Abstract
In an attempt to determine whether mutations in Gag in human immunodeficiency virus type 1 (HIV-1) variants selected with a protease inhibitor (PI) affect the development of resistance to the same or a different PI(s), we generated multiple infectious HIV-1 clones carrying mutated Gag and/or mutated protease proteins that were identified in amprenavir (APV)-selected HIV-1 variants and examined their virological characteristics. In an HIV-1 preparation selected with APV (33 passages, yielding HIVAPVp33), we identified six mutations in protease and six apparently critical mutations at cleavage and non-cleavage sites in Gag. An infectious recombinant clone carrying the six protease mutations but no Gag mutations failed to replicate, indicating that the Gag mutations were required for the replication of HIVAPVp33. An infectious recombinant clone that carried wild-type protease and a set of five Gag mutations (rHIVWTpro12/75/219/390/409gag) replicated comparably to wild-type HIV-1; however, when exposed to APV, rHIVWTpro12/75/219/390/409gag rapidly acquired APV resistance. In contrast, the five Gag mutations significantly delayed the acquisition of HIV-1 resistance to ritonavir and nelfinavir (NFV). Recombinant HIV-1 clones containing NFV resistance-associated mutations, such as D30N and N88S, had increased susceptibilities to APV, suggesting that antiretroviral regimens including both APV and NFV may bring about favorable antiviral efficacy. The present data suggest that the preexistence of certain Gag mutations related to PI resistance can accelerate the emergence of resistance to the PI and delay the acquisition of HIV resistance to other PIs, and these findings should have clinical relevance in the therapy of HIV-1 infection with PI-including regimens.
Combination antiretroviral therapy using reverse transcriptase inhibitors and protease inhibitors (PIs) produces substantial suppression of viral replication in human immunodeficiency virus type 1 (HIV-1)-infected patients (3, 27, 28, 42). However, the emergence of drug-resistant HIV-1 variants in such patients has limited the efficacy of combination chemotherapy. HIV-1 variants resistant to all of the currently available antiretroviral therapeutics have emerged both in vitro and in vivo (6, 16, 27, 30). Of note, a number of PI resistance-associated amino acid substitutions in the active site of protease have been identified, and such substitutions have considerable impact on the catalytic activity of protease. This impact is reflected by impaired processing of Gag precursors in mutated-protease-carrying virions and by decreased catalytic efficiency of the protease toward peptides with natural cleavage sites (7, 29, 31, 43).
However, the highly PI-resistant viruses frequently have amino acid substitutions at the p7-p1 and p1-p6 cleavage sites in Gag. These mutations have been identified in PI-resistant HIV-1 variants selected in vitro (2, 5, 8, 29) and in HIV-1 isolated from patients with AIDS for whom chemotherapy including PIs was failing (26, 40, 47, 48). These mutations are known to compensate for the enzymatic impairment of protease, per se, resulting from the acquisition of PI resistance-conferring mutations within the protease-encoding region. Moreover, certain mutations at non-cleavage sites in Gag have been shown previously to be essential for the replication of HIV-1 variants in the presence of PIs (14, 15). Although a few amino acid substitutions at cleavage and non-cleavage sites in Gag have been shown to be associated with resistance to PIs, the roles and impact of amino acid substitutions in Gag for the HIV-1 acquisition of PI resistance remain to be elucidated.
In the present study, we identified novel Gag non-cleavage site mutations in addition to multiple mutations in the protease gene during in vitro selection of HIV-1 variants highly resistant to amprenavir (APV). We show that the non-cleavage site mutations were important for not only the replication of the mutated-protease-carrying HIV-1 but also the accelerated acquisition of HIV-1 resistance to APV and an unrelated PI, nelfinavir (NFV). We also show that recombinant HIV-1 clones containing NFV resistance-associated mutations, such as D30N and N88S, had increased susceptibility to APV, suggesting that antiretroviral regimens including both APV and NFV may bring about favorable antiviral efficacy.
MATERIALS AND METHODS
Cells and antiviral agents.
MT-2 and MT-4 cells were grown in RPMI 1640-based culture medium, and 293T cells were propagated in Dulbecco's modified Eagle's medium. These media were supplemented with 10% fetal calf serum (HyClone, Logan, UT), 50 U/ml penicillin, and 50 μg/ml streptomycin. APV was kindly provided by GlaxoSmithKline, Research Triangle Park, NC. Saquinavir (SQV) and ritonavir (RTV) were provided by Roche Products Ltd. (Welwyn Garden City, United Kingdom) and Abbott Laboratories (Abbott Park, IL), respectively. NFV and indinavir (IDV) were kindly provided by Japan Energy Inc., Tokyo.
Generation of PI-resistant HIV-1 in vitro.
For the generation of PI-resistant HIV-1, various PI-resistant HIV-1 strains were propagated in the presence of increasing concentrations of a drug in a cell-free fashion as described previously (44, 45). In brief, on the first passage, MT-2 or MT-4 cells (5 × 105) were exposed to 500 50% tissue culture infective doses (TCID50) of each infectious molecular HIV-1 clone and cultured in the presence of various PIs at initial concentrations of 0.01 to 0.06 μM. On the last day of each passage (approximately day 7), 1 ml of the cell-free supernatant was harvested and transferred to a culture of fresh uninfected cells in the presence of increased concentrations of the drug for the following round of culture. In this round of culture, three drug concentrations (increased by one-, two-, and threefold compared to the previous concentration) were employed. When the replication of HIV-1 in the culture was confirmed by substantial Gag protein production (greater than 200 ng/ml), the highest drug concentration among the three concentrations was used to continue the selection (for the next round of culture). This protocol was repetitively used until the drug concentration reached the targeted concentration. Proviral DNA from the lysates of infected cells at various passages was subjected to nucleotide sequencing.
Determination of nucleotide sequences.
Molecular cloning and the determination of nucleotide sequences of HIV-1 passaged in the presence of each PI were performed as described previously (44, 45). In brief, high-molecular-weight DNA was extracted from HIV-1-infected MT-2 and MT-4 cells by using the InstaGene matrix (Bio-Rad Laboratories, Hercules, CA) and was subjected to molecular cloning, followed by sequence determination. The primers used for the first-round PCR amplification of the entire Gag- and protease-encoding regions of the HIV-1 genome were LTR F1 (5′-GAT GCT ACA TAT AAG CAG CTG C-3′) and PR12 (5′-CTC GTG ACA AAT TTC TAC TAA TGC-3′). The first-round PCR mixture consisted of 5 μl of proviral DNA solution, 2.0 U of premix Taq (Ex Taq version; Takara Bio Inc., Otsu, Japan), and 12.5 pmol of each of the first-round PCR primers in a total volume of 50 μl. The PCR conditions used were an initial 2-min step at 94°C, followed by 30 cycles of 30 s at 94°C, 30 s at 58°C, and 3 min at 72°C, with a final 8 min of extension at 72°C. The first-round PCR products (1 μl) were used directly in the second round of PCR with primers LTR F2 (5′-GAG ACT CTG GTA ACT AGA GAT C-3′) and Ksma2.1 (5′-CCA TCC CGG GCT TTA ATT TTA CTG GTA C-3′) under the same PCR conditions described above. The second-round PCR products were purified with spin columns (MicroSpin S-400 HR; Amersham Biosciences Corp., Piscataway, NJ), cloned directly, and subjected to sequencing with a model 377 automated DNA sequencer (Applied Biosystems, Foster City, CA).
Generation of recombinant HIV-1 clones.
