Abstract
Axonal transport of herpes simplex virus (HSV-1) is essential for viral infection and spread in the peripheral nervous system of the host. Therefore, the virus probably utilizes existing active transport and targeting mechanisms in neurons for virus assembly and spread from neurons to skin. In the present study, we used transmission immnunoelectron microscopy to investigate the nature and origin of vesicles involved in the anterograde axonal transport of HSV-1 tegument and envelope proteins and of vesicles surrounding partially and fully enveloped capsids in growth cones. This study aimed to elucidate the mechanism of virus assembly and exit from axons of human fetal dorsal root ganglia neurons. We demonstrated that viral tegument and envelope proteins can travel in axons independently of viral capsids and were transported to the axon terminus in two types of transport vesicles, tubulovesicular membrane structures and large dense-cored vesicles. These vesicles and membrane carriers were derived from the trans-Golgi network (TGN) and contained key proteins, such as Rab3A, SNAP-25, GAP-43, and kinesin-1, involved in the secretory and exocytic pathways in axons. These proteins were also observed on fully and partially enveloped capsids in growth cones and on extracellular virions. Our findings provide further evidence to the subassembly model of separate transport in axons of unenveloped capsids from envelope and tegument proteins with final virus assembly occurring at the axon terminus. We postulate that HSV-1 capsids invaginate tegument- and envelope-bearing TGN-derived vesicles and utilize the large secretory vesicle pathway of exocytosis for exit from axons.
Herpes simplex virus type 1 (HSV-1) is a member of the alphaherpesvirus subfamily which includes human and animal viruses such as human varicella-zoster virus and porcine pseudorabies virus (45). Herpesviruses have the ability to infect and remain dormant or latent in neurons in the peripheral nervous system of the host (45). In humans, HSV-1 enters via the intact mucosa or via breaks in the skin. After replication in the epithelial cells, the virions infect the nerve endings of dorsal root ganglion (DRG) neurons innervating the infected tissue. The virus is then transported via retrograde axonal transport to the neuronal cell body where a lifelong latent infection is established. Reactivation of HSV-1 from latency results in the anterograde axonal transport of the virus from the cell body to the nerve terminals to reinfect cells in the skin or mucosa. Reactivation of HSV-1 during a patient's lifetime is frequent, resulting in either recurrent symptomatic disease or asymptomatic viral shedding (15, 45).
DRG neurons in vitro have a highly polarized structure consisting of a cell body, short neurites, and one long axon. Most of the proteins (membrane and synaptic vesicle proteins) needed for axon outgrowth and function of growth cones and synapses are synthesized in the cell body and transported along the axon by fast axonal flow (1, 26). Newly synthesized synaptic vesicle and plasma membrane proteins have been shown to be transported by pleiomorphic tubulovesicular organelles and dense-cored vesicles from the trans-Golgi network (TGN) in the cell body to the growth cones and synapses (26, 39, 54). Neurotropic viruses such as HSV-1 probably utilize existing transport machinery in neurons for production of virus progeny and virus spread from neuron to neuron.
All herpesviruses have four structural components (45). The virion consists of an electron-dense core containing double-stranded DNA enclosed within an icosahedral capsid. The capsid is surrounded by the tegument, which consists of approximately 23 proteins. A host cell-derived lipid envelope containing an estimated 16 membrane proteins, including 13 different glycoproteins, encloses the virus (33, 45). The exact mechanism of anterograde axonal transport, assembly, and exit from axons of alphaherpesviruses, especially HSV-1 and pseudorabies virus, is the subject of considerable debate. Two models have been proposed. The subassembly model proposes that there is separate transport of virus components or subassemblies. In this model, unenveloped viral capsids are transported directly along microtubules to the axon terminus while the envelope and tegument proteins travel in association with transport vesicles. Final assembly of enveloped capsids and exit of virus then occurs either along the axon shaft or at the axon terminus (18, 27, 29, 30, 31, 37, 38, 40, 41, 44, 46, 49, 50, 56, 57). The alternative or “married” model proposes that fully assembled capsids in the cell body are transported down the length of the axon within host cell vesicles prior to exocytosis (3, 7, 9, 11, 20).
Using an in vitro culture of human fetal axons, we demonstrated the presence of partially and fully enveloped capsids within vesicles in axonal varicosities and growth cones. This suggested that axonal varicosities and growth cones are the probable sites of envelopment of at least a proportion of HSV-1 capsids in mid to distal human axons in vitro (46). We have also shown that HSV-1 tegument and envelope proteins can be transported in axons in association with transport vesicles separately from viral capsids (27, 37, 38, 46). However, little is known about the origin, nature, and fate of these transport vesicles and the distribution of viral tegument and envelope proteins on these vesicles. The work presented here follows up on our previous study (46) and utilizes transmission immunoelectron microscopy (TIEM) to examine the vesicles carrying HSV-1 tegument and envelope proteins in human fetal axons and the vesicles surrounding partially and fully enveloped capsids in axonal varicosities and growth cones. This study shows that HSV-1 tegument and envelope proteins are transported in axons to the axon termini either together or separately. We also show that their transport is associated with tubulovesicular membrane structures and large dense-cored vesicles derived from the TGN. In particular, HSV-1 tegument and envelope proteins and viral particles associate with TGN-46, GAP-43, Rab3A, SNAP-25, and kinesin-1, which are key cellular proteins involved in the secretory and exocytic pathways in neurons (10, 24, 25, 26, 32, 39, 51, 52, 62). This study also provides further support to the subassembly model of HSV-1 transport in axons and proposes that the final envelopment of HSV-1 capsids in varicosities and growth cones occurs by invagination of TGN-derived vesicles bearing tegument and envelope proteins. Our findings suggest that HSV-1 utilizes the large secretory vesicle pathway of exocytosis for exit from axons.
MATERIALS AND METHODS
Cells and viruses.
Two viruses were used in parallel in this study, a clinical isolate of HSV-1 (CW1) and vUL37-green fluorescent protein (GFP)-labeled HSV-1 (strain 17). These viruses were grown and passaged in Hep-2 cells as previously described (46). The findings reported in this study were the same for both viruses used.
Antibodies.
Antibodies as specified were kindly provided by the following investigators: rabbit antibodies against VP5 and gB (R69) were from Gary Cohen and Roselyn Eisenberg, University of Pennsylvania, Philadelphia, PA (8); rabbit antibody against VP22 was from Peter O'Hare, Marie Curie Research Institute, Oxted, United Kingdom (17); and mouse antibodies against VP16 (LP1) were from Tony Minson, University of Cambridge, United Kingdom (36). The following commercially available antibodies were used: rabbit polyclonal against SNAP-25 (Affinity BioReagents), rabbit polyclonal against Rab3A (Santa Cruz Biotechnology), sheep anti-human TGN46 (AbD Serotec, United Kingdom), rabbit polyclonal against GAP-43 (Chemicon International), and mouse monoclonal antibody against kinesin-1 (Chemicon International). The gold-conjugated antibodies were obtained from British Biocell International (United Kingdom), and the electron microscope-silver enhancement kit was obtained from Aurion (The Netherlands).
Preparation of human fetal DRG explants.
DRGs were prepared from human fetal tissue obtained at therapeutic termination following the informed consent of the mother. The University of Sydney and Sydney West Area Health Services Human Research Ethics Committees approved these protocols. The DRGs were dissected, cleansed of connective tissue, placed onto Matrigel-coated plastic coverslips or on Aclar film (Electron Microscopy Sciences) and cultured for 5 to 7 days to allow axon outgrowth as previously described (46).
HSV-1 infection of DRG cultures.
DRGs explant cultures were infected with either CW1 (5 × 105 PFU/0.5 ml/ganglion) or vUL37 GFP virus (5 × 105 PFU/0.5 ml/ganglion). After 2 h, the inoculum was removed. The cultures were washed twice, and fresh medium was added. The infected cultures were incubated in 5% CO2 at 37°C for 28 h. The cultures were washed three times in phosphate-buffered saline and processed for TIEM. Mock-infected cultures were incubated in medium and fixed at 30 hpi.
TIEM.
