Abstract
Adenovirus fiber knobs are the capsid components that interact with binding receptors on cells, while an Arg-Gly-Asp (RGD) sequence usually found in the penton base protein is important for the interaction of most adenoviruses with integrin entry receptors. Mouse adenovirus type 1 (MAV-1) lacks an RGD sequence in the virion penton base protein. We tested whether an RGD sequence found in the MAV-1 fiber knob plays a role in infection. Treatment of cells with a competitor RGD peptide or a purified recombinant RGD-containing fiber knob prior to infection resulted in reduced virus yields compared to those of controls, indicating the importance of the RGD sequence for infection. An investigation of the role of integrins as possible receptors showed that MAV-1 yields were reduced in the presence of EDTA, an inhibitor of integrin binding, and in the presence of anti-αv integrin antibody. Moreover, mouse embryo fibroblasts that were genetically deficient in αv integrin yielded less virus, supporting the hypothesis that αv integrin is a likely receptor for MAV-1. We also investigated whether glycosaminoglycans play a role in MAV-1 infection. Preincubation of MAV-1 with heparin, a heparan sulfate glycosaminoglycan analog, resulted in a decrease in MAV-1 virus yields. Reduced MAV-1 infectivity was also found with cells that genetically lack heparan sulfate or cells that were treated with heparinase I. Cumulatively, our data demonstrate that the RGD sequence in the MAV-1 fiber knob plays a role in infection by MAV-1, αv integrin acts as a receptor for the virus, and cell surface heparin sulfate glycosaminoglycans are important in MAV-1 infection.
Mouse adenovirus type 1 (MAV-1) primarily infects endothelial cells and cells of the monocyte lineage (13, 21, 28, 31), but little is known about the receptors it uses to enter cells. Interaction of a virus with host cell surface molecules is the initial step in infection, and these interactions can directly or indirectly affect pathogenesis. Surface receptors can mediate attachment and/or entry of viruses; adenoviruses were among the first viruses for which distinct receptors for attachment or internalization were identified (42). The primary attachment receptor for most human adenoviruses (hAds) is the coxsackie and adenovirus receptor (CAR), a transmembrane immunoglobulin superfamily glycoprotein (9, 55). For many of the species B hAds, the primary attachment receptor is CD46, a complement regulatory factor (19, 48, 52, 63), while for other species B hAds, the primary attachment receptor remains unknown (56); it is CD80 and CD86 in some cell types (51). The three domains in fiber protein are the N-terminal tail, associated with the penton base; the shaft, which can be of various lengths, depending on the adenovirus serotype; and the globular C-terminal knob. The mature fiber in virions is a trimer of the fiber monomer proteins. The knob domain of the fiber binds the primary attachment receptor in most hAds, and the interaction of knob and receptor is the primary determinant of the subsequent intracellular trafficking of the virus (50). After attachment, internalization of hAds is generally mediated by another capsid protein, the penton base, interacting with a second cell surface receptor, αv integrin (62). The virus interacts with integrin via an Arg-Gly-Asp (RGD) sequence in the penton base.
Not all adenoviruses conform to the usage of the attachment and internalization receptors described above. For example, hAd41 does not have an RGD sequence in its penton base, and this is postulated to be partly responsible for inefficient internalization of the virus (1). Canine adenovirus type 2 lacks any known integrin-interacting motif in its capsid, yet it is internalized with kinetics similar to those of hAd5 (14). The internalization processes for both porcine adenovirus type 3 and bovine adenovirus type 3 are independent of CAR or αv integrins, and these two viruses also apparently do not share primary receptors (6, 7). Ovine atadenovirus 7 has CAR-independent tropism, and its penton base does not contain an RGD sequence; its fiber has been suggested to be involved in tropism, though it lacks an RGD or any other identifiable integrin-binding domain (65).
Additional molecules have been reported to play a role in adenovirus-cell interactions, including major histocompatibility class I (23), plasma proteins such as coagulation factors VII, IX, and X, and complement-binding protein (29, 46, 49, 59) and sialic acid moieties (3). hAd2 and hAd5 utilize heparan sulfate glycosaminoglycans (HS-GAGs) as initial binding receptors in vitro (16, 17), and infection of cells and mice by hAd5 is increased in the presence of HS-GAGs (45, 49). A basic motif of Lys-Lys-Thr-Lys (KKTK), which can bind HS-GAGs (22), is found in the hAd5 fiber shaft near the penton base. There are data indicating that HS-GAGs may serve as receptors by indirect and direct binding to hAds and that in some cases this may involve the fiber shaft KKTK for binding to HS-GAGs and/or subsequent virus trafficking (8, 29, 32, 45, 46, 49, 59). MAV-1 does not utilize the mouse homolog of CAR, mCAR (34), although mCAR is functional as a receptor for hAds (10, 55). It is not surprising that MAV-1 does not use mCAR, since the MAV-1 fiber lacks conserved residues involved in CAR binding (33, 34). Also, mCAR mRNA is not expressed in some mouse cell lines (55) that we have found are productively infected by MAV-1 (K. R. Spindler, unpublished data). MAV-1 has recently been shown to associate with mouse coagulation factors IX and X, but this does not lead to enhanced cellular attachment to hepatocytes (35). It is not known what MAV-1 uses as attachment and/or internalization receptors. MAV-1 does not have an RGD sequence in its penton base (39), but it has an RGD in its fiber, in a sequence of 50 amino acids (aa) not found in hAd fiber knobs, in a position corresponding to the DE loop (Fig. 1) (64). The presence of RGD in the MAV-1 fiber knob raises the possibility that MAV-1 may use an integrin not only as a receptor for internalization but also as the primary attachment receptor. To begin to address this hypothesis, we investigated virus-host interactions through competition experiments and by using cell lines deficient in αv integrin. We demonstrate that the RGD sequence of the fiber knob is important for infectivity by MAV-1 and that αv integrin serves as a primary receptor for the virus. MAV-1 fiber does not have a basic motif like KKTK, which is found in the hAd5 fiber shaft. However, the MAV-1 penton base has a similar basic sequence, KKPR, in a region near where the hAd RGD is found (the MAV-1 penton base has no RGD), which we postulate might interact with HS-GAGs. In this study, we also report experiments testing whether HS-GAGs are important for MAV-1 infection. Heparin treatment of virus inhibited infection, and MAV-1 infectivity was reduced in cells that were pretreated with heparinase or that do not express heparan sulfate, indicating a role for HS-GAGs in infection by MAV-1.
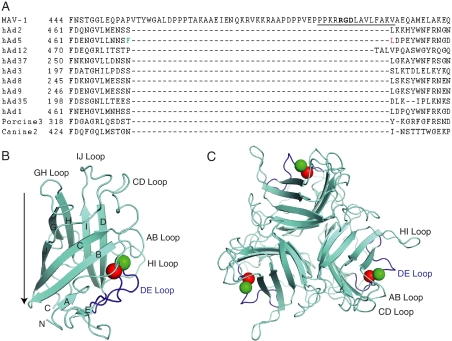
FIG. 1.
