Abstract
Cytotoxic CD8 T cells exert their antiviral and antitumor activity primarily through the secretion of cytotoxic granules. Degranulation activity and cytotoxic granules (perforin plus granzymes) generally define CD8 T cells with cytotoxic function. In this study, we have investigated the expression of granzyme K (GrmK) in comparison to that of GrmA, GrmB, and perforin. The expression of the cytotoxic granules was assessed in virus-specific CD8 T cells specific to influenza virus, Epstein-Barr virus (EBV), cytomegalovirus (CMV), or human immunodeficiency virus type 1 (HIV-1). We observed a dichotomy between GrmK and perforin expression in virus-specific CD8 T cells. The profile in influenza virus-specific CD8 T cells was perforin− GrmB− GrmA+/− GrmK+; in CMV-specific cells, it was perforin+ GrmB+ GrmA+ GrmK−/+; and in EBV- and HIV-1-specific cells, it was perforin−/+ GrmB+ GrmA+ GrmK+. On the basis of the delineation of memory and effector CD8 T cells with CD45RA and CD127, the GrmK+ profile was associated with early-stage memory CD8 T-cell differentiation, the perforin+ GrmB+ GrmA+ profile with advanced-stage differentiation, and the GrmB+ GrmA+ Grmk+ profile with intermediate-stage differentiation. Furthermore, perforin and GrmB but not GrmA and GrmK correlated with cytotoxic activity. Finally, changes in antigen exposure in vitro and in vivo during primary HIV-1 infection and vaccination modulated cytotoxic granule profiles. These results advance our understanding of the relationship between distinct profiles of cytotoxic granules in memory CD8 T cells and function, differentiation stage, and antigen exposure.
The primary function of cytotoxic T lymphocytes and natural killer cells is to detect and eliminate virus-infected or transformed cells, thus playing a key role in the so-called immunosurveillance (6, 8, 43). Cytotoxic cells mediate their lytic activity through two mechanisms: granule-dependent cytotoxicity and death-receptor-dependent cytotoxicity (19, 43). The death-receptor-dependent pathway is activated by the interaction between CD95 (FAS), tumor necrosis factor, or TRAIL and the cognate ligands expressed on the surfaces of cytotoxic cells. The principal cytotoxic mechanism, however, is represented by granule-dependent cytotoxicity. The cytotoxic granules contain perforin (pore-forming protein) and several granule-associated proteases, including the granzymes (3, 11, 19, 35). Five different granzymes have been identified in humans: granzyme A (GrmA), GrmB, GrmH, GrmK, and GrmM (3, 11, 19, 22, 35). Following recognition of the target cells by cytotoxic lymphocytes, secretion of the cytotoxic granules is induced, leading to the death of the target cells. It has been proposed that perforin either destabilizes the plasma membrane, facilitating the internalization of the cytotoxic granules into endosomes and the subsequent release in the cytoplasm, or colocalizes with the granzymes into the endosomes and destabilizes the vesicles to allow release of the granzymes in the cytoplasm (14, 42, 57, 58). The granzymes found in humans are all able to induce cell death in different experimental models, and they differ in their intracellular substrates (11, 19, 35, 36, 51). GrmB is a serine protease, and GrmA and -K belong to the tryptase family (11, 18, 19, 35, 36, 52, 63). With regard to GrmK, a series of studies with mice has shown that GrmK may induce cell death by a mechanism that involves the generation of reactive oxygen species (37), that GzmK may cause mitochondrial damage (62), and that GrmK mimics GrmA DNA damage by inducing caspase-independent nuclear fragmentation and nuclear condensation and single-stranded DNA breaks (62). Furthermore, another study analyzing cytolytic transcriptional profiles (including that of GrmK) in influenza virus- and herpes simplex virus type 1-specific CD8 T cells showed increased expression of all the granzymes during acute infection (38). GrmB induces caspase-dependent apoptosis (49), while GrmA-, GrmH-, GrmK- and GrmM-induced death is independent of caspase activation (27, 32, 37). Experiments performed with mice have provided a direct demonstration of the key role of perforin and GrmB in the cytotoxic activity mediated by CD8 T cells (30, 31, 47, 54). Along the same lines, studies performed with human CD8 T cells support the important role of perforin (61).
Cytotoxic CD8 T cells play a fundamental role in protection against virus infections, and the induction of vigorous CD8 T-cell responses following vaccination is thought to be a key component of protective immunity (29, 34, 39, 40, 46, 48). For these reasons, several studies have centered on the functional characterization of CD8 T cells with the objective of identifying functional profiles that may correlate with protective CD8 T-cell responses (9, 26, 33, 39, 40, 56). In this regard, extensive phenotypic and functional characterization of Ag-specific memory CD8 T cells has been made with different models of virus infection (1, 2, 33, 34, 40, 46, 48, 56, 60), and the term polyfunctional has been used to define T-cell immune responses that, in addition to typical effector functions such as secretion of gamma interferon, tumor necrosis factor alpha, or MIP-1β and cytotoxic activity, comprise distinct T-cell populations also able to secrete interleukin 2 (IL-2) and which retain proliferation capacity (9, 26, 39, 40). The polyfunctional profile of CD8 T cells has been associated with protective antiviral immunity (9, 26, 39, 40). Of note, it has been suggested that antigen (Ag) exposure/persistence or Ag levels substantially influenced the functional and phenotypic profiles of T-cell responses (26, 39, 40).
Several studies have previously analyzed the distribution of cytotoxic granules, and it has been shown that perforin and GrmB are predominantly expressed in CD8 T cells with effector function whereas GrmA expression appears to be more ubiquitous (2, 8, 16, 35, 50, 57). However, the distribution of GrmK has never been investigated, nor has the simultaneous expression of perforin and GrmA, GrmB, and GrmK in several models of virus infection, including influenza virus, Epstein-Barr virus (EBV), cytomegalovirus (CMV), and human immunodeficiency virus type 1 (HIV-1).
In the present study, we have performed a comprehensive characterization of virus-specific CD8 T-cell responses against HIV-1, CMV, EBV, and influenza virus in order to carry out the following: (i) analysis of the profiles of cytotoxic granules, including perforin and granzymes (GrmA, -B, and -K), in different models of virus-specific CD8 T-cell responses, (ii) analysis of the relationship between cytotoxic granule profiles and cytotoxic activity, proliferation capacity, and differentiation stage of CD8 T cells, and (iii) kinetic studies of the distribution of cytotoxic granules in HIV-1-specific CD8 T-cell responses during primary infection and following vaccination.
MATERIALS AND METHODS
Study groups.
Seventeen subjects with progressive chronic or primary HIV-1 infection were investigated prior to their enrolment in clinical trials including antiviral therapy with nucleoside and protease inhibitors (7). In patients with chronic HIV-1 infection, at baseline (prior to the initiation of antiviral therapy), CD4 T-cell counts (mean ± standard error) were 790 ± 241 cells/μl and plasma viremia was 4.39 ± 043 log10 HIV-1 RNA copies/ml. Regarding patients with primary HIV-1 infection, primary HIV-1 infection was diagnosed on the basis of the presence of an acute clinical syndrome, a negative HIV-1 antibody test, a positive test for HIV-1 RNA in plasma, and a presence of fewer than three positive bands in a Western blot. In addition, blood from 39 HIV-negative subjects was obtained either from the local blood bank (Lausanne, Switzerland) or from lab coworkers. Five volunteers enrolled in the EV02 HIV vaccine clinical trial were also studied (24). These studies were approved by the Institutional Review Board of the Centre Hospitalier Universitaire Vaudois.