DRG cultures were processed by modified freeze substitution as previously described (38, 46).
Immunolabeling.
Tissue sections on Formvar/Pioloform-coated gilded nickel grids were immunolabeled as previously described (38, 46). Briefly, primary antibodies were incubated together overnight at 4°C, followed by incubation with secondary antibodies conjugated to gold particles of different sizes (5, 10, 15, and 20 nm). In some experiments, secondary antibodies conjugated with 2-nm gold particles were used in combination with sequential silver enhancement performed to obtain two different sizes of gold particles. Steps following primary antibody incubation, including washing steps, secondary antibody incubation, and silver enhancement, were performed using a Leica EM IGL Immunostainer (Leica Microsystems, Austria). After immunolabeling, the sections were double stained using 1% uranyl acetate (in 50% ethanol) and Reynolds lead citrate and examined with a Philips CM120 BioTWIN transmission electron microscope at 100 kV. Images were collected using a SIS Morada digital camera.
For all our immunolabeling experiments, a blocking step with 10% normal serum and acetylated bovine serum albumin was performed in order to assist in the reduction of nonspecific binding reactions. Immunolabeling of mock-infected cells was also performed in parallel as a control for viral antigens. In addition, primary antibody omission and incubation with irrelevant antibodies (e.g., rabbit polyclonal anti-GFP [eBioscience] and sheep polyclonal anti-human immunodeficiency virus type 1 p24 [Aalto Bio Reagents, Ireland]) were used as controls for cellular and viral antigens. Antibodies to GFP labeled only cells and viral particles from DRG cultures infected with the vUL-37 GFP-labeled virus but not those from DRG cultures infected with the clinical isolate of HSV-1 (CW1). Mock-infected cultures did not label with this antibody. Antibodies to human immunodeficiency virus type 1 p24 did not label either mock-infected or HSV-1-infected DRG cultures.
RESULTS
In this study, the distribution and colocalization of tegument proteins VP16 and VP22, envelope protein gB, and key cellular proteins involved in the secretory and exocytic pathways in neurons (TGN-46, Rab3A, SNAP-25, GAP-43, and kinesin-1) were examined by single and dual gold labeling of longitudinal sections of mid and distal regions of human fetal axons cultured in vitro. This technique allowed us to visualize long regions of the same axon and clearly identify varicosities and growth cones (46). In order to identify and distinguish viral capsids from membranous vesicles in axons, the following set of criteria was followed. Unenveloped capsids were distinguished from axonal vesicles on the basis of their size (approximately 125 nm in diameter) (63), thicker structure of the viral capsid compared to vesicle walls, and electron-dense DNA cores. A viral capsid has a complex internal structure, whereas a membranous axonal vesicle has a homogeneously dense center surrounded by a clear halo and is enclosed in a single membrane (27). Enveloped capsids were identified based on the following criteria including size (170 to 220 nm in diameter) (23), an electron-dense core or capsid, a well-developed tegument layer, and two distinct membranes, representing the viral envelope and the membrane of the enclosing vesicle. It is possible to encounter enveloped capsids without a visible core in a given plane of section. In this situation, viral capsids were identified if they contained dual immunogold labeling for viral capsid, tegument, or envelope proteins. Electron micrographs shown here in support of our findings are representative of multiple observations.
HSV-1 tegument and envelope proteins are transported in axons in tubulovesicular structures and large dense-cored vesicles. (i) Tegument proteins (VP16 and VP22).
Immunogold label for tegument proteins VP16 and VP22 was commonly observed on mid to large dense-cored vesicles and tubulovesicular membrane structures in mid axons, varicosities, and growth cones (Fig. 1). These dense-cored vesicles varied in size from 90 nm up to 170 nm in length and from 60 nm to 130 nm in width. The tubulovesicular membrane structures were quite large and varied from 230 nm to 670 nm in length. Label for VP16 and VP22 was observed either in separate vesicles or together in the same vesicle along the length of axons and in varicosities and growth cones (Fig. 1A to D) (Table 1). Label for both VP16 and VP22 was also present decorating unenveloped and enveloped capsids which accumulated in varicosities and growth cones (Fig. 1D to G) (Table 1). Similarly, label for both tegument proteins densely decorated extracellular virions adjacent to axons and growth cones (Fig. 1B, D, and G) (Table 1). In addition, label for both tegument proteins could also be seen in close apposition to the axonal plasma membrane (Fig. 2A and B).
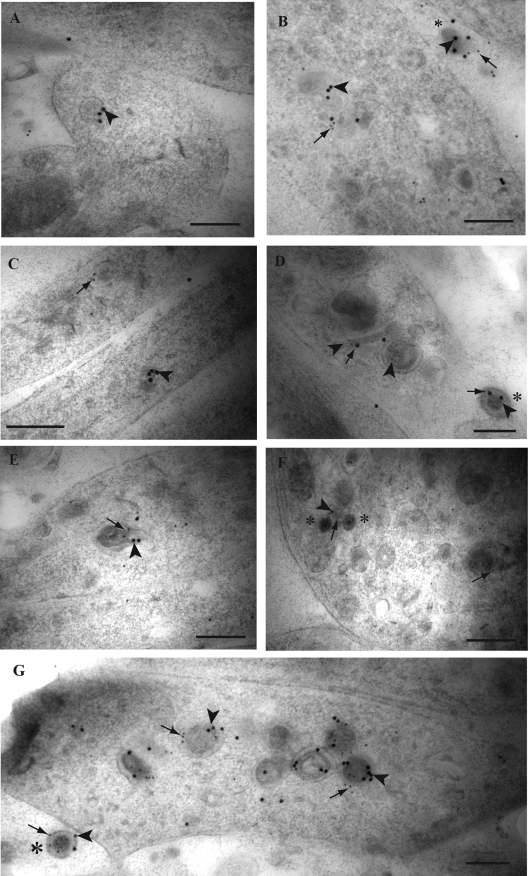
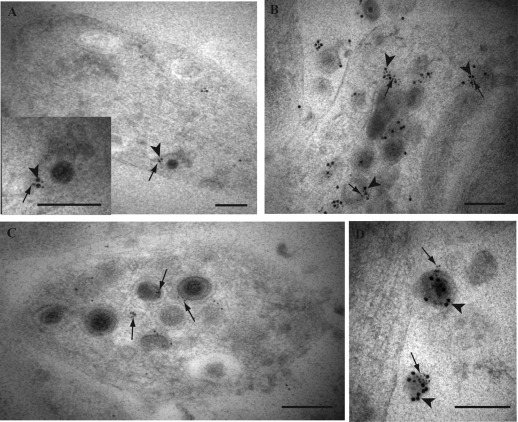
FIG. 1.
Double immunogold labeling for HSV-1 tegument proteins VP16 and VP22 in axons of HSV-1-infected human DRG. Coverslips with DRG cultures were processed to Lowicryl HM20, and dual immunogold labeling of ultrathin sections of mid and distal axons containing varicosities and growth cones was performed as described in Materials and Methods. (A) Label for VP22 (arrowhead) on a dense-cored vesicle in a growth cone. (B) Double label for VP16 (10-nm gold particles; arrows) and VP22 (15-nm gold particles; arrowheads) on dense-cored vesicles in a varicosity and decorating an extracellular virion (indicated by the asterisk). (C) Label for VP16 (arrow) and VP22 (arrowhead) on separate dense-cored vesicles in adjacent axons. (D) Label for VP22 (5-nm gold particles; arrowheads) and VP16 (20-nm gold particles; arrows) is present on a tubulovesicular membrane structure and multivesicle aggregate in between two enveloped capsids in a varicosity. Label for VP22 is decorating both viral particles inside the varicosity, while label for both VP16 and VP22 decorates the extracellular virion (indicated by the asterisk). (E) Dual label for VP16 (10-nm gold particles; arrow) and VP22 (15-nm gold particles; arrowhead) on a partially enveloped viral capsid in a growth cone. (F) Label for both VP16 (10-nm gold particles; arrows) and VP22 (15-nm gold; arrowhead) in proximity to unenveloped capsids (indicated by the asterisk) and on a large dense-cored vesicle in a growth cone. (G) Accumulation of enveloped capsids in a growth cone, densely labeled for both VP16 (arrows) and VP22 (arrowheads). Label for both tegument proteins also decorates an extracellular viral particle (indicated by the asterisk) adjacent to the growth cone. Bars, 200 nm.
