Abstract
Defining the antiviral efficacy of CD8 T cells is important for immunogen design, and yet most current assays do not measure the ability of responses to neutralize infectious virus. Here we show that human immunodeficiency virus (HIV)-specific cytotoxic T-lymphocyte (CTL) clones and cell lines derived from infected persons and targeting diverse epitopes differ by over 1,000-fold in their ability to retard infectious virus replication in autologous CD4 T cells during a 7-day period in vitro, despite comparable activity as assessed by gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assay. Cell lines derived from peripheral blood mononuclear cells stimulated in vitro with peptides representing targeted Gag epitopes consistently neutralized HIV better than Env-specific lines from the same person, although ineffective inhibition of virus replication is not a universal characteristic of Env-specific responses at the clonal level. Gag-specific cell lines were of higher avidity than Env-specific lines, although avidity did not correlate with the ability of Gag- or Env-specific lines to contain HIV replication. The greatest inhibition was observed with cell lines restricted by the protective HLA alleles B*27 and B*57, but stimulation with targeted Gag epitopes resulted in greater inhibition than did stimulation with targeted Env epitopes even in non-B*27/B*57 subjects. These results assessing functional virus neutralization by HIV-specific CD8 T cells indicate that there are marked epitope- and allele-specific differences in virus neutralization by in vitro-expanded CD8 T cells, a finding not revealed by standard IFN-γ ELISPOT assay currently in use in vaccine trials, which may be of critical importance in immunogen design and testing of candidate AIDS vaccines.
Many current human immunodeficiency virus (HIV) vaccine strategies are focused not on preventing infection but on preventing disease progression by induction of virus-specific CD8 T-cell responses (16). As such, there is a great urgency to define the relative contribution of cytotoxic T lymphocytes (CTLs) to viral control during chronic infection. However, numerous studies have failed to show a relationship between breadth or magnitude of CD8 T-cell responses, as measured by gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assay, and viral load (1, 5, 12), a major predictor of disease progression. Moreover, in a recent phase IIB trial of a recombinant HIV-adenovirus vaccine, CD8 T-cell responses to HIV as measured by IFN-γ were induced in vaccinees but failed to lower set point viral loads in immunized persons who subsequently became infected, raising concerns that this entire approach may be untenable (16). The ability of vaccine-induced responses to inhibit primary virus replication in autologous CD4 T cells, the ultimate function likely required of these cells, was not measured and has rarely been measured.
IFN-γ ELISPOT approaches to defining CD8 T-cell efficacy may be of limited value in defining effector function, as IFN-γ is the main cytokine that continues to be expressed throughout the pathway to T-cell exhaustion (3, 4, 33). The ability of CD8 T cells to secrete multiple cytokines has been associated with long-term nonprogressive infection but also has not been directly linked to viral control (6, 24, 39). Most of these studies measure the ability of uninfected cells pulsed with supraphysiologic concentrations of synthetic viral peptide to trigger cytokine production by T cells and thus fail to account for differences that may be associated with antigen processing and presentation (22, 31) and kinetics of T-cell lysis in relation to new progeny virion production (36). Indeed, recent studies indicate that preformed viral Gag protein processed in the cytoplasm upon viral entry can sensitize target cells for lysis by Gag-specific CTLs within 4 h of infection, whereas endogenous envelope synthesis over a 24-h period is required for cells to be targeted by envelope-specific CTLs (29). Detailed IFN-γ ELISPOT studies of breadth and specificity of HIV-specific CD8 T cells from HIV controllers who maintain levels of viremia below the limit of detection by current assays indicate that these cells are actually of lower response magnitude and more narrowly directed than those in persons with progressive infection (27), although the ability of cells from these persons to inhibit virus replication in autologous CD4 T cells appears to be enhanced (30). Previous limited studies in humans using CTL clones and HIV infection of HLA-matched cell lines infected with laboratory virus strains indicated superior inhibition of viral replication by Gag- and Nef-specific CTL clones, compared to reverse transcriptase (RT)-specific clones (37, 38). Recent studies of viral suppression by CTL clones derived from simian immunodeficiency virus-infected monkeys have shown marked differences in activity among multiple clones specific for the same epitopes and that in vitro suppression of virus replication does not correlate with the ability to produce IFN-γ, tumor necrosis factor alpha, or interleukin-2 (IL-2) (9). Similar studies in humans have shown that the detection of cross-clade CD8 T-cell reactivity by IFN-γ ELISPOT assay does not predict the ability to neutralize viruses containing the same variant sequences (3). Together, these studies suggest differences in antiviral efficacy that depend on the epitope targeted and other as-yet-undefined properties of the CTLs. To date, no studies have examined the ability of CD8 T cells of differing specificities derived from the same person to inhibit virus in infected autologous CD4 T cells.
Here we address the relative antiviral efficacy of CD8 T-cell responses in a functional assay that measures the ability to neutralize virus in autologous CD4 T cells. HIV-specific CD8 T-cell lines and clones from infected persons were tested for the ability to inhibit replication of primary HIV isolates over 7 to 10 days in vitro, using an adaptation of a previously reported assay (37). By expanding peripheral blood mononuclear cells (PBMCs) through stimulation with peptides representing optimal epitopes targeted in vivo, we were able to assess the relative antiviral efficacy of different responses within a single individual. This approach incorporates numerous steps that influence virus replication in vivo, including viral entry, antigen processing, epitope presentation, epitope recognition by CD8 T cells, infected cell lysis, and subsequent spread of infection to uninfected cells. Given recent population-based IFN-γ ELISPOT studies indicating that Gag-specific responses are associated with lower viral loads and Env-specific responses with higher viral load (20), we concentrated our studies on epitopes targeted within these two proteins. In order to examine the polyclonal populations that exist in vivo and also to compare the relative efficacies of multiple responses present in vivo in individual infected persons, we established effector cells of differing specificities by short-term cultures by stimulation with epitopic peptides shown by IFN-γ ELISPOT assay to be targeted in each person. Our results indicate marked differences in antiviral efficacy of CTL responses induced in HIV infection and significant superiority of in vitro-expanded Gag-specific cell lines over Env-specific cell lines.
MATERIALS AND METHODS
Study subjects.
HIV-infected individuals were recruited from outpatient clinics at local Boston hospitals following institutional review board approval and written informed consent. PBMCs were obtained and cryopreserved as previously described (27). CD4 counts and viral loads were determined as described previously (27). All subjects were not on anti-HIV therapy at the time of testing. HLA types of the subjects are shown in Table 1.
TABLE 1.
Gag- and Env-specific CTL lines generated from HIV-1-infected individuals
| Patient | CTL line generated
|
|
|---|---|---|
| Gag specific (n = 14) | Env specific (n = 12) | |
| 013646A (HLA-A3/26 and HLA-B15/27) | A3-RY10 | A3-TK10 |
| A3-KK9 | A3-RR11 | |
| A3-RK9 | B27-GY10 | |
| B27-KK10 | ||
| 013196G (HLA-A2/30 and HLA-B44/57) | A2-SL9 | A2-SAV10 |
| B57-IW9 | A2-RA9 | |
| B57-KF11 | A30-KQY9 | |
| B57-QW9 | A30-IY9 | |
| A01 (HLA-A2/3 and HLA-B35/55) | A3-RY10 | A3-TK10 |
| A3-KK9 | A3-RR11 | |
| A3-RK9 | ||
| CR0023W (HLA-A2/11 and HLA-B35/40) | A11-AK11 | A11-SK9 |
| B35-PY9 | B35-VL11 | |
| B35-WF9 | B35-DL9 | |
Viruses.
