Abstract
Human cytomegalovirus (HCMV), a member of the β subgroup of the family Herpesviridae, causes serious health problems worldwide. HCMV gene expression in host cells is a well-defined sequential process: immediate-early (IE) gene expression, early-gene expression, DNA replication, and late-gene expression. The most abundant IE gene, major IE (MIE) gene pre-mRNA, needs to be spliced before being exported to the cytoplasm for translation. In this study, the regulation of MIE gene splicing was investigated; in so doing, we found that polypyrimidine tract binding proteins (PTBs) strongly repressed MIE gene production in cotransfection assays. In addition, we discovered that the repressive effects of PTB could be rescued by splicing factor U2AF. Taken together, the results suggest that PTBs inhibit MIE gene splicing by competing with U2AF65 for binding to the polypyrimidine tract in pre-mRNA. In intron deletion mutation assays and RNA detection experiments (reverse transcription [RT]-PCR and real-time RT-PCR), we further observed that PTBs target all the introns of the MIE gene, especially intron 2, and affect gene splicing, which was reflected in the variation in the ratio of pre-mRNA to mRNA. Using transfection assays, we demonstrated that PTB knockdown cells induce a higher degree of MIE gene splicing/expression. Consistently, HCMV can produce more viral proteins and viral particles in PTB knockdown cells after infection. We conclude that PTB inhibits HCMV replication by interfering with MIE gene splicing through competition with U2AF for binding to the polypyrimidine tract in MIE gene introns.
Human cytomegalovirus (HCMV) is a leading cause of birth defects and transplantation failures, especially in individuals with compromised immunity (28). The viral genome is about 235 kbp long (which is variable among different strains or due to serial propagation in the laboratory in cell culture) and putatively encodes about 200 proteins that are produced sequentially (8, 10, 30, 51). In the presence of protein synthesis inhibitors, HCMV-infected cells express the first viral genes, i.e., the immediate-early (IE) genes. Among them, IE1 and IE2 are the most abundant, leading to their being named the major IE (MIE) genes (46). IE1 and IE2 encode two phosphorylated proteins, IE72 and IE86, respectively; these transcripts result from the differential splicing of the pre-mRNA. MIE genes consist of five exons and four introns (48). The first exon contains the initiation site but does not encode any amino acids. In order for the exons to fuse and produce IE1 and IE2, the introns must be spliced out of the pre-mRNA; the resultant genes share exons 2 and 3. In both transfection with the entire MIE gene construct and infection by HCMV in cell culture, IE1 is always produced at much higher levels than IE2 (an intriguing fact that is the basis for our interest in MIE gene splicing) at an early stage of infection. The virus must use the cellular splicing machinery, and viral-gene splicing must also be regulated by cellular splicing regulation factors.
In eukaryotic cells, mRNA maturation in the nucleus requires three major steps: capping, polyadenylation, and pre-mRNA splicing. The mature mRNA is exported to the cytoplasm for translation. Most transcripts of mammalian cells contain introns and exons, and the introns must be removed before the nuclear export of mRNA for translation. Alternative splicing of pre-mRNA is a common process in mammalian cells by which one gene can produce multiple gene products (23, 38). This is also true for some viral-gene expression, because viruses have a limited genome size and use cellular pre-mRNA-processing machinery. The CMV MIE gene can yield at least five distinct proteins by splicing: IE1, IE2, IE18, IE19, and IE55 (and possibly IE9). IE1 and IE2 share the same promoter, the MIE promoter, and the first three exons and introns. Their gene structures and regulation of gene expression have been intensively investigated (2, 16, 22, 48, 50, 52). However, the splicing regulation of MIE genes remains unclear, and studies of this topic are limited.
Pre-mRNA splicing takes place within a highly congregated site in the nucleus called the spliceosome, a large molecular complex composed of four small nuclear ribonucleoproteins (snRNPs) (U1, U2, U4/U6, and U5) and about 50 to 100 non-snRNP splicing factors (3, 13, 20, 25, 64). Many cellular factors are involved in RNA processing. snRNP complexes are essential to define exons and introns and to enhance both alternative splicing and polyadenylation; therefore, they are splicing enhancers (12, 24, 57). U2 auxiliary factor (U2AF) is essential in defining the splicing site by recognizing a polypyrimidine (Py) tract nonconsensus sequence near the 3′ splice site. Spliceosome assembly follows an ordered sequence of events that begins with the recognition of the 5′ splice site by the U1 snRNP and the binding of U2AF to the Py tract and the 3′ splice site. Human U2AF is a heterodimer composed of a 65-kDa subunit (U2AF65), which binds to the Py tract, and a 35-kDa subunit (U2AF35), which interacts with the AG dinucleotide at the 3′ splice site (56, 64). Assembly of U2AF with the pre-mRNA requires an interaction with the U1 snRNP and is important for the subsequent recruitment of the U2 snRNP to the spliceosome (29).
Conversely, most hnRNPs (heterogeneous nuclear ribonucleoproteins) are splicing repressors. Recently, Py tract binding protein (PTB), also called hnRNPI, has been widely studied. It has been reported to be involved in the repression of the pre-mRNA splicing of many important genes, including exons in actinin, tropomyosin, troponin, c-src, fibronectin, and FGF receptors 1 and 2 (26, 36, 41, 44). PTB represses gene splicing by competing in the Py tract in introns with U2AF65 and/or interacting with U2AF65 to inhibit the function of U2AF65 (44). The Py tract for binding with splicing factors in yeast consists of the same nucleotide sequence for all genes. However, in mammalian genes, the Py tract sequences are different for each individual intron (18). The idea that the procedures for viral-gene and cellular splicing might be the same is based on the fact that viruses use the cellular machinery for pre-mRNA splicing. The Py tracts in the individual introns of the viral genes should be different from each other, which provided a specific target for studies leading to the production of some small molecules that could specifically inhibit viral-gene splicing and production.
This study marks the first time that the effects of different PTB isoforms on MIE gene splicing, expression, and production have been investigated. In so doing, we found that PTBs are gene-splicing inhibitors, inhibiting HCMV MIE gene expression and hence viral-protein production, as well as blocking virus production by competing with U2AF, a splicing enhancer.
MATERIALS AND METHODS
Cell culture and viruses.
HEp-2 (ATCC) and 293-T (ATCC) cells and human foreskin fibroblasts (HFF) (ATCC) were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and 1% penicillin-streptomycin. To make the PTB knockdown (kdPTB) cell line, we first cotransfected 293-T cells with a lentiviral plasmid expressing short hairpin RNA (shRNA) against PTB (pLKO.1-puro-PTB; Sigma) and a packaging plasmid (pHR′8.2deltaR) (49) at an 8:1 ratio with the envelope plasmid (pCMV-VSV-G) (49). The transfection was performed using Metafectene Pro (Biontex, Martinsried/Planegg, Germany), according to the manufacturer's instructions. Seventy-two hours after transfection, the medium from the 293-T cells was collected and filtered (using a 0.45-μm syringe filter to remove any 293-T cells). The supernatant was used to infect HEp-2 cells, and 2 μg/ml of puromycin (Sigma) was added at 24 h postinfection (p.i.); the cells resistant to puromycin were selected, and the knockdown purity was detected by immunofluorescence using anti-PTB antibody (Abcam, Cambridge, MA). The cells were labeled HEp-2-kdPTB. A control cell line was set up using the same protocol in which pLKO.1-puro (Sigma) was used instead of pLKO.1-puro-PTB. These cells were labeled HEp-2-pLKO. The cell lines were maintained in minimal essential medium (MEM) with puromycin (1 μg/ml), which was then removed before the experiments. For immunohistochemical staining, cells were grown on round coverslips (Corning Glass, Inc., Corning, NY) in 24-well plates (BD Falcon; Becton Dickinson Labware, Lincoln Park, NJ). The HCMV Towne strain was obtained from the ATCC, and HCMV BADrUL131 was provided by T. Shenk (60).
Molecular cloning and plasmids.
