Abstract
Human T-cell leukemia virus type 1 (HTLV-1) is an oncogenic retrovirus etiologically causal of adult T-cell leukemia (ATL). The virus encodes a Tax oncoprotein that functions in transcriptional regulation, cell cycle control, and transformation. ATL is a highly virulent cancer that is resistant to chemotherapeutic treatments. To understand this disease better, it is important to comprehend how HTLV-1 promotes cellular growth and survival. Tax activation of NF-κB is important for the proliferation and transformation of virus-infected cells. We show here that prolyl isomerase Pin1 is over expressed in HTLV-1 cell lines; Pin1 binds Tax and regulates Tax-induced NF-κB activation.
Many of the molecular alterations associated with carcinogenesis occur in cell signaling pathways that regulate cell proliferation and differentiation. Human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent for adult T-cell leukemia (ATL), an aggressive human T-cell malignancy (15, 23, 43, 44, 51). The mechanisms of ATL leukemogenesis are not yet fully understood. However, accumulating evidence suggests that viral protein expression early in infection plays a major role for disease development (17, 44, 72). HTLV-1 encodes a 40-kDa nuclear oncoprotein, Tax (7, 26, 42). A current view is that cellular transformation by HTLV-1 is linked to Tax's capacity to deregulate mitotic checkpoints (1, 8, 19, 25, 31, 35, 48, 49, 52), to activate cellular signaling pathways (18, 25, 50, 53, 54, 60), and to inactivate tumor suppressors (25) and perturb cellular gene expression in part through transcription factors such as nuclear factor-κB (NF-κB) (24, 37, 63). Untimely activation of these processes and their downstream mediators can provoke uncontrolled cell growth and malignant transformation (28, 33, 36, 44).
The recent identification of the enzyme Pin1 that specifically isomerizes the phosphorylated Ser/Thr-Pro bonds in some proteins has led to the discovery of a new signaling mechanism whereby, after phosphorylation, prolyl isomerization induces conformational protein changes (11, 32, 41, 59, 62, 65). The human Pin1 gene was originally identified in a yeast genetic screen (41a) for proteins involved in mitotic regulation and was shown to be the first peptidylproline cis-trans isomerase (PPIase) that is essential for cell division in yeast and human cells (59, 71). PPIases catalyze the intrinsically slow cis-trans isomerization of peptide bonds that are N-terminal to proline residues to influence protein folding (41, 71). The two best-characterized families of PPIases are the cyclophilins and the FK506-binding proteins, which are involved in various cellular processes. In part, they function in the immune system, where they act as cellular receptors for several clinically relevant immunosuppressive drugs. In contrast to other PPIases, Pin1 has a unique substrate specificity: it binds and isomerizes phosphorylated Ser/Thr-Pro motifs (11, 32, 41, 59, 62). Depending on the substrate protein, Pin1-induced conformational changes can affect enzymatic activities, phosphorylation status, protein-protein interactions, subcellular localization, and/or protein stability (11, 32, 41, 59, 62). Functionally, Pin1 can regulate cell cycle progression (14, 41, 69, 73), transcription (45), and the response to DNA damage (5, 69). Moreover, Pin1 has been shown to be involved in the pathogenesis of human ailments such as Alzheimer's disease (3, 32, 41) and cancers (2, 4, 41, 56, 67, 68, 71).
Much of Tax's activity inside cells arises from its protein-protein interaction with cellular factors (10, 70). How these protein-protein interactions are regulated has not been studied in detail. Here, we report that Pin1 expression is enhanced by Tax in HTLV-1-transformed cells. More importantly, inhibition of Pin1 suppressed Tax's signaling through NF-κB and Tax's transforming phenotype. Collectively, these new findings indicate that Pin1 acts as a molecular switch to regulate Tax activity through protein conformational changes.
MATERIALS AND METHODS
Reagents, antibodies, and plasmids.
Anti-Pin1 was purchased from R&D Systems and Cell Signaling Technology. Tax was detected using either rabbit anti-Tax or mouse monoclonal anti-Tax (NIH AIDS Research and Reference Reagent Program) (29, 38). Anti-hemagglutinin (HA), anti-actin, and anti-FLAG (Sigma) were used at dilutions of 1:5,000. The Pin1 cDNA was cloned by reverse transcription-PCR from a human lymphocyte cDNA library (Clontech) using the following primers: Forward, 5′-GTTGAATTCATGGCGGACGAG-3′; Reverse, 5′-GTTCTCGAGTCACTCAGTGCG-3′. pCAG-FLAG-Pin1, an expression plasmid for FLAG-tagged Pin1 in mammalian cells, and pGEX-Pin1, an expression plasmid for glutathione S-transferase (GST)-fused Pin1 in Escherichia coli, were constructed by insertion of the EcoRI-XhoI fragment of a PCR product into pCAG-FLAG and pGEX-6P1 vectors (Clontech), respectively. The expression plasmids for Pin1 with a point mutation of tryptophan at position 34 or lysine at position 63 changed to alanine [Pin1(W34A) and Pin1(K63A), respectively] were generated by oligonucleotide-directed mutagenesis (65). pCAG-HA-Tax and pCAG-FLAG-Tax expression vectors were generated by insertion of the EcoRI-XhoI fragment of a PCR product into the pCAG-FLAG and pCAG-HA vectors, respectively.
Cell culture, transfection, and reporters assays.
Jurkat, H9, C8166-45, MT4, HUT102, and JPX9 cells were propagated in RPMI 1640 medium with 10% fetal calf serum (FCS). JPX9 cells, derived from a Jurkat cell line expressing Tax under the control of the metallothionein-inducible promoter, were activated by addition of cadmium chloride (CdCl2). Mouse embryonic fibroblasts (MEF Pin1 wild type [WT] and MEF Pin1 knockout [KO] cells), and a human kidney cell line (HEK 293T) were propagated in Dulbecco's modified Eagle's medium (DMEM) with 10% FCS and transfected according to the manufacturer's protocol using Lipofectamine 2000. MEF Pin1 WT and MEF Pin1 KO cells were generous gifts from Anthony R. Means (Duke University Medical Center). To assay luciferase (Luc) activity, cells were transfected with a plasmid DNA mixture containing reporter plasmids, 100 ng of NF-κB-Luc or 100 ng of HTLV-1-long terminal repeat (LTR)-Luc and 100 ng of Rous sarcoma virus-β-galactosidase. Total amounts of plasmid DNA were normalized by addition of pCDNA3.0. Cells were washed twice with 1× phosphate-buffered saline and then lysed in 1× Luc lysis buffer (Promega). Luc assay substrate (Promega) was used according to the manufacturer's protocol, and activity was measured in an Opticom II luminometer (MGM Instruments). β-Galactosidase activity was measured using Galacto-Star (Tropix) as described by the manufacturer. Luc activities were normalized for transfection efficiency based on galactosidase readings. All transfections were performed at least three times. Error bars represent standard deviations.
Immunoprecipitations.
For coimmunoprecipitations, 48 h after transfection, cells were harvested using cell-removing buffer (40 mM Tris, pH 7.5, 1 mM EDTA, and 150 mM NaCl) and pelleted by centrifugation. The cells were washed twice with phosphate-buffered saline and resuspended into radioimmunoprecipitation assay buffer (50 mM HEPES, pH 7.5, 0.5% Nonidet P-40, 150 mM NaCl, 2 mM EDTA, 20 mM β-glycerophosphate, 0.1 mM sodium orthovanadate [Na3VO4], 1 mM sodium fluoride [NaF], 0.5 mM dithiothreitol, and protease cocktail from Roche). Total cell lysates were immunoprecipitated using anti-HA, anti-FLAG, or anti-Myc agarose beads (Sigma) overnight at 4°C. After samples were washed in radioimmunoprecipitation assay buffer four times, the immunoprecipitates were eluted with either three copies of FLAG peptide (150 ng/ml) (Sigma) or HA peptide (100 μg/ml) (Sigma) and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with the indicated antibodies.