In addition to the CXCR4-utilizing HIV-1 strain NL4-3, the primary isolate X4 92HT599 and the primary CCR5-utilizing HIV-1 strain R5 91US056, were obtained from the AIDS Research and Reagent Program, Division of AIDS, NIAID, NIH (Bethesda, MD). CCR5-utilizing HIV-1 isolates SE and JGC were isolated from HIV-infected patients as described previously (14). HIV-1 laboratory strain NL4-3 was also modified to express one or more mutations in p24gag as previously described (8).
Virus sequencing.
Nested PCR for viral DNA or RNA was performed as previously described (2). PCR fragments were population sequenced to identify regions of sequence variation. All fragments were sequenced bidirectionally on an ABI 3100 PRISM automated sequencer (Applied Biosystems, Foster City, CA).
ELISPOT assay.
IFN-γ ELISPOT assays were performed as described, using overlapping peptides spanning the designated viral proteins, or optimally defined epitopes (1). Input cells ranged from 10,000 to 100,000. To calculate the number of specific spot-forming cells (SFC), the number of spots in the negative control wells was subtracted from the counted number of spots in each well. The magnitude of epitope-specific response was calculated as SFC per million cells.
Effector cell preparation.
PBMCs were isolated from whole blood of HIV-infected individuals by Ficoll-Hypaque density gradient centrifugation. To generate HIV-specific CD8 T-cell lines, one aliquot (2 to 4 M PBMCs) was stimulated with 10 μg/ml of specific peptides for 90 min and irradiated. After washing, the cells were incubated with a second aliquot of unstimulated autologous PBMCs and 10 to 20 M irradiated allogeneic PBMCs in RPMI 1640 medium containing 50 U/ml of IL-2 in addition to 0.5 μg/ml of a CD3 CD4-bispecific monoclonal antibody, which results in the selective expansion of CD8 cells (17). These cultures were maintained at 37°C and 5% CO2 for 10 to 12 days. Epitope-specific cell lines were progressively enriched by stimulation with specific epitope peptides presented by irradiated autologous PBMCs. Bulk CD8 T cells were generated from freshly isolated PBMCs by the addition of CD3 CD4-bispecific monoclonal antibody or by positive selection with anti-CD8 antibody-coated beads (30). The generated CD8 T-cell lines/clones were stimulated in vitro in the presence of peptides representing epitopes that were identified in IFN-γ ELISPOT assays, as described previously (1). The degree of enrichment in specificity of cell lines is reported as the “percent of response,” defined as the magnitude of response of the expanded cell line to the stimulating peptide, divided by the sum of all epitope specificities (stimulating peptide and all other peptides targeted at baseline) detectable in the resultant cell line. T-cell clones were prepared by limiting dilution cloning and screening with recombinant HIV-vaccinia viruses as described previously (32), and clonality was determined by T-cell receptor sequencing, as described previously (23).
Target cell preparation.
Primary CD4 T cells were generated from freshly isolated PBMCs by the addition of CD3 CD8-bispecific monoclonal antibody (34, 35) or by positive selection with anti-CD4 antibody-coated beads (30). Greater than 95% of these primary cells coexpressed CD3 and CD4 by flow cytometric analysis. These CD4 T cells were stimulated with PHA for 3 days before infection with HIV.
Viral inhibition assay.
Inhibition of viral replication was assessed in a previously established assay system (36, 37). Autologous CD4 lymphocytes were stimulated with PHA at 1 μg/ml and infected at day 3 with the designated HIV-1 isolates at a multiplicity of infection of 0.1 or as otherwise specified for 4 h at 37°C, washed twice, resuspended in medium, and plated at 5 × 105 cells per well in a 24-well plate. To assess inhibition, effector cells then were added at a ratio of 1:1 or as otherwise specified in a total of 2 ml of medium in the presence of IL-2 at 50 U/ml. At 2- to 4-day intervals, the cocultures were fed by removing and replacing one-half of the culture supernatant with fresh medium. The removed supernatant was cryopreserved for later p24 antigen quantitation by a standard quantitative enzyme-linked immunosorbent assay (commercial kit; Dupont, Boston, MA). Log inhibition units were calculated as −log10 (p24 with CTL/p24 without CTL) at day 7 in culture.
Statistical analyses.
Spearman rank-correlation, Mann-Whitney, and Wilcoxon matched-pairs tests were performed using GraphPad Prism version 4.0a. All tests were two-tailed, and P values of P < 0.05 were considered significant.
RESULTS
HIV-specific CD8 T cells can potently suppress HIV replication in vitro.
In initial experiments, we examined the ability of bulk CD8 T cells from HIV-infected persons to inhibit virus replication. In contrast to previous studies examining the ability of CTLs to inhibit laboratory strains of HIV replication in HLA-matched cell lines (36-38), here we tested multiple primary viruses, including both X4 and R5 viruses, and used autologous CD4 T cells that were expanded and infected in vitro. Initial experiments were performed using cells from elite controllers, persons who spontaneously control HIV without the need for medication (27). Since outgrowth of autologous virus in CD4 T cells is markedly delayed in these individuals (data not shown), we were able to use controlled inoculums of primary HIV isolates to infect these CD4 T cells.
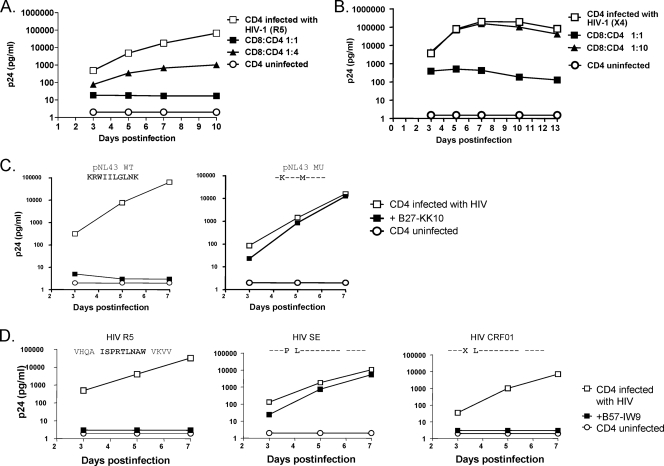
Using PBMCs obtained from an elite controller (subject 013196g) that were expanded nonspecifically in vitro by stimulation with a CD3:CD4-bispecific monoclonal antibody, which results in selective expansion of CD8 T cells, marked inhibition of replication was observed at CD8/CD4 T-cell ratios of 1:1 and 1:4 (Fig. 1A). Similar experiments were performed with CD8 T cells directly isolated from fresh peripheral blood in the absence of any IL-2 or initial exogenous stimulation, again revealing marked inhibition of virus replication in autologous CD4 T cells at cell ratios present in vivo and marked diminution of inhibition when the added CD8 T cells were diluted 10-fold (Fig. 1B), providing clear evidence of active virus neutralization of the reference viruses by circulating CD8 T cells.
FIG. 1.
