cellubrevin alterations and Mycobacterium tuberculosis phagosome maturation arrest. J. Biol. Chem. 27717320-17326. [DOI] [PubMed] [Google Scholar]
- 12.Glaser, P., C. Rusniok, C. Buchrieser, F. Chevalier, L. Frangeul, T. Msadek, M. Zouine, E. Couve, L. Lalioui, C. Poyart, P. Trieu-Cuot, and F. Kunst. 2002. Genome sequence of Streptococcus agalactiae, a pathogen causing invasive neonatal disease. Mol. Microbiol. 451499-1513. [DOI] [PubMed] [Google Scholar]
- 13.Graham, M. R., L. M. Smoot, C. A. Migliaccio, K. Virtaneva, D. E. Sturdevant, S. F. Porcella, M. J. Federle, G. J. Adams, J. R. Scott, and J. M. Musser. 2002. Virulence control in group A Streptococcus by a two-component gene regulatory system: global expression profiling and in vivo infection modeling. Proc. Natl. Acad. Sci. USA 9913855-13860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halfmann, A., M. Kovacs, R. Hakenbeck, and R. Bruckner. 2007. Identification of the genes directly controlled by the response regulator CiaR in Streptococcus pneumoniae: five out of 15 promoters drive expression of small non-coding RNAs. Mol. Microbiol. 66110-126. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton, A., D. L. Popham, D. J. Carl, X. Lauth, V. Nizet, and A. L. Jones. 2006. Penicillin-binding protein 1a promotes resistance of group B streptococcus to antimicrobial peptides. Infect. Immun. 746179-6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heath, L. S., H. E. Heath, P. A. LeBlanc, S. R. Smithberg, M. Dufour, R. S. Simmonds, and G. L. Sloan. 2004. The streptococcolytic enzyme zoocin A is a penicillin-binding protein. FEMS Microbiol. Lett. 236205-211. [DOI] [PubMed] [Google Scholar]
- 17.Hoch, J. A. 2000. Two-component and phosphorelay signal transduction. Curr. Opin. Microbiol. 3165-170. [DOI] [PubMed] [Google Scholar]
- 18.Ibrahim, Y. M., A. R. Kerr, J. McCluskey, and T. J. Mitchell. 2004. Control of virulence by the two-component system CiaR/H is mediated via HtrA, a major virulence factor of Streptococcus pneumoniae. J. Bacteriol. 1865258-5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeng, A., V. Sakota, Z. Li, V. Datta, B. Beall, and V. Nizet. 2003. Molecular genetic analysis of a group A Streptococcus operon encoding serum opacity factor and a novel fibronectin-binding protein, SfbX. J. Bacteriol. 1851208-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang, S. M., M. J. Cieslewicz, D. L. Kasper, and M. R. Wessels. 2005. Regulation of virulence by a two-component system in group B streptococcus. J. Bacteriol. 1871105-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kadowaki, T., R. Takii, K. Yamatake, T. Kawakubo, T. Tsukuba, and K. Yamamoto. 2007. A role for gingipains in cellular responses and bacterial survival in Porphyromonas gingivalis-infected cells. Front. Biosci. 124800-4809. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan, A., K. Chung, H. Kocak, C. Bertolotto, A. Uh, C. J. Hobel, C. F. Simmons, K. Doran, G. Y. Liu, and O. Equils. 2008. Group B streptococcus induces trophoblast death. Microb. Pathog. 45231-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karlyshev, A. V., M. J. Pallen, and B. W. Wren. 2000. Single-primer PCR procedure for rapid identification of transposon insertion sites. BioTechniques 281078, 1080, 1082. [DOI] [PubMed] [Google Scholar]
- 24.Kim, K. J., S. J. Elliott, F. Di Cello, M. F. Stins, and K. S. Kim. 2003. The K1 capsule modulates trafficking of E. coli-containing vacuoles and enhances intracellular bacterial survival in human brain microvascular endothelial cells. Cell. Microbiol. 5245-252. [DOI] [PubMed] [Google Scholar]
- 25.Lamy, M. C., M. Zouine, J. Fert, M. Vergassola, E. Couve, E. Pellegrini, P. Glaser, F. Kunst, T. Msadek, P. Trieu-Cuot, and C. Poyart. 2004. CovS/CovR of group B streptococcus: a two-component global regulatory system involved in virulence. Mol. Microbiol. 541250-1268. [DOI] [PubMed] [Google Scholar]
- 26.Lehrer, R. I., and T. Ganz. 1999. Antimicrobial peptides in mammalian and insect host defence. Curr. Opin. Immunol. 1123-27. [DOI] [PubMed] [Google Scholar]
