Abstract
Mucosal surfaces play a major role in human immunodeficiency virus type 1 (HIV-1) transmission and pathogenesis, and yet the role of lamina propria macrophages in mucosal HIV-1 infection has received little investigative attention. We report here that vaginal and intestinal macrophages display distinct phenotype and HIV-1 permissiveness profiles. Vaginal macrophages expressed the innate response receptors CD14, CD89, CD16, CD32, and CD64 and the HIV-1 receptor/coreceptors CD4, CCR5, and CXCR4, similar to monocytes. Consistent with this phenotype, green fluorescent protein-tagged R5 HIV-1 entered macrophages in explanted vaginal mucosa as early as 30 min after inoculation of virus onto the epithelium, and purified vaginal macrophages supported substantial levels of HIV-1 replication by a panel of highly macrophage-tropic R5 viruses. In sharp contrast, intestinal macrophages expressed no detectable, or very low levels of, innate response receptors and HIV-1 receptor/coreceptors and did not support HIV-1 replication, although virus occasionally entered macrophages in intestinal tissue explants. Thus, vaginal, but not intestinal, macrophages are monocyte-like and permissive to R5 HIV-1 after the virus has translocated across the epithelium. These findings suggest that genital and gut macrophages have different roles in mucosal HIV-1 pathogenesis and that vaginal macrophages play a previously underappreciated but potentially important role in mucosal HIV-1 infection in the female genital tract.
Human immunodeficiency virus type 1 (HIV-1) enters the host in virtually all infections, excluding those acquired parenterally, through the mucosal surfaces of either the genital tract or the gastrointestinal tract (1, 36, 42). After translocation across the mucosal epithelium, HIV-1 encounters potential target cells in the dense lymphocyte and macrophage populations of the lamina propria. In human and macaque cervical mucosa, lamina propria CD4+ T lymphocytes are early target cells for HIV-1 and simian immunodeficiency virus (SIV) and support viral replication (4, 12, 62). In macaque vaginal mucosa, CD4+ T cells are rapidly depleted after intravenous inoculation of SIV (56). In the vaginal mucosa of humans, HIV-1 target cell populations have not been fully characterized, although HIV-1 has been detected in CD4+ T cells in vaginal epithelium in a modified organ culture system by electron microscopy (13) and in subepithelial vaginal macrophages in a biopsy system by immunohistochemistry (9). In human and macaque gastrointestinal mucosa, most attention has focused on the small intestine, where lamina propria CD4+ T cells are prominent HIV-1 and SIV target cells and undergo profound depletion shortly after infection (3, 10, 11, 18, 21-23, 44, 53).
In contrast to CD4+ T cells, little is known about the role of macrophages in genital and gut HIV-1 infections due largely to difficulties in isolating and purifying macrophages from mucosal tissues. Although the mucosa is the largest reservoir of macrophages in the body (16) and mucosal macrophages play an important role in host defense (46, 49), studies of monocytes/macrophages in HIV-1 infection have focused on blood monocytes and lymph node macrophages, which have been implicated in the establishment, persistence, and pathogenesis of HIV-1 infection (5, 14, 29-32, 41, 60). However, the striking and well-defined phenotypic and functional differences between blood monocytes and mucosal macrophages, in particular macrophages in the gastrointestinal mucosa (46, 48, 51), preclude the simple extrapolation from findings in HIV-1-infected monocytes to HIV-1 infection of mucosal macrophages. In this regard, we and others have shown that, in contrast to monocytes and monocyte-derived macrophages, intestinal macrophages do not express many innate response receptors (48, 51) and are downregulated for triggering receptor expressed on monocytes (i.e., TREM-1) (39, 40) and costimulatory molecules (37, 51). Consistent with this unique phenotype, intestinal macrophages are profoundly inflammation anergic (51), and yet they retain potent phagocytic and microbicidal activity (45, 46, 51). We also have reported that intestinal macrophages, unlike monocyte-derived macrophages, are resistant to infection with HIV-1 (17, 24). Therefore, to elucidate the role of macrophages in different mucosal compartments in the pathogenesis of HIV-1 infection, we isolated lamina propria macrophages from normal human vaginal and intestinal mucosa and analyzed the cells for differentiation markers, innate response receptors, HIV-1 receptor and coreceptors, and HIV-1 permissiveness to highly macrophage-tropic R5 viruses. We report that vaginal, but not intestinal, mucosal macrophages retain a monocyte-like phenotype and support R5 HIV-1 replication.
MATERIALS AND METHODS
Mucosal tissues and blood.
Human intestinal (jejunal) tissue and blood were obtained from otherwise healthy subjects undergoing gastric bypass for obesity, and vaginal tissue was obtained from women undergoing reconstructive pelvic surgery. All specimens were obtained in accordance with Institutional Review Board approved protocols.
HIV-1 molecular clones and viruses.
Replication-competent clones of R5 viruses, including YU2 (19) and the highly macrophage-tropic NA420 B33, NA20 B59, and NA353 B27 (33), were transfected into 293T cells by Fugene 6 (Roche, Indianapolis, IN), according to the manufacturer's protocol. After 60 h, the supernatants were harvested, clarified by low-speed centrifugation (1,000 × g, 10 min), filtered through a 0.45-μm-pore-size filter, divided into aliquots, and stored at −80°C. The viruses were titrated as previously described (25) using JC53BL cells derived from CXCR4+ HeLa cells transduced to express CCR5 and CXCR4 (6). To generate green fluorescent protein (GFP)-tagged viral particles, the pLR2PHIVgag-GFP plasmid was transfected with pCMV-ENV/HIV-YU2 (R5) into 293T cells by Fugene 6 (Roche), as previously described (61). Supernatants were harvested after 60 h, clarified by low-speed centrifugation, filtered, and ultracentrifuged as described above to concentrate the GFP-labeled virus-like particles. The pellets were resuspended in Dulbecco modified Eagle medium, divided into aliquots, and frozen at −80°C. To prepare a GFP-expressing reporter virus, YU2 envelope (Env)-pseudotyped HIV-1 that expresses GFP upon replication and supports only one round of replication (15) was constructed as follows. The env gene was deleted, and the gfp gene was inserted between the env and nef genes of the NL4-3 clone. The internal ribosome entry site element was inserted between the gfp and nef genes to rescue nef gene expression. To generate the GFP-YU2 Env-pseudotyped virus, the clone was cotransfected with the YU2 Env expression plasmid into 293T cells, as described above.
Isolation and purification of mucosal cells and blood monocytes.