GST pull-down assay.
Recombinant GST and GST-Pin1 were produced in BL21 cells (Invitrogen) following treatment of cells with 1 mM isopropyl-β-d-thiogalactopyranoside. After the binding of the appropriate proteins to glutathione-Sepharose resins (Amersham Biosciences), the protein-bound resins were incubated with cell lysates overnight at 4°C in immunoprecipitation buffer consisting of 50 mM HEPES, 150 mM NaCl, 10% glycerol, 1% NP-40, 1.5 mM MgCl2, 1 mM dithiothreitol, 0.2 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, 20 mM NaF, and protease inhibitor (complete; Roche). The resins were then washed five times, and resin-bound proteins were eluted and resolved by SDS-PAGE and were detected by immunoblotting with anti-Tax antibody.
Cell proliferation and apoptosis assay.
Cell proliferation was determined using Cell Counting Kit-8 (CCK-8; Dojindo) according to the manufacturer's protocol. Briefly, after transfection 50,000 to 100,000 cells/well were plated in a 96-well plate. After 24, 48, or 72 h, 10 μl of CCK-8 solution [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt; Dojindo] was added to each well and incubated for 1 to 4 h. The cell metabolism in each well was determined by reading the optical density at 450 nm. After treatment with the Pin1 inhibitor juglone (5-hydroxy-1,4-naphthoquinone; 10 μM) (Sigma) for 8 h, the cells were harvested, and apoptosis was determined using an annexin V/7-amino-actinomycin apoptosis detection kit (BD Pharmingen) according to the manufacturer's protocol.
Focus formation assays.
Soft-agar focus formation assays were performed with a slight modification from that described previously (58). Briefly, NIH 3T3 or MEF Pin1 KO cells were transfected with 0.5 μg of Tax alone or in combination with 0.5 μg of Pin1 WT expression plasmid. At 48 h posttransfection, cells were removed from the confluent layer with trypsin and plated at 1 × 105 cells per dish coated with 1% soft agar in DMEM-2% FCS. The cultures were maintained in the same medium, with changes every 3 days, until the appearance of cell foci (between 21 and 30 days after transfection). Colonies were then stained with methylene blue.
RESULTS
Pin1 expression is enhanced in Tax-expressing cells.
Alterations of signaling pathways that regulate cell proliferation are important markers of transformation. Phosphorylation of proteins on serine or threonine residues that precede prolines (Ser/Thr-Pro) is an important regulatory mechanism in cellular proliferation and transformation (41, 71). Interestingly, the phosphorylated Ser/Thr-Pro motifs in proteins exist in distinct cis and trans conformers whose conversion rates are induced by phosphorylation and are catalyzed by the prolyl isomerase Pinl. The Pin1-catalyzed changes can affect protein-protein interaction, subcellular localization, and/or turnover of phosphorylated proteins (41, 71).
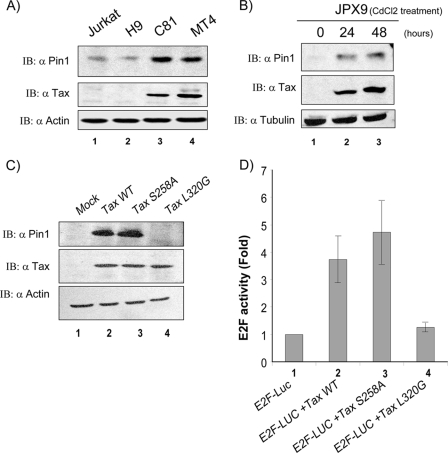
Because Pin1 has been shown to be important in cancers, we wished to explore if Pin1 plays a role in ATL, perhaps by influencing the protein-protein interaction between Tax and cellular factors. We tested our hypothesis by first comparing the expression of Pin1 in two control T-cell lines (Jurkat and H9) (Fig. 1A, lanes 1 and 2) and two Tax-expressing HTLV-1-transformed T-cell lines (C8166-45 and MT4) (Fig. 1A, lanes 3 and 4) using immunoblotting with affinity-purified anti-Pin1 antibody. Compared to control cells (Fig. 1A, lanes 1, 2), Pin1 expression was enhanced in the Tax-expressing cells (Fig. 1A, lanes 3 and 4). To test whether this effect was due to a direct transcriptional effect of Tax on Pin1 gene expression, we compared Pin1 levels in the presence or absence of Tax. This experiment was conducted using the JPX9 cell line which has an integrated Tax cDNA driven by a heavy metal ion (ZnCl2 or CdCl2)-inducible promoter (46). As shown in Fig. 1B, the level of Pin1 was increased in JPX9 cells treated with CdCl2, consistent with the notion that Tax increases Pin1 expression in cells.
FIG. 1.
Pin1 is overexpressed in Tax-expressing cell lines. (A) Cell-endogenous Pin1 from Jurkat (lane 1), H9 (lane 2), C8166-45 (C81; lane 3), and MT4 (lane 4) cells was assayed using anti-Pin1. Tax protein was assessed using a monoclonal anti-Tax. Equal loading of the cell extracts was verified with anti-actin. (B) Induction of Pin1 expression in JPX9 cells. Whole-cell lysates from JPX9 cells treated with CdCl2 for 24 h (lane 2) and 48 h (lane 3) were analyzed by immunoblotting with anti-Pin1 and anti-Tax. Equal loading was verified with anti-tubulin. (C) Whole-cell lysates of 293T cells transfected with different GFP-Tax plasmids were immunoblotted for Pin1 with anti-Pin1 and for Tax with anti-Tax. Equal loading of cell extracts was verified with anti-actin. Tax and Tax S258A, a point mutant that cannot activate NF-κB, induced Pin1 expression (lanes 2 and 3), while Tax L320G, a CREB activation-deficient mutant, did not induce Pin1 expression (lane 4). (D) 293T cells were cotransfected with pCDNA 3.0 and E2F-Luc plasmid (lane 1), plus 2 μg of Tax (lane 2), Tax S258A (lane 3), or Tax L320G (lane 4). The same amounts of cellular extracts were assayed for Luc. Tax and Tax S258A activated E2F-Luc (lanes 2 and 3), but Tax L320G did not (lane 4). α, anti: IB, immunoblotting.
Tax can activate the CREB and NF-κB pathways in cells (27, 44, 57). Several Tax mutants have been constructed that restrict activation to either CREB or NF-κB (57). To understand if Tax's effect on Pin1 segregates with CREB or NF-κB, we checked two green fluorescent protein (GFP)-tagged Tax point mutants. GFP-Tax S258A is phenotypically NF-κB negative and CREB positive (NF-κB−/CREB+), and GFP-Tax L320G is NF-κB+/CREB− (54) (Fig. 1C). We transfected 293T cells with GFP-Tax, GFP-Tax S258A, or GFP-Tax L320G, and we then checked Pin1 levels 48 h later by Western blotting. Indeed, we observed that GFP-Tax WT and GFP-Tax S258A (Fig. 1C, lanes 2 and 3), but not GFP-Tax L320G (Fig. 1C, lane 4), elevated Pin1 expression.
The above results for GFP-Tax, GFP-Tax S258A, and GFP-Tax L320G suggest that signaling through CREB but not NF-κB is needed to promote Pin1 expression. However, the Pin1 promoter, when carefully examined, does not contain any obvious CREB binding sites but does have several E2F-cognate sites (55). Because elsewhere Tax has been found to activate E2F-1-dependent transcription (39) and because Pin1 expression has been shown to be regulated by E2F (55), we next asked if Tax-induced Pin1 expression might arise from Tax's E2F activity. To answer this question, we transfected 293T cells with an E2F-Luc reporter alone (Fig. 1D, lane 1) or with the reporter plus Tax WT (Fig. 1D, lane 2), the reporter plus Tax S258A (Fig. 1D, lane 3), or the reporter plus Tax L320G (Fig. 1D, lane 4). Tax and Tax S258A (Fig. 1D, lanes 2 and 3), but not Tax L320G (Fig. 1D, lane 4), activated E2F, correlating Tax's E2F activity with elevated Pin1 expression in HTLV-1 cells.
