Abstract
Nonstructural protein 3 (NS3) is an essential replicative component of the hepatitis C virus (HCV) and a member of the DExH/D-box family of proteins. The C-terminal region of NS3 (NS3hel) exhibits RNA-stimulated NTPase and helicase activity, while the N-terminal serine protease domain of NS3 enhances RNA binding and unwinding by NS3hel. The nonstructural protein 4A (NS4A) binds to the NS3 protease domain and serves as an obligate cofactor for NS3 serine protease activity. Given its role in stimulating protease activity, we sought to determine whether NS4A also influences the activity of NS3hel. Here we show that NS4A enhances the ability of NS3hel to bind RNA in the presence of ATP, thereby acting as a cofactor for helicase activity. This effect is mediated by amino acids in the C-terminal acidic domain of NS4A. When these residues are mutated, one observes drastic reductions in ATP-coupled RNA binding and duplex unwinding by NS3. These same mutations are lethal in HCV replicons, thereby establishing in vitro and in vivo that NS4A plays an important role in the helicase mechanism of NS3 and its function in replication.
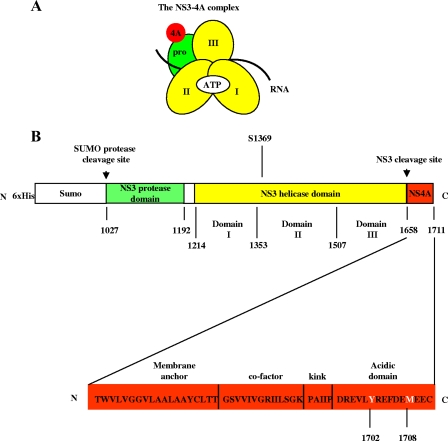
Nonstructural protein 3 (NS3) is a multifunctional enzyme with serine protease, NTPase, and RNA helicase activities. It is an essential replicative component of the hepatitis C virus (HCV) (16, 20) and is a member of the DExH/D-box family of proteins (6). NS3 exhibits NTPase and helicase activity from its C-terminal helicase domain (NS3hel) (9, 10, 28) and serine protease activity from its N-terminal protease domain when it is bound by the HCV NS4A cofactor, which is a 54-amino-acid peptide that intercalates with the NS3 protease domain (7, 10, 26, 29) (Fig. 1A).
FIG. 1.
Composition of NS3-4A and the NS3-4A expression construct. (A and B) The NS3-4A complex organization and construct design is illustrated schematically. In panel A, “pro” refers to the serine protease domain, and the Roman numerals indicate the respective NS3 helicase subdomains. The regions where ATP, RNA, and the NS4A cofactor bind are indicated as well. The protein construct expressed in E. coli is depicted in panel B. The numbers below the map refer to the HCV polyprotein numbering of the amino acids of NS3-4A. A detailed map of NS4A (i.e., HCV polyprotein residues 1658 to 1711) (18) is shown below the His-SUMO-NS3-4A expression construct. Residues Y1702 and M1708 are indicated in white font.
The influence of the NS4A cofactor on the activities of NS3hel has not been fully investigated. The C terminus of NS4A contains a conserved, acidic domain (Fig. 1B) that has been demonstrated to be essential for HCV replicon replication (18). Importantly, mutations in the NS4A acidic domain that reduce or prevent replicon replication do not affect NS3-4A proteolytic processing of the HCV polyprotein (18). It is therefore likely that these mutations affect other NS3-4A activities, such as functional RNA binding, ATP hydrolysis, or RNA unwinding.
Although it is not known whether NS4A influences interactions between NS3 and RNA, many DExH/D-box proteins require a cofactor to stimulate their RNA binding and/or ATPase activities. For example, the ATPase activity of RhlB is greatly stimulated when RhlB is bound to RNase E (31), which also serves to localize target mRNAs to RhlB (25, 31). Another example is yeast Dbp8, which displays enhanced ATPase activity enhanced in the presence of cofactor Esf2. Furthermore, Esf2 may help Dbp8 to specifically recognize pre-rRNAs (11).