TABLE 1.
Quantification of vesicles and HSV-1 particles containing immunogold label for tegument and envelope proteins in mid to distal axons
| Vesicles or viral particles in mid and distal axons | % of vesicles or HSV-1 particles (no. labeled/total) containing:
|
||
|---|---|---|---|
| Tegument label only | Envelope label only | Tegument and envelope label | |
| Vesicles in mid axons | 36.84 (14/38) | 52.63 (20/38) | 10.53 (4/38) |
| Vesicles in growth cones and varicosities | 33.33 (14/42) | 52.38 (22/42) | 14.29 (6/42) |
| Enveloped capsids in growth cones and varicosities | 28.58 (2/7) | 28.58 (2/7) | 42.85 (3/7) |
| Unenveloped capsids in growth cones and varicosities | 58.33 (7/12) | 0.00 (0/12) | 0.00 (0/12) |
| Extracellular virions | 21.28 (20/94) | 8.51 (8/94) | 70.21 (66/94) |
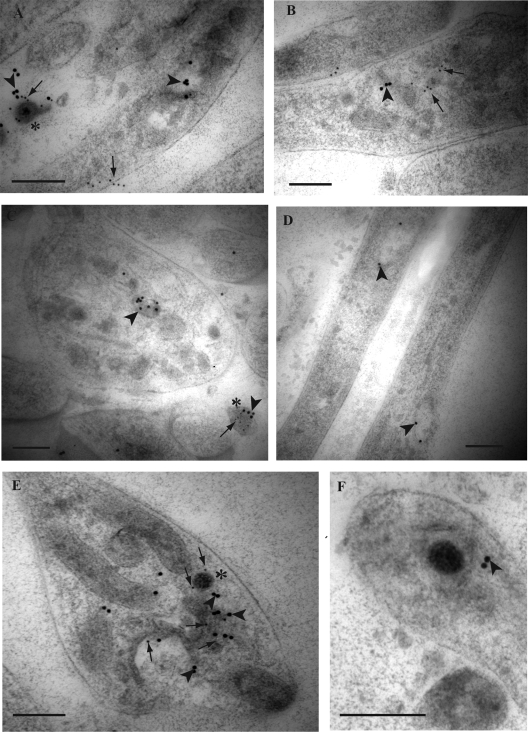
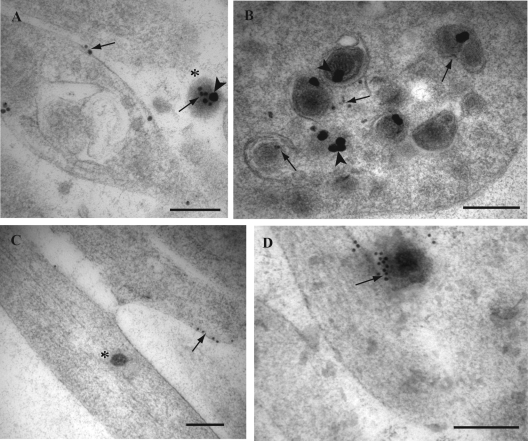
FIG. 2.
Double immunogold labeling for HSV-1 tegument protein VP16 and envelope gB in axons of HSV-1-infected human DRG. Coverslips with DRG cultures were processed to Lowicryl HM20, and dual immunogold labeling of ultrathin sections of mid and distal axons containing varicosities and growth cones was performed as described in Materials and Methods. Label for VP16 was detected with a 10-nm gold conjugate and for gB with a 15-nm gold conjugate. (A) Label for gB (15-nm gold particles; arrowheads) is associated with a large clear-cored vesicle in a varicosity. Label for VP16 (10-nm gold particles; arrows) is found on the plasma membrane separately from label for gB (arrowhead). Note the label for both gB (arrowhead) and VP16 (arrow) is found on the extracellular viral particle (indicated by the asterisk) adjacent to the varicosity. (B) Label for gB (arrowhead) and VP16 (arrows) on separate dense-cored vesicles in a varicosity. (C) Label for gB (arrowhead) in a large dense-cored vesicle in a growth cone. Note the extracellular viral particle (indicated by the asterisk) adjacent to the growth cone is decorated with label for both gB (arrowhead) and VP16 (arrow). (D) Label for gB (arrowheads) on two separate large tubulovesicles in mid axons. (E) Label for gB (arrowheads) and VP16 (arrows) is present on tubulovesicular membrane structures in close proximity to an unenveloped capsid (indicated by the asterisk) in a growth cone. Label for VP16 (arrows) is also present on the unenveloped capsid. (F) Unenveloped capsid partially surrounded by a tubulovesicular membrane structure with associated label for gB (arrowhead). Bars, 200 nm.
(ii) Tegument and envelope proteins (VP16 and gB).
Label for envelope glycoprotein B in mid axons, varicosities, and growth cones was present on large clear and dense-cored vesicles, tubulovesicular structures, and in close apposition to the plasma membrane but was usually not associated with label for tegument protein VP16 (Fig. 2A to E and 3B) (Table 1). In growth cones, label for gB and VP16 was usually found together decorating enveloped capsids, tubulovesicular membrane structures, and vesicles adjacent to unenveloped or enveloped capsids (Fig. 2E and F) (Table 1). The label for VP16 frequently decorated the viral capsids (Fig. 2E), while label for gB was found on the vesicles that partially or completely surrounded capsids (Fig. 2F). In addition, extracellular virions adjacent to the axons and growth cones showed dense labeling for both gB and VP16 (Fig. 2A and C) (Table 1).
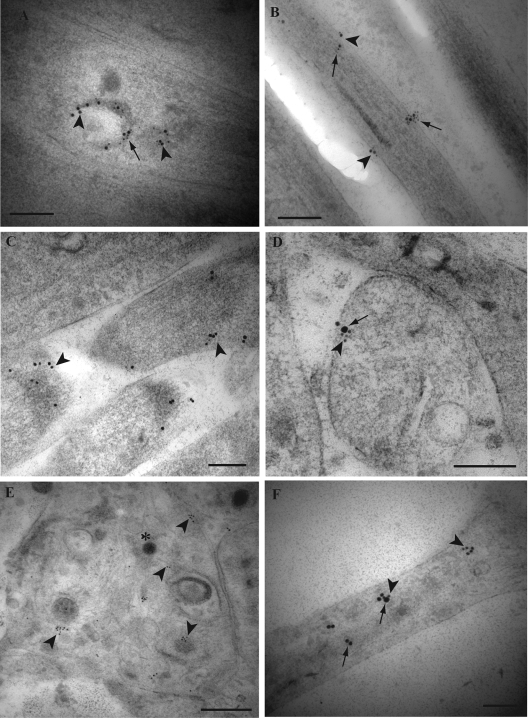
FIG. 3.
Immunogold labeling for TGN-46, GAP-43, Rab3A, tegument proteins VP16 and VP22, and envelope gB in mid and distal axons of HSV-1-infected human DRG. Ultrathin sections were simultaneously incubated with primary antibodies overnight. Detection of primary antibodies was obtained either by incubation with gold-conjugated antibodies of different sizes of gold particles (5 and 10 nm) or by incubation with secondary antibodies conjugated with 2-nm gold particles and sequential silver enhancement to produce two different sizes of gold particles. (A) Label for VP22 (arrowheads) and TGN-46 (arrows) together on tubulovesicular structures in a mid axon. (B) Label for gB (arrowheads) together with label for TGN-46 (arrows) on the plasma membrane in a mid axon. (C) Label for GAP-43 (arrowheads) is present on the plasma membrane of the tips of axons. (D) Label for GAP-43 (small gold particles) (arrowhead) is present in association with label for VP16 (large gold particles; arrow) on the plasma membrane of a growth cone. (E) Label for Rab3A (arrowheads) is found on clear and dense-cored vesicles as well as on the plasma membrane in a growth cone. Note that the label for Rab3A does not associate with the unenveloped capsid (indicated by the asterisk). (F) Label for VP16 (arrows) is present together with or separately from label for Rab3A (arrowheads) in an axon. Bars, 200 nm.