Pin1 interacts with Tax.
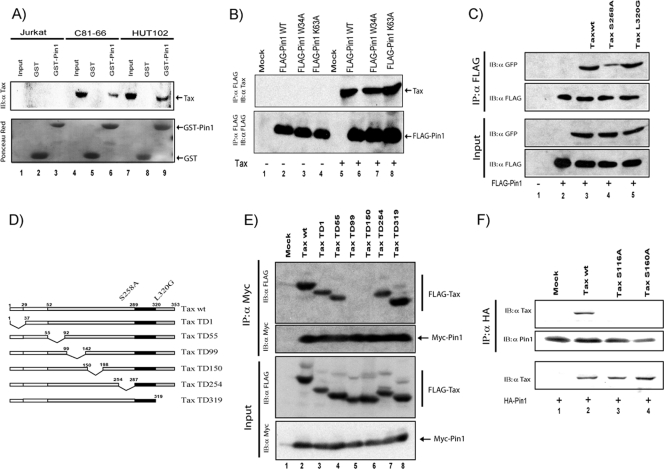
Pin1 recognizes phosphorylated proteins. Tax is a phosphoprotein, which is phosphorylated on serine residue(s) in both mouse and human cells (12, 13, 27). We wondered next whether Pin1 might interact with Tax. To address this possibility, we first checked for Tax-Pin1 binding (Fig. 2). Using GST-fused Pin1 (GST-Pin1) pull-down assays, we could indeed capture the Tax protein expressed in C8166-45 and HUT102 cell lysates (Fig. 2A, lanes 6 and 9). These pull-down results were extended by coimmunoprecipitation assays employing cells that were transfected with Tax and WT FLAG-Pin1 or two FLAG-Pin1 point mutants [FLAG-Pin1 WT, FLAG-Pin1(W34A), and FLAG-Pin1(K63A)] (Fig. 2B, lanes 6, 7, and 8). Tax coimmunoprecipitated with all three versions of Pin1. The Tax-Pin1 interaction was next checked using two Tax point mutants, GFP-Tax S258A (phenotypically NF-κB−/CREB+) and GFP-Tax L320G (phenotypically NF-κB+/CREB−). Interestingly GFP-Tax S258A, which does not activate NF-κB, interacted weakly with Pin1 (Fig. 2C, lane 4), while GFP-Tax and GFP-Tax L320G, which do activate NF-κB, coimmunoprecipitated well with Pin1 (Fig. 2C, lanes 3 and 5). These results suggest that Tax-Pin1 interaction might contribute to Tax-NF-κB activity.
FIG. 2.
Tax binds Pin1. (A) In vitro interaction between Tax and Pin1. GST pull-down assays were performed with control Jurkat and HTLV-1 (C8166-45 and HUT102) cell lysates using the indicated recombinant proteins, Pin1 fused to GST (GST-Pin1) or GST alone. The input lane shows approximately one-fifth the amount of protein material used in the pull-down assays. Immunoblotting for Tax is shown in the top panel, while the bottom panel shows Ponceau red staining of the blotted membrane. (B) Coimmunoprecipitation of Tax and Pin1. 293T cells were cotransfected with FLAG-Pin1 WT, FLAG-Pin1(W34A) (mutated in the phosphorylated S/T-P binding domain), or FLAG-Pin1(K63A) (mutated in the catalytic domain) without (lanes 1 to 4) or with (lanes 5 to 8) Tax. Pin1 was immunoprecipitated with anti-FLAG agarose beads, and the immunoprecipitates were immunoblotted with anti-Tax (upper panel) or anti-FLAG (lower panel). (C) 293T cells were transfected with FLAG-Pin1 vector (from lane 2 to lane 5) and WT Tax (lane 3) or different Tax point mutants as indicated (lanes 4 and 5). FLAG-Pin1 was immunoprecipitated using anti-FLAG agarose beads. GFP-tagged protein was detected using anti-GFP. Amounts of FLAG-Pin1 in the immunoprecipitation and GFP-Tax in cell extracts were verified using anti-FLAG or anti-GFP. (D) A schematic overview of the FLAG-tagged deletion mutants TD1, TD55, TD99, TD150, TD254, and TD319. (E) Mapping the interaction between Tax and Pin1. 293T cells were transfected with a Myc-Pin1 expression vector and different Tax deletion mutants (lanes 3 to 8). Myc-Pin1 was immunoprecipitated with anti-Myc agarose beads (Sigma). FLAG-bound protein was detected by anti-FLAG (upper panels). The amount of Myc-Pin1 in the immunoprecipitate and the amount of FLAG-Tax in the cell extracts were checked using anti-Myc and anti-FLAG (bottom panels). (F) Coimmunoprecipitation assay of Tax and the indicated Tax mutants with Pin1. 293 T cells were cotransfected with HA-Pin1 alone (lane 1) or with 2 μg of Tax WT (lane 2), 6 μg of Tax S116A (lane 3), or 6 μg of Tax S160A (lane 4). Pin1 was immunoprecipitated with anti-HA agarose beads, and the immunoprecipitates were resolved by SDS-PAGE and immunoblotted with anti-Tax (upper panel). The amount of Pin1 in the immunoprecipitates and the amount of Tax in the cell extracts were checked using anti-Pin1 and anti-Tax (bottom panels). IB, immunoblotting; IP, immunoprecipitation; α, anti.
The amino acid sequence for Tax has seven Ser/Thr-Pro motifs. To understand which of these S/T-P motifs might be important, we attempted next to map the Tax-Pin1 interaction. We constructed six FLAG-tagged Tax deletion mutants (20) (Fig. 2D) and separately transfected these plasmids into 293T cells with a Myc-tagged Pin1 plasmid. Transfected cell lysates were immunoprecipitated using anti-Myc beads and then assayed for coimmunoprecipitated Tax (Fig. 2E). Four Tax deletion mutants, TD1 (a deletion of Tax WT residues 1 to 37 [Δ1-37]), TD55 (Δ55-92), TD254 (Δ254-287), and TD319 (Δ319-353), captured Pin1 strongly (Fig. 2E, lanes 3, 4, 7, and 8), while two other Tax mutants, TD99 (Δ99-142) and TD150 (Δ150-198) (Fig. 2D, lanes 5 and 6), did not. These results map the Pin1 interaction to the NF-κB-activating domain of Tax (amino acids 99 to 198).
To extend the findings from the Tax deletion mutants (Fig. 2D and E), we next used two Tax point mutants, S116A and S160A (Fig. 2F). S116A has a serine at position 116 mutated to alanine, while S160A has a serine at position 160 mutated to alanine. Both point mutations map within the respective deletions contained in TD99 and TD150. Moreover, previous results have shown that both Tax S116A and Tax S160A are incapable of NF-κB activation (54). Interestingly, neither Tax point mutant coimmunoprecipitated with Pin1 (Fig. 2F, lanes 3 and 4), which coimmunoprecipitated well with the control WT Tax protein (Fig. 2F, lane 2). These findings further correlate Tax-Pin1 interaction with Tax-NF-κB activation.
Pin1 contributes to Tax signaling through NF-κB.