Macrophages and lymphocytes were isolated from tissue segments by enzyme digestion, as described previously (45, 52). Briefly, sections of vaginal and intestinal tissue were dissected to mechanically remove the submucosa. The resulting mucosa was rinsed in Ca2+-and Mg2+-free phosphate-buffered saline (PBS) and washed in Hanks balanced salt solution containing 200 μg of dithiothreitol/ml to remove residual mucus and then in Hanks balanced salt solution with 0.2 M EDTA and 10 mM β-mercaptoethanol to remove the epithelium. Sections were then minced and digested with 1 mg of collagenase (Sigma-Aldridge, Milwaukee, WI)/ml to release the lamina propria mononuclear cells (MNLs). After gradient sedimentation, macrophages and lymphocytes were purified from the intestinal MNL population by counterflow centrifugal elutriation (45, 52, 59). Because the quantity of vaginal tissue was often smaller than that of the intestinal tissue, the number of vaginal MNLs was insufficient to separate by elutriation. Therefore, magnetic cell sorting (MACS; Miltenyi Biotec, Auburn, CA) was used to purify macrophages and lymphocytes from the vaginal MNL populations. For the purification of vaginal macrophages, the MNLs were incubated with the myeloid-specific CD13-phycoerythrin (PE) antibody (BD Pharmingen, San Jose, CA) for 15 min, after which anti-PE magnetic beads were added to the mixture, the mixture was incubated for 15 min, and the macrophages were selected by using a MACS column. For the purification of vaginal lymphocytes, lymphocytes were selected from the CD13-depleted vaginal MNLs using anti-CD3 magnetic beads. Lymphocytes were isolated on the basis of CD3, rather than CD4, expression because the monocytes and macrophages also expressed CD4. Purified vaginal and intestinal CD13+ macrophage and CD3+ lymphocyte populations contained <3% contaminating leukocytes. Circulating blood monocytes were purified by gradient sedimentation, followed by magnetic anti-PE bead isolation of anti-CD13-PE-treated cells. Preliminary studies showed that macrophages isolated from the same mucosal MNL population by elutriation or magnetic bead isolation in parallel yielded phenotypically identical CD13+ populations of macrophages.
RNA analysis.
CD4, CCR5, and CXCR4 gene expression in vaginal and intestinal macrophages was assessed by real-time PCR. RNA was extracted from purified macrophages and reverse transcribed into cDNA, and real-time PCR was performed using primer-probe pairs specific for CD4, CCR5, and CXCR4 gene (Applied Biosystems, Foster City, CA), as previously described (58). Relative mRNA expression levels were calculated from normalized ΔCT (cycle threshold) values and reported as the fold change. CT values correspond to the cycle number at which the fluorescence signal exceeded background fluorescence (threshold). In this analysis, the CT value for the housekeeping gene (GAPDH [glyceraldehyde-3-phosphate dehydrogenase]) was subtracted from the CT value of the target gene for each sample for normalization. For the detection of changes in gene expression in the two macrophage populations, the RNA levels for each gene in vaginal macrophages was compared to the levels in the intestinal macrophages and calculated as follows: increase = 2−ΔΔCT (User Bulletin 2, ABI Prism 7700 Sequence Detection System; Applied Biosystems). The data are presented as the fold change in the expression of each gene in vaginal macrophages compared to intestinal macrophages.
To compare the level of HIV-1 entry into intestinal and vaginal mucosa, infectious YU2 was inoculated onto the apical surface of intestinal and vaginal explants, and the explants were incubated for 2 h. Explants then were harvested, washed three times with PBS, treated with trypsin for 10 min, and washed three more times with PBS. Next, the total DNA-free RNA isolated from the mucosa using the Qiagen RNeasey Plus Minikit was transcribed into cDNA by using an iScript cDNA synthesis kit (Bio-Rad), which uses both oligo(dT) and random primers, and the gag gene was amplified by using TaqMan Universal PCR master mix (Applied Biosystems) (30). The gag-specific primers were 5′-ACATCAAGCAGCCATGCAAAT-3′ and 5′-CTATGTCACTTCCCCTTGGTTCTCT-3′. The gag-specific probe was 5′-6-FAM-ACCATCAATGAGGAAGCTGCAGAATGGG-TAMRA-3′. Expression of the housekeeping gene 18S rRNA was determined simultaneously, and standard curves for gag and 18S rRNA were obtained using 10-fold dilutions of a reference cDNA obtained from HEK293 cells infected with pYU2. Relative copy numbers were calculated from the standard curve and normalized against 18S rRNA.
Flow cytometric analysis.
To phenotype the vaginal and intestinal macrophages, mucosal macrophages were purified as described above and then incubated with optimal concentrations of PE-, allophycocyanin-, PE-Cy5-, or fluorescein isothiocyanate-conjugated antibodies to HLA-DR, CD13, CD14, CD16, CD32, CD64, CD89, CD4, CCR5, and CXCR4 (BD Pharmingen) at 4°C for 20 min. Irrelevant monoclonal antibodies of the same isotype were included in each experiment. After staining, cells were washed with PBS, fixed with 1% paraformaldehyde, and analyzed by flow cytometry, as previously described (51). The data were analyzed by using CellQuest software (BD Pharmingen).
Tissue explant and HIV-1 infection of explanted mucosa.
To construct tissue explants, the submucosa was removed from fresh segments of vaginal and intestinal mucosa by mechanical dissection at the muscularis mucosa. The mucosa was then divided into 1-cm pieces and attached to a disc of filter paper with a 0.5-cm- diameter circular perforation in the middle, with the intact apical surface in the superior position to maintain tissue polarity. The filter paper was then sealed at the outer edge with surgical glue to a nylon mesh in a 40-μm-pore-size cell strainer (BD Falcon, Bedford, MA). A polystyrene cylinder (1 cm high, 0.6 cm wide), hereafter referred to as the upper chamber, was attached with surgical glue to the apical surface of the mucosa. The resulting mucosal explant was placed in a TC six-well plate to which RPMI containing 10% human AB serum (Atlanta Biological, Norcross, GA) was added to the upper and lower chambers, avoiding any air bubbles at the lower and upper surfaces of the explant. Explants were warmed to 37°C, and 7.5 × 108 GFP-tagged YU2 virions were inoculated onto the apical surface in 50 μl of RPMI. After 30 min, the virus-containing medium was aspirated, and the explants were washed twice with trypsin to remove virus still bound to the epithelial surface. The tissue then was snap-frozen in optimal cutting temperature compound (Sakura Finetek, Torrance, CA), cut into 5-μm sections, and analyzed by immunofluorescence and confocal microscopy. Control explants were treated identically but were inoculated with medium alone.
Immunohistochemistry.
Serial sections (5 μm) of formalin-fixed, paraffin-embedded tissue from vaginal and intestinal explants were placed on lysine-coated slides, deparaffinized, and rehydrated. Antigen retrieval was achieved by immersion of the sections in 10 mM sodium citrate buffer (pH 6.0), heated to 95°C for 15 min, and allowed to cool for 20 min. Slides were transferred to water (5 min) and then treated with H2O2 (3%, 30 min) to block endogenous peroxidase, rinsed in Tris-buffered saline (TBS) for 5 min, blocked (casein protein, 60 min; Dako, Carpinteria, CA), rinsed again in TBS, and then incubated with either mouse monoclonal antibody to the macrophage marker HAM56 (0.05 mg/ml, 30 min; Dako), mouse monoclonal antibody to CD3 (0.05 mg/ml, 60 min; B&D Biosciences), or irrelevant isotype-matched antibody. Sections were then washed in TBS (5 min), incubated with anti-mouse polymer (horseradish peroxidase, 30 min; Dako), followed by diaminobenzidene-positive AB+ substrate-chromogen solution (Dako), and counterstained with hematoxylin.
Immunofluorescence and confocal microscopy.