Adenovirus fiber knob sequences and structures. (A) Alignment of a portion of the MAV-1 fiber knob sequence (aa 444 to 517) with fiber knob sequences from representative hAds of different hAd species and animal adenoviruses. Species A, adenovirus 12; B, adenovirus 3; C, adenoviruses 2 and 5; D, adenoviruses 8 and 9. The MAV-1 RGD sequence is in bold. Dashes indicate gaps in the sequence; numbers indicate the amino acid sequence number. The sequence of the synthetic RGD peptide used in Fig. 2A is underlined. Phe (green) and Leu (red) residues in hAd5 flanking the gap are colored as in panel B. The T-Coffee program was used for the alignment (44). (B) The hAd5 fiber knob monomer protein structure is depicted, based on the structure determined by Xia et al. (64). The structure data (1KNB.pdb) were downloaded from the RCSB Protein Data Bank, and aa 396 to 581 were displayed using MacPyMOL software in cyan, with the DE loop in blue. Amino (N) and carboxyl (C) termini and β-strand regions are indicated by letters; loops between β-strand regions are also shown. Green and red spheres represent Phe 472 and Leu 473, respectively. These are the residues in the DE loop that flank the insertion found in the MAV-1 fiber knob sequence relative to other knob sequences shown in panel A. A side view is depicted: the arrow indicates the axis of the fiber shaft (pointing toward the capsid). (C) Top view of the hAd5 trimer, viewed looking toward the capsid; colors as in panel B.
MATERIALS AND METHODS
Cells and virus.
Mouse NIH 3T6 fibroblasts were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% heat-inactivated calf serum. HeLa cells were maintained in DMEM with 10% heat-inactivated calf serum. Mouse embryo fibroblasts (MEFs) from mice whose αv integrin gene was knocked out (4) and wild-type (wt) control MEFs were kind gifts from Richard Hynes and Joe McCarty. MEFs were grown in DMEM with 10% fetal bovine serum on plates that had been treated with a 10 μg/ml solution of laminin for 1 h at 37°C. Mouse gro2C cells (deficient in heparan sulfate [20]) were originally isolated by Frank Tufaro. The gro2C and parental L929 cells were obtained from Gary Cohen and maintained in DMEM with 10% heat-inactivated calf serum. MAV-1 was the standard MAV-1 stock originally obtained from S. Larsen (5). It was propagated and titrated by plaque assay on 3T6 cells as described previously (12). hAd5 was propagated and titrated on HeLa cells.
Fiber knob peptides and recombinant proteins.
Synthetic 15-mer peptides were obtained from GenScript Corporation (Piscataway, NJ) and were reported to be >90% pure by the manufacturer. The wt fiber knob peptide, corresponding to aa 492 to 506 of the MAV-1 fiber, was PPKRRGDLAVLFAKV, and the negative control peptide, which had KGE in place of RGD, was PPKRKGELAVLFAKV (the RGD and KGE are indicated in bold). The peptides were dissolved in water and diluted in phosphate-buffered saline (PBS) prior to incubation with cells.
To express recombinant fiber knob protein, a pQE30-based plasmid (Qiagen) containing the knob domain sequence of hAd35, pQE30F35 (50), was obtained from Dmitry Shayakhmetov. The construct has the fiber knob domain contained as a BamHI-HindIII insert with a sequence encoding a six-His tag N terminal to the knob. To make a MAV-1 fiber knob construct, named pMAV-1FK, the MAV-1 sequence from 26,528 to 27,253 (corresponding to fiber knob aa 373 to 613) was amplified with primers MAVL26528 and MAV27253 that included a BamHI site or a HindIII site, respectively (Table 1). The PCR product was isolated, digested with BamHI and HindIII, and inserted in place of the BamHI-HindIII insert of pQE30F35. The RGD of pMAV-1FK was mutated to KGE or AAA by overlap PCR as follows. Primers corresponding to the pQE30 vector were used in conjunction with primers introducing the mutation of interest (Table 1); amplification was with Herculase polymerase (Stratagene), and pMAV-1FK was the template. To create the KGE mutant, a fragment amplified with PQEL64 and MAV26886kge was mixed with a fragment amplified with pQE225 and MAVL26886kge, and the mixture was amplified with PQEL64 and PQE225. To create the AAA mutant, a fragment amplified with PQEL64 and MAV26886aaa was mixed with a fragment amplified with PQE225 and MAVL26886aaa, and the mixture was amplified with PQEL64 and PQE225. The final amplification products containing either the putative KGE or the AAA mutation were digested with BamHI and HindIII and inserted in place of the BamHI-HindIII insert of pMAV-1FK. The resulting plasmids were named pMAV-1FKKGE and pMAV-1FKAAA, respectively. All constructs were verified by sequencing.
TABLE 1.
Oligonucleotide primers used in this study
| Name | Sequencea | Notes |
|---|---|---|
| MAVL26528 | 5′ tttaaggatccGGTTTTCATGCTCACAGGGGC 3′ | Amplify wt fiber knob |
| MAV27253 | 5′ tatataagcTTAATAGTCTTCAGCATAGTACC 3′ | Amplify wt fiber knob |
| PQEL64 | 5′ gtgagcggataacaatttcacacag 3′ | Overlap PCR, sequencing |
| PQE225 | 5′ gatggagttctgaggtcattactgg 3′ | Overlap PCR, sequencing |
| MAV26886kge | 5′ GAGAACTGCTAGCTCTCCTTTTCGTTTGGGCG 3′ | Overlap PCR |
| MAV26886aaa | 5′ GAGAACTGCTAGAGCTGCTGCTCGTTTGGGCG 3′ | Overlap PCR |
| MAVL26886kge | 5′ CGCCCAAACGAAAAGGAGAGCTAGCAGTTCTC 3′ | Overlap PCR |
| MAVL26886aaa | 5′ CGCCCAAACGAGCAGCAGCTCTAGCAGTTCTC 3′ | Overlap PCR |
MAV-1 nucleotides are uppercase, and vector or random nucleotides are lowercase; mutated nucleotides are underlined.
Escherichia coli M15[pRep4] (Qiagen) cultures containing the wt and mutant fiber knob constructs were grown in LB broth containing 100 μg/ml ampicillin and 25 μg/ml kanamycin to an optical density at 600 nm of 0.6 to 1.0, induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside), and incubated for four additional hours. The His-tagged recombinant fiber knob proteins were isolated using Qiagen Ni-NTA agarose, following the manufacturer's instructions for native purification, with minor modifications and with all steps at 4°C. Briefly, cells were lysed in 50 mM NaH2PO4, 436 mM NaCl, 50 mM imidazole, pH 8.0, containing 1.6% Tween 20, 1 mg/ml lysozyme, and a 1:5,000 dilution of protease inhibitor cocktail (Sigma; catalog no. 8849). Lysates were sonicated and centrifuged at 10,000 × g, and supernatants were incubated with Ni-NTA agarose in 50 mM NaH2PO4, 300 mM NaCl, and 50 mM imidazole, pH 8.0, for 1 h, washed in the same buffer, and eluted in 50 mM NaH2PO4, 436 mM NaCl, and 1 M imidazole, pH 8.0. The proteins were analyzed on sodium dodecyl sulfate-polyacrylamide gels with Coomassie blue staining; a single band of the appropriate molecular weight for the fiber knob fragment was seen and estimated to be 99% pure (data not shown). A single band was detected on a Western blot with rabbit anti-6X His antibody (Abcam). The concentration of protein was quantitated by Bradford assay.