Viral proteins are transported in axons to growth cones in vesicles derived from the TGN: TGN and viral proteins VP22 and envelope glycoprotein gB.
Several studies have shown that vesicles or carriers that deliver synaptic and membrane proteins to the axon terminus originate from the TGN (39, 54). In order to identify the origin of the tubulovesicular structures and dense-cored vesicles carrying viral proteins, we performed single and dual immunogold labeling for TGN-46 protein with either tegument VP22 or envelope gB proteins. TGN-46 is a resident membrane protein of the TGN. Single labeling for TGN-46 of mock-infected and infected axons showed label for TGN-46 in clusters on the plasma membrane along mid axons, growth cones, and at the tips of axons (without visible growth cones). Label for TGN-46 was also seen in large dense-cored vesicles and tubulovesicular membrane structures in mid axons and growth cones (data not shown). Dual labeling of infected axons showed the presence of label for TGN-46 in association with either label for VP22 (Fig. 3A) or label for gB (Fig. 3B) on large dense-cored vesicles, tubulovesicular structures, and plasma membrane in mid axons and in growth cones (Table 2).
TABLE 2.
Quantification of viral particles and vesicles carrying viral proteins in axons containing immunogold label for TGN-46, Rab3A, GAP-43, SNAP-25, and kinesin-1
| Vesicles or HSV-1 particles with label for VP16, VP22, or gB | % of viral particles or vesicles (no. marked/total) carrying indicated cellular markera
|
||||
|---|---|---|---|---|---|
| TGN-46 | Rab3A | GAP-43 | SNAP-25 | Kinesin-1 | |
| Vesicles in mid axons | 80.0 (24/30) | 50.0 (12/24) | 52.2 (12/23) | 40.0 (10/25) | 35.5 (22/62) |
| Vesicles in growth cones and varicosities | 85.7 (12/14) | 62.5 (10/16) | 65.2 (15/23) | 46.1 (6/13) | 53.6 (15/28) |
| Enveloped capsids in growth cones and varicosities | 68.0 (17/25) | 50 (5/10) | 62.5 (5/8) | 80.0 (12/15) | 72.2 (13/18) |
| Extracellular virions | 80.3 (61/76) | 35.1 (13/37) | 71.4 (15/21) | 73.7 (59/80) | 74.4 (64/86) |
Apart from kinesin-1 and SNAP-25, immunogold label for TGN-46, Rab3A, and GAP-43 was not observed on unenveloped capsids in axons.
The vesicles carrying HSV-1 tegument and envelope proteins also transport GAP-43, Rab3A, and SNAP-25, three key proteins in the secretory pathway in neurons.
To further characterize the vesicles carrying the viral proteins along axons, we performed single and dual immunogold labeling experiments for viral proteins in axons with markers for key proteins involved in the secretory and exocytic pathways in axons, including GAP-43, Rab3A, and SNAP-25.
In order to determine if viral proteins travel in axons with proteins known to be destined for transport to and accumulation at axon termini, we performed single and dual labeling of viral tegument proteins with the growth cone-associated protein GAP-43. This neuron-specific GAP-43 protein is localized to axons and plasma membrane of growth cones and presynaptic terminals (21, 39, 58). In this study, label for GAP-43 was found on or adjacent to the plasma membrane of axon tips, varicosities, and growth cones in mock-infected (data not shown) and infected (Fig. 3C and D) axons. Dual labeling for GAP-43 and VP16 showed the presence of both labels together on the plasma membrane of growth cones (Fig. 3D). Label for both was also observed on large dense-cored vesicles and tubulovesicular structures in mid axons, varicosities, and growth cones (Table 2).
Rab3A is a member of a large set of GTP-binding proteins that regulate intracellular trafficking by controlling fusion and transport, and it plays a key role in the regulated exocytosis of hormones and neurotransmitters (10). Label for Rab3A was present on a variety of vesicles, including large dense-cored vesicles, tubulovesicular structures, and also small clear-cored vesicles found along mid axons, varicosities, and growth cones of mock-infected (data not shown) and infected (Fig. 3E) neurons. In addition, label for Rab3A was also found on or adjacent to the plasma membrane along axons, varicosities, and growth cones of both mock-infected and infected axons (Fig. 3E). In infected axons, label for Rab3A was observed in association with label for tegument VP16 on the same tubulovesicular structures and large dense-cored vesicles in mid axons and growth cones (Fig. 3F) (Table 2). However, label for Rab3A and VP16 could also be found on separate vesicles along the same axon (Fig. 3F).
Another important protein involved in the exocytic pathway in neurons is SNAP-25 (synaptosomal-associated protein of 25 kDa). SNAP-25 is a soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor (SNARE) that localizes on the plasma membrane (51, 52, 62). SNAP-25 plays a role in the docking and fusion of vesicles to the plasma membrane and is essential for calcium-triggered exocytosis (51, 52, 62). Label for SNAP-25 was present in axons on large dense-cored vesicles and along the plasma membrane in mid axons, growth cones, and axon tips in both mock-infected (data not shown) and infected cultures (see Fig. 6A) (Table 2). In infected axons, label for SNAP-25 was found either together on the same vesicle with or separately from label for tegument VP16 (Table 2).
FIG. 6.
Immunogold labeling for SNAP-25 in HSV-1-infected axons. (A and B) Label for SNAP-25 (arrows) is present on enveloped capsids within vesicles as well as on the plasma membrane in axon tips. (C) Label for SNAP-25 (arrows) associates with an unenveloped capsid in an axon. (D) Extracellular viral particles with dense label for SNAP-25 (arrows). Bars, 200 nm.
HSV-1 capsids in growth cones invaginate TGN-derived vesicles carrying viral tegument and envelope proteins.
The nature and origin of the vesicles which HSV-1 capsids invaginate to acquire an envelope are not known. Vesicles derived from the TGN have been shown to play a key role in the axonal transport of synaptic and membrane proteins to growth cones (39). Therefore, we examined the distribution of label for TGN and viral proteins on HSV-1 capsids (enveloped and unenveloped) accumulating in the growth cones.
Label for TGN-46 together with either envelope glycoprotein gB or tegument protein VP22 was found decorating enveloped capsids within growth cones or axon tips (Fig. 4B and C) (Table 2). The membranes of tubulovesicular structures partially surrounding capsids in growth cones were also found to contain dual label for TGN-46 and either gB or VP22 (Fig. 4A). In addition, extracellular viral particles in close proximity to axons contain dual label for TGN-46 and either gB or VP22 (Fig. 4D), respectively (Table 2).
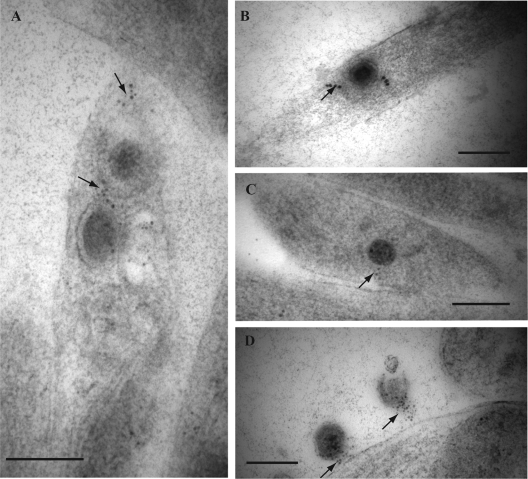
FIG. 4.