Frozen tissue sections from HIV-1-infected and control explants were thawed, fixed, washed in PBS, and blocked with casein (Dako) for 60 min. Sections were then stained sequentially with (i) mouse anti-human HAM56, a macrophage marker (1:50; Dako), in dilution buffer (Dako) for 1 h at room temperature, washed with PBS-0.5% Tween 20, and incubated with Cy3-conjugated donkey anti-mouse (1:600; Jackson Immunoresearch, West Grove, PA) for 1 h at room temperature; (ii) anti-GFP Alexa 488 (1:100; Invitrogen Molecular Probes, Eugene, OR) for 30 min at room temperature to detect and amplify the GFP signal on HVI-1 virions; and (iii) DAPI (4′,6′-diamidino-2-phenylindole; 1:500; Calbiochem, San Diego, CA) for 5 min at room temperature to identify cell nuclei. Controls included consecutive tissue sections stained with irrelevant or no first antibody and sections from tissue explants not exposed to HIV-1 but stained as described above. Sections were also stained with anti-GFP Far Red (1:100; Alexa Fluor 647; Invitrogen Molecular Probes) to confirm that the GFP signal corresponded to GFP+ virions and not to autofluorescing background particles. Imaging was performed by using a laser-scanning confocal microscope equipped with UV, argon, krypton, and helium/neon lasers.
Detection of HIV-1 replication in mucosal MNLs and purified mucosal macrophages and lymphocytes.
Suspensions of unfractionated vaginal and intestinal MNLs were inoculated with GFP-expressing YU2 at a multiplicity of infection (MOI) of 0.2, cultured for 2 days at 37°C in RPMI plus macrophage colony-stimulating factor (R&D Systems, Minneapolis, MN) and serum, and analyzed by fluorescence-activated cell sorting for cells that express GFP. For infection of mucosal macrophages and lymphocytes that were purified as described above, lamina propria macrophages, at 2 × 105 cells/well in RPMI plus macrophage colony-stimulating factor and serum, were cultured in 96-well plates in parallel with autologous, purified lamina propria lymphocytes at the same concentration in RPMI plus PHA (5 μg/ml; Sigma, St. Louis, MO) and interleukin-2 (24 U/ml; R&D Systems) for 2 days. The cultures were then inoculated in triplicate with R5 viruses at an MOI of 1 and cultured for 16 days, with 100 μl of supernatant harvested every 4 days and stored at −70°C until p24 determination by enzyme-linked immunosorbent assay (Perkin-Elmer, Boston, MA).
RESULTS
Vaginal macrophages display a monocyte-like phenotype.
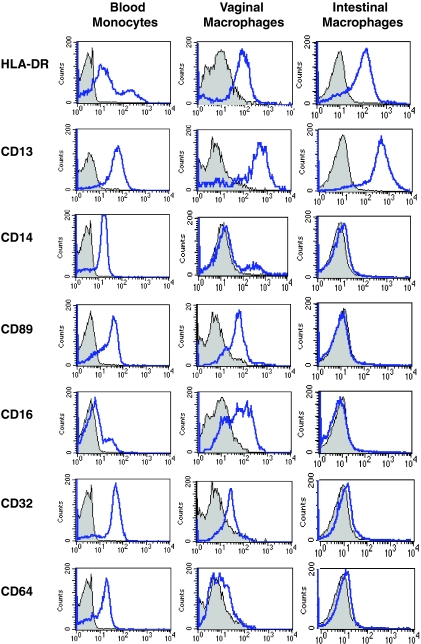
To characterize the expression levels of differentiation and innate response receptors on genital and gut macrophages, we analyzed blood monocytes and mucosal macrophages from normal vagina and jejunum by flow cytometry. The majority of blood monocytes and vaginal and intestinal macrophages expressed high levels of the major histocompatibility complex class II antigen HLA-DR and the myeloid marker CD13 (aminopeptidase N) (Fig. 1). Blood monocytes and vaginal macrophages also expressed variable levels of CD14, the receptor for complexes of lipopolysaccharide and lipopolysaccharide-binding protein; CD89, the Fcα receptor (FcαR); CD16 (FcγRIII); CD32 (FcγRII); and CD64 (FcγRI) (Fig. 1). Thus, vaginal macrophages display a phenotype characteristic of monocytes (48, 51). The mean percentage of vaginal macrophages that expressed these receptors ranged between 10.2 and 34.4% in four separate donors, which represent a 2.4- to 7.2-fold reduction in receptor expression level compared to that of blood monocytes (Fig. 1 and Table 1). In our studies of mucosal macrophages, we have shown previously that resident intestinal macrophages are profoundly downregulated for the expression of innate response and growth factor receptors (48, 51). In sharp contrast to blood monocytes and vaginal macrophages, intestinal macrophages expressed no detectable, or barely detectable, CD14, CD89, CD16, CD32, or CD64, reflecting a 25- to 83-fold decrease in receptor expression compared to that of blood monocytes (Fig. 1 and Table 1). Thus, although intestinal macrophages, and likely vaginal macrophages, are derived from blood monocytes (50), vaginal macrophages express variable levels of key innate response receptors typical of monocytes and monocyte-derived macrophages, whereas intestinal macrophages do not express such receptors.
FIG. 1.
Vaginal, but not intestinal, macrophages retain a monocyte-like phenotype. Gradient sedimentation purified blood and mucosal MNLs were stained with fluorescence-conjugated antibodies to the myeloid markers HLA-DR and CD13 and the innate receptors CD14, CD89, CD16, CD32, CD64 and analyzed by flow cytometry by gating on the macrophage population. Profiles are representative of cells from four separate vaginal and intestinal specimens.
TABLE 1.
Expression of myeloid-specific antigen and innate response receptors on blood monocytes and vaginal and intestinal macrophagesa
| Receptor | Blood monocytes
|
Vaginal macrophages
|
Intestinal macrophages
|
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| CD13 | 89.9 | 12.6 | 80.7 | 7.6 | 95.8 | 2.4 |
| CD14 (LPS-R) | 85.2 | 6.6 | 18.6 | 3.4 | 1.1 | 1.2 |
| CD89 (FcαR) | 83.2 | 11.3 | 34.4 | 23.1 | 1.0 | 0.8 |
| CD16 (FcγRIII) | 54.5 | 34.0 | 19.7 | 24.3 | 2.2 | 2.4 |
| CD32 (FcγRII) | 92.8 | 6.2 | 15.1 | 3.3 | 1.5 | 1.4 |
| CD64 (FcγRI) | 73.4 | 22.2 | 10.2 | 5.6 | 1.1 | 0.5 |
Values refer to the mean percentage of positive cells (n = 4).
Vaginal macrophages express higher levels of CD4, CCR5, and CXCR4 than intestinal macrophages.