HIV-specific CD8 T cells can potently suppress replication of primary HIV isolates in autologous CD4 cells. (A) Bulk CD8 T cells expanded in vitro from an elite controller (subject 013196g) by stimulation of PBMCs with CD3:CD4-bispecific monoclonal antibody inhibit HIV R5 replication in autologous CD4 T cells at the indicated effector/target cell ratios. The control of uninfected CD4 T cells showed that there were no autologous viruses grown out from the tested subject during the period of the assay. (B) Bulk CD8 T cells directly isolated from peripheral blood of the same subject by positive selection with anti-CD8 antibody-coated magnetic beads suppressed HIV X4 replication in autologous CD4 T cells at a 1:1 ratio of CD8 to CD4 T cells. (C) The Gag epitope KK10-specific, HLA-B*27-restricted CD8 T-cell clone recognized pNL4-3 wild-type virus but did not recognize an engineered escape variant which contains R-to-K and L-to-M mutations within the KK10 epitope and thus abrogates HLA class I binding with the peptide. (D) The B*57-restricted cell line specific for the epitope IW9 in Gag inhibited the R5 and CRF_01 viruses over time, whereas it had no demonstrable effect on HIV SE virus, which contains a single-amino-acid substitution, A-to-P, known to alter the peptide processing.
The above studies examined bulk CD8 T cells. To further define potential contribution of HIV-specific CD8 T cells to the observed inhibition, we next established HIV-specific CD8 T-cell lines and clones by repeated in vitro stimulation with synthetic peptides representing immunodominant HIV epitopes. Given the reported superiority of Gag-specific CTLs in disease outcome and the enrichment for HLA-B*27 and -B*57 in persons who spontaneously control HIV replication, we first focused on responses to known B*27 and B*57 epitopes. There was essentially complete suppression of wild-type NL4-3 virus replication by a Gag-specific, HLA-B*27-restricted CD8 T-cell clone specific for the KK10 wild-type epitope (KRWIILGLNK, residues 263 to 272). In contrast, infection with a virus engineered to contain two mutations (R264K and L268M) within the KK10 epitope that arise in vivo and abrogate HLA class I binding (15) led to complete loss of antiviral effect by the CD8 T-cell clone specific for the wild-type epitope (Fig. 1C). Likewise, using a cell line from a second donor (subject 013196g) that recognize the Gag IW9 epitope (ISPRTLNAW, residues 147 to 155) in the context of HLA-B*57, complete suppression of wild-type virus was observed, whereas infection with a virus containing a single-amino-acid substitution (A146P) known to arise just proximal to the epitope in vivo and alter the epitope processing (11) in the setting of HLA-B*57 resulted in 1,000-fold-higher p24 antigen in the supernatant at 7 days. In contrast, a common conservative mutation (I147L) within the epitope did not compromise the antiviral efficacy of these cells (Fig. 1D). The assay thus accurately replicates specific steps known to influence recognition of infected cells by HIV-specific CTLs that are not assessed in standard IFN-γ ELISPOT assays. Together, these data indicate that circulating HIV-specific CD8 T cells can markedly inhibit HIV replication in autologous CD4 T cells, in an assay that is sensitive to both the sequence of the infecting virus and critical steps in antigen processing.
HIV-specific CD8 T-cell clones differ in their antiviral efficacy, depending on antigen specificity.
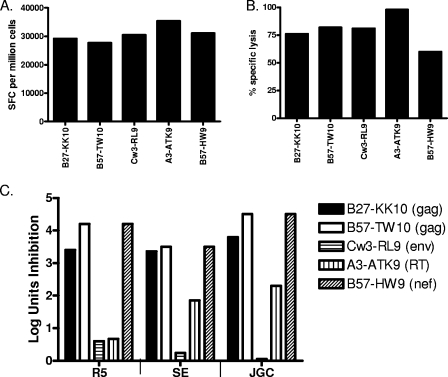
We next examined the ability of HIV-specific CD8 T-cell clones specific for both structural and accessory proteins and restricted by different HLA class I alleles to inhibit virus replication. Clones were established by limiting dilution using a CD3-specific monoclonal antibody as a stimulus for T-cell proliferation and had comparable potency by IFN-γ ELISPOT and comparable killing in cytotoxicity assays with exogenous peptide-pulsed autologous or HLA-matched B-lymphoblastoid cell lines (B-LCL) (Fig. 2A and B). Next, we evaluated the production of p24 antigen in exogenously infected autologous CD4 T cells in the presence or absence of added CD8 T cells, and expressed the data as log inhibition units (38). CD8 T-cell clones differed markedly in their ability to inhibit virus production, with results strikingly similar for three different primary isolates (Fig. 2C), all of which had been sequenced and were known to present the relevant cognate epitopes (data not shown). Of note, the least antiviral activity was observed for an Env-specific CD8 T-cell clone and an RT-specific clone despite exhibiting robust IFN-γ production in ELISPOT assay and potent killing in cytotoxic assay triggered by exogenously added peptides. These data indicate that at a clonal level, using primary viruses and infected autologous CD4 cell lines, there are marked differences in the ability of CD8 T cells to inhibit virus replication, despite comparable activity by the IFN-γ ELISPOT assay.
FIG. 2.
CD8 T-cell clones differ in antiviral efficacy depending on antigen specificity. (A and B) HIV-specific CD8 T-cell clones were isolated from HIV-infected individuals' peripheral blood by limiting dilution. The B27-KK10-specific clone was isolated from an elite controller with 1,060 SFC per million PBMCs assessed directly ex vivo in an IFN-γ ELISPOT assay, a B57-TW10-specific clone from a chronic progressor who did not recognize TW10 peptide in the IFN-γ ELISPOT assay at that time, a Cw3-RL9-specific clone from an elite controller with 1,360 SFC per million PBMCs, an A3-ATK-specific clone from an HIV-infected individual during a time the IFN-γ ELISPOT assay was technically not available, and a B57-HW9-specific clone from an elite controller with 1,640 SFC per million PBMCs. These clones were tested in a standard IFN-γ ELISPOT assay (A) and a 4-h chromium release assay with peptide-pulsed autologous or HLA-matched B-LCL targets (B). (C) The same clones were tested for antiviral function using autologous CD4 T cells infected with different primary HIV isolates. Peptide-specific CD8 T-cell clones differed in their antiviral efficacy with similar potency against the R5, SE, and JGC HIV isolates. Data are expressed as log inhibition units, calculated as −log10 (p24 with CTL/p24 without CTL) at day 7 in culture.
Simulation of PBMCs with Gag epitopes results in more effective virus neutralization than stimulation with Env epitopes.
Previous IFN-γ ELISPOT studies have shown that broader Gag-specific CD8 T-cell responses are associated with lower viral load in vivo, whereas broader Env-specific responses are associated with higher viral loads (20), and that cells are sensitized for lysis by Gag-specific CTLs much earlier after infection than Env-specific CTLs (29). We therefore compared the ability of Gag-specific and Env-specific cell lines established from four HIV-infected individuals (Table 1) to inhibit replication of a primary X4 virus and a primary R5 virus. For each subject, PBMCs were stimulated in vitro in the presence of peptides representing epitopes shown to be targeted by that individual in an IFN-γ ELISPOT assay (data not shown). For each subject, 5 to 8 individual epitope-specific cell lines were established, for a total of 26 different cell lines tested.
