Abstract
Human metapneumovirus (hMPV) is associated with respiratory tract infections among children and adults. Because hMPV induces significant morbidity and mortality in the elderly, a model of hMPV infection in aged BALB/c mice was established. Young (8 weeks old) and aged (18 months old) mice were intranasally inoculated with hMPV. The infected mice showed respiratory dysfunction, as measured by plethysmography, a marked loss in weight (up to 24%), and severe histopathological abnormalities including bronchiolitis obliterans organizing pneumonia. However, clinical severity was far higher in the aged mice, and none of the young infected mice died. Although virus replication in the lung was greater in the older mice, clearance of virus was not delayed compared to young mice. Production of virus-specific antibody as well as neutralizing antibody was lower. Gamma interferon and monocyte chemotactic protein-1 levels in bronchoalveolar lavage fluid were significantly lower in older mice, whereas interleukin-6 and interleukin-4 levels were significantly higher. We observed by flow cytometry a significant increase in the CD4+ T lymphocytes (P < 0.05) of the aged mice and no difference in CD8+ T-cell recruitment to the respiratory tract between the two groups. The present study investigated the effects of aging on the immunopathogenesis of hMPV infection and suggests that CD4+ T lymphocytes, the cytokine response, or a defect in humoral response may be associated with the increased disease severity observed in the aged mice.
Human metapneumovirus (hMPV) was first isolated in The Netherlands in 2001 (53) from nasopharyngeal aspirates of young children. hMPV has since been reported in many parts of the world and identified in patients of all ages. Two major genotypes of hMPV circulate during community outbreaks. More than 90% of children become infected before the age of 5, and the seroprevalence of hMPV-specific antibody in adults is nearly 100% (35, 53). hMPV causes both upper and lower respiratory tract diseases in infants and young children and is frequently associated with a clinical diagnosis of bronchiolitis in hospitalized children. Healthy middle-aged adults suffer from an influenza-like illness, whereas both asthma (58) and chronic obstructive pulmonary disease (15, 20, 40, 51) are exacerbated in immunocompromised adults. Moreover, the virus is a cause of prolonged, serious respiratory infections with associated mortality in adults with underlying disease or hematological malignancies (58) and following hematopoietic stem cell (8, 13, 27, 29, 41) or lung transplantation (33, 44, 48, 52). Few studies are available for elderly populations, but it has been shown that hMPV is a major causative agent of infections in this population (5, 25, 38). Elderly subjects frequently suffer from bronchitis and pneumonia, and hMPV is responsible for many hospitalized cases of respiratory infection in this population (3, 16, 24, 30). hMPV infections in the elderly with underlying disease are associated with high morbidity and mortality (5, 6, 9). In a group of six elderly institutionalized persons, three died during an outbreak of hMPV infection (6). Another study reported the death of two out of six elderly patients (44).
The reasons for the more severe clinical manifestations of hMPV infection in the elderly, however, have not yet been established. Although BALB/c mice have been used to study hMPV-induced pathogenesis (1, 10, 21, 39), there are currently no animal models to study the effect of aging on hMPV infection. In this study, we sought to characterize the age-related aspects of the clinical manifestations including pulmonary inflammation and airway obstruction following hMPV infection in aged (18 months old) BALB/c mice. Our results showed that aged mice exhibited more severe clinical disease than young mice and that disease was accompanied by a deficit in the humoral response and an increase in CD4+ T lymphocytes in bronchoalveolar lavage (BAL) fluid. The study of the response of aged mice to viral infection is a key issue to understand the pathogenesis of hMPV infection in the elderly.
MATERIALS AND METHODS
Mice.
Experiments were performed in two groups of specific-pathogen-free female BALB/c mice: 6- to 8-week-old mice (n = 74) and 18- to 19-month-old mice (n = 77). Mice were purchased from Charles River (France), and the mice intended for the aged mouse group were housed in a certified pathogen-free facility at the University of Dijon until the age of 18 to 19 months. Throughout the study, mice were allowed access to food and water ad libitum. This work was approved by the local ethics committee of Burgundy University, France.
Cells and virus.
LLC-MK2 cells were maintained in Eagles' minimal essential medium (Gibco/BRL) supplemented with 2 mM l-glutamine, 2 mg/ml sodium bicarbonate, 102 U of penicillin per liter, 0.1 mg of streptomycin per liter, 1% nonessential amino acids, and 5% fetal calf serum. The hMPV strain C4-CJP05, a subgroup A2 virus, was a clinical strain isolated in our laboratory that was passed through LLC-MK2 cells in Eagles' minimal essential medium containing 0.3% bovine serum albumin (BSA) and 0.025% trypsin (infection medium) nine times.
At full cytopathic effect, the cell monolayer was disrupted with sterile glass beads, and the resulting cell suspension was divided into aliquots, snap frozen, and stored at −80°C until required. Uninfected LLC-MK2 cell lysate was prepared similarly to the virus preparation to be used as the mock suspension.
Experimental model.
At the time of infection, the virus and mock suspensions were rapidly thawed at room temperature and immediately inoculated into the mice. Both the young and aged mice were divided into two main groups: (i) hMPV-infected mice challenged intranasally with 1 × 106 PFU/mouse of hMPV on day zero, and (ii) mock-infected mice inoculated with an uninfected cell preparation prepared as described above. Before inoculation, both infected and mock-infected mice were anesthetized by intraperitoneal administration of a mixture of 80 mg of ketamine/kg (Imalgene; Merial, Lyon, France) and 16 mg of xylazine/kg (Rompun; Bayer, Puteaux, France) of body weight, diluted in phosphate-buffered saline (PBS) to a final volume of 200 μl/mouse. The mice were weighed daily and observed for signs of illness. Blood was collected by retroorbital puncture on days 0, 6, 14, and 25 postinoculation (p.i.). Airway obstruction (AO) was measured daily from day 0 to day 22 p.i. At the indicated time points described below, the mice were killed by pentothal injection as follows: on days 3, 5, 7, and 9 p.i. to assess the level of viral replication; and on days 2, 6, 14, and 25 p.i. to measure the quantity of cytokine in BAL fluids, to determine and analyze cell infiltration in BAL fluid, or for lung histopathological analysis.
Illness severity score, body weight, and temperature.
Clinical illness was scored daily by a blinded observer using a grading system as follows: 0, healthy; 0.5, huddling; 0.5, reduced activity; 1, inactive; 1, ruffled fur; 2, emaciated; 6, dead. In addition, body weight and temperature were measured daily to monitor the progression of disease. Temperature for the four groups of mice was measured using a rectal thermometer. Every day, the mean temperature of the mock-infected mice was considered the reference for interpretation of the results.
hMPV quantification and virus titration in lungs.
LLC-MK2 cells were seeded into 24-well plates 24 h prior to titration. Immediately before titration, the medium was aspirated from the cells, which were then washed with PBS before inoculation with serial 10-fold dilutions of the virus in the infection medium. The plates were incubated at 37°C for 2 h. The inoculum was subsequently removed, and the cells were covered with infection medium containing 0.5% agarose. The virus infection was left to propagate for 3 days. Detection of hMPV cytopathic plaques by immunostaining was as follows: the agarose was removed, and the cells were fixed in cold methanol containing 10% acetone (between each step, the cells were washed with PBS). A human anti-hMPV serum diluted to 1:100 in PBS was added to the cells, and the plates were incubated at 37°C for 30 min. The cells were then incubated with a horseradish peroxidase-labeled anti-human immunoglobulin G (IgG) diluted to 1:500 (Southern Biotechnology Associates, Birmingham, AL) at 37°C for 30 min before the addition of an aminoethylcarbazole substrate for peroxidase (Vector Laboratories, Burlingame, CA). They were then left for 10 min at room temperature. The plates were finally rinsed with water. Cytopathic plaques were counted after aminoethylcarbazole staining. To quantify virus replication, the lungs were individually homogenized with 1-mm glass beads in a Mini-BeadBeater homogenizer (Biospec Products). The suspension was centrifuged at 10,000 × g for 1 min at 4°C, and the resulting supernatant was titrated as described above. Virus titer was expressed as PFU/g of lung. The sensitivity of this plaque assay is ∼66 PFU/g of lung.