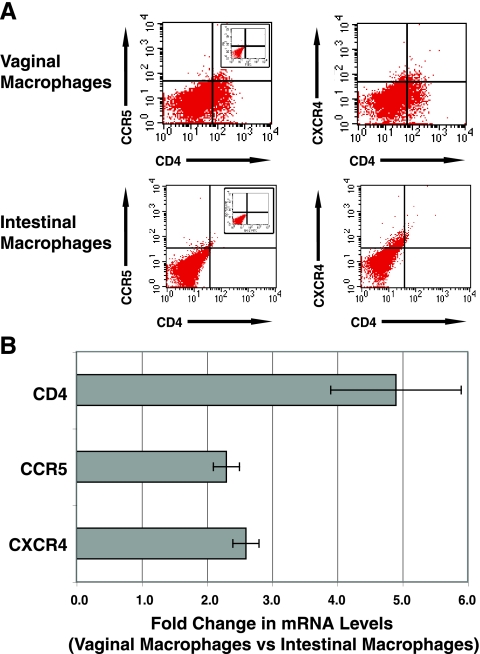
Vaginal macrophages were analyzed next for expression of the HIV-1 primary receptor CD4 and the coreceptors CCR5 and CXCR4. As shown in Fig. 2A and Table 2, normal vaginal macrophages expressed low levels of CD4 (6.4%), CCR5 (1.6%), and CXCR4 (6.7%), and 1.0% of the macrophages were CD4+ CCR5+. In contrast to vaginal macrophages, intestinal macrophages from normal mucosa expressed even lower, albeit detectable, levels of CD4 (1.0%), CCR5 (0.9%), and CXCR4 (4.4%), and only 0.3% of the intestinal macrophages were CD4+ CCR5+, threefold fewer than for the vaginal macrophages (Fig. 2 and Table 2). Consistent with their higher levels of surface CD4, CCR5, and CXCR4 protein, vaginal macrophages constitutively expressed 4.9-, 2.3-, and 2.6-fold-higher levels of CD4, CCR5, and CXCR4 mRNA, respectively, compared to intestinal macrophages (Fig. 2B), suggesting that the increased expression of these receptors on vaginal macrophages was regulated at the level of gene transcription.
FIG. 2.
Vaginal macrophages express higher levels of CD4, CCR5, and CXCR4 protein and mRNA than intestinal macrophages. (A) Gradient sedimentation purified mucosal MNLs were stained with the indicated fluorescence-conjugated antibodies, and the macrophages were analyzed by flow cytometry by gating on the macrophage population. Insets show staining with the isotype control antibodies. Profiles are representative of macrophages in four vaginal and four intestinal tissue specimens. (B) Purified vaginal and intestinal macrophages were analyzed by real-time PCR for CD4, CCR5, and CXCR4 mRNA. The mean (± the standard deviation) levels of CD4, CCR5, and CXCR4 mRNA were 4.9-, 2.3-, and 2.6-fold higher, respectively, in vaginal macrophages than in intestinal macrophages (n = 3).
TABLE 2.
HIV-1 receptor and coreceptor expression on vaginal and intestinal macrophagesa
| Receptor | Vaginal macrophages
|
Intestinal macrophages
|
P | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| CD4 | 6.4 | 3.7 | 1.0 | 0.8 | 0.001 |
| CCR5 | 1.6 | 0.3 | 0.9 | 0.7 | 0.023 |
| CXCR4 | 6.7 | 6.9 | 4.4 | 6.5 | 0.274 |
| CD4+CCR5 | 1.0 | 0.2 | 0.3 | 0.3 | 0.001 |
| CD4+CXCR4 | 1.6 | 0.4 | 0.5 | 0.6 | 0.005 |
Values refer to the mean percentage of positive cells (n = 5).
To determine whether the markedly reduced level of detectable CCR5 on intestinal macrophages was the consequence of receptor ligation, we analyzed intestinal macrophages for the presence of surface macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and RANTES, the natural ligands for CCR5. Intestinal macrophages did not display detectable levels of these ligands, and acidification of the cells to remove ligand using our previously described protocol (25) did not facilitate the detection of surface CCR5 (data not shown), suggesting that the near absence of CCR5 on intestinal macrophages was not caused by CCR5 ligation or ligand-induced internalization of CCR5. Also, we did not detect MIP-1α, MIP-1β, or RANTES in the intestinal (or vaginal) lamina propria extracellular matrix that was isolated using our previously described protocol (51), and culture of the extracellular matrix did not result in the release of ligand into the culture supernatant (data not shown). Finally, culturing intestinal macrophages for up to 3 days did not promote CD4, CCR5, or CXCR4 expression, indicating that the absence of the receptors was not due to transient loss induced by the isolation procedure. Thus, vaginal macrophages display a characteristic monocyte-like phenotype and express significantly higher levels of CD4 and CCR5 protein and 4.9- and 2.3-fold more CD4- and CCR5-specific mRNA, respectively, than intestinal macrophages.
Vaginal and intestinal macrophages take up R5 HIV-1.
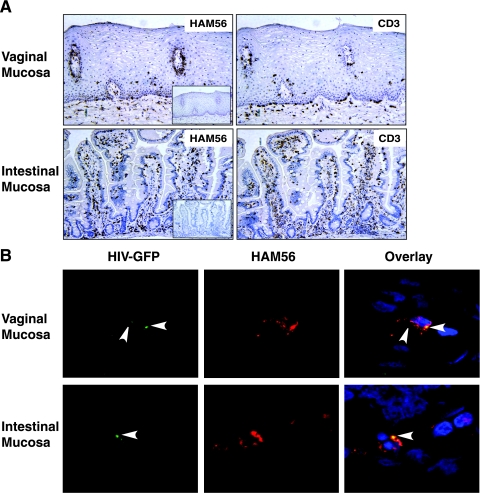
We first located potential HIV-1 target MNLs in genital and gut mucosa. In vaginal mucosa, HAM56+ macrophages were detected in the lamina propria and occasionally in the basal region of the squamous epithelium (Fig. 3A, upper left panel). CD3+ T cells were scattered throughout the lamina propria and infrequently at the basal region of the squamous epithelium (Fig. 3A, upper right panel). Both macrophages and lymphocytes were detected at the edges of dermal papillae. In the intestinal mucosa, dense populations of HAM56+ macrophages and CD3+ T cells were present throughout the lamina propria and near the basal region of the columnar epithelium; CD3+ T cells, but not HAM56+ macrophages, were also present in the epithelium (Fig. 3A, lower panels).
FIG. 3.
Localization of potential mononuclear target cells and detection of HIV-1 in macrophages in vaginal and intestinal mucosa. (A) In noninflamed vaginal mucosa, HAM56+ macrophages were detected in the basal region of the epithelium and bordering the dermal papillae and scattered throughout the lamina propria; few CD3+ lymphocytes were identified in the epithelium, concentrating mainly at the dermal papillae, but were present predominantly in the lamina propria. In noninflamed intestinal mucosa, dense populations of HAM56+ macrophages and CD3+ lymphocytes were present throughout the lamina propria. Insets show sections stained with irrelevant, isotype-matched control antibodies. (Sections representative of vaginal and intestinal tissues were from four separate donors [magnification, ×20].) (B) GFP-tagged YU2 particles were inoculated onto the apical surface of vaginal and intestinal mucosal explants and, after 30 min, the explants were harvested, sectioned, stained, and analyzed by confocal microscopy for HAM56+ macrophages containing GFP-YU2. GFP-tagged viruses were stained with anti-GFP Alexa Fluor 488, macrophages were stained with anti-HAM56-Cy3, and cell nuclei were stained with DAPI.