Synthetic peptides and peptide-major histocompatibility complex (MHC) class I tetramer complexes.
All the peptides used in this study were high-performance liquid chromatography purified (>80% purity). HLA-A2-GILGFVFTL (influenza virus), -GLCTLVAML (EBV), -NLVPMVATV (CMV), -SLYNTVATL (HIV Gag), and -ILKEPVHGV (HIV Pol) and HLA-A*0101-YSENSSEYY (HIV Env) peptide-MHC class I tetramer complexes were purchased from Beckman Coulter (Fullerton, CA); HLA-B*0701/02-GPGHKARVL (HIV Gag) and HLA-B*0801-RAFKQLL (EBV) peptide-MHC class I tetramers complexes were produced as described previously (15).
Antibodies.
The following antibodies were used in different combinations. CD8-PerCPCy5.5, CD45RA-PECy5, and GrmB-AF700 were purchased from BD (Becton Dickinson, San Diego, CA), GrmA-PB from Biolegend (San Diego, CA), CD27-APC and CD127-PECy7 from eBioscience Inc. (San Diego, CA), CCR7-FITC and CD127-APC from R&D Systems (Minneapolis, MN), CD3-Qdot655 from Invitrogen (Carlsbad, CA), and GrmK-FITC from Santa Cruz Biotechnology (Santa Cruz, CA).
Ex vivo analysis of virus-specific CD8 T cells.
Cryopreserved blood mononuclear cells (1 × 106 to 2 × 106) were stained with appropriate titers of peptide-MHC class I tetramer complexes at 4°C for 15 min and then either permeabilized (Cytofix/Cytoperm; BD) and stained at room temperature for 20 min with GrmK, GrmA, GrmB, perforin, CD45RA, CD127, and CD8 or directly stained at 4°C for 20 min with CD3, CD8, CD45RA, CD127, CD27, and CCR7. Cells were then fixed with CellFix (BD), acquired on an LSRII SORP flow cytometer (four lasers), and analyzed using the FlowJo 8.7.1 (Tree star Inc., Ashland, OR) and SPICE 4.1.5 (developed by Mario Roederer, Vaccine Research Center, NIAID, NIH) software programs. The number of lymphocyte-gated events ranged between 6 × 105 and 106 in the flow cytometry experiments.
Ex vivo proliferation assay.
Cryopreserved blood mononuclear cells (1 × 106 in 1 ml of complete medium) were cultured in the presence of anti-CD28 antibody (0.5 μg/ml; BD), 50 UI of IL-2 (Proleukin; Novartis International, Basel, Switzerland) and 1 μg/ml of peptide as described previously (25, 64). At day 7, cells were harvested, stained with appropriate titers of peptide-MHC class I tetramer complexes at 4°C for 15 min, and then permeabilized (Cytofix/Cytoperm; BD) and stained at room temperature for 20 min with GrmK, GrmA, GrmB, perforin, CD45RA, CD127 and CD8. Cells were then acquired on an LSRII SORP flow cytometer (4 lasers) and analyzed using FlowJo 8.7.1 and SPICE 4.1.5 (developed by Mario Roederer, Vaccine Research Center, NIAID, NIH).
Chromium release assay.
T2 cells were used as target cells in standard 51Cr release assays as described previously (21). 51Cr labeling and pulsing with cognate peptide (or an irrelevant HIV peptide for a control) was performed for 1 h at 37°C. Two thousand, five hundred target cells were aliquoted into microtiter plates. CD4- and CD19-depleted peripheral blood mononuclear CD8 T cells were added to the targets at 50:1, 25:1, 12:1, and 6:1 ratios. Assay plates were incubated for 4 h at 37°C in 5% CO2 before harvest. Specific 51Cr release was calculated from the following equation: [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100%.
Statistical analysis.
The statistical significance (P values) of the results was calculated by using a two-tailed Student t test using either the Excel (Microsoft, Redmond, WA) or SPICE 4.1.5 software program. A two-tailed P value of less than 0.05 was considered significant. The correlations among variables were tested by simple regression analysis.
RESULTS
Profile of cytotoxic-granule distribution in virus-specific CD8 T cells.
To determine the profiles of cytotoxic-granule distribution, influenza virus-, EBV-, CMV-, and HIV-1-specific CD8 T cells were detected by peptide-HLA tetramer complexes and simultaneously stained with perforin, GrmA, GrmB, and GrmK antibodies using polychromatic flow cytometry. The analysis was limited to GrmA, -B and -K, since monoclonal antibodies for GrmH and -M are not available. In the representative examples shown in Fig. 1A, perforin and GrmB expression was almost absent in influenza virus-specific CD8 T cells whereas a larger percentage, ranging between 60 and 80%, expressed GrmA and GrmK. About 10% of EBV-specific CD8 T cells expressed perforin, and larger percentages expressed GrmB (about 50%) and GrmA and -K (about 80%) (Fig. 1A). About 40% of CMV-specific CD8 T cells expressed perforin, about 60% expressed GrmB and GrmA, and about 40% expressed GrmK (Fig. 1A). About 10% of HIV-1-specific CD8 T cells expressed perforin, whereas a larger percentage of cells expressed GrmB (about 60%), GrmA (about 80%), and GrmK (about 50%). The profile of perforin and the percentages of positive cells observed in HIV-1- and CMV-specific CD8 T cells in our study (Fig. 1A) are identical to those shown in two previous publications (2, 61). Perforin unfortunately does not consistently have a bimodal distribution, particularly in virus-specific CD8 T cells, and this is the reason why in our report and the two previous reports the gates for perforin appear in the middle of a population in some cases. With regard to the different percentages of cytotoxic-granule expression in different populations of virus-specific CD8 T cells, it is also important to underscore that these changes truly reflect immunologically relevant changes. In this regard, it is worth mentioning that all the experiments with tetramers were performed using Ca2+-free medium and all our tetramers have been tested using both the 4°C and 37°C experimental conditions. As shown in the additional data attached (see Data S1 in the supplemental material), the percentages of tetramer-positive cells for HIV-, CMV-, EBV-, and influenza virus-specific CD8 T cells are almost identical under the two experimental conditions. Therefore, the changes in cytotoxic-granule profiles among the virus-specific CD8 T-cell populations are not the result of unstable tetramer expression due to T-cell receptor downregulation or to modifications in the content of cytotoxic granules following degranulation.
FIG. 1.
Perforin and GrmB, -A, and -K expression in virus-specific CD8 T-cells. (A) Representative flow cytometry profiles of perforin and GrmB, -A, and -K expression in influenza virus- (Flu), EBV-, CMV-, and HIV-1-specific CD8 T cells. (B) Simultaneous analysis of the cytotoxic-granule composition of virus-specific CD8 T cells on the basis of perforin, GrmB, GrmA, and GrmK expression. All the possible combinations of the different markers are shown on the x axis, whereas the percentage of the perforin/granzyme distinct cell subsets within virus-specific CD8 T-cell populations are shown on the y axis. The pie charts summarize the data, and each slice corresponds to the proportion of virus-specific CD8 T cells positive for a certain combination of markers. Only significant differences of a given virus-specific CD8 T-cell response versus all the others are shown. At least 600,000 live gated events were acquired.