To add green fluorescent protein (GFP) to the N termini of HCMV MIE-1 and -2 genes (IE1/2) based on an IE1/2-expressing plasmid, pSVH (from R. Stenberg) (47), and thereby generate pgfpSVH, we used overlapping PCR. This generated a GFP-IE overlapping fragment, which was subsequently ligated at the NdeI and ApaI sites of pSVH. The primers used were as follows: 5′-AAG TGT ATC ATA TGC-3′ and 5′-CTC GCC CTT GCT CAC CAT CGT GTC AAG GAC GGT GAC-3′ for IE1 containing a GFP C-terminal short fragment and 5′-GGA AGG GCC CTC GTC AGG ATT ATC AGG GTC CAT CTT TCT CTT GGC AGA GGA CTC CAT CTT GTA CAG CTC GTC CAT-3′ and 5′-GTC ACC GTC CTT GAC ACG ATG GTG AGC AAG GGC GAG-3′ for GFP containing an IE1 N-terminal short fragment. To construct GFP-tagged IE1 (pgfpIE1), we removed exon 5 from pgfpSVH. Intron deletion mutants (see Fig. 3A) were generated using pgfpIE1 as the original plasmid; different PCR or overlapping PCR fragment inserts were added at the ApaI and BglII sites. The primers used for the PCR were as follows: 5′-ATG AGT CAG GAG GAC GGA TAC TTA TAT GTG TTG TTA TCC TCC TCT AC-3′, 5′-GTA GAG GAG GAT AAC AAC ACA TAT AAG TAT CCG TCC TCC TGA CTC AT-3′, 5′-TAA AGA TTA ACT CTT GCA TGT GAG CGG GGC ATC GAG ATA GCG ATA A-3′, and 5′-TTA TCG CTA TCT CGA TGC CCC GCT CAC ATG CAA GAG TTA ATC TTT A-3′. The plasmids used in this study (pPTB1-X, pPTB2, and pPTB4-X) were provided by C. W. J. Smith of the University of Cambridge, Cambridge, United Kingdom (43, 44), and the expression of PTB genes was under the control of the CMV promoter. pBIU2AF (Bicistronic) expressing both U2AF65 and U2AF35 was a generous gift from M. Carmo-Fonseca of the Universidade de Lisboa, Lisbon, Portugal (32), and the U2AF65 gene expression was under the control of the CMV promoter.
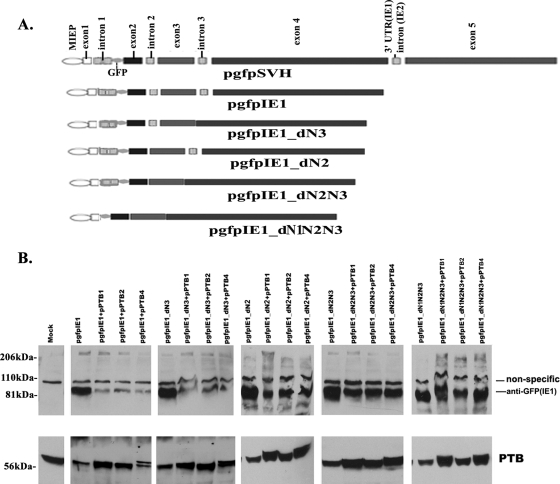
FIG. 3.
Effects of PTBs on different IE1 gene-expressing constructs. (A) Diagram describing the mutants made in this study. pgfpSVH (top) shows all the introns and exons of the MIE gene, as indicated. UTR, untranslated region. (B) Western blot to detect the production of IE1 and PTBs, as indicated. The samples are the whole-cell lysates made 24 h after transfection in HEp-2 cells. Above the IE1 band there is a nonspecific band, which can also be seen in the mock sample. The nonspecific band could be used as a sample-loading control.
To construct a PTB1 expression plasmid that could produce PTB in HEP-2-kdPTB cells, we mutated the nucleotide corresponding to the shRNA (sequence, 5′-CCG GCT CAA CGT CAA GTA CAA CAA TCT CGA GAT TGT TGT ACT TGA CGT TGA GTT TTT-3′) used for knocking down PTB in the cells, but the amino acid sequences were the same. pcDNA3 cut with ApaI and BamHI was used as a vector, and the fragment synthesized by overlapping PCR was recovered and inserted into the vector. The primers used for cloning were as follows: 5′-CGG GAT CCG ACT ACA AAG ACG ATG ACG ACA AGA TGG ACG GCA TTG TC-3′, 5′-CTT AAT GTA AAA TAT AAT AAC GAC AAG AGC CGT GAC-3′, 5′-GTT ATT ATA TTT TAC ATT AAG GCT GGT GAG CTT GGA-3′, and 5′-GCC TCG AGG AAT TCC TAG ATG GTG GAC TTG GA-3′. The generated plasmids were named pcDNA3PTB and pcDNA3PTB-reverse.
Transfection.
Transfection was performed using Metafectene Pro (Biontex, Martinsried/Planegg, Germany) according to the manufacturer's instructions. Briefly, the cells were washed with MEM (without serum and penicillin/streptomycin) when they were 75% confluent, and the DNA-lipid complexes formed at room temperature were added to the cells and incubated at 37°C in a CO2 incubator for 2 h. Finally, the supernatant of the cells was replaced with complete MEM (with 10% fetal bovine serum and 1% penicillin/streptomycin) and incubated at 37°C in a CO2 incubator overnight.
Coimmunoprecipitation (co-IP) assay.
Nuclear extracts were obtained essentially as described previously (54). Antibodies were coupled to protein G-Sepharose beads (Amersham Pharmacia Biotech AB, Uppsala, Sweden), according to the manufacturer's instructions. After being blocked with PBS-0.1% bovine serum albumin, the beads were incubated overnight at 4°C with clarified nuclear extracts, washed repeatedly in 0.1% bovine serum in buffered saline, and resuspended in a mixture of phosphate-buffered saline (PBS) and 2× Laemmli buffer. After being heated at 95°C for 5 min, the beads were isolated by centrifugation; proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and analyzed by Western blotting, as described below.
Immunohistochemistry to detect PTB.
HEp-2-kdPTB cells were seeded on coverslips and 24 h later were washed twice with PBS (PBS), fixed in 1% paraformaldehyde for 10 min at room temperature, washed twice with PBS, and permeabilized with 0.2% Triton X-100 on ice for 20 min. Primary antibody (anti-PTB; 1:500) was added and incubated for 30 min at room temperature. The cells were then washed twice with PBS. Either anti-rabbit or anti-mouse immunoglobulin G (IgG) secondary antibody labeled with Texas Red or fluorescein isothiocyanate (green) was added and incubated for an additional 30 min at room temperature. After a final wash with PBS, the cells were stained with Hoechst 33258. The cells were examined with a Leica TCS SPII confocal laser scanning system.
Immunoblot analysis.
Proteins were separated by sodium dodecyl sulfate (SDS)-7.5% polyacrylamide gel electrophoresis (10 to 20 μg loaded in each lane), transferred to nitrocellulose membranes (Amersham, Piscataway, NJ), and blocked with 5% nonfat milk for 60 min at room temperature. The membranes were incubated at room temperature for 1 h with primary antibody, followed by incubation with a horseradish peroxidase-coupled secondary antibody (Amersham) and detection by enhanced chemiluminescence (Pierce, Rockford, IL) according to standard methods. The membranes were stripped with stripping buffer (100 mM β-mercaptoethanol, 2% SDS, 62.5 mM Tris-HCl, pH 6.8), washed with PBS-0.1% Tween 20, and used to detect additional proteins. The following antibodies (and dilutions) were used: anti-IE1/2 (MAB810; Chemicon, Temecula, CA, 1:2,000), polyclonal antibody anti-HCMV IE1 (from H. Zhu at the university of Medicine and Density of New Jersey 1:2,000), monoclonal antibody anti-tubulin (Sigma-Aldrich, St. Louis, MO; 1:1,000), monoclonal antibodies anti-HCMV pp65 and anti-MCP (from W. Britt at Alabama University; 1:1,000), monoclonal antibody anti-GFP and anti-U2AF65 (MC3) (Santa Cruz Biotechnology, Santa Cruz, CA; 1:1,000); anti-PTB monoclonal antibody (Abcam, Cambridge, MA; 1:1,000), and anti-nPTB polyclonal antibody (from D. Black at the University of California—Los Angeles; 1:1,000). To quantitatively analyze the relative increase or decrease of HCMV proteins or PTB, we compared the intensities of the specific bands using densitometry (Quantity One 4.5.0 software; Bio-Rad Laboratories, Richmond, CA).