The PCR products obtained as described above were digested with two of the three enzymes BssHII, ApaI, and SmaI, and the obtained fragments were introduced into pHIV-1NLSma, designed to have a SmaI site by changing two nucleotides (2590 and 2593) of pHIV-1NL4-3 (15, 19). To generate HIV-1 clones carrying the mutations, site-directed mutagenesis using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) was performed, and the mutation-containing genomic fragments were introduced into pHIV-1NLSma. Determination of the nucleotide sequences of plasmids confirmed that each clone had the desired mutations but no unintended mutations. 293T cells were transfected with each recombinant plasmid by using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA), and the thus-obtained infectious virions were harvested 48 h after transfection and stored at −80°C until use.
Drug sensitivity assays.
Assays for HIV-1 p24 Gag protein production were performed with MT-4 cells as described previously (1, 20, 24). In brief, MT-4 cells (105/ml) were exposed to 100 TCID50 of infectious molecular HIV-1 clones in the presence or absence of various concentrations of drugs and were incubated at 37°C. On day 7 of culture, the supernatant was harvested and the amounts of p24 Gag protein were determined by using a fully automated chemiluminescent enzyme immunoassay system (Lumipulse F; Fujirebio Inc., Tokyo). The drug concentrations that suppressed the production of p24 Gag protein by 50% (50% inhibitory concentrations [IC50]) were determined by comparing the levels of p24 production with that in a drug-free control cell culture. All assays were performed in triplicate.
Replication kinetic assay.
MT-2 or MT-4 cells (105) were exposed to each infectious HIV-1 clone (5 ng of p24 Gag protein/ml) for 3 h, washed twice with phosphate-buffered saline, and cultured in 10 ml of complete medium as described previously (1, 14). Culture supernatants (50 μl) were harvested every other day, and the p24 Gag amounts were determined as described above.
CHRA.
Two titrated infectious clones to be compared for their replicative capabilities or fitness in the competitive HIV-1 replication assay (CHRA) were combined and added to freshly prepared MT-4 cells (2 × 105) in the presence or absence of various concentrations of PIs as described previously (21, 36). Briefly, a fixed amount (200 TCID50) of one infectious clone was combined with three different amounts (100, 200, and 300 TCID50) of the other infectious clone, and the mixture was added to the culture of MT-4 cells. On the following day, one-third of infected MT-4 cells were harvested and washed twice with phosphate-buffered saline, and cellular DNA was extracted and subjected to nested PCR and sequencing as described above. The HIV-1 coculture that best approximated a 50:50 mixture on day 1 was further propagated, and the remaining cultures were discarded. Every 7 days, the cell-free supernatant of the virus coculture was transmitted to fresh uninfected MT-4 cells. The cells harvested at the end of each passage were subjected to direct DNA sequencing, and viral population changes were determined. The persistence of the original amino acid substitutions was confirmed for all infectious clones used in this assay.
Statistical analysis of selection profiles of infectious HIV-1 clones.
The selection profiles of various infectious HIV-1 clones were compared as follows. The logarithms of the concentrations were modeled as normally distributed variables with possible left censoring. The mean was assumed to be a quadratic function of the passage number. The difference between two curves was assessed by combining the estimated covariance-weighted differences of the linear and quadratic coefficients and comparing the result to computer simulations for the same quantity generated under the specific null hypothesis for that difference. SAS 9.1.3 (SAS Institute, Cary, NC) was used for all the computations. All P values are two tailed, and for figures with more than two curves, the values were corrected by the Hochberg method for multiple pairwise comparisons.
RESULTS
Amino acid sequences of Gag and protease of HIV-1 passaged in the presence of APV.
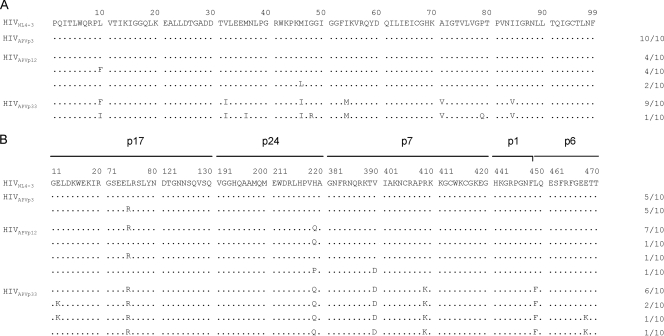
A wild-type HIV-1 strain (HIVWT) was propagated in MT-2 cells in the presence of increasing concentrations of APV, and the proviral DNA sequences in those MT-2 cells were determined at passages 3, 12, and 33 (Fig. 1). By passage 3, when HIV-1 was propagating in the presence of 0.04 μM APV (yielding HIVAPVp3), no amino acid substitutions in protease were identified but 5 of 10 clones had acquired the substitution of arginine for leucine at position 75 (L75R) in Gag. By passage 12 (at 0.18 μM APV), two APV-related resistance mutations (L10F and M46L) in protease had emerged and one mutation (H219Q) in Gag had been added. By passage 33 (at 10 μM; yielding HIVAPVp33), six APV-related amino acid substitutions, one primary mutation (I84V) and five secondary mutations (L10F, V32I, M46I, I54M, and A71V), in protease had emerged (Fig. 1A). In addition, a p1-p6 cleavage site mutation in Gag (L449F) was identified in all 10 HIV-1 clones of HIVAPVp33 examined, and five non-cleavage site mutations (E12K, L75R, H219Q, V390D, and R409K) were seen in Gag of HIVAPVp33 (Fig. 1B). Cleavage site mutations have been known to emerge when amino acid substitutions in protease are accumulated and HIV-1 develops resistance to PIs both in vitro and in vivo (5, 8). Intriguingly, the present data suggest that certain amino acid substitutions in non-cleavage sites of Gag (i.e., L75R and H219Q) may emerge earlier and in greater numbers than amino acid substitutions in protease, at least in the case of HIV-1 selection with APV. The amino acid substitutions that emerged in the virus and the pattern and order of such substitutions were largely in agreement with the data in the previous report by Gatanaga et al. (15). The present results suggested that the non-cleavage site mutations observed may play a key role in the development of HIV-1 resistance against PIs and that especially the two Gag mutations H219Q and R409K may be required for the development of PI resistance.
FIG. 1.
Amino acid sequences deduced from the nucleotide sequences of protease (A)- and Gag (B)-encoding regions of proviral DNA isolated at the indicated passages (p3, p12, and p33) from HIV-1NL4-3 variants selected in the presence of APV. The amino acid sequences of the protease and Gag proteins of wild-type HIV-1NL4-3 are shown at the top as a reference. Identity to the sequence at individual amino acid positions is indicated by dots. The numbers of clones with the given amino acid substitutions among a total of 10 clones are listed.
Mutations in Gag are required for the replication of HIVAPVp33.