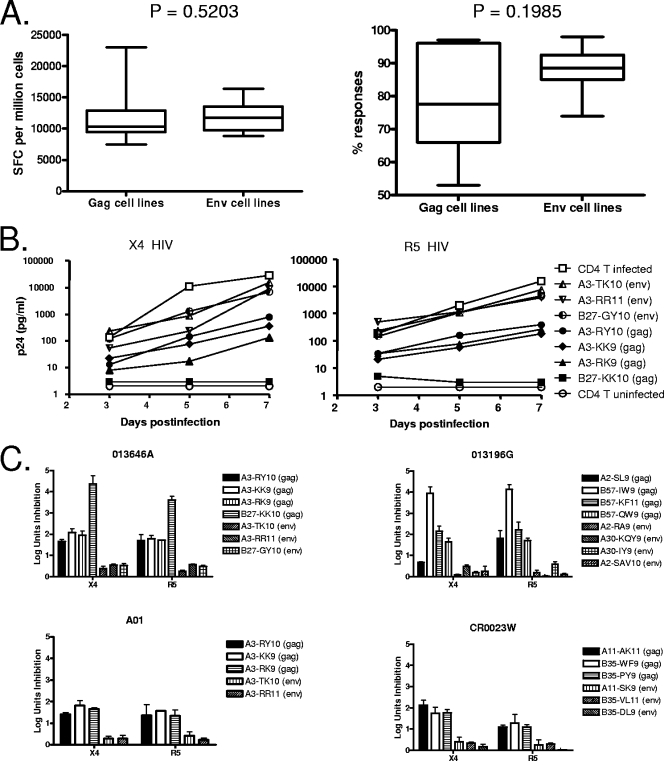
For each cell line generated, the magnitude and specificity of these epitope-specific CD8 T-cell lines were first evaluated by IFN-γ ELISPOT assay (Fig. 3A), the standard assay used to assess anti-HIV responses in vaccine trials. Although there were differences in the number of rounds of restimulation that each of the lines had received in establishing the epitope-specific lines, there were no differences among the lines in terms of the magnitude of Gag-specific responses versus Env-specific responses (11,684 ± 3,868 versus 11,793 ± 2,388 SFC per million cells; P = 0.5203) or in terms of the percentage of the enriched response that was specific for the stimulating Gag versus Env epitopes (78.43% ± 14.99% versus 87.42% ± 7.452%; P = 0.1985) when they were assessed in an IFN-γ ELISPOT assay. These data indicate comparable in vitro expansion of PBMCs with Gag and Env epitopes, as assessed by IFN-γ production.
FIG. 3.
Different antiviral efficacies of epitope-specific CD8 T-cell lines enriched in vitro from PBMCs. (A) The generated cell lines were stimulated in vitro in the presence of peptides representing epitopes in an IFN-γ ELISPOT assay. There was no difference among all lines in terms of the magnitude (SFC per million cells) or specificity (% response) of response specific for Gag (n = 14) versus Env epitopes (n = 12) and calculated as the percentage of the epitope-specific response of the overall HIV-specific CD8 T-cell response. Statistical comparisons were made using the Mann-Whitney test. (B) Example of differences in the antiviral efficacy of epitope-specific CD8 T-cell lines generated from a single subject (013646a) against X4 and R5 viruses during a 7-day period. The control uninfected CD4 T cells showed that there were no autologous viruses grown out from the tested subjects during the period of the assay. (C) Summary of data demonstrating different antiviral efficacies for Gag- and Env-specific cell lines against X4 and R5 in log units of inhibition after 7 days of culture. Inhibition was evaluated in multiple assays for each cell line at least twice (mean ± standard deviation).
We next evaluated the ability of each of these 26 cell lines to inhibit the outgrowth of primary HIV isolates in autologous CD4 T cells infected with different strains of HIV. For each subject, we attempted to grow HIV-specific lines to epitopes known to be restricted by the subject's HLA alleles and shown to be targeted by the bulk expanded CD8 T-cell lines. An example of neutralization of an X4 and R5 virus using multiple cell lines derived from one HIV-infected individual (subject 013646a) is shown in Fig. 3B, demonstrating over 1,000-fold differences in p24 antigen production using the enriched cell lines by day 7 in culture, depending on the antigenic specificity of the cell lines tested. Furthermore, Gag-specific CD8 T-cell lines in this individual were more effective than Env-specific cell lines in suppression of both R5 and X4 virus replication.
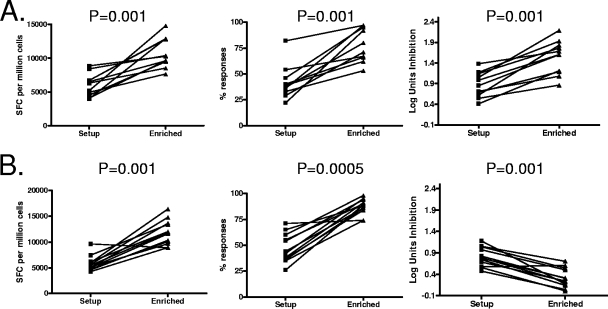
After expansion of these detailed studies to all Gag and Env epitopes targeted by four persons (two elite controllers, 013646a and 013196g; one person with treated acute infection, A01; and one person with chronic untreated infection, CR0023w, all of whom had no autologous viruses grown out during the period of the assay), consistent superiority in the antiviral efficacy of Gag- versus Env-specific responses was observed, with similar results obtained when infecting with R5 and X4 viruses (Fig. 3C). Of note, even in cases where the same HLA allele presented both Gag and Env epitopes, in each case the Gag-specific cell lines derived by repeated peptide-specific stimulation resulted in greater inhibition than the Env-specific cell lines. Moreover, serial assessment of cell lines that were repeatedly stimulated revealed that progressive increase in magnitude and specificity for Gag-specific responses was associated with greater antiviral efficacy (Fig. 4A), whereas progressive enrichment for Env-specific responses always resulted in diminished antiviral efficacy (Fig. 4B). Although there were differences in the number of rounds of stimulations that each of the lines underwent in establishing the epitope-specific lines, overall there was no difference in the number of rounds of stimulations when comparing Gag- and Env-specific cell lines (data not shown), suggesting that the enrichment process itself did not account for the difference in antiviral efficacy of Gag-specific cell lines compared to Env-specific cell lines.
FIG. 4.
Enrichment for Gag-specific responses enhances neutralization, whereas enrichment for Env-specific responses diminishes neutralization. PBMCs were stimulated in vitro with the cognate HIV Env or Gag epitope (Table 1). After a single round of stimulation starting with bulk PBMCs to establish epitope-specific cell lines (setup), the resultant cell lines were assessed for HIV-specific activity by IFN-γ ELISPOT and in the viral inhibition assay. After progressive enrichment of the setup lines for Gag or Env specificities using targeted epitopes as a stimulus (enriched), assays were repeated. (A) The progressive increase in magnitude (SFC per million cells) and specificity (% responses) for Gag-specific responses enhanced their ability to inhibit viral replication, as measured by log inhibition units (n = 11). (B) The progressive increase in magnitude and specificity for Env-specific responses diminished their ability to inhibit viral replication (n = 12). Statistical comparisons were made using the Wilcoxon matched-pairs test.