RNA isolation, treatment with DNase I (RNase free), reverse transcription (RT)-PCR, and real-time RT-PCR.
Total RNA was isolated using Tri reagent (Ambion, Inc., Austin, TX) and treated with DNase I (RNase free; Invitrogen catalog no. 18047-019) according to the manufacturer's instructions; the DNase I was inactivated by extraction of the RNA sample with phenol and chloroform. RT was carried out using a kit (Invitrogen, Carlsbad, CA) and a specific primer (pRev [5′-CAT CCT CCC ATC ATA TTA-3′]), according to the manufacturer's protocol. PCR was performed using three different forward primers and a reverse primer (pRev). The forward primers were 5′-ATG TCC TGG CAG AAC-3′ (pX3S, from the 3′ terminus of exon 3), 5′-GAC GTT CCT GCA GAC-3′ (pX3L, from the 5′ terminus of exon 3), and 5′-GAC CCT GAT AAT CCT-3′ (pX2, from the middle of exon 2).
To detect the mRNA levels in total-RNA samples from the pgfpIE1-transfected Hep-2-kdPTB and Hep-2-pLKO cells, real-time RT-PCR was undertaken using the QuantiTect SYBR Green RT-PCR kit (Qiagen, Valencia, CA). A total of 1 μg of total RNA and 0.2 μM of sense and antisense primers—pX3X4 (CCT CCA AGG TGC CAC GGC CCG A), which can amplify only cDNA as it transverses the two exons, exon 3 and exon 4, and pRev—were used in a final 25-μl master mixture volume. An RT step of 20 min at 50°C was included prior to PCR. The PCRs consisted of 50 cycles with optimal conditions as follows: 94°C for 20 s, 50°C for 1 min, 72°C for 30 s, and an optimized data collection step of 80°C for 5 s. Fluorescence captured at 80°C was determined to be lacking signal generated by primer dimers. All samples were run in triplicate; data were collected and recorded by iCycler iQ software (Bio-Rad) and expressed as a function of the threshold cycle (CT), which represented the number of cycles at which the fluorescence intensity of the SYBR green dye was significantly greater than the background fluorescence. The CT is directly correlated with the log10 copy number of the RNA standards. RNA copies were extrapolated from standard curves (CT versus the log10 copy number) representing at least seven-point serial dilutions of standard RNA (101 to 107 copies/μl). RNA standards were used as calibrators for the relative quantification of product generated in the exponential phase of the amplification curve for real-time RT-PCR. The results for standard curves with correlation coefficients greater than 0.95 were accepted. A melting-temperature curve analysis was obtained by measuring the fluorescence during a period of warming from 60°C to 95°C, after the amplification cycles.
Plaque assay.
To detect the viral growth curve, HEp-2-kdPTB or HEp-2-pLKO cells were infected with BADrUL131 virus (with a repaired UL131 open reading frame) at a multiplicity of infection of 1 PFU/cell. For infection in HEp-2 cells, a multiplicity of infection of 1 PFU/cell was needed because HEp-2 cell cultures could not be maintained for the extended period of time needed for an analysis of a low-multiplicity infection. Media and cells from infected cultures were collected on different days p.i., and virus was obtained by three freeze-thaw cycles of the collected culture. Virus titers were determined on HFF after the PFU was analyzed. Student's t test was used to statistically analyze the difference between the two groups; a P value of <0.05 indicated a significant difference.
Infection and transfection efficiency assays.
For detection of infection efficiency, cells were infected for 12 h with virus, washed with PBS, and stained with anti-IE1/2 antibody and secondary antibody-fluorescein isothiocyanate (FITC). For detection of transfection efficiency, cells were transfected with plasmid (pgfpIE1) and then trypsinized and suspended in PBS. The cells were then analyzed with a FACSCalibur system with two lasers and four channels (Becton Dickinson) to detect the total cell number and cells with fluorescence. Uninfected or nontransfected cells were prepared during the same experiments as background controls.
RESULTS
The splicing of pre-mRNA is regulated by different nuclear proteins, including PTBs (which are splicing inhibitors) and U2AFs (splicing enhancers). PTB proteins consist of four isoforms, PTB1, -2, and -4 and nPTB, which result from differential splicing. nPTB is more abundantly produced in neural cells (7, 34, 44, 45). PTB binds to single-stranded Py RNA, with a high preference for UCUU, CUCU, and UUCU in introns. U2AF consists of two subunits, U2AF65 and U2AF35. U2AF65 binds to Py, and U2AF35 binds to the 3′ splice site of the intron; the binding of U2AF35 to the 3′ end of the intron strengthens the binding of U2AF65 to Py (40). Both PTB and U2AF are abundant nuclear proteins. Their existence in the nucleus is critical in order to maintain the balance of cellular-gene expression. Since viruses use the cellular machinery for gene expression, it is reasonable to speculate that PTBs could repress the expression of viral genes that are to be spliced; however, this has never been tested on HCMV MIE genes.
Overexpression of PTB (PTB1, PTB2, or PTB4) inhibits the production of IE1/2.
In this study, we set out to test whether HCMV MIE genes could be regulated by gene-splicing regulators, i.e., PTBs. The HCMV MIE gene comprises four introns that need to be spliced before the MIE pre-mRNA is processed into mRNA and the mRNA is exported to the cytoplasm for translation. If the production of IE1/2 is affected negatively by the overexpression of PTB, MIE gene regulation at the splicing level would be suggested. We cotransfected an IE1/2-GFP (IE1/2 tagged with GFP in front of exon 2) plasmid (pgfpSVH), together with a PTB-expressing plasmid (PTB1-Xpress, PTB2, or PTB4-Xpress), into HEp-2 cells. A Western blot assay was used to detect the production of IE1/2 and PTB, using anti-GFP antibody to label IE1/2 proteins and anti-PTB antibody to label PTB proteins (Fig. 1, left). It was clear that IE1/2 production was strongly repressed when PTB was overexpressed. We could see two bands in both PTB1 and PTB4 because PTB1 and PTB4 were tagged with Xpress. To further demonstrate our observations, we repeated the cotransfection of pgfpSVH with different doses of PTB-expressing plasmid. We found that this inhibition was PTB plasmid DNA dose dependent (Fig. 1, right), suggesting that HCMV IE1/2 gene expression is regulated by splicing inhibitors. In the Western blot assay results, the nonspecific bands (which could also be seen in the “Mock” lanes) were kept for the control of sample loading. To quantitatively analyze the relative repression of IE1/2 by PTB or relative overexpression of PTB, we compared the intensities of the specific bands using densitometry (Quantity One 4.5.0 software; Bio-Rad Laboratories, Richmond, CA). First, we compared the intensities of the IE1/2 or PTB bands (in pgfpSVH with or without pPTB) with their own nonspecific bands for normalization. Then, the normalized IE1/2 (from pgfpSVH without PTB) was compared to normalized IE1/2 (from the cotransfected group); the ratio was the amount of repression of IE1/2 by PTB [ratio of IE1/2 from pgfpSVH alone to that from pgfpSVH and pPTBs = (intensity of IE1/2 in pgfpSVH without PTB/its nonspecific band)/(intensity of IE1/2 in pgfpSVH with PTB/its nonspecific band)]. Similarly, we obtained the amount of PTB overexpression [PTB overexpression = (intensity of PTB in pgfpSVH with pPTB/its nonspecific band)/(intensity of PTB in pgfpSVH without pPTB/its nonspecific band)].
FIG. 1.