In order to examine the effects of the mutations identified in Gag as described above on the replication profile of HIV-1, we generated infectious recombinant HIV-1 clones containing the six mutations (L10F, V32I, M46I, I54V, A71V, and I84V) in protease seen in HIVAPVp33. A recombinant HIV-1 clone containing the protease of HIVAPVp33 plus a wild-type Gag (rHIVAPVp33proWTgag) or the L449F cleavage site mutation-containing Gag (rHIVAPVp33pro449gag) failed to replicate in MT-2 cells over the 7-day period of culture (Fig. 2A), indicating that these Gag species do not support the growth of HIVAPVp33. Therefore, we next generated a recombinant HIV-1 clone containing the protease of HIVAPVp33 and the Gag protein with the five non-cleavage site mutations (E12K, L75R, H219Q, V390D, and R409K; rHIVAPVp33pro12/75/219/390/409gag), which replicated moderately under the same conditions (Fig. 2A). The addition of the cleavage site mutation L449F, generating rHIVAPVp33pro12/75/219/390/409/449gag, further improved the replication of the virus. In MT-4 cells, in which HIV-1 generally replicates more quickly and efficiently than in MT-2 cells, rHIVAPVp33proWTgag and rHIVAPVp33pro449gag replicated moderately; however, both rHIVAPVp33pro12/75/219/390/409gag and rHIVAPVp33pro12/75/219/390/409/449gag replicated comparably to HIVWT (Fig. 2B), due presumably to the greater replication of HIV-1 in MT-4 cells, making the difference relatively indistinct. These data clearly indicate that both non-cleavage site and cleavage site mutations in Gag contribute to the robust fitness of HIVAPVp33. We also attempted to examine the effects of combined Gag mutations on the replication of HIV-1 containing wild-type protease and generated three recombinant HIV clones, rHIVWTpro75/219gag, rHIVWTpro219/409gag, and rHIVWTpro12/75/219/390/409gag. The replication rates of these three recombinant clones turned out to be comparable to that of HIVWT when examined in MT-2 and MT-4 cells (Fig. 2C and D), unlike the finding by Doyon and his colleagues that the cleavage site mutation L449F compromised the replication of HIV-1 containing wild-type protease (8).
FIG. 2.
Replication kinetics of Gag mutant clones with or without protease mutations. MT-2 cells (A and C) and MT-4 cells (B and D) were exposed to Gag mutant clones with (A and B) or without (C and D) protease mutations. Virus replication was monitored by the amounts of p24 Gag produced in the culture supernatants. The results shown are representative of results from three independent experiments. HIVAPVp33 variants had six mutations (L10F, V32I, M46I, I54M, A71V, and I84V) in the viral protease.
Gag mutations predispose HIV-1 to rapidly acquire APV resistance.
The appearance of two non-cleavage site mutations (L75R and H219Q) in Gag prior to the emergence of mutations in protease (Fig. 1) prompted us to examine whether these two Gag mutations predisposed the virus to the acquisition of APV resistance-associated mutations in protease. We thus attempted to select APV-resistant HIV-1 by propagating HIVNL4-3 (HIVWT) and rHIVWTpro75/219gag in the presence of increasing concentrations of APV (Fig. 3). When we compared the selection curves of these two viruses, there was no significant difference (P, 0.53 and 0.65 for propagation in MT-2 and MT-4 cells, respectively). We then examined the effects of two mutated Gag species containing two and five mutations (H219Q and R409K and E12K, L75R, H219Q, V390D, and R409K [yielding mGag12/75/219/390/409gag], respectively) on the selection curves. The selection profile of a newly generated recombinant HIV clone (rHIVWTpro219/409gag) was not different from that of HIVWT in MT-2 cells (P = 0.22); however, rHIVWTpro219/409gag acquired resistance to APV much earlier than HIVWT when propagated in MT-4 cells (P < 0.0001). The recombinant clone with five non-cleavage site mutations (rHIVWTpro12/75/219/390/409gag) started to propagate in both cell lines in the presence of APV significantly earlier than HIVWT, with P values of 0.0080 and <0.0001 for MT-2 and MT-4 cells, respectively (Fig. 3).
FIG. 3.
In vitro selection of APV-resistant variants using HIV-1 carrying Gag mutations. HIVWT (▪) and three infectious HIV clones, rHIVWTpro75/219gag (▵), rHIVWTpro219/409gag (⋄), and rHIVWTpro12/75/219/390/409gag (○), were propagated in the presence of increasing concentrations of APV (starting at 0.03 μM) in MT-2 cells (A) or MT-4 cells (B). The selection was carried out in a cell-free manner for a total of 14 to 29 passages. The results of statistical evaluation of the selection profiles are as follows: panel A, HIVWT versus rHIVWTpro75/219gag, P = 0.53; HIVWT versus rHIVWTpro12/75/219/390/409gag, P = 0.0080; HIVWT versus rHIVWTpro219/409gag, P = 0.22; rHIVWTpro75/219gag versus rHIVWTpro12/75/219/390/409gag, P = 0.0065; rHIVWTpro75/219gag versus rHIVWTpro219/409gag, P = 0.15; and rHIVWTpro12/75/219/390/409gag versus rHIVWTpro219/409gag, P = 0.0018, and panel B, HIVWT versus rHIVWTpro75/219gag, P = 0.65; HIVWT versus rHIVWTpro12/75/219/390/409gag, P < 0.0001; HIVWT versus rHIVWTpro219/409gag, P < 0.0001; rHIVWTpro75/219gag versus rHIVWTpro12/75/219/390/409gag, P < 0.0001; rHIVWTpro75/219gag versus rHIVWTpro219/409gag, P < 0.0001; and rHIVWTpro12/75/219/390/409gag versus rHIVWTpro219/409gag, P = 0.088.
We then asked whether additional amino acid substitutions occurred and accelerated the acquisition of APV resistance by the virus when the Gag mutations were present. To investigate this issue, we determined the nucleotide sequence of the protease-encoding gene of each virus. Only one protease mutation (L10F) was seen by passage 20 when HIVWT and rHIVWTpro75/219gag were propagated in MT-2 cells in the presence of APV (Fig. 4A and B). In contrast, two mutations (M46L and I84V) had been acquired by rHIVWTpro219/409gag by passage 20. Of note, when rHIVWTpro12/75/219/390/409gag was propagated in MT-2 cells in the presence of APV, a mutation (L10F) had occurred by an early passage (passage 5) and four mutations (L10F, V32I, M46I, and I84V) had emerged by passage 17 (Fig. 4D). When examined in MT-4 cells, HIVWT and rHIVWTpro75/219gag had acquired two mutations (L10F and I84V and M46L and I84V, respectively) by passage 10, although rHIVWTpro219/409gag and rHIVWTpro12/75/219/390/409gag had acquired three and four mutations (L10F, M46I, and I84V and L10F, V32I, M46I, and I84V, respectively) by the same passage (Fig. 4E to H). These data, taken together, indicate that the two sets of Gag mutations (H219Q and R409K and E12K, L75R, H219Q, V390D, and R409K) clearly predisposed the virus to rapidly acquire APV resistance-associated mutations in protease and begin to propagate in the presence of APV.
FIG. 4.
Number of amino acid substitutions corresponding to the protease-encoding region of each infectious HIV-1 clone selected in the presence of APV. Nucleotide sequences of proviral DNA of HIVWT (A and E) and three infectious HIV-1 clones, rHIVWTpro75/219gag (B and F), rHIVWTpro219/409gag (C and G), and rHIVWTpro12/75/219/390/409gag (D and H), were determined using lysates of HIV-1-infected MT-2 cells (A to D) and MT-4 cells (E to H) at the termination of each passage and compared to the nucleotide sequence of HIV-1NL4-3. The number within each symbol represents the number of mutations identified in the protease when each infectious HIV-1 clone was selected in the presence of APV.
Gag mutations in HIVAPVp33 delay viral acquisition of resistance to other PIs.