NS3 requires at least one cofactor to stimulate its RNA-binding and unwinding activities: the NS3 protease domain greatly enhances NS3hel RNA binding and is required for NS3hel to unwind RNA (4). The protease domain does not bind RNA, rather it allosterically influences NS3hel RNA binding and unwinding (4). NS4A may act as a second cofactor to modulate the allosteric effect of the protease domain and to further stimulate RNA binding and unwinding by NS3hel.
In contrast to previous studies of NS3-4A, we examined here the influence of NS4A on NS3hel using untagged, wild-type NS3-4A. Previous work on NS3-4A has been performed with His-tagged NS3 protein (9, 23) and a sequence-modified NS4A cofactor (10). Recent work has shown that His tags on NS3 can enhance NS3 RNA binding (4). Thus, observed differences between NS3 and NS3-4A activities may have been the result of protein construct design. In addition, changing the NS4A amino acid sequence can prevent the replication of a subgenomic version of HCV (i.e., an HCV replicon) (18), suggesting that wild-type NS4A should be used in in vitro studies to maintain physiological relevance.
To determine whether NS3hel activities are enhanced by the NS4A cofactor, we compared the ligand interactions and enzymatic functions of NS3 and NS3-4A using filter-binding, ATPase, and RNA-unwinding assays. Here we show that the NS4A cofactor tightly couples RNA binding with ATP hydrolysis and that it promotes strong binding between RNA and NS3 in the ATP-bound state. These effects are mediated by the C-terminal acidic domain of NS4A, and mutational studies, coupled with kinetic analyses, reveal that mutation of specific amino acids within this domain attenuate the function of NS3. Because these same residues are known to be essential for replication in HCV replicon assays (18), the present study integrates our understanding of NS3 and NS4A reaction mechanisms in vitro with their replicative roles in vivo.
MATERIALS AND METHODS
Materials.
RNA and DNA oligonucleotides were obtained from Dharmacon and Invitrogen, respectively. All other reagents were obtained from Fisher unless otherwise indicated.
Cloning.
The NS3+ and NS3/4A+ genes were PCR amplified from the HCV genotype 1b (version N) (from plasmid pNNeo/3-5B [13], kindly donated by Stan Lemon) and cloned into the pET-SUMO vector (Invitrogen) using ExTaq PCR (Takara), followed by ligation with linear pET-SUMO according to the manufacturer's protocol. The DNA oligonucleotides used were NS3 5′SUMO (5′-TCC GCG CCT ATT ACG GCC TAC TC-3′) and NS3 3′SUMO (5′-TTA GGT GAC GAC TTC CAG GTC GG-3′).
We also cloned and purified mutant forms of NS3-4A from HCV genotype 1b (version N). The S1369R NS3-4A-expesssing plasmid was created by modifying the pET-SUMO-NS3-4A(1b)+ plasmid using a Stratagene QuikChange kit along with the DNA oligonucleotides S1369R-1 and S1369R-2 (5′-GAG GAG GTG GGC CCT GCG CAA CAC TGG AGA GAT C-3′ and 5′-GAT CTC TCC AGT GTT GCG CAG GGC CAC CTC CTC-3′). For the M1708A NS3-4A-expressing plasmid, the DNA oligonucleotides for the QuikChange reaction were M1708A-1 and M1708A-2 (5′-CTC TAC CGG GAG TTC GAT GAA GCC GAA GAA TGC TAG GAT CCG CGA-3′ and 5′-TCG CGG ATC CTA GCA TTC TTC GGC TTC ATC GAA CTC CCG GTA GAG-3′). For the Y1702A NS3-4A-expressing plasmid, the DNA oligonucleotides for the QuikChange reaction, Y1702A-1 and Y1702A-2, were used (5′-GAT AGG GAA GTC CTC GCC CGG GAG TTC GAT GAA-3′ and 5′-TTC ATC GAA CTC CCG GGC GAG GAC TTC CCT ATC-3′).
All constructs were verified initially through PCR screening as necessary and subsequently sequenced for accuracy (Keck Facility, Yale University).