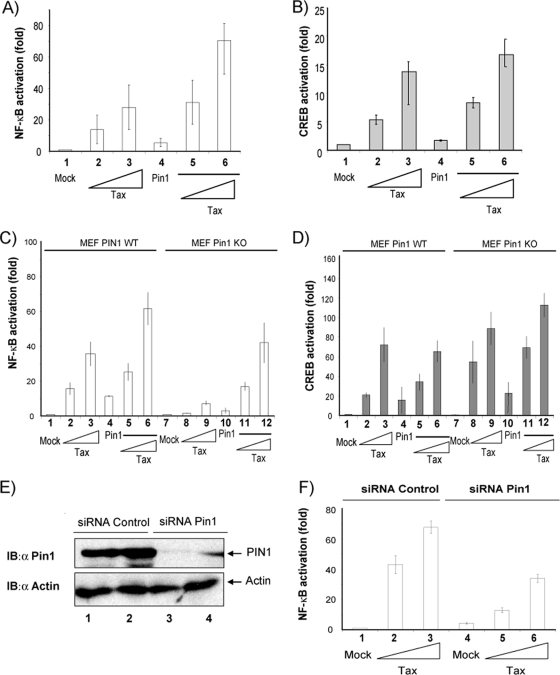
The functional impact of phosphorylation-dependent prolyl isomerization is complex and not fully understood (66). The above results prompted us to consider whether Pin1 could affect Tax's transcriptional activities. To address this possibility, we measured Luc activity in 293T cells transfected with a Luc reporter under the control of either an NF-κB-responsive promoter (Fig. 3A) or the HTLV-1-LTR (a CREB-responsive promoter) (Fig. 3B). The reporter plasmids (HTLV-LTR-Luc or NF-κB-Luc) were either transfected individually into cells or were cotransfected with increasing amounts of Tax and/or FLAG-Pin1 expression vectors. We found that expression of FLAG-Pin1 increased up to threefold Tax's transcriptional activity on the NF-κB-Luc (Fig. 3A, lanes 3 and 6) but not the HTLV-1-LTR-Luc promoter (Fig. 3B, lanes 3 and 6).
FIG. 3.
Tax activation of NF-κB is impaired in Pin1−/− MEFs. (A) 293T cells were transfected with 3κb-Luc plasmid (an NF-κB dependent Luc reporter) and an empty control plasmid (lane 1), 3κb-Luc with Tax in increasing concentrations (1 μg and 3 μg in lanes 2 and 3, respectively), 3κb-Luc with a Pin1 expressing plasmid (1 μg; lane 4), or 3κb-Luc with a Pin1 expressing plasmid plus increasing amounts of Tax (1 μg and 3 μg in lanes 5 and 6, respectively). The same amounts of cell extract from each transfected sample were assayed for Luc. (B) 293T cells were cotransfected with HTLV-1-LTR-Luc and control plasmid (lane 1), HTLV-1-LTR-Luc with Tax in increasing concentrations (1 μg and 3 μg in lanes 2 and 3, respectively), 3κb-Luc with Pin1 (1 μg; lane 4), or 3κb-Luc with Pin1 plus increasing amounts of Tax (1 μg and 3 μg in lanes 5 and 6, respectively). The same amounts of cellular extract from each transfected sample were subjected to Luc assay (see Materials and Methods). (C) WT MEFs (lanes 1 to 6) and Pin1 KO MEFs (lanes 7 to 12) were transfected with 3κb-Luc plasmid and control plasmid (lanes 1 and 7), 3κb-Luc with Tax in increasing concentrations (1 μg in lanes 2 and 8 and 3 μg in lanes 3 and 9), 3κb-Luc with Pin1 (1 μg; lanes 4 and 10), or 3κb-Luc with Pin1 plus increasing amounts of Tax vector (1 μg in lanes 5 and 11 and 3 μg in lanes 6 and 12). The same amounts of cell extracts were subjected to Luc assay. (D) WT MEFs (lanes 1 to 6) and Pin1 KO MEFs (lanes 7 to 12) were transfected with HTLV-1-LTR-Luc plasmid and control plasmid (lanes 1 and 7), 3κb-Luc with Tax in increasing concentrations (1 μg in lanes 2 and 8 and 3 μg in lanes 3 and 9), or 3κb-Luc with Pin1 (1 μg; lanes 4 and 10) plus increasing amounts of Tax vector (1 μg in lanes 5 and 11 and 3 μg in lanes 6 and 12). The same amounts of cell extracts were subjected to Luc assay. (E) 293T cells were transfected with 2 μg of control siRNA (two replications in lanes 1 and 2) or an siRNA mixture targeted against Pin1 (two replications in lanes 3 and 4). The same amounts of cell extracts were analyzed by Western blotting using anti-Pin1 and anti-actin. The amounts of extract used for Western blotting detection in this experiment were higher than those used in previous immunoblotting assays (Fig. 1C) due to the low expression level of cell-endogenous Pin1. (F) 293T cells were transfected with control siRNA (lanes 1, 2, and 3) or siRNA against Pin1 (lanes 4, 5, and 6) plus 3κb-Luc plus the control plasmid (lane 1) or 3κb-Luc plus Tax in increasing concentrations (1 μg in lanes 2 and 5 and 3 μg in lanes 3 and 6). The same amounts of cell extracts were subjected to Luc assay.
The above experiments relied on the overexpression of transfected plasmids and cannot directly assess the effect of cell-endogenous Pin1 on Tax function. To check the effect of endogenous Pin1, we exploited the availability of MEF Pin1 KO cells. We next assessed Tax's activity in Pin1+/+ and Pin1−/− MEFs. Using two Luc reporters, we found that the NF-κB-responsive reporter was activated ∼10-fold less by Tax in Pin1 −/− than in Pin1 +/+ MEFs (Fig. 3C, lanes 3 and 9). In contrast, the control HTLV-1-LTR-Luc plasmid, which is a CREB-responsive reporter, was unaffected (Fig. 3D, lanes 3 and 9). We also performed a reconstitution experiment to demonstrate that the effect is specific to Pin1 and not due to other cryptic differences between the two MEF lines. Thus, we observed that when exogenous Pin1 was introduced into the Pin1−/− MEFs, Tax-induced activation of NF-κB was restored (Fig. 3C, lanes 11 and 12).
The above results with KO cells support a role for Pin1 in Tax-NF-κB activation in mouse cells. To extend the findings from mouse to human, we performed small interfering RNA (siRNA)-mediated knockdown of Pin1 endogenous to 293T cells (Fig. 3E). 293T cells were cotransfected with a NF-κB-responsive reporter and increasing amounts of Tax and a Pin1-specific siRNA or an irrelevant control siRNA. NF-κB activation was assessed by Luc assay (Fig. 3F). Compared to control siRNA transfection, the Pin1 siRNA-knockdown cells showed an approximately threefold decrease in Tax-induced NF-κB activity (Fig. 3F, lanes 3 and 6). These results verified a role for Pin1 in Tax activation of NF-κB in human cells.
Pin1 contributes to Tax interaction with IKKγ.
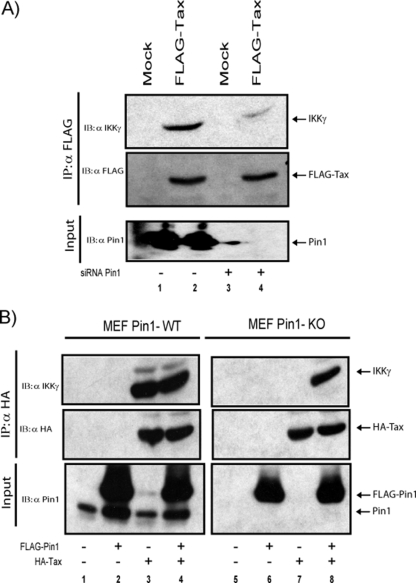
Tax activates NF-κB in part through the direct binding of IκB kinase γ ([IKKγ] also known as the NF-κB essential modulator, or NEMO) (9, 21, 24, 30). To further explore the mechanistic link between Pin1 and Tax-induced NF-κB activation, we wondered if the former might influence the protein-protein binding of Tax and IKKγ. To investigate this possibility, 293T cells were cotransfected with FLAG-Tax and either a Pin1-specific siRNA or an irrelevant control siRNA. Transfected cell lysates were immunoprecipitated using anti-FLAG agarose beads, and we checked for the coimmunoprecipitation of IKKγ (Fig. 4A). Compared to cells transfected with the control siRNA, Pin1 siRNA-knockdown cells showed diminished Tax binding to IKKγ (Fig. 4A, lanes 2 and 4). Tax binding to IKKγ was also examined in the Pin1−/− MEFs (Fig. 4B). Here, Tax interaction with IKKγ was also impaired (Fig. 4B, lanes 3 and 7), and this impairment was restored by the transfection of exogenous FLAG-Pin1 (Fig. 4B, lanes 8). These results indicate that Pin1 can affect Tax's NF-κB activity through the modulation of Tax-IKKγ binding.