We next asked whether the presence of the five Gag mutations (E12K, L75R, H219Q, V390D, and R409K) accelerated the viral acquisition of resistance to other currently available PIs (SQV, IDV, RTV, and NFV) (Fig. 5). To this end, we propagated two HIV-1 strains (HIVWT and rHIVWTpro12/75/219/390/409gag) in MT-4 cells in the presence of increasing concentrations of each PI and compared the replication profiles. The initial drug concentrations used were 0.01 μM for SQV, 0.03 μM for IDV and NFV, and 0.06 μM for RTV, and each virus was selected by a concentration of up to 5 μM. The selection was carried out in a cell-free manner for a total of 13 to 32 passages as described previously (44, 45). There was no significant difference in the selection profiles of the two strains when they were passaged in the presence of SQV (P = 0.8) or IDV (P = 0.22) (Fig. 5A and B). However, rHIVWTpro12/75/219/390/409gag started to replicate significantly later in the presence of RTV (P = 0.0001 (Fig. 5C). The selection profiles of HIVWT and rHIVWTpro12/75/219/390/409gag in the presence of NFV were examined in two independent experiments. Both curves in the first and second sets depicted in Fig. 5D showed statistically significant difference, with P values of <0.0001 and 0.0016, respectively. These data strongly suggest that the Gag mutations seen in HIVAPVp33 predispose HIV-1 to the rapid acquisition of APV resistance; however, such Gag mutations delay the viral acquisition of resistance to other PIs.
FIG. 5.
In vitro selection of PI-resistant variants using HIV-1 carrying Gag mutations. HIVWT (▪ and ⧫) and rHIVWTpro12/75/219/390/409gag (○ and ▵) were propagated in MT-4 cells in the presence of increasing concentrations of SQV (A), IDV (B), RTV (C), or NFV (D). The initial drug concentrations used were 0.01 μM for SQV, 0.03 μM for IDV and NFV, and 0.06 μM for RTV, and each virus was selected by up to a 5 μM concentration of each PI. The selection was carried out in a cell-free manner for a total of 13 to 32 passages. NFV selection was performed twice. Data from the first selection are shown with a solid line; the second selection was started using the HIV-1 from passage 10 of the first selection (with NFV at 0.7 μM), and the data are shown with a dashed line. The results of statistical evaluation of the selection profiles are as follows: panel A, P = 0.80; panel B, P = 0.22; panel C, P = 0.0001; and panel D, first selection, P < 0.0001, and second selection, P = 0.0016.
Gag mutations seen in HIVAPVp33 do not affect viral susceptibilities to PIs.
Since the Gag mutations seen in HIVAPVp33 were found to contribute to the rapid acquisition of viral resistance to APV but they delayed the emergence of viral resistance to other PIs, we examined whether such Gag mutations affected the susceptibilities of HIV-1 to various PIs in the HIV-1 drug susceptibility assay. As shown in Table 1, none of three sets of Gag mutations, as examined in the context of rHIVWTpro75/219gag, rHIVWTpro219/409gag, and rHIVWTpro12/75/219/390/409gag, affected the susceptibility of HIV-1 to any of five PIs (APV, SQV, IDV, RTV, and NFV). Indeed, the IC50s for HIVWT were highly comparable to those for any of the three recombinant clones carrying combined Gag mutations.
TABLE 1.
Sensitivities of infectious HIV-1 clones with Gag mutations to various PIs
| Infectious HIV-1 clone | IC50a (μM) of:
|
||||
|---|---|---|---|---|---|
| APV | SQV | IDV | RTV | NFV | |
| HIVWT | 0.031 ± 0.0008 | 0.021 ± 0.002 | 0.032 ± 0.002 | 0.032 ± 0.0005 | 0.028 ± 0.002 |
| rHIVWTpro75/219gag | 0.031 ± 0.003 | 0.017 ± 0.003 | 0.032 ± 0.003 | 0.031 ± 0.0007 | 0.029 ± 0.003 |
| rHIVWTpro219/409gag | 0.029 ± 0.003 | 0.020 ± 0.01 | 0.032 ± 0.001 | 0.031 ± 0.004 | 0.028 ± 0.002 |
| rHIVWTpro12/75/219/390/409gag | 0.032 ± 0.0001 | 0.023 ± 0.005 | 0.032 ± 0.003 | 0.032 ± 0.0001 | 0.028 ± 0.002 |
Data shown are mean values (with 1 standard deviation) derived from the results of three independent experiments conducted in triplicate. The IC50s were determined by employing MT-4 cells exposed to each infectious HIV-1 clone (50 TCID50) in the presence of each PI, with the inhibition of p24 Gag protein production as an end point.
Replication rate difference is not the cause of the contrasting resistance acquisition patterns.
Our observations of the contrasting resistance acquisition patterns, in which rHIVWTpro12/75/219/390/409gag acquired resistance to APV more rapidly than HIVWT when selected with APV (Fig. 3) and rHIVWTpro12/75/219/390/409gag significantly delayed the acquisition of resistance to other PIs compared to HIVWT (Fig. 5), prompted us to ask whether the replication rates of rHIVWTpro12/75/219/390/409gag and HIVWT were differentially affected by the presence of PIs. We therefore compared the replication rates of rHIVWTpro12/75/219/390/409gag and HIVWT in the presence or absence of APV, SQV, IDV, RTV, or NFV by using the CHRA (21). As shown in Fig. 6, rHIVWTpro12/75/219/390/409gag outgrew HIVWT regardless of the absence or presence of PIs. Comparing the divergence patterns of the curves for rHIVWTpro12/75/219/390/409gag and HIVWT in the absence and presence of APV (Fig. 6A and B) revealed that those for growth in the presence of APV diverged more quickly than those for growth in the absence of APV (Fig. 6B). However, similar divergence patterns were seen with SQV, IDV, RTV, and NFV (Fig. 6C, D, E, and F), suggesting that the replication advantage of rHIVWTpro12/75/219/390/409gag seen in the CHRA was not the cause for the observed contrasting resistance acquisition patterns.
FIG. 6.
Results from the CHRA for HIVWT and rHIVWTpro12/75/219/390/409gag in the absence or presence of each drug. Replication profiles of HIVWT (▪) and rHIVWTpro12/75/219/390/409gag (○) in the absence (A) or presence of 0.03 μM APV (B), 0.02 μM SQV (C), 0.03 μM IDV (D), 0.03 μM RTV (E), or 0.03 μM NFV (F) were examined by the CHRA. The cell-free supernatant was transferred to fresh MT-4 cells every 7 days. High-molecular-weight DNA extracted from infected cells at the end of each passage was subjected to nucleotide sequencing, and the proportions of Arg and Lys at position 409 in Gag were determined.
NFV resistance-conferring protease mutations increase HIV-1 susceptibility to APV.
There have been reports that an NFV-related resistance mutation, N88S, renders HIV-1 susceptible to APV (33, 49). Since the acquisition of viral resistance to PIs such as NFV was significantly delayed when HIV-1 had the Gag mutations seen in HIVAPVp33, we asked if another NFV-related resistance mutation (D30N) would render HIV-1 more susceptible to APV. We also asked whether the presence of multiple NFV resistance-associated mutations (D30N, M46I, and V77I) would make HIV-1 susceptible to APV. Moreover, we examined the effects of the Gag mutations seen in HIVAPVp33 on HIV-1 susceptibilities to APV and NFV.