Detection of hMPV-specific IgG, IgG1, and IgG2a antibodies in serum by ELISA.
Wells of Maxisorp plates (Nunc, Denmark) were coated with hMPV-infected or uninfected LLC-MK2 cells. The plates were blocked with PBS containing 10% milk. Serial dilutions of serum (starting dilution of 1:100) in PBS with 5% BSA were incubated at 37°C for 1 h and then added to the wells. Total IgG, IgG1, and IgG2a were detected by the subsequent addition of biotinylated goat anti-mouse antibody (anti-IgG, 100 ng/ml; anti-IgG1, 50 ng/ml; anti-IgG2a, 100 ng/ml) (Southern Biotechnology Associates, Birmingham, AL), followed by neutralite avidin-horseradish peroxidase (Southern Biotechnology Associates). Color was developed by adding Sure Blue TMB (tetramethylrhodamine isothiocyanate) peroxidase substrate (KPL, Gaithersburg, MD), and the optical density (OD) was read at 450 nm. Titers in enzyme-linked immunosorbent assays (ELISAs) were expressed as the reciprocal of the final dilution with an OD of 0.2, which was at least twice that of the negative control.
Neutralizing antibodies.
Neutralizing antibody responses in serum of hMPV- and mock-infected mice collected at days 0, 14, and 25 after the challenge were assessed using a 50% cytopathic plaque reduction assay. hMPV (2 × 102 PFU/ml) was mixed 1:1 (vol/vol) with serial twofold dilutions of serum in the infection medium. Reaction mixtures were incubated for 1 h at 37°C and added to LLC-MK2 cells in 24-well plates for 2 h at 37°C. The cells were subsequently covered with infection medium containing 0.5% agarose, and the virus infection was left to propagate for 3 days. Detection of hMPV cytopathic plaques was performed using the same method of immunostaining as for virus titration. The neutralizing antibody titers were calculated as the reciprocal of the highest serum dilution that inhibited 50% of virus plaques (relative to the virus control). This assay was performed in duplicate.
BAL.
Lavage of the airways was performed twice via a trachea cannula with 1 ml of PBS. The resulting fluid was immediately centrifuged (at 500 × g for 5 min) (56). Supernatants were removed and stored at −80°C for cytokine quantification, and pellets of BAL cells were resuspended in 500 μl of RPMI medium.
Quantification of leukocytes in BAL fluid.
BAL cells were pelleted onto glass slides (Cytospin; ThermoShandon, Pittsburgh, PA) by cytocentrifugation (400 × g for 4 min at low speed). Specific cell populations were distinguished using eosin methylene blue (RAL555 kit; RAL Reagents, Martillac, France). A minimum of 200 cells/slide were counted.
Quantification of cytokines in BAL fluid.
Interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), and soluble TNF receptor 1 (sTNF-R1) were detected simultaneously using a commercial DuoSet ELISA Development System (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. The lower limits of detection were 15 pg/ml, 60 pg/ml, and 8 pg/ml, respectively. Monocyte chemotactic protein-1 (MCP-1) was detected with BD OptEIA Set Mouse MCP-1 (BD Biosciences, San Diego, CA) with a limit of detection of 15 pg/ml. IL-4 and gamma interferon (IFN-γ) were quantified by sandwich ELISA using commercially available antibodies (Becton Dickinson, San Jose, CA) and avidin-peroxidase (SIGMA-Aldrich, Steinheim, Germany), in accordance with the manufacturer's protocol. Color was developed by adding Sure Blue TMB peroxidase substrate (KPL, Gaithersburg, MD), and the OD was read at 450 nm. Detection limits were 80 pg/ml for IFN-γ and 15 pg/ml for IL-4. All concentrations of cytokines were calculated from a standard curve by using recombinant mouse protein as a standard.
Flow cytometric analysis of cell surface and intracellular antigens.
Single-cell suspensions of BAL cells (0.5 × 106) were surface stained for 30 min at 4°C with anti-CD3-fluorescein isothiocyanate (FITC) or anti-CD45-FITC (BD PharMingen, Le pont de Claix, France). Samples were then washed with PBS containing 1% BSA and 0.1% sodium azide. Lymphocytes were identified by their forward and side scatter properties. Data were analyzed on a Beckman Coulter flow cytometer on 40,000 events per sample.
To detect intracellular cytokines, freshly isolated cells (0.5 × 106) were stimulated for 4 h at 37°C with 50 ng/ml phorbol myristate acetate (Sigma, Steinheim, Germany), 500 ng/ml ionomycin (Sigma, Steinheim, Germany), and 2 μl/3 ml monensin (Golgi stop; BD PharMingen). The cells were then washed with buffer (PBS containing 1% BSA and 0.1% sodium azide), incubated for 30 min at 4°C in buffer with rat anti-mouse CD16/CD32 antibodies (Fc Block; BD PharMingen). Cells were then fixed and permeabilized with Cytofix/Cytoperm (BD PharMingen) for 20 min at 4°C and washed in buffer. Single-cell suspensions of BAL cells (0.5 × 106) were then incubated for 30 min at 4°C with the following antibody combinations: (i) anti-CD4-FITC (clone RMA4-5), anti-CD8-biotine-ECD (energy-coupled dye; clone 53-6.7), and anti-IFN-γ-phycoerythrin (PE) (clone XMG1.2; BD PharMingen); (ii) anti-CD4-FITC (clone RMA4-5), anti-CD8-biotine-ECD (clone 53-6.7), and anti-IL-4-PE (clone 11B1; BD PharMingen); (iii) anti-CD3-FITC (clone 17A2), anti-CD49b/pan-NK-biotine (clone DX5), and anti-IFN-γ-PE (clone XMG1.2; BD PharMingen). For group iii, 2 μl of streptavidin-ECD (Beckman-Coulter) was added, and the mixture was left in the dark at room temperature for 15 min. Conjugated isotype-matched control antibodies were used to define background gates.
Determination of AO.
Whole-body, unrestrained plethysmography (Buxco Electronics Inc., Sharon, CT) was used to monitor the respiratory dynamics (the enhanced pause, or Penh value) of mice in a quantitative manner. Penh, as measured by plethysmography, has already been validated in animal models of infection-associated AO (22). The mice were allowed to acclimatize to the plethysmograph chamber, and then baseline readings were recorded to determine AO. Airway function was evaluated daily until day 22 p.i. for hMPV- and mock-infected mice.
Pulmonary histopathology.
Lung tissue of mice from both hMPV- and mock-infected groups was fixed in 4% buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Histopathology was assessed on days 2, 6, 14, and 25 p.i. by a pathologist in a blind test. Each section was graded from 0 to 3 for six parameters: bronchitis, peribronchiolar and bronchial infiltrates of lymphocytes, perivascular infiltrates of lymphocytes, parenchymal pneumonia (presence of neutrophils and monocytes), alveolitis (inflammatory cells within alveolar spaces), and organizing pneumonia (presence of polypoid fibroblastic and collagenic tissue in alveolar duct and alveoli). A cumulative score was then calculated for each mouse (the greater the value, the greater the inflammatory changes in the lung). Masson's trichrome stain was used to visualize collagen deposition in hMPV- and mock-infected mice.
Statistical analysis.