Dual immunogold labeling for tegument VP22 with label for TGN-46 in mid and distal axons of HSV-1-infected human DRG. Ultrathin sections were incubated with sheep polyclonal antibody against TGN-46 and with rabbit polyclonal antibody to VP22. Label for TGN-46 was detected with a 10-nm gold conjugate and for VP22 with a 15-nm gold conjugate. (A) Label for both TGN-46 (10-nm gold particles; arrow) and VP22 (15-nm gold particles; arrowhead) is associated with a tubulovesicular membrane structure partially surrounding a viral capsid in a growth cone. Inset shows higher magnification of the unenveloped capsid and label for both TGN-46 and VP22. (B) Label for both TGN-46 (arrows) and VP22 (arrowheads) is associated with vesicles surrounding fully enveloped viral capsids in a growth cone. (C) Label for TGN-46 (arrows) on enveloped viral capsids within vesicles accumulating in a growth cone. (D) Label for both TGN-46 (arrows) and VP22 (arrowheads) decorating extracellular viral particles. Bars, 200 nm.
Enveloped viral capsids in growth cones associate with GAP-43, Rab3A, and SNAP-25, key cellular proteins involved in the secretory and exocytic pathways in neurons.
To further investigate the mechanism of envelopment of HSV-1 particles in growth cones, we examined the distribution of label for GAP-43, Rab3A, and SNAP-25 in growth cones containing viral particles.
Label for GAP-43 was observed mainly on or in close apposition to the plasma membrane of axon tips, varicosities, and growth cones (Fig. 5A). Label for GAP-43 was also present in most of the extracellular viral particles adjacent to varicosities and growth cones (Fig. 5A) (Table 2). The label for GAP-43 was frequently present on enveloped capsids in varicosities and growth cones and accumulated in proximity to enveloped particles in growth cones (Fig. 5B) (Table 2). The association of GAP-43 label to viral capsids was exclusive to enveloped capsids in growth cones as it did not associate with unenveloped capsids (Fig. 5C) in mid axons or in growth cones (Table 2).
FIG. 5.
Immunogold labeling for GAP-43 and Rab3A in HSV-1-infected mid and distal human axons. Ultrathin sections were either single labeled for GAP-43 or Rab3A or dual labeled with monoclonal antibody to VP5 and rabbit polyclonal antibody for GAP-43 incubated simultaneously overnight. For double labeling, sequential incubation with secondary antibodies conjugated with 2-nm gold particles and silver enhancement was performed to produce two different sizes of gold particles. (A) Label for GAP-43 (small gold particles; arrows) is present on the plasma membrane of a varicosity and on the extracellular viral particle (indicated by the asterisk). The extracellular viral particle also carries label for VP5 (large gold particle; arrowhead). (B) Label for GAP-43 (arrows) is present on vesicles surrounding enveloped capsids in a growth cone while label for VP5 (arrowheads) decorates the enveloped capsids. (C) Label for GAP-43 along the plasma membrane of axons. Note that label for GAP-43 (arrow) does not associate with the unenveloped capsid (indicated by the asterisk) found in mid axon. (D) Label for Rab3A (10-nm gold particles; arrow) is present decorating an extracellular viral particle. Bars, 200 nm.
Label for Rab3A was observed on about half of the enveloped capsids within vesicles in growth cones and in less than half of the extracellular viral particles (Fig. 5D) (Table 2). Like label for GAP-43, label for Rab3A was not detected on unenveloped capsids in mid axons and growth cones (Fig. 3E) (Table 2).
Label for SNAP-25, like GAP-43, accumulated and decorated enveloped capsids in growth cones and axon tips (Fig. 6A and B) (Table 2). But unlike GAP-43 and Rab3A, label for SNAP-25 was present on unenveloped capsids in mid axons and growth cones (Fig. 6C). In addition, the majority of extracellular viral particles adjacent to axons labeled densely for SNAP-25 (Fig. 6D) (Table 2). In particular, a “trail” of SNAP-25 label could be seen extending from the axonal membrane to the extracellular viral particle when the viral particle was in close proximity to, but detached from, the axon (Fig. 6D).
The motor protein kinesin-1 is present on vesicles carrying HSV-1 tegument proteins and on enveloped capsids in varicosities and growth cones and on extracellular virions.
Kinesin-1 (KIF5) is a member of the kinesin superfamily of microtubule-associated motor proteins that are important for neurite polarization and outgrowth. Kinesin-1 is involved in the anterograde transport of organelles, vesicles, and various cargoes, such as GAP-43, in axons (13, 26). Given the importance of kinesin-1 in axonal transport, we sought to examine the localization of kinesin-1 with viral tegument proteins as well as viral particles in axons.
Label for kinesin-1 was found associated with tubulovesicular structures and large dense-cored vesicles carrying label for VP22 in mid axons, varicosities, and growth cones (Fig. 7A). Label for kinesin-1 was also seen in association with label for VP22 in mid axons in apposition to microtubules (data not shown). In particular, dense label for kinesin-1 was found decorating enveloped capsids located inside varicosities and growth cones as well as on extracellular virions adjacent to axons (Fig. 7B, C, and D) (Table 2).
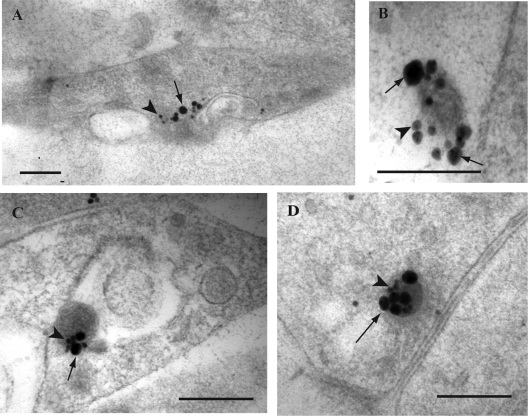
FIG. 7.
Dual immunogold labeling for kinesin-1 and tegument protein VP22 in HSV-1-infected mid and distal human axons. Ultrathin sections were simultaneously incubated with monoclonal antibody to kinesin-1 and rabbit polyclonal antibody for VP22 overnight. This was followed by sequential incubation with secondary antibodies conjugated with 2-nm gold particles and sequential silver enhancement to produce two different sizes of gold particles. Labels for kinesin-1 (large gold particles; arrows) and VP22 (small gold particles; arrowheads) were present together on the same tubulovesicular membrane structure in a varicosity (A), on an extracellular viral particle (B), and decorating enveloped viral capsids in a varicosity (C) and a growth cone (D). Bars, 200 nm.
DISCUSSION
In the present study, we continue our investigation into the mechanisms of transport of HSV-1 in axons of human DRG neurons. Using TIEM, we sought to investigate the nature and origin of the vesicles or carriers involved in the axonal transport of viral tegument and envelope proteins to the axon terminus and the possible mechanism(s) of virus assembly prior to exit from axons. The key findings of this study are: (i) HSV-1 tegument and envelope proteins can travel in axons independently of viral capsids; (ii) viral tegument proteins are transported either independently (on different vesicles) or together (on the same vesicle) with other tegument or envelope proteins; (iii) viral proteins are transported in mid axons to varicosities and growth cones in multiple types of vesicles or carriers, including pleiomorphic tubulovesicular structures and large clear- or dense-cored vesicles; (iv) these vesicles or carriers are derived from the TGN and contain key proteins involved in the secretory and exocytic pathways in axons, which include GAP-43, Rab3A, and SNAP-25; (v) viral capsids in varicosities and growth cones invaginate vesicles or membrane carriers containing viral tegument and envelope proteins, GAP-43, Rab3A, and SNAP-25; and (vi) the anterograde molecular motor kinesin-1 is present on these vesicles or carriers involved in the transport of viral proteins and those in which the viral capsids invaginate during final virus assembly in the growth cones.
In neurons, newly synthesized axonal proteins such as synaptic vesicle proteins or plasma membrane proteins have been shown to travel down axons to growth cones or synapses using membrane carriers derived from the TGN. These carriers include dense-cored vesicles, tubulovesicular membrane structures, and pleiomorphic vesicles (2, 39, 42, 54, 61). These membrane carriers have been shown to be heterogeneous in shape and size. Tubulovesicular membrane structures may vary in shape from spheres to tubules and can be up to 1.5 μm long, whereas dense-cored vesicles measure between 70 and 200 nm in diameter (35, 39).
