Abstract
Wild-type (wt) vesicular stomatitis virus (VSV) strains stimulate plasmacytoid dendritic cells (pDC) through Toll-like receptor 7 (TLR7) and its adaptor molecule, MyD88. Granulocyte-macrophage colony-stimulating factor-derived DC (G-DC), which do not express TLR7, are unresponsive to wt VSV due to inhibition of cellular gene expression by the matrix (M) protein. In contrast to its recombinant wt (rwt) counterpart, an M protein mutant of VSV, rM51R-M virus, stimulates maturation of G-DC independently of MyD88. These results suggest that, as in the case of G-DC, rM51R-M virus may stimulate pDC by mechanisms distinct from that by rwt virus. Studies presented here demonstrate that both rwt and rM51R-M viruses induced maturation of TLR7-positive DC derived by culture in the presence of Flt3L (F-DC), with the subsequent expression of type I interferon (IFN). F-DC are a mixture of myeloid (CD11b+) and plasmacytoid (B220+) DC, both of which respond to TLR7 ligands. Separated CD11b+ and B220+ F-DC responded to both rwt and rM51R-M viruses. Both viruses were also defective at inhibiting host gene expression in F-DC, including the expression of genes involved in the antiviral response. The data from F-DC generated from IFN receptor knockout mice demonstrated that the maturation of F-DC induced by rwt virus was dependent on the type I IFN response, while maturation induced by rM51R-M virus was partially dependent on this molecule. Therefore, activation of the type I IFN pathway appears to be important for not only inducing an antiviral response but also for stimulating maturation of F-DC upon virus infection. Importantly, F-DC from TLR7 and MyD88 knockout mice did not undergo maturation in response to rwt virus, while maturation induced by rM51R-M virus was largely independent of both molecules. These results indicate that although both viruses induce F-DC maturation, F-DC detect and respond to rM51R-M virus by means that are distinct from rwt virus. Specifically, this mutant virus appears capable of inducing DC maturation in a wide variety of DC subsets through TLR-dependent and independent mechanisms.
Dendritic cells (DC), the most potent antigen-presenting cells, play a key role in the induction of adaptive immune responses to a variety of different pathogens (reviewed in reference 15). DC have been categorized into several subsets with distinct phenotypes and functional capacities (31). Resident DC identified in the secondary lymphoid tissues can be divided into conventional (or myeloid) DC (consisting of cells expressing CD11c and combinations of CD8α and/or CD11b+) and a CD11c+ B220+ subset that has been referred to as plasmacytoid DC (pDC) or interferon (IFN)-producing cells. These subsets can also be generated in vitro by culturing bone marrow cells in the presence of Flt3L (referred to here as F-DC). Shortman and Naik also describe an inflammatory DC subset that is recruited in vivo upon infection (31). DC with corresponding phenotypic and functional characteristics to inflammatory DC can also be generated upon culture of mouse bone marrow in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) (these DC are referred to here as G-DC). These DC subsets respond to different sets of stimuli and play distinct roles in innate and adaptive immunity in vivo. The goal of the experiments presented here was to determine the differences in responsiveness of F-DC versus G-DC to vesicular stomatitis virus (VSV).
Studies have shown that pDC often play important roles in the innate response to virus infection. For example, pDC are the major type I IFN-producing cell type in mice infected with wild-type (wt) VSV (6). Although the role of pDC in antiviral immunity in vivo is controversial, it is believed that pDC are generally less effective than myeloid DC (mDC) in the direct activation of T cells (8). In addition to their known role as sources of type I IFN to stimulate mDC to prime T cells, pDC provide initial CD40L signals to mDC to promote their ability to present antigen to CD4+ and CD8+ T cells (39). In fact, studies indicate that cooperation between subtypes is important for immunity against a variety of pathogens (30).
DC are known to recognize the presence of many invading organisms via receptors belonging to the Toll-like receptor (TLR) family (reviewed in references 22 and 38). TLRs are important for the recognition of pathogen-specific molecular patterns derived from both viral and bacterial species. Engagement of a TLR by a pathogen initiates signal transduction events, leading to the induction of inflammatory cytokines and costimulatory molecules by DC (reviewed in reference 32). Although TLR signaling is differentially regulated in a TLR-specific manner, many TLR transduce signals through the adaptor protein MyD88. TLR7, a MyD88-dependent TLR, is required for responsiveness to wt VSV as well as another negative-strand RNA virus, influenza virus, by pDC (11, 29).
We have previously reported that wt VSV suppresses maturation of G-DC due to inhibition of host gene expression by the wt matrix (M) protein (1). In contrast, an oncolytic M protein mutant of VSV, rM51R-M virus, stimulates the maturation of G-DC independently of MyD88. rM51R-M virus contains an methionine to arginine substitution at position 51 of the M protein. This mutation renders the virus defective in the ability to inhibit host gene expression but does not compromise the expression of viral genes or the production of infectious progeny (4, 21). Because of its inability to shut off host gene expression, rM51R-M virus induces the expression of genes involved in antiviral responses, including the type I IFN response, in infected cells (4). Therefore, induction of the IFN pathway by M protein mutant VSV most likely provides an activating signal for G-DC. These results led us to postulate that, as in the case of G-DC, rM51R-M virus may stimulate pDC by a mechanism distinct from that by wt virus.
In the experiments presented here, we tested the ability of rM51R-M virus to induce the maturation of F-DC compared to its isogenic recombinant wt counterpart, rwt virus. Both rwt and rM51R-M viruses induced maturation of F-DC, as indicated by an increase in the expression of the DC costimulatory molecules CD40, CD80, and CD86, with the subsequent expression of type I IFN. Separated CD11b+ and B220+ F-DC underwent maturation in response to both rwt and rM51R-M viruses. Both viruses were also defective at inhibiting host gene expression in F-DC, including the expression of genes involved in the antiviral response. The data from F-DC generated from IFN-α receptor knockout (IFNAR−/−) mice demonstrated that the maturation of F-DC by rwt virus was dependent on the type I IFN response, while maturation by rM51R-M virus was only partially dependent on this molecule. Furthermore, whereas F-DC maturation induced by rwt virus was dependent on TLR7 and MyD88, maturation induced by rM51R-M virus was largely independent of MyD88 and TLR7. These results indicate that although both viruses induce F-DC maturation, F-DC detect and respond to rM51R-M virus by means that are distinct from rwt virus.
MATERIALS AND METHODS
Mice.
C57/BL6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). MyD88−/− mice were kindly provided by D. Golenbock (University of Massachusetts), with the permission of S. Akira. IFN receptor-negative (IFNAR−/−) mice were a generous gift of S. Mizel (Wake Forest University), with the permission of C. Schindler (Columbia University). TLR7−/− mice were provided by R. Flavell (Yale University). All mice were maintained and bred in the animal facility at Wake Forest University School of Medicine.
Cells and viruses. (i) DC propagation.
G-DC were generated as previously described (17). Briefly, bone marrow was removed from the tibias and femurs of 8- to 10-week-old mice. After red cell lysis and washing, progenitor cells (5 × 105/ml) were resuspended and plated in RPMI containing 10% fetal calf serum supplemented with 20 ng of GM-CSF/ml (without antibiotics) for 6 days. F-DC were generated from mouse bone marrow as previously described (14). Progenitor cells (1.2 × 106/ml), obtained as described above, were resuspended and plated in RPMI containing 10% fetal calf serum supplemented with 100 ng of Flt3L/ml for 9 days.
(ii) Separation of CD11b+ and B220+ cells.
CD11b+ and B220+ cells were separated from F-DC using the MACS separation method (Miltenyi Biotec) with some modifications. Briefly, F-DC were incubated with B220 MicroBeads for 15 min at 4°C, washed, and placed over a MACS column inside the magnetic field of a MACS separator. The column was rinsed with MACS buffer, and B220− cells (CD11b+ cells) were eluted and collected. B220+ cells were collected by elution through the column with MACS buffer in the absence of a magnetic field. The CD11b+ cells were run through a second MACS column to remove any contaminating B220+ cells. Cells were counted and resuspended in RPMI containing 10% fetal calf serum. An aliquot of each subset was taken for staining with CD11b or B220 antibodies to check purity of cells after selection.
(iii) Virus growth and preparation.
The recombinant viruses, rwt and rM51R-M, were isolated from infectious VSV cDNA clones, and virus stocks were prepared as described previously (21). Briefly, viruses were grown on BHK cell monolayers. Supernatants containing progeny virions were harvested, titrated, and stored at −80°C. To rule out the possibility of endotoxin contamination in the stocks, DC were treated with supernatants from parallel cultures of uninfected BHK monolayers, and costimulatory molecule expression and cytokine levels were measured.