Protein purification.
All proteins were purified according to the methods described in Beran et al. (4). Briefly, His6 and His6-SUMO fusion proteins were purified through a nickel column (Qiagen), treated with 10 U of SUMO protease (Invitrogen) where necessary to create untagged protein, and subsequently passed through a gel filtration column (HiLoad Superdex 200 16/60). Protein preparations were divided into 10-μl aliquots and stored at −80°C.
Helicase assays.
Unwinding assays were performed and analyzed using gel shift methods, as previously described (2, 23). Protein (100 nM) and duplex substrate (0.2 nM) were preincubated in a volume of 100 μl of NS3 helicase assay buffer (25 mM morpholinepropanesulfonic acid [MOPS]-NH4+ [pH 6.5], 3 mM MgCl2, 1% glycerol, 2 mM dithiothreitol [DTT], 30 mM NaCl, 0.2% Triton X-100 [vol/vol]). After 1 h of preincubation at 37°C, 6 μl of preincubation mix was added to 2 μl of ATP-trap mix (25 mM MOPS-NH4+, [pH 6.5], 3 mM MgCl2, 30 mM NaCl, 1 μM top-strand oligonucleotide, 16 mM ATP) such that the final concentration of ATP during each reaction was 4 mM. The RNA unwinding substrate RNA2 is a 34-bp duplex with a 20-nucleotide 3′ overhang. The top strand (RNA2-TS34) is 5′-GCC UCG CUG CCG UCG CCA GCA UAU GUA CAC UAG U-3′. The bottom strand (RNA2-BS54) is 5′-ACU AGU GUA CAU AUG CUG GCG ACG GCA GCG AGG CAG AGG AGC AGA GGG AGC AUG-3′. The sequence of RNA1 has been described (2).
Filter-binding assays.
Filter-binding assays were used to determine the affinity of the various NS3 constructs for RNA. The RNA oligonucleotide used in this assay was TS34 5′-C UGU GGC AUG UCC UAG CGU CGU AUC GAU CUG GUC-3′ (2), which was 5′ end labeled with 32P using T4 polynucleotide kinase (New England Biolabs) and [γ-32P]ATP (150 μCi/μl, 6,000 Ci/mmol; Perkin-Elmer). Labeled TS34 was purified by electrophoresis (15% acrylamide-8 M urea), visualized using an InstantImager (Perkin-Elmer), excised from the gel, and eluted by shaking for 4 h at 4°C in 300 mM sodium acetate (pH 5.2). After isopropanol precipitation, labeled TS34 was quantified by using a scintillation counter (Beckman).
Filter-binding assays were performed as previously described (4). Briefly, various protein concentrations were incubated in a final volume of 100 μl for 1 h with 0.2 nM labeled RNA oligonucleotide at 37°C in NS3 helicase assay buffer before being loaded onto a membrane sandwich, which consisted of a nitrocellulose membrane top layer (0.45 μm; Pierce) and a Nytran N nylon membrane bottom layer (0.2 μm; Whatman). An aliquot of each incubation mix (50 μl) was loaded into the lanes of a dot blot apparatus, with a continuous application of vacuum. The samples in each lane were subsequently washed using 150 μl of ice-cold NS3 helicase assay buffer. The blots were exposed to a PhosphorImager plate for ∼1 h and quantified by PhosphorImager scanning (Molecular Dynamics). The percentage of the total RNA bound to protein was subsequently calculated, and the resulting binding data were fit to the Hill equation in order to obtain Kd values (Ys = [S]n/Kd + [S]n) (32) using SigmaPlot (Systat Software).
ATPase assays.