Data are presented as means ± standard errors of the means (SEM). Mean values were compared by one-way analysis of variance and multiple comparison tests (Scheffe) in order to determine significant differences between the groups at the same time point. Bartlett's test was used to assess the homogeneity of variance. A nonparametric test (Kruskal-Wallis) was performed when the data were not normally distributed, when the variance was heterogeneous, or when the sample size was too small. A P value of <0.05 was considered significant. This initial threshold was increased to take into account the number of tests performed. All analyses were performed with STATA software (version 8). The recovery phase estimated by weight changes from day 10 to day 15 p.i. was compared across hMPV-infected groups by applying generalized estimating equations (GEEs) with an exchangeable correlation matrix and a robust variance estimator (26).
RESULTS
Disease. (i) Weight.
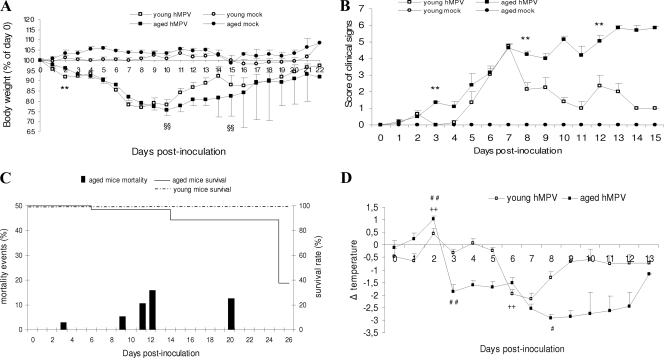
On day 2 p.i., young hMPV-infected mice started to lose weight (young mice, −8.1%; aged mice, −4%; P < 0.01); then both groups of infected mice lost weight to a greater extent: up to −24% around days 8 to 10 p.i. However, the young mice started to recover earlier than the surviving aged mice (P < 0.01) (Fig. 1A).
FIG. 1.
Kinetics of signs of disease after hMPV infection in aged and young mice. (A) Weight curves. Weight was calculated as a percentage of the starting weight (100%). **, P < 0.01, young versus aged hMPV-infected mice; §§, difference in recovery phase of the two groups of hMPV-infected mice estimated by applying GEEs on weight changes from days 10 and 15 p.i. (B) Clinical score evaluated with the following parameters: ruffled fur, reduced activity, huddling, emaciation, death. **, P < 0.01, young versus aged hMPV-infected mice. (C) Survival and mortality rate in hMPV-infected mice. (D) Evolution of temperature of hMPV-infected BALB/c mice, with the temperature of mock-infected mice used as a reference to reduce biases due to circadian rhythms. For aged hMPV-infected mice, significant temperature change is indicated as follows: #, P < 0.05 for day 8 versus day 3; # #, P < 0.01 for day 2 versus day 0 and day 3 versus day 0. For young hMPV-infected mice significant temperature change is indicated as follows: ++, P < 0.01 for day 2 versus day 0 and day 6 versus day 0. Data are shown as the mean ± SEM.
(ii) Clinical signs.
A cohort was observed for signs of disease such as ruffled fur, tendency to huddle, or being less active, inactive, hunched and emaciated, or dead. As early as day 2 p.i., infected mice exhibited ruffled fur and a tendency to huddle. The clinical signs score (CSS) of aged mice then increased at day 3 (1.33 compared to 0 for the young; P < 0.01); then the CSS of both groups of infected mice increased to reach a value of 4.7 at day 7 p.i. (Fig. 1B). Afterwards, while the CSS of the young mice decreased (P < 0.01 at day 8 p.i.) and was followed by recovery, the CSS of the aged mice increased. The majority of deaths occurred at days 11, 12, and 20 p.i., and most of the aged mice were dead at day 25 p.i. (62.5%, i.e., 5/8) (Fig. 1C). None of the young hMPV-infected mice died during the study. None of the mock-infected mice showed signs of illness or died.
(iii) Rectal temperature.
To further investigate the clinical manifestations, we measured rectal temperature daily (Fig. 1D). No statistical difference was observed at day 0 among the four groups of mice. Intranasal inoculation of hMPV induced fever at day 2 p.i. in the aged mice (+1.03 ± 0.13 °C) and young mice (+0.45 ± 0.21°C; P < 0.01, compared to temperature before infection). By day 3 p.i., the rectal temperature of the aged mice decreased dramatically to reach a value as low as −2.9 ± 0.13°C (P < 0.01) at day 8. The temperature returned to normal levels only at day 13 p.i. (measured on a unique surviving aged mouse). In contrast, hypothermia in the young mice lasted 2 only days, days 6 and 7 p.i., and their temperature had returned to normal by day 8 p.i.
(iv) Determination of AO.
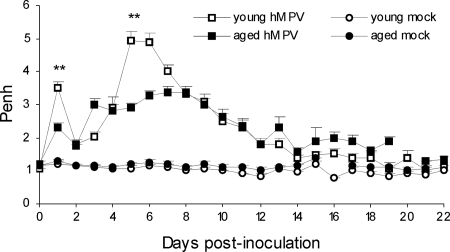
AO increased with biphasic kinetics peaking at day 1 and from day 3 to 13 p.i., and then it declined (Fig. 2). At day 1 p.i., AO was significantly higher in the young hMPV-infected mice than in the aged mice: 3.5 ± 0.19 and 2.31 ± 0.14, respectively (P < 0.01). On days 5 and 6 p.i., AO peaked at 4.95 ± 0.28 in the young hMPV-infected mice compared to 2.91 ± 0.08 in the aged mice (P < 0.01). Although less severe in the aged mice, the AO in both groups of infected mice was far greater than that in the mock-infected mouse groups from day 1 until day 13 p.i. (P < 0.05).
FIG. 2.
AO following hMPV infection of young and aged mice. AO, reported as enhanced pause (Penh) values, was determined using whole-body unrestrained plethysmography. AO was higher in young hMPV-infected mice (n = 3 to 32) than in aged mice (n = 1 to 35) at days 1 and 5. **, P < 0.01, young versus aged hMPV-infected BALB/c mice.
Viral load.
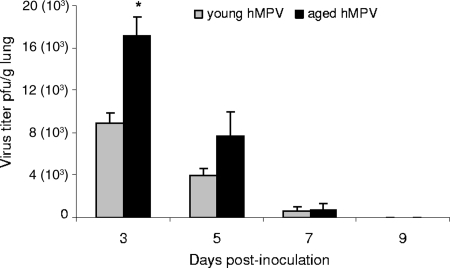
Virus replication in the lung was assessed at days 3, 5, 7, and 9 p.i. Virus titers in aged mice were significantly higher than in young mice at day 3 (mean virus titer in young mice, 8.9 × 103 ± 1.0 × 103 PFU/g; mean virus titer in old mice, 17.1 × 103 ± 1.8 × 103 PFU/g; P < 0.05) (Fig. 3). Virus titers remained higher on day 5 in aged mice but were not significantly different from titers in young mice. Clearance of the virus in aged mice was not delayed compared to that in young mice as at day 9 the virus was cleared from the lungs in both groups of mice.
FIG. 3.
hMPV replication in the lungs of infected aged (n = 3 to 5) and young (n = 3 to 5) mice. Virus titer was assessed at 3, 5, 7, and 9 days after inoculation; values are expressed in PFU/g of lung tissue. hMPV replication in aged hMPV-infected mice at day 3 was twice as high as the level in young hMPV-infected mice (*, P < 0.05).
Ig response. (i) IgG, IgG1, IgG2a.
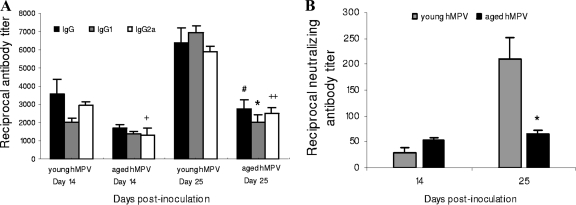
Virus-specific antibody titers were determined at days 6, 14, and 25 p.i. At day 6 p.i., IgG antibody levels were not detectable by ELISA. In the young mice, at day 14 IgG, IgG1, and IgG2a antibodies increased significantly, and at day 25 hMPV induced prominent specific IgG, IgG1, and IgG2a responses (Fig. 4A). In contrast, in the aged mice, hMPV induced a low antibody response (IgG; P < 0.05 compared to young mice), and there was no enhancement of IgG from day 14 to day 25 p.i. Although both IgG2a, a typical Th1 IgG isotype, and IgG1, which is related to the Th2-type response, were detected in the aged mice, these levels were lower than those in young mice (IgG1, P < 0.05; IgG2a, P < 0.01) at day 25 p.i.