As shown in Table 2, the N88S mutant clone rHIVN88SproWTgag was more susceptible to APV than HIVWT by a factor of 20, in agreement with the reports by Ziermann et al. and Resch et al. (33, 49). We found that the D30N mutation in rHIVD30NproWTgag also made HIV-1 more susceptible to APV, by a factor of 10. Interestingly, rHIV10/30/45/71proWTgag, with the four mutations L10F, D30N, K45I, and A71V, was more resistant to NFV than HIVWT by a factor of 9; however, the recombinant virus remained more susceptible to APV than HIVWT (Table 2). The introduction of the five Gag mutations (E12K, L75R, H219Q, V390D, and R409K) into rHIV10/30/45/71proWTgag, generating rHIV10/30/45/71pro12/75/219/390/409gag, did not change the susceptibility profile (Table 2). Another recombinant HIV-1 clone with three protease mutations (D30N, M46I, and V77I), rHIV30/46/77proWTgag, was also more resistant to NFV (by a factor of 9) and more susceptible to APV than HIVWT. The introduction of the five Gag mutations, generating rHIV30/46/77pro12/75/219/390/409gag, did not affect the susceptibility of rHIV30/46/77proWTgag to APV or NFV (Table 2).
TABLE 2.
Phenotypic sensitivities of recombinant HIV-1 clones passaged with NFVa
| Infectious HIV-1 clone | IC50 (μM) ± SD (change, n-fold) of:
|
|
|---|---|---|
| APV | NFV | |
| HIVWT | 0.031 ± 0.0008 (1) | 0.028 ± 0.002 (1) |
| rHIVN88SproWTgag | 0.0015 ± 0.0007 (0.05) | 0.028 ± 0.001 (1) |
| rHIVD30NproWTgag | 0.0031 ± 0.0001 (0.1) | 0.045 ± 0.001 (1.6) |
| rHIV10/30/45/71proWTgag | 0.014 ± 0.0021 (0.45) | 0.26 ± 0.03 (9) |
| rHIV10/30/45/71pro12/75/219/390/409gag | 0.020 ± 0.002 (0.64) | 0.32 ± 0.03 (11) |
| rHIV30/46/77proWTgag | 0.0069 ± 0.0024 (0.22) | 0.25 ± 0.04 (9) |
| rHIV30/46/77pro12/75/219/390/409gag | 0.0046 ± 0.0019 (0.15) | 0.21 ± 0.06 (8) |
Recombinant HIV clones rHIV10/30/45/71proWTgag and rHIV10/30/45/71pro12/75/219/390/409gag were generated to have a set of four protease mutations (L10F, D30N, K45I, and A71V) and wild-type Gag or Gag with five mutations, while other clones, rHIV30/46/77proWTgag and rHIV30/46/77pro12/75/219/390/409gag, were generated with three protease mutations (D30N, M46I, and V77I) and wild-type Gag or Gag with five mutations. Both sets of protease mutations were seen when HIV-1 was propagated in the presence of NFV. The IC50s were determined by employing MT-4 cells exposed to each recombinant HIV-1 clone (50 TCID50) in the presence of each PI, with the inhibition of p24 Gag protein production as an end point. All values were determined in triplicate, and the data are shown as mean values ±1 standard deviation of results from two or three independent experiments. The numbers in parentheses are changes (n-fold) compared to the IC50 of each PI for HIVWT.
Taken together, the data suggest that, as seen in the case of the lamivudine (3TC) resistance-associated mutation M184V that restores zidovudine (ZDV) sensitivity (37), NFV resistance-associated mutations paradoxically render HIV-1 more susceptible to APV.
DISCUSSION
Certain amino acid substitutions in Gag are known to occur in common with resistance to PIs (11, 15, 32, 36); however, no salient features such as patterns and orders of the occurrence have been identified for a number of amino acid substitutions seen in Gag in PI-resistant HIV-1 variants. The roles and impact of such amino acid substitutions in Gag for the replication of HIV-1 have not been delineated, either. These limitations have been worsened since the functions and tertiary structures of entire HIV-1 Gag proteins remain to be determined, although some structures of certain parts of Gag proteins have been lately elucidated (13, 34, 41).
In the present study, we attempted to determine the effects of non-cleavage site mutations in Gag which emerged during the in vitro selection of HIV-1 in the presence of APV on the viral acquisition of resistance to APV and other currently existing PIs. When we selected HIV-1 in vitro in the presence of increasing concentrations of APV, six amino acid substitutions apparently critical for the development of APV resistance emerged. Such substitutions included five non-cleavage site mutations (E12K, L75R, H219Q, V390D, and R409K) and one cleavage site mutation, L449F (Fig. 1B).
HIV-1 variants containing PI resistance-conferring amino acid substitutions in protease plus wild-type Gag often have highly limited replicative abilities (7, 31). Indeed, in the present study, the recombinant HIV-1 clone containing the protease of HIVAPVp33 plus a wild-type Gag (rHIVAPVp33proWTgag) or the L449F cleavage site mutation-containing Gag (rHIVAPVp33pro449gag) failed to replicate in MT-2 cells (Fig. 2A), indicating that neither of the two Gag species supported the growth of HIVAPVp33. However, a recombinant HIV-1 clone containing the protease of HIVAPVp33 and the five Gag non-cleavage site mutations, rHIVAPVp33pro12/75/219/390/409gag, replicated moderately under the same conditions (Fig. 2A), an observation in agreement with reports by others that some PI resistance-associated mutations compromise the catalytic activity of protease and/or alter polyprotein processing, often leading to slower viral replication (29, 36, 43). Since some of the five non-cleavage site mutations emerged before mutations in protease developed, we examined the effects of three sets of non-cleavage site amino acid mutations upon the emergence of APV resistance. Interestingly, HIV-1 with either of two sets of Gag mutations (rHIVWTpro219/409gag and rHIVWTpro12/75/219/390/409gag) acquired APV resistance significantly faster than HIVWT (Fig. 3), while such mutations alone did not alter the susceptibilities of HIV to the PIs examined (Table 1), a finding providing the first report that Gag mutations expedite the emergence of PI-resistant HIV-1 variants. At this time, it is apparently unknown whether certain Gag mutations associated with viral resistance to PIs persist when highly active antiretroviral therapy (HAART) regimens including a PI(s) are interrupted or changed to regimens containing no PIs. However, the non-cleavage site mutations in Gag examined in this study did not reduce the viral fitness (Fig. 2 and 6), suggesting that Gag mutations may persist longer in circulation and/or in the HIV-1 reservoir in the body than mutations in protease when antiretroviral therapy including a PI(s) is interrupted. Such persisting Gag mutations may enable HIV-1 to rapidly acquire resistance to that very PI when treatment with the PI is resumed. It is of note that on the other hand, two sets of Gag non-cleavage site mutations seen in HIVAPVp33 (H219Q and R409K and E12K, L75R, H219Q, V390D, and R409K) significantly delayed the emergence of resistance to other PIs such as RTV and NFV (Fig. 5). These data suggest that if a HAART regimen including APV is changed to an alternative regimen, the inclusion of a different PI in the alternative regimen is likely to delay the emergence of resistance to the different PI.
It is known that the L449F cleavage site mutation renders recombinant HIV-1 carrying a protease mutation (I50V) more resistant to APV (25). In the present study, a recombinant HIV-1 clone containing the protease of HIVAPVp33 plus the L449F cleavage site mutation-containing Gag (rHIVAPVp33pro449gag) failed to replicate (Fig. 2A). These data strongly suggest that the L449F mutation alone prevents HIVAPVp33 from replicating, although HIVAPVp33 did not contain the I50V mutation. The observation in the present study that the addition of five non-cleavage site mutations to rHIVAPVp33pro449gag, generating rHIVAPVp33pro12/75/219/390/409/449gag, restored the replicative ability of the virus indicates that the presence of non-cleavage site Gag mutations plays an important role in the replication of APV-resistant HIV-1 variants.