ATP hydrolysis was monitored as described previously (4). The assays were performed at 37°C in 20-μl volumes of ATPase reaction buffer (25 mM MOPS-NH4+ [pH 6.5], 0.75 mM MgCl2, 1% glycerol, 2 mM DTT, 30 mM NaCl, 0.2% [vol/vol] Triton X-100) containing ∼1 mM ATP, 50 nM protein, and various amounts of TS34 RNA1 oligonucleotide as indicated. All tubes containing protein and RNA were preincubated at 37°C for 1 h before the addition of ATP. Free Pi was separated from ATP by using thin-layer chromatography, and the plates were subsequently quantified by PhosphorImager scanning (Molecular Dynamics). The percentage of hydrolyzed ATP was determined for each time point, at each RNA concentration, and the amount of ATP hydrolyzed (in nmol) versus time was plotted. Initial velocities were determined from a linear regression of the data (SigmaPlot). Subsequently, the initial reaction velocities versus the RNA concentration were plotted and fit to the Michaelis-Menten equation (v0 = Vmax[S]/Km + [S]) (32). The RNA stimulation factors were calculated in each case by dividing the ATPase velocities determined in the presence of saturating levels of RNA (i.e., the Vmax value) by the basal ATPase velocity in the absence of RNA.
Protease assays.
Protease assays were performed at 37°C using the resonance energy transfer-S1 substrate (RET-S1; Anaspec) designed by Taliani et al. (29). RET-S1 is an NS4A-NS4B junction mimic that fluoresces upon cleavage. All assays were performed in 60-μl reaction volumes containing 40 nM NS3-4A and 5 μM RET-S1. The buffer conditions were the same as those normally used for helicase assays (4): 25 mM MOPS-NH4+ (pH 6.5), 3 mM MgCl2, 1% glycerol, 2 mM DTT, 30 mM NaCl, and 0.2% (vol/vol) Triton X-100. The data were collected by using a Cary Eclipse spectrophotometer (Varian) with a temperature-controlled cuvette holder. The excitation wavelength was 350 nm, and the emission wavelength was 440 nm. The initial background value (the value at time zero, just before substrate addition) was subtracted from all subsequent time points.
RESULTS
NS4A enhances the coupling between RNA binding and ATPase activity of NS3.
Because the activities of many SF2 helicases (e.g., RhlB, Dbp5, and Dbp8) are stimulated by cofactor proteins (11, 31, 33), we set out to determine whether NS4A serves as a cofactor for promoting NS3 helicase activity. Specifically, we wanted to explore the influence of NS4A on diverse aspects of helicase function, including the kinetics and thermodynamics of RNA binding to NS3, ATPase activity and its coupling with RNA binding, and the kinetic parameters of RNA unwinding.
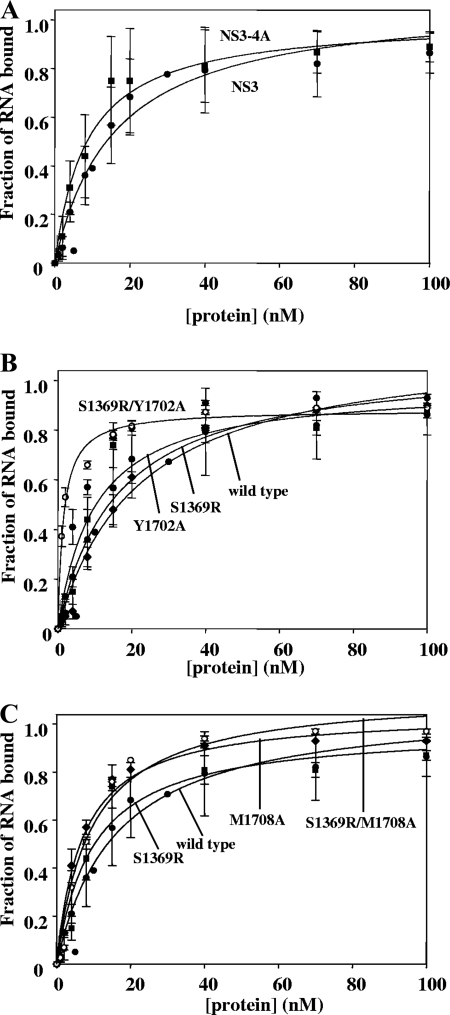
To compare the overall affinities of NS3 and NS3-4A for RNA, each protein was subjected to a direct binding experiment in which 32P-labeled single-stranded RNA (ssRNA) was titrated with increasing concentrations of excess, unlabeled protein and the fraction of bound complex was determined by filter binding. NS3 was observed to bind ssRNA with a Kd of 9 ± 2 nM and NS3-4A bound ssRNA with a Kd of 10 ± 1 nM (Table 1 and Fig. 2A), which indicated that NS4A does not affect the overall affinity of NS3 for RNA. Similarly, NS4A did not significantly influence NS3 binding to RNA1 (2), which is a 34-bp RNA duplex unwinding substrate (for NS3, Kd = 14 ± 1 nM; for NS3-4A, Kd = 19 ± 2 nM) (data not shown).