FIG. 4.
Ig response following hMPV infection in serum from young and aged mice. (A) hMPV infection induced a higher level of specific IgG, IgG1, and IgG2a responses at day 25 in young hMPV-infected mice (n = 5) than in aged mice (n = 5). #, P < 0.05 compared to total IgG; *, P < 0.05 for IgG1; +, P < 0.05 for IgG2a; and ++, P < 0.01 for IgG2a in young versus aged hMPV-infected mice. (B) At day 25, young mice (n = 5) had developed three times as many neutralizing antibodies as aged mice (n = 5 to 8). *, P < 0.05, comparison between young and aged hMPV-infected groups. Data are shown as the mean ± SEM.
(ii) Neutralizing antibody.
We measured in vitro serum neutralizing titers in both hMPV-infected groups. The young animals developed a statistically significant rise in hMPV-neutralizing titers by day 25 (means, 1:29 at day 14 and 1:210 at day 25 p.i.) (Fig. 4B). This titer was three times higher than the mean serum neutralizing titer of 1:64 we observed in the aged mice at day 25 p.i.
Characterization of cells in BAL fluids after hMPV infection. (i) Total and differential cell counts in the BAL cell population.
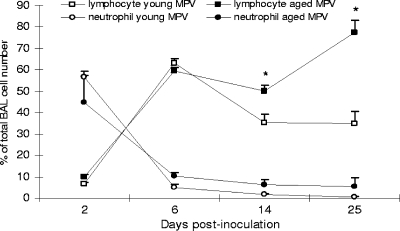
BAL cells were evaluated on days 2, 6, 14, and 25 p.i. for numbers of lymphocytes and neutrophils (eosinophils were rare). There was no significant difference in cell numbers between the aged and young hMPV-infected mice. Cell numbers in the BAL fluids in hMPV-infected mice increased approximately fivefold at day 2 p.i. compared to cell numbers recovered from mock-infected mice, and a maximum increase of approximately sevenfold was noted at 6 days p.i. The cellular infiltration of the airways remained elevated over the course of the hMPV infection. Early in the course of infection, hMPV induced an acute neutrophilic response that declined by day 6 p.i. (Fig. 5). The cellular infiltrate was associated with a peak in the number of lymphocytes in the BAL fluid at day 6. Lymphocyte numbers increased at 6, 14, and 25 days p.i. due to hMPV infection and were significantly greater in the aged mice on days 14 and 25 of infection than in the young mice (P < 0.05). At day 25, lymphocytes represented the main population in BAL cells in the aged mice.
FIG. 5.
Cellular infiltration in BAL fluid after hMPV infection. Neutrophils appeared at day 2 in the two groups (young, n = 4 to 6; aged, n = 3 to 5) of hMPV mice, whereas lymphocytes accumulated at day 6. At day 25, there were more lymphocytes in the BAL fluid of aged mice. *, P < 0.05, for percentages of lymphocytes in BAL fluids of young versus aged hMPV-infected mice. Data are shown as the mean ± SEM.
(ii) Analysis of chemokines and cytokines in the BAL fluids following hMPV infection.
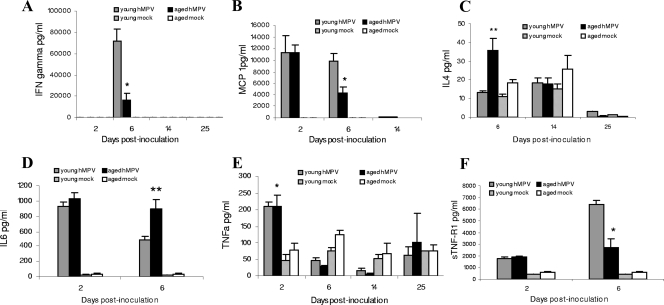
We assessed the production of the Th1-type cytokine, IFN-γ, the Th2-type cytokine, IL-4, the proinflammatory cytokine IL-6, and the chemokine MCP-1 in the BAL fluids of the aged and young mice as well as TNF-α and sTNF-R1 (Fig. 6). At day 2 p.i., similarly high production levels of MCP-1 and IL-6 were measured in the young and aged mice. At day 6 p.i., however, levels of IL-6 in aged mice were greater than in young mice (P < 0.01) (Fig. 6D) while levels of MCP-1 were lower (P < 0.05) (Fig. 6B). TNF-α, a cytokine associated with inflammation, fever, and antiviral defense, was also detected at the same level in BAL fluids 2 days after infection in both infected groups (P < 0.05 compared to mock-infected groups) (Fig. 6E). Because sTNF-R1 often functions as a TNF-α antagonist (23, 55), we measured sTNF-R1; the level was significantly lower in aged mice (P < 0.05) than in young hMPV-infected mice at day 6 p.i. (Fig. 6F). IFN-γ was produced in both groups only at day 6 p.i., with a higher concentration in young hMPV-infected mice (Fig. 6A). In contrast, a higher level of IL-4 was detected in BAL fluids of infected aged mice (P < 0.01) (Fig. 6C). All these molecules were at basal levels in mock-infected mice.
FIG. 6.
Cytokine/chemokine levels in BAL fluid in young and aged mice at different days after inoculation. At day 2, MCP-1 (B), TNF-α (E), and IL-6 (D) were produced by the two groups of infected mice. At day 6, IFN-γ (A), MCP-1, and sTNF-R1 (F) levels were greater for young mice whereas IL-4 (C) and IL-6 levels were greater for aged mice. *, P < 0.05; **, P < 0.01, comparison of young (n = 3 to 10) versus aged hMPV-infected mice (n = 2 to 8). Data are shown as the mean ± SEM.
(iii) Analysis of the cells recruited to the BAL fluids by flow cytometry.
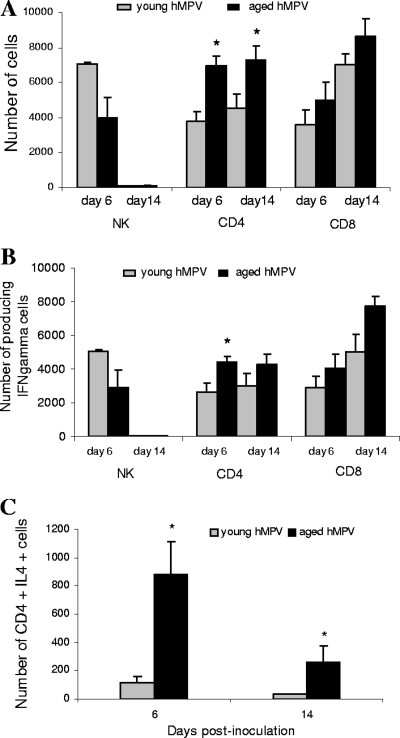
Flow cytometry was performed at days 6 and 14 p.i. because day 6 corresponded to the time of maximal cell infiltrate in the BAL fluid and maximal IFN-γ production, and on day 14, aged mice had a high CSS while young mice had a low CSS. The cellular infiltrate in the BAL fluid of hMPV-infected mice was analyzed for T-cell subsets (CD3+, CD4+, and CD8+) and NK cells (pan-NK). The NK response to hMPV infection was elevated at day 6 and minimal at day 14 p.i. Although there was a trend toward a higher level in the numbers of NK cells in young mice at day 6 than in aged mice, the difference did not reach statistical significance (Fig. 7A). The number of CD4+ T cells was higher in aged mice at 6 days p.i. and at day 14 p.i. For both groups, a substantial increase in the percentage of CD8+ T cells was detected between day 6 p.i. and day 14 p.i., but there were no significant differences with regard to CD8+ T-cell recruitment to the respiratory tract in the two hMPV-infected groups, suggesting that CD8+ T cells may not be the main factor associated with the increased disease severity observed in aged mice. There was no significant difference between the two hMPV-infected groups concerning the number of IFN-γ-secreting populations of NK and CD8+ T cells, but CD4+ at day 6 (Fig. 7B). In contrast, a higher number of CD4+ T cells producing IL-4 was observed at day 6 and 14 p.i. in the aged group (P < 0.05) (Fig. 7C).