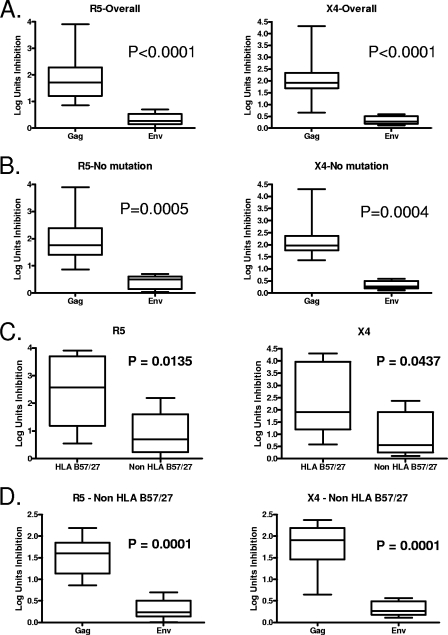
One possible explanation for the observed differences in antiviral efficacy between Gag and Env specificities is that mutations might be more likely to arise in envelope epitopes, due to the known greater plasticity of HIV Env over other expressed proteins (21). This could result in stimulation with a peptide that did not match the autologous peptide or in vitro selection for virus escape mutants that were not present at baseline. To address these possibilities, autologous virus was sequenced from each person, revealing that baseline mutations (compared to the X4 and R5 virus strains used in these assays) were present within the targeted epitope for a minority of the cell lines (one Gag epitope mutant and five Env epitope mutants in R5 virus and two Gag epitope mutants and five Env epitope mutants in X4 virus [data not shown]). Whether the antiviral efficacy of all cell lines was examined (Fig. 5A; P < 0.0001), or just those for which the autologous virus sequence was homologous to the experimental X4 and R5 viruses (Fig. 5B; P = 0.0004 and P = 0.0005, respectively), there were highly significant differences observed in the ability of Gag-specific versus Env-specific CD8 T-cell lines to inhibit virus replication. Sequencing of culture supernatants revealed there was no sequence change in the viruses that grew out despite CTL selection pressure during the 7-day coculture period (data not shown). Together these data indicate that viral escape mutations in Env protein did not account for the consistently lower antiviral efficacy of Env-specific CD8 T-cell lines compared to Gag-specific lines from the same person.
FIG. 5.
Gag-specific CD8 T-cell lines are more effective than Env-specific lines in control of HIV replication. (A) Significant differences in antiviral efficacy against both R5 and X4 virus were observed between Gag-specific (n = 14) and Env-specific (n = 12) cell lines, using either an R5 virus (left panel) or X4 virus (right panel). (B) Similar significant differences in the ability to inhibit virus replication were detected when we compared Gag-specific (n = 13 for R5; n = 12 for X4) and Env-specific (n = 7 for either R5 or X4) cell lines for which the autologous viral sequences were homologous to the experimental R5 or X4 virus. (C) Antiviral efficacy against both R5 and X4 virus appeared greater for HLA B*27/B*57-restricted lines (n = 5) compared to non-HLA B*27/B*57-restricted lines (n = 21). (D) Among non-HLA B*27/B*57-restricted cell lines, we observed that Gag-specific (n = 10) responses inhibited R5 or X4 virus replication significantly better than Env-specific (n = 11) responses. Statistical comparisons were made using the Mann-Whitney test.
The strong correlation between HIV viral control and certain HLA class I alleles, particularly HLA-B*57 and -B*27 (19, 20, 26), supports the hypothesis that CTLs recognizing epitopes restricted by these HLA molecules provide an important antiviral function. Given more HLA-B*27 and -B*57-restricted cell lines specific for Gag epitopes than specific for Env epitopes, another possible explanation for the observed differences is enhanced antiviral potency of the HLA-B*27 and -B*57-restricted cell lines. To address this possibility, statistical analysis was performed between HLA-B*27/B*57-restricted lines and non-HLA-B*27/B*57-restricted lines, as well as among those non-HLA-B*27/B*57-restricted cell lines. HLA-B*27/B*57-restricted cell lines were more effective than non-HLA-B*27/B*57-restricted lines in inhibition of virus, with similar results obtained when infecting with R5 and X4 viruses (Fig. 5C; P = 0.0135 and P = 0.0437, respectively). Furthermore, among the non-HLA-B*27/B*57-restricted cell lines, there were highly significant differences observed in the ability of Gag-specific compared to Env-specific CD8 T cells to inhibit virus replication (Fig. 5D; P = 0.0001). These data provide a link between protective HLA alleles and virus neutralization but also indicate that the significantly different antiviral efficacy of Gag-specific versus Env-specific CD8 T-cell lines derived from repeated in vitro stimulation of PBMCs is not due to certain protective alleles but is antigen specific.
In vitro-derived Gag-specific CD8 T lines are of higher avidity than Env-specific lines.
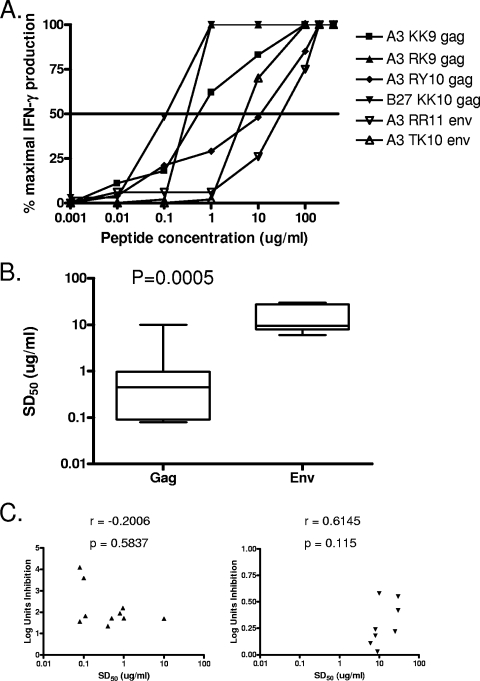
In order to address possible mechanisms of lack of control, we next determined the functional avidity of the Gag and Env-specific CTL lines. An example is shown in Fig. 6A, demonstrating 1,000-fold differences in the sensitizing dose of peptides required to yield 50% maximal CTL triggering of IFN-γ production (SD50), depending on the antigenic specificity of the cell lines tested. Furthermore, Env-specific cell lines were of significantly lower avidity than Gag-specific cell lines (Fig. 6B; P = 0.0005), despite the fact that these cell lines had been established with equal amounts of stimulating peptide. Consistent with previous studies (38), there was no significant correlation between functional avidity and antiviral activity when Gag-specific cell lines were analyzed as a group, and this was also the case when the Env-specific cell lines were examined together (Fig. 6C; P = 0.5837 and P = 0.115, respectively). These results, extended here by the use of primary HIV isolates and autologous infected CD4 T cells, indicate that specificity rather than avidity is critical for the ability of HIV-specific CD8 T cells to inhibit virus replication in vitro, although there may be an avidity threshold for optimal antiviral activity (38).
FIG. 6.
Gag-specific cell lines are of higher avidity than Env-specific cell lines. (A) An example demonstrates that the indicated cell lines differed markedly in functional avidity by peptide titration in the IFN-γ ELISPOT assay. (B) Summary data demonstrate that Gag-specific responses (n = 10) were of higher avidity than Env-specific responses (n = 8). Statistical comparisons were made using the Mann-Whitney test. (C) Avidity did not correlate with the ability to control viral replication for either Gag-specific responses (left panel; n = 10) or Env-specific responses (right panel; n = 8). Correlation statistics were analyzed using the Spearman correlation.