We next evaluated the ability of genital and gut lamina propria macrophages to take up R5 HIV-1 inoculated onto the mucosa. GFP-tagged YU2 viral particles were inoculated onto the apical surface of explanted vaginal and intestinal mucosa, and 30 min later the explants were harvested, sectioned, stained, and analyzed by confocal microscopy for macrophages that contain GFP-YU2. GFP-YU2 particles were identified in HAM56+ macrophages, which were readily detected in the lamina propria of the vaginal tissue (Fig. 3B, upper panels). In contrast, GFP-YU2 particles were detected only occasionally in HAM56+ intestinal macrophages (Fig. 3B, lower panels). The low numbers of GFP+ HAM56+ cells precluded quantitative comparison of infected cells in the two tissues. Therefore, vaginal and intestinal explants were inoculated onto the apical surface with equivalent amounts of infectious YU2, and after 2 h the explants were harvested, washed three times, treated with trypsin, and washed again three times. The total RNA was isolated, and virus that had entered the mucosae was quantified by real-time reverse transcription-PCR, which revealed fourfold more viral RNA in vaginal than intestinal mucosa (n = 5 each; P = 0.013). GFP-YU2 particles also were detected in HAM56− (nonmacrophage) cells in explants of both tissues. These findings indicate that HIV-1 translocated across the epithelium in both vaginal and intestinal mucosa and then was taken up by subepithelial macrophages. The entry of HIV-1 into vaginal macrophages in our explant system is consistent with the findings of Greenhead et al. (9), who used immunohistochemistry to show that the majority of HIV-1-infected cells in the subepithelial space in cultured genital mucosa were macrophages.
Vaginal, not intestinal, macrophages support R5 HIV-1 replication.
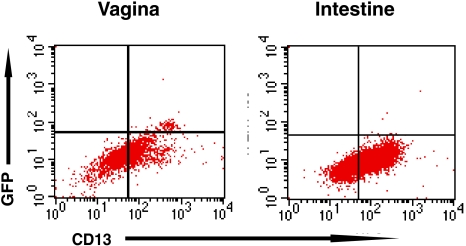
We next investigated the ability of mucosal macrophages to support R5 HIV-1 replication. Vaginal and intestinal MNLs were exposed to GFP-expressing YU2, incubated at 37°C for 2 days, and analyzed by fluorescence-activated cell sorting for YU2 replication in CD13+ macrophages, using anti-GFP Alexa Fluor 488 to amplify the GFP signal. As shown in Fig. 4, macrophages in the vaginal MNL population supported YU2 replication, reflected in the presence of GFP+CD13+ cells. The percentage of GFP-expressing macrophages was low, since only one round of replication is possible with this reporter virus. Together with the findings in Fig. 3B, these results indicate that intestinal macrophages supported HIV-1 entry but not replication, which is consistent with our previous detection of HIV-1 DNA (but not RNA) in cultured primary intestinal macrophages (17).
FIG. 4.
Vaginal, not intestinal, macrophages in isolated mucosal MNLs support R5 HIV-1 replication. Cultures of gradient sedimentation-purified vaginal and intestinal MNLs were inoculated with YU-2env pseudotyped GFP-expressing HIV-1 and incubated at 37°C. The ability of macrophages to support virus replication was analyzed 2 days postinfection by GFP expression using flow cytometry. The results are representative of two separate experiments.
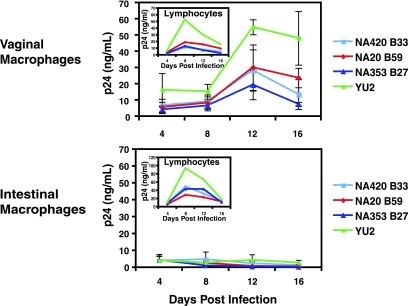
To confirm that vaginal, but not intestinal, macrophages support HIV-1 replication, mucosal macrophages and lymphocytes were purified from normal vaginal MNLs by MACS column using anti-CD13 and anti-CD3 antibodies, respectively, and cultures of each population were inoculated (MOI = 1) with macrophage-tropic viruses and monitored for p24 production at 4-day intervals for 16 days. The macrophage-tropic viruses included NA420B33, NA20B59, and NA353B27, which are highly fusigenic and tropic for macrophages that express low levels of CD4 and/or CCR5 (33). Vaginal macrophages supported replication by all four R5 viruses, displaying peak p24 production at day 12 (Fig. 5). In sharp contrast, purified intestinal macrophages did not support replication by any of the R5 viruses (Fig. 5). Importantly, lymphocytes from both vaginal and intestinal mucosa supported replication by all four viruses with peak p24 production occurring earlier (day 8) and at higher levels in the intestinal lymphocytes compared to vaginal lymphocytes (Fig. 5). These findings indicate that the inability of intestinal macrophages to support R5 replication was not due to the isolation procedure, since intestinal lymphocytes, which were isolated in parallel from the same tissue and by the same protocol as the macrophages, supported viral replication. Thus, both macrophages and lymphocytes from the vagina support R5 HIV-1 replication, but only lymphocytes from the intestine support R5 HIV-1 replication.
FIG. 5.
Purified vaginal, but not intestinal, macrophages support HIV-1 replication. Cultures of purified vaginal and intestinal macrophages were inoculated with YU2 and three highly macrophage-tropic viruses (NA420 B33, NA20 B59, and NA353 B27) at an MOI of 1. The levels of p24 production in media were assayed by ELISA at the indicated time points postinoculation. The inset shows cultures of purified vaginal and intestinal lymphocytes that had been isolated from the same tissue specimens as the macrophages were inoculated in parallel with the same viruses and analyzed for p24 production. Values are mean ± the standard deviation p24 levels for two to four wells/time point/virus in a representative experiment (n = 3).
DISCUSSION
We investigated the biological parameters of HIV-1 infection in vaginal and intestinal macrophages. We report that a substantial proportion of human vaginal macrophages expressed the differentiation markers HLA-DR and CD13 and the innate response receptors CD14, CD89, CD16, CD32, and CD64, characteristic of monocytes. Importantly, a subset of vaginal macrophages also displayed CD4, CCR5, and CXCR4, and ca. 1% were CD4+ CCR5+. Consistent with this phenotype, vaginal macrophages supported R5 virus entry in explanted vaginal mucosa, and purified vaginal macrophages supported substantial levels of R5 HIV-1 replication. Intestinal macrophages also expressed HLA-DR and CD13 but, in sharp contrast to vaginal macrophages, the intestinal macrophages did not express (or expressed barely detectable levels of) innate response receptors, displayed markedly reduced CD4 and CCR5 protein and mRNA compared to vaginal macrophages, and did not support HIV-1 replication, although virus was detected in macrophages in explanted intestinal mucosa.
The presence of monocyte-like macrophages in the vaginal lamina propria and the ability of vaginal macrophages to support HIV-1 entry and replication indicate that vaginal macrophages may be important target cells in the genital transmission of HIV-1 and genital HIV-1 infection. That HIV-1 was detected in macrophages in explanted vaginal mucosa 30 min after inoculation of virus onto the epithelial surface underscores the potential role of macrophages in the early events in mucosal HIV-1 infection in the female genital tract. In this connection, CCR5 inhibitors delivered vaginally (54, 57), as well as orally (55), have been shown to block or diminish vaginal transmission of CCR5-receptor-using simian-human immunodeficiency virus (SHIV) infection. The HIV-1 permissiveness of vaginal macrophages raises the possibility that the ability of CCR5 inhibitors to block SHIV infection involves the inhibition of infection of vaginal macrophages, as well as the inhibition of CCR5-mediated translocation of virus across the genital epithelium.