TABLE 1.
Summary of RNA filter-binding data for NS3-4A variants
| Protein | Avg ± SDa
|
|
|---|---|---|
| Kd (nM) for binding to ssRNA | Maximal fraction of bound RNA | |
| His-NS3 | 1.0 ± 0.4 | 0.99 ± 0.01 |
| His-NS3-4A | 3 ± 1 | 0.99 ± 0.01 |
| NS3 | 9 ± 2 | 0.98 ± 0.02 |
| NS3-4A | 10 ± 1 | 0.84 ± 0.03 |
| NS3-4A S1369R | 10 ± 3 | 0.98 ± 0.01 |
| NS3-4A Y1702A | 13 ± 1 | 0.90 ± 0.03 |
| NS3-4A M1708A | 5 ± 1 | 0.90 ± 0.03 |
| NS3-4A S1369R/Y1702A | 2.0 ± 0.1 | 0.88 ± 0.02 |
| NS3-4A S1369R/M1708A | 7 ± 1 | 0.96 ± 0.02 |
These data are averages from three experiments.
FIG. 2.
The NS4A cofactor does not affect direct RNA binding by NS3. The parameters for each binding curve are listed in Table 1. In all cases, the Hill equation with a cooperativity value of n = 1 provided the best-fit curve (see Materials and Methods). In panel A, the curves are NS3 (solid circles) and NS3-4A (solid squares). In panel B, the curves are NS3-4A (solid circles), NS3-4A S1369R (solid squares), NS3-4A Y1702A (solid diamonds), and NS3-4A S1369R/Y1702A (hatched circles). In panel C, the curves are NS3-4A (solid circles), NS3-4A S1369R (solid squares), and NS3-4A M1708A (solid diamonds), NS3-4A S1369R/M1708A (hatched circles). These data are the averages of three experiments, and the error represents the standard deviation.
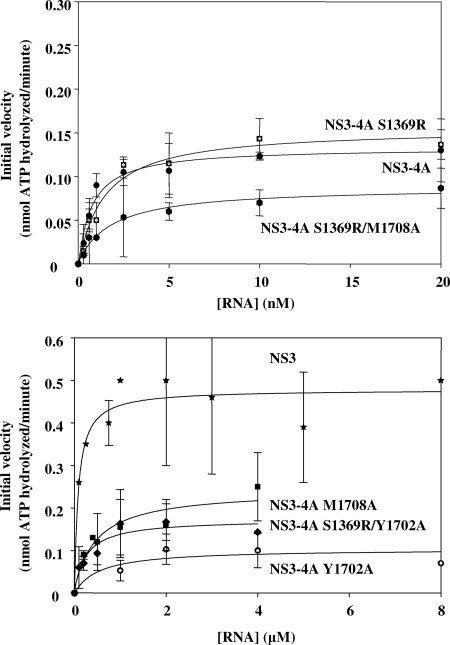
NS3 is a highly basic protein, with multiple RNA-binding sites, and it is hypothesized to interact with RNA during numerous processes that may be unrelated to helicase activity, such as viral packaging (35). Nonspecific RNA binding by helicases is a common phenomenon that can greatly complicate the interpretation of direct binding assays (4, 23). To circumvent this problem and monitor RNA binding that is relevant to helicase function of NS3 and NS3-4A, we measured the RNA dependence of ATPase activity by the two proteins. This experiment provides a functional metric of protein-RNA affinity in the presence of ATP. When the initial velocity of ATP hydrolysis was monitored as a function of the ssRNA concentration, we observed that NS3-4A reached its maximal ATP-hydrolysis velocity at much lower concentrations of RNA than for NS3. Indeed, the Km for RNA-stimulated ATPase activity by NS3-4A was 1.0 ± 0.1 nM (see Fig. 4 and Table 2), while for NS3 the Km was 100 ± 1 nM (Table 2) (4). This result indicates that a functionally important ATP-bound state of NS3 binds RNA much more tightly in the presence of NS4A, effectively coupling RNA binding to ATPase activity.