DISCUSSION
These data, employing an assay that measures the ability of CD8 T cells to limit growth of primary HIV isolates in autologous infected CD4 T cells, indicate that there are marked differences in antiviral efficacy of CTLs induced by natural infection, based on the protein and epitope targeted. Moreover, they demonstrate for the epitopes tested here that in vitro expansion of Gag-specific cell lines from infected persons results in far greater virus neutralization than in vitro expansion of Env-specific cell lines. Importantly, these differences were not detectable using current approaches to assess immune function, including IFN-γ ELISPOT assays and cytotoxicity assays using recombinant vaccinia viruses to sensitize target cells for lysis. These data also provide a link between protective HLA alleles and their functional ability to neutralize HIV in vitro, showing greater neutralization by HLA-B*57- and HLA-B*27-restricted cells. Results were consistent using bulk expanded CD8 T cells, CD8 T cells directly isolated from infected persons, CTL clones, and CD8 T-cell lines enriched for HIV-specific responses, as well as primary HIV isolates, showing that substantial inhibition of HIV can be observed at CD8/CD4 T-cell ratios present in vivo. Although the response magnitude of these cell lines/clones was relatively low as measured in IFN-γ ELISPOT assays with stimulation of peptides, the specificity in the enriched epitope-specific responses represented greater than 80% of the overall HIV-specific CD8 T-cell responses in most cell lines (Fig. 3A). Moreover, a progressive increase in magnitude and specificity always resulted in greater antiviral efficacy for Gag-specific responses and diminished antiviral efficacy for Env-specific responses (Fig. 4).
These data provide a possible functional explanation for population-based studies showing that Gag-specific CD8 T cells, as measured by IFN-γ ELISPOT assays, are associated with lower viral load, whereas Env-specific responses are associated with higher viral load (20). Although we found it difficult to establish viable CTL clones and lines specific for many Env epitopes, we have been able to establish one Env gp41-specific clone that displays robust neutralizing activity in this assay (18); however, this is the only Env epitope for which we have been able to generate antiviral clones and cell lines that have similar antiviral potency to what we routinely observe for many Gag-specific clones and cell lines (data not shown). The data are also consistent with the recent report that HIV Gag-specific CD8 T-cell responses are superior to Env-specific responses in control of viral load in immunized and subsequently challenged monkeys (28). The mechanism accounting for these differences is not clear but may have to do with the fact that preformed Gag protein introduced into the cytoplasm at the time of initial viral entry can be processed and presented for recognition within 4 h of infection, whereas Env protein has to be synthesized de novo before it can sensitize infected cells for lysis, a process that takes up to 24 h (29). Another possible contribution to differential neutralization is epitope- or antigen-specific differences in lytic granule loading (25). The pathway of epitope processing for Env may also play a role, in that Env is cotranslationally translocated into the endoplasmic reticulum and then undergoes posttranslational modification and reverse transport into the cytosol, where it finally gains access to a transporter associated with antigen processing-dependent class I processing pathway (13). Differential processing of HIV epitopes has been reported recently (22), and assessing the relative kinetics of Gag and Env epitope processing will be important for future studies.
One of the striking differences between Env- and Gag-specific responses is that the Env-specific responses generated in vivo by natural infection were consistently of lower avidity than Gag-specific responses, despite the fact that we used similar peptide concentrations to generate all of the lines. However, consistent with previous reports using laboratory strains of virus and immortalized CD4-bearing cell lines as target cells, we observed no significant correlation between functional avidity and antiviral activity, when either Gag-specific cell lines or Env-specific cell lines were assessed separately. These data support the finding by Yang and his colleagues that the suppression of HIV replication by CTLs depends more on antigen specificity than functional avidity (38) and support the concept of an avidity threshold for effective immune containment (4). Additional future studies will be needed to address functional avidity of CTL lysis of peptide-pulsed uninfected cells compared with CTL recognition of virus-infected cells.
There are a number of limitations to these studies that must be acknowledged and should guide future experiments. Although similar techniques were used to generate the Env- and Gag-specific cell lines tested, and these lines were comparable when assessed by IFN-γ ELISPOT, the abilities of these cell lines to subsequently expand in vitro and maintain effector functions may be different. Although testing of CTL clones used here revealed a lack of virus inhibition by an Env-specific clone compared to two Gag-specific clones, we have previously reported the marked antiviral activity of an HLA-B*14-restricted CTL clone specific for a relatively conserved epitope in gp41 (18), indicating that ineffective inhibition of virus replication is not a universal characteristic of Env-specific responses. Attempts to further address this issue were problematic, in that we found it extremely difficult and in most cases impossible to generate long-term CTL clones specific for the envelope epitopes targeted here, despite the fact that we could readily generate Gag-specific clones. This would suggest that Env-specific CTL clones might be more terminally differentiated. We have previously reported PD-1 as an exhaustion marker in a majority of HIV-specific CD8 T cells during progressive disease (10). Using quantitative RT-PCR, we evaluated Gag- versus Env-specific CTL lines from subject 013196g for expression of PD-1. PD-1 mRNA levels trended higher in the Env-specific CTL lines, but this was not significant for the small number of lines tested (unpublished data). These studies need to be further assessed in the context of survival and/or proliferation capacity of Gag- versus Env-specific CTL lines over the course of the virus inhibition assays. Sequencing of autologous virus revealed that we were using the same peptide for in vitro stimulation that was present in vivo, but it is possible that the circulating Env epitopes actually differed from the epitopes that induced these responses, which may have mutated previously. As shown in Fig. 5, the data that Env-specific cell lines were of significantly lower avidity than Gag-specific lines could be consistent with a scenario in which the tested epitope sequences represent escape (partial or complete) variants that have been selected in vivo for Env, but not for Gag. Along these lines, it is also possible that rapid escape from Env-specific responses, as has been reported in acute infection (7), leads to less effective induction of long-term memory responses with the ability to expand in vitro.
These studies also need to be examined in the context of an increasing number of studies in humans and monkeys regarding the antiviral efficacy of CTL lines and clones. Monkey studies have shown differences in the antiviral ability of multiple CTL clones from a single individual and specific for the same epitope (9), but a similar consistent difference in Env- versus Gag-specific responses has not been reported. Our own earlier data using laboratory strains of virus and infected cell lines rather than primary lymphocytes and CTL clones revealed consistent differences in antiviral efficacy depending on the protein targeted, with responses to the RT protein consistently less effective than those to Gag or Nef (38). The mechanistic underpinnings of these differences remain to be defined.
Taken together, our findings support the hypothesis that, notwithstanding the existence of individual epitope-specific differences, overall CD8 T-cell-mediated control of HIV infection during steady-state viremia in chronic infection is protein specific. Moreover, they indicate that a sizeable fraction of responses detectable in infected persons have little antiviral efficacy. These results suggest that the antiviral efficacy of vaccine-induced HIV-specific CD8 T-cell responses should be assessed in terms of ability to neutralize HIV in vitro and the development of high-throughput mechanisms to assess functional activity of vaccine-induced CTL responses should be a priority.