FIG. 7.
Characterization by flow cytometry of BAL cells after hMPV infection. There was no significant difference in cell numbers between the aged and young hMPV-infected mice. Numbers of NK+ (DX5+/CD3−), CD4+, and CD8+ cells (A), IFN-γ-producing cells (B), and IL-4-producing CD4+ cells (C) were determined by fluorescence-activated cell sorter analysis of BAL cells from aged (n = 1 to 3) and young (n = 2 to 3) hMPV-infected BALB/c mice. The experiment was repeated three times or twice (NK) with similar results. The numbers of CD4+ T cells were higher in aged mice at day 6 and at day 14 as well as CD4+ T cells producing IL-4. *, P < 0.05 for a comparison of young and aged hMPV-infected groups. Data are shown as the mean ± SEM.
Histology.
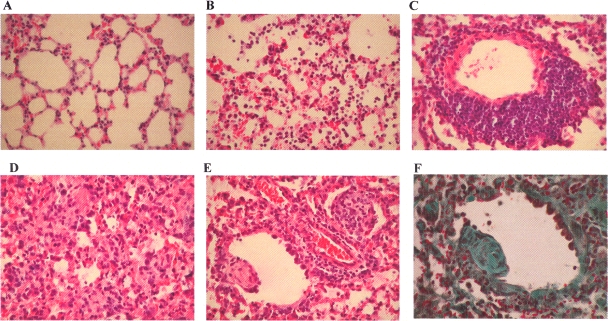
At day 2 p.i. in hMPV-infected mice, fairly small histopathological changes were seen. Parenchymal pneumonia with diffuse histiocytic and neutrophilic infiltration was observed from day 2 through to day 25 p.i., with numerous foci of neutrophils at day 14 in young mice and day 25 p.i. in aged hMPV-infected mice (Fig. 8). Alveolitis appeared at day 6 and declined at day 14 in both groups of hMPV-infected mice (Fig. 8B). The lungs of hMPV-infected mice showed large histopathological abnormalities characterized by abundant perivascular lymphocytic infiltrate (Fig. 8C); these inflammatory changes, discrete at day 2, increased at day 6 and were persistent from day 6 to day 25 p.i. A peribronchiolar and bronchiolar lymphocytic infiltrate was seen. It was discrete in young mice and moderate in aged mice. At day 14 p.i., while alveolitis tended to disappear, lung analysis revealed the development of bronchiolitis obliterans organizing pneumonia (BOOP) (14). Polypoid fibroblastic and collagenic buds (highlighted by Masson's trichrome stain) were seen in the airways of hMPV-infected mice at day 14 (Fig. 8E and F). At day 25 p.i., these fibrotic lesions were either absent or discrete.
FIG. 8.
Lung infiltration after hMPV infection. Large histopathological changes were seen in the lungs, and these changes were of a similar nature in both age groups for the lesions examined. Representative lung sections at day 14 p.i. stained with hematoxylin and eosin are shown. Panel A shows a lung section from a mock-infected animal. Other panels show histopathological changes in lung from hMPV-infected mice as follows: alveolitis (B), perivascular infiltrate (C), parenchymal pneumonia (D), BOOP (E), and BOOP visualized with Masson's trichrome stain(F).
Although both groups of infected mice presented histopathological abnormalities similar in nature for the lesions examined, aged hMPV-infected mice had a higher histopathological score than young mice at day 6 p.i. (4.25 versus 6.75; P < 0.05). The higher score was observed at day 14 p.i. in both groups (8 for the young mice versus 9 for the aged mice). In aged mock mice, only a low level of perivascular lymphocytic and peribronchiolar infiltration was observed at day 25 p.i. (data not shown).
DISCUSSION
hMPV is a frequent cause of hospital admission for frail elderly persons during winter. However, the mechanisms that are associated with more severe illness in the elderly have not been studied. hMPV promotes significant morbidity and mortality. The risk factors include underlying disease, immunodepression, and advanced age. In the present study, we compared the extent of clinical disease, viral replication, and pulmonary inflammation in young and aged mice after primary virus infection to evaluate the age-associated immunopathogenesis of hMPV infection. Intranasal challenge with hMPV resulted in infection of 100% of aged and young mice, but aged mice were far more susceptible than young mice to hMPV infection, as pointed out by the clinical expression and the mortality rate. To better understand the clinical manifestations of hMPV infection in aged and young mice, we determined whether hMPV stimulated overproduction of inflammatory cytokines during acute hMPV infection and whether it was associated with the aggravation of symptoms observed in aged mice. In the first two days p.i., the clinical manifestations in the aged and young mice were similar (apart from a higher fever in aged mice) as were the extent of neutrophil migration in the airways and the levels of inflammatory cytokines IL-6, MCP-1, and TNF-α. No IL-10 (data not shown) or IFN-γ was detected at day 2 p.i. Our results are similar to previous findings showing that hMPV only slightly activates IFN-γ in the early phase of infection and does not induce IL-10 in BALB/c mice (19). Inflammatory cytokines in respiratory secretions of the elderly have not been reported. In infants, however, one report (32) showed that hMPV elicits low levels of inflammatory cytokines including TNF-α and IL-6 in primo-infected infants and that levels of cytokines in sicker, hospitalized infants infected with hMPV were not higher than those in infants infected with hMPV who were treated as outpatients, which suggests that these inflammatory cytokines were not associated with severity. The main differences in the clinical manifestations between the two groups were observed after day 3 p.i. as were the differences in the analyzed cytokines that were assessed at day 6 p.i. Indeed, in the BAL fluids of aged mice, we observed lower levels of MCP-1 and higher levels of IL-6, which has a cachectic activity. There was no difference in TNF-α, which also has a cachectic activity, but lower levels of sTNF-R1, an antagonist of the TNF, were measured at day 6 p.i. in the aged mice.
IL-4 and IFN-γ are known to be important in the cross-regulation of CD4+ Th cell activation. Cytokine analysis revealed that significantly lower levels of IFN-γ were produced in the aged mice but that IL-4 as well as IL-4-producing CD4+ cells were detected. This contrasts with infections in the young mice, which induced high IFN-γ levels and no IL-4. Moreover, hMPV induced high antibody responses in the young mice, but we did not see a similar robust level of antibody response in the old mice. This poor antibody response to hMPV in the aged mice was not due to lower replication of the virus, as shown by virus load at day 3 p.i. This impaired antibody response particularly concerned IgG2a, which is induced by Th1 cytokines. This is in accordance with the fact that IFN-γ decreased and IL-4 increased in the aged mice, suggesting that these mice had a more Th2-like profile. Data from our study support an age-related shift from a Th1-like (IFN-γ) to a Th2-like (IL-4 and IL-6) cytokine response. Altered cytokine production with lower IFN-γ and higher IL-4 and IL-10 responses occurs in the elderly (49). Such modifications in the context of an hMPV infection in elderly humans have not been studied. Similarly, the antibody response to hMPV in the elderly has not been measured. Although a seroprevalence of 100% has been reported in adults, mean antibody titers in the elderly have not been detailed (53). In the case of respiratory syncytial virus (RSV), the antibody response displayed by elderly individuals is comparable to that of young adults, suggesting that humoral immunity to RSV does not appear to be responsible for the increased severity of RSV disease found in the elderly (17, 18). We also determined the effect of age on hMPV neutralizing antibody titers. As for the IgG response, an age-dependent defect was seen in mice in their neutralizing antibody responses to hMPV. Our data suggest that the hMPV-specific antibody response may contribute to protection against or reduction of the severity of the illness. This hypothesis is in accordance with the results from Alvarez and Tripp (2), who showed that the passive transfer of hMPV-immune serum protected naïve BALB/c mice to a certain degree from challenge, and with the results from Williams et al. (57), who reported that a neutralizing monoclonal antibody to the F protein conferred protection against challenge (2, 57).