This study shows that HSV-1 tegument and envelope proteins appear to travel along axons either independently from other tegument and envelope proteins in different large dense-cored vesicles or tubulovesicular membrane structures or together in the same vesicle or membrane carrier. In addition, our study shows that these large dense-cored vesicles and tubulovesicular membrane carriers associated with tegument and envelope proteins also carry TGN-46 protein, which is a resident membrane protein of the TGN. These results confirm that these vesicle carriers are derived from the TGN in the cell body and are consistent with the role that TGN plays in the sorting of viral proteins destined for the axon terminus. In addition, kinesin-1 protein was found associated with these large dense-cored vesicles and tubulovesicular structures carrying tegument and envelope proteins, indicating that kinesin-1 is probably involved in their transport. Further examination of the large dense-cored vesicles and tubulovesicular structures carrying these viral proteins revealed that they also contained key axonal proteins involved in the secretory and exocytic pathways in axons. These proteins include the synaptosomal-associated protein SNAP-25, the GTP binding protein Rab3A, and the growth cone-associated protein GAP-43 (10, 24, 25, 26, 28, 32, 39, 51, 52, 62).
SNAP-25 is a Q-SNARE protein, which localizes to the plasma membrane and plays multiple roles in membrane trafficking, axon elongation, and synaptogenesis (19, 32, 51, 52). SNARE proteins are a family of membrane-tethered coiled-coil proteins that regulate fusion reactions and target specificity in vesicle trafficking (34, 51, 52, 62). SNAP-25 plays a role in the docking and fusion of vesicles to the plasma membrane and together with synaptotagmin is essential for calcium-triggered exocytosis (51, 52, 62). Another key protein in the calcium-dependent regulated exocytosis of hormones and neurotransmitters in neurons is Rab3A (5, 10, 53). Rab3A is a member of a large set of GTP-binding proteins that regulate intracellular trafficking. Rab3A has been implicated in the regulation of membrane trafficking by controlling fusion and transport including transport and sequestration of secretory vesicles at the release site (5, 10, 53). In addition to SNAP-25 and Rab3A, GAP-43 has also been shown to be involved in the calcium-dependent fusion of synaptic vesicles (24). GAP-43 (also known as B-50, neuromodulin, and F1) is a neuron-specific protein, which localizes to axons and plasma membrane of growth cones and presynaptic terminals (21, 39, 58). GAP-43 has been shown to interact with the synaptic core complex, including SNAP-25, and to play an important role in the regulation of exocytosis and release of both small clear and large dense-cored vesicles (24, 25).
The secretion of neurotransmitters is mediated by the regulated exocytosis of two classes of vesicles, small clear synaptic vesicles and large dense-cored vesicles (52, 62). Small clear-cored vesicles store neurotransmitters such as glutamate, γ-amino-butyric acid, and acetylcholine. These vesicles are released at the active zones and undergo multiple cycles of exocytosis and endocytosis within the nerve terminal (25). On the other hand, large dense-cored vesicles, which contain catecholamines or neuropeptides, cannot be refilled after exocytosis and are therefore recycled via the TGN (25). The fusion of these vesicles with the plasma membrane occurs in response to increased calcium concentration and utilizes similar mechanisms in which SNARE proteins like SNAP-25 play a key function (34, 51, 53, 62). Our findings indicate that HSV-1 tegument and envelope proteins associate in axons mainly with large dense-cored vesicles. We found no evidence in this study that viral tegument or envelope proteins associated with the small clear synaptic vesicles.
The large dense-cored vesicles and tubulovesicular structures observed in axons and growth cones in this study, carrying HSV-1 tegument and envelope proteins, are similar in morphology and size to those already described for the transport of axonal, plasma membrane, and synaptic vesicle proteins such as GAP43, synaptophysin, and SNAP-25 in axons (16, 39, 54). Both types of membrane carriers were found to be derived from the TGN and to carry TGN-38 protein (mouse homolog of human TGN-46) (39, 54). In addition, these tubulovesicular membrane carriers have been shown to be transported by rapid axonal transport (up to 1 μm/s) (39), which is consistent with the colocalization of kinesin-1 on these vesicles. Most of the long-range intracellular transport occurs via the microtuble network. Transport of cargo along microtubules is driven by two families of motor proteins, kinesins and dyneins (22, 26). Kinesin-1 (also known as KIF5 or conventional kinesin) is a member of the kinesin superfamily. Most kinesin motor proteins, with a few exceptions, transport cargo toward the plus end of the microtubules. The transport of axonal proteins and presynaptic components are dependent on specific kinesin motors. Kinesin-1 is involved in the transport of organelles, vesicles, and various cargoes down axons (15, 22, 26). Kinesin-1 has been shown to interact with SNAP-25 (13) and to mediate the transport down axons of organelles containing GAP-43 (5, 26). Hence, our findings strongly suggest that HSV-1 utilizes the existing cellular pathways used for delivery of synaptic vesicle proteins and membrane proteins to the axon terminus or synapses and for the regulated exocytosis of neurotransmitters and hormones.
It is possible that viral proteins could be recycled and transported from varicosities and growth cones into late endosomes and multivesicular bodies (MVBs) by pleiomorphic vesicles, including tubulovesicular structures, which can be found in axons as part of the retrograde vesicular transport pathway (43, 60). However, the cellular markers used in this study have been previously shown to associate with the large vesicle secretory pathway (5, 10, 21, 24, 25, 34, 39, 51, 52, 53, 58, 62). In addition, TGN-46, SNAP-25, and GAP-43 do not usually traffic into late endosomes, lysosomes, or MVBs (4, 12, 55, 59). The presence of TGN-46, SNAP-25, and GAP-43 and to a lesser degree Rab3A is indicative of a TGN origin. The presence of all of these markers in association with viral proteins and viral particles is not consistent with a predominance of the viral proteins and viral particles being recycled in organelles such as lysosomes or MVBs. Furthermore, TGN-46 and kinesin-1 as well as the putative receptor for kinesin, amyloid precursor protein, have been reported to associate with VP16-GFP-labeled HSV-1 particles moving in the anterograde direction after injection into the giant axon of the squid (48). Thus, the complement of markers including TGN46, the presence of kinesin-1, and the size of the vesicles involved indicate that at least the vast majority of the viral proteins and viral particles in our study were associated with the anterograde vesicular transport pathway rather than with retrograde vesicular transport.
A major finding of this study is the association of TGN-46, GAP-43, SNAP-25, and kinesin-1 with fully or partially enveloped capsids in varicosities and growth cones and with extracellular viral particles. However, these cellular proteins were not identified in purified extracellular viruses analyzed by mass spectrometry (33). This discrepancy could be due to the different techniques and cell types used in our study and in that of Loret and colleagues (33). Both mass spectrometry and immunoelectron microscopy have technical limitations, and certain proteins due to their biochemical properties, low abundance, or accessibility may not be readily detected by either technique, especially when dealing with cellular proteins which are present in low abundance compared to viral proteins. It is also important to note that this study uses primary human neurons and that the cellular protein composition in viruses may differ according to the cell or region of the cell (e.g., axons) of origin (6, 47). Hence, viruses produced in neurons may differ from the viruses produced in cell lines such as HeLa and baby hamster kidney cells used in the Loret et al. study (33). Furthermore, some of the proteins identified in our study, such as GAP-43, SNAP-25, and Rab3A, are enriched in neurons and hence will not be expected to be present in the viruses produced from cells used in the study by Loret et al. (33).