Inhibition of infection.
The synthetic peptides or recombinant proteins corresponding to the fiber knob sequence, containing either the wt RGD or mutant KGE or AAA sequences, were incubated with 3T6 cells in suspension for 1 h at 4°C with shaking. MAV-1 that had been dialyzed versus PBS was then added at a multiplicity of infection (MOI) of 5 for 1 h at 4°C with shaking. The cells were washed three times with PBS, growth medium (DMEM containing 2% heat-inactivated calf serum) was added, and the virus yield after incubation for five days was determined. To test the requirement for divalent cations in infection of MAV-1, we preincubated cells in suspension with 5 mM EDTA for 1 h at 4°C with shaking and then washed them three times with PBS. Virus that had been dialyzed against PBS (to remove divalent cations from the virus stock) was added to the cells at a MOI of 5 for 1 h at 4°C with shaking. The cells were washed three times with PBS, growth medium was added, and the virus yield after incubation for 5 days was determined. Incubation of cells or virus with 5 mM EDTA did not adversely affect cell viability or virus titer, respectively (data not shown).
The rat monoclonal antibody to mouse αv integrin (CD51) and the rat immunoglobulin G1 (IgG1) isotype control were purchased from eBioscience (San Diego, CA). Inhibition experiments were carried out as described for the peptide/recombinant protein inhibition experiments above, except that instead of peptide or protein, 100 μg of antibody was added to the cells for the preincubation.
Heparin, chondroitin sulfate A, and chondroitin sulfate B (Sigma) were dissolved in water and diluted in PBS and then incubated with virus for 1 h at 37°C. Virus was then added at a MOI of 5 to cells in monolayers that had been precooled for 15 min on ice and then was incubated for 1 h at 4°C. The plates were then washed with cold DMEM, warmed growth media was added, and the plates were harvested after 24 h (hAd5) or 48 h (MAV-1) of incubation at 37°C.
To remove heparan sulfate from the cell surface, monolayer 3T6 cells (2 × 105 to 3 × 105 cells in 16-mm-diameter wells) were treated with heparinase I (Sigma) dissolved in heparinase buffer (20 mM Tris, 50 mM NaCl, 4 mM CaCl2, 0.01% bovine serum albumin [BSA], pH 7.5) and added at 10 U per well in 0.25 ml. The cells were incubated for 1 h at 37°C, washed twice with PBS, and then either processed for flow cytometry or infected with MAV-1 at a MOI of 5.
Flow cytometry analysis.
3T6 cells were seeded in monolayers and treated with 0 or 10 U of heparinase I per well for 1 h as described above. The cells were washed with PBS and treated with 25 mM EDTA/PBS to detach them from the wells. The cells were pelleted at 200 × g and fixed for 15 min in 3.7% formaldehyde/PBS at room temperature. The efficiency of removal of heparan sulfate was assayed by fluorescence-activated cell sorter (FACS) analysis using an anti-heparan sulfate antibody (F58-10E4; Seikagaku America). Pelleted cells were resuspended in antibody diluted 1:200 in PBS/1% BSA and incubated for 30 min on ice. Cells were washed once with PBS, resuspended in fluorescein isothiocyanate-goat anti-mouse IgM (Invitrogen) diluted 1:50 in PBS/1% BSA, and incubated on ice for 30 min. Cells were pelleted, resuspended in PBS/1% BSA, and analyzed by FACSCanto (Becton-Dickinson). An average of 20,000 cells was counted per sample. Results were analyzed using FlowJo software (Tree Star, Inc.).
Statistical analysis.
Statistical analyses were carried out using SPSS v. 16 software (Chicago, IL) in consultation with the University of Michigan Center for Statistical Consultation and Research.
RESULTS
Competitive inhibition of MAV-1 infection with RGD peptide or recombinant protein.
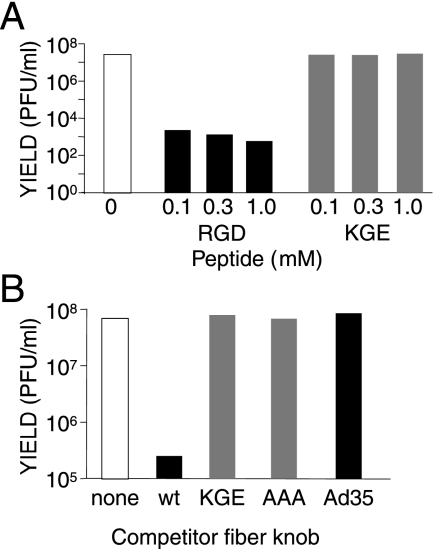
Most hAds use an integrin as an entry receptor through interaction with an RGD sequence in the virion penton base protein. MAV-1 penton base lacks an RGD sequence, but an RGD is found in the fiber knob of MAV-1 in a stretch of 50 aa not found in hAd fiber knobs (Fig. 1). This 50-aa sequence is predicted to be an insertion in the fiber knob DE loop between the D and E β-strands (64). We tested whether this RGD is important for MAV-1 infectivity. We pretreated mouse 3T6 fibroblasts with a 15-mer synthetic peptide encompassing the RGD of the MAV-1 fiber knob (Fig. 1A) or a control peptide with the amino acids KGE instead of RGD for 1 h at 4°C. MAV-1 was then added at a MOI of 5 and incubated for 1 h at 4°C. The cells were washed to remove unbound peptide and virus, plated in growth media, and incubated for 5 days at 37°C. Virus yield was determined by plaque assay (Fig. 2A). In cells infected in the presence of three concentrations of competitor RGD peptide, the virus yield was reduced ∼4.4 log units compared to that in the absence of peptide. No reduction in yield was seen with the control KGE peptide.
FIG. 2.
The RGD of the fiber knob is required for efficient MAV-1 infection. Mouse 3T6 cells were preincubated with the indicated concentrations of RGD peptide or control KGE peptide (A) or 0.1 μg/μl of purified fiber knob (B) (aa 373 to 613 of MAV-1 wt or mutant fibers; aa 122 to 323 of hAd35 fiber), or with no competitor, prior to addition of virus at a MOI of 5 for 1 h at 4°C. The cells were washed, growth media was added, and they were incubated at 37°C for 5 days. Virus yields were determined by plaque assay. Cells treated with no peptide (A) or no competitor polypeptide (B) served as controls. The data are representative of two experiments.
The fiber knob peptide competition results were confirmed with purified recombinant fiber knob. We expressed the MAV-1 fiber knob in a pQE30 vector with an N-terminal six-His tag for use in purification with Ni-NTA affinity columns. We also used overlap PCR to create mutant fiber knobs with the RGD mutated to KGE or AAA in the pQE30 vector. The His-tagged proteins were purified and eluted. They electrophoresed as single bands of the appropriate molecular weights on Coomassie blue-stained gels, and we estimated they were at least 99% pure (data not shown). As seen in Fig. 2B, preincubation of 3T6 cells with the wt MAV-1 fiber knob recombinant protein caused a >2-log unit reduction in MAV-1 yield, whereas the KGE and AAA mutant knobs and a control hAd35 knob did not decrease the viral yield. Taken together with the peptide data, we conclude that the RGD sequence of the MAV-1 fiber knob is required for efficient viral infection.