- 27.Lewis, A. L., V. Nizet, and A. Varki. 2004. Discovery and characterization of sialic acid O-acetylation in group B Streptococcus. Proc. Natl. Acad. Sci. USA 10111123-11128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, G. Y., K. S. Doran, T. Lawrence, N. Turkson, M. Puliti, L. Tissi, and V. Nizet. 2004. Sword and shield: linked group B streptococcal beta-hemolysin/cytolysin and carotenoid pigment function to subvert host phagocyte defense. Proc. Natl. Acad. Sci. USA 10114491-14496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maisey, H. C., M. Hensler, V. Nizet, and K. S. Doran. 2007. Group B streptococcal pilus proteins contribute to adherence to and invasion of brain microvascular endothelial cells. J. Bacteriol. 1891464-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maisey, H. C., D. Quach, M. E. Hensler, G. Y. Liu, R. L. Gallo, V. Nizet, and K. S. Doran. 2008. A group B streptococcal pilus protein promotes phagocyte resistance and systemic virulence. FASEB J. 221715-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mascher, T., J. D. Helmann, and G. Unden. 2006. Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol. Mol. Biol. Rev. 70910-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nizet, V. 2006. Antimicrobial peptide resistance mechanisms of human bacterial pathogens. Curr. Issues Mol. Biol. 811-26. [PubMed] [Google Scholar]
- 33.Nizet, V., and R. L. Gallo. 2003. Cathelicidins and innate defense against invasive bacterial infection. Scand. J. Infect. Dis. 35670-676. [DOI] [PubMed] [Google Scholar]
- 34.Nizet, V., K. S. Kim, M. Stins, M. Jonas, E. Y. Chi, D. Nguyen, and C. E. Rubens. 1997. Invasion of brain microvascular endothelial cells by group B streptococci. Infect. Immun. 655074-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nizet, V., T. Ohtake, X. Lauth, J. Trowbridge, J. Rudisill, R. A. Dorschner, V. Pestonjamasp, J. Piraino, K. Huttner, and R. L. Gallo. 2001. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414454-457. [DOI] [PubMed] [Google Scholar]
- 36.Peschel, A. 2002. How do bacteria resist human antimicrobial peptides? Trends Microbiol. 10179-186. [DOI] [PubMed] [Google Scholar]
- 37.Phares, C. R., R. Lynfield, M. M. Farley, J. Mohle-Boetani, L. H. Harrison, S. Petit, A. S. Craig, W. Schaffner, S. M. Zansky, K. Gershman, K. R. Stefonek, B. A. Albanese, E. R. Zell, A. Schuchat, and S. J. Schrag. 2008. Epidemiology of invasive group B streptococcal disease in the United States, 1999-2005. JAMA 2992056-2065. [DOI] [PubMed] [Google Scholar]
- 38.Poyart, C., M. C. Lamy, C. Boumaila, F. Fiedler, and P. Trieu-Cuot. 2001. Regulation of d-alanyl-lipoteichoic acid biosynthesis in Streptococcus agalactiae involves a novel two-component regulatory system. J. Bacteriol. 1836324-6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poyart, C., E. Pellegrini, M. Marceau, M. Baptista, F. Jaubert, M. C. Lamy, and P. Trieu-Cuot. 2003. Attenuated virulence of Streptococcus agalactiae deficient in d-alanyl-lipoteichoic acid is due to an increased susceptibility to defensins and phagocytic cells. Mol. Microbiol. 491615-1625. [DOI] [PubMed] [Google Scholar]
- 40.Pritzlaff, C. A., J. C. Chang, S. P. Kuo, G. S. Tamura, C. E. Rubens, and V. Nizet. 2001. Genetic basis for the beta-haemolytic/cytolytic activity of group B Streptococcus. Mol. Microbiol. 39236-247. [DOI] [PubMed] [Google Scholar]
- 41.Qi, F., J. Merritt, R. Lux, and W. Shi. 2004. Inactivation of the ciaH gene in Streptococcus mutans diminishes mutacin production and competence development, alters sucrose-dependent biofilm formation, and reduces stress tolerance. Infect. Immun. 724895-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rallu, F., A. Gruss, S. D. Ehrlich, and E. Maguin. 2000. Acid- and multistress-resistant mutants of Lactococcus lactis: identification of intracellular stress signals. Mol. Microbiol. 35517-528. [DOI] [PubMed] [Google Scholar]
- 43.Rawlings, N. D., and A. J. Barrett. 1995. Evolutionary families of metallopeptidases. Methods Enzymol. 248183-228. [DOI] [PubMed] [Google Scholar]
- 44.Riani, C., K. Standar, S. Srimuang, C. Lembke, B. Kreikemeyer, and A. Podbielski. 2007. Transcriptome analyses extend understanding of Streptococcus pyogenes regulatory mechanisms and behavior toward immunomodulatory substances. Int. J. Med. Microbiol. 297513-523. [DOI] [PubMed] [Google Scholar]