Overall, our findings provide evidence supporting that fully enveloped viral capsids in growth cones and varicosities utilize the large vesicle pathway of exocytosis to exit the axons. These findings strongly suggest that unenveloped HSV-1 capsids invaginate large dense-cored vesicles and tubulovesicular organelles derived from the TGN carrying viral tegument and envelope proteins for envelopment in varicosities and growth cones. The incorporation of the cellular proteins TGN-46, SNAP-25, GAP-43, and kinesin-1 into enveloped capsids may be derived from the invaginating vesicle or tubulovesicular membrane although SNAP-25 and kinesin-1 (14) are already present on the surface of unenveloped capsids prior to envelopment. Hence, our findings give further support to the subassembly model of separate transport in axons of unenveloped HSV-1 capsids from envelope and tegument proteins with final virus assembly occurring in growth cones at the axon termini. The invagination of TGN-derived vesicles by tegument-coated capsids in growth cones and varicosities is probably analogous to the assembly and envelopment of viral capsids at the TGN in the cell body of the neuron. Further studies are required to determine whether all envelope and tegument proteins are conveyed in the same membrane vesicle carrier or whether there is heterogeneity with various combinations of envelope and tegument proteins. For successful virus assembly, all of the key tegument and envelope proteins need to be present on the vesicle into which the unenveloped capsid invaginates. Alternatively, the capsid could invaginate two or more vesicles to acquire a full complement of tegument and envelope proteins. If so, whether such vesicles fuse prior to or at the same time as the invagination of the capsid will need to be determined. Further studies will reveal whether some vesicles bearing an incomplete complement of viral proteins are present, whether unenveloped capsids invaginate into them, or whether they are incompetent for such final viral assembly.
Acknowledgments
We thank David McNab, MRC Virology Unit, Institute of Virology, United Kingdom, for excellent technical assistance with vUL37 GFP. We also thank Carol Robinson, Levina Dear, and Gayle Versace-Avis, Electron Microscope Laboratory, ICPMR, Westmead Hospital, for assistance with electron microscopy.
This project was supported by project grant no. 402457 to A. L. Cunningham, R. J. Diefenbach, and M. Miranda-Saksena from the National Health and Medical Research Council of Australia.
Footnotes
Published ahead of print on 28 January 2009.
REFERENCES
- 1.Aguado, F., E. Pozas, and J. Blasi. 1999. Colchicine administration in the rat central nervous system induces SNAP-25 expression. Neuroscience 93275-283. [DOI] [PubMed] [Google Scholar]
- 2.Ahmari, S. E., J. Buchanan, and S. J. Smith. 2000. Assembly of presynaptic active zones from cytoplasmic transport vesicles packets. Nat. Neurosci. 3445-451. [DOI] [PubMed] [Google Scholar]
- 3.Antinone, S. E., and G. A. Smith. 2006. Two modes of herpesvirus trafficking in neurons: membrane acquisition directs motion. J. Virol. 8011235-11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beuret, N., H. Stettler, A. Renold, J. Rutishauser, and M. Spiess. 2004. Expression of regulated secretory proteins is sufficient to generate granule-like structures in constitutively secreting cells. J. Biol. Chem. 27920242-20249. [DOI] [PubMed] [Google Scholar]
- 5.Bonanomi, D., F. Benfenati, and F. Valtorta. 2006. Protein sorting in the synaptic vesicle life cycle. Prog. Neurobiol. 80177-217. [DOI] [PubMed] [Google Scholar]
- 6.Cantin, R., S. Méthot, and M. J. Tremblay. 2005. Plunder and stowaways: incorporation of cellular proteins by enveloped viruses. J. Virol. 796577-6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ch'ng, T. H., and L. W. Enquist. 2005. Neuron-to-cell spread of pseudorabies virus in a compartmented neuronal culture system. J. Virol. 7910875-10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen, G. H., M. Ponce de Leon, H. Diggelmann, W. C. Lawrence, R. J. Vernon, and R. J. Eisenberg. 1980. Structural analysis of the capsid polypeptides of herpes simplex virus type 1 and 2. J. Virol. 34521-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coller, K. E., and G. A. Smith. 2008. Two viral kinases are required for sustained long-distance axon transport of a neuroinvasive herpesvirus. Traffic 91458-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darchen, F., and B. Goud. 2000. Multiple aspects of Rab protein action in the secretory pathway: focus on Rab3 and Rab6. Biochimie 82375-384. [DOI] [PubMed] [Google Scholar]
- 11.del Rio, T., T. H. Ch'ng, E. A. Flood, S. P. Gross, and L. W. Enquist. 2005. Heterogeneity of a fluorescent tegument component in single pseudorabies virus virions and enveloped axonal assemblies. J. Virol. 793903-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denny, J. B. 2006. Molecular mechanisms, biological actions, and neuropharmacology of the growth-associated protein GAP-43. Curr. Neuropharmacol. 4293-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diefenbach, R., E. Diefenbach, M. W. Douglas, and A. L. Cunningham. 2002. The heavy chain of conventional kinesin interacts with the SNARE proteins SNAP25 and SNAP23. Biochemistry 4114906-14915. [DOI] [PubMed] [Google Scholar]
- 14.Diefenbach, R., M. Miranda-Saksena, E. Diefenbach, D. J. Holland, R. A. Boadle, P. J. Armati, and A. L. Cunningham. 2002. Herpes simplex virus tegument US11 interacts with conventional kinesin heavy chain. J. Virol. 763282-3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diefenbach, R., M. Miranda-Saksena, M. W. Douglas, and A. L. Cunningham. 2008. Transport and egress of herpes simplex virus in neurons. Rev. Med. Virol. 1835-51. [DOI] [PubMed] [Google Scholar]
- 16.Duc, C., and S. Catsicas. 1995. Ultrastructural localization of SNAP-25 within the rat spinal cord and peripheral nervous system. J. Comp. Neurol. 356152-163. [DOI] [PubMed] [Google Scholar]
- 17.Elliott, G., G. Mouzakitis, and P. O'Hare. 1995. VP16 interacts via its activation domain with VP22, a tegument protein of herpes simplex virus, and is relocated to a novel macromolecular assembly in coexpressing cells. J. Virol. 697932-7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enquist, L. W., M. J. Tomishima, S. Gross, and G. A. Smith. 2002. Directional spread of an alpha-herpesvirus in the nervous system. Vet. Microbiol. 865-16. [DOI] [PubMed] [Google Scholar]
- 19.Fasshauer, D., R. B. Sutton, A. T. Brunger, and R. Jahn. 1998. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc. Natl. Acad. Sci. USA 9515781-15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feierbach, B., M. Bisher, J. Goodhouse, and L. W. Enquist. 2007. In vitro analysis of transneuronal spread of an alphaherpesvirus infection in peripheral nervous system neurons. J. Virol. 816846-6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frey, D., T. Laux, L. Xu, C. Schneider, and P. Caroni. 2000. Shared and unique roles of CAP23 and GAP43 in actin regulation, neurite outgrowth, and anatomical plasticity. J. Cell Biol. 1491443-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldstein, L. S., and Z. Yang. 2000. Microtubule-based transport systems in neurons: the roles of kinesins and dyneins. Annu. Rev. Neurosci. 2339-71. [DOI] [PubMed] [Google Scholar]
- 23.Grünewald, K., P. Desai, D. C. Winkler, J. B. Heymann, D. M. Belnap, W. Baumeister, and A. C. Steven. 2003. Three-dimensional structure of herpes simplex virus from cryo-electron tomography. Science 3021396-1398. [DOI] [PubMed] [Google Scholar]
- 24.Haruta, T., N. Takami, M. Ohmura, Y. Misumi, and Y. Ikehara. 1997. Ca2+ dependent interaction of the growth-associated protein GAP-43 with the synaptic core complex. Biochem. J. 325455-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hens, J. J., W. E. Ghijsen, U. Weller, H. A. Spierenburg, F. Boomsma, A. B. Oestreicher, F. H. Lopes da Silva, and P. N. De Graan. 1998. Anti-B-50 (GAP-43) antibodies decrease exocytosis of glutamate in permeated synaptosomes. Eur. J. Pharmacol. 363229-240. [DOI] [PubMed] [Google Scholar]