Role of integrin in MAV-1 infection.
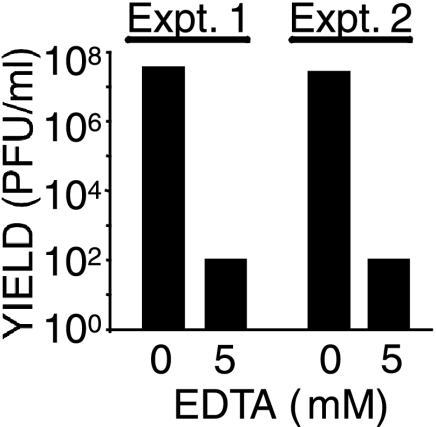
Because RGD sequences interact with integrins, we tested whether MAV-1 uses integrins as receptors. Integrin binding requires divalent cations (reviewed in references 2 and 47), so we assayed MAV-1 infection in the presence of 5 mM EDTA, a divalent cation chelator. Virus yield was reduced ∼5.5 log units compared to the virus yield in the absence of EDTA (Fig. 3). To eliminate the possibility that the yield reduction resulted from the virus capsid being unstable in EDTA, we exposed MAV-1 to 5 mM EDTA for 1 h prior to infection. Infectivity was reduced only 0.1 log unit compared to virus not exposed to EDTA (data not shown). Infectivity was also not decreased when EDTA was added to cultures after a 1-h entry step at 37°C, indicating that EDTA did not affect a postentry step (data not shown).
FIG. 3.
Inhibition of MAV-1 infection by EDTA. Mouse 3T6 cells were preincubated with the indicated concentration of EDTA for 1 h at 4°C prior to the addition of virus at a MOI of 5 for 1 h at 4°C. The cells were washed, growth media was added, and they were incubated at 37°C for 5 days. Virus yields were determined by plaque assay. The results of two experiments are shown.
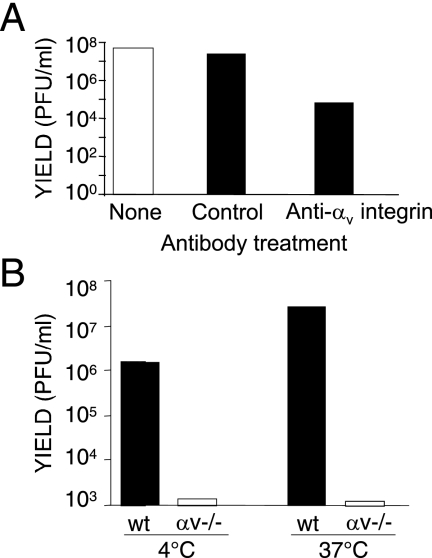
Since the data indicated that MAV-1 uses integrins as a receptor and that the RGD sequence in the fiber is required for infection, we used two methods to examine whether MAV-1 uses αv integrin, an RGD-binding integrin, as a receptor. First, we measured viral yield after preincubating cells with anti-αv antibody or control antibody. Anti-αv antibody reduced virus yield ∼2.8 log units compared to the level in controls (Fig. 4A). Second, we compared the infection of MEFs from mice whose αv integrin gene had been knocked out to the infection of MEFs from wt control mice (4). We infected the αv−/− MEFs and controls with MAV-1, performing the virus adsorption step at either 4°C or 37°C. In both cases, the virus yield from the αv−/− MEFs was reduced by ∼3.5 log units in two independent experiments (Fig. 4B). Together with the results of the antibody interference experiments, these results indicate that αv integrin is involved in MAV-1 infection.
FIG. 4.
αv integrin is required for efficient MAV-1 infection. (A) Mouse 3T6 cells were preincubated with no antibody, rat IgG1 isotype control, or rat monoclonal anti-αv integrin for 1 h at 4°C. MAV-1 was then added at a MOI of 5 for 1 h at 4°C. The cells were washed, growth media was added, and they were incubated at 37°C for 5 days. Virus yields were determined by plaque assay. (B) Infection of cells lacking αv integrin. Wild-type control or αv−/− MEFs were adsorbed with 5 PFU/cell of MAV-1 at the indicated temperature for 1 h and washed, media was added, and the cells were incubated at 37°C for 5 days. The data are representative of two experiments.
Role of heparan sulfate in infection.
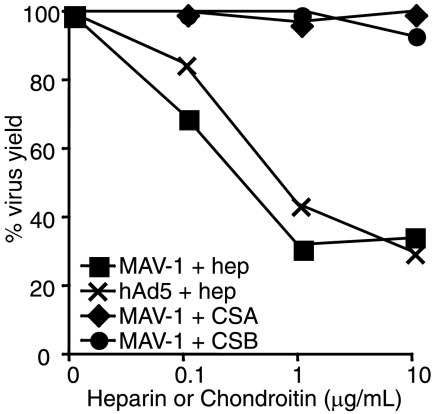
Because HS-GAGs have been shown to be involved in hAd2/5 and other virus infections, we tested whether MAV-1 interacts with heparan sulfate by incubating MAV-1 with various concentrations of heparin, a HS-GAG analog, for 1 h at 37°C. The virus was then added at an MOI of 5 to mouse 3T6 cells on ice and allowed to adsorb for 1 h. The cells were washed, growth medium was added, and the cells were incubated for 44 h at 37°C. Virus yield was determined by plaque assay. As a control, hAd5 was similarly treated with heparin and incubated with human HeLa cells, and the virus yield was determined at 24 h postinfection. The results of three experiments showed that MAV-1 was inhibited to the same extent as hAd5 (Fig. 5); our results with hAd5 were similar in magnitude to the results of Dechecchi et al. (17). In two of the heparin experiments, we also treated virus in parallel with chondroitin sulfate A and B to determine whether other sulfated glycosaminoglycans serve as MAV-1 receptors. There was no inhibition by either chondroitin sulfate A or B (Fig. 5). Thus, MAV-1 appears to utilize HS-GAGs, but not chondroitin sulfate, as a receptor.
FIG. 5.
Inhibition of MAV-1 infection by heparin. Aliquots of MAV-1 or hAd5 were incubated with the indicated concentrations of heparin (hep) or chondroitin sulfate A or B (CSA, CSB) at 37°C for 1 h and then added at a MOI of 5 to 3T6 cells (MAV-1) or HeLa cells (hAd5) on ice and incubated for 1 h at 4°C. After virus adsorption, the cells were then washed and incubated with media for 24 h at 37°C (hAd5) or 44 h (MAV-1). Virus yields were determined by plaque assay and normalized to samples not treated with glycosaminoglycans. The results shown are representative of three experiments with heparin and two with chondroitin sulfates A and B.