Acknowledgments
We thank all study participants for their contributions, J. Lieberman, M. Altfeld, M. Feeney, G. Alter, and Z. Brumme for comments on the manuscript, and Jill Gilmour for helpful discussions.
This work was supported by the Harvard University Center for AIDS Research and the International AIDS Vaccine Initiative and by grants from the Bill and Melinda Gates Foundation (B.D.W), the Doris Duke Charitable Foundation (B.D.W.), the NIH (B.D.W. and T.M.A.), the Howard Hughes Medical Institute (B.D.W.), and the Mark and Lisa Schwartz Foundation.
We declare that we have no competing financial interests.
Footnotes
Published ahead of print on 21 January 2009.
REFERENCES
- 1.Addo, M. M., X. G. Yu, A. Rathod, D. Cohen, R. L. Eldridge, D. Strick, M. N. Johnston, C. Corcoran, A. G. Wurcel, C. A. Fitzpatrick, M. E. Feeney, W. R. Rodriguez, N. Basgoz, R. Draenert, D. R. Stone, C. Brander, P. J. Goulder, E. S. Rosenberg, M. Altfeld, and B. D. Walker. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 772081-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, T. M., M. Altfeld, X. G. Yu, K. M. O'Sullivan, M. Lichterfeld, S. Le Gall, M. John, B. R. Mothe, P. K. Lee, E. T. Kalife, D. E. Cohen, K. A. Freedberg, D. A. Strick, M. N. Johnston, A. Sette, E. S. Rosenberg, S. A. Mallal, P. J. R. Goulder, C. Brander, and B. D. Walker. 2004. Selection, transmission, and reversion of an antigen-processing cytotoxic T-lymphocyte escape mutation in human immunodeficiency virus type 1 infection. J. Virol. 787069-7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett, M. S., H. L. Ng, A. Ali, and O. O. Yang. 2008. Cross-clade detection of HIV-1-specific cytotoxic T lymphocytes does not reflect cross-clade antiviral activity. J. Infect. Dis. 197390-397. [DOI] [PubMed] [Google Scholar]
- 4.Bennett, M. S., H. L. Ng, M. Dagarag, A. Ali, and O. O. Yang. 2007. Epitope-dependent avidity thresholds for cytotoxic T-lymphocyte clearance of virus-infected cells. J. Virol. 814973-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betts, M. R., D. R. Ambrozak, D. C. Douek, S. Bonhoeffer, J. M. Brenchley, J. P. Casazza, R. A. Koup, and L. J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 7511983-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betts, M. R., M. C. Nason, S. M. West, S. C. De Rosa, S. A. Migueles, J. Abraham, M. M. Lederman, J. M. Benito, P. A. Goepfert, M. Connors, M. Roederer, and R. A. Koup. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 1074781-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borrow, P., H. Lewicki, X. Wei, M. S. Horwitz, N. Peffer, H. Meyers, J. A. Nelson, J. E. Gairin, B. H. Hahn, M. B. Oldstone, and G. M. Shaw. 1997. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 3205-211. [DOI] [PubMed] [Google Scholar]
- 8.Brockman, M. A., A. Schneidewind, M. Lahaie, A. Schmidt, T. Miura, I. DeSouza, F. Ryvkin, C. A. Derdeyn, S. Allen, E. Hunter, J. Mulenga, P. A. Goepfert, B. D. Walker, and T. M. Allen. 2007. Escape and compensation from early HLA-B57-mediated cytotoxic T-lymphocyte pressure on human immunodeficiency virus type 1 Gag alter capsid interactions with cyclophilin A. J. Virol. 8112608-12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung, C., W. Lee, J. T. Loffredo, B. Burwitz, T. C. Friedrich, J. P. Giraldo Vela, G. Napoe, E. G. Rakasz, N. A. Wilson, D. B. Allison, and D. I. Watkins. 2007. Not all cytokine-producing CD8+ T-cells suppress simian immunodeficiency virus replication. J. Virol. 811517-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Day, C. L., D. E. Kaufmann, P. Kiepiela, J. A. Brown, E. S. Moodley, S. Reddy, E. W. Mackey, J. D. Miller, A. J. Leslie, C. DePierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E. J. Wherry, H. M. Coovadia, P. J. Goulder, P. Klenerman, R. Ahmed, G. J. Freeman, and B. D. Walker. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443350-354. [DOI] [PubMed] [Google Scholar]
- 11.Draenert, R., S. Le Gall, K. J. Pfafferott, A. J. Leslie, P. Chetty, C. Brander, E. C. Holmes, S. C. Chang, M. E. Feeney, M. M. Addo, L. Ruiz, D. Ramduth, P. Jeena, M. Altfeld, S. Thomas, Y. Tang, C. L. Verrill, C. Dixon, J. G. Prado, P. Kiepiela, J. Martinez-Picado, B. D. Walker, and P. J. Goulder. 2004. Immune selection for altered antigen processing leads to cytotoxic T lymphocyte escape in chronic HIV-1 infection. J. Exp. Med. 199905-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards, B. H., A. Bansal, S. Sabbaj, J. Bakari, M. J. Mulligan, and P. A. Goepfert. 2002. Magnitude of functional CD8+ T-cell responses to the Gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J. Virol. 762298-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferris, R. L., C. Hall, N. V. Sipsas, J. T. Safrit, A. Trocha, R. A. Koup, R. P. Johnson, and R. F. Siliciano. 1999. Processing of HIV-1 envelope glycoprotein for class I-restricted recognition: dependence on TAP1/2 and mechanisms for cytosolic localization. J. Immunol. 1621324-1332. [PubMed] [Google Scholar]
- 14.Gartner, S., and M. Popovic. 1990. Virus isolation and production, p. 53-66. In A. Aldovini and B. D. Walker (ed.), Techniques in HIV research. Stockton Press, New York, NY.