Whole-body plethysmography has been used to assess whether acute hMPV infection results in increased AO. We previously showed that infection with hMPV NL-001 induced AO on days 1 and 2 p.i. only (10). With the clinical strain C4-CJP05 (subtype A2) cultured with a low passage number, an increase in AO occurred on day 1 and then again from day 3 to day 13 p.i. The viral load or, more probably, the genotype of the strain influenced the extent and the kinetics of the infection. In this study, the kinetics of AO were similar in the aged and young mice infected by hMPV, but the development in young mice was greater. At the same time, the pulmonary levels of IFN-γ and MCP-1 were significantly greater in the young mice than in the aged mice. These results are in accordance with those of Deffranes et al. (12), who reported a lesser degree of airway obstruction concomitant with lower levels of IFN-γ, MCP-1, and RANTES in mice treated with a peptide derived from the hMPV fusion protein (12). Two studies on RSV infection with high inoculum titers described a significant increase in AO associated with a high IFN-γ concentration and cellular infiltration of the lungs (28, 54). Together, these data suggest a potential pathogenic role of IFN-γ in causing AO. Moreover, concurrent depletion of CD4+ and CD8+ T cells was shown to completely inhibit airway obstruction induced by hMPV (31).
Gross examination of the lung revealed severe lesions (data not shown). Large histopathological changes were then seen in the lungs, which were of a similar nature in both age groups for the lesions examined. However, the histologic score was higher at day 6 p.i. in aged mice. Infiltration was more perivascular than peribronchiolar, and both groups presented organizing pneumonia (BOOP) at day 14 p.i., which parallels what is found in humans. Indeed, a histopathological investigation of an 89-year-old woman who died from a respiratory failure after hMPV infection revealed the presence of acute and organizing diffuse alveolar damage characterized by hyaline membranes and interstitial fibrosis as well as some peribronchiolar inflammation (6).
Aging is associated with considerable decreases in CD4+ and CD8+ T-cell-mediated responses (36). Cellular immune responses probably play an important role in controlling hMPV infection. Indeed, in immunosuppressed and transplant patients, who present defects in cellular immune responses, hMPV disease is prolonged and often leads to severe respiratory failure and sometimes death. We therefore studied T-lymphocyte subsets in the BAL fluid of mice and showed at day 6 p.i. a trend toward lower levels of NK cells in the aged mice than in young mice and a higher number of CD4+ T cells. These results suggest that CD4+ T cells and potentially a defect in NK cells may have a role in the increased disease severity observed in the aged mice. Functional analysis of T cells was estimated by IFN-γ production following nonspecific stimulation. At day 6 or 14 p.i., there was no significant difference between the two hMPV-infected groups with regard to the number of IFN-γ-secreting populations (NK and CD8+ T cells). In contrast, a higher number of CD4+ T cells producing IL-4 was observed at day 6 and 14 p.i. in the aged group (P < 0.05). There was no difference between the two hMPV-infected groups concerning CD8+ T-cell recruitment to the respiratory tract. These findings underline the potential role of CD4+ lymphocytes rather than CD8+ T cells in the aggravation of disease. Other investigators have mentioned the role of T cells in the control of replication (1). More recently, depletion of CD4+ cells in young BALB/c mice has been shown to result in significantly less body weight loss and a considerably lower lung pathology score while depletion of CD8+ cells was less effective (31). At day 14 p.i., we did not detect IL-4 and CD8+ (data not shown), which contrasts with the findings of Alvarez and Tripp, who reported that 2 to 4 weeks after hMPV infection, lung T cells producing IL-4 and IL-5 were mainly CD8+ (2).
A number of reports have described declining immunity with advancing age in humans (36, 45). It has been suggested that during RSV infections, aging is associated with a smaller number of RSV-specific CD8+ memory T cells (11) or a reduction in the IL-10/IFN-γ ratio (34). In the case of influenza, a decline in cellular immunity was found to make elderly individuals more susceptible to severe infections (42, 47). In an attempt to understand the relationship between aging and responses to primary respiratory virus infection, models using aged animal have been developed (43, 50). Reduced CD8+ T-cell responses in BALB/c mice are associated with age for influenza (4, 37, 46) or RSV (59). More recently, differences in pulmonary cytokine response were also related to age after RSV infection (7). To date, no studies have evaluated hMPV infection in an aged-animal model. We showed that age has a striking effect on clinical manifestations, and our results suggest that CD4+ and an antibody response deficit may contribute to the severity of hMPV disease. Our model demonstrated that susceptibility to hMPV disease is age related. The model may provide some clues to the mechanisms underlying hMPV disease and information that could lead to anti-inflammatory or immunomodulatory treatment of hMPV disease in the elderly.
Acknowledgments
We are indebted to Valérie Lepagnole for flow cytometry experiments, to Jean-Baptiste Bour for helpful comments, and to Philip Bastable for editorial assistance.
This work was supported by the Centre Hospitalier Universitaire of Dijon and the Conseil Régional de Bourgogne, France. C.P. is supported by Sixth Framework grant LSHM-CT-2006-037276 from the European Union.
Footnotes
Published ahead of print on 14 January 2009.