FIG. 4.
RNA-stimulated ATPase activity of NS3-4A variants. The Michaelis-Menten kinetic parameters for each fit are listed in Table 2. The data plotted are NS3 (stars), NS3-4A (solid circles), NS3-4A (S1369R) (hatched squares), NS3-4A (Y1702A) (hatched circles), NS3-4A (M1708A) (solid squares), NS3-4A (S1369R/Y1702A) (solid diamonds), and NS3-4A (S1369R/M1708A) (solid hexagons). The data shown are the averages of three assays, and the error represents the standard deviation.
TABLE 2.
Summary of RNA-stimulated ATPase activities of NS3-4A variantsa
| Protein | Avg ± SD
|
kcat/Kmd | RNA stimulation factor | ||
|---|---|---|---|---|---|
| Km (μM) | Vmaxb | kcatc | |||
| NS3 | 0.100 ± 0.001 | 0.48 ± 0.03 | 0.80 ± 0.02 | 8 | 3.0 |
| NS3-4A | 0.0010 ± 0.0001 | 0.14 ± 0.01 | 0.14 ± 0.01 | 140 | 5.0 |
| S1369R | 0.0010 ± 0.0002 | 0.16 ± 0.01 | 0.16 ± 0.01 | 160 | 4.0 |
| Y1702A | 0.5 ± 0.1 | 0.10 ± 0.02 | 0.10 ± 0.02 | 0.2 | 2.0 |
| M1708A | 0.5 ± 0.2 | 0.23 ± 0.02 | 0.23 ± 0.02 | 0.5 | 2.0 |
| S1369R/Y1702A | 0.3 ± 0.1 | 0.17 ± 0.02 | 0.17 ± 0.02 | 0.6 | 2.0 |
| S1369R/M1708A | 0.0020 ± 0.0003 | 0.090 ± 0.003 | 0.090 ± 0.003 | 45 | 2.0 |
These data are averages from three experiments.
Values are expressed in nmol of ATP hydrolyzed/min.
Values are expressed in nmol of ATP hydrolyzed/min·pmol of protein.
Values are expressed in nmol of ATP hydrolyzed/min·pmol of protein/μM RNA.
Given that NS4A dramatically enhances the apparent affinity between RNA and the functional ATPase site of NS3, it was important to determine whether NS4A influences the basal ATPase activity of NS3 in the absence of RNA binding. However, we found that the unstimulated ATPase activity of NS3-4A was actually somewhat lower than that of NS3 in the absence of RNA stimulation (0.030 ± 0.004 nmol of ATP/min and 0.16 ± 0.03 nmol of ATP/min, respectively). This difference in basal ATPase activities was due at least in part to the fact that ∼20% of our NS3-4A preparation is catalytically inactive due to the lack of autocleavage between NS3 and NS4A (3). In addition, the extent to which RNA stimulates maximal ATPase activity did not differ greatly between the two constructs. We observed that RNA stimulated ATPase activity of NS3-4A by fivefold (Table 2), while RNA stimulated ATPase activity by NS3 by threefold, as described previously (4) (Table 2). Therefore, the NS4A cofactor did not increase the maximal level of basal NS3 ATPase activity. In addition, the maximal rate constants for both NS3-4A and NS3 ATPase activities were stimulated to a similar extent by RNA binding. These data indicate that NS4A does not function by altering the inherent ATPase activity of NS3.
NS4A does not influence the kinetic parameters for RNA unwinding by NS3.