The profiles of cytotoxic-granule distribution within virus-specific CD8 T cells were confirmed by the analysis of 49 individual virus-specific CD8 T-cell responses, and we then analyzed the simultaneous expression of perforin and granzymes in the different virus-specific CD8 T cells. This analysis showed first that about 40% of influenza virus-specific CD8 T cells did not express either perforin or granzymes, compared to 10 to 20% of the other virus-specific CD8 T cells (Fig. 1B). These differences were statistically significant (P < 0.01). The remaining influenza virus-specific CD8 T cells expressed single GrmK+ or dual GrmK+ GrmA+ cells (>90% of cells) (Fig. 1B). EBV- and HIV-1-specific CD8 T cells had similar profiles of perforin and granzyme expression (Fig. 1B); about 50% of CD8 T cells were singly GrmK+ or dually GrmK+ GrmA+, about 30% triply GrmK+ GrmA+ GrmB+; only a minority (about 10%) were perforin+ (Fig. 1B). Of note, about 75% of CMV-specific CD8 T cells contained cell populations expressing perforin and/or GrmB (Fig. 1B). The profiles of cytotoxic granules in the different virus-specific CD8 T cells were significantly different (P < 0.01), with the exception of those for EBV and HIV-1 (P > 0.05).
Taken together, these results indicate substantial differences in the profiles of cytotoxic-granule distribution among different virus-specific CD8 T cells.
Relationship between cytotoxic granules and differentiation stage of memory CD8 T cells.
We next addressed the relationship between the profiles of cytotoxic-granule distribution and the stage of differentiation of virus-specific CD8 T cells. For these purposes, virus-specific CD8 T cells were stained with a panel of markers used to identify functionally distinct populations of memory CD8 T cells at different stages of differentiation. These markers included CD45RA, CD28, CD27, CD127 and CCR7 (15, 33, 34, 44, 45, 56, 64).
As previously shown (1, 17, 26), this analysis confirmed the lack of an overlap between the different markers, and up to 10 different populations of virus-specific CD8 T cells were identified when all markers were analyzed simultaneously (data not shown). Based on this observation, we investigated which combination of markers was the most suitable to better identify differences in the differentiation stages of the CD8 T cells specific to HIV, EBV, CMV, and influenza virus. In particular, we wanted to identify differentiation markers that can discriminate between memory CD8 T-cell populations specific to viruses that are efficiently cleared, such as influenza virus, and to those (predominantly effector CD8 T-cell populations) specific to viruses that persist and are poorly controlled, such as HIV-1.
Consistent with previous studies (1, 26, 33), the large majority (>70%) of influenza virus-, EBV-, and HIV-1-specific CD8 T cells were CD45RA− CD27+ while the distribution of these markers was quite heterogeneous in CMV-specific CD8 T cells, which were characterized by a large percentage of CD45RA+ cells (Fig. 2A; see Data S2 in the supplemental material). All the four virus-specific CD8 T-cell populations were CCR7−, and with the exception of CMV-specific CD8 T cells, of which a substantial percentage were CD45RA+ (about 50%), were also CD45RA− (Fig. 2A; see Data S2 in the supplemental material). The large majority (about 80%) of influenza virus-specific CD8 T cells were CD127+, while about 80% of HIV-1-specific CD8 T cells were CD127− (Fig. 2A and B). CD127 was expressed on about 50% and 30% of EBV- and CMV-specific CD8 T cells, respectively (Fig. 2A and B). Consistent with previous studies, our results indicated that CD127 is very instrumental for discriminating between memory and effector CD8 T-cell populations at different stages of differentiations, the expression being associated with an early stage and the lack of expression to a more advanced stage (4, 10, 28, 55, 59).
FIG. 2.
Phenotypic analyses of virus-specific CD8 T cells, defined by CD27, CCR7, CD127, and CD45RA expression. (A) Representative flow cytometry profiles of MHC class I peptide tetramer complexes specific to influenza virus (Flu), EBV, CMV, or HIV-1 epitope (top panel) and expression of CD45RA versus that of CD27, CCR7, or CD127 in total CD8 T cells (left panels) or in virus-specific CD8 T cells (right panels). (B) Cumulative data on the distribution of virus-specific CD8 T cells in the different T-cell subsets defined by CD45RA and CD127. Asterisks indicate significant differences of a given virus-specific CD8 T-cell response versus all the others. (C) Cumulative analysis of the simultaneous expression of perforin and GrmB, -A, and -K in different virus-specific CD8 T cells defined by the expression of CD45RA and CD127. Only relevant CD8 T-cell populations are shown for each virus-specific CD8 T-cell response. Each slice corresponds to the proportion of virus-specific CD8 T cells positive for a certain combination of perforin/granzyme expression. Only the populations positive for at least one marker (i.e., perforin or GrmB, -A, or -K) are shown. At least 600,000 live gated events were acquired.
Therefore, the CD127 and CD45RA combination appeared to be more suitable for assessing the diversity of the differentiation stage of the different memory virus-specific CD8 T cells, and for these reasons, this combination was used in the subsequent experiments.
Cytotoxic granule distribution was then determined within the three CD8 T-cell memory populations defined by the expression of CD127 and CD45RA. The preliminary analyses were performed with total blood CD8 T cells. The analysis of the expression of GrmA versus that of GrmB and of that of GrmK versus that of perforin within the CD127+ CD45RA−, CD127− CD45RA−, and CD127− CD45RA+ CD8 T-cell populations indicated that a large proportion of memory CD8 T cells coexpressed GrmA and GrmB and that the percentage of cells positive for these cytotoxic granules increased with the progression of cell differentiation (see Data S3 in the supplemental material). GrmA and GrmB were coexpressed in about 80% of terminally differentiated CD127−CD45RA+ CD8 T cells (see Data S3 in the supplemental material). Furthermore, GrmK and perforin were expressed in memory CD8 T-cell populations at different stages of differentiation but were virtually never coexpressed (see Data S3 in the supplemental material). The simultaneous analysis of granzymes and perforin allowed a better appreciation of the relationship between the profiles of cytotoxic-granule distribution and memory CD8 T-cell differentiation. The early-stage-differentiation CD127+ CD45RA− CD8 T-cell population contained predominantly GrmK and GrmA, whereas GrmB and perforin were almost absent (see Data S3 in the supplemental material). The intermediate-stage-differentiation CD127− CD45RA− CD8 T-cell population contained higher levels of GrmA (about 80%) and GrmB (about 70%) than of GrmK (about 50%) and perforin (about 25%) (see Data S3 in the supplemental material). The terminally differentiated CD127− CD45RA+ cells contained comparable high levels of perforin, GrmA, and GrmB (about 50 to 60%), consistent with recently published results from a study using CD57 as a marker of differentiated cells (16). However, GrmK was almost absent in differentiated cells (see Data S3 in the supplemental material).