Reagents.
Recombinant mouse GM-CSF and human Flt3L were purchased from Invitrogen (Camarillo, CA). Fluorescence-labeled antibodies to mouse cell surface antigens (CD40-phycoerythrin [CD40-PE; clone 3/23], CD80-PE [clone 16-10A1], CD86-PE [clone GL1], CD11c-allophycocyanin [CD11c-APC; clone HL3], CD11b-APC [clone M1/70], B220-APC [CD45R/B220-APC; clone RA3-6B2], recombinant immunoglobulin G-PE [rIgG-PE], and rIgG-APC) were purchased from BD Biosciences/Pharmingen (San Diego, CA). For use as TLR agonists, lipopolysaccharide (LPS) from Salmonella enterica serovar Minnesota was purchased from Sigma (St. Louis, MO) and used at a concentration of 100 to 300 ng/ml; poly(I-C) (used at 25 μg/ml) and loxoribine (Lox; used at 200 μM) were purchased from InvivoGen (San Diego, CA).
Viral infection of DC.
F-DC or G-DC, cultured as described above, were harvested, plated in 48-well plates at a density of 5 × 105/well, and infected with either rwt or rM51R-M virus at the indicated multiplicity of infection (MOI) for 1 h in a small volume. The volume was then restored to 1 ml, and the cells were cultured for an additional 23 h to allow time for maturation.
Measurement of costimulatory molecule and cytokine expression by DC after viral infection.
F-DC (or CD11b+ or B220+ subsets) were plated in 48-well plates at a density of 5 × 105 DC/ml in antibiotic-free medium. The cells were then infected with virus at the indicated MOIs or treated with the indicated TLR agonist and then incubated for 24 h. Culture supernatants were removed, and the presence of interleukin-6 (IL-6) and IL-12 p40 was measured by using OptiEIA kits (BD Pharmingen). Type I IFN in the supernatants was measured by a bioassay or using an IFN-β enzyme-linked immunosorbent assay (ELISA) kit (PBL). The cells were harvested and stained with PE-conjugated antibodies to murine CD40, CD80, and CD86. Nonspecific background staining was assessed using isotype control antibodies (rat IgG-PE for CD40, CD80, and CD86). DC were costained with antibodies to CD11c or to CD11b and B220 to enable analysis of costimulatory molecules expressed by the distinct DC populations. In addition, dead cells were excluded from analysis based on forward- and side-scatter gating.
IFN assays. (i) IFN bioassay.
To determine the IFN activity produced by cells infected with wt and mutant viruses, supernatants (500 μl) were collected from F-DC infected with rwt or rM51R-M virus or treated with poly(I-C) or Lox for 24 h. The bioassay was carried out as previously described (1). Briefly, infectious virus was inactivated by acid treatment (pH 2), the acid was neutralized, and serial dilutions were incubated overnight with L929 cells in 96-well plates at 37°C. To construct a standard dose-response curve, cells were incubated with serial fivefold dilutions of IFN (universal type I IFN; PBL Biomedical Laboratories, New Brunswick, NJ). The samples were aspirated, and cells were challenged with wt VSV at an MOI of 5 PFU/cell in 100 μl of medium. Controls included cells infected with VSV alone and cells that were not challenged with VSV. Cells were incubated overnight at 37°C, the medium was aspirated, and the cells were fixed with 95% ethanol. Cells were then stained with a 0.1% crystal violet solution in methanol. The absorbance was read at 550 nm on an ELISA reader. IFN levels of >500 IU/ml were detectable by this bioassay.
(ii) IFN-β ELISA.
IFN-β levels in the supernatants of infected or treated DC were determined by using a VeriKine Mouse Interferon Beta ELISA kit (PBL Biomedical Laboratories) according to the manufacturer's directions.
35S radiolabeling of infected cells.
To analyze protein synthesis during virus infections, G-DC and F-DC (5 × 105 cells) were infected with rwt or rM51R-M virus at an MOI of 10 PFU/cell in RPMI containing 10% fetal bovine serum (FBS). At different times postinfection, cells were labeled with a 15-min pulse of [35S]methionine (100 μCi/ml) in a total volume of 0.3 ml of methionine-free medium. Cells were washed with phosphate-buffered saline and harvested in radioimmunoprecipitation assay buffer (0.15 M NaCl, 1% deoxycholate, 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS], 10 mM Tris [pH 7.4]). Extracts normalized for protein levels (detected by the Lowry protein assay) were electrophoresed by SDS-10% polyacrylamide gel electrophoresis (PAGE) and were analyzed by phosphorescence imaging as described previously (21).
Growth curves.
G-DC and F-DC were infected with rwt or rM51R-M viruses in six-well dishes at the indicated MOIs in RPMI containing 10% FBS. Cells were washed at 1 h postinfection and recultured in 3 ml of fresh medium. At 24 h postinfection, 100 μl of medium was removed from each dish and stored at −70°C. The yields of virus were determined by plaque assays on BHK cells and were expressed as PFU/ml.
Microarray analysis.
Cells were infected with rwt or rM51R-M viruses at an MOI of 1 PFU/cell in RPMI containing 10% FBS. At 6 h postinfection, cells were harvested, and total RNA was isolated by using TRIzol reagent (Invitrogen). Each RNA sample was processed according the manufacturer's protocol (Affymetrix) and hybridized to the Affymetrix Mouse Genome MOE 430A array representing 14,000 well-characterized mouse genes. Each chip was scaled to a target intensity of 500, normalized to the 100 control probe sets present on each chip, and then expressed as a ratio to the nonspecific background on a per-gene basis. Analysis of data was carried out by using Affymetrix Data Mining Tool software (Affymetrix).
RESULTS
wt and M protein mutant viruses induce maturation of Flt3L DC.
We have previously demonstrated that rwt virus kills myeloid-like bone marrow-derived G-DC, while rM51R-M virus stimulates the maturation of these cells (1). The difference in response to these two viruses is due to the inhibition of host gene expression by wt M protein, while the mutant M protein is defective in the inhibition of host gene expression. In contrast to G-DC, it has been demonstrated by studies from other groups that pDC produce type I IFN in response to both wt and M protein mutant viruses (29, 37), suggesting that these cells are protected from the shutoff of host gene expression and killing by wt VSV. To determine whether pDC undergo maturation in response to both wt and mutant viruses, we tested the effect of these viruses on the expression of costimulatory molecules on DC generated from murine bone marrow cells cultured in the presence of Flt3L. These culture conditions give rise to two populations of DC resembling those found resident in the spleen, including B220+ pDC, as well as CD11b+ conventional DC. Both of these DC populations respond to the TLR7 agonist loxirubine (lox) and are also effective producers of type 1 IFN (data not shown).
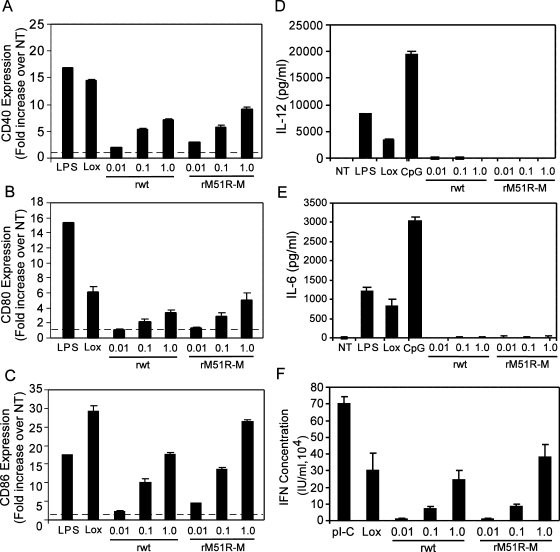
F-DC were infected with either rwt or rM51R-M viruses at different MOIs. Cells treated with LPS or the TLR7 agonist, Lox, served as positive controls, while untreated, immature DC served as a negative control. At 24 h, cells were harvested and stained with antibodies to the costimulatory molecules CD40, CD80, and CD86. Cells were costained with the DC cell surface marker CD11c, and flow cytometry was performed to determine the cell surface expression of CD40, CD80, and CD86 on the CD11c+ cells. The data were quantitated by determining the fold increase in costimulatory molecule expression on infected or treated cells compared to untreated, immature DC. Our results demonstrate that both rwt and rM51R-M viruses enhanced cell surface expression of CD40, CD80, and CD86 in a dose-dependent manner (Fig. 1). Specifically, we observed an ∼5-fold increase in CD40 expression, a 4-fold increase in CD80 expression, and a 20-fold increase in CD86 expression in F-DC upon infection with 1 PFU of rwt virus/cell (Fig. 1A, B and C, respectively). Increases in costimulatory molecule expression were slightly greater in rM51R-M virus-infected cells than on those infected with rwt virus, especially in the case of CD86 (Fig. 1C). However, in contrast to our previous findings in G-DC, in which only the rM51R-M virus induced costimulatory molecule expression, the F-DC responded strongly to both viruses by upregulating the expression of CD40, CD80, and CD86.