- 15.Goulder, P. J., R. E. Phillips, R. A. Colbert, S. McAdam, G. Ogg, M. A. Nowak, P. Giangrande, G. Luzzi, B. Morgan, A. Edwards, A. J. McMichael, and S. Rowland-Jones. 1997. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat. Med. 3212-217. [DOI] [PubMed] [Google Scholar]
- 16.Johnston, M. I., and A. S. Fauci. 2007. An HIV vaccine—evolving concepts. N. Engl. J. Med. 3562073-2081. [DOI] [PubMed] [Google Scholar]
- 17.Jones, N., D. Agrawal, M. Elrefaei, A. Hanson, V. Novitsky, J. T. Wong, and H. Cao. 2003. Evaluation of antigen-specific responses using in vitro enriched T cells. J. Immunol. Methods 274139-147. [DOI] [PubMed] [Google Scholar]
- 18.Kalams, S. A., R. P. Johnson, A. K. Trocha, M. J. Dynan, H. S. Ngo, R. T. D'Aquila, J. T. Kurnick, and B. D. Walker. 1994. Longitudinal analysis of T cell receptor (TCR) gene usage by human immunodeficiency virus 1 envelope-specific cytotoxic T lymphocyte clones reveals a limited TCR repertoire. J. Exp. Med. 1791261-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiepiela, P., A. J. Leslie, I. Honeyborne, D. Ramduth, C. Thobakgale, S. Chetty, P. Rathnavalu, C. Moore, K. J. Pfafferott, L. Hilton, P. Zimbwa, S. Moore, T. Allen, C. Brander, M. M. Addo, M. Altfeld, I. James, S. Mallal, M. Bunce, L. D. Barber, J. Szinger, C. Day, P. Klenerman, J. Mullins, B. Korber, H. M. Coovadia, B. D. Walker, and P. J. Goulder. 2004. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature 432769-775. [DOI] [PubMed] [Google Scholar]
- 20.Kiepiela, P., K. Ngumbela, C. Thobakgale, D. Ramduth, I. Honeyborne, E. Moodley, S. Reddy, C. de Pierres, Z. Mncube, N. Mkhwanazi, K. Bishop, M. van der Stok, K. Nair, N. Khan, H. Crawford, R. Payne, A. Leslie, J. Prado, A. Prendergast, J. Frater, N. McCarthy, C. Brander, G. H. Learn, D. Nickle, C. Rousseau, H. Coovadia, J. I. Mullins, D. Heckerman, B. D. Walker, and P. Goulder. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 1346-53. [DOI] [PubMed] [Google Scholar]
- 21.Kusumi, K., B. Conway, S. Cunningham, A. Berson, C. Evans, A. K. N. Iversen, D. Colvin, M. V. Gallo, S. Coutre, E. G. Shpaer, D. V. Faulkner, A. deRonde, S. Volkman, C. Williams, M. S. Hirsch, and J. I. Mullins. 1992. Human immunodeficiency virus type 1 envelope gene structure and diversity in vivo and after cocultivation in vitro. J. Virol. 66875-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Gall, S., P. Stamegna, and B. D. Walker. 2007. Portable flanking sequences modulate CTL epitope processing. J. Clin. Investig. 1173563-3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer-Olson, D., K. W. Brady, M. T. Bartman, K. M. O'Sullivan, B. C. Simons, J. A. Conrad, C. B. Duncan, S. Lorey, A. Siddique, R. Draenert, M. Addo, M. Altfeld, E. Rosenberg, T. M. Allen, B. D. Walker, and S. A. Kalams. 2006. Fluctuations of functionally distinct CD8+ T-cell clonotypes demonstrate flexibility of the HIV-specific TCR repertoire. Blood 1072373-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Migueles, S. A., A. C. Laborico, W. L. Shupert, M. S. Sabbaghian, R. Rabin, C. W. Hallahan, D. Van Baarle, S. Kostense, F. Miedema, M. McLaughlin, L. Ehler, J. Metcalf, S. Liu, and M. Connors. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 31061-1068. [DOI] [PubMed] [Google Scholar]
- 25.Migueles, S. A., C. M. Osborne, C. Royce, A. A. Compton, R. P. Joshi, K. A. Weeks, J. E. Rood, A. M. Berkley, J. B. Sacha, N. A. Cogliano-Shutta, M. Lloyd, G. Roby, R. Kwan, M. McLaughlin, S. Stallings, C. Rehm, M. A. O'Shea, J. Mican, B. Z. Packard, A. Komoriya, S. Palmer, A. P. Wiegand, F. Maldarelli, J. M. Coffin, J. W. Mellors, C. W. Hallahan, D. A. Follman, and M. Connors. 2008. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity 291009-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Migueles, S. A., M. S. Sabbaghian, W. L. Shupert, M. P. Bettinotti, F. M. Marincola, L. Martino, C. W. Hallahan, S. M. Selig, D. Schwartz, J. Sullivan, and M. Connors. 2000. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. USA 972709-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereyra, F., M. M. Addo, D. E. Kaufmann, Y. Liu, T. Miura, A. Rathod, B. Baker, A. Trocha, R. Rosenberg, E. Mackey, P. Ueda, Z. Lu, D. Cohen, T. Wrin, C. J. Petropoulos, E. S. Rosenberg, and B. D. Walker. 2008. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J. Infect. Dis. 197563-571. [DOI] [PubMed] [Google Scholar]
- 28.Peut, V., and S. J. Kent. 2007. Utility of human immunodeficiency virus type 1 envelope as a T-cell immunogen. J. Virol. 8113125-13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sacha, J. B., C. Chung, E. G. Rakasz, S. P. Spencer, A. K. Jonas, A. T. Bean, W. Lee, B. J. Burwitz, J. J. Stephany, J. T. Loffredo, D. B. Allison, S. Adnan, A. Hoji, N. A. Wilson, T. C. Friedrich, J. D. Lifson, O. O. Yang, and D. I. Watkins. 2007. Gag-specific CD8+ T lymphocytes recognize infected cells before AIDS-virus integration and viral protein expression. J. Immunol. 1782746-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sáez-Cirión, A., C. Lacabaratz, O. Lambotte, P. Versmisse, A. Urrutia, F. Boufassa, F. Barré-Sinoussi, J.-F. Delfraissy, M. Sinet, G. Pancino, and A. Venet. 2007. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc. Natl. Acad. Sci. USA 1046776-6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valentine, L. E., S. M. Piaskowski, E. G. Rakasz, N. L. Henry, N. A. Wilson, and D. I. Watkins. 2008. Recognition of escape variants in ELISPOT does not always predict CD8+ T-cell recognition of simian immunodeficiency virus-infected cells expressing the same variant sequences. J. Virol. 82575-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker, B. D., C. Flexner, K. Birch-Limberger, L. Fisher, T. J. Paradis, A. Aldovini, R. Young, B. Moss, and R. T. Schooley. 1989. Long-term culture and fine specificity of human cytotoxic T-lymphocyte clones reactive with human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 869514-9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wherry, E. J., and R. Ahmed. 2004. Memory CD8 T-cell differentiation during viral infection. J. Virol. 785535-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson, C. C., J. T. Wong, D. D. Girard, D. P. Merrill, M. Dynan, D. D. An, S. A. Kalams, R. P. Johnson, M. S. Hirsch, R. T. D'Aquila, et al. 1995. Ex vivo expansion of CD4 lymphocytes from human immunodeficiency virus type 1-infected persons in the presence of combination antiretroviral agents. J. Infect. Dis. 17288-96. [DOI] [PubMed] [Google Scholar]
- 35.Wong, J. K., M. Hezareh, H. F. Gunthard, D. V. Havlir, C. C. Ignacio, C. A. Spina, and D. D. Richman. 1997. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 2781291-1295. [DOI] [PubMed] [Google Scholar]
- 36.Yang, O. O., S. A. Kalams, M. Rosenzweig, A. Trocha, N. Jones, M. Koziel, B. D. Walker, and R. P. Johnson. 1996. Efficient lysis of human immunodeficiency virus type 1-infected cells by cytotoxic T lymphocytes. J. Virol. 705799-5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang, O. O., S. A. Kalams, A. Trocha, H. Cao, A. Luster, R. P. Johnson, and B. D. Walker. 1997. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J. Virol. 713120-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang, O. O., P. T. Sarkis, A. Trocha, S. A. Kalams, R. P. Johnson, and B. D. Walker. 2003. Impacts of avidity and specificity on the antiviral efficiency of HIV-1-specific CTL. J. Immunol. 1713718-3724. [DOI] [PubMed] [Google Scholar]
- 39.Zimmerli, S. C., A. Harari, C. Cellerai, F. Vallelian, P. A. Bart, and G. Pantaleo. 2005. HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc. Natl. Acad. Sci. USA 1027239-7244. [DOI] [PMC free article] [PubMed] [Google Scholar]