FIG. 4.
Pin1 contributes to Tax interaction with IKKγ. (A) 293T cells were cotransfected with 1.5 μg of control siRNA (lanes 1 and 2) or 1.5 μg of siRNA targeted against Pin1 (lanes 3 and 4) and FLAG-tagged Tax (lanes 2 and 4). FLAG-Tax was immunoprecipitated using anti-FLAG agarose beads. IKKγ was detected using anti-IKKγ (upper panel). Amounts of FLAG-Tax and IKKγ in the immunoprecipitate and Pin1 in the cell extract were verified using anti-FLAG, anti-IKKγ, and anti-Pin1, respectively. (B) WT MEFs (lanes 1 to 4) and Pin1 KO MEFs (lanes 5 to 8) were cotransfected with control plasmid (4 μg; lanes 1 and 5), with HA-Tax (3 μg; lanes 3, 4, 7, and 8), or with FLAG-Pin1-expressing vector (1 μg; lanes 2, 4, 6, and 8). HA-Tax was immunoprecipitated using anti-HA agarose beads. HA-Tax and IKKγ in the immunoprecipitate and Pin1 in the cell extract were detected using anti-HA, anti-IKKγ, and anti-Pin1, respectively. IB, immunoblotting; IP, immunoprecipitation; α, anti.
Pin1 cooperates with Tax to enhance cellular proliferation.
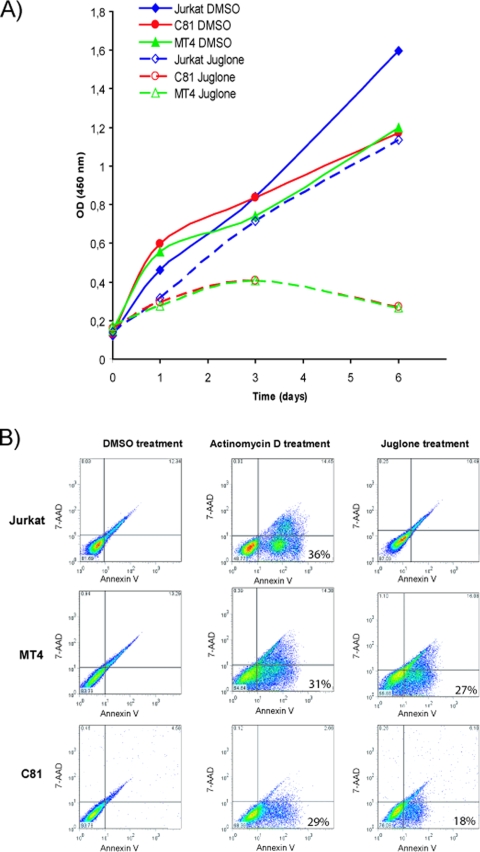
Because others have shown that ectopic expression of Pin1 alone can transform mouse fibroblasts (4, 71), we wondered about the biological impact of Pin1 on Tax's transforming activity. Thus, we assessed how loss of Pin1 function could affect cellular proliferation and viability. We used juglone, an inhibitor of the parvulin PPIase family (22) that is not wholly specific to inhibiting only Pin1, to query its potential antiproliferative effects on control Jurkat T cells and the HTLV-1-transformed cell lines MT4 and C8166-45. In this approach, we first measured the cell proliferation of juglone-treated cells (as described in the Materials and Methods). Juglone treatment at the concentration used did not noticeably affect the proliferation of Jurkat cells (Fig. 5A) while it did induce a significant inhibition of C8166-45 and MT4 proliferation (Fig. 5A). As a positive control, we also treated cells with actinomycin D, which has been shown to potently induce cellular apoptosis (16, 61). Annexin V staining (a measure of apoptosis) of juglone- or actinomycin D-treated cells (Jurkat, MT4, and C8166-45) was then assessed (Fig. 5B). We observed that while actinomycin D induced apoptosis in all three cell lines, juglone treatment was specifically antiproliferative for the Tax-expressing C8166-45 and MT4 cells but not the Jurkat cells (Fig. 5). Indeed, juglone treatment induced apoptosis in 27% and 17.5% of MT4 and C8166-45 cells, respectively (Fig. 5B, lower right panels). With the caveat that juglone can inhibit other parvulin PPIases, these results are consistent with the interpretation that Pin1 function could contribute to Tax-induced survival/proliferation of HTLV-1-transformed T-cells.
FIG. 5.
Juglone inhibited the proliferation and induced the apoptosis of Tax-expressing MT4 and C8166-45 cells. (A) Juglone inhibited cell growth of Tax-expressing MT4 and C8166-45 (C81) cells. Juglone-treated cells were monitored for proliferation using the CCK-8 coloration assay (Dojindo) according to the manufacturer's protocol. (B) Jurkat, C8166-45 (C81), and MT4 cells were treated with either dimethyl sulfoxide (DMSO), actinomycin D (1 mg/ml), or juglone (10 μM) (right column) for 8 h. Apoptosis was determined by annexin V and 7-amino-actinomycin (7-AAD) staining.
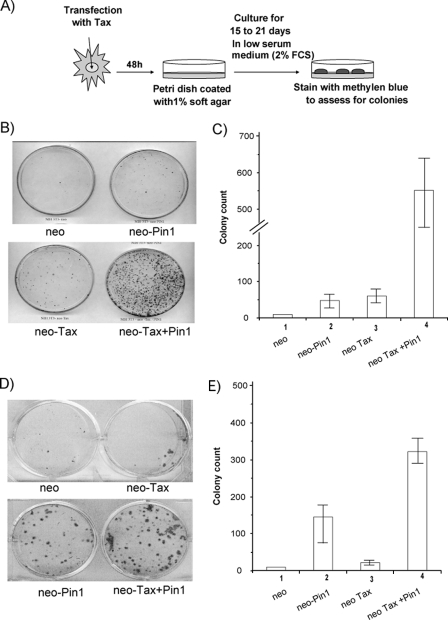
To complement the above loss-of-function assay, we performed a gain-of-Pin1 function experiment (Fig. 6A). We introduced with a neomycin resistance plasmid Pin1 alone, Tax alone, or Pin1 plus Tax into NIH 3T3 fibroblasts. We then selected for stable cell lines that expressed these proteins. The integration of Pin1 and Tax DNA in stable transfectants was confirmed by PCR, and protein expression was verified by Western blotting (data not shown). NIH 3T3 cells are normally contact inhibited when cultured in reduced serum (2% FCS), and we asked if cell foci that were no longer contact inhibited would develop from our transfected cells (Fig. 6B). Indeed, when anchorage-independent growth on soft agar was monitored, NIH 3T3 cells that overexpressed Pin1 plus Tax showed a five- to sixfold increase in the number of foci formed over cells that expressed either Pin1 alone or Tax alone (Fig. 6B and C).
FIG. 6.
Pin1 is important for Tax-mediated cell proliferation. (A) A schematic summary of the focus-forming assay procedure (see Materials and Methods). (B) Tax-induced focus formation in NIH 3T3 cells. NIH 3T3 cells were transfected with either 0.5 μg of FLAG-Pin1 alone, Tax alone, or FLAG Pin1 plus Tax (0.5 μg). At 48 h posttransfection, cells were plated at 1,000 cells/ml in 5-cm dishes coated with 1% soft agar and cultured in DMEM-2% FCS supplemented with G418 (0.5 mg/ml). After visible colonies were observed (21 days after transfection), the plates were washed and stained with methylene blue. Plates from representative experiments are shown. (C) Colonies, as indicated, were counted after 21 days. The mean values from three different experiments are shown. (D) Tax-induced focus formation of cells in Pin1 KO MEFs. Pin1 KO MEFs were transfected with either 0.5 μg of FLAG-Pin1 alone, Tax alone, or FLAG-Pin1 plus Tax (0.5 μg). At 48 h after transfection, cells were plated at a concentration of 1,000 cells/ml in 5-cm dishes coated with 1% soft agar and maintained in DMEM-2% FCS supplemented with G418 (0.3 mg/ml). After visible colonies were observed (21 days after transfection), the plates were washed and stained with methylene blue. Plates from representative experiments are shown. (E) Colonies, as indicated, were counted after 21 days. The values shown are means ± standard deviations from three independent experiments. neo, neomycin.