REFERENCES
- 1.Alvarez, R., K. S. Harrod, W. J. Shieh, S. Zaki, and R. A. Tripp. 2004. Human metapneumovirus persists in BALB/c mice despite the presence of neutralizing antibodies. J. Virol. 7814003-14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez, R., and R. A. Tripp. 2005. The immune response to human metapneumovirus is associated with aberrant immunity and impaired virus clearance in BALB/c mice. J. Virol. 795971-5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastien, N., D. Ward, P. Van Caeseele, K. Brandt, S. H. Lee, G. McNabb, B. Klisko, E. Chan, and Y. Li. 2003. Human metapneumovirus infection in the Canadian population. J. Clin. Microbiol. 414642-4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender, B. S., M. P. Johnson, and P. A. Small. 1991. Influenza in senescent mice: impaired cytotoxic T-lymphocyte activity is correlated with prolonged infection. Immunology 72514-519. [PMC free article] [PubMed] [Google Scholar]
- 5.Boivin, G., Y. Abed, G. Pelletier, L. Ruel, D. Moisan, S. Cote, T. C. Peret, D. D. Erdman, and L. J. Anderson. 2002. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J. Infect. Dis. 1861330-1334. [DOI] [PubMed] [Google Scholar]
- 6.Boivin, G., G. De Serres, M. E. Hamelin, S. Cote, M. Argouin, G. Tremblay, R. Maranda-Aubut, C. Sauvageau, M. Ouakki, N. Boulianne, and C. Couture. 2007. An outbreak of severe respiratory tract infection due to human metapneumovirus in a long-term care facility. Clin. Infect. Dis. 441152-1158. [DOI] [PubMed] [Google Scholar]
- 7.Boukhvalova, M. S., K. C. Yim, K. H. Kuhn, J. P. Hemming, G. A. Prince, D. D. Porter, and J. C. Blanco. 2007. Age-related differences in pulmonary cytokine response to respiratory syncytial virus infection: modulation by anti-inflammatory and antiviral treatment. J. Infect. Dis. 195511-518. [DOI] [PubMed] [Google Scholar]
- 8.Cane, P. A., B. G. van den Hoogen, S. Chakrabarti, C. D. Fegan, and A. D. Osterhaus. 2003. Human metapneumovirus in a haematopoietic stem cell transplant recipient with fatal lower respiratory tract disease. Bone Marrow Transplant. 31309-310. [DOI] [PubMed] [Google Scholar]
- 9.Chiu, C. Y., A. A. Alizadeh, S. Rouskin, J. D. Merker, E. Yeh, S. Yagi, D. Schnurr, B. K. Patterson, D. Ganem, and J. L. DeRisi. 2007. Diagnosis of a critical respiratory illness caused by human metapneumovirus by use of a pan-virus microarray. J. Clin. Microbiol. 452340-2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darniot, M., T. Petrella, S. Aho, P. Pothier, and C. Manoha. 2005. Immune response and alteration of pulmonary function after primary human metapneumovirus (hMPV) infection of BALB/c mice. Vaccine 234473-4480. [DOI] [PubMed] [Google Scholar]
- 11.de Bree, G. J., J. Heidema, E. M. van Leeuwen, G. M. van Bleek, R. E. Jonkers, H. M. Jansen, R. A. van Lier, and T. A. Out. 2005. Respiratory syncytial virus-specific CD8+ memory T cell responses in elderly persons. J. Infect. Dis. 1911710-1718. [DOI] [PubMed] [Google Scholar]
- 12.Deffrasnes, C., M. E. Hamelin, G. A. Prince, and G. Boivin. 2008. Identification and evaluation of a highly effective fusion inhibitor for human metapneumovirus. Antimicrob. Agents Chemother. 52279-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Englund, J. A., M. Boeckh, J. Kuypers, W. G. Nichols, R. C. Hackman, R. A. Morrow, D. N. Fredricks, and L. Corey. 2006. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann. Intern. Med. 144344-349. [DOI] [PubMed] [Google Scholar]
- 14.Epler, G. R. 2001. Bronchiolitis obliterans organizing pneumonia. Arch. Intern. Med. 161158-164. [DOI] [PubMed] [Google Scholar]
- 15.Falsey, A. R., M. C. Criddle, and E. E. Walsh. 2006. Detection of respiratory syncytial virus and human metapneumovirus by reverse transcription polymerase chain reaction in adults with and without respiratory illness. J. Clin. Virol. 3546-50. [DOI] [PubMed] [Google Scholar]
- 16.Falsey, A. R., D. Erdman, L. J. Anderson, and E. E. Walsh. 2003. Human metapneumovirus infections in young and elderly adults. J. Infect. Dis. 187785-790. [DOI] [PubMed] [Google Scholar]
- 17.Falsey, A. R., and E. E. Walsh. 1992. Humoral immunity to respiratory syncytial virus infection in the elderly. J. Med. Virol. 3639-43. [DOI] [PubMed] [Google Scholar]
- 18.Falsey, A. R., E. E. Walsh, R. J. Looney, J. E. Kolassa, M. A. Formica, M. C. Criddle, and W. J. Hall. 1999. Comparison of respiratory syncytial virus humoral immunity and response to infection in young and elderly adults. J. Med. Virol. 59221-226. [DOI] [PubMed] [Google Scholar]
- 19.Guerrero-Plata, A., A. Casola, and R. P. Garofalo. 2005. Human metapneumovirus induces a profile of lung cytokines distinct from that of respiratory syncytial virus. J. Virol. 7914992-14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamelin, M. E., S. Cote, J. Laforge, N. Lampron, J. Bourbeau, K. Weiss, R. Gilca, G. DeSerres, and G. Boivin. 2005. Human metapneumovirus infection in adults with community-acquired pneumonia and exacerbation of chronic obstructive pulmonary disease. Clin. Infect. Dis. 41498-502. [DOI] [PubMed] [Google Scholar]
- 21.Hamelin, M. E., K. Yim, K. H. Kuhn, R. P. Cragin, M. Boukhvalova, J. C. Blanco, G. A. Prince, and G. Boivin. 2005. Pathogenesis of human metapneumovirus lung infection in BALB/c mice and cotton rats. J. Virol. 798894-8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamelmann, E., J. Schwarze, K. Takeda, A. Oshiba, G. L. Larsen, C. G. Irvin, and E. W. Gelfand. 1997. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am. J. Respir. Crit. Care Med. 156766-775. [DOI] [PubMed] [Google Scholar]
- 23.Herbein, G., and W. A. O'Brien. 2000. Tumor necrosis factor (TNF)-alpha and TNF receptors in viral pathogenesis. Proc. Soc. Exp. Biol. Med. 223241-257. [DOI] [PubMed] [Google Scholar]
- 24.High, K. P. 2005. Pneumonia in older adults. New categories add complexity to diagnosis and care. Postgrad. Med. 11818-20, 25-28. [DOI] [PubMed] [Google Scholar]
- 25.Honda, H., J. Iwahashi, T. Kashiwagi, Y. Imamura, N. Hamada, T. Anraku, S. Ueda, T. Kanda, T. Takahashi, and S. Morimoto. 2006. Outbreak of human metapneumovirus infection in elderly inpatients in Japan. J. Am. Geriatr. Soc. 54177-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horton, N. J., Lipsitz, S. R. 1999. Review of software to fit generalized estimating equation regression models. Am. Stat. 53245-248. [Google Scholar]
- 27.Huck, B., M. Egger, H. Bertz, G. Peyerl-Hoffman, W. V. Kern, D. Neumann-Haefelin, and V. Falcone. 2006. Human metapneumovirus infection in a hematopoietic stem cell transplant recipient with relapsed multiple myeloma and rapidly progressing lung cancer. J. Clin. Microbiol. 442300-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jafri, H. S., S. Chavez-Bueno, A. Mejias, A. M. Gomez, A. M. Rios, S. S. Nassi, M. Yusuf, P. Kapur, R. D. Hardy, J. Hatfield, B. B. Rogers, K. Krisher, and O. Ramilo. 2004. Respiratory syncytial virus induces pneumonia, cytokine response, airway obstruction, and chronic inflammatory infiltrates associated with long-term airway hyperresponsiveness in mice. J. Infect. Dis. 1891856-1865. [DOI] [PubMed] [Google Scholar]
- 29.Kamble, R. T., C. Bollard, G. Demmler, P. R. LaSala, and G. Carrum. 2007. Human metapneumovirus infection in a hematopoietic transplant recipient. Bone Marrow Transplant. 40699-700. [DOI] [PubMed] [Google Scholar]