FIG. 1.
wt and M protein mutant viruses induce maturation of F-DC. F-DC were infected with rwt or rM51R-M virus at multiplicities of 0.01, 0.1, and 1 PFU/cell or were treated with LPS, Lox, or pI-C. At 24 h postinfection or posttreatment, the cell surface expression of CD40, CD80, and CD86 was measured by flow cytometry. The geometric mean fluorescence of each sample was determined and used to quantitate the increase in CD40 (A), CD80 (B), and CD86 (C) expression over that in untreated cells (NT). Culture supernatants from infected or treated cells were collected and the presence of IL-12 p40 (D) and IL-6 (E) was measured by ELISA. The data are expressed in pg of cytokine secreted/ml into the supernatant. (F) Type I IFN in the supernatant was measured by a bioassay based on the reduction of VSV cytopathic effects. The IFN concentration (in IU/ml) was quantitated by comparing the results to those in cells incubated with serial dilutions of an IFN standard. The data were normalized to the percentage of viable cells and are the means ± the standard errors for three experiments.
To determine how the infection of F-DC with VSV affects the production of cytokines, supernatants from VSV-infected F-DC were collected 24 h after treatment. The secretion of IL-12p40 and IL-6 was measured by ELISA, and the production of type I IFN was determined by an IFN bioassay. The data were normalized to the percentage of viable cells in the culture. Interestingly, our results indicate that neither IL-12p40 nor IL-6 (Fig. 1D and E, respectively) was induced by infection with either rwt or rM51R-M viruses, whereas each of the positive controls—LPS, Lox, and the TLR9 agonist CpG—induced secretion of these cytokines. However, both viruses stimulated production of type I IFN (Fig. 1F) to levels comparable to that obtained with Lox. These data indicate that both wt and mutant viruses induce the maturation of F-DC.
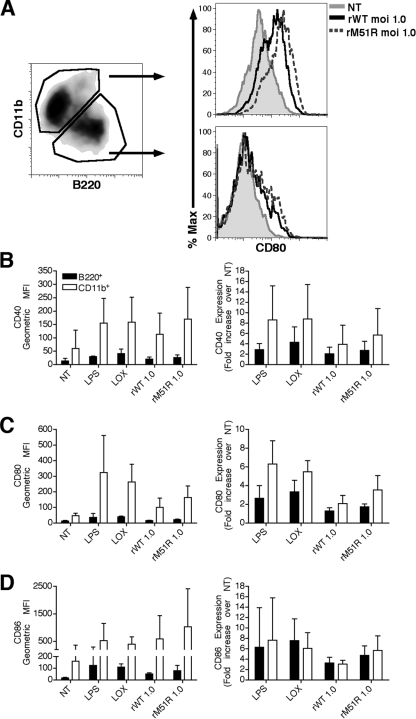
Both B220+ and CD11b+ populations in F-DC respond to wt and M protein mutant viruses.
As stated previously, treatment of murine bone marrow with Flt3L generates two distinct subsets of DC. As illustrated in the left panel of Fig. 2A, at day 9 of culture ca. 60% of the cells were CD11b+ B220−, resembling conventional DC, and ca. 40% of the cells were CD11b− B220+, correlating with pDC. To determine how both subsets of cells responded to infection with each virus, we monitored the expression of costimulatory molecules in combination with the subset markers CD11b or B220 after infection. As controls, cells were also treated with the TLR agonists LPS and Lox. The level of expression of CD80 on each gated population is depicted in the right panels of Fig. 2A. In general, the level of expression of CD40, CD80, and CD86 on the B220+ cells was markedly lower than that of the CD11b+ cells in both resting and stimulated conditions (Fig. 2B, C, and D, respectively). We observed that in both populations there was an increase in the expression of costimulatory molecules after infection with either rwt or rM51R-M virus, although the response to rM51R-M virus was stronger. Although the relative level of expression of costimulatory molecules was much higher in the CD11b+ than in the B220+ cells after infection, the background or nonstimulated level of expression was also higher on the CD11b+ cells. Thus, we also calculated the relative fold increase in costimulatory molecule expression for each cell type over its unstimulated background (Fig. 2, right panels of B, C, and D). This analysis revealed that the B220+ DC showed comparable fold upregulation of CD86 as the CD11b+ cells, but their fold upregulation of CD80 and CD40 was weaker than the CD11b+ DC. These data argue that the high level of costimulatory molecule expression observed in the mixed population was primarily due to the CD11b+ cells. In terms of patterns of response to stimulation, we observed a strong upregulation of CD86 in both populations in response to Lox, but a more robust increase in response to LPS in CD11b+ than in B220+ cells. This finding is consistent with previous findings that CD11b+ DC express higher levels of TLR4 than the B220+ cells (18).
FIG. 2.
B220+ and CD11b+ populations in F-DC respond to rwt and rM51R-M viruses. F-DC were infected with rwt or rM51R-M virus at a multiplicity of 1 PFU/cell or treated with Lox or LPS for 24 h. After infection, the expression of costimulatory molecules was measured in combination with the subset markers CD11b or B220 by flow cytometry. The level of expression of CD80 on each gated population is depicted in the histograms shown in panel A. The cell surface expression of CD40 (B), CD80 (C), and CD86 (D) in each sample is shown as the geometric mean fluorescence (left side) and as the increase in costimulatory molecule expression over that in untreated cells (NT) (right side). The data are the means ± the standard errors for three experiments.
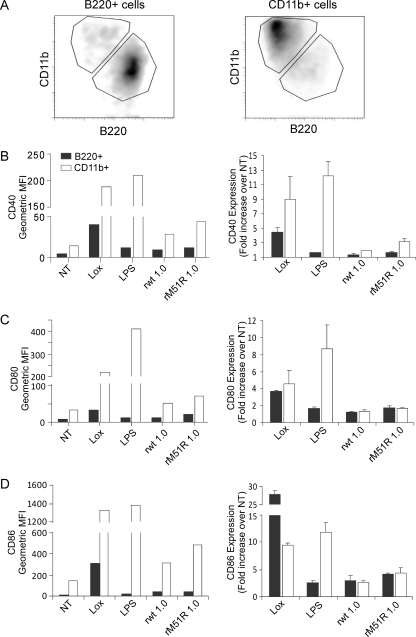
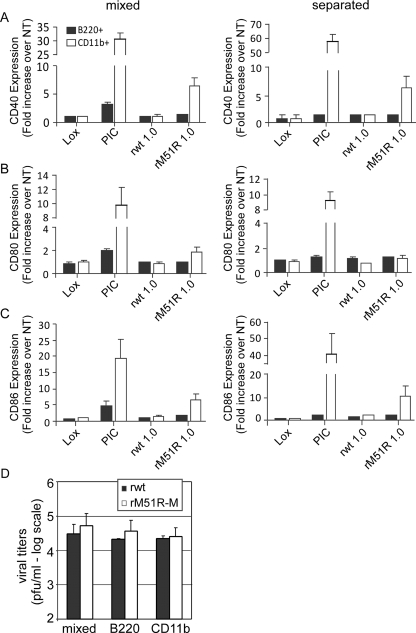
To determine whether the results obtained in Fig. 2 reflect the responses of the individual subsets to virus infection, CD11b+ and B220+ subsets were separated from F-DC, and each population was infected with rwt or rM51R-M viruses or treated with LPS or Lox, as described above. After infection or treatment, the expression of costimulatory molecules was determined in combination with the subset markers CD11b or B220 (Fig. 3). From our separation method, we obtained CD11b+ and B220+ populations with >90% purity (Fig. 3A). As in the mixed population, the level of expression of CD40, CD80, and CD86 on the separated B220+ cells was lower than that of the separated CD11b+ cells under all conditions (Fig. 3B, C, and D, respectively, left panels). We also observed an increase in the levels of costimulatory molecule expression in both populations in response to the TLR agonists, Lox and LPS. Although both cell types responded to rwt and rM51R-M viruses, the magnitude of the response was not as great as that observed in the mixed population, especially in the case of rwt virus. When we calculated the fold increase in costimulatory molecule expression versus untreated cells (Fig. 3B, C, and D, right panels), the data revealed that upon infection with both rwt and rM51R-M viruses, the B220+ cells showed comparable fold upregulation of CD80 and CD86 as the CD11b+ DC, while their level of expression of CD40 was lower than in the CD11b+ DC. We observed a strong upregulation of CD40, CD80, and CD86 in both populations in response to Lox but, as seen in the mixed population, the levels of these molecules in response to LPS was greater in CD11b+ cells than in B220+ cells. Overall, these results indicate that the separated B220+ and CD11b+ subsets respond to VSV and to stimulation with Lox and LPS in a manner that is similar to that observed in the mixed population. In addition, the cross talk between the two subsets in the mixed cultures appears to enhance the maturation of both populations. Due to the similarity in responses between the mixed and separated populations we chose, in subsequent experiments, to further analyze the response of the mixed F-DC population to VSV.