The profiles of cytotoxic-granule distribution observed in total memory CD8 T-cell populations were then confirmed with virus-specific CD8 T cells. On the basis of the analysis of 35 virus-specific CD8 T-cell responses specific to influenza virus, EBV, CMV, and HIV-1, GrmK was contained predominantly within the CD127+ CD45RA− CD8 T-cell population (Fig. 2C). In addition, CD127+ CD45RA− CMV-specific CD8 T cells also contained perforin (Fig. 2C). GrmA and GrmB were contained predominantly within the CD127− CD45RA− CD8 T-cell population (Fig. 2C). CD127− CD45RA− CMV-specific CD8 T-cell populations had higher percentages of perforin+ cells (Fig. 2C). Finally, the CD127−CD45RA+ CMV-specific CD8 T-cell population contained the highest percentage (about 90%) of CD8 T cells expressing perforin and/or GrmB (Fig. 2C). The profiles of cytotoxic granules between the different virus-specific CD8 T cells within the CD127+ CD45RA− and CD127− CD45RA− cell populations were significantly different (P < 0.01), with the exception of those for EBV and HIV-1 (P > 0.05).
Relationship between profiles of cytotoxic granules and Ag exposure in vitro and in vivo.
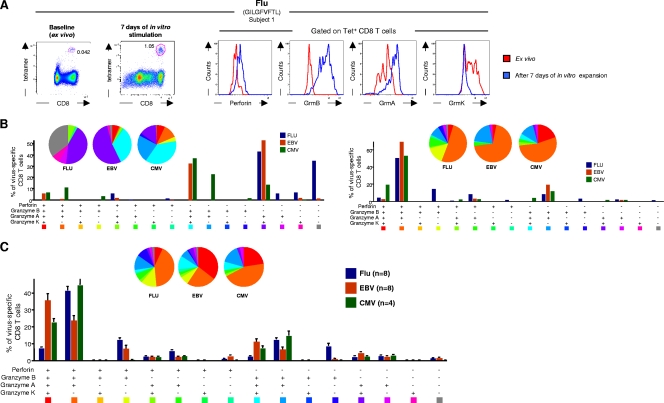
The relationship between Ag exposure and the profiles of cytotoxic granules was then investigated and was first determined in vitro. For this purpose, the profile of cytotoxic granules was compared in the same samples either ex vivo in unstimulated cell populations or in vitro 7 days after Ag-specific stimulation. Subject no. 1 was very instrumental, since he had CD8 T cells specific to influenza virus-, EBV-, and CMV-derived peptides restricted by HLA-A2. Simultaneous analysis of the cytotoxic granules in the influenza virus-, EBV-, and CMV-specific CD8 T-cell populations showed the typical profile of higher GrmK and GrmA expression in influenza virus-specific CD8 T cells, higher perforin and GrmB expression in CMV-specific CD8 T cells, and comparable GrmA, -B, and -K expression and lower perforin expression in EBV-specific CD8 T cells (Fig. 3A and B). Flow cytometry profiles of cytotoxic granules are shown only for influenza virus-specific CD8 T cells (Fig. 3A).
FIG. 3.
Relationship between the profiles of cytotoxic granules and Ag exposure in vitro.(A) Representative flow cytometry profiles of the expression of perforin, GrmB, GrmA, and GrmK on tetramer+ CD8 T cells from subject no. 1 directly ex vivo (red histograms) or after 7 days of in vitro expansion (blue histograms). Flu, influenza virus specificity. (B) Analysis of the simultaneous expression of GrmA, -B, -K, and perforin in influenza virus-, EBV-, or CMV-specific CD8 T cells from subject no. 1 directly ex vivo (left panel) or after 7 days of in vitro stimulation (right panel). (C) Cumulative data on the simultaneous expression of perforin and GrmB, -A, and -K in virus-specific CD8 T cells after 7 days of in vitro stimulation. All the possible combinations of the different markers are shown on the x axis, whereas the percentages of perforin/granzyme distinct cell populations within virus-specific CD8 T cells are shown on the y axis. The pie charts summarize the data, and each slice corresponds to the proportion of virus-specific CD8 T cells positive for a certain combination of perforin/granzymes.
Blood mononuclear cells from subject no. 1 were then stimulated for 7 days with the influenza virus-, EBV-, and CMV-derived peptides, and the stimulated cultures were assessed for the profile of cytotoxic granules at the end of the stimulation period. Simultaneous analysis of the cytotoxic granules in the influenza virus-, EBV-, and CMV-specific CD8 T-cell populations showed major changes in the expression of the different cytotoxic granule profiles. As shown in Fig. 3A, in vitro expansion of the influenza virus-specific CD8 T cells induced an important upregulation of perforin, GrmA, and GrmB and a downregulation of GrmK. Of note, similar changes occurred for EBV- and CMV-specific CD8 T cells (Fig. 3B). Furthermore, the changes in the profiles of cytotoxic granules after Ag-specific stimulation were confirmed by the analysis of 20 virus-specific CD8 T-cell responses (Fig. 3C). The profiles of cytotoxic granules were significantly different after expansion from those analyzed directly ex vivo (P < 0.01 for all three viruses).
These results clearly indicated that Ag reexposure/stimulation in vitro was associated with the increased expression of perforin, GrmA, and GrmB and with the decreased expression of GrmK.
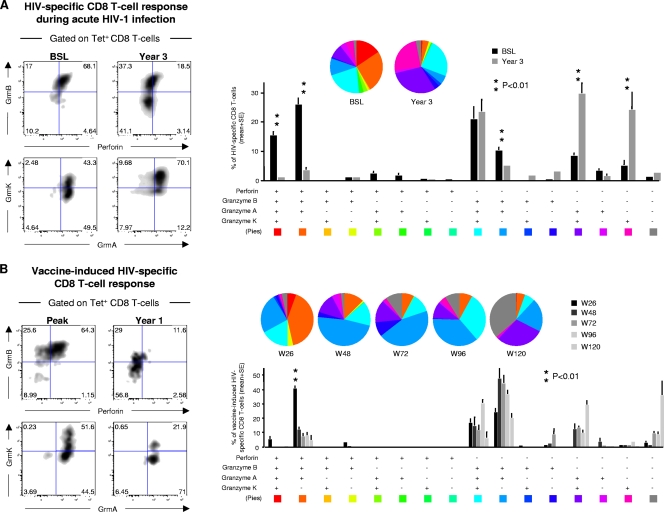
We then investigated whether similar changes in the cytotoxic-granule profiles occurred in vivo. For these purposes, we monitored the changes in cytotoxic granules occurring during primary HIV-1 infection and following vaccination with the experimental DNA C/NYVAC C candidate HIV vaccine combination (24). At the time of the diagnosis of primary HIV-1 infection, patient no. 1016 had high levels of viremia (2,320,000 HIV-1 RNA copies per ml). At this time point, the large majority (about 70%) of HIV-1-specific CD8 T cells recognizing the Gag SLYNTVATL epitope restricted by HLA-A*0201 coexpressed perforin and GrmB and lower (40 to 50%) levels of GrmK and -A (Fig. 4A). The patient was treated with antiviral therapy, and the viremia levels have remained stably below 50 HIV-1 RNA copies per ml of plasma. Three years later, after constant virus suppression, perforin expression was substantially reduced (18%) and only 37% of CD8 T cells were GrmB positive, whereas there was a substantial increase (70%) in Gag-specific CD8 T cells coexpressing GrmK and -A (Fig. 4A). These observations were confirmed for several subjects, and the cumulative analysis clearly show a significant (P < 0.01) downregulation of perforin and/or GrmB and an upregulation of GrmA- and/or GrmK-positive HIV-1-specific CD8 T cells after viral suppression (Fig. 4A).