Since rHIVWTpro12/75/219/390/409gag acquired resistance to APV more rapidly than HIVWT (Fig. 3), while rHIVWTpro12/75/219/390/409gag significantly delayed the acquisition of resistance to other PIs (Fig. 5), we examined whether the replication rates of rHIVWTpro12/75/219/390/409gag and HIVWT were associated with the observed contrasting resistance acquisition patterns by using the CHRA (21). We found that rHIVWTpro12/75/219/390/409gag outgrew HIVWT regardless of the presence or absence of PIs (Fig. 6), suggesting that the difference in the replication rates of rHIVWTpro12/75/219/390/409gag and HIVWT was not the cause for the contrasting resistance acquisition patterns. As for the reason why rHIVWTpro12/75/219/390/409gag outgrew HIVWT, it is well explained by the presence of the H219Q mutation. His-219 is located within the cyclophilin A (CypA) binding loop of p24 Gag protein. It is thought that CypA plays an essential role in the HIV-1 replication cycle (4, 35) by destabilizing the capsid (p24 Gag protein) shell during viral entry and uncoating (12) and/or by performing an additional chaperone function, thus facilitating correct capsid condensation during viral maturation (17, 39). CypA is also known to support the replication of HIV-1 by binding to the Ref-1 restriction factor and/or TRIM5α, the human cellular inhibitors that impart resistance to retroviral infection (18, 38). It has also been demonstrated previously that the effect of CypA on HIV-1 replicative ability is bimodal: both high and low CypA contents limit HIV-1 replication (14). We have demonstrated previously that certain human cells, such as MT-2 and H9 cells, contain large amounts of CypA (14). We have determined more recently that MT-2 cells contain more CypA by about fivefold and that MT-4 cells contain about three times more than peripheral blood mononuclear cells (PBMCs) (unpublished data). In fact, HIV-1 produced in MT-4 cells contains large amounts of CypA, presumably resulting in compromised replication of the HIV-1. However, the H219Q mutation apparently reduces the incorporation of CypA into the virions through significantly distorting the CypA binding loop and restores the replicative ability of virions produced in MT-4 cells (14). Therefore, H219Q should contribute at least in part to the replication advantage of rHIVWTpro12/75/219/390/409gag. It is noteworthy that of 156 different HIV-1 strains whose sequences were compiled in the HIV Sequence Compendium 2008 (22), 95 and 45 strains had histidine and glutamine, respectively, at position 219. Hence, position 219 is a polymorphic amino acid site, and it is thought that this polymorphic position is associated with the acquisition of resistance to certain PIs. Indeed, we have observed that rHIVWTpro219gag overgrew rHIVWTproWTgag in the CHRA using fresh phytohemagglutinin-stimulated PBMCs (14). Since H219Q confers a replication advantage on HIV-1 in PBMCs, it is likely that HIV-1 with H219Q may acquire resistance more rapidly than HIV-1 without H219Q.
Two groups, Ziermann et al. and Resch et al., have reported that an NFV-related resistance mutation, N88S, renders HIV-1 susceptible to APV (33, 49), and indeed, Zachary et al. have reported an anecdotal finding that the infection of an individual with HIV-1 containing N88S was successfully managed with an ensuing APV-based regimen (46). Therefore, we examined the effect of another NFV resistance-associated mutation, D30N, in addition to that of the N88S mutation on HIV-1 susceptibility to APV. It was found that the mutations (D30N and N88S) clearly increased the susceptibility of HIV-1 to APV by 10- and 20-fold, respectively. These data are reminiscent of the observation that the 3TC resistance-associated mutation M184V in a background of mutations conferring resistance to ZDV restores ZDV sensitivity (37) and that ZDV-3TC combination therapy has proven to be more beneficial than ZDV monotherapy in patients harboring HIV-1 with the M184V mutation (9, 23), although the structural mechanism of the restoration of ZDV sensitivity by M184V is not clear. When a set of four protease mutations (L10F, D30N, K45I, and A71V), which had emerged by passage 10 when HIVWT was selected with NFV, were introduced into HIVWT, generating rHIV10/30/45/71proWTgag, the recombinant HIV-1 clone was more resistant to NFV than HIVWT by a factor of 9 while the clone was slightly more sensitive to APV (Table 2). When we introduced mGag12/75/219/390/409gag into HIV-1 containing a set of three NFV resistance-associated protease mutations (D30N, M46I, and V77I), generating rHIV30/46/77pro12/75/219/390/409gag, the recombinant clone was more resistant to NFV by a factor of 8 but more sensitive to APV by a factor of 6.7 (Table 2).
There has been a report that dual PI therapy with APV plus NFV is generally safe and well tolerated and that the combination of APV with NFV may have the most beneficial pharmacokinetic interactions, based on the results of a phase II clinical trial of dual PI therapies, APV in combination with IDV, NFV, or SQV, although this phase II trial was handicapped by the presence of substantial PI resistance at the baseline and the small number of patients in the study, precluding conclusions about the relative activities or toxicities of the dual PI combinations (10). The hypothesis that a HAART regimen combining APV with NFV may bring about more favorable antiviral efficacy for HIV-1-infected individuals should merit further study.
Acknowledgments
This work was supported in part by a grant-in-aid for scientific research (priority areas) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Monbu-Kagakusho); a grant to the Cooperative Research Project on Clinical and Epidemiological Studies of Emerging and Reemerging Infectious Diseases (Renkei Jigyo; no. 78, Kumamoto University) of Monbu-Kagakusho (H.M.); a grant for the promotion of AIDS research from the Ministry of Health, Welfare, and Labor of Japan (Kosei-Rohdosho; H15-AIDS-001); a grant-in-aid from the Institute of Health Sciences, Kumamoto Health Science University (M.A.); and in part by the intramural research program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Footnotes
Published ahead of print on 28 January 2009.