Gut macrophages, and likely genital macrophages, originate from blood monocytes that recruit to the mucosal lamina propria (50). The differences in phenotype and HIV-1 permissiveness between vaginal and intestinal macrophages reported here may therefore reflect differences in the local microenvironment, since mucosa-derived cytokines, including transforming growth factor β, regulate the phenotype and function of blood monocytes after their recruitment to the mucosa, at least in the intestinal mucosa (51). Importantly, despite their likely common embryological origin, the genital and intestinal tracts display an array of distinct features relevant to HIV-1 infection (26). In the genital tract, immunoglobulin G (IgG) is the dominant immunoglobulin isotype but, in the gut, IgA is the main immunoglobulin isotype (27, 38). Moreover, genital tract antibodies originate locally and systemically, whereas gut antibodies originate almost exclusively in the gut mucosa. Furthermore, systemic immunization induces specific IgG antibodies in cervicovaginal fluids (27, 28, 38) but limited IgA responses in gut fluids (2). Thus, the dominance of IgG antibodies in the genital tract and the presence of FcγR-bearing macrophages in vaginal mucosa, as we have shown here, raise the important possibility that local IgG anti-HIV-1 antibodies, including those induced by systemic or mucosal vaccines, could promote HIV-1 infection of vaginal macrophages through more efficient internalization of IgG-HIV-1 immune complexes (7). The reduced level of IgG in the gastrointestinal tract and the absence of detectable FcγRs on intestinal macrophages, however, diminish the likelihood of immune complex-mediated HIV-1 entry into intestinal macrophages. Similarly, the marked reduction in surface CD4 and CCR5 may contribute to the reduced permissiveness of intestinal macrophages to HIV-1 reported here.
In the human vagina, CD4+ T cells have been identified in the epithelium and the lamina propria in an ex vivo model created by suction blistering the mucosa (13). In this model, R5 HIV-1 applied to the apical surface entered the mucosa via the epithelial CD4+ T cells through CD4 and CCR5 and replicated in these cells (13). Along with others (35), we detected few lymphocytes in the squamous epithelium of normal, noninflamed vaginal mucosa, and these lymphocytes were always located in the basal region of the epithelium, close to the lamina propria. Importantly, in the mucosal explant system used here, the architectural relationship between the epithelium and the underlying lamina propria remained intact. Using this system and purified cell populations, we confirmed and extended the immunohistochemical findings of Greenhead et al. (9) by showing that vaginal macrophages not only express CD4 and CCR5 but also support robust R5 HIV-1 replication. In contrast, intestinal macrophages were threefold less frequently CD4+ CCR5+ than vaginal macrophages, and yet virus was detected in intestinal macrophages, indicating low-level receptor-mediated entry and/or endocytosis. However, intestinal macrophages did not support viral replication. The mechanism by which virus enters but does not replicate in intestinal macrophages is currently under investigation, but preliminary studies suggest that it is not due to increased expression of APOBEC3G (R. Shen, unpublished data).
In summary, we show that vaginal, but not intestinal, macrophages are phenotypically similar to monocytes and are permissive to R5 HIV-1 infection, suggesting that genital and gut macrophage populations have different roles in mucosal HIV-1 pathogenesis. Robust HIV-1 replication in vaginal macrophages indicates the cells are a likely source of virus in the female genital tract during HIV-1 infection. In addition, the susceptibility of macrophages in the vaginal mucosa to HIV-1 suggests the potential contribution of vaginal macrophages to heterosexual HIV-1 transmission, particularly in the presence of genital infections that enhance CCR5 expression (8, 60, 43), HIV-1 replication (32), and cytokine production that in turn could enhance HIV-1 replication (20, 34, 47). Finally, the findings presented here suggest that the cellular reservoir of HIV-1 during chronic infection may extend to vaginal macrophages. Thus, vaginal macrophages appear to play a previously underappreciated but potentially important role in mucosal HIV-1 infection in the female genital tract.
Acknowledgments
This study was supported by the National Institutes of Health (DK-74033, DK-47322, DK-54495, DK-61297, T32 AI-007051, and RR-20136), the Mucosal HIV and Immunobiology Center (DK-64400), the Center for HIV/AIDS Vaccine Immunology (AI-67854), the Crohn's and Colitis Foundation of America, and the Research Service of the Veterans Administration.
Footnotes
Published ahead of print on 19 January 2009.
REFERENCES
- 1.Alexander, N. J. 1990. Sexual transmission of human immunodeficiency virus: virus entry into the male and female genital tract. Fertil. Steril. 541-18. [DOI] [PubMed] [Google Scholar]
- 2.Boyaka, P. N., J. R. McGhee, C. Czerkinsky, and J. Mestecky. 2005. Mucosal vaccines: an overview, p. 855-873. In J. Mestecky, J. Bienenstock, M. E. Lamm, L. Mayer, J. R. McGhee, and W. Strober (ed.), Mucosal Immunology, 3rd ed. Elsevier/Academic Press, Amsterdam, The Netherlands.
- 3.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T-cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins, K. B., B. K. Patterson, G. J. Naus, D. V. Landers, and P. Gupta. 2000. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat. Med. 6475-479. [DOI] [PubMed] [Google Scholar]
- 5.Crowe, S., T. Zhu, and W. A. Muller. 2003. The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection. J. Leukoc. Biol. 74635-641. [DOI] [PubMed] [Google Scholar]
- 6.Derdeyn, C. A., J. M. Decker, J. N. Sfakianos, X. Wu, W. A. O'Brien, L. Ratner, J. C. Kappes, G. M. Shaw, and E. Hunter. 2000. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J. Virol. 748358-8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forthal, D. N., G. Landucci, J. Bream, L. P. Jacobson, T. B. Phan, and B. Montoya. 2007. FcγRIIa genotype predicts progression of HIV infection. J. Immunol. 1797916-7923. [DOI] [PubMed] [Google Scholar]
- 8.Fraziano, M., G. Cappelli, M. Santucci, F. Mariani, M. Amicosante, M. Casarini, S. Giosue, A. Bisetti, and V. Colizzi. 1999. Expression of CCR5 is increased in human monocyte-derived macrophages and alveolar macrophages in the course of in vivo and in vitro Mycobacterium tuberculosis infection. AIDS Res. Hum. Retrovir. 15869-874. [DOI] [PubMed] [Google Scholar]