The expression of cell-endogenous Pin1 in NIH 3T3 cells complicates the interpretation of the above experiments. To clarify this issue better, we introduced with a neomycin resistance plasmid Pin1 alone, Tax alone, or Pin1 plus Tax into Pin1 KO MEFs and selected for stable cell lines that express these proteins. The integration of Pin1 and Tax DNA in stable transfectants was confirmed by PCR, and protein expression was again verified by Western blotting (data not shown). Reconstitution of Pin1 did induce foci formation in Pin1−/− MEF cells, and coexpression of Pin1 plus Tax WT increased greatly the number of foci achieved with Tax WT alone (Fig. 6D and E). These results illustrate that Pin1 alone and Tax alone are separately sufficient to initiate focus formation but that the coexpression of Pin1 plus Tax increases the robustness of anchorage-independent cell growth. Taken together, our findings support an important, though not absolutely essential, contribution of Pin1 to the activity of Tax in cellular proliferation.
DISCUSSION
NF-κB activity contributes to multiple biological processes including cell growth, cellular transformation, and cell death (6, 34, 40). The IKK complex (IKKαβγ) has been shown to be critical for NF-κB activation through the canonical pathway (6, 34, 40). The current model suggests that IKKγ is a converging nexus for stimuli that activate NF-κB signaling. In this regard, the transformation of cells by Tax has been shown to require its binding to IKKγ (17, 24, 47, 64). Here, we have studied a cellular factor that influences Tax-IKKγ interaction. We have determined that the PPIase Pin1 contributes to Tax signaling through NF-κB but not CREB/ATF. Tax interacts with Pin1 both in transfected cells and in HTLV-1-transformed cell lines. While our data are consistent with a direct Tax-Pin1 protein-protein interaction, we currently cannot exclude the possibility that the intracellular interaction could be indirect and require the mediation of other factors. In the absence of Pin1, Tax is impaired for IKKγ binding and attenuated for its induction of anchorage-independent cellular focus formation. Taken together, our findings suggest that Tax is a Pin1 substrate and that Pin1-mediated isomerization is an important regulator of Tax's protein-protein interaction.
An unexpected finding from our study is the overexpression of Pin1 in ATL cells. This observation is consistent with previous characterizations that Pin1 expression is highly regulated and is correlated with oncogenesis. Pin1 is an E2F downstream target gene whose expression is regulated during the cell cycle in normal cells (41, 71). However, in cancer cells, Pin1 levels are elevated and do not change with the cell cycle (41, 71). The prevalent deregulation of E2F/Rb pathways in many human cancers (41, 71) may play a critical role in the upregulation of Pin1 in human cancers, and Pin1 overexpression can then activate multiple oncogenic pathways and contribute to cell transformation (41, 71). Hence, in breast cancers, Pin1 upregulates cyclin D1 function at the transcriptional and posttranslational levels in cooperation with Ras and Wnt/β-catenin (41, 71). Furthermore, Pin1 overexpression enhances and facilitates transformation by oncogenic Neu and Ras, while Pin1 inhibition suppresses Neu/Ras oncogenesis (41, 71). These results indicate a biological role for Pin1 in Neu/Ras-induced transformation. Finally, Pin1 levels are excellent prognosticators of disease progression in prostate cancers (41, 71). Considered with the extant literature, our current results expand the range of Pin1's oncogenic involvement to include ATL.
Our experiments describe a positive Tax-Pin1 feedback circuit. In HTLV-1 cells, we envision that expression of viral Tax protein first activates E2F to enhance the transcription and expression of Pin1. In return, Pin1 recognizes phosphorylated Tax to regulate the latter's interaction with IKKγ, enhancing Tax's transformation potential through NF-κB. Indeed, we documented that Pin1 contributes substantially to Tax's ability to elicit anchorage-independent cell growth. These characterizations could hold implications for the treatment of ATL. Currently, despite advances in the chemotherapy of many cancers, ATL prognosis remains poor, with an overall survival period after diagnosis averaging only 6 months. One wonders if Pin1 inhibitors such as juglone and others could be considered for treating ATL. Because Pin1 is normally expressed at very low levels in most tissues and because Pin1 KO mice do reach adulthood, it could be that there exists a reasonable treatment window in which anti-Pin1 therapy might be effective against cancers without creating a significant general toxicity to normal cells. This concept merits further exploration for its potential in ATL treatment.
Acknowledgments
We thank Anthony R. Means for the generous gift of MEF Pin1 KO cells, members of the Jeang laboratory for critical reading of manuscript, and Ron Plishka and Alicia Buckler-White for assistance with DNA sequencing.
This work was supported by intramural funds from the National Institute of Allergy and Infectious Diseases, the National Institutes of Health.
Footnotes
Published ahead of print on 21 January 2009.
REFERENCES
- 1.Afonso, P. V., A. Zamborlini, A. Saib, and R. Mahieux. 2007. Centrosome and retroviruses: the dangerous liaisons. Retrovirology 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayala, G. 2003. The prolyl isomerase Pin1 is a novel prognostic marker in human prostate cancer. Cancer Res. 636244-6251. [PubMed] [Google Scholar]
- 3.Balastik, M., J. Lim, L. Pastorino, and K. P. Lu. 2007. Pin1 in Alzheimer's disease: multiple substrates, one regulatory mechanism? Biochim. Biophys. Acta 1772422-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao, L., A. Kimzey, G. Sauter, J. M. Sowadski, K. P. Lu, and D.-G. Wang. 2004. Prevalent overexpression of prolyl isomerase Pin1 in human cancers. Am. J. Pathol. 1641727-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger, M., N. Stahl, G. Del Sal, and Y. Haupt. 2005. Mutations in proline 82 of p53 impair its activation by Pin1 and Chk2 in response to DNA damage. Mol. Cell. Biol. 255380-5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beyaert, R., K. Heyninck, and S. Van Huffel. 2000. A20 and A20-binding proteins as cellular inhibitors of nuclear factor-kappa B-dependent gene expression and apoptosis. Biochem. Pharmacol. 601143-1151. [DOI] [PubMed] [Google Scholar]
- 7.Boxus, M., J. C. Twizere, S. Legros, J. F. Dewulf, R. Kettmann, and L. Willems. 2008. The HTLV-1 Tax interactome. Retrovirology 576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ching, Y. P., S. F. Chan, K. T. Jeang, and D. Y. Jin. 2006. The retroviral oncoprotein Tax targets the coiled-coil centrosomal protein TAX1BP2 to induce centrosome overduplication. Nat. Cell Biol. 8717-724. [DOI] [PubMed] [Google Scholar]
- 9.Chu, Z. L., Y. A. Shin, J. M. Yang, J. A. DiDonato, and D. W. Ballard. 1999. IKKγ mediates the interaction of cellular IκB kinases with the Tax transforming protein of human T cell leukemia virus type 1. J. Biol. Chem. 27415297-15300. [DOI] [PubMed] [Google Scholar]