REFERENCES
- 1.Amano, M., Y. Koh, D. Das, J. Li, S. Leschenko, Y. F. Wang, P. I. Boross, I. T. Weber, A. K. Ghosh, and H. Mitsuya. 2007. A novel bis-tetrahydrofuranylurethane-containing nonpeptidic protease inhibitor (PI), GRL-98065, is potent against multiple-PI-resistant human immunodeficiency virus in vitro. Antimicrob. Agents Chemother. 512143-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bally, F., R. Martinez, S. Peters, P. Sudre, and A. Telenti. 2000. Polymorphism of HIV type 1 gag p7/p1 and p1/p6 cleavage sites: clinical significance and implications for resistance to protease inhibitors. AIDS Res. Hum. Retrovir. 161209-1213. [DOI] [PubMed] [Google Scholar]
- 3.Bhaskaran, K., O. Hamouda, M. Sannes, F. Boufassa, A. M. Johnson, P. C. Lambert, and K. Porter. 2008. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA 30051-59. [DOI] [PubMed] [Google Scholar]
- 4.Braaten, D., and J. Luban. 2001. Cyclophilin A regulates HIV-1 infectivity, as demonstrated by gene targeting in human T cells. EMBO J. 201300-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrillo, A., K. D. Stewart, H. L. Sham, D. W. Norbeck, W. E. Kohlbrenner, J. M. Leonard, D. J. Kempf, and A. Molla. 1998. In vitro selection and characterization of human immunodeficiency virus type 1 variants with increased resistance to ABT-378, a novel protease inhibitor. J. Virol. 727532-7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clavel, F., and A. J. Hance. 2004. HIV drug resistance. N. Engl. J. Med. 3501023-1035. [DOI] [PubMed] [Google Scholar]
- 7.Colonno, R., R. Rose, C. McLaren, A. Thiry, N. Parkin, and J. Friborg. 2004. Identification of I50L as the signature atazanavir (ATV)-resistance mutation in treatment-naive HIV-1-infected patients receiving ATV-containing regimens. J. Infect. Dis. 1891802-1810. [DOI] [PubMed] [Google Scholar]
- 8.Doyon, L., G. Croteau, D. Thibeault, F. Poulin, L. Pilote, and D. Lamarre. 1996. Second locus involved in human immunodeficiency virus type 1 resistance to protease inhibitors. J. Virol. 703763-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eron, J. J., S. L. Benoit, J. Jemsek, R. D. MacArthur, J. Santana, J. B. Quinn, D. R. Kuritzkes, M. A. Fallon, and M. Rubin for the North American HIV Working Party. 1995. Treatment with lamivudine, zidovudine, or both in HIV-positive patients with 200 to 500 CD4+ cells per cubic millimeter. N. Engl. J. Med. 3331662-1669. [DOI] [PubMed] [Google Scholar]
- 10.Eron, J. J., R. Haubrich, W. Lang, G. Pagano, J. Millard, J. Wolfram, W. Snowden, L. Pedneault, and M. Tisdale. 2001. A phase II trial of dual protease inhibitor therapy: amprenavir in combination with indinavir, nelfinavir, or saquinavir. J. Acquir. Immune Defic. Syndr. 26458-461. [DOI] [PubMed] [Google Scholar]
- 11.Gallego, O., C. de Mendoza, A. Corral, and V. Soriano. 2003. Changes in the human immunodeficiency virus p7-p1-p6 gag gene in drug-naive and pretreated patients. J. Clin. Microbiol. 411245-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gamble, T. R., F. F. Vajdos, S. Yoo, D. K. Worthylake, M. Houseweart, W. I. Sundquist, and C. P. Hill. 1996. Crystal structure of human cyclophilin A bound to the amino-terminal domain of HIV-1 capsid. Cell 871285-1294. [DOI] [PubMed] [Google Scholar]
- 13.Ganser-Pornillos, B. K., A. Cheng, and M. Yeager. 2007. Structure of full-length HIV-1 CA: a model for the mature capsid lattice. Cell 13170-79. [DOI] [PubMed] [Google Scholar]
- 14.Gatanaga, H., D. Das, Y. Suzuki, D. D. Yeh, K. A. Hussain, A. K. Ghosh, and H. Mitsuya. 2006. Altered HIV-1 Gag protein interactions with cyclophilin A (CypA) on the acquisition of H219Q and H219P substitutions in the CypA binding loop. J. Biol. Chem. 2811241-1250. [DOI] [PubMed] [Google Scholar]
- 15.Gatanaga, H., Y. Suzuki, H. Tsang, K. Yoshimura, M. F. Kavlick, K. Nagashima, R. J. Gorelick, S. Mardy, C. Tang, M. F. Summers, and H. Mitsuya. 2002. Amino acid substitutions in Gag protein at non-cleavage sites are indispensable for the development of a high multitude of HIV-1 resistance against protease inhibitors. J. Biol. Chem. 2775952-5961. [DOI] [PubMed] [Google Scholar]
- 16.Grabar, S., C. Pradier, E. Le Corfec, R. Lancar, C. Allavena, M. Bentata, P. Berlureau, C. Dupont, P. Fabbro-Peray, I. Poizot-Martin, and D. Costagliola. 2000. Factors associated with clinical and virological failure in patients receiving a triple therapy including a protease inhibitor. AIDS 14141-149. [DOI] [PubMed] [Google Scholar]
- 17.Gross, I., H. Hohenberg, C. Huckhagel, and H. G. Krausslich. 1998. N-terminal extension of human immunodeficiency virus capsid protein converts the in vitro assembly phenotype from tubular to spherical particles. J. Virol. 724798-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatziioannou, T., D. Perez-Caballero, S. Cowan, and P. D. Bieniasz. 2005. Cyclophilin interactions with incoming human immunodeficiency virus type 1 capsids with opposing effects on infectivity in human cells. J. Virol. 79176-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koh, Y., S. Matsumi, D. Das, M. Amano, D. A. Davis, J. Li, S. Leschenko, A. Baldridge, T. Shioda, R. Yarchoan, A. K. Ghosh, and H. Mitsuya. 2007. Potent inhibition of HIV-1 replication by novel non-peptidyl small molecule inhibitors of protease dimerization. J. Biol. Chem. 28228709-28720. [DOI] [PubMed] [Google Scholar]
- 20.Koh, Y., H. Nakata, K. Maeda, H. Ogata, G. Bilcer, T. Devasamudram, J. F. Kincaid, P. Boross, Y. F. Wang, Y. Tie, P. Volarath, L. Gaddis, R. W. Harrison, I. T. Weber, A. K. Ghosh, and H. Mitsuya. 2003. Novel bis-tetrahydrofuranylurethane-containing nonpeptidic protease inhibitor (PI) UIC-94017 (TMC114) with potent activity against multi-PI-resistant human immunodeficiency virus in vitro. Antimicrob. Agents Chemother. 473123-3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosalaraksa, P., M. F. Kavlick, V. Maroun, R. Le, and H. Mitsuya. 1999. Comparative fitness of multi-dideoxynucleoside-resistant human immunodeficiency virus type 1 (HIV-1) in an in vitro competitive HIV-1 replication assay. J. Virol. 735356-5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuiken, C., B. Foley, P. Marx, S. Wolinsky, T. Leitner, B. Hahn, F. McCuthan, and B. Korber. 2008. HIV sequence compendium 2008. LA-UR 08-03719. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, NM.
- 23.Larder, B. A., S. D. Kemp, and P. R. Harrigan. 1995. Potential mechanism for sustained antiretroviral efficacy of AZT-3TC combination therapy. Science 269696-699. [DOI] [PubMed] [Google Scholar]
- 24.Maeda, K., K. Yoshimura, S. Shibayama, H. Habashita, H. Tada, K. Sagawa, T. Miyakawa, M. Aoki, D. Fukushima, and H. Mitsuya. 2001. Novel low molecular weight spirodiketopiperazine derivatives potently inhibit R5 HIV-1 infection through their antagonistic effects on CCR5. J. Biol. Chem. 27635194-35200. [DOI] [PubMed] [Google Scholar]
- 25.Maguire, M. F., R. Guinea, P. Griffin, S. Macmanus, R. C. Elston, J. Wolfram, N. Richards, M. H. Hanlon, D. J. Porter, T. Wrin, N. Parkin, M. Tisdale, E. Furfine, C. Petropoulos, B. W. Snowden, and J. P. Kleim. 2002. Changes in human immunodeficiency virus type 1 Gag at positions L449 and P453 are linked to I50V protease mutants in vivo and cause reduction of sensitivity to amprenavir and improved viral fitness in vitro. J. Virol. 767398-7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mammano, F., C. Petit, and F. Clavel. 1998. Resistance-associated loss of viral fitness in human immunodeficiency virus type 1: phenotypic analysis of protease and gag coevolution in protease inhibitor-treated patients. J. Virol. 727632-7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitsuya, H., and J. W. Erickson. 1999. Discovery and development of antiretroviral therapeutics for HIV infection, p. 751-780. In T. C. Merigan, J. G. Bartlet, and D. Bolognes (ed.), Textbook of AIDS medicine. The Williams & Wilkins Co., Baltimore, MD.