- 9.Greenhead, P., P. Hayes, P. S. Watts, K. G. Laing, G. E. Griffin, and R. J. Shattock. 2000. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J. Virol. 745577-5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guadalupe, M., E. Reay, S. Sankaran, T. Prindiville, J. Flamm, A. McNeil, and S. Dandekar. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 7711708-11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guadalupe, M., S. Sankaran, M. D. George, E. Reay, D. Verhoeven, B. L. Shacklett, J. Flamm, J. Wegelin, T. Prindiville, and S. Dandekar. 2006. Viral suppression and immune restoration in the gastrointestinal mucosa of human immunodeficiency virus type 1-infected patients initiating therapy during primary or chronic infection. J. Virol. 808236-8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta, P., K. B. Collins, D. Ratner, S. Watkins, G. J. Naus, D. V. Landers, and B. K. Patterson. 2002. Memory CD4+ T cells are the earliest detectable human immunodeficiency virus type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. J. Virol. 769868-9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hladik, F., P. Sakchalathorn, L. Ballweber, G. Lentz, M. Fialkow, D. Eschenbach, and M. J. McElrath. 2007. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity 26257-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kedzierska, K., S. M. Crowe, S. Turville, and A. L. Cunningham. 2003. The influence of cytokines, chemokines and their receptors on HIV-1 replication in monocytes and macrophages. Rev. Med. Virol. 1339-56. [DOI] [PubMed] [Google Scholar]
- 15.Kutsch, O., E. N. Benveniste, G. M. Shaw, and D. N. Levy. 2002. Direct and quantitative single-cell analysis of human immunodeficiency virus type 1 reactivation from latency. J. Virol. 768776-8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, S. H., P. M. Starkey, and S. Gordon. 1985. Quantitative analysis of total macrophage content in adult mouse tissues: immunochemical studies with monoclonal antibody F4/80. J. Exp. Med. 161475-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li, L., G. Meng, M. F. Graham, G. M. Shaw, and P. D. Smith. 1999. Intestinal macrophages display reduced permissiveness to human immunodeficiency virus 1 and decreased surface CCR5. Gastroenterology 1161043-1053. [DOI] [PubMed] [Google Scholar]
- 18.Li, Q., L. Duan, J. D. Estes, Z.-M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 4341148-1152. [DOI] [PubMed] [Google Scholar]
- 19.Li, Y., J. C. Kappes, J. A. Conway, R. W. Price, G. M. Shaw, and B. H. Hahn. 1991. Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured human brain tissue: identification of replication-competent and -defective viral genomes. J. Virol. 653973-3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maheshwari, A., L. E. Smythies, X. Wu, L. Novak, R. Clements, D. Eckhoff, A. J. Lazenby, W. J. Britt, and P. D. Smith. 2006. Cytomegalovirus blocks intestinal stroma-induced down-regulation of macrophage HIV-1 infection. J. Leukoc. Biol. 801111-1117. [DOI] [PubMed] [Google Scholar]
- 21.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 4341093-1097. [DOI] [PubMed] [Google Scholar]
- 22.Mehandru, S., M. A. Poles, K. Tenner-Racz, A. Horowitz, A. Hurley, C. Hogan, D. Boden, P. Racz, and M. Markowitz. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehandru, S., M. A. Poles, K. Tenner-Racz, V. Manuelli, P. Jean-Pierre, P. Lopez, A. Shet, A. Low, H. Mohri, D. Boden, P. Racz, and M. Markowitz. 2007. Mechanisms of gastrointestinal CD4+ T-cell depletion during acute and early human immunodeficiency virus type 1 infection. J. Virol. 81599-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng, G., M. Sellers, M. Mosteller-Barnum, T. Rogers, G. Shaw, and P. Smith. 2000. Lamina propria lymphocytes, not macrophages, express CCR5 and CXCR4 and are the likely target cell for human immunodeficiency virus type 1 in the intestinal mucosa. J. Infect. Dis. 182785-791. [DOI] [PubMed] [Google Scholar]
- 25.Meng, G., X. Wei, X. Wu, M. T. Sellers, J. M. Decker, Z. Moldoveanu, J. M. Orenstein, M. F. Graham, J. C. Kappes, J. Mestecky, G. M. Shaw, and P. D. Smith. 2002. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ cells. Nat. Med. 8150-156. [DOI] [PubMed] [Google Scholar]
- 26.Mestecky, J. 2006. Humoral immune responses to the human immunodeficiency virus type-1 (HIV-1) in the genital tract compared to other mucosal sites. J. Reprod. Immunol. 721-17. [DOI] [PubMed] [Google Scholar]
- 27.Mestecky, J., Z. Moldoveanu, and M. W. Russell. 2005. Immunologic uniqueness of the genital tract: challenge for vaccine development. Am. J. Reprod. Immunol. 53208-214. [DOI] [PubMed] [Google Scholar]
- 28.Moldoveanu, Z., W. Q. Huang, R. Kulhavy, M. S. Pate, and J. Mestecky. 2005. Human male genital tract secretions: both mucosal and systemic immune compartments contribute to the humoral immunity. J. Immunol. 1754127-4136. [DOI] [PubMed] [Google Scholar]
- 29.Montaner, L. J., S. M. Crowe, S. Aquaro, C. F. Perno, M. Stevenson, and R. G. Collman. 2006. Advances in macrophage and dendritic cell biology in HIV-1 infection stress key understudied areas in infection, pathogenesis, and analysis of viral reservoirs. J. Leukoc. Biol. 80961-964. [DOI] [PubMed] [Google Scholar]
- 30.Moroney, S. M., L. C. Heller, and R. H. Widen. 2006. Evaluation of two TaqMan PCR assays for the detection of HIV-1 proviral DNA in blood samples. J. Microbiol. Methods 65350-353. [DOI] [PubMed] [Google Scholar]
- 31.Newman, G. W., G. H. Kelley, H. Gan, O. Kandil, M. J. Newman, P. Pinkston, R. M. Rose, and H. G. Remold. 1993. Concurrent infection of human macrophages with HIV-1 and Mycobacterium avium results in decreased cell viability, increased M. avium multiplication and altered cytokine production. J. Immunol. 1512261-2272. [PubMed] [Google Scholar]
- 32.Orenstein, J. M., C. Fox, and S. M. Wahl. 1997. Macrophages as a source of HIV during opportunistic infections. Science 2761857-1861. [DOI] [PubMed] [Google Scholar]
- 33.Peters, P. J., J. Bhattacharya, S. Hibbitts, M. T. Dittmar, G. Simmons, J. Bell, P. Simmonds, and P. R. Clapham. 2004. Biological analysis of human immunodeficiency virus type 1 R5 envelopes amplified from brain and lymph node tissues of AIDS patients with neuropathology reveals two distinct tropism phenotypes and identifies envelopes in the brain that confer an enhanced tropism and fusigenicity for macrophages. J. Virol. 786915-6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson, P. K., G. Gekker, C. C. Chao, S. Hu, C. Edelman, H. H. Balfour, and J. Verhoef. 1992. Human cytomegalovirus-stimulated peripheral blood mononuclear cells induce HIV-1 replication via a tumor necrosis factor-α-mediated mechanism. J. Clin. Investig. 89574-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pudney, J., A. J. Quayle, and D. J. Anderson. 2005. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol. Reprod. 731253-1263. [DOI] [PubMed] [Google Scholar]
- 36.Royce, R. A., A. Sena, W. Cates, Jr., and M. S. Cohen. 1997. Sexual transmission of HIV. N. Engl. J. Med. 3361072-1078. [DOI] [PubMed] [Google Scholar]