FIG. 4.
Modulation of the cytotoxic granule profiles by Ag exposure in vivo. (A) Flow cytometry profiles of perforin and granzyme expression, perforin versus GrmB and GrmK versus GrmA, in HIV-1 Gag-specific CD8 T cells at the time of the diagnosis of primary HIV-1 infection and 3 years later (after effective control of HIV-1 replication). Cumulative analysis of the simultaneous expression of perforin, GrmB, GrmA, and GrmK in different HIV-1-specific CD8 T cells is also shown (n = 5). SE, standard error. (B) Flow cytometry profiles of perforin and granzyme expression, perforin versus GrmB and GrmK versus GrmA, in HIV-1 Env-specific CD8 T cells at different time points following vaccination with the DNA C/NYVAC C candidate vaccine combination. Cumulative analysis of the simultaneous expression of perforin, GrmB, GrmA, and GrmK in different HIV-1 Env-specific CD8 T cells is also shown. Each slice corresponds to the proportion of virus-specific CD8 T cells positive for a certain combination of perforin/granzyme expression. Asterisks indicate significant differences for a given Env-specific CD8 T-cell population at a certain time point versus all the others.
CD8 T-cell responses specific to an HIV envelope peptide (YSENSSEYY) restricted by HLA-A*0101 were characterized in a group of healthy volunteers enrolled in the EV02 phase I clinical trial evaluating the safety and immunogenicity of a candidate HIV vaccine combination (DNA C at weeks 0 and 4, followed by NYVAC C at weeks 20 and 24) (24). Representative (one out of five) flow cytometry profiles of volunteer no. 16 are shown in Fig. 4B. At week 26, i.e., 2 weeks after the last immunization, about 65% of Env-specific CD8 T cells coexpressed perforin and GrmB and about 40 to 50% GrmK and GrmA (Fig. 4B). At week 72, i.e., 48 weeks after the last immunization, perforin expression was very low and GrmB expression substantially reduced (Fig. 4B). The simultaneous analysis of the cytotoxic granules at different time points after vaccination showed that there was a progressive shift from perforin/GrmB-positive Env-specific CD8 T cells to perforinlow/GrmBlow GrmA/GrmKhigh cells (Fig. 4B).
These results indicated that Ag exposure modulated cytotoxic granule profiles both in vitro and in vivo.
Relationship between profiles of cytotoxic granules and cytotoxic activity.
The relationship between the profiles of cytotoxic granules and cytotoxic activity was determined ex vivo on freshly isolated blood mononuclear cells using the conventional 51Cr release assay. Thus, cytotoxic activity was determined on HIV-1-negative subjects with known influenza virus-, EBV-, and CMV-specific CD8 T-cell responses. As shown in Fig. 5A, cytotoxic activity was determined for subjects no. 1 and no. 2 for EBV- and CMV-specific CD8 T-cell responses, respectively. The profiles of cytotoxic granules for these subjects were typical of CMV-specific (i.e., large amounts of cells expressing perforin, GrmB, and GrmA and a few cells expressing GrmK) and EBV-specific (i.e., large amounts of cells expressing GrmA and GrmK but a few cells expressing GrmB and virtually no cells expressing perforin) CD8 T-cell responses (Fig. 5A). Of interest, direct cytotoxic activity was detected only against target cells pulsed with CMV- but not EBV-derived peptides (Fig. 5B).
FIG. 5.
Correlation between perforin/granzyme expression and cytotoxic function. (A) Flow cytometry profiles of perforin and granzyme expression, perforin versus GrmB and GrmK versus GrmA, in CMV-specific (left panels) or EBV-specific (right panels) CD8 T cells. (B) Cytotoxic activity of EBV- and CMV-specific CD8 T cells from freshly isolated blood mononuclear cells from subjects 1 and 2 (shown in panel A) in a 51Cr release assay. E:T ratio, effector-to-target-cell ratio. (C) Correlations between cytotoxic activity and expression of perforin, GrmB, GrmA, and GrmK. The percentage of specific lysis is shown on the x axis and is correlated with the expression of each of the different markers.
These results clearly indicated that expression of perforin and GrmB was associated with cytotoxic activity. Based on these results, we determined whether there was a hierarchy among the cytotoxic granules in conferring better cytotoxic activity to CD8 T cells. We found a strong correlation between perforin expression and the magnitude of cytotoxic activity mediated by CD8 T cells (Fig. 5C). A good correlation was found between GrmB and the magnitude of cytotoxic activity (Fig. 5C). The correlation was not found for GrmA, and a negative correlation was found between GrmK expression and cytotoxic activity (Fig. 5C).
DISCUSSION
Cytotoxic CD8 T cells play a fundamental protective role in immunosurveillance against viral infections and tumors and are thought to represent a key component of the protective immunity induced by vaccines (6, 40, 43, 47). The cytotoxic function is the primary mechanism of CD8 T cells to mediate protection, and cytotoxic function is exerted predominantly through cytotoxic granules (3, 6, 11, 14, 19, 35). For these reasons, it is important to delineate the relationship between cytotoxic granules and cytotoxic activity. In this regard, CD8 T cells from perforin-deficient mice have impaired cytotoxic function, and these mice are severely immunosuppressed (30). Furthermore, granzyme-deficient mice have also shown abnormalities of the CD8 T-cell cytotoxic function and association with various degrees of immunodeficiency (19).
A series of studies has previously investigated the profiles of cytotoxic-granule distribution in human CD8 T cells, and some patterns of cytotoxic-granule distribution have been shown to preferentially associate with certain stages of differentiation and cytotoxic activity (2, 5, 12, 16, 23, 35, 50, 61).
In the present study, we have investigated the distribution of GrmK in different populations of virus-specific CD8 T cells, the relationship between Grmk, GrmA, GrmB, and perforin expression, the relationship between GrmK and cytotoxic activity, the relationship with the differentiation stage of CD8 T cells, and the modulation of cytotoxic-granule expression and Ag exposure in vitro and ex vivo.
Distinct profiles of perforin and granzyme distribution were associated with the different virus-specific CD8 T-cell responses. Influenza virus- and CMV-specific CD8 T cells had very divergent profiles, with the former being characterized by high levels of expression of GrmK and almost no perforin and GrmB and the latter by high perforin/GrmB expression and low GrmK. HIV-1- and EBV-specific CD8 T cells had an intermediate (compared to influenza virus and CMV) cytotoxic-granule profile, with high levels of expression of GrmA, -B and -K and low levels of perforin. According to previous studies, perforin expression was deficient in HIV-1-specific T cells compared to that in CMV-specific CD8 T cells (2).