- 45.Rosenberger, C. M., R. L. Gallo, and B. B. Finlay. 2004. Interplay between antibacterial effectors: a macrophage antimicrobial peptide impairs intracellular Salmonella replication. Proc. Natl. Acad. Sci. USA 1012422-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidtchen, A., I. M. Frick, E. Andersson, H. Tapper, and L. Bjorck. 2002. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol. Microbiol. 46157-168. [DOI] [PubMed] [Google Scholar]
- 47.Schnupf, P., and D. A. Portnoy. 2007. Listeriolysin O: a phagosome-specific lysin. Microbes Infect. 91176-1187. [DOI] [PubMed] [Google Scholar]
- 48.Sebert, M. E., L. M. Palmer, M. Rosenberg, and J. N. Weiser. 2002. Microarray-based identification of htrA, a Streptococcus pneumoniae gene that is regulated by the CiaRH two-component system and contributes to nasopharyngeal colonization. Infect. Immun. 704059-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simmonds, R. S., W. J. Simpson, and J. R. Tagg. 1997. Cloning and sequence analysis of zooA, a Streptococcus zooepidemicus gene encoding a bacteriocin-like inhibitory substance having a domain structure similar to that of lysostaphin. Gene 189255-261. [DOI] [PubMed] [Google Scholar]
- 50.Spellerberg, B., E. Rozdzinski, S. Martin, J. Weber-Heynemann, and R. Lutticken. 2002. rgf encodes a novel two-component signal transduction system of Streptococcus agalactiae. Infect. Immun. 702434-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stins, M. F., N. V. Prasadarao, J. Zhou, M. Arditi, and K. S. Kim. 1997. Bovine brain microvascular endothelial cells transfected with SV40-large T antigen: development of an immortalized cell line to study pathophysiology of CNS disease. In Vitro Cell. Dev. Biol. Anim. 33243-247. [DOI] [PubMed] [Google Scholar]
- 52.Stock, A. M., V. L. Robinson, and P. N. Goudreau. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69183-215. [DOI] [PubMed] [Google Scholar]
- 53.Tenenbaum, T., C. Bloier, R. Adam, D. J. Reinscheid, and H. Schroten. 2005. Adherence to and invasion of human brain microvascular endothelial cells are promoted by fibrinogen-binding protein FbsA of Streptococcus agalactiae. Infect. Immun. 734404-4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tenenbaum, T., B. Spellerberg, R. Adam, M. Vogel, K. S. Kim, and H. Schroten. 2007. Streptococcus agalactiae invasion of human brain microvascular endothelial cells is promoted by the laminin-binding protein Lmb. Microbes Infect. 9714-720. [DOI] [PubMed] [Google Scholar]
- 55.Tettelin, H., V. Masignani, M. J. Cieslewicz, J. A. Eisen, S. Peterson, M. R. Wessels, I. T. Paulsen, K. E. Nelson, I. Margarit, T. D. Read, L. C. Madoff, A. M. Wolf, M. J. Beanan, L. M. Brinkac, S. C. Daugherty, R. T. DeBoy, A. S. Durkin, J. F. Kolonay, R. Madupu, M. R. Lewis, D. Radune, N. B. Fedorova, D. Scanlan, H. Khouri, S. Mulligan, H. A. Carty, R. T. Cline, S. E. Van Aken, J. Gill, M. Scarselli, M. Mora, E. T. Iacobini, C. Brettoni, G. Galli, M. Mariani, F. Vegni, D. Maione, D. Rinaudo, R. Rappuoli, J. L. Telford, D. L. Kasper, G. Grandi, and C. M. Fraser. 2002. Complete genome sequence and comparative genomic analysis of an emerging human pathogen, serotype V Streptococcus agalactiae. Proc. Natl. Acad. Sci. USA 9912391-12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Throup, J. P., K. K. Koretke, A. P. Bryant, K. A. Ingraham, A. F. Chalker, Y. Ge, A. Marra, N. G. Wallis, J. R. Brown, D. J. Holmes, M. Rosenberg, and M. K. Burnham. 2000. A genomic analysis of two-component signal transduction in Streptococcus pneumoniae. Mol. Microbiol. 35566-576. [DOI] [PubMed] [Google Scholar]
- 57.Wessels, M. R., V.-J. Benedi, D. L. Kasper, L. M. Heggen, and C. E. Rubens. 1991. Type III capsule and virulence of group B streptococci, p. 219-223. In G. M. Dunny, P. P. Cleary, and L. L. McKay (ed.), Genetics and molecular biology of streptococci, lactococci, and enterococci. American Society for Microbiology, Washington, DC.
- 58.Zähner, D., K. Kaminski, M. van der Linden, T. Mascher, M. Meral, and R. Hakenbeck. 2002. The ciaR/ciaH regulatory network of Streptococcus pneumoniae. J. Mol. Microbiol. Biotechnol. 4211-216. [PubMed] [Google Scholar]