FIG. 3.
Separated B220+ and CD11b+ populations in F-DC respond to rwt and rM51R-M viruses. The B220+ and CD11b+ populations in F-DC were separated and infected with rwt or rM51R-M virus at an MOI of 1 PFU/cell or treated with Lox or LPS for 24 h. After infection, the expression of costimulatory molecules was measured in combination with the subset markers CD11b or B220 by flow cytometry. The efficiency of separation of the CD11b+ and B220+ DC populations is shown in panel A. The cell surface expression of CD40 (B), CD80 (C), and CD86 (D) in each sample is shown as the geometric mean fluorescence (left side) and is representative of two individual experiments. The increase in costimulatory molecule expression over that in untreated cells (NT) was quantitated (right side). The data are the means ± the standard deviations for two experiments.
F-DC are resistant to shutoff of host protein synthesis by wt and M protein mutant VSV.
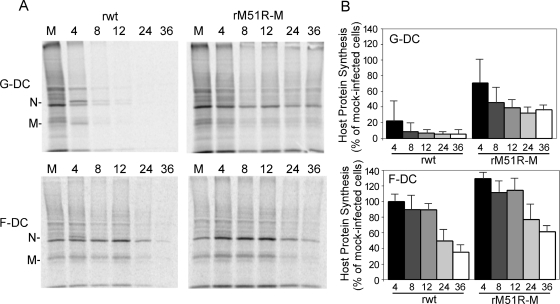
Our earlier observations in G-DC indicated that the ability of rM51R-M virus to induce maturation of virus-infected cells is related to its reduced ability to inhibit host gene expression (1). To determine the rates of protein synthesis in F-DC compared to G-DC infected with wt and mutant viruses, cells were infected at an MOI of 10 PFU/ml and pulse-labeled with [35S]methionine for 15 min at different times postinfection. Proteins were solubilized, and equivalent amounts of protein were subjected to SDS-PAGE and phosphorescence imaging. Representative images are shown in Fig. 4A. The positions of the viral proteins are indicated to the left in the images. As observed previously, rwt virus effectively inhibited host translation in G-DC over time compared to mock-infected cells. This is clearly seen in the regions of the gel that are devoid of viral proteins. However, rM51R-M virus was less effective at shutting off host protein synthesis. In contrast to results obtained in G-DC, F-DC were relatively resistant to shutoff of host protein synthesis by both rwt and rM51R-M viruses. Host protein synthesis in infected cells at different times postinfection was quantitated from images similar to that shown in Fig. 4A. The data in Fig. 4B are shown as percentages of the values for the mock-infected control. rwt virus effectively inhibited G-DC translation at early times postinfection, whereas host protein synthesis in F-DC infected with this virus was not markedly inhibited at 8 h postinfection and was still 40 to 60% that of the control at 24 h. F-DC were also more resistant than G-DC to shutoff of host protein synthesis by rM51R-M virus. Based on these data, we can conclude that both wt and M protein mutant viruses effectively stimulate the maturation of F-DC, most likely due to their inability to shut off host gene expression in these cells.
FIG. 4.
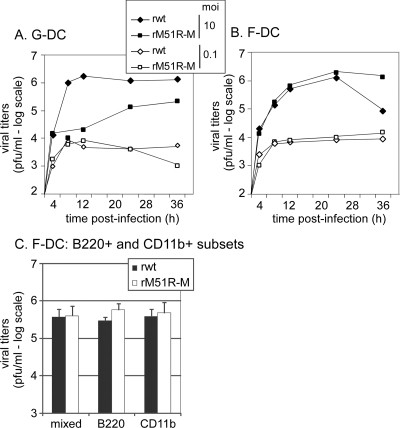
F-DC are more resistant to shutoff of host gene expression by VSV than G-DC. G-DC and F-DC were infected with rwt or rM51R-M virus at an MOI of 10 PFU/cell or were mock infected as a control. At different times postinfection, cells were labeled with a 15-min pulse of [35S]methionine (100 μCi/ml) and harvested. Lysates were subjected to SDS-PAGE, and labeled proteins were quantitated by phosphorimaging. (A) Representative images from analysis of rwt and rM51R-M virus-infected G-DC and F-DC. (B) Host protein synthesis was determined from images similar to that shown in panel A for regions of the gel devoid of viral proteins. The results are shown as percentages of the mock-infected control value and are the means ± the standard errors of three independent experiments.
F-DC are equally permissive for rwt and rM51R-M viruses.
To determine whether F-DC were permissive to infection with rwt and rM51R-M viruses, yields of progeny virus were determined by a plaque assay, and titers were compared to those in VSV-infected G-DC. G-DC infected with rwt virus at an MOI of 10 PFU/cell produced progeny virus at ∼106 PFU/ml by 8 h postinfection (Fig. 5A). Virus titers from cells infected with rM51R-M virus were consistently 1 to 2 logs lower than those in G-DC infected with rwt virus. As observed previously, these results indicate that G-DC are more susceptible to infection with rwt virus than with rM51R-M virus. However, at the lower MOI (0.1 PFU/cell), both viruses were attenuated for growth in G-DC and produced progeny virus at 104 PFU/ml.
FIG. 5.
Viral growth analysis in DC. G-DC (A) or F-DC (B) were infected with rwt or rM51R-M virus at MOIs of 10 and 0.1 PFU/cell. At different times postinfection, supernatants were collected to determine the amounts of progeny virus by a plaque assay. The data are the means of two to three experiments. (C) Virus titers in F-DC and the B220+ and CD11b+ subsets. B220+ and CD11b+ cells were separated and infected with rwt or rM51R-M viruses at an MOI of 1 PFU/cell. Virus titers were determined at 24 h postinfection and compared to those obtained in the mixed F-DC culture. The data are the means ± the standard deviations for two experiments.
In contrast to G-DC, F-DC were equally permissive for both rwt and rM51R-M viruses at an MOI of 10 PFU/cell (Fig. 5B). However, while titers were maintained at 106 PFU/ml in rM51R-M virus-infected F-DC at later times postinfection, supernatants of cells infected with rwt virus displayed decreasing titers. Further studies indicated that this was due to a loss in viability in rwt virus-infected cells at later time points (data not shown). At the lower MOI, both viruses grew to similar titers and results were very similar to those observed in VSV-infected G-DC. These data indicate that both rwt and rM51R-M viruses grow with similar kinetics in F-DC.
To determine which population in F-DC is the major producer of viral progeny upon infection with rwt or rM51R-M virus, the B220+ and CD11b+ subsets were separated and infected at an MOI of 1 PFU/ml. Virus titers were determined at 24 h postinfection, and the results were compared to titers obtained from the mixed population (Fig. 5C). As observed in Fig. 5B, F-DC (mixed population) infected with either rwt or rM51R-M viruses produced similar levels of progeny virus. Furthermore, there was no difference between the mixed and the B220+ and CD11b+ subsets in their ability to support the growth of rwt or rM51R-M virus by 24 h. Together with the data obtained in Fig. 3, we can conclude that the B220+ and CD11b+ populations are equally permissive and responsive to rwt and rM51R-M viruses.
F-DC respond to wt and M protein mutant viruses by upregulating expression of genes in the type I IFN pathway.