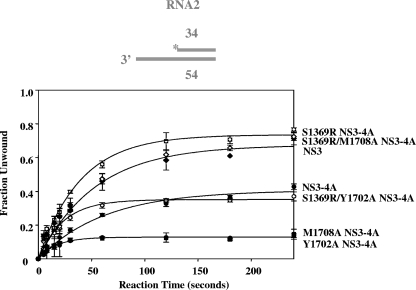
In addition to its role in coupling RNA binding with ATPase activity, NS4A might be expected to influence the kinetic parameters of RNA unwinding by modulating parameters such as the overall rate constant for NS3 unwinding, processivity, or the rate constant for functional NS3-RNA complex formation. To address the first issue, we compared the unwinding by NS3 and NS3-4A of RNA duplexes of various lengths (12 to 34 bp). In each case, NS3-4A unwound the RNA duplex with a rate constant and amplitude that was similar to that of NS3. For example, NS3 unwound the 34-bp RNA substrate RNA2 (see Materials and Methods) to a final amplitude of 67% ± 2% with an observed rate constant of 0.020 ± 0.002 s−1 (see Fig. 5), while NS3-4A unwound RNA2 to a final amplitude of 41% ± 2% with an observed rate constant of 0.020 ± 0.002 s−1 (Fig. 5). The slightly lower amplitude for unwinding by NS3-4A is attributable to the fact that the NS3-4A protein preparation contains ∼20% unreactive, uncleaved NS3/4A polyprotein (3) and is unlikely to reflect any innate differences in reactivity by the NS3-4A complex. Furthermore, NS3-4A did not display enhanced unwinding of longer duplexes relative to short ones (data not shown), which is consistent with previous work demonstrating the NS4A is not a processivity factor for NS3 (23).
FIG. 5.
RNA unwinding by NS3-4A variants. The unwinding parameters of the 34-bp RNA duplex RNA2 for NS3 (solid diamonds) are A = 0.67 ± 0.02 and kobs = 0.020 ± 0.002 s−1; for NS3-4A (solid circles), they are A = 0.41 ± 0.02 and kobs = 0.020 ± 0.002 s−1; for NS3-4A Y1702A (solid squares), they are A = 0.13 ± 0.01 and kobs = 0.06 ± 0.01 s−1; for NS3-4A M1708A (crossed circles), they are A = 0.13 ± 0.01 and kobs = 0.06 ± 0.01 s−1; for NS3-4A S1369R (hatched squares), they are A = 0.74 ± 0.02 and kobs = 0.03 ± 0.01 s−1; for NS3-4A S1369R/M1708A (hatched diamonds), they are A = 0.70 ± 0.02 and kobs = 0.02 ± 0.01 s−1; and for NS3-4A S1369R/Y1702A (hatched circles), they are A = 0.35 ± 0.01 and kobs = 0.04 ± 0.01 s−1. These data are the averages of three experiments, and the error represents the standard deviation.
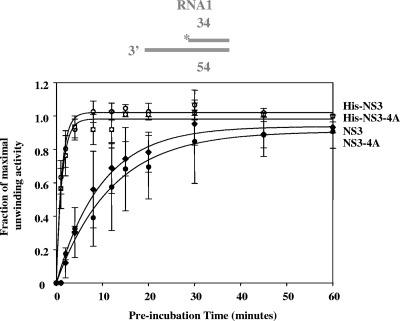
Next, we set out to determine whether NS4A influences the rate constant for the formation of NS3-RNA complexes that are capable of unwinding (functional helicase complexes) (23). In these experiments, NS3-4A or NS3 was incubated with a double-stranded, 34 bp RNA substrate (RNA1) (2) for various amounts of time before initiating unwinding with the addition of ATP. Based on the rate constants for functional complex formation by NS3 and NS3-4A, NS4A did not stimulate the rate constant for functional complex formation (kr) between NS3 and an RNA substrate (for NS3, kr = 0.05 ± 0.03 min−1 and for NS3-4A, kr = 0.02 ± 0.01 min−1) (Fig. 3).
FIG. 3.