- 26.Hirokawa, N., and R. Takemura. 2005. Molecular motors and mechanisms of directional transport in neurons. Nat. Rev. Neurosci. 6201-214. [DOI] [PubMed] [Google Scholar]
- 27.Holland, D. J., M. Miranda-Saksena, R. A. Boadle, P. Armati, and A. L. Cunningham. 1999. Anterograde transport of herpes simplex virus proteins in axons of peripheral human foetal neurons: an immunoelectron microscopy study. J. Virol. 738503-8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jahn, R., and R. H. Scheller. 2006. SNAREs—engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 7631-643. [DOI] [PubMed] [Google Scholar]
- 29.LaVail, J. H., A. N. Tauscher, E. Aghaian, O. Harrabi, and S. S. Sidhu. 2003. Axonal transport and sorting of herpes simplex virus components in a mature mouse visual system. J. Virol. 776117-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LaVail, J. H., A. N. Tauscher, J. W. Hicks, O. Harrabi, G. T. Melroe, and D. M. Knipe. 2005. Genetic and molecular in vivo analysis of herpes simplex virus assembly in murine visual system neurons. J. Virol. 7911142-11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LaVail, J. H., A. N. Tauscher, A. Sucher, O. Harrabi, and R. Brandimarti. 2007. Viral regulation of the long distance axonal transport of herpes simplex virus nucleocapsid. Neuroscience 146974-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linial, M., and D. Parnas. 1996. Deciphering neuronal secretion: tools of the trade. Biochim. Biophys. Acta 1286117-152. [DOI] [PubMed] [Google Scholar]
- 33.Loret, S., G. Guay, and R. Lippé. 2008. Comprehensive characterization of extracellular herpes simplex virus type 1 virions. J. Virol. 828605-8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martens, S., and H. T. McMahon. 2008. Mechanisms of membrane fusion: disparate players and common principles. Nat. Rev. Mol. Cell Biol. 9543-556. [DOI] [PubMed] [Google Scholar]
- 35.Matteoli, M., S. Coco, U. Schenk, and C. Verderio. 2004. Vesicle turnover in developing neurons: how to build a presynaptic terminal. Trends Cell Biol. 14133-140. [DOI] [PubMed] [Google Scholar]
- 36.McLean, C., A. Buckmaster, D. Hancock, A. Buchan, A. Fuller, and A. Minson. 1982. Monoclonal antibodies to three non-glycosylated antigens of herpes simplex virus type 2. J. Gen. Virol. 63297-305. [DOI] [PubMed] [Google Scholar]
- 37.Miranda-Saksena, M., P. J. Armati, R. A. Boadle, D. J. Holland, and A. L. Cunningham. 2000. Anterograde transport of herpes simplex virus type 1 in cultured, dissociated human and rat dorsal root ganglion neurons. J. Virol. 741827-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miranda-Saksena, M., R. A. Boadle, P. J. Armati, and A. L. Cunningham. 2002. In rat dorsal root ganglion neurons, herpes simplex virus type 1 tegument forms in the cytoplasm of the cell body. J. Virol. 769934-9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakata, T., S. Terada, and N. Hirokawa. 1998. Visualization of the dynamics of synaptic vesicle and plasma membrane proteins in living axons. J. Cell Biol. 140659-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohara, P. T., M. S. Chin, and J. H. LaVail. 2000. The spread of herpes simplex virus type 1 from trigeminal neurons to the murine cornea: an immunoelectron microscopy study. J. Virol. 744776-4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohara, P. T., A. N. Tauscher, and J. H. LaVail. 2001. Two paths for dissemination of herpes simplex virus from infected trigeminal ganglion to the murine cornea. Brain Res. 899260-263. [DOI] [PubMed] [Google Scholar]
- 42.Okada, Y., H. Yamazaki, Y. Sekine-Aizawa, and N. Hirokawa. 1995. The neuron-specific kinesin superfamily protein KIF1A is a unique monomeric motor for anterograde axonal transport of synaptic vesicles precursors. Cell 81769-780. [DOI] [PubMed] [Google Scholar]
- 43.Overly, C. C., and P. J. Hollenbeck. 1996. Dynamic organization of endocytic pathways in axons of cultured sympathetic neurons. J. Neurosci. 166056-6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Penfold, M. E., P. Armati, and A. L. Cunningham. 1994. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc. Natl. Acad. Sci. USA 916529-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roizman, B., and D. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2460. In D. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 46.Saksena, M. M., H. Wakisaka, B. Tijono, R. A. Boadle, F. Rixon, H. Takahashi, and A. L. Cunningham. 2006. Herpes simplex virus type 1 accumulation, envelopment and exit in growth cones and varicosities in mid-distal regions of axons. J. Virol. 803592-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato, K., J. Aoki, N. Misawa, E. Daikoku, K. Sano, Y. Tanaka, and Y. Koyanagi. 2008. Modulation of human immunodeficiency virus type 1 infectivity through incorporation of tetraspanin proteins. J. Virol. 821021-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Satpute-Krishnan, P., J. A. DeGiorgis, and E. L. Bearer. 2003. Fast anterograde transport of herpes simplex virus: role for the amyloid precursor protein of Alzheimer's disease. Aging Cell 2305-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snyder, A., T. W. Wisner, and D. C. Johnson. 2006. Herpes simplex virus capsids are transported in neuronal axons without an envelope containing the viral glycoproteins. J. Virol. 8011165-11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snyder, A., B. Bruun, H. M. Browne, and D. C. Johnson. 2007. A herpes simplex virus gD-YFP fusion glycoprotein is transported separately from viral capsids in neuronal axons. J. Virol. 818337-8340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Söllner, T. H. 2003. Regulated exocytosis and SNARE function. Mol. Membr. Biol. 20209-220. [DOI] [PubMed] [Google Scholar]
- 52.Söllner, T. H., S. W. Whiteheart, M. Brunner, H. Erdjument-Bromage, S. Geromanos, P. Tempst, and J. E. Rothman. 1993. SNAP receptors implicated in vesicle targeting and fusion. Nature 362318-324. [DOI] [PubMed] [Google Scholar]
- 53.Sudhof, T. C. 2004. The synaptic vesicle cycle. Annu. Rev. Neurosci. 27509-547. [DOI] [PubMed] [Google Scholar]
- 54.Sytnyk, V., I. Leshchyns'ka, A. Dityatev, and M. Schachner. 2004. Trans-Golgi network delivery of synaptic proteins in synaptogenesis. J. Cell Sci. 117381-388. [DOI] [PubMed] [Google Scholar]
- 55.Tao-Cheng, J. H., J. Du, and C. J. McBain. 2000. Snap-25 is polarized to axons and abundant along the axolemma: an immunogold study of intact neurons. J. Neurocytol. 2967-77. [DOI] [PubMed] [Google Scholar]
- 56.Tomishima, M. J., and L. W. Enquist. 2001. A conserved alpha-herpesvirus protein necessary for axonal localization of viral membrane proteins. J. Cell Biol. 154741-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tomishima, M. J., and L. W. Enquist. 2002. In vivo egress of an alphaherpesvirus from axons. J. Virol. 768310-8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Lookeren Campagne, M., C. G. Dotti, E. R. A. Jap Tjoen San, A. J. Verkleu, W. H. Gispen, and A. B. Oestreicher. 1992. B-50/GAP-43 localization in polarized hippocampal neurons in vitro: an ultrastructural quantitative study. Neuroscience 5035-52. [DOI] [PubMed] [Google Scholar]
- 59.van Meel, E., and J. Klumperman. 2008. Imaging and imagination: understanding the endo-lysosomal system. Histochem. Cell Biol. 129253-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weible, M. N., II, and I. A. Hendry. 2004. What is the importance of multi-vesicular bodies in retrograde axonal transport in vivo? J. Neurobiol. 58230-243. [DOI] [PubMed] [Google Scholar]
- 61.Zhai, R. G., H. Vardinon-Friedman, C. Cases-Langhoff, B. Becker, E. D. Gundelfinger, N. E. Ziv, and C. C. Garner. 2001. Assembling the presynaptic active zone: a characterization of an active one precursor vesicle. Neuron 29131-143. [DOI] [PubMed] [Google Scholar]
- 62.Zhang, X., M. J. Kim-Miller, M. Fukuda, J. A. Kowalchyk, and T. F. J. Martin. 2002. Ca2+-dependent synaptotagmin binding to SNAP-25 is essential for Ca2+-triggered exocytosis. Neuron 34599-611. [DOI] [PubMed] [Google Scholar]
- 63.Zhou, Z. H., M. Dougherty, J. Jakana, J. He, F. J. Rixon, and W. Chiu. 2000. Seeing the herpesvirus capsid at 8.5 Å. Science 288877-880. [DOI] [PubMed] [Google Scholar]