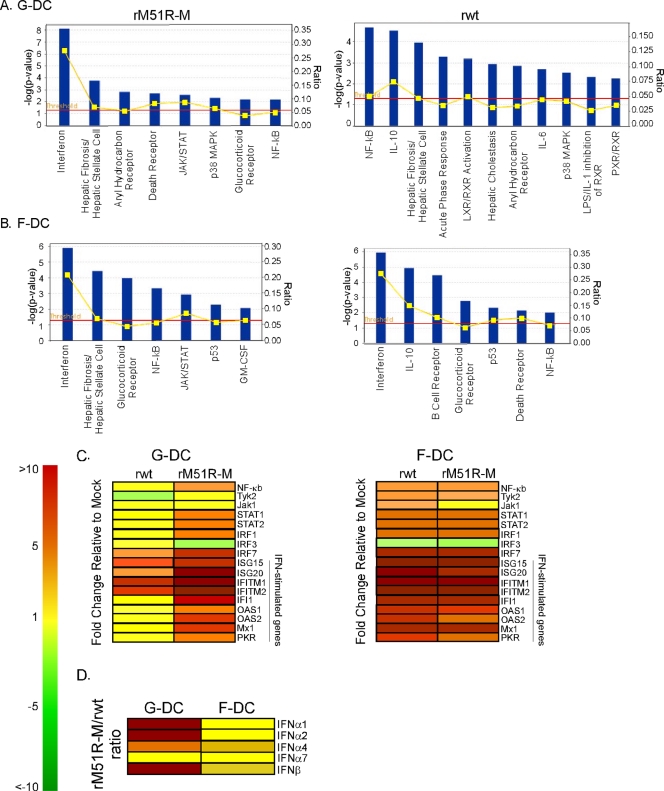
A consequence of the inhibition of host gene expression by wt VSV is the suppression of the host antiviral response (4). Therefore, F-DC resistance to both wt and mutant VSV may be due to the inability of these viruses to block the type I IFN response in infected cells. This hypothesis was tested by microarray analysis of mRNAs encoding antiviral proteins expressed in mock-infected or in rwt- and rM51R-M virus-infected G-DC and F-DC. Cells were mock infected or VSV infected for 6 h, an early time of infection allowing analysis of the initial response of cells to virus infection. Total RNA was isolated, and the expression of mRNAs for more than 33,000 characterized genes was analyzed. In order to generate an analysis that was not biased toward a particular pathway, all mRNAs detected in either G-DC or F-DC were tested for statistical association with a data set of canonical pathways using Ingenuity Pathways Analysis software. In this analysis, 80 different signaling pathways were evaluated for the fraction of genes associated with each pathway in the database that were differentially expressed (either up- or downregulated) and for the statistical probability that the effect on the pathway would be due to random chance. The data were analyzed as the ratio of expression of each gene in VSV-infected cells versus mock-infected cells (rwt/mock or rM51R-M/mock), with the criteria for whether a gene was differentially expressed set at a ratio of ≥4.0 or ≤−4.0. These criteria included the average number of differentially expressed genes recommended by the software (≤500 out of ≥6,000 eligible for pathway analysis) and excluded nearly all genes whose expression was not significantly different in repeated experiments.
The signaling pathways statistically associated with the difference in gene expression between VSV-infected cells and mock-infected cells in G-DC (Fig. 6A) and F-DC (Fig. 6B) were sorted according to the statistical significance of the association shown on the y axis (dark blue bars; the dashed line shows P = 0.05). Also shown on the alternative y axis is the fraction of genes associated with each pathway in the database that were differentially expressed (yellow line). In G-DC infected with rM51R-M virus (Fig. 6A), the IFN signaling pathway had the highest fraction of differentially expressed genes (8/29 = 0.28) and a high level of statistical significance [P = 7.65 × 10−9, −log(P) = 8.12]. In fact, each of the differentially expressed genes associated with this pathway was upregulated. These included STAT1 and STAT2, transcription factors in the type I IFN signaling pathway, and OAS1, an IFN-responsive antiviral gene. Similar results were obtained in F-DC infected with both rwt and rM51R-M viruses (Fig. 6B). In contrast, genes in the type I IFN pathway were not significantly altered in G-DC infected with rwt virus (note the difference in scale). These results indicate that G-DC infected with rM51R-M virus and F-DC infected with both viruses respond by activating the antiviral IFN signaling pathway, whereas rwt virus-infected G-DC are unable to mount an antiviral response, most likely due to suppression of host gene expression by wt M protein.
FIG. 6.
Analysis of mRNA expression in VSV-infected G-DC and F-DC. Microarray analysis of rwt and rM51R-M virus-infected cells was performed and mRNA levels were analyzed by Ingenuity Pathway Analysis software (A and B). Results, indicated by dark blue bars, for genes that were expressed differentially between rwt or rM51R-M virus-infected cells and mock-infected cells in G-DC (A) and F-DC (B) were analyzed for association with any of 80 canonical pathways and are sorted according to their statistical significance of association (P < 0.05, indicated by the red line). An alternate axis (yellow line) shows the fraction of genes associated with each pathway in the database that were differentially expressed between VSV-infected and mock-infected cells. (C) Genes in the IFN signaling pathway. Genes in red were overexpressed in VSV-infected cells relative to mock-infected cells. Genes in yellow were present in similar levels in VSV-infected and mock-infected cells, while genes in green were expressed at higher levels in mock-infected cells relative to VSV-infected cells. (D) Expression of IFN-α and IFN-β genes in virus-infected cells.
Figure 6C shows data for specific genes in the type I IFN pathway whose expression was significantly altered (P < 0.05) upon infection with rwt or rM51R-M viruses compared to mock-infected cells. In G-DC, rM51R-M virus upregulated expression of genes in the IFN signaling pathways (i.e., NF-κB, STAT1, and STAT2) to higher levels than those observed by rwt virus. Furthermore, rM51R-M virus also promoted expression of a number of IFN-inducible genes, including OAS2, Mx1, and PKR, while expression of these antiviral genes in rwt virus-infected G-DC was not detected above the signal from mock-infected cells. These results are consistent with the observation that rwt virus suppresses the host antiviral response in G-DC as a result of inhibition of host gene expression (Fig. 4), while rM51R-M virus promotes this response. However, in F-DC, both viruses enhanced the expression of genes in the type I IFN pathway over mock-infected cells. In fact, in some cases, F-DC infected with rwt virus expressed these genes at levels higher than those observed in rM51R-M virus-infected cells (i.e., OAS2 and PKR).
Figure 6D shows the expression of IFN-α and βgenes as the ratio in rM51R-M-infected cells to rwt-infected cells (these mRNAs were not detected in mock-infected cells). Interestingly, both viruses induced expression of IFN-α and -β genes in G-DC. However, the levels of stimulation of several IFN-α genes and the IFN-β gene were substantially greater in rM51R-M virus-infected G-DC than in cells infected with rwt virus. In contrast, both viruses induced expression of these genes to similar levels in F-DC. These results are consistent with our data indicating that F-DC respond to both rwt and rM51R-M viruses by mounting an antiviral response, further suggesting that the activation of the type I IFN pathway is important for VSV-induced maturation of F-DC.
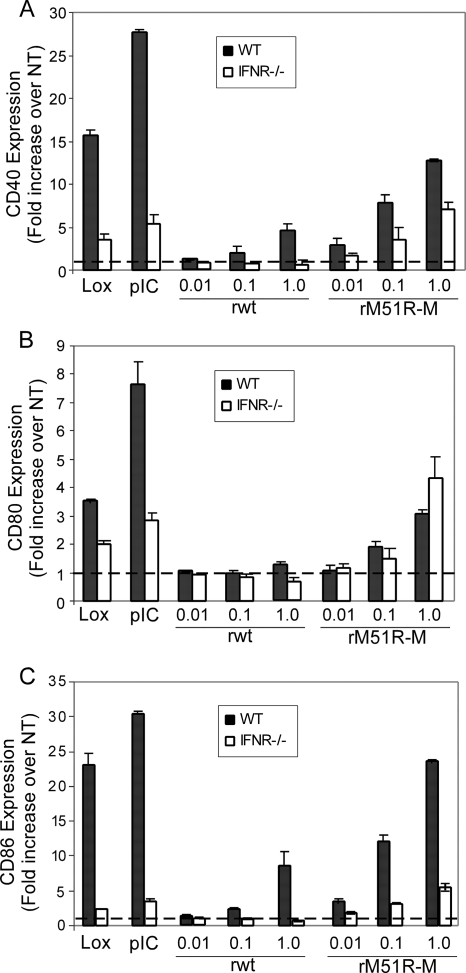
Maturation of Flt3L DC by VSV is largely dependent on the type I IFN response.