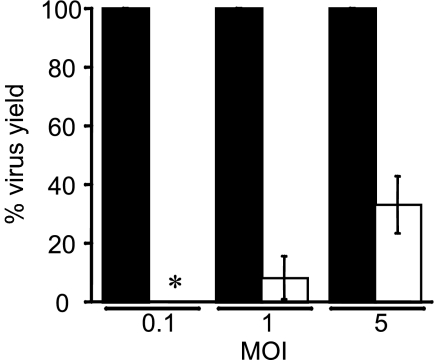
To confirm the result suggesting that MAV-1 binds to heparan sulfate, we used two approaches to test whether cells lacking heparan sulfate yielded less virus upon infection by MAV-1. First, we infected mouse gro2C cells, which are L929 cell derivatives that have a >99.95% reduction of cell surface expression of heparan sulfate due to a defect in the biosynthesis pathway for heparan sulfate (20). MAV-1 infection of the gro2C cells indicated that there was a significant reduction in virus yield compared to that for parental L929 cells (Fig. 6). The virus yield was below the level of detection for gro2C cells at a MOI of 0.1, and there were ∼90% and 65% decreases with infection at MOIs of 1 and 5, respectively. The MAV-1 yield in gro2C cells was significantly different from control L929 cells at both MOIs (P < 0.001). Second, we treated mouse 3T6 cells with heparinase to remove cell surface heparan sulfate. Heparinase treatment did not reduce cell viability (data not shown), and FACS analysis of heparinase-treated uninfected cells showed that the treatment reduced cell surface expression of heparan sulfate by 75% ± 4% (data not shown). After incubation with enzyme, cells were washed and infected at a MOI of 5 for 44 to 48 h. In six independent infections, the mean reduction in viral yield in cells treated with heparinase I compared to that for untreated cells was 23% ± 14%; this was a statistically significant reduction (P = 0.01). These results are consistent with the finding that cells lacking heparan sulfate had reduced viral yield and support the hypothesis that heparan sulfate serves as a receptor for MAV-1.
FIG. 6.
MAV-1 yield is reduced in the absence of heparan sulfate. Mouse gro2c (white bars) or control L929 (black bars) cells were infected with MAV-1 at the indicated MOIs for 1 h at 37°C. The cells were washed and then incubated with growth media at 37°C for 48 h. Virus yields were determined by plaque assay on 3T6 cells and were normalized to virus yields from L929 cells. *, for infections at a MOI of 0.1, virus yields from gro2C cells were below the limit of detection. Mean virus yields of five experiments for three MOIs are shown; error bars, standard deviations of the mean. A one-sample t test with a null hypothesis that the mean was 100% yield indicated that the mean virus yields of MAV-1-infected gro2c cells versus L929 cells were significantly different for infections at both a MOI of 1 and of 5 (P < 0.001).
DISCUSSION
To further our understanding of MAV-1 pathogenesis, we have undertaken this study to identify the receptor(s) the virus uses to attach to and enter cells. MAV-1 causes a widely disseminated infection in mice, targeting endothelial cells and cells of the monocyte lineage (13, 21, 28, 31) and, after intranasal inoculation, respiratory epithelial cells (61). Defining the factors involved in the tropism of MAV-1 will include identifying the receptors used by the virus. For hAds the primary attachment receptor, whether it be CAR, CD46, or another molecule, has been identified as a key determinant of tropism in vivo (reviewed in references 43 and 53). Virus-integrin interactions are also important for infection in vivo, particularly in cell types in which CAR expression is low or undetectable (reviewed in reference 53).
Since MAV-1 does not use mCAR as a receptor (34), and because its fiber knob has an RGD sequence, we investigated whether the RGD was important for MAV-1 infection of mouse 3T6 fibroblasts. We showed that peptide and protein sequences containing the RGD of the fiber knob inhibited infection, whereas identical molecules with the RGD mutated to KGE or AAA did not. We then investigated whether αv integrin, for which RGD is a ligand, was involved. Divalent cations are involved in integrin-ligand interactions (2, 47), and EDTA, a divalent cation chelator, inhibited MAV-1 infection. A specific involvement of αv integrin was shown using antibody to αv integrin, which inhibited MAV-1 infection. MEFs lacking αv integrin produced reduced yields of MAV-1 upon infection. Taken together, these data indicate that the RGD of the MAV-1 fiber knob is important for infection of fibroblasts, most likely via interaction with αv integrin, which is itself necessary for infection. The assays we used here were not able to distinguish whether these molecules (the fiber knob and αv integrin) are required for attachment, entry, or both. Studies are in progress to answer these questions. We also do not know which integrin β-chains are used. For hAds, αvβ1, αvβ3, and αvβ5 promote entry into cells (36, 62), and β5 specifically mediates endosomal rupture, a necessary requirement for virions to be released into the cytoplasm (60). To our knowledge, porcine adenovirus type 4 (NADC-1) is the only other adenovirus reported to have an RGD in the fiber knob sequence (30), and whether the RGD interacts with integrin or is used for attachment or entry of that virus has not been described.
It is interesting to note that hAd vectors that have been constructed with an RGD in the fiber knob can utilize integrins and efficiently infect cells lacking CAR, thus expanding the vectors’ host range (18, 41). Viruses that do not use CAR as their primary receptor have also been modified by insertion of an RGD into the fiber knob, producing vectors that show promise for therapy in human cancers due to their ability to infect malignant cells (11, 57). Integrins are capable of mediating attachment of hAds. For example, αmβ2 integrin promotes attachment of hAd2 to human monocytes and macrophages, which lack CAR (25). In these cells another integrin, αv integrin, mediates entry of the hAd2 virus, and increasing integrin expression on lymphocytes and monocytes increases infection by the vectors (24, 26). Thus, hAd2 depends entirely on integrins for attachment and entry into monocytes and macrophages (25). Based on such findings with hAds, we suggest that MAV-1, which lacks interaction with CAR but has RGD in its fiber knob, utilizes integrin as an attachment and entry receptor. Thus, integrins may be a determinant of cell tropism of MAV-1.
HS-GAGs have been shown to be important for infection by some hAd serotypes (16, 17, 45, 46, 49). We also found evidence that suggests MAV-1 binds to heparan sulfate but not chondroitin sulfate on mouse 3T6 fibroblasts. Infection was inhibited when virus was pretreated with heparin (but not chondroitin sulfate) and in cells whose heparan sulfate was enzymatically removed with heparinase. Mouse L929 fibroblast derivatives that do not express heparan sulfate yielded only 10% of virus compared to the level of L929 parental cells infected with MAV-1 at a MOI of 0.1. We do not know whether the interaction with heparan sulfate is required for attachment, entry, or both, because our assay measures infectious virus produced at the end of infection. We had postulated that the KKPR sequence in the MAV-1 penton base is important as a heparan sulfate-binding moiety. However, when we used two synthetic peptides for the penton base that included the KKPR sequence in experiments like those in Fig. 2A for fiber knob peptides, the penton peptides did not inhibit MAV-1 infection in multiple experiments performed in parallel with experiments in which the fiber knob did inhibit infection (data not shown). While there could be other reasons for the negative results, at face value the data are consistent with the KKPR sequence not being important for MAV-1 infection. A mutational analysis of penton would confirm this. Models have been proposed whereby soluble factors such as coagulation factor IX and X and complement component C4-binding protein serve as bridging molecules between the adenovirus capsid and HS-GAGs (45, 46, 49). In the case of factor X, the capsid component involved for liver tropism of hAd5 is hexon (29, 59). It will be of interest to determine whether MAV-1 interaction with HS-GAGs is direct or mediated by soluble factors. Recent surface plasmon resonance experiments by Lenaerts et al. demonstrated that MAV-1 binds to mouse factors IX and X (35). However, the addition of factor X at physiologic concentrations did not increase the attachment of MAV-1 to mouse hepatocytes. Endothelial cells (particularly in the brain and spinal cord), rather than hepatocytes, are the major target cell of MAV-1 in immunocompetent mice (13, 28). It is possible that factor X or other soluble plasma factors bridge MAV-1 to HS-GAGs on endothelial cells.