- 30.Kaye, M., S. Skidmore, H. Osman, M. Weinbren, and R. Warren. 2006. Surveillance of respiratory virus infections in adult hospital admissions using rapid methods. Epidemiol. Infect. 134792-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolli, D., E. L. Bataki, L. Spetch, A. Guerrero-Plata, A. M. Jewell, P. A. Piedra, G. N. Milligan, R. P. Garofalo, and A. Casola. 2008. T lymphocytes contribute to antiviral immunity and pathogenesis in experimental human metapneumovirus infection. J. Virol. 828560-8569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laham, F. R., V. Israele, J. M. Casellas, A. M. Garcia, C. M. Lac Prugent, S. J. Hoffman, D. Hauer, B. Thumar, M. I. Name, A. Pascual, N. Taratutto, M. T. Ishida, M. Balduzzi, M. Maccarone, S. Jackli, R. Passarino, R. A. Gaivironsky, R. A. Karron, N. R. Polack, and F. P. Polack. 2004. Differential production of inflammatory cytokines in primary infection with human metapneumovirus and with other common respiratory viruses of infancy. J. Infect. Dis. 1892047-2056. [DOI] [PubMed] [Google Scholar]
- 33.Larcher, C., C. Geltner, H. Fischer, D. Nachbaur, L. C. Muller, and H. P. Huemer. 2005. Human metapneumovirus infection in lung transplant recipients: clinical presentation and epidemiology. J. Heart Lung Transplant. 241891-1901. [DOI] [PubMed] [Google Scholar]
- 34.Lee, F. E., E. E. Walsh, A. R. Falsey, N. Liu, D. Liu, A. Divekar, J. E. Snyder-Cappione, and T. R. Mosmann. 2005. The balance between influenza- and RSV-specific CD4 T cells secreting IL-10 or IFNγ in young and healthy-elderly subjects. Mech. Ageing Dev. 1261223-1229. [DOI] [PubMed] [Google Scholar]
- 35.Leung, J., F. Esper, C. Weibel, and J. S. Kahn. 2005. Seroepidemiology of human metapneumovirus (hMPV) on the basis of a novel enzyme-linked immunosorbent assay utilizing hMPV fusion protein expressed in recombinant vesicular stomatitis virus. J. Clin. Microbiol. 431213-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linton, P. J., and K. Dorshkind. 2004. Age-related changes in lymphocyte development and function. Nat. Immunol. 5133-139. [DOI] [PubMed] [Google Scholar]
- 37.Liu, B., and Y. Kimura. 2007. Local immune response to respiratory syncytial virus infection is diminished in senescence-accelerated mice. J. Gen. Virol. 882552-2558. [DOI] [PubMed] [Google Scholar]
- 38.Louie, J. K., D. P. Schnurr, C. Y. Pan, D. Kiang, C. Carter, S. Tougaw, J. Ventura, A. Norman, V. Belmusto, J. Rosenberg, and G. Trochet. 2007. A summer outbreak of human metapneumovirus infection in a long-term-care facility. J. Infect. Dis. 196705-708. [DOI] [PubMed] [Google Scholar]
- 39.MacPhail, M., J. H. Schickli, R. S. Tang, J. Kaur, C. Robinson, R. A. Fouchier, A. D. Osterhaus, R. R. Spaete, and A. A. Haller. 2004. Identification of small-animal and primate models for evaluation of vaccine candidates for human metapneumovirus (hMPV) and implications for hMPV vaccine design. J. Gen. Virol. 851655-1663. [DOI] [PubMed] [Google Scholar]
- 40.Martinello, R. A., F. Esper, C. Weibel, D. Ferguson, M. L. Landry, and J. S. Kahn. 2006. Human metapneumovirus and exacerbations of chronic obstructive pulmonary disease. J. Infect. 53248-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martino, R., R. P. Porras, N. Rabella, J. V. Williams, E. Ramila, N. Margall, R. Labeaga, J. E. Crowe, Jr., P. Coll, and J. Sierra. 2005. Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biol. Blood Marrow Transplant. 11781-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mbawuike, I. N., C. L. Acuna, K. C. Walz, R. L. Atmar, S. B. Greenberg, and R. B. Couch. 1997. Cytokines and impaired CD8+ CTL activity among elderly persons and the enhancing effect of IL-12. Mech. Ageing Dev. 9425-39. [DOI] [PubMed] [Google Scholar]
- 43.Murasko, D. M., and J. Jiang. 2005. Response of aged mice to primary virus infections. Immunol. Rev. 205285-296. [DOI] [PubMed] [Google Scholar]
- 44.O'Gorman, C., E. McHenry, and P. V. Coyle. 2006. Human metapneumovirus in adults: a short case series. Eur. J. Clin. Microbiol. Infect. Dis. 25190-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pawelec, G., and A. Larbi. 2008. Immunity and ageing in man: annual review 2006/2007. Exp. Gerontol. 4334-38. [DOI] [PubMed] [Google Scholar]
- 46.Po, J. L., E. M. Gardner, F. Anaraki, P. D. Katsikis, and D. M. Murasko. 2002. Age-associated decrease in virus-specific CD8+ T lymphocytes during primary influenza infection. Mech. Ageing Dev. 1231167-1181. [DOI] [PubMed] [Google Scholar]
- 47.Powers, D. C. 1993. Influenza A virus-specific cytotoxic T lymphocyte activity declines with advancing age. J. Am. Geriatr. Soc. 411-5. [DOI] [PubMed] [Google Scholar]
- 48.Raza, K., S. B. Ismailjee, M. Crespo, S. M. Studer, S. Sanghavi, D. L. Paterson, E. J. Kwak, C. R. Rinaldo, Jr., J. M. Pilewski, K. R. McCurry, and S. Husain. 2007. Successful outcome of human metapneumovirus (hMPV) pneumonia in a lung transplant recipient treated with intravenous ribavirin. J. Heart Lung Transplant. 26862-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rink, L., I. Cakman, and H. Kirchner. 1998. Altered cytokine production in the elderly. Mech. Ageing Dev. 102199-209. [DOI] [PubMed] [Google Scholar]
- 50.Roberts, A., C. Paddock, L. Vogel, E. Butler, S. Zaki, and K. Subbarao. 2005. Aged BALB/c mice as a model for increased severity of severe acute respiratory syndrome in elderly humans. J. Virol. 795833-5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rohde, G., I. Borg, U. Arinir, J. Kronsbein, R. Rausse, T. T. Bauer, A. Bufe, and G. Schultze-Werninghaus. 2005. Relevance of human metapneumovirus in exacerbations of COPD. Respir. Res. 6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sumino, K. C., E. Agapov, R. A. Pierce, E. P. Trulock, J. D. Pfeifer, J. H. Ritter, M. Gaudreault-Keener, G. A. Storch, and M. J. Holtzman. 2005. Detection of severe human metapneumovirus infection by real-time polymerase chain reaction and histopathological assessment. J. Infect. Dis. 1921052-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van den Hoogen, B. G., J. C. de Jong, J. Groen, T. Kuiken, R. de Groot, R. A. Fouchier, and A. D. Osterhaus. 2001. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 7719-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Schaik, S. M., N. Obot, G. Enhorning, K. Hintz, K. Gross, G. E. Hancock, A. M. Stack, and R. C. Welliver. 2000. Role of interferon gamma in the pathogenesis of primary respiratory syncytial virus infection in BALB/c mice. J. Med. Virol. 62257-266. [DOI] [PubMed] [Google Scholar]
- 55.Van Zee, K. J., T. Kohno, E. Fischer, C. S. Rock, L. L. Moldawer, and S. F. Lowry. 1992. Tumor necrosis factor soluble receptors circulate during experimental and clinical inflammation and can protect against excessive tumor necrosis factor alpha in vitro and in vivo. Proc. Natl. Acad. Sci. USA 894845-4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waris, M. E., C. Tsou, D. D. Erdman, S. R. Zaki, and L. J. Anderson. 1996. Respiratory syncytial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J. Virol. 702852-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams, J. V., Z. Chen, G. Cseke, D. W. Wright, C. J. Keefer, S. J. Tollefson, A. Hessell, A. Podsiad, B. E. Shepherd, P. P. Sanna, D. R. Burton, J. E. Crowe, Jr., and R. A. Williamson. 2007. A recombinant human monoclonal antibody to human metapneumovirus fusion protein that neutralizes virus in vitro and is effective therapeutically in vivo. J. Virol. 818315-8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams, J. V., J. E. Crowe, Jr., R. Enriquez, P. Minton, R. S. Peebles, Jr., R. G. Hamilton, S. Higgins, M. Griffin, and T. V. Hartert. 2005. Human metapneumovirus infection plays an etiologic role in acute asthma exacerbations requiring hospitalization in adults. J. Infect. Dis. 1921149-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang, Y., Y. Wang, X. Gilmore, K. Xu, P. R. Wyde, and I. N. Mbawuike. 2002. An aged mouse model for RSV infection and diminished CD8(+) CTL responses. Exp. Biol. Med. (Maywood) 227133-140. [DOI] [PubMed] [Google Scholar]