Our data indicate that the type I IFN response pathway is a major arm of the response elicited by both rwt and rM51R-M viruses in these DC. To directly determine the requirement for signaling through the type I IFN receptor in the maturation of F-DC induced by rwt and rM51R-M viruses, we compared the response of these cells lacking this molecule (IFNAR−/−) to that of wt F-DC upon infection with VSV (Fig. 7). As expected, the expression of CD40, CD80, and CD86 induced by both Lox and poly(I-C) was strongly inhibited in the absence of the type I IFN receptor (Fig. 7A, B, and C, respectively). The induction of costimulatory molecules CD40 and CD86 by rwt virus was completely ablated in IFNAR−/− F-DC. However, in rM51R-M virus-infected F-DC, the upregulation of CD40 and CD86 was only partially reduced in the IFNAR−/− F-DC, while CD80 expression was slightly enhanced. These data indicate that while the expression of costimulatory molecules induced by rM51R-M virus is not completely dependent on the type I IFN response, the response of F-DC to rwt virus is almost entirely dependent on this molecule.
FIG. 7.
Expression of costimulatory molecules on IFNR−/− F-DC by rwt and rM51R-M virus. F-DC derived from wt or IFNR−/− mice were infected with rwt and rM51R-M virus and treated with Lox or poly(I-C) for 24 h. The cell surface expression of CD40 (A), CD80 (B), and CD86 (C) was determined by flow cytometry. The data represent the means ± the standard errors of three experiments.
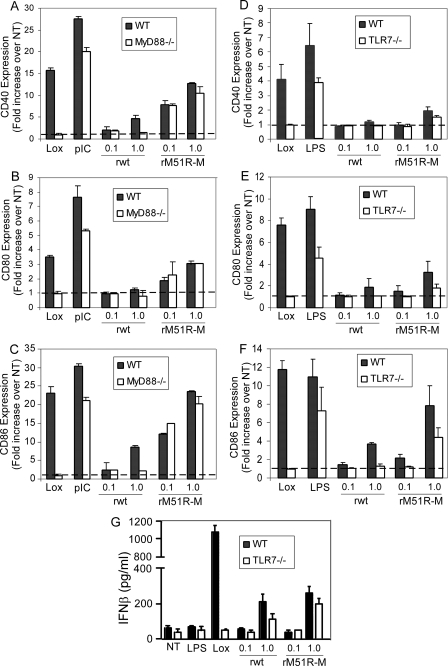
Differential requirement for MyD88 and TLR7 in the response of F-DC to rM51R-M or rwt viruses.
Our results thus far demonstrate that both rwt and rM51R-M viruses stimulate the maturation of F-DC. Other studies have demonstrated that recognition of wt VSV by pDC is mediated by TLR7, a MyD88-dependent TLR. To test whether the maturation of F-DC by both viruses was dependent on MyD88 and TLR7, F-DC from MyD88−/− and TLR7−/− mice were infected with wt and mutant viruses and costimulatory molecule expression was compared to that of wt DC (Fig. 8). As expected, the response to Lox, a TLR7-dependent stimulus, was completely ablated in the MyD88−/− (Fig. 8A to C) and TLR7−/− (Fig. 8D to F) DC. However, the response to poly(I-C), a MyD88-independent stimulus, was not greatly affected in the absence of MyD88 (Fig. 8A to C), and LPS, a TLR4 agonist, was not significantly affected in the absence of TLR7 (Fig. 8D to F). In rwt virus-infected F-DC, we observed that the induction of CD40 and CD86 was completely ablated in the absence of both molecules. However, the upregulation of these costimulatory molecules stimulated by rM51R-M virus was not affected by the loss of MyD88 and was only partially diminished in TLR7−/− cells. We also examined the requirement for TLR7 in expression of IFN-β induced by both viruses (Fig. 8G). Similar to the costimulatory molecule data, we observed that the IFN-β induced by the TLR7 agonist, Lox, was completely abolished in the absence of TLR7. However, IFN-β induced by the rwt virus was diminished by about half while that induced by the rM51R-M was not significantly diminished in the absence of TLR7. These data suggest that there may be alternative pathways for the induction of type I IFN by VSV in the absence of TLR7 in these cells. Taken together, these data indicate that the response to rwt virus in F-DC is mediated through a TLR7-MyD88-dependent pathway. This may explain why G-DC, which are nonresponsive to TLR7 agonists, fail to mature upon infection with rwt virus. In contrast to rwt virus, rM51R-M virus induces maturation of F-DC in a manner that does not require MyD88 or TLR7. These results suggest that rwt and rM51R-M viruses use distinct mechanisms to induce maturation of F-DC.
FIG. 8.
Response of MyD88−/− and TLR7−/− F-DC to rwt and rM51R-M virus. F-DC generated from wt and MyD88−/− (A) or TLR7−/− (B) mice were infected with VSV at MOIs of 0.1 and 1 PFU/cell and treated with Lox, LPS, or poly(I-C) for 24 h. The cell surface expression of CD40 (A and D), CD80 (B and E), and CD86 (C and F) was determined by flow cytometry. The data represent the means ± the standard errors of three experiments. (G) IFN-β production in the supernatants of wt versus TLR7−/− F-DC.
One possible mechanism by which rM51R-M virus may stimulate F-DC in the absence of MyD88 or TLR7 is through stimulation of CD11b+ cells in a MyD88-independent manner, leading to the subsequent activation of B220+ cells in the mixed culture. To determine the individual responses of the B220+ versus CD11b+ subsets in MyD88−/− F-DC, these subsets were separated by magnetic labeling and infected with rwt or rM51R-M viruses or treated with Lox or poly(I-C). Costimulatory molecule expression was determined and expressed as the fold increase in CD40, CD80, and CD86 expression over the unstimulated background in the mixed population (Fig. 9A, B, and C, respectively, left panels), and the separated B220+ and CD11b+ DC (right panels). As expected, the response to Lox was ablated in each population, as was the response to rwt virus. Furthermore, the upregulation of costimulatory molecule expression upon treatment with poly(I-C) was greatest in the CD11b+ DC in either the mixed or separated cultures. Similarly, upon infection with rM51R-M virus, we observed a robust increase in expression of costimulatory molecules, especially CD40 and CD86, in the CD11b+ population, whereas the response of B220+ to rM51R-M virus was significantly lower in each subset regardless of whether they were mixed or separated. The low response of MyD88−/− B220+ cells to rM51R-M virus was in contrast to the response obtained in wt B220+ cells (Fig. 3). These data indicate that rM51R-M virus stimulates MyD88−/− F-DC through activation of CD11b+ cells and that while there appears to be cooperation between DC subsets in the wt cultures, this cross talk is not MyD88 dependent.
FIG. 9.
CD11b+ cells from MyD88−/− F-DC respond to rM51R-M virus. The B220+ and CD11b+ populations in MyD88−/− F-DC were separated and infected with rwt or rM51R-M virus at an MOI of 1 PFU/cell or treated with Lox or poly(I-C) for 24 h. After infection, the expression of costimulatory molecules was measured by flow cytometry. The increase in CD40 (A), CD80 (B), and CD86 (C) expression over that in untreated cells (NT) was quantitated for the mixed population (left side) and the separated B220+ and CD11b+ subsets (right side). The data are the means ± the standard deviations for two experiments. (D) Virus titers in mixed and separated MyD88−/− F-DC. B220+ and CD11b+ cells from MyD88−/− F-DC were separated and infected with rwt or rM51R-M viruses at an MOI of 1 PFU/cell. Virus titers were determined at 24 h postinfection and compared to those obtained in the mixed MyD88−/− F-DC culture. The data are the means ± the standard deviations for two experiments.
To determine which population supports virus replication, supernatants of rwt versus rM51R-M virus-infected cells were collected for determination of virus titers. Interestingly, the mixed and the B220+ and CD11b+ subsets were equally permissive for rwt and rM51R-M viruses in the absence of MyD88 as indicated by similar virus titers at 24 h postinfection (Fig. 9D). Therefore, we can conclude that regardless of their response to virus infection, there is no difference in the ability of the different F-DC subsets to support rwt or rM51R-M virus replication.