Inhibition of IE1/2 production by Py tract binding proteins. (Left) An IE1/2-expressing plasmid, pgfpSVH, was transfected into HEp-2 cells in the absence or presence of three different types of PTB-expressing plasmid for 24 h. Western blotting was performed to detect IE1/2 (using anti-GFP antibody) and PTB (using anti-PTB antibody). (Right) pgfpSVH was transfected into HEp-2 cells together with different amounts of three different types of PTB-expressing plasmid for 24 h. Western blotting was performed to detect IE1/2 (using anti-GFP antibody) and PTB (using anti-PTB antibody). Quantitative analysis of IE1/2 and PTB by densitometry (Quantity One 4.5.0 software; Bio-Rad Laboratories, Richmond, CA) showed the following relationships: ratio of IE1/2 from pgfpSVH alone to that from pgfpSVH and pPTBs = (intensity of IE1/2 in pgfpSVH without PTB/its nonspecific band)/(intensity of IE1/2 in pgfpSVH with PTB/its nonspecific band) and ratio of PTB from pgfpSVH and pPTBs to that from pgfpSVH alone = (intensity of PTB in pgfpSVH with pPTB/its nonspecific band)/(intensity of PTB in pgfpSVH without pPTB/its nonspecific band).
The inhibition of HCMV IE1/2 production by PTB was rescued by U2AF65.
At least three different models have been proposed to explain the inhibitory effect of PTB on splicing. The first and most accepted model is that PTB simply competes with the splicing factor U2AF65 for binding to Py. A second possibility is that the repressed exons loop out as a result of the binding of PTB to flanking sites. Structural analysis of PTB bound to RNA suggests that PTB monomers can induce loops, but recent studies have indicated that repression by PTB involves more than just binding to RNA (19, 44, 45, 59, 62). A third model suggests that PTB can interact with U2AF65 so that U2AF65 can be isolated from binding to Py (39, 45). In order to determine which of these models fits with our observation that PTB inhibits HCMV IE1/2 gene expression, we cotransfected HEp-2 cells with U2AF and PTB, together with IE1/2 plasmids. A Western blot assay (Fig. 2A) showed that the cotransfection of the PTB1 plasmid with the U2AF expression plasmid could rescue the repression of IE1/2, which implies that the inhibitory effect on HCMV IE1/2 gene expression by PTB results from either competition or interaction with U2AF65. We noticed that there were two bands for U2AF65 when there was higher expression of IE1/2, which might have resulted from modification by IE1/2; we will further investigate this interesting observation.
FIG. 2.
The repressive effect of PTB can be rescued by U2AF. (A) The IE1/2-expressing plasmid pgfpSVH was transfected into HEp-2 cells with the DNA amount control plasmid pcDNA3 (lane 2 from left) or together with pPTB1 (lanes 3, 4, and 5) or pPTB1 and pU2AF65 (lane 6) for 24 h. Whole-cell lysates were collected, and Western blotting was performed to detect IE1/2 (using anti-GFP antibody), PTB (using anti-PTB antibody), U2AF65 (with anti-U2AF65 antibody), and tubulin (as a sample-loading control). (B) Co-IP. The nuclear extracts from HEp-2 cells were prepared and divided into two aliquots: one was treated with RNase, and the other was not treated. Antibodies (mouse anti-PTB and anti-U2AF65) and pre-immune mouse IgG were used to react with the nuclear extracts for 2 h, and then Sepharose-protein G (after being washed) was added to the reaction mixture for another 2 h at 4°C, and the samples were analyzed by Western blot (WB) assay with anti-PTB.
It has been reported that PTB interacts with U2AF65, and we wondered whether the interaction is mediated by RNA. An RNA-mediated interaction of PTB with U2AF65 could suggest that the activity of U2AF65 on the inhibitory effect of PTB on MIE gene expression is due to competition with PTB for the binding site in pre-mRNA rather than segregation of PTB by direct interaction. It can be detected by adding RNase to treat the nuclear extract both before and during the co-IP assay. As shown in Fig. 2B for the co-IP assay, we used both anti-PTB and anti-U2AF65 antibodies to pull down the nuclear extract of HEp-2 cells with (bottom) and without (top) RNase treatment. RNase activity was detected before the assays by running an agarose gel and checking it under UV light (not shown). First, anti-U2AF65 antibody could pull down both PTB and U2AF65, which represented an interaction between PTB and U2AF65. Second, no significant difference was seen between PTBs pulled down by anti-U2AF65 in the two groups of co-IP assays (with and without RNase treatment). Therefore, two possible mechanisms exist for U2AF65 to repress the inhibitory effect of PTB on MIE gene expression: competing with PTB for binding to the Py tract or segregating PTB from binding to the Py tract.
Intron 2 of the MIE gene is involved in the repressive effect of PTB on IE1 gene production.
If the repressive effects of PTB on MIE gene products are a result of competing with U2AF65, as we found (described above), it is highly likely that the cis elements for those PTBs that are on pre-mRNA are located in introns. We searched the sequences of the MIE introns and noticed that there were Py-like sequences (UCUC, UCCU, or CUCC) in all of them. Therefore, we wondered whether PTBs act on all the introns or on only some of them. The sequences between exons 4 and 5 contain both the 3′-terminal untranslated region of IE1 and the intron of IE2; it is difficult to interpret the activity of PTB on the sequence in this area. To simplify the experiments, we removed exon 5 and kept only the IE1 gene intact, resulting in pgfpIE1 (Fig. 3A), which has only three introns. Intron 1 is the most complicated because it overlaps with other open reading frames, such as UL124, UL125, and UL126. We mutated pgfpIE1 by removing intron 2 only (fusing exons 2 and 3; pgfpIE1_dN2), intron 3 only (fusing exon 3 with exon 4; pgfpIE1_dN3), both introns 2 and 3 (fusing exons 2, 3, and 4; pgfpIE1_dN2N3), and all three introns (fusing exons 1, 2, 3, and 4; pgfpIE1_dN1N2N3) (Fig. 3A).
After cotransfecting the IE1-expressing plasmids with the introns deleted with PTBs, we detected IE1 production using a Western blot assay. We observed that intron 3 was not necessary for the repression by PTB of IE1 production (Fig. 3B), since reductions in IE1 production by PTBs were the same in pgfpIE1_dN3 as in pgfpIE1. However, upon the removal of either intron 2 (alone) or both introns 2 and 3, we observed that the repressive effects of PTBs had been reduced; however, it should be noted that PTBs still reduced IE1 production. This information implies that intron 2 rather than intron 3 is important for the inhibitory effects of PTBs. Intron 1 could also be involved in the effects of PTBs on MIE gene splicing because PTBs lost their repressive effects on MIE gene expression when introns 1, 2, and 3 were all removed. Investigations to identify the minimum length of the sequences in introns that interact with PTB will also be undertaken. As before, the nonspecific band was employed for controlling sample loading.
MIE gene expression after transfection in PTB knockdown cell lines.
We have repeatedly observed that PTBs inhibit MIE gene production and that their effects on MIE gene production were accomplished through competing or interacting with U2AF65. These data were obtained from experiments dealing with the overexpression of PTBs by the cotransfection of plasmids. We wondered whether the results could be confirmed in cell cultures if we knocked down the PTB gene (Fig. 4).
FIG. 4.
IE1 gene expression in PTB knockdown cell lines. (A and B) Cells before (A) and after (B) purification. Cells were seeded on coverslips. After 24 h, the cells were washed twice with PBS, fixed in 1% paraformaldehyde for 10 min at room temperature, again washed twice with PBS, and permeabilized with 0.2% Triton X-100 on ice for 20 min. The primary antibody was added, and the cells were incubated for 30 min at room temperature and then (again) washed twice with PBS. Either anti-rabbit or anti-mouse IgG secondary antibody, labeled with FITC (green), for PTB or U2AF65 was added, and the cells were incubated for an additional 30 min at room temperature. (C) IE1 gene production in PTB knockdown cell lines after transfection. Western blotting was performed to detect the production of IE1 and PTBs, as indicated. The samples were the whole-cell lysates made 24 h after transfection in HEp-2 cells. Tubulin was used as a sample-loading control. (D) Transfection and infection efficiency assays. For detection of infection efficiency (right), cells (as indicated) were infected for 12 h with HCMV BADrUL131, washed with PBS, and stained with anti-IE1/2 antibody and secondary antibody-FITC. For detection of transfection efficiency (left), cells were transfected with the plasmid pgfpIE1, trypsinized, and suspended in PBS. The cells were then analyzed by FACSCalibur (Becton Dickinson) in order to detect the total number of cells and the total number of GFP/FITC-positive cells. Uninfected or nontransfected cells (Mock) were prepared in the same experiments as background controls.