- 37.Rugtveit, J., A. Bakka, and P. Brandtzaeg. 1997. Differential distribution of B7.1 (CD80) and B7.2 (CD86) costimulatory molecules on mucosal macrophage subsets in human inflammatory bowel disease (IBD). Clin. Exp. Immunol. 110104-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russell, M. W., and J. Mestecky. 2002. Humoral immune responses to microbial infections in the genital tract. Microbes Infect. 4667-677. [DOI] [PubMed] [Google Scholar]
- 39.Schenk, M., A. Bouchon, S. Tirrer, M. Colonna, and C. Mueller. 2005. Macrophages expressing triggering receptor expressed on myeloid cells-1 are underrepresented in the human intestine. J. Immunol. 174517-524. [DOI] [PubMed] [Google Scholar]
- 40.Schenk, M., A. Bouchon, F. Seibold, and C. Mueller. 2007. TREM-1-expressing intestinal macrophages crucially amplify chronic inflammation in experimental colitis and inflammatory bowel diseases. J. Clin. Investig. 1173097-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharova, N., C. Swingler, M. Sharkey, and M. Stevenson. 2005. Macrophages archive HIV-1 virions for dissemination in trans. EMBO J. 242481-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shattock, R. J., and J. P. Moore. 2003. Inhibiting sexual transmission of HIV-1 infection. Nat. Rev. Microbiol. 125-34. [DOI] [PubMed] [Google Scholar]
- 43.Sheffield, J. S., G. D. Wendel, Jr., D. D. McIntire, and M. V. Norgard. 2007. Effect of genital ulcer disease on HIV-1 coreceptor expression in the female genital tract. J. Infect. Dis. 1961509-1516. [DOI] [PubMed] [Google Scholar]
- 44.Smit-McBride, Z., J. J. Mattapallil, M. McChesney, D. Ferrick, and S. Dandekar. 1998. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4+ T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. J. Virol. 726646-6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith, P. D., E. N. Janoff, M. Mosteller-Barnum, M. Merger, J. M. Orenstein, J. F. Kearney, and M. F. Graham. 1997. Isolation and purification of CD14-negative mucosal macrophages from normal human small intestine. J. Immunol. Methods 2021-11. [DOI] [PubMed] [Google Scholar]
- 46.Smith, P. D., C. Ochsenbauer-Jambor, and L. E. Smythies. 2005. Intestinal macrophages: unique effector cells of the innate immune system. Immunol. Rev. 206149-159. [DOI] [PubMed] [Google Scholar]
- 47.Smith, P. D., S. S. Saini, M. Raffeld, J. F. Manischewitz, and S. M. Wahl. 1992. Cytomegalovirus induction of tumor necrosis factor-α by human monocytes and mucosal macrophages. J. Clin. Investig. 901642-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith, P. D., L. E. Smythies, M. Mosteller-Barnum, D. A. Sibley, M. W. Russell, M. Merger, M. F. Graham, T. Shimada, and H. Kubagawa. 2001. Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS- and IgA-mediated activities. J. Immunol. 1672651-2656. [DOI] [PubMed] [Google Scholar]
- 49.Smith, P. D., L. E. Smythies, and S. M. Wahl. 2001. Macrophage effector function, p. 19.1-19.9. In R. R. Rich, T. A. Fleisher, W. T. Sherarer, B. Kotzin, and H. W. J. Schroeder (ed.), Clinical immunology, 2nd ed., vol. 1. Harcourt Health Sciences, London, England. [Google Scholar]
- 50.Smythies, L. E., A. Maheshwari, R. H. Clements, D. Eckhoff, L. Novak, M. Mosteller-Barnum, M. Sellers, and P. D. Smith. 2006. Mucosal IL-8 and TGF-β recruit blood monocytes: evidence for cross-talk between the lamina propria stroma and myeloid cells. J. Leukoc. Biol. 80492-499. [DOI] [PubMed] [Google Scholar]
- 51.Smythies, L. E., M. Sellers, R. H. Clements, M. Mosteller-Barnum, G. Meng, W. H. Benjamin, J. M. Orenstein, and P. D. Smith. 2005. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bactericidal activity. J. Clin. Investig. 11566-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smythies, L. E., L. M. Wahl, and P. D. Smith. 2006. Isolation and purification of human intestinal macrophages, p. 7.6B.1-7.6B.19. In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, and W. Strober (ed.), Current protocols in immunology. John Wiley & Sons, Inc., New York, NY. [DOI] [PubMed]
- 53.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T-cell depletion and viral replication in SIV infection. Science 280427-431. [DOI] [PubMed] [Google Scholar]
- 54.Veazey, R. S., P. J. Klasse, T. J. Ketas, J. D. Reeves, M. Piatak, Jr., K. Kunstman, S. E. Kuhmann, P. A. Marx, J. D. Lifson, J. Dufour, M. Mefford, I. Pandrea, S. M. Wolinsky, R. W. Doms, J. A. DeMartino, S. J. Siciliano, K. Lyons, M. S. Springer, and J. P. Moore. 2003. Use of a small molecule CCR5 inhibitor in macaques to treat simian immunodeficiency virus infection or prevent simian-human immunodeficiency virus infection. J. Exp. Med. 1981551-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Veazey, R. S., P. J. Klasse, S. M. Schader, Q. Hu, T. J. Ketas, M. Lu, P. A. Marx, J. Dufour, R. J. Colonno, R. J. Shattock, M. S. Springer, and J. P. Moore. 2005. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature 43899-102. [DOI] [PubMed] [Google Scholar]
- 56.Veazey, R. S., P. A. Marx, and A. A. Lackner. 2003. Vaginal CD4+ T cells express high levels of CCR5 and are rapidly depleted in simian immunodeficiency virus infection. J. Infect. Dis. 187769-776. [DOI] [PubMed] [Google Scholar]
- 57.Veazey, R. S., M. S. Springer, P. A. Marx, J. Dufour, P. J. Klasse, and J. P. Moore. 2005. Protection of macaques from vaginal SHIV challenge by an orally delivered CCR5 inhibitor. Nat. Med. 111293-1294. [DOI] [PubMed] [Google Scholar]
- 58.Verhoeven, D., S. Sankaran, M. Silvey, and S. Dandekar. 2008. Antiviral therapy during primary simian immunodeficiency virus infection fails to prevent acute loss of CD4+ T cells in gut mucosa but enhances their rapid restoration through central memory T cells. J. Virol. 824016-4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wahl, L., S. M. Wahl, L. E. Smythies, and P. D. Smith. 2006. Isolation of human monocyte populations, p. 7.6A.1-7.6A.10. In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, and W. Strober (ed.), Current protocols in immunology. John Wiley & Sons, Inc., New York, NY. [DOI] [PubMed]
- 60.Wahl, S. M., T. Greenwell-Wild, G. Peng, H. Hale-Donze, T. M. Doherty, D. Mizel, and J. M. Orenstein. 1998. Mycobacterium avium complex augments macrophage HIV-1 production and increases CCR5 expression. Proc. Natl. Acad. Sci. USA 9512574-12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu, X., J. K. Wakefield, L. Hongmei, H. Xiao, R. Kralovics, J. T. Prchal, and J. C. Kappes. 2000. Development of a novel trans-lentiviral vector that afford predictable safety. Mol. Ther. 247-55. [DOI] [PubMed] [Google Scholar]
- 62.Zhang, Z., T. Schuler, M. Zupancic, S. Wietgrefe, K. A. Staskus, K. A. Reimann, T. A. Reinhart, M. Rogan, W. Cavert, C. J. Miller, R. S. Veazey, D. Notermans, S. Little, S. A. Danner, D. D. Richman, D. Havlir, J. Wong, H. L. Jordan, T. W. Schacker, P. Racz, K. Tenner-Racz, N. L. Letvin, S. Wolinsky, and A. T. Haase. 1999. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 2861353-1357. [DOI] [PubMed] [Google Scholar]