DISCUSSION
Our data highlight the extent to which viral suppressors of host responses can modify the response of DC to virus infection. In the case of VSV, the M protein is the primary suppressor of host antiviral responses (4, 33). The suppression of host responses by M protein is due to a general shutoff of host gene expression at the level of transcription, nuclear-cytoplasmic transport, and translation (4, 33). Although the mechanism by which M protein inhibits host gene expression is not completely clear, it appears to involve interaction of M protein with host nuclear factors such as Rae1 and Nup98 (12, 35). In contrast to wt M protein, M51R mutant M protein is defective in its ability to inhibit host gene expression (7, 13). As a result, rM51R-M virus is a potent inducer of antiviral responses in infected cells, while rwt virus suppresses host antiviral responses in most cell types. As shown here, the inability of rM51R-M virus to suppress host responses has a profound effect on the response of DC to virus infection, both in terms of the DC subsets which are able to respond, as well as the pathways by which these responses are initiated. Specifically, rM51R-M virus induces maturation in a broader range of DC subsets than rwt virus, including both G-DC and F-DC, while rwt virus induces maturation of F-DC, but not G-DC (Fig. 1). Likewise, rM51R-M virus induces DC maturation by both TLR7-dependent and TLR7-independent pathways, while the response to rwt is primarily dependent on TLR7 (Fig. 8).
The data presented here indicate that, unlike our previous findings with G-DC, F-DC possess the machinery required to recognize and respond to the wt virus by undergoing maturation. These results are consistent with previous reports demonstrating the ability of pDC to respond to infection with wt VSV both in cell culture and in vivo (6, 29, 37). The difference between F-DC and G-DC is due in part to the delay in the inhibition of host gene expression in F-DC (Fig. 4), which allows these cells to respond to virus infection by upregulating antiviral genes (Fig. 6). Two pieces of evidence suggest that the expression of TLR7 by F-DC is the key to their response to VSV. First, in contrast to G-DC, F-DC respond to the TLR7 agonist, Lox, by upregulating costimulatory molecules and type I IFN (Fig. 1). Second, in the absence of TLR7 or its signaling adaptor, MyD88, rwt virus failed to induce maturation of F-DC (Fig. 8). These results are similar to studies indicating that TLR7/MyD88 signaling by pDC is required for responsiveness to wt VSV, as well as another negative-stranded RNA virus, influenza virus (11, 29). In contrast to virus with wt M protein, the M protein mutant virus, rM51R-M virus, induced costimulatory molecule expression in F-DC regardless of their expression of MyD88 and partially in the absence of TLR7. Similarly, rM51R-M virus was able to stimulate production of type I IFN in the absence of TLR7. These results indicate that this virus is detected by other sensors in F-DC, leading to the activation of the type I IFN signaling pathway. Likely candidates are members of the RNA helicase family, such as retinoic acid-inducible gene I (RIG-I), which regulates virus-induced antiviral immunity by the activation of IRF-3 and NF-κB and the enhanced production of type I IFN (16, 40). Although studies have suggested that the production of type I IFN by pDC can occur independently of RIG-I (19), it is possible that rM51R-M virus stimulates F-DC primarily through this signaling pathway.
Although most of the response of F-DC to M protein mutant virus was independent of TLR7, we did observe a decrease in CD86 levels in TLR7−/− F-DC in response to rM51R-M virus. However, there was no effect of lack of MyD88 in the CD86 response to this virus. The elimination of MyD88 effectively cuts off signaling through several TLRs, as well as IL-1/IL-18R, and thus has more effects on signaling than the elimination of TLR7 alone. Thus, a likely explanation is that the potential for compensatory mechanisms may be greater in MyD88−/− cells than in TLR7−/− cells.
We have shown that the main response of F-DC to virus infection is the upregulation of expression of genes in the type I IFN pathway (Fig. 6). Additional pathways also showed some significance, but only 10% of their genes were affected at most. Furthermore, we found that IFNAR−/− DC failed to upregulate costimulatory molecule expression in response to infection with rwt virus. Thus, activation of the type I IFN pathway is important not only for stimulating an antiviral response but also for inducing the maturation of F-DC upon virus infection. However, we observed that, in the absence of a type I IFN response, F-DC were partially responsive to rM51R-M virus. Thus, rM51R-M virus stimulates expression of costimulatory molecule genes independently of type I IFN signaling. However, it is likely that full maturation of F-DC by this virus, including the ability to activate T cells, may require intact IFN signaling. In fact, numerous studies have indicated that type I IFN has important stimulatory effects on the adaptive immune response, including the induction of CD8+ T-cell responses by cross-priming (25, 27). Although DC represent one direct target of type I IFN (23, 25), it is clear that this cytokine enhances cross-priming through effects on other cell types as well. For example, studies have indicated that optimal induction of cross-priming was dependent on the direct stimulation of T cells by IFN-α/β (20, 24). Type I IFN has also been shown to augment antibody responses (26). Therefore, type I IFN is a potent stimulator of adaptive immunity through effects on DC, T cells, and B cells.
M protein mutant viruses, such as rM51R-M virus, are promising candidates as vaccine vectors for the delivery of foreign antigens and as oncolytic viruses for antitumor therapies because they offer the promise of more effectively activating DC as means of initiating antigen- or tumor-specific T-cell responses. We have recently shown that rM51R-M virus induces strong antibody responses in vivo without causing disease, thus demonstrating the potential of viruses that stimulate innate immunity as successful delivery vehicles for target antigens (3). Furthermore, M protein mutant viruses have been shown to selectively and effectively kill tumor cells in vivo without causing disease in normal tissues (2, 33). In some cases, this may be due to enhanced induction of antitumor immunity as a result of virus infection within the tumor (10). Although the combination of immune cell and viral infection within the tumor has the potential to prime antitumor immune responses, host responses may severely limit viral replication and tumor cell infection, thus limiting anticancer efficacy (9). Therefore, despite the promising results of virus-based cancer therapies, it will be necessary to improve methods of delivery and utilize therapeutic combinations for the effective treatment of cancers. Results similar to those obtained from M protein mutant VSV have been obtained with modified vaccinia virus Ankara, a highly attenuated vaccinia virus strain that is being studied as a replication-deficient vaccine vector against various infections and tumor diseases (36). Modified vaccinia virus Ankara induces type I IFN production in F-DC through TLR-dependent and independent mechanisms, indicating that, although attenuated, such viruses possess the capacity to potently stimulate DC to induce robust immune responses.
Our in vitro results demonstrate that wt VSV is more limited than rM51R-M virus in its ability to stimulate different DC populations. Nevertheless, wt VSV strains induce potent T-cell responses in vivo (5, 41). Adoptive-transfer studies indicate that by releasing small amounts of VSV particles within local lymphoid tissues, mDC rapidly induce anti-VSV and T-cell responses (28). In addition, CD8+ T-cell activation by VSV-derived antigens has been shown to occur in DC-rich regions of lymph nodes (34). It is possible that wt VSV activates these T-cell-stimulatory DC indirectly via bystander activity upon activation of TLR7+ pDC (i.e., through IFN produced by VSV-responsive pDC). Future studies will seek to determine the mechanisms by which wt and mutant VSVs induce DC maturation and how different DC subsets cooperate in vivo to activate T cells.
Acknowledgments
This study was supported by NIH program project grant P01-AI060642 and RO1-AI057770 (to E.M.H.) and has complied with all relevant federal guidelines and institutional policies related to animal care and use.
Footnotes
Published ahead of print on 14 January 2009.