The present results indicate a dichotomy in the expression of GrmK versus that of perforin. We were therefore interested in determining the profile of expression of GrmK versus that of perforin in virus-specific CD8 T cells at different stages of differentiation. We assessed a series of memory markers, including CD27, CCR7, and CD127, in combination with CD45RA in order to define different populations of memory and effector CD8 T cells. We selected CD127 in combination with CD45RA since CD127 was the only marker, compared to CD27 and CCR7, providing a clear, distinct expression profile in influenza virus-specific CD8 T cells, which are a remnant of efficient virus clearance, and HIV-1-specific CD8 T cells, which are associated with persistence and uncontrolled virus replication. On the basis of previous studies with mice and humans, it appears that the CD45RA/CD127 combination provides a better definition of the differentiation stage of memory virus-specific CD8 T cells compared to the different combinations of CD45RA with CD27 or CCR7 (28, 29, 34, 46, 60).
Our findings indicate a clear distinct profile of GrmK expression versus perforin expression in memory and effector CD8 T-cell populations at different stages of differentiation as defined by CD45RA and CD127 of CD8 T cells (28, 29, 34, 46, 60). The GrmK+ profile was associated with memory CD8 T cells at early-stage differentiation (CD127+ CD45RA−), while the perforin+ (and also GrmB+) profile was associated with cells at advanced-stage differentiation (CD127− CD45RA+). The GrmA+, -B+ and -K+ profile was found in CD8 T cells at intermediate-stage differentiation (CD127− CD45RA−). Perforin expression was barely detected in CD8 T cells at early-stage differentiation and was found at low levels at intermediate-stage differentiation, while GrmA was expressed at all differentiation stages. Of note, according to recent studies, perforin expression was enriched in differentiated cells (16, 50).
Having established the relationship between distinct profiles of cytotoxic granules and differentiation stages, we determined their correlation with the cytotoxic function. The results obtained contribute to further consolidating the relationship between cytotoxic-granule profiles and CD8-T-cell-mediated cytotoxic function and demonstrate a hierarchy among the cytotoxic granules. Perforin was the most powerful correlate of cytotoxic function, followed by GrmB. Interestingly, GrmA did not correlate with cytotoxic function, whereas GrmK was negatively correlated. GrmK, however, correlated with the percentage of CD127+ CD8 T cells, which in turn is correlated with the proliferation capacity (data not shown).
Of interest, the results obtained in vitro and in vivo clearly demonstrated that Ag exposure influenced cytotoxic-granule profiles and thus also cytotoxic activity of CD8 T-cells. Poorly cytotoxic profiles were observed in models of low or absent Ag load, such as influenza virus infection or vaccine-induced T-cell responses or after effective control of HIV-1 replication. These situations were associated with high expression levels of CD127 and therefore with a high proliferation capacity (data not shown). In contrast, highly cytotoxic profiles were observed in vitro following Ag reexposure or in vivo during acute HIV-1 infection or at the peak of the immune response following immunization. Therefore, these observations should be taken into account for a correct evaluation of the cytotoxic function of CD8 T cells. However, it is important to underscore that in certain virus infections, such as HIV infection, defective cytotoxic activity and low perforin levels are found despite high Ag levels and may result from mechanisms such as skewed maturation (15), senescence (13), and exhaustion (20, 41, 53).
In conclusion, these data provide two distinct markers to delineate noncytotoxic, i.e., GrmK-expressing, and cytotoxic, i.e., perforin-expressing, CD8 T cells and are therefore instrumental for developing appropriate strategies to monitor memory cytotoxic CD8 T-cell responses in viral infections and cancer and following vaccination.
Supplementary Material
Acknowledgments
Research was conducted as part of the Poxvirus T Cell Vaccine Discovery Consortium (PTVDC) and the Vaccine Immune Monitoring Consortium (VIMC) as part of the Collaboration for AIDS Vaccine Discovery with support from the Bill & Melinda Gates Foundation. This work was also supported by Swiss National Science Foundation research grant FN-310000-116342.
We thank Kim Ellefsen-Lavoie and Emmanuelle Medjitna-Rais for providing some peptide-MHC class I tetramer complexes.
Supplemental material for this article may be found at http://jvi.asm.org/.
Footnotes
Published ahead of print on 28 January 2009.
REFERENCES
- 1.Appay, V., P. R. Dunbar, M. Callan, P. Klenerman, G. M. Gillespie, L. Papagno, G. S. Ogg, A. King, F. Lechner, C. A. Spina, S. Little, D. V. Havlir, D. D. Richman, N. Gruener, G. Pape, A. Waters, P. Easterbrook, M. Salio, V. Cerundolo, A. J. McMichael, and S. L. Rowland-Jones. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8379-385. [DOI] [PubMed] [Google Scholar]
- 2.Appay, V., D. F. Nixon, S. M. Donahoe, G. M. Gillespie, T. Dong, A. King, G. S. Ogg, H. M. Spiegel, C. Conlon, C. A. Spina, D. V. Havlir, D. D. Richman, A. Waters, P. Easterbrook, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 19263-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashton-Rickardt, P. G. 2005. The granule pathway of programmed cell death. Crit. Rev. Immunol. 25161-182. [DOI] [PubMed] [Google Scholar]
- 4.Bachmann, M. F., P. Wolint, K. Schwarz, P. Jager, and A. Oxenius. 2005. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J. Immunol. 1754686-4696. [DOI] [PubMed] [Google Scholar]
- 5.Bade, B., H. E. Boettcher, J. Lohrmann, C. Hink-Schauer, K. Bratke, D. E. Jenne, J. C. Virchow, Jr., and W. Luttmann. 2005. Differential expression of the granzymes A, K and M and perforin in human peripheral blood lymphocytes. Int. Immunol. 171419-1428. [DOI] [PubMed] [Google Scholar]
- 6.Barry, M., and R. C. Bleackley. 2002. Cytotoxic T lymphocytes: all roads lead to death. Nat. Rev. Immunol. 2401-409. [DOI] [PubMed] [Google Scholar]
- 7.Bart, P. A., G. P. Rizzardi, G. Tambussi, J. P. Chave, A. G. Chapuis, C. Graziosi, J. M. Corpataux, N. Halkic, J. Y. Meuwly, M. Munoz, P. Meylan, W. Spreen, H. McDade, S. Yerly, L. Perrin, A. Lazzarin, and G. Pantaleo. 2000. Immunological and virological responses in HIV-1-infected adults at early stage of established infection treated with highly active antiretroviral therapy. AIDS 141887-1897. [DOI] [PubMed] [Google Scholar]