A more complex picture than the paradigm of the two-step mechanism of attachment and internalization for adenovirus infection emerges when we consider hAd vectors administered in vivo (reviewed in references 43 and 49) and adenoviruses that lack apparent binding to an established primary receptor or involvement with integrins. These include hAd41, bovine adenovirus type 3, porcine adenovirus type 3, and ovine adenovirus type 7, discussed above (1, 6, 7, 65). Manipulations of hAds to alter tropism also support a more complex model of the requirements for adenovirus infection. For example, hAd5 that has been mutated so that only fiberless particles are produced is nevertheless capable of infecting monocytes via an integrin interaction with the penton base (58). The finding by Lenaerts et al. (34) that MAV-1 does not require CAR, and our results demonstrating that MAV-1 infection is dependent on αv integrin and is enhanced by interaction with heparan sulfate, provide further examples of the variation from the standard two-step model of entry for adenoviruses. We do not know whether integrin and HS-GAGs act additively or synergistically as MAV-1 receptors. Given the level of sensitivity of our plaque assays (2 × 103 PFU/ml) and the 3- to 4.5-log unit reduction in virus yield in the presence of the RGD peptide or recombinant knob protein alone, it would be difficult to make such a determination. We expect that even an additive effect of heparin inhibition, on the order of one log unit, would reduce the virus yield to the limit of detection of our assay. In addition to possible combined effects of integrin and HS-GAGs, other cellular molecules may be involved in the MAV-1-cell interaction. There may be significant interactions with other capsid components known to be involved in hAd-cell interactions, i.e., penton and hexon.
Numerous studies indicate that administration of adenovirus vectors in vivo (in people and in mice) causes an acute inflammatory response with extensive cytokine production (reviewed in reference 15). Evidence suggests that this response is at least in part due to viral attachment and entry; for induction of some cytokines, endosomal escape is required. Viral gene expression is not required for at least some of the response, because the response is rapid (within the first 60 min) and occurs when there is no detectable viral mRNA synthesis (15, 40). Downstream activation of cell signaling that triggers virus escape into the cytosol and transit to the nucleus may contribute to inflammatory responses (reviewed in reference 15). Virus interactions with integrins that ultimately result in endocytosis occur via activation of phosphatidylinositol-3-OH kinase and Rho family GTPases (37, 38). Adenoviruses activate at least two cellular signaling pathways that aid in nuclear targeting of the virus, one that activates protein kinase A and one that activates p38/MAP kinase and MAPKAP kinase 2 (54). The type of entry pathway used by adenoviruses influences the nature of the innate immune response: CD46-utilizing hAds induce higher levels of cytokine expression and NF-κB activation than do CAR-utilizing hAds (27). Because MAV-1 utilizes integrin for infection, it is possible that many of these same pathways are stimulated by MAV-1 infection, resulting in innate responses to the virus. Manipulation of virus capsid and host cell components involved in the MAV-1-receptor interaction by genetic and biochemical means will allow the study of this critical step in the virus infection process both in cell culture and in the natural animal host.
Acknowledgments
We thank Michelle Ozbun for the gift of anti-heparin antibody, Richard Hynes and Joe McCarty for αv integrin-deficient MEFs, Frank Tufaro and Gary Cohen for heparan sulfate- and chondroitin sulfate-deficient cells, and Dmitri Shayakhmetov for the hAd35 fiber plasmid. We thank Mike Imperiale, Glen Nemerow, Dmitry Shayakhmetov, Clay Brown, Saul Silverstein, Terry Jackson, Esteban Domingo, and Malini Raghavan for helpful discussions. We thank Yujing Lin for technical assistance. Clay Brown and John Tesmer kindly provided help in generating the structure figures. We thank the University of Michigan Center for Statistical Consultation and Research for statistical advice. We are grateful to Mike Imperiale, Glen Nemerow, and Jason Weinberg for comments on the manuscript.
This work was supported by NIH R01 AI023762 to K.R.S., by NIH T32 AI007528 to S.R., and by a University of Michigan Rackham Merit Fellowship to T.-H.H.
Footnotes
Published ahead of print on 28 January 2009.
REFERENCES
- 1.Albinsson, B., and A. H. Kidd. 1999. Adenovirus type 41 lacks an RGD αv-integrin binding motif on the penton base and undergoes delayed uptake in A549 cells. Virus Res. 64125-136. [DOI] [PubMed] [Google Scholar]
- 2.Arnaout, M. A., S. L. Goodman, and J. P. Xiong. 2002. Coming to grips with integrin binding to ligands. Curr. Opin. Cell Biol. 14641-651. [DOI] [PubMed] [Google Scholar]
- 3.Arnberg, N., A. H. Kidd, K. Edlund, J. Nilsson, P. Pring-Åkerblom, and G. Wadell. 2002. Adenovirus type 37 binds to cell surface sialic acid through a charge-dependent interaction. Virology 30233-43. [DOI] [PubMed] [Google Scholar]
- 4.Bader, B. L., H. Rayburn, D. Crowley, and R. O. Hynes. 1998. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all αv integrins. Cell 95507-519. [DOI] [PubMed] [Google Scholar]
- 5.Ball, A. O., C. W. Beard, P. Villegas, and K. R. Spindler. 1991. Early region 4 sequence and biological comparison of two isolates of mouse adenovirus type 1. Virology 180257-265. [DOI] [PubMed] [Google Scholar]
- 6.Bangari, D. S., and S. K. Mittal. 2005. Porcine adenovirus serotype 3 internalization is independent of CAR and αvβ3 or αvβ5 integrin. Virology 332157-166. [DOI] [PubMed] [Google Scholar]
- 7.Bangari, D. S., A. Sharma, and S. K. Mittal. 2005. Bovine adenovirus type 3 internalization is independent of primary receptors of human adenovirus type 5 and porcine adenovirus type 3. Biochem. Biophys. Res. Commun. 3311478-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bayo-Puxan, N., M. Cascallo, A. Gros, M. Huch, C. Fillat, and R. Alemany. 2006. Role of the putative heparan sulfate glycosaminoglycan-binding site of the adenovirus type 5 fiber shaft on liver detargeting and knob-mediated retargeting. J. Gen. Virol. 872487-2495. [DOI] [PubMed] [Google Scholar]
- 9.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 2751320-1323. [DOI] [PubMed] [Google Scholar]