- 10.Chun, A. C., Y. Zhou, C. M. Wong, H. F. Kung, K. T. Jeang, and D. Y. Jin. 2000. Coiled-coil motif as a structural basis for the interaction of HTLV type 1 Tax with cellular cofactors. AIDS Res. Hum. Retrovir. 161689-1694. [DOI] [PubMed] [Google Scholar]
- 11.Crenshaw, D. G., J. Yang, A. R. Means, and S. Kornbluth. 1998. The mitotic peptidyl-prolyl isomerase, Pin1, interacts with Cdc25 and Plx1. EMBO J. 171315-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durkin, S. S., M. D. Ward, K. A. Fryrear, and O. J. Semmes. 2006. Site-specific phosphorylation differentiates active from inactive forms of the human T-cell leukemia virus type 1 Tax oncoprotein. J. Biol. Chem. 28131705-31712. [DOI] [PubMed] [Google Scholar]
- 13.Fontes, J. D., J. M. Strawhecker, N. D. Bills, R. E. Lewis, and S. H. Hinrichs. 1993. Phorbol esters modulate the phosphorylation of human T-cell leukemia virus type I Tax. J. Virol. 674436-4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujimori, F., K. Takahashi, C. Uchida, and T. Uchida. 1999. Mice lacking Pin1 develop normally, but are defective in entering cell cycle from G0 arrest. Biochem. Biophys. Res. Commun. 265658-663. [DOI] [PubMed] [Google Scholar]
- 15.Gallo, R. C. 2005. The discovery of the first human retrovirus: HTLV-1 and HTLV-2. Retrovirology 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glynn, J. M., T. G. Cotter, and D. R. Green. 1992. Apoptosis induced by actinomycin D, camptothecin or aphidicolin can occur in all phases of the cell cycle. Biochem. Soc. Trans. 2084S. [DOI] [PubMed] [Google Scholar]
- 17.Grassmann, R., M. Aboud, and K. T. Jeang. 2005. Molecular mechanisms of cellular transformation by HTLV-1 Tax. Oncogene 245976-5985. [DOI] [PubMed] [Google Scholar]
- 18.Hall, W. W., and M. Fujii. 2005. Deregulation of cell-signaling pathways in HTLV-1 infection. Oncogene 245965-5975. [DOI] [PubMed] [Google Scholar]
- 19.Haller, K., K. V. Kibler, T. Kasai, Y. H. Chi, J. M. Peloponese, V. S. Yedavalli, and K. T. Jeang. 2006. The N-terminus of rodent and human MAD1 confers species-specific stringency to spindle assembly checkpoint. Oncogene 252137-2147. [DOI] [PubMed] [Google Scholar]
- 20.Haller, K., Y. Wu, E. Derow, I. Schmitt, K. T. Jeang, and R. Grassmann. 2002. Physical interaction of human T-cell leukemia virus type 1 Tax with cyclin-dependent kinase 4 stimulates the phosphorylation of retinoblastoma protein. Mol. Cell. Biol. 223327-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harhaj, E. W., and S. C. Sun. 1999. IKKγ serves as a docking subunit of the IκB kinase (IKK) and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J. Biol. Chem. 27422911-22914. [DOI] [PubMed] [Google Scholar]
- 22.Hennig, L. 1998. Selective inactivation of parvulin-like peptidyl-prolyl cis/trans isomerases by juglone. Biochemistry 375953-5960. [DOI] [PubMed] [Google Scholar]
- 23.Hinuma, Y., K. Nagata, M. Hanaoka, M. Nakai, T. Matsumoto, K. I. Kinoshita, S. Shirakawa, and I. Miyoshi. 1981. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA 106476-6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iha, H., K. V. Kibler, V. R. Yedavalli, J. M. Peloponese, K. Haller, A. Miyazato, T. Kasai, and K. T. Jeang. 2003. Segregation of NF-κB activation through NEMO/IKKγ by Tax and TNFα: implications for stimulus-specific interruption of oncogenic signaling. Oncogene 228912-8923. [DOI] [PubMed] [Google Scholar]
- 25.Ishioka, K., M. Higuchi, M. Takahashi, S. Yoshida, M. Oie, Y. Tanaka, S. Takahashi, L. Xie, P. L. Green, and M. Fujii. 2006. Inactivation of tumor suppressor Dlg1 augments transformation of a T-cell line induced by human T-cell leukemia virus type 1 Tax protein. Retrovirology 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeang, K. T. 2001. Functional activities of the human T-cell leukemia virus type I Tax oncoprotein: cellular signaling through NF-κB. Cytokine Growth Factor Rev. 12207-217. [DOI] [PubMed] [Google Scholar]
- 27.Jeang, K. T., I. Boros, J. Brady, M. Radonovich, and G. Khoury. 1988. Characterization of cellular factors that interact with the human T-cell leukemia virus type I p40x-responsive 21-base-pair sequence. J. Virol. 624499-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeang, K. T., C. Z. Giam, F. Majone, and M. Aboud. 2004. Life, death, and Tax: role of HTLV-I oncoprotein in genetic instability and cellular transformation. J. Biol. Chem. 27931991-31994. [DOI] [PubMed] [Google Scholar]
- 29.Jeang, K. T., C. Z. Giam, M. Nerenberg, and G. Khoury. 1987. Abundant synthesis of functional human T-cell leukemia virus type I p40x protein in eucaryotic cells by using a baculovirus expression vector. J. Virol. 61708-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin, D. Y., V. Giordano, K. V. Kibler, H. Nakano, and K. T. Jeang. 1999. Role of adapter function in oncoprotein-mediated activation of NF-κB. Human T-cell leukemia virus type I Tax interacts directly with IκB kinase gamma. J. Biol. Chem. 27417402-17405. [DOI] [PubMed] [Google Scholar]
- 31.Jin, D. Y., F. Spencer, and K. T. Jeang. 1998. Human T cell leukemia virus type I oncoprotein Tax targets the human mitotic checkpoint protein MADI. Cell 9381-91. [DOI] [PubMed] [Google Scholar]
- 32.Joseph, J. D., E. S. Yeh, K. I. Swenson, A. R. Means, and Winkler. 2003. The peptidyl-prolyl isomerase Pin1. Prog. Cell Cycle Res. 5477-487. [PubMed] [Google Scholar]
- 33.Karin, M. 2006. Nuclear factor-κB in cancer development and progression. Nature 441431-436. [DOI] [PubMed] [Google Scholar]
- 34.Karin, M., Y. Cao, F. R. Greten, and Z. W. Li. 2002. NF-κB in cancer: from innocent bystander to major culprit. Nat. Rev. Cancer 2301-310. [DOI] [PubMed] [Google Scholar]
- 35.Kasai, T., Y. Iwanaga, H. Iha, and K. T. Jeang. 2002. Prevalent loss of mitotic spindle checkpoint in adult T-cell leukemia confers resistance to microtubule inhibitors. J. Biol. Chem. 2775187-5193. [DOI] [PubMed] [Google Scholar]
- 36.Kehn, K., R. Berro, C. de la Fuente, K. Strouss, E. Ghedin, S. Dadgar, M. E. Bottazzi, A. Pumfery, and F. Kashanchi. 2004. Mechanisms of HTLV-1 transformation. Front. Biosci. 92347-2372. [DOI] [PubMed] [Google Scholar]
- 37.Lacoste, J., J. Lanoix, N. Pepin, and J. Hiscott. 1994. Interactions between HTLV-I Tax and NF-κB/Rel proteins in T cells. Leukemia 8(Suppl. 1)S71-S76. [PubMed] [Google Scholar]
- 38.Langton, B. C., M. Sliwkowski, K. V. Tran, S. Knapp, E. Keitelman, C. Smith, S. Wallingford, H.-L. Liu, J. S. Ralston, J. Brandis, and S. Coates. 1988. Development and characterization of monoclonal antibodies to the HTLV-I Tax (P40X) protein. Med. Virol. 8295. [Google Scholar]
- 39.Lemasson, I., S. Thebault, C. Sardet, C. Devaux, and J. M. Mesnard. 1998. Activation of E2F-mediated transcription by human T-cell leukemia virus type I Tax protein in a p16INK4A-negative T-cell line. J. Biol. Chem. 27323598-23604. [DOI] [PubMed] [Google Scholar]
- 40.Li, Q., S. Withoff, and I. M. Verma. 2005. Inflammation-associated cancer: NF-κB is the lynchpin. Trends Immunol. 26318-325. [DOI] [PubMed] [Google Scholar]