First, we set up the knockdown cells from HEp-2 cells (for an unknown reason, setting up PTB knockdown cell lines failed in human fibroblasts) using an shRNA against all PTBs (PTB1, -2, and -4, but not nPTB). Using a lentivirus packaging system and a plasmid that expressed shRNA of PTB (see Materials and Methods), we successfully made lentiviruses that expressed shRNAs against PTBs; then, we infected the HEp-2 cells with the lentivirus and selected the cells that could grow in the presence of puromycin. We successfully generated PTB knockdown cell lines from HEp-2 cells. The PTB knockdown cell line in Fig. 4A (top) is shown before purification, with a purity of about 60 to 70%. After purification with limited cell dilution, a PTB knockdown cell line with 100% purity, HEp-2-kdPTB (shown in Fig. 4B, right), was obtained; DAPI (4′,6′-diamidino-2-phenylindole) (Fig. 4B, left) was used to show all the cells. To control the specificity of shRNA, we generated cell lines containing the empty retroviral vector in which the PTBs were at normal levels and which were also resistant to puromycin; we labeled them HEp-2-pLKO. To exclude any interfering effect of the puromycin used for the selection of cell lines, we removed it from the cells before the experiments. Furthermore, the comparison was between the two cell lines that were both resistant to puromycin. The knockdown cells were convenient, not just for the transfection assay, but also for the infection assay, because infection after transfection affects the infection efficiency, as we observed.
We transfected pgfpIE1 into HEp-2-kdPTB and HEp-2-pLKO cells to compare the IE1 production levels, as shown in Fig. 4C; Western blotting showed that IE1 production was higher in HEp-2-kdPTB than in HEp-2-pLKO cells. Meanwhile, we also observed that PTB could still be detected by Western blotting, even though it was not detectable by immunofluorescence.
To eliminate the possibility that the constructed (HEp-2-kdPTB) cells would have a higher infection or transfection efficiency than the control (HEp-2-pLKO) cells, we performed infection and transfection efficiency assays using FACSCalibur. We transfected pgfpIE1 into both types of cell overnight, and the cells were trypsinized and directly applied to detect total cell numbers and GFP-positive cell numbers, and the nontransfected cells were used as controls. For detection of infection efficiency, we infected cells with HCMV for 12 h, after which the cells were fixed and stained with anti-IE1/2 antibody and FITC-labeled secondary antibody. The cells were suspended in PBS and used to detect the total number of cells and the total number of FITC-positive cells; uninfected cells were used as controls. As can be seen in Fig. 4D, there was no significant difference between the infection and transfection efficiencies in the two cell lines. Therefore, our constructed cells were appropriate for use in the studies.
So far, we have demonstrated that PTBs have repressive effects on MIE gene products that are all at the protein production level. It is also necessary to demonstrate that PTBs have effects on MIE gene splicing. MIE gene splicing should be enhanced when the PTB gene is knocked down, which would result not only in higher gene expression and production, but also in a higher ratio of mRNA to pre-mRNA than in nonknockdown cells. RT-PCR and real-time RT-PCR were undertaken to test this speculation. First, we performed RT-PCR. The total RNA isolated from two different groups of cells (pgfpIE1-transfected HEp-2-kdPTB and pgfpIE1-transfected HEp-2-pLKO) was used to do the RT-PCR, using a specific primer (pREV) in exon 4. Then, the RT products were used for PCR, using the same reverse primer as was used for the RT-PCR; the forward primer was as indicated in Fig. 5B, top. For the RT-PCR assay, we designed three different pairs of primers, using the same reverse primer (which allowed the transverse of the introns). By doing so, we were able to amplify cDNA from both pre-mRNA and mRNA. Since the primers rest in different sites, the amplified cDNAs reverse transcribed from pre-mRNA and mRNA had different lengths. The designing, sites, and directions of the primers are indicated in the diagram (Fig. 5A). pgfpIE1 DNA was used as a positive control for the PCR, which produced bands of the same size as those of cDNAs from pre-mRNA. As shown in Fig. 5B, bottom, in which pIE1cDNAwas transfected into the two cell lines, the RT-PCR results showed that there were no differences in the production of cDNA. Taking the data together, we showed that the amount of IE1 mRNA was greater in HEp-2-kdPTB cells than it was in HEp-2-pLKO cells, which suggests that PTB is involved in the splicing of the IE1 gene.
FIG. 5.
Detection of IE1 gene expression at the RNA level. (A) Diagram of the IE1 gene containing exon 2 (X2), exon 3 (X3), and exon 4 (X4); introns are between the exons. The positions of primers pX2, pX3L, pX3S, pX3X4, and pRev are shown. (B) RT-PCR. Total RNAs from HEp-2-kdPTB (pgfpIE1-transfected) and HEp-2-pLKO (pgfpIE1-transfected) cells (top) or from HEp-2-kdPTB (pgfpIE1cDNA-transfected) and HEp-2-pLKO (pgfpIE1cDNA-transfected) cells (bottom), as described previously, were transcribed using a specific primer, pRev, with an RT kit (Invitrogen). The cDNAs were then amplified using different pairs of primers, as indicated. pgfpIE1 DNA was used as a control (as it produces a product the same size as the cDNAs from pre-mRNA), and the total RNA (treated with RNase-free DNase I) was used as a negative control for the PCR. The bands below single asterisks represent cDNA amplified from pre-mRNA; the bands above double asterisks represent the cDNA amplified from spliced RNA. (C) Real-time RT-PCR. Total RNA (1 μg) isolated from HEp-2-kdPTB (pgfpIE1-transfected) and HEp-2-pLKO (pgfpIE1-transfected) cells, as described previously, were analyzed by one-step real-time RT-PCR using the QuantiTect SYBR Green RT-PCR kit (Qiagen, Valencia, CA). Water was used as a negative control for subtraction of the background. The three curves in each sample represent triplicate experiments.
To further detect the effects of PTB on MIE gene expression at the mRNA level, we employed real-time RT-PCR, the method of choice in the quantitative detection of mRNA. The same amount of HEp-2-kdPTB or HEp-2-pLKO cells was seeded in a six-well plate and transfected with the same amount of pgfpIE1 when the cells were at 75% confluence. Total RNA was isolated at 20 h posttransfection. One microgram of the total RNA was used for real-time RT-PCR. The forward primer, pX3X4 (Fig. 5A) (5′-GTC CTG GCA GAA CTC GTC AA-3′, with the first 15 nucleotides from the end of exon 3 and the last 5 nucleotides from the beginning of exon 4), can amplify only cDNA from mRNA. As shown in Fig. 5C, the fluorescence absorption symbolizing the PCR products in HEp-2-kdPTB cells was approximately two to three times stronger than it was in HEp-2-pLKO cells, which is consistent with our observations of protein production by Western blot assay.
Knockdown of PTB enhances HCMV gene production and viral replication but has no effect on permissiveness.
Transfection systems can detect protein function only on specific cis elements; it still remained unclear whether the PTBs could affect HCMV infection in vitro. It was reasonable to speculate that PTB has a repressive effect on HCMV infection, because PTB interferes with HCMV IE1/2 gene splicing and represses IE1/2 production; to test this, we needed to infect the HEp-2-kdPTB and HEp-2-pLKO cell lines with HCMV. The laboratory strains of HCMV, including Towne, AD169, and Toledo, are not able to infect endothelial or epithelial cells (Fig. 6C); therefore, we used the repaired HCMV, vDW215-BADrUL131 virus (60, 61), in this study. By comparing viral-protein production levels using the Western blot assay, we showed that the knockdown of PTB could increase the production of different HCMV proteins (Fig. 6A). To detect viral replication, we infected the HEp-2-kdPTB and HEp-2-pLKO cell lines with HCMV, vDW215-BADrUL131 virus, at a multiplicity of infection of 1 for up to 8 days. Viruses were collected every day after infection. The collected virus samples were frozen and thawed three times, and the supernatants were analyzed by PFU assay in HFF. We observed a three- to sevenfold increase of viral particles by PFU assay (Fig. 6B) when PTB was knocked down. We also noticed in the viral growth curve that the viral titer went down after 6 days p.i., which might be because PTB knockdown cells died earlier after viral infection. However, the knockdown of PTB did not influence the permissiveness of epithelial cells regarding HCMV; the laboratory strains failed to infect HEp-2-kdPTB cells (Fig. 6C).