- 10.Bergelson, J. M., A. Krithivas, L. Celi, G. Droguett, M. S. Horwitz, T. Wickham, R. L. Crowell, and R. W. Finberg. 1998. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J. Virol. 72415-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borovjagin, A. V., A. Krendelchtchikov, N. Ramesh, D. C. Yu, J. T. Douglas, and D. T. Curiel. 2005. Complex mosaicism is a novel approach to infectivity enhancement of adenovirus type 5-based vectors. Cancer Gene Ther. 12475-486. [DOI] [PubMed] [Google Scholar]
- 12.Cauthen, A. N., A. R. Welton, and K. R. Spindler. 2007. Construction of mouse adenovirus type 1 mutants. Methods Mol. Med. 13041-59. [DOI] [PubMed] [Google Scholar]
- 13.Charles, P. C., J. D. Guida, C. F. Brosnan, and M. S. Horwitz. 1998. Mouse adenovirus type-1 replication is restricted to vascular endothelium in the CNS of susceptible strains of mice. Virology 245216-228. [DOI] [PubMed] [Google Scholar]
- 14.Chillon, M., and E. J. Kremer. 2001. Trafficking and propagation of canine adenovirus vectors lacking a known integrin-interacting motif. Hum. Gene Ther. 121815-1823. [DOI] [PubMed] [Google Scholar]
- 15.Cotter, M. J., and D. A. Muruve. 2005. The induction of inflammation by adenovirus vectors used for gene therapy. Front. Biosci. 101098-1105. [DOI] [PubMed] [Google Scholar]
- 16.Dechecchi, M. C., P. Melotti, A. Bonizzato, M. Santacatterina, M. Chilosi, and G. Cabrini. 2001. Heparan sulfate glycosaminoglycans are receptors sufficient to mediate the initial binding of adenovirus types 2 and 5. J. Virol. 758772-8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dechecchi, M. C., A. Tamanini, A. Bonizzato, and G. Cabrini. 2000. Heparan sulfate gycosaminoglycans are involved in adenovirus type 5 and 2-host cell interactions. Virology 268382-390. [DOI] [PubMed] [Google Scholar]
- 18.Dmitriev, I., V. Krasnykh, C. R. Miller, M. Wang, E. Kashentseva, G. Mikehhva, N. Belousova, and D. T. Curiel. 1998. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J. Virol. 729706-9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaggar, A., D. M. Shayakhmetov, and A. Lieber. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 91408-1412. [DOI] [PubMed] [Google Scholar]
- 20.Gruenheid, S., L. Gatzke, H. Meadows, and F. Tufaro. 1993. Herpes simplex virus infection and propagation in a mouse L cell mutant lacking heparan sulfate proteoglycans. J. Virol. 6793-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guida, J. D., G. Fejer, L.-A. Pirofski, C. F. Brosnan, and M. S. Horwitz. 1995. Mouse adenovirus type 1 causes a fatal hemorrhagic encephalomyelitis in adult C57BL/6 but not BALB/c mice. J. Virol. 697674-7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hileman, R. E., J. R. Fromm, J. M. Weiler, and R. J. Linhardt. 1998. Glycosaminoglycan-protein interactions: definition of consensus sites in glycosaminoglycan binding proteins. Bioessays 20156-167. [DOI] [PubMed] [Google Scholar]
- 23.Hong, S. S., L. Karayan, J. Tournier, D. T. Curiel, and P. A. Boulanger. 1997. Adenovirus type 5 fiber knob binds to MHC class I α2 domain at the surface of human epithelial and B lymphoblastoid cells. EMBO J. 162294-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang, S., R. I. Endo, and G. R. Nemerow. 1995. Upregulation of integrins αvβ3 and αvβ5 on human monocytes and T lymphocytes facilitates adenovirus-mediated gene delivery. J. Virol. 692257-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang, S., T. Kamata, Y. Takada, Z. M. Ruggeri, and G. R. Nemerow. 1996. Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells. J. Virol. 704502-4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang, S., D. Stupack, P. Mathias, Y. Wang, and G. Nemerow. 1997. Growth arrest of Epstein-Barr virus immortalized B lymphocytes by adenovirus-delivered ribozymes. Proc. Natl. Acad. Sci. USA 948156-8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iacobelli-Martinez, M., and G. R. Nemerow. 2007. Preferential activation of Toll-like receptor nine by CD46-utilizing adenoviruses. J. Virol. 811305-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kajon, A. E., C. C. Brown, and K. R. Spindler. 1998. Distribution of mouse adenovirus type 1 in intraperitoneally and intranasally infected adult outbred mice. J. Virol. 721219-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalyuzhniy, O., N. C. Di Paolo, M. Silvestry, S. E. Hofherr, M. A. Barry, P. L. Stewart, and D. M. Shayakhmetov. 2008. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc. Natl. Acad. Sci. USA 1055483-5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleiboeker, S. B. 1995. Sequence analysis of the fiber genomic region of a porcine adenovirus predicts a novel fiber protein. Virus Res. 39299-309. [DOI] [PubMed] [Google Scholar]
- 31.Kring, S. C., C. S. King, and K. R. Spindler. 1995. Susceptibility and signs associated with mouse adenovirus type 1 infection of adult outbred Swiss mice. J. Virol. 698084-8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kritz, A. B., C. G. Nicol, K. L. Dishart, R. Nelson, S. Holbeck, D. J. Von Seggern, L. M. Work, J. H. McVey, S. A. Nicklin, and A. H. Baker. 2007. Adenovirus 5 fibers mutated at the putative HSPG-binding site show restricted retargeting with targeting peptides in the HI loop. Mol. Ther. 15741-749. [DOI] [PubMed] [Google Scholar]
- 33.Law, L. K., and B. L. Davidson. 2005. What does it take to bind CAR? Mol. Ther. 12599-609. [DOI] [PubMed] [Google Scholar]
- 34.Lenaerts, L., D. Daelemans, N. Geukens, E. De Clercq, and L. Naesens. 2006. Mouse adenovirus type 1 attachment is not mediated by the coxsackie-adenovirus receptor. FEBS Lett. 5803937-3942. [DOI] [PubMed] [Google Scholar]
- 35.Lenaerts, L., J. H. McVey, A. H. Baker, L. Denby, S. Nicklin, E. Verbeken, and L. Naesens. 8 December 2008, posting date. Mouse adenovirus type 1 and human adenovirus type 5 differ in endothelial cell tropism and liver targeting. J. Gene Med. doi: 10.1002/jgm.1283. [DOI] [PubMed]
- 36.Li, E., S. L. Brown, D. G. Stupack, X. S. Puente, D. A. Cheresh, and G. R. Nemerow. 2001. Integrin αvβ1 is an adenovirus coreceptor. J. Virol. 755405-5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li, E., D. Stupack, G. M. Bokoch, and G. R. Nemerow. 1998. Adenovirus endocytosis requires actin cytoskeleton reorganization mediated by rho family GTPases. J. Virol. 728806-8812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, E., D. Stupack, R. Klemke, D. A. Cheresh, and G. R. Nemerow. 1998. Adenovirus endocytosis via αv integrins requires phosphoinositide-3-OH kinase. J. Virol. 722055-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meissner, J. D., G. N. Hirsch, E. A. LaRue, R. A. Fulcher, and K. R. Spindler. 1997. Completion of the DNA sequence of mouse adenovirus type 1: sequence of E2B, L1, and L2 (18-51 map units). Virus Res. 5153-64. [DOI] [PubMed] [Google Scholar]