REFERENCES
- 1.Ahmed, M., K. L. Brzoza, and E. M. Hiltbold. 2006. Matrix protein mutant of vesicular stomatitis virus stimulates maturation of myeloid dendritic cells. J. Virol. 802194-2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed, M., S. D. Cramer, and D. S. Lyles. 2004. Sensitivity of prostate tumors to wild type and M protein mutant vesicular stomatitis viruses. Virology 33034-49. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed, M., T. R. Marino, S. Puckett, N. D. Kock, and D. S. Lyles. 2008. Immune response in the absence of neurovirulence in mice infected with M protein mutant vesicular stomatitis virus. J. Virol. 829273-9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed, M., M. O. McKenzie, S. Puckett, M. Hojnacki, L. Poliquin, and D. S. Lyles. 2003. Ability of M protein of vesicular stomatitis virus to suppress interferon beta gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J. Virol. 774646-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachmann, M. F., T. M. Kundig, G. Freer, Y. Li, C. Y. Kang, D. H. Bishop, H. Hengarner, and R. M. Zinkernagel. 1994. Induction of protective cytotoxic T cells with viral proteins. Eur. J. Immunol. 242228-2236. [DOI] [PubMed] [Google Scholar]
- 6.Barchet, W., M. Cella, B. Odermatt, C. Asselin-Paturel, M. Colonna, and U. Kalinke. 2002. Virus-induced interferon α production by a dendritic cell subset in the absence of feedback signaling in vivo. J. Exp. Med. 195507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black, B. L., R. B. Rhodes, M. McKenzie, and D. S. Lyles. 1993. The role of vesicular stomatitis virus matrix protein in inhibition of host-directed gene expression is genetically separable from its function in virus assembly. J. Virol. 674814-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brawand, P., D. R. Fitzpatrick, B. W. Greenfield, K. Brasel, C. R. Maliszewski, and T. De Smedt. 2002. Murine plasmacytoid pre-dendritic cells generated from Flt3 ligand-supplemented bone marrow cultures are immature APCs. J. Immunol. 1696711-6719. [DOI] [PubMed] [Google Scholar]
- 9.Chiocca, E. A. 2008. The host response to cancer virotherapy. Curr. Opin. Mol. Ther. 1038-45. [PubMed] [Google Scholar]
- 10.Diaz, R. M., F. Galivo, T. Kottke, P. Wongthida, J. Qiao, J. Thompson, M. Valdes, G. Barber, and R. G. Vile. 2007. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 672840-2848. [DOI] [PubMed] [Google Scholar]
- 11.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 3031529-1531. [DOI] [PubMed] [Google Scholar]
- 12.Faria, P. A., P. Chakraborty, A. Levay, G. N. Barber, H. J. Ezelle, J. Enninga, C. Arana, J. van Deursen, and B. M. A. Fontoura. 2005. VSV disrupts the Rae1/mrnp41 mRNA nuclear export pathway. Mol. Cell 1793-102. [DOI] [PubMed] [Google Scholar]
- 13.Ferran, M. C., and J. M. Lucas-Lenard. 1997. The vesicular stomatitis virus matrix protein inhibits transcription from the human beta interferon promoter. J. Virol. 71371-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilliet, M., A. Boonstra, C. Paturel, S. Antonenko, X. L. Xu, G. Trinchieri, A. O'Garra, and Y. J. Liu. 2002. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 195953-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heath, W. R., G. T. Belz, G. M. Behrens, C. M. Smith, S. P. Forehan, I. A. Parish, G. M. Davey, N. S. Wilson, F. R. Carbone, and J. A. Villadangos. 2004. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol. Rev. 1999-26. [DOI] [PubMed] [Google Scholar]
- 16.Heim, M. H. 2005. RIG-I: an essential regulator of virus-induced interferon production. J. Hepatol. 42431-433. [DOI] [PubMed] [Google Scholar]
- 17.Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Muramatsu, and R. M. Steinman. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1761693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadowaki, N., S. Ho, S. Antonenko, R. de Waal Malefyt, R. A. Kastelein, F. Bazan, and Y.-J. Liu. 2001. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194863-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato, H., S. Sato, M. Yoneyama, M. Yamamoto, S. Uematsu, K. Matsui, T. Tsujimura, K. Takeda, T. Fujita, O. Takeuchi, and S. Akira. 2005. Cell type-specific involvement of RIG-I in antiviral response. Immunity 2319-28. [DOI] [PubMed] [Google Scholar]
- 20.Koluman, G. A., S. Thomas, L. J. Thompson, J. Sprent, and K. Murali-Krishna. 2005. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection J. Exp. Med. 202637-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopecky, S. A., M. C. Willingham, and D. S. Lyles. 2001. Matrix protein and another viral component contribute to induction of apoptosis in cells infected with vesicular stomatitis virus. J. Virol. 7512169-12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kopp, E., and R. Medzhitov. 2003. Recognition of microbial infection by Toll-like receptors. Curr. Opin. Immunol. 15396-401. [DOI] [PubMed] [Google Scholar]
- 23.Lapenta, S. M., M. Santini, M. Spada, S. Donati, F. Urbani, and D. Accapezzato. 2006. IFN-alpha-conditioned dendritic cells are highly efficient in inducing cross-priming CD8+ T cells against exogenous viral antigens. Eur. J. Immunol. 362046-2060. [DOI] [PubMed] [Google Scholar]
- 24.Le Bon, A., V. Durand, E. Kamphuis, C. Thompson, S. Bulfone-Paus, and C. Rossman. 2006. Direct stimulation of T cells by type I IFN enhances the CD8+ T-cell response during cross-priming. J. Immunol. 1764682-4689. [DOI] [PubMed] [Google Scholar]
- 25.Le Bon, A., N. Etchart, C. Rossman, M. Ashton, S. Hou, D. Gewert, P. Borrow, and D. F. Tough. 2003. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 41009-1015. [DOI] [PubMed] [Google Scholar]
- 26.Le Bon, A., G. Schiavoni, G. D'Agostino, I. Gresser, F. Belardelli, and D. F. Tough. 2001. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 14461-470. [DOI] [PubMed] [Google Scholar]
- 27.Le Bon, A., and D. F. Tough. 2008. Type I interferon as a stimulus for cross-priming. Cytokine Growth Factor Rev. 1933-40. [DOI] [PubMed] [Google Scholar]
- 28.Ludewig, B., K. J. Maloy, C. López-Macías, B. Odermatt, H. Hengartner, and R. M. Zinkernagel. 2000. Induction of optimal antiviral neutralizing B-cell responses by dendritic cells requires transport and release of virus particles in secondary lymphoid organs. Eur. J. Immunol. 30185-196. [DOI] [PubMed] [Google Scholar]
- 29.Lund, J. M., L. Alexopoulou, A. Sato, M. Karow, N. C. Adams, N. W. Gale, A. Iwasaki, and R. A. Flavell. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 1015598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pulendran, B. 2006. Division of labor and cooperation between dendritic cells. Nat. Immunol. 7699-700. [DOI] [PubMed] [Google Scholar]
- 31.Shortman, K., and S. H. Naik. 2007. Steady-state and inflammatory dendritic-cell development. 719-30. [DOI] [PubMed] [Google Scholar]
- 32.Smith, P. L., G. Lombardi, and G. R. Foster. 2005. Type I interferons and the innate immune response—more than just antiviral cytokines. Mol. Immunol. 42869-877. [DOI] [PubMed] [Google Scholar]
- 33.Stojdl, D. F., B. D. Lichty, B. R. tenOever, J. M. Paterson, A. T. Power, S. Knowles, R. Marius, J. Reynard, L. Poliquin, H. Atkins, E. G. Brown, R. K. Durbin, J. E. Durbin, J. Hiscott, and J. C. Bell. 2003. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4263-275. [DOI] [PubMed] [Google Scholar]
- 34.Turner, D. L., L. S. Cauley, K. M. Khanna, and L. Lefrancois. 2007. Persistent antigen presentation after acute vesicular stomatitis virus infection. J. Virol. 812039-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Kobbe, C., J. M. A. van Deursen, J. P. Rodrigues, D. Sitterlin, A. Bachi, X. Wu, M. Wilm, M. Carmo-Fonseca, and E. Izaurralde. 2000. Vesicular stomatitis virus matrix protein inhibits host cell gene expression by targeting the nucleoporin Nup98. Mol. Cell 61243-1252. [DOI] [PubMed] [Google Scholar]
- 36.Waibler, Z., M. Anzaghe, H. Ludwig, S. Akira, S. Weiss, G. Sutter, and U. Kalinke. 2007. Modified vaccinia virus Ankara induces Toll-like receptor-independent type I interferon responses. J. Virol. 8112102-12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waibler, Z., C. N. Detje, J. C. Bell, and U. Kalinke. 2007. Matrix protein mediated shutdown of host cell metabolism limits vesicular stomatitis virus-induced interferon-alpha responses to plasmacytoid dendritic cells. Immunobiology 212887-894. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto, M., K. Takeda, and S. Akira. 2004. TIR domain-containing adaptors define the specificity of TLR signaling. Mol. Immunol. 40861-868. [DOI] [PubMed] [Google Scholar]
- 39.Yoneyama, H., K. Matsuno, E. Toda, T. Nishiwaki, N. Matsuo, A. Nakano, S. Narumi, B. Lu, C. Gerard, S. Ishikawa, and K. Matsushima. 2005. Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs. J. Exp. Med. 202425-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagashi, K. Taira, S. Akira, and T. Fujiita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5730-737. [DOI] [PubMed] [Google Scholar]
- 41.Zammit, D. J., L. S. Cauley, Q.-M. Pham, and L. Lefrancois. 2005. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity 22561-570. [DOI] [PMC free article] [PubMed] [Google Scholar]