- 28.Murphy, E. L., A. C. Collier, L. A. Kalish, S. F. Assmann, M. F. Para, T. P. Flanigan, P. N. Kumar, L. Mintz, F. R. Wallach, and G. J. Nemo. 2001. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann. Intern. Med. 13517-26. [DOI] [PubMed] [Google Scholar]
- 29.Nijhuis, M., N. M. van Maarseveen, S. Lastere, P. Schipper, E. Coakley, B. Glass, M. Rovenska, D. de Jong, C. Chappey, I. W. Goedegebuure, G. Heilek-Snyder, D. Dulude, N. Cammack, L. Brakier-Gingras, J. Konvalinka, N. Parkin, H. G. Krausslich, F. Brun-Vezinet, and C. A. Boucher. 2007. A novel substrate-based HIV-1 protease inhibitor drug resistance mechanism. PLoS Med. 4e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paredes, R., A. Mocroft, O. Kirk, A. Lazzarin, S. E. Barton, J. van Lunzen, T. L. Katzenstein, F. Antunes, J. D. Lundgren, and B. Clotet. 2000. Predictors of virological success and ensuing failure in HIV-positive patients starting highly active antiretroviral therapy in Europe: results from the EuroSIDA study. Arch. Intern. Med. 1601123-1132. [DOI] [PubMed] [Google Scholar]
- 31.Perrin, V., and F. Mammano. 2003. Parameters driving the selection of nelfinavir-resistant human immunodeficiency virus type 1 variants. J. Virol. 7710172-10175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peters, S., M. Munoz, S. Yerly, V. Sanchez-Merino, C. Lopez-Galindez, L. Perrin, B. Larder, D. Cmarko, S. Fakan, P. Meylan, and A. Telenti. 2001. Resistance to nucleoside analog reverse transcriptase inhibitors mediated by human immunodeficiency virus type 1 p6 protein. J. Virol. 759644-9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Resch, W., R. Ziermann, N. Parkin, A. Gamarnik, and R. Swanstrom. 2002. Nelfinavir-resistant, amprenavir-hypersusceptible strains of human immunodeficiency virus type 1 carrying an N88S mutation in protease have reduced infectivity, reduced replication capacity, and reduced fitness and process the Gag polyprotein precursor aberrantly. J. Virol. 768659-8666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saad, J. S., S. D. Ablan, R. H. Ghanam, A. Kim, K. Andrews, K. Nagashima, F. Soheilian, E. O. Freed, and M. F. Summers. 2008. Structure of the myristylated human immunodeficiency virus type 2 matrix protein and the role of phosphatidylinositol-(4,5)-bisphosphate in membrane targeting. J. Mol. Biol. 382434-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinkasserer, A., R. Harrison, A. Billich, F. Hammerschmid, G. Werner, B. Wolff, P. Peichl, G. Palfi, W. Schnitzel, E. Mlynar, et al. 1995. Mode of action of SDZ NIM 811, a nonimmunosuppressive cyclosporin A analog with activity against human immunodeficiency virus type 1 (HIV-1): interference with early and late events in HIV-1 replication. J. Virol. 69814-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamiya, S., S. Mardy, M. F. Kavlick, K. Yoshimura, and H. Mistuya. 2004. Amino acid insertions near Gag cleavage sites restore the otherwise compromised replication of human immunodeficiency virus type 1 variants resistant to protease inhibitors. J. Virol. 7812030-12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tisdale, M., S. D. Kemp, N. R. Parry, and B. A. Larder. 1993. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3′-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc. Natl. Acad. Sci. USA 905653-5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Towers, G. J., T. Hatziioannou, S. Cowan, S. P. Goff, J. Luban, and P. D. Bieniasz. 2003. Cyclophilin A modulates the sensitivity of HIV-1 to host restriction factors. Nat. Med. 91138-1143. [DOI] [PubMed] [Google Scholar]
- 39.Turner, B. G., and M. F. Summers. 1999. Structural biology of HIV. J. Mol. Biol. 2851-32. [DOI] [PubMed] [Google Scholar]
- 40.Verheyen, J., E. Litau, T. Sing, M. Daumer, M. Balduin, M. Oette, G. Fatkenheuer, J. K. Rockstroh, U. Schuldenzucker, D. Hoffmann, H. Pfister, and R. Kaiser. 2006. Compensatory mutations at the HIV cleavage sites p7/p1 and p1/p6-gag in therapy-naive and therapy-experienced patients. Antivir. Ther. 11879-887. [PubMed] [Google Scholar]
- 41.Verli, H., A. Calazans, R. Brindeiro, A. Tanuri, and J. A. Guimaraes. 2007. Molecular dynamics analysis of HIV-1 matrix protein: clarifying differences between crystallographic and solution structures. J. Mol. Graph. Model. 2662-68. [DOI] [PubMed] [Google Scholar]
- 42.Walensky, R. P., A. D. Paltiel, E. Losina, L. M. Mercincavage, B. R. Schackman, P. E. Sax, M. C. Weinstein, and K. A. Freedberg. 2006. The survival benefits of AIDS treatment in the United States. J. Infect. Dis. 19411-19. [DOI] [PubMed] [Google Scholar]
- 43.Watkins, T., W. Resch, D. Irlbeck, and R. Swanstrom. 2003. Selection of high-level resistance to human immunodeficiency virus type 1 protease inhibitors. Antimicrob. Agents Chemother. 47759-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshimura, K., R. Kato, M. F. Kavlick, A. Nguyen, V. Maroun, K. Maeda, K. A. Hussain, A. K. Ghosh, S. V. Gulnik, J. W. Erickson, and H. Mitsuya. 2002. A potent human immunodeficiency virus type 1 protease inhibitor, UIC-94003 (TMC-126), and selection of a novel (A28S) mutation in the protease active site. J. Virol. 761349-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshimura, K., R. Kato, K. Yusa, M. F. Kavlick, V. Maroun, A. Nguyen, T. Mimoto, T. Ueno, M. Shintani, J. Falloon, H. Masur, H. Hayashi, J. Erickson, and H. Mitsuya. 1999. JE-2147: a dipeptide protease inhibitor (PI) that potently inhibits multi-PI-resistant HIV-1. Proc. Natl. Acad. Sci. USA 968675-8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zachary, K. C., G. J. Hanna, and R. T. D'Aquila. 2001. Human immunodeficiency virus type 1 hypersusceptibility to amprenavir in vitro can be associated with virus load response to treatment in vivo. Clin. Infect. Dis. 332075-2077. [DOI] [PubMed] [Google Scholar]
- 47.Zennou, V., F. Mammano, S. Paulous, D. Mathez, and F. Clavel. 1998. Loss of viral fitness associated with multiple Gag and Gag-Pol processing defects in human immunodeficiency virus type 1 variants selected for resistance to protease inhibitors in vivo. J. Virol. 723300-3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang, Y. M., H. Imamichi, T. Imamichi, H. C. Lane, J. Falloon, M. B. Vasudevachari, and N. P. Salzman. 1997. Drug resistance during indinavir therapy is caused by mutations in the protease gene and in its Gag substrate cleavage sites. J. Virol. 716662-6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ziermann, R., K. Limoli, K. Das, E. Arnold, C. J. Petropoulos, and N. T. Parkin. 2000. A mutation in human immunodeficiency virus type 1 protease, N88S, that causes in vitro hypersensitivity to amprenavir. J. Virol. 744414-4419. [DOI] [PMC free article] [PubMed] [Google Scholar]