- 41.Lu, K. P., and X. Z. Zhou. 2007. The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signalling and disease. Nat. Rev. Mol. Cell Biol. 8904-916. [DOI] [PubMed] [Google Scholar]
- 41a.Lu, K. P., S. D. Hanes, and T. Hunter. 1996. A human peptidyl-prolyl isomerase essential for regulation of mitosis. Nature 380544-547. [DOI] [PubMed] [Google Scholar]
- 42.Marriott, S. J., F. J. Lemoine, and K. T. Jeang. 2002. Damaged DNA and miscounted chromosomes: human T cell leukemia virus type I tax oncoprotein and genetic lesions in transformed cells. J. Biomed. Sci. 9292-298. [DOI] [PubMed] [Google Scholar]
- 43.Matsuoka, M. 2003. Human T-cell leukemia virus type I and adult T-cell leukemia. Oncogene 225131-5140. [DOI] [PubMed] [Google Scholar]
- 44.Matsuoka, M., and K. T. Jeang. 2007. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat. Rev. Cancer 7270-280. [DOI] [PubMed] [Google Scholar]
- 45.Monje, P., J. Hernandez-Losa, R. J. Lyons, M. D. Castellone, and J. S. Gutkind. 2005. Regulation of the transcriptional activity of c-Fos by ERK. A novel role for the prolyl isomerase PIN1. J. Biol. Chem. 28035081-35084. [DOI] [PubMed] [Google Scholar]
- 46.Nagata, K., K. Ohtani, M. Nakamura, and K. Sugamura. 1989. Activation of endogenous c-fos proto-oncogene expression by human T-cell leukemia virus type I-encoded p40tax protein in the human T-cell line, Jurkat. J. Virol. 633220-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nerenberg, M., S. H. Hinrichs, R. K. Reynolds, G. Khoury, and G. Jay. 1987. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science 2371324-1329. [DOI] [PubMed] [Google Scholar]
- 48.Neuveut, C., and K. T. Jeang. 2002. Cell cycle dysregulation by HTLV-I: role of the tax oncoprotein. Front. Biosci. 7d157-d163. [DOI] [PubMed] [Google Scholar]
- 49.Peloponese, J. M., K. Haller, A. Miyazato, and K. T. Jeang. 2005. Abnormal centrosome amplification in cells through the targeting of Ran-binding protein-1 by the human T cell leukemia virus type-1 Tax oncoprotein. Proc. Natl. Acad. Sci. USA 10218974-18979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peloponese, J. M., M. L. Yeung, and K. T. Jeang. 2006. Modulation of nuclear factor-κB by human T cell leukemia virus type 1 Tax protein: implications for oncogenesis and inflammation. Immunol. Res. 341-12. [DOI] [PubMed] [Google Scholar]
- 51.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 127415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pumfery, A., C. de la Fuente, and F. Kashanchi. 2006. HTLV-1 Tax: centrosome amplification and cancer. Retrovirology 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qu, Z., G. Qing, A. Rabson, and G. Xiao. 2004. Tax deregulation of NF-κB2 p100 processing involves both beta-TrCP-dependent and -independent mechanisms. J. Biol. Chem. 27944563-44572. [DOI] [PubMed] [Google Scholar]
- 54.Rosin, O., C. Koch, I. Schmitt, O. J. Semmes, K. T. Jeang, and R. Grassmann. 1998. A human T-cell leukemia virus Tax variant incapable of activating NF-κB retains its immortalizing potential for primary T-lymphocytes. J. Biol. Chem. 2736698-6703. [DOI] [PubMed] [Google Scholar]
- 55.Ryo, A. 2002. PIN1 is an E2F target gene essential for Neu/Ras-induced transformation of mammary epithelial cells. Mol. Cell. Biol. 225281-5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ryo, A., Y. C. Liou, K. P. Lu, and G. Wulf. 2003. Prolyl isomerase Pin1: a catalyst for oncogenesis and a potential therapeutic target in cancer. J. Cell Sci. 116773-783. [DOI] [PubMed] [Google Scholar]
- 57.Semmes, O. J., and K. T. Jeang. 1992. Mutational analysis of human T-cell leukemia virus type 1 Tax: regions necessary for function determined with 47 mutant proteins. J. Virol. 667183-7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheleg, S. V., J.-M. Peloponese, Y.-H. Chi, Y. Li, M. Eckhaus, and K.-T. Jeang. 2007. Evidence for cooperative transforming activity of human pituitary tumor transforming gene and human T-cellleukemia virus type 1 Tax. J. Virol. 817894-7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen, M., P. T. Stukenberg, M. W. Kirschner, and K. P. Lu. 1998. The essential mitotic peptidyl-prolyl isomerase Pin1 binds and regulates mitosis-specific phosphoproteins. Genes Dev. 12706-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith, M. R., and W. C. Greene. 1990. Identification of HTLV-I tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 41875-1885. [DOI] [PubMed] [Google Scholar]
- 61.Sobell, H. M. 1985. Actinomycin and DNA transcription. Proc. Natl. Acad. Sci. USA 825328-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stukenberg, P. T., and M. W. Kirschner. 2001. Pin1 acts catalytically to promote a conformational change in Cdc25. Mol. Cell 71071-1083. [DOI] [PubMed] [Google Scholar]
- 63.Sun, S. C., and D. W. Ballard. 1999. Persistent activation of NF-κB by the tax transforming protein of HTLV-1: hijacking cellular IkappaB kinases. Oncogene 186948-6958. [DOI] [PubMed] [Google Scholar]
- 64.Tanaka, A., C. Takahashi, S. Yamaoka, T. Nosaka, M. Maki, and M. Hatanaka. 1990. Oncogenic transformation by the tax gene of human T-cell leukemia virus type 1 in vitro. Proc. Natl. Acad. Sci. USA 871071-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watashi, K., M. Khan, V. R. K. Yedavalli, M. L. Yeung, K. Strebel, and K.-T. Jeang. 2008. Human immunodeficiency virus type 1 replication and regulation of APOBEC3G by peptidyl prolyl isomerase Pin1. J. Virol. 829928-9936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wulf, G., G. Finn, F. Suizu, and K. P. Lu. 2005. Phosphorylation-specific prolyl isomerization: is there an underlying theme? Nat. Cell Biol. 7435-441. [DOI] [PubMed] [Google Scholar]
- 67.Wulf, G., A. Ryo, Y. C. Liou, and K. P. Lu. 2003. The prolyl isomerase Pin1 in breast development and cancer. Breast Cancer Res. 576-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wulf, G. M. 2001. Pin1 is overexpressed in breast cancer and cooperates with Ras signaling in increasing the transcriptional activity of c-Jun towards cyclin D1. EMBO J. 203459-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wulf, G. M., Y. C. Liou, A. Ryo, S. W. Lee, and K. P. Lu. 2002. Role of Pin1 in the regulation of p53 stability and p21 transactivation, and cell cycle checkpoints in response to DNA damage. J. Biol. Chem. 27747976-47979. [DOI] [PubMed] [Google Scholar]
- 70.Xie, L., B. Yamamoto, A. Haoudi, O. J. Semmes, and P. L. Green. 2006. PDZ binding motif of HTLV-1 Tax promotes virus-mediated T-cell proliferation in vitro and persistence in vivo. Blood 1071980-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yeh, E. S., and A. R. Means. 2007. PIN1, the cell cycle and cancer. Nat. Rev. Cancer 7381-388. [DOI] [PubMed] [Google Scholar]
- 72.Yoshida, M. 2005. Discovery of HTLV-1, the first human retrovirus, its unique regulatory mechanisms, and insights into pathogenesis. Oncogene 245931-5937. [DOI] [PubMed] [Google Scholar]
- 73.You, H. 2002. IGF-1 induces Pin1 expression in promoting cell cycle S-phase entry. J. Cell. Biochem. 84211-216. [DOI] [PubMed] [Google Scholar]