FIG. 6.
Effects of PTB on HCMV infection. (A) HEp-2-kdPTB and HEp-2-pLKO cell lines were infected with HCMV (vDW215-BADrUL131), and samples were collected at 24 h p.i. and at 72 h p.i. After an SDS-polyacrylamide gel electrophoresis gel was run, the proteins were transferred to a membrane for detection with specific antibodies (as shown on the right). Mock, not infected; 24 h p.i. and 72 h p.i., samples collected at 24 and 72 h p.i. Quantitative analysis of viral proteins by densitometry (Quantity One 4.5.0 software; Bio-Rad Laboratories, Richmond, CA) showed the following relationship: enhancement of HCMV proteins with knockdown of PTB = (intensity of viral proteins in HEp-2-kdPTB/corresponding tubulin)/(intensity of viral poteins in HEp-2-pLKO/corresponding tubulin). (B) Plaque assays. HEp-2-kdPTB and HEp-2-pLKO cell lines were infected with HCMV (vDW215-BADrUL131). The cells and medium were collected on different days p.i., as indicated. The viral particles were released from the cells by three cycles of freezing and thawing, and the virus particles in the supernatants were assayed by PFU in HFF. Each experiment was performed in triplicate, and the averages are shown on the curves with standard errors. (C) HCMV strain Towne was used to infect the cells, as indicated. The whole-cell lysates were obtained by Western blot assay to detect viral protein (IE1) and cellular protein (PTB). The nonspecific band at the top could be used as a sample-loading control.
Rescued expression of PTB resulted in repressive effects in PTB knockdown cells.
To demonstrate that the effects of PTB on MIE gene expression (shown in PTB knockdown cell lines) were specific to PTB, we constructed the PTB1 expression plasmid pcDNA3PTB-reverse, which could produce PTB in HEP-2-kdPTB cells, by mutating the nucleotides corresponding to the shRNA sequence (though the amino acid sequences remained the same) (Fig. 7A). We transfected the HEp-2-kdPTB cells with pIE1 (no GFP fusion), together with pcDNA3 (as a control), pcDNA3PTB, or pcDNA3PTB-reverse, and performed Western blotting to detect IE1 production in order to determine whether expression of PTB in the HEp-2-kdPTB cells could rescue the repressive effects of PTB on IE gene expression. As can be seen in Fig. 7B, pcDNA3PTB-reverse expressed PTB in the knockdown cells and pcDNA3PTB did not. In addition, it is also shown (lane 4) that rescued expression of PTB in HEp-2-kdPTB repressed IE gene expression.
FIG. 7.
Rescued PTB expression resulted in repressive effects of PTB in PTB knockdown cells. (A) Diagram of the mutation in pcDNA3PTB-reverse. Nucleotides were changed (original to modified), but the amino acid sequence (middle) was the same in the constructed pcDNA3PTB-reverse. (B) Western blot. Twenty hours after the transfection of pIE1 with pcDNA3PTB or pcDNA3PTB-reverse in HEp-2-kdPTB cell lines, the cell lysates were assayed by Western blotting and probed with antibodies as indicated.
DISCUSSION
The studies discussed in this article are the first to show that PTB represses HCMV MIE gene splicing, IE1/2 gene expression and production, and, subsequently, viral-protein production and replication. Since the MIE gene products IE1 and IE2 are both critical for HCMV replication, study of their regulation might provide a new clue leading to the design of a strategy to specifically target HCMV infection. This is the first step in our ongoing project to determine HCMV gene-splicing regulation. Previous studies by Adair et al. (1) found that HCMV infection can induce a response from cellular splicing factors, including PTB, and implied that PTB might be involved in the interaction of HCMV and cells. Other studies of PTB showed that it has positive effects on viral infection by activating the internal ribosome entry site (4, 42). Therefore, PTBs are a group of multifunctional proteins and are important in maintaining the equilibrium of gene regulation.
The fact that PTB has a clear repressive effect on HCMV replication (as shown in this study) and that the PTB level changes after HCMV infection (demonstrated by Adair et al. [1]) implies that PTB might be one of the factors activated during the cellular defense response to viral infection. In recent years, intrinsic defenses against viral infection have been extensively studied, and several different cellular proteins have been identified as defensive molecules. Proteins related to PML bodies congregate in the nuclei, and many viruses evolve measures to destroy these congregates in order to induce a productive infection; the proteins studied include PML, Daxx, SP100, and HP1 (6, 11, 14, 35, 37, 53-55). Histone deacetylases are a second group of gene expression suppressors that have inhibitory effects on CMV infection, which is evidenced by the fact that histone deacetylase inhibitors can enhance virus production (27, 31, 33, 54, 58). All the defensive cellular proteins investigated previously inhibit virus production, either by repressing gene expression or by inducing cell apoptosis. In this study, we report that PTBs negatively affect MIE gene expression, and hence HCMV replication, by interfering with splicing. Therefore, PTBs may be another type of defensive cellular protein, one that acts at the gene-splicing level.
Regulation of alternative splicing is mediated by RNA-binding proteins, including SR (serine/arginine-rich) and hnRNP family proteins (5). The hnRNPs have mostly been considered splicing inhibitors. PTB (also called hnRNPI) is a widely expressed protein (59) that has been found to be involved in much gene-splicing regulation (44). In nonneural cells, PTB exists in three different isoforms, PTB1, PTB2, and PTB4, all of which result from alternative splicing. They differ by the insertion of 19 to 26 amino acids (63). In addition to having repressive effects on splicing, PTB is also involved in 3′-end processing, internal initiation of translation, and RNA localization, in which PTBs exert positive effects (9, 15, 17, 21). We were curious whether PTB could also be involved in MIE gene-splicing regulation, since the MIE gene is essential for HCMV replication. A general cotransfection study (previously described) showed that PTB can strongly repress MIE gene expression. This study also demonstrated that all three PTB isoforms appear to have the same repressive effect (Fig. 1). Interestingly, this finding differs from what has been observed in other gene-splicing experiments, such as those done with α-tropomyosin (63), in which PTB4 had a stronger effect than either PTB1 or PTB2. Consistently, when PTB was knocked down, MIE gene expression was greatly promoted (Fig. 4, 5, and 6).
PTB is, in general, a splicing inhibitor and could affect the expression of many other cellular genes, implying that the effects of PTB on MIE gene expression might also be indirect. However, RT-PCR showed that PTB affected MIE gene splicing in that the ratio of mRNA to pre-mRNA was greater when PTB was depleted (Fig. 5). This is the first evidence supporting the idea that PTB might have a direct effect on MIE gene splicing.
In further studies, we deleted either intron 2 or 3, both introns 2 and 3, or all three introns in pgfpIE1. The mutation studies revealed that intron 2 was involved in interaction with PTB because the repressive effect was much less when both introns 2 and 3 or all three introns were removed, but not when only intron 3 was removed (Fig. 3). Taken together, our results support the conclusion that the effect of PTB on HMCV MIE gene splicing is a direct interaction between PTB and the cis elements in the pre-mRNA of the MIE gene. We are currently using an in vitro RNA-protein interaction assay and an in vitro splicing assay to further determine the interaction of PTB and U2AF65 with MIE gene introns.
Currently proposed models for gene-splicing suppression by PTB are threefold, as described in Results. Our results suggest that the activity of PTB on HCMV is through competition with U2AF65 for binding to Py, by the direct binding of PTB to U2AF65 to inhibit the binding of U2AF65 to Py, or both, since U2AF65 can rescue the repressive activity of PTB on IE1/2 production (Fig. 2).
In our studies, we also noticed that the repressive effects of PTBs on HCMV replication were not as dramatic as those on MIE gene expression. That could be because (i) the HEp-2-kdPTB cells can live for a limited time in cell culture after infection of HCMV and (ii) PTBs could have an active effect on other viral genes, since PTBs were found to activate the 3′ addition of poly(A) and the internal initiation of translation.