- 8.Berke, G. 1995. The CTL's kiss of death. Cell 819-12. [DOI] [PubMed] [Google Scholar]
- 9.Betts, M. R., M. C. Nason, S. M. West, S. C. De Rosa, S. A. Migueles, J. Abraham, M. M. Lederman, J. M. Benito, P. A. Goepfert, M. Connors, M. Roederer, and R. A. Koup. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 1074781-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boettler, T., E. Panther, B. Bengsch, N. Nazarova, H. C. Spangenberg, H. E. Blum, and R. Thimme. 2006. Expression of the interleukin-7 receptor alpha chain (CD127) on virus-specific CD8+ T cells identifies functionally and phenotypically defined memory T cells during acute resolving hepatitis B virus infection. J. Virol. 803532-3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bots, M., and J. P. Medema. 2006. Granzymes at a glance. J. Cell Sci. 1195011-5014. [DOI] [PubMed] [Google Scholar]
- 12.Bratke, K., M. Kuepper, B. Bade, J. C. Virchow, Jr., and W. Luttmann. 2005. Differential expression of human granzymes A, B, and K in natural killer cells and during CD8+ T cell differentiation in peripheral blood. Eur. J. Immunol. 352608-2616. [DOI] [PubMed] [Google Scholar]
- 13.Brenchley, J. M., N. J. Karandikar, M. R. Betts, D. R. Ambrozak, B. J. Hill, L. E. Crotty, J. P. Casazza, J. Kuruppu, S. A. Migueles, M. Connors, M. Roederer, D. C. Douek, and R. A. Koup. 2003. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 1012711-2720. [DOI] [PubMed] [Google Scholar]
- 14.Catalfamo, M., and P. A. Henkart. 2003. Perforin and the granule exocytosis cytotoxicity pathway. Curr. Opin. Immunol. 15522-527. [DOI] [PubMed] [Google Scholar]
- 15.Champagne, P., G. S. Ogg, A. S. King, C. Knabenhans, K. Ellefsen, M. Nobile, V. Appay, G. P. Rizzardi, S. Fleury, M. Lipp, R. Forster, S. Rowland-Jones, R. P. Sekaly, A. J. McMichael, and G. Pantaleo. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 410106-111. [DOI] [PubMed] [Google Scholar]
- 16.Chattopadhyay, P. K., M. R. Betts, D. A. Price, E. Gostick, H. Horton, M. Roederer, and S. C. De Rosa. 2009. The cytolytic enzymes granzyzme A, granzyme B, and perforin: expression patterns, cell distribution, and their relationship to cell maturity and bright CD57 expression. J. Leukoc. Biol. 8588-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chattopadhyay, P. K., D. A. Price, T. F. Harper, M. R. Betts, J. Yu, E. Gostick, S. P. Perfetto, P. Goepfert, R. A. Koup, S. C. De Rosa, M. P. Bruchez, and M. Roederer. 2006. Quantum dot semiconductor nanocrystals for immunophenotyping by polychromatic flow cytometry. Nat. Med. 12972-977. [DOI] [PubMed] [Google Scholar]
- 18.Chowdhury, D., P. J. Beresford, P. Zhu, D. Zhang, J. S. Sung, B. Demple, F. W. Perrino, and J. Lieberman. 2006. The exonuclease TREX1 is in the SET complex and acts in concert with NM23-H1 to degrade DNA during granzyme A-mediated cell death. Mol. Cell 23133-142. [DOI] [PubMed] [Google Scholar]
- 19.Chowdhury, D., and J. Lieberman. 2008. Death by a thousand cuts: granzyme pathways of programmed cell death. Annu. Rev. Immunol. 26389-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day, C. L., D. E. Kaufmann, P. Kiepiela, J. A. Brown, E. S. Moodley, S. Reddy, E. W. Mackey, J. D. Miller, A. J. Leslie, C. DePierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E. J. Wherry, H. M. Coovadia, P. J. Goulder, P. Klenerman, R. Ahmed, G. J. Freeman, and B. D. Walker. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443350-354. [DOI] [PubMed] [Google Scholar]
- 21.Dutoit, V., V. Rubio-Godoy, P. Y. Dietrich, A. L. Quiqueres, V. Schnuriger, D. Rimoldi, D. Lienard, D. Speiser, P. Guillaume, P. Batard, J. C. Cerottini, P. Romero, and D. Valmori. 2001. Heterogeneous T-cell response to MAGE-A10(254-262): high avidity-specific cytolytic T lymphocytes show superior antitumor activity. Cancer Res. 615850-5856. [PubMed] [Google Scholar]
- 22.Grossman, W. J., P. A. Revell, Z. H. Lu, H. Johnson, A. J. Bredemeyer, and T. J. Ley. 2003. The orphan granzymes of humans and mice. Curr. Opin. Immunol. 15544-552. [DOI] [PubMed] [Google Scholar]
- 23.Grossman, W. J., J. W. Verbsky, B. L. Tollefsen, C. Kemper, J. P. Atkinson, and T. J. Ley. 2004. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood 1042840-2848. [DOI] [PubMed] [Google Scholar]
- 24.Harari, A., P. A. Bart, W. Stohr, G. Tapia, M. Garcia, E. Medjitna-Rais, S. Burnet, C. Cellerai, O. Erlwein, T. Barber, C. Moog, P. Liljestrom, R. Wagner, H. Wolf, J. P. Kraehenbuhl, M. Esteban, J. Heeney, M. J. Frachette, J. Tartaglia, S. McCormack, A. Babiker, J. Weber, and G. Pantaleo. 2008. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J. Exp. Med. 20563-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harari, A., C. Cellerai, F. B. Enders, J. Kostler, L. Codarri, G. Tapia, O. Boyman, E. Castro, S. Gaudieri, I. James, M. John, R. Wagner, S. Mallal, and G. Pantaleo. 2007. Skewed association of polyfunctional antigen-specific CD8 T cell populations with HLA-B genotype. Proc. Natl. Acad. Sci. USA 10416233-16238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harari, A., V. Dutoit, C. Cellerai, P. A. Bart, R. A. Du Pasquier, and G. Pantaleo. 2006. Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunol. Rev. 211236-254. [DOI] [PubMed] [Google Scholar]
- 27.Johnson, B. J., E. O. Costelloe, D. R. Fitzpatrick, J. B. Haanen, T. N. Schumacher, L. E. Brown, and A. Kelso. 2003. Single-cell perforin and granzyme expression reveals the anatomical localization of effector CD8+ T cells in influenza virus-infected mice. Proc. Natl. Acad. Sci. USA 1002657-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaech, S. M., J. T. Tan, E. J. Wherry, B. T. Konieczny, C. D. Surh, and R. Ahmed. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 41191-1198. [DOI] [PubMed] [Google Scholar]
- 29.Kaech, S. M., E. J. Wherry, and R. Ahmed. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2251-262. [DOI] [PubMed] [Google Scholar]
- 30.Kagi, D., B. Ledermann, K. Burki, P. Seiler, B. Odermatt, K. J. Olsen, E. R. Podack, R. M. Zinkernagel, and H. Hengartner. 1994. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 36931-37. [DOI] [PubMed] [Google Scholar]
- 31.Kagi, D., B. Ledermann, K. Burki, R. M. Zinkernagel, and H. Hengartner. 1996. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu. Rev. Immunol. 14207-232. [DOI] [PubMed] [Google Scholar]
- 32.Kelly, J. M., N. J. Waterhouse, E. Cretney, K. A. Browne, S. Ellis, J. A. Trapani, and M. J. Smyth. 2004. Granzyme M mediates a novel form of perforin-dependent cell death. J. Biol. Chem. 27922236-22242. [DOI] [PubMed] [Google Scholar]