- 40.Muruve, D. A., M. J. Barnes, I. E. Stillman, and T. A. Libermann. 1999. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum. Gene Ther. 10965-976. [DOI] [PubMed] [Google Scholar]
- 41.Nagel, H., S. Maag, A. Tassis, F. O. Nestle, U. F. Greber, and S. Hemmi. 2003. The αvβ5 integrin of hematopoietic and nonhematopoietic cells is a transduction receptor of RGD-4C fiber-modified adenoviruses. Gene Ther. 101643-1653. [DOI] [PubMed] [Google Scholar]
- 42.Nemerow, G. R. 2000. Cell receptors involved in adenovirus entry. Virology 2741-4. [DOI] [PubMed] [Google Scholar]
- 43.Nicklin, S. A., E. Wu, G. R. Nemerow, and A. H. Baker. 2005. The influence of adenovirus fiber structure and function on vector development for gene therapy. Mol. Ther. 12384-393. [DOI] [PubMed] [Google Scholar]
- 44.Notredame, C., D. G. Higgins, and J. Heringa. 2000. T-Coffee: a novel method of fast and accurate multiple sequence alignment. J. Mol. Biol. 302205-217. [DOI] [PubMed] [Google Scholar]
- 45.Parker, A. L., J. H. McVey, J. H. Doctor, O. Lopez-Franco, S. N. Waddington, M. J. Havenga, S. A. Nicklin, and A. H. Baker. 2007. Influence of coagulation factor zymogens on the infectivity of adenoviruses pseudotyped with fibers from subgroup D. J. Virol. 813627-3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parker, A. L., S. N. Waddington, C. G. Nicol, D. M. Shayakhmetov, S. M. Buckley, L. Denby, G. Kemball-Cook, S. Ni, A. Lieber, J. H. McVey, S. A. Nicklin, and A. H. Baker. 2006. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood 1082554-2561. [DOI] [PubMed] [Google Scholar]
- 47.Plow, E. F., T. A. Haas, L. Zhang, J. Loftus, and J. W. Smith. 2000. Ligand binding to integrins. J. Biol. Chem. 27521785-21788. [DOI] [PubMed] [Google Scholar]
- 48.Segerman, A., J. P. Atkinson, M. Marttila, V. Dennerquist, G. Wadell, and N. Arnberg. 2003. Adenovirus type 11 uses CD46 as a cellular receptor. J. Virol. 779183-9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shayakhmetov, D. M., A. Gaggar, S. Ni, Z. Y. Li, and A. Lieber. 2005. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J. Virol. 797478-7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shayakhmetov, D. M., Z. Y. Li, V. Ternovoi, A. Gaggar, H. Gharwan, and A. Lieber. 2003. The interaction between the fiber knob domain and the cellular attachment receptor determines the intracellular trafficking route of adenoviruses. J. Virol. 773712-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Short, J. J., A. V. Pereboev, Y. Kawakami, C. Vasu, M. J. Hoterman, and D. T. Curiel. 2004. Adenovirus serotype 3 utilizes CD80 (B7.1) and CD86 (B7.2) as cellular attachment receptors. Virology 322349-359. [DOI] [PubMed] [Google Scholar]
- 52.Sirena, D., B. Lilienfeld, M. Eisenhut, S. Kalin, K. Boucke, R. R. Beerli, L. Vogt, C. Ruedl, M. F. Bachmann, U. F. Greber, and S. Hemmi. 2004. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J. Virol. 784454-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stewart, P. L., T. S. Dermody, and G. R. Nemerow. 2003. Structural basis of nonenveloped virus cell entry. Adv. Protein Chem. 64455-491. [DOI] [PubMed] [Google Scholar]
- 54.Suomalainen, M., M. Y. Nakano, K. Boucke, S. Keller, and U. F. Greber. 2001. Adenovirus-activated PKA and p38/MAPK pathways boost microtubule-mediated nuclear targeting of virus. EMBO J. 201310-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tomko, R. P., R. Xu, and L. Philipson. 1997. HCAR and MCAR: The human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA 943352-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tuve, S., H. Wang, C. Ware, Y. Liu, A. Gaggar, K. Bernt, D. Shayakhmetov, Z. Li, R. Strauss, D. Stone, and A. Lieber. 2006. A new group B adenovirus receptor is expressed at high levels on human stem and tumor cells. J. Virol. 8012109-12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tyler, M. A., I. V. Ulasov, A. Borovjagin, A. M. Sonabend, A. Khramtsov, Y. Han, P. Dent, P. B. Fisher, D. T. Curiel, and M. S. Lesniak. 2006. Enhanced transduction of malignant glioma with a double targeted Ad5/3-RGD fiber-modified adenovirus. Mol. Cancer Ther. 52408-2416. [DOI] [PubMed] [Google Scholar]
- 58.Von Seggern, D. J., C. Y. Chiu, S. K. Fleck, P. L. Stewart, and G. R. Nemerow. 1999. A helper-independent adenovirus vector with E1, E3, and fiber deleted: structure and infectivity of fiberless particles. J. Virol. 731601-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Waddington, S. N., J. H. McVey, D. Bhella, A. L. Parker, K. Barker, H. Atoda, R. Pink, S. M. Buckley, J. A. Greig, L. Denby, J. Custers, T. Morita, I. M. Francischetti, R. Q. Monteiro, D. H. Barouch, N. van Rooijen, C. Napoli, M. J. Havenga, S. A. Nicklin, and A. H. Baker. 2008. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 132397-409. [DOI] [PubMed] [Google Scholar]
- 60.Wang, K., T. Guan, D. A. Cheresh, and G. R. Nemerow. 2000. Regulation of adenovirus membrane penetration by the cytoplasmic tail of integrin β5. J. Virol. 742731-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weinberg, J. B., G. S. Stempfle, J. E. Wilkinson, J. G. Younger, and K. R. Spindler. 2005. Acute respiratory infection with mouse adenovirus type 1. Virology 340245-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell 73309-319. [DOI] [PubMed] [Google Scholar]
- 63.Wu, E., S. A. Trauger, L. Pache, T. M. Mullen, D. J. von Seggern, G. Siuzdak, and G. R. Nemerow. 2004. Membrane cofactor protein is a receptor for adenoviruses associated with epidemic keratoconjunctivitis. J. Virol. 783897-3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xia, D., L. J. Henry, R. D. Gerard, and J. Deisenhofer. 1994. Crystal structure of the receptor-binding domain of adenovirus type 5 fiber protein at 1.7 A resolution. Structure 21259-1270. [DOI] [PubMed] [Google Scholar]
- 65.Xu, Z. Z., and G. W. Both. 1998. Altered tropism of an ovine adenovirus carrying the fiber protein cell binding domain of human adenovirus type 5. Virology 248156-163. [DOI] [PubMed] [Google Scholar]