Two questions remain: could the ratio of IE1 to IE2 also result from alternative splicing, and are there any other genes besides the MIE gene (for example, UL36/UL37 or UL112/113) involved in regulation by PTB? A further study that is interesting to contemplate is the investigation of whether MIE gene splicing could be involved in HCMV latency and cross-species infection. In future studies, we will not only identify whether intron 1 could be involved in interaction with PTB, but also determine the minimal elements in the introns that interact with PTB. In addition, HCMV is an evolved pathogen and has adopted different measures against cellular defensive mechanisms; we will explore what kind of strategy is used by HCMV to evade the repressive effect of PTB.
Acknowledgments
This study was supported by the Research Center for Minority Institutes (RCMI) program (grant G12RR003050) of the Ponce School of Medicine and a startup fund from the Ponce School of Medicine to Q.T.
We thank W. Britt, D. Black, M. Carmo-Fonseca, R. Stenberg, G. Maul, T. Shenk, and H. Zhu for reagents. We acknowledge Bob Ritchie for English editing. We also thank Ann Campbell and Julie Kerry for their critical reviews of the article before publication and Richard J. Noel, Jr., Pablo Lopez Colon, and Omayra De Jesus Matos for technical assistance.
Footnotes
Published ahead of print on 14 January 2009.
REFERENCES
- 1.Adair, R., G. W. Liebisch, Y. Su, and A. M. Colberg-Poley. 2004. Alteration of cellular RNA splicing and polyadenylation machineries during productive human cytomegalovirus infection. J. Gen. Virol. 853541-3553. [DOI] [PubMed] [Google Scholar]
- 2.Awasthi, S., J. A. Isler, and J. C. Alwine. 2004. Analysis of splice variants of the immediate-early 1 region of human cytomegalovirus. J. Virol. 788191-8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battle, D. J., M. Kasim, J. Yong, F. Lotti, C. K. Lau, J. Mouaikel, Z. Zhang, K. Han, L. Wan, and G. Dreyfuss. 2006. The SMN complex: an assembly machine for RNPs. Cold Spring Harbor Symp. Quant. Biol. 71313-320. [DOI] [PubMed] [Google Scholar]
- 4.Bieleski, L., C. Hindley, and S. J. Talbot. 2004. A polypyrimidine tract facilitates the expression of Kaposi's sarcoma-associated herpesvirus vFLIP through an internal ribosome entry site. J. Gen. Virol. 85615-620. [DOI] [PubMed] [Google Scholar]
- 5.Caceres, J. F., and A. R. Kornblihtt. 2002. Alternative splicing: multiple control mechanisms and involvement in human disease. Trends Genet. 18186-193. [DOI] [PubMed] [Google Scholar]
- 6.Cantrell, S. R., and W. A. Bresnahan. 2006. Human cytomegalovirus (HCMV) UL82 gene product (pp71) relieves hDaxx-mediated repression of HCMV replication. J. Virol. 806188-6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, K. S., and G. Luo. 2006. The polypyrimidine tract-binding protein (PTB) is required for efficient replication of hepatitis C virus (HCV) RNA. Virus Res. 1151-8. [DOI] [PubMed] [Google Scholar]
- 8.Chee, M. S., A. T. Bankier, S. Beck, R. Bohni, C. M. Brown, R. Cerny, T. Horsnell, C. A. Hutchison III, T. Kouzarides, J. A. Martignetti, et al. 1990. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr. Top. Microbiol. Immunol. 154125-169. [DOI] [PubMed] [Google Scholar]
- 9.Cote, C. A., D. Gautreau, J. M. Denegre, T. L. Kress, N. A. Terry, and K. L. Mowry. 1999. A Xenopus protein related to hnRNP I has a role in cytoplasmic RNA localization. Mol. Cell 4431-437. [DOI] [PubMed] [Google Scholar]
- 10.Davison, A. J., A. Dolan, P. Akter, C. Addison, D. J. Dargan, D. J. Alcendor, D. J. McGeoch, and G. S. Hayward. 2003. The human cytomegalovirus genome revisited: comparison with the chimpanzee cytomegalovirus genome. J. Gen. Virol. 8417-28. [DOI] [PubMed] [Google Scholar]
- 11.Everett, R. D., S. Rechter, P. Papior, N. Tavalai, T. Stamminger, and A. Orr. 2006. PML contributes to a cellular mechanism of repression of herpes simplex virus type 1 infection that is inactivated by ICP0. J. Virol. 807995-8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu, X. D. 1995. The superfamily of arginine/serine-rich splicing factors. RNA 1663-680. [PMC free article] [PubMed] [Google Scholar]
- 13.Green, M. R. 1991. Biochemical mechanisms of constitutive and regulated pre-mRNA splicing. Annu. Rev. Cell Biol. 7559-599. [DOI] [PubMed] [Google Scholar]
- 14.Groves, I. J., and J. H. Sinclair. 2007. Knockdown of hDaxx in normally non-permissive undifferentiated cells does not permit human cytomegalovirus immediate-early gene expression. J. Gen. Virol. 882935-2940. [DOI] [PubMed] [Google Scholar]
- 15.Hunt, S. L., and R. J. Jackson. 1999. Polypyrimidine-tract binding protein (PTB) is necessary, but not sufficient, for efficient internal initiation of translation of human rhinovirus-2 RNA. RNA 5344-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isomura, H., M. F. Stinski, A. Kudoh, T. Daikoku, N. Shirata, and T. Tsurumi. 2005. Two Sp1/Sp3 binding sites in the major immediate-early proximal enhancer of human cytomegalovirus have a significant role in viral replication. J. Virol. 799597-9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaminski, A., S. L. Hunt, J. G. Patton, and R. J. Jackson. 1995. Direct evidence that polypyrimidine tract binding protein (PTB) is essential for internal initiation of translation of encephalomyocarditis virus RNA. RNA 1924-938. [PMC free article] [PubMed] [Google Scholar]
- 18.Kielkopf, C. L., S. Lucke, and M. R. Green. 2004. U2AF homology motifs: protein recognition in the RRM world. Genes Dev. 181513-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim, S. M., and Y. S. Jeong. 2006. Polypyrimidine tract-binding protein interacts with the 3′ stem-loop region of Japanese encephalitis virus negative-strand RNA. Virus Res. 115131-140. [DOI] [PubMed] [Google Scholar]
- 20.Kramer, A. 1996. The structure and function of proteins involved in mammalian pre-mRNA splicing. Annu. Rev. Biochem. 65367-409. [DOI] [PubMed] [Google Scholar]
- 21.Lou, H., D. M. Helfman, R. F. Gagel, and S. M. Berget. 1999. Polypyrimidine tract-binding protein positively regulates inclusion of an alternative 3′-terminal exon. Mol. Cell. Biol. 1978-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lukac, D. M., J. R. Manuppello, and J. C. Alwine. 1994. Transcriptional activation by the human cytomegalovirus immediate-early proteins: requirements for simple promoter structures and interactions with multiple components of the transcription complex. J. Virol. 685184-5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maniatis, T., and R. Reed. 2002. An extensive network of coupling among gene expression machines. Nature 416499-506. [DOI] [PubMed] [Google Scholar]
- 24.Manley, J. L., and R. Tacke. 1996. SR proteins and splicing control. Genes Dev. 101569-1579. [DOI] [PubMed] [Google Scholar]
- 25.Maquat, L. E., and G. G. Carmichael. 2001. Quality control of mRNA function. Cell 104173-176. [DOI] [PubMed] [Google Scholar]
- 26.Matlin, A. J., F. Clark, and C. W. Smith. 2005. Understanding alternative splicing: towards a cellular code. Nat. Rev. Mol. Cell Biol. 6386-398. [DOI] [PubMed] [Google Scholar]
- 27.Michaelis, M., T. A. Ha, H. W. Doerr, and J. Cinatl, Jr. 2007. Valproic acid interferes with antiviral treatment in human cytomegalovirus-infected endothelial cells. Cardiovasc.Res. 77544-550. [DOI] [PubMed] [Google Scholar]
- 28.Mocarski, E S., Jr., and C. T. Courcelle. 2001. Cytomegaloviruses and their replication, p. 2629-2673. In D. M. Knipe and P. M. Howley (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, PA.