- 33.Klenerman, P., and A. Hill. 2005. T cells and viral persistence: lessons from diverse infections. Nat. Immunol. 6873-879. [DOI] [PubMed] [Google Scholar]
- 34.Lanzavecchia, A., and F. Sallusto. 2005. Understanding the generation and function of memory T cell subsets. Curr. Opin. Immunol. 17326-332. [DOI] [PubMed] [Google Scholar]
- 35.Lieberman, J. 2003. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat. Rev. Immunol. 3361-370. [DOI] [PubMed] [Google Scholar]
- 36.Lord, S. J., R. V. Rajotte, G. S. Korbutt, and R. C. Bleackley. 2003. Granzyme B: a natural born killer. Immunol. Rev. 19331-38. [DOI] [PubMed] [Google Scholar]
- 37.MacDonald, G., L. Shi, C. Vande Velde, J. Lieberman, and A. H. Greenberg. 1999. Mitochondria-dependent and -independent regulation of Granzyme B-induced apoptosis. J. Exp. Med. 189131-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mintern, J. D., C. Guillonneau, F. R. Carbone, P. C. Doherty, and S. J. Turner. 2007. Cutting edge: tissue-resident memory CTL down-regulate cytolytic molecule expression following virus clearance. J. Immunol. 1797220-7224. [DOI] [PubMed] [Google Scholar]
- 39.Pantaleo, G., and A. Harari. 2006. Functional signatures in antiviral T-cell immunity for monitoring virus-associated diseases. Nat. Rev. Immunol. 6417-423. [DOI] [PubMed] [Google Scholar]
- 40.Pantaleo, G., and R. A. Koup. 2004. Correlates of immune protection in HIV-1 infection: what we know, what we don't know, what we should know. Nat. Med. 10806-810. [DOI] [PubMed] [Google Scholar]
- 41.Petrovas, C., J. P. Casazza, J. M. Brenchley, D. A. Price, E. Gostick, W. C. Adams, M. L. Precopio, T. Schacker, M. Roederer, D. C. Douek, and R. A. Koup. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 2032281-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pipkin, M. E., and J. Lieberman. 2007. Delivering the kiss of death: progress on understanding how perforin works. Curr. Opin. Immunol. 19301-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russell, J. H., and T. J. Ley. 2002. Lymphocyte-mediated cytotoxicity. Annu. Rev. Immunol. 20323-370. [DOI] [PubMed] [Google Scholar]
- 44.Sallusto, F., J. Geginat, and A. Lanzavecchia. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22745-763. [DOI] [PubMed] [Google Scholar]
- 45.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401708-712. [DOI] [PubMed] [Google Scholar]
- 46.Seder, R. A., and R. Ahmed. 2003. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat. Immunol. 4835-842. [DOI] [PubMed] [Google Scholar]
- 47.Smyth, M. J., K. Y. Thia, S. E. Street, D. MacGregor, D. I. Godfrey, and J. A. Trapani. 2000. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J. Exp. Med. 192755-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sprent, J., and C. D. Surh. 2002. T cell memory. Annu. Rev. Immunol. 20551-579. [DOI] [PubMed] [Google Scholar]
- 49.Sutton, V. R., M. E. Wowk, M. Cancilla, and J. A. Trapani. 2003. Caspase activation by granzyme B is indirect, and caspase autoprocessing requires the release of proapoptotic mitochondrial factors. Immunity 18319-329. [DOI] [PubMed] [Google Scholar]
- 50.Takata, H., and M. Takiguchi. 2006. Three memory subsets of human CD8+ T cells differently expressing three cytolytic effector molecules. J. Immunol. 1774330-4340. [DOI] [PubMed] [Google Scholar]
- 51.Trapani, J. A., and M. J. Smyth. 2002. Functional significance of the perforin/granzyme cell death pathway. Nat. Rev. Immunol. 2735-747. [DOI] [PubMed] [Google Scholar]
- 52.Trapani, J. A., and V. R. Sutton. 2003. Granzyme B: pro-apoptotic, antiviral and antitumor functions. Curr. Opin. Immunol. 15533-543. [DOI] [PubMed] [Google Scholar]
- 53.Trautmann, L., L. Janbazian, N. Chomont, E. A. Said, S. Gimmig, B. Bessette, M. R. Boulassel, E. Delwart, H. Sepulveda, R. S. Balderas, J. P. Routy, E. K. Haddad, and R. P. Sekaly. 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 121198-1202. [DOI] [PubMed] [Google Scholar]
- 54.van den Broek, M. E., D. Kagi, F. Ossendorp, R. Toes, S. Vamvakas, W. K. Lutz, C. J. Melief, R. M. Zinkernagel, and H. Hengartner. 1996. Decreased tumor surveillance in perforin-deficient mice. J. Exp. Med. 1841781-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Leeuwen, E. M., G. J. de Bree, E. B. Remmerswaal, S. L. Yong, K. Tesselaar, I. J. ten Berge, and R. A. van Lier. 2005. IL-7 receptor alpha chain expression distinguishes functional subsets of virus-specific human CD8+ T cells. Blood 1062091-2098. [DOI] [PubMed] [Google Scholar]
- 56.van Lier, R. A., I. J. ten Berge, and L. E. Gamadia. 2003. Human CD8(+) T-cell differentiation in response to viruses. Nat. Rev. Immunol. 3931-939. [DOI] [PubMed] [Google Scholar]
- 57.Voskoboinik, I., M. J. Smyth, and J. A. Trapani. 2006. Perforin-mediated target-cell death and immune homeostasis. Nat. Rev. Immunol. 6940-952. [DOI] [PubMed] [Google Scholar]
- 58.Voskoboinik, I., and J. A. Trapani. 2006. Addressing the mysteries of perforin function. Immunol. Cell Biol. 8466-71. [DOI] [PubMed] [Google Scholar]
- 59.Wherry, E. J., C. L. Day, R. Draenert, J. D. Miller, P. Kiepiela, T. Woodberry, C. Brander, M. Addo, P. Klenerman, R. Ahmed, and B. D. Walker. 2006. HIV-specific CD8 T cells express low levels of IL-7Rα: implications for HIV-specific T cell memory. Virology 353366-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wherry, E. J., V. Teichgraber, T. C. Becker, D. Masopust, S. M. Kaech, R. Antia, U. H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4225-234. [DOI] [PubMed] [Google Scholar]
- 61.Zhang, D., P. Shankar, Z. Xu, B. Harnisch, G. Chen, C. Lange, S. J. Lee, H. Valdez, M. M. Lederman, and J. Lieberman. 2003. Most antiviral CD8 T cells during chronic viral infection do not express high levels of perforin and are not directly cytotoxic. Blood 101226-235. [DOI] [PubMed] [Google Scholar]
- 62.Zhao, T., H. Zhang, Y. Guo, and Z. Fan. 2007. Granzyme K directly processes bid to release cytochrome c and endonuclease G leading to mitochondria-dependent cell death. J. Biol. Chem. 28212104-12111. [DOI] [PubMed] [Google Scholar]
- 63.Zhao, T., H. Zhang, Y. Guo, Q. Zhang, G. Hua, H. Lu, Q. Hou, H. Liu, and Z. Fan. 2007. Granzyme K cleaves the nucleosome assembly protein SET to induce single-stranded DNA nicks of target cells. Cell Death Differ. 14489-499. [DOI] [PubMed] [Google Scholar]
- 64.Zimmerli, S. C., A. Harari, C. Cellerai, F. Vallelian, P. A. Bart, and G. Pantaleo. 2005. HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc. Natl. Acad. Sci. USA 1027239-7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.