- 29.Mollet, I., N. L. Barbosa-Morais, J. Andrade, and M. Carmo-Fonseca. 2006. Diversity of human U2AF splicing factors. FEBS J. 2734807-4816. [DOI] [PubMed] [Google Scholar]
- 30.Murphy, E., I. Rigoutsos, T. Shibuya, and T. E. Shenk. 2003. Reevaluation of human cytomegalovirus coding potential. Proc. Natl. Acad. Sci. USA 10013585-13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nevels, M., C. Paulus, and T. Shenk. 2004. Human cytomegalovirus immediate-early 1 protein facilitates viral replication by antagonizing histone deacetylation. Proc. Natl. Acad. Sci. USA 10117234-17239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pacheco, T. R., L. F. Moita, A. Q. Gomes, N. Hacohen, and M. Carmo-Fonseca. 2006. RNA interference knockdown of hU2AF35 impairs cell cycle progression and modulates alternative splicing of Cdc25 transcripts. Mol. Biol. Cell 174187-4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park, J. J., Y. E. Kim, H. T. Pham, E. T. Kim, Y. H. Chung, and J. H. Ahn. 2007. Functional interaction of the human cytomegalovirus IE2 protein with histone deacetylase 2 in infected human fibroblasts. J. Gen. Virol. 883214-3223. [DOI] [PubMed] [Google Scholar]
- 34.Petoukhov, M. V., T. P. Monie, F. H. Allain, S. Matthews, S. Curry, and D. I. Svergun. 2006. Conformation of polypyrimidine tract binding protein in solution. Structure 141021-1027. [DOI] [PubMed] [Google Scholar]
- 35.Preston, C. M., and M. J. Nicholl. 2006. Role of the cellular protein hDaxx in human cytomegalovirus immediate-early gene expression. J. Gen. Virol. 871113-1121. [DOI] [PubMed] [Google Scholar]
- 36.Robinson, F., and C. W. Smith. 2006. A splicing repressor domain in polypyrimidine tract-binding protein. J. Biol. Chem. 281800-806. [DOI] [PubMed] [Google Scholar]
- 37.Saffert, R. T., and R. F. Kalejta. 2006. Inactivating a cellular intrinsic immune defense mediated by Daxx is the mechanism through which the human cytomegalovirus pp71 protein stimulates viral immediate-early gene expression. J. Virol. 803863-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandri-Goldin, R. M. 2004. Viral regulation of mRNA export. J. Virol. 784389-4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sauliere, J., A. Sureau, A. Expert-Bezancon, and J. Marie. 2006. The polypyrimidine tract binding protein (PTB) represses splicing of exon 6B from the beta-tropomyosin pre-mRNA by directly interfering with the binding of the U2AF65 subunit. Mol. Cell. Biol. 268755-8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sickmier, E. A., K. E. Frato, and C. L. Kielkopf. 2006. Crystallization and preliminary X-ray analysis of a U2AF65 variant in complex with a polypyrimidine-tract analogue by use of protein engineering. Acta Crystallogr. F 62457-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith, C. W. 2005. Alternative splicing—when two's a crowd. Cell 1231-3. [DOI] [PubMed] [Google Scholar]
- 42.Song, Y., E. Tzima, K. Ochs, G. Bassili, H. Trusheim, M. Linder, K. T. Preissner, and M. Niepmann. 2005. Evidence for an RNA chaperone function of polypyrimidine tract-binding protein in picornavirus translation. RNA 111809-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spellman, R., M. Llorian, and C. W. Smith. 2007. Crossregulation and functional redundancy between the splicing regulator PTB and its paralogs nPTB and ROD1. Mol. Cell 27420-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spellman, R., A. Rideau, A. Matlin, C. Gooding, F. Robinson, N. McGlincy, S. N. Grellscheid, J. Southby, M. Wollerton, and C. W. Smith. 2005. Regulation of alternative splicing by PTB and associated factors. Biochem. Soc. Trans. 33457-460. [DOI] [PubMed] [Google Scholar]
- 45.Spellman, R., and C. W. Smith. 2006. Novel modes of splicing repression by PTB. Trends Biochem. Sci. 3173-76. [DOI] [PubMed] [Google Scholar]
- 46.Stenberg, R. M. 1996. The human cytomegalovirus major immediate-early gene. Intervirology 39343-349. [DOI] [PubMed] [Google Scholar]
- 47.Stenberg, R. M., J. Fortney, S. W. Barlow, B. P. Magrane, J. A. Nelson, and P. Ghazal. 1990. Promoter-specific trans activation and repression by human cytomegalovirus immediate-early proteins involves common and unique protein domains. J. Virol. 641556-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stenberg, R. M., D. R. Thomsen, and M. F. Stinski. 1984. Structural analysis of the major immediate early gene of human cytomegalovirus. J. Virol. 49190-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stewart, S. A., D. M. Dykxhoorn, D. Palliser, H. Mizuno, E. Y. Yu, D. S. An, D. M. Sabatini, I. S. Chen, W. C. Hahn, P. A. Sharp, R. A. Weinberg, and C. D. Novina. 2003. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA 9493-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stinski, M. F., and H. Isomura. 2007. Role of the cytomegalovirus major immediate early enhancer in acute infection and reactivation from latency. Med. Microbiol. Immunol. 197223-231. [DOI] [PubMed] [Google Scholar]
- 51.Stinski, M. F., E. S. Mocarski, and D. R. Thomsen. 1979. DNA of human cytomegalovirus: size heterogeneity and defectiveness resulting from serial undiluted passage. J. Virol. 31231-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stinski, M. F., D. R. Thomsen, R. M. Stenberg, and L. C. Goldstein. 1983. Organization and expression of the immediate early genes of human cytomegalovirus. J. Virol. 461-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang, Q., L. Li, A. M. Ishov, V. Revol, A. L. Epstein, and G. G. Maul. 2003. Determination of minimum herpes simplex virus type 1 components necessary to localize transcriptionally active DNA to ND10. J. Virol. 775821-5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang, Q., and G. G. Maul. 2003. Mouse cytomegalovirus immediate-early protein 1 binds with host cell repressors to relieve suppressive effects on viral transcription and replication during lytic infection. J. Virol. 771357-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tavalai, N., P. Papior, S. Rechter, and T. Stamminger. 2008. Nuclear domain 10 components promyelocytic leukemia protein and hDaxx independently contribute to an intrinsic antiviral defense against human cytomegalovirus infection. J. Virol. 82126-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valcarcel, J., R. K. Gaur, R. Singh, and M. R. Green. 1996. Interaction of U2AF65 RS region with pre-mRNA branch point and promotion of base pairing with U2 snRNA. Science 2731706-1709. [DOI] [PubMed] [Google Scholar]
- 57.Valcarcel, J., and M. R. Green. 1996. The SR protein family: pleiotropic functions in pre-mRNA splicing. Trends Biochem. Sci. 21296-301. [PubMed] [Google Scholar]
- 58.Vanniasinkam, T., H. Ertl, and Q. Tang. 2006. Trichostatin-A enhances adaptive immune responses to DNA vaccination. J. Clin. Virol. 36292-297. [DOI] [PubMed] [Google Scholar]
- 59.Wagner, E. J., and M. A. Garcia-Blanco. 2001. Polypyrimidine tract binding protein antagonizes exon definition. Mol. Cell. Biol. 213281-3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang, D., and T. Shenk. 2005. Human cytomegalovirus UL131 open reading frame is required for epithelial cell tropism. J. Virol. 7910330-10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang, D., and T. Shenk. 2005. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc. Natl. Acad. Sci. USA 10218153-18158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Waysbort, A., S. Bonnal, S. Audigier, J. P. Esteve, and A. C. Prats. 2001. Pyrimidine tract binding protein and La autoantigen interact differently with the 5′ untranslated regions of lentiviruses and oncoretrovirus mRNAs. FEBS Lett. 49054-58. [DOI] [PubMed] [Google Scholar]
- 63.Wollerton, M. C., C. Gooding, F. Robinson, E. C. Brown, R. J. Jackson, and C. W. Smith. 2001. Differential alternative splicing activity of isoforms of polypyrimidine tract binding protein (PTB). RNA 7819-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yong, J., L. Wan, and G. Dreyfuss. 2004. Why do cells need an assembly machine for RNA-protein complexes? Trends Cell Biol. 14226-232. [DOI] [PubMed] [Google Scholar]