The NS4A cofactor does not affect the rate of functional helicase formation on an RNA unwinding substrate. The rate constant for functional complex formation for NS3 was 0.05 ± 0.03 min−1 (solid diamonds); for NS3-4A, it was 0.02 ± 0.01 min−1 (solid circles); for His-NS3, it was 0.7 ± 0.1 min−1 (hatched circles); and for His-NS3-4A, it was 1.0 ± 0.3 min−1 (hatched squares). The data shown are the averages of three experiments, and the error represents the standard deviation.
The addition of charged residues (e.g., His6 tags) to the NS3 N terminus greatly increases the affinity of NS3 for RNA (4). Because previous studies on the influence of NS4A utilized His-tagged proteins (23), we sought to determine whether an N-terminal His tag would increase the rate constant for functional complex formation between NS3-4A and an RNA substrate. NS4A did not significantly influence the observed rate constants for functional complex formation in the context of His-tagged proteins (Fig. 3). However, His-NS3 formed a functional complex with RNA substrate 14-fold faster than NS3 and His-NS3-4A formed a functional complex ∼50-fold faster than NS3-4A (for His-NS3, kr = 0.7 ± 0.1 min−1; for NS3-4A, kr = 1.0 ± 0.3 min−1) (Fig. 3). There is also an increase in the direct RNA-binding affinity of NS3-4A in the presence of the N-terminal His tag (Table 1).
NS4A mutants that are defective in ATP-coupled RNA binding are lethal in vivo.
Although our findings indicate that NS4A helps couple ATP utilization with strong RNA binding by NS3 in vitro, it was important to establish whether this behavior contributes to NS4A function in vivo. In addition, we wanted to determine whether a specific region of NS4A was involved in this function. To this end, we examined the properties of NS4A mutants that are known to be defective in replication.
It was previously shown that the C terminus of NS4A (the “acidic domain”) (Fig. 1B) contains conserved residues that are essential for replication by HCV replicons (18). Two of these highly conserved, essential residues are Y1702 and M1708. When these residues were mutated to alanines, replication was severely reduced (18). A spontaneous second-site mutation (S1369R, located in NS3 helicase subdomain 2) was found to suppress the M1708A mutation (18). These effects were not due to defects in NS3-4A protease activity because proteolysis assays revealed that Y1702A and M1708A are slightly more efficient proteases than wild-type NS3-4A (the kcat/Km values for wild-type NS3-4A, Y1702A, and M1708A are 0.12 ± 0.01, 0.70 ± 0.02, and 0.45 ± 0.14 pmol of RET-S1 cleaved/s·pmol of enzyme/μM substrate, respectively [see Materials and Methods]). This observation is in agreement with the fact that the HCV polyprotein was fully proteolytically processed in cells transfected with replicons containing these mutations (18).
To explore a more direct role for the NS4A acidic domain during replication, we investigated the ability of the Y1702A and M1708A mutants to bind and unwind RNA in vitro. When filter-binding assays were used to evaluate the overall affinity of NS3-4A mutants for ssRNA, only small variations in affinity were observed (Kd = 10 ± 1 nM for wild-type NS3-4A, Kd = 13 ± 1 for Y1702A, and Kd = 5 ± 1 for M1708A) (Fig. 2 and Table 1). However, when we examined RNA binding that was functionally coupled to ATPase activity (the Km for RNA), we observed that the Y1702A and M1708A mutations reduced functional NS3-4A binding affinity for RNA by 500-fold relative to the wild type (Km = 500 ± 100 nM and 500 ± 200 nM) (Fig. 4 and Table 2). Strikingly, the S1369R suppressor mutant displayed an ATP-coupled RNA affinity that was identical to that of wild-type NS3-4A (Fig. 4 and Table 2), and the reduced ATP-coupled RNA affinity of the M1708A mutant was suppressed by the addition of the S1369R mutation (for wild-type NS3-4A, Km = 1.0 ± 0.1 nM; for S1369R/M1708A, Km = 2.0 ± 0.3 nM) (Fig. 4 and Table 2).
Because the Y1702A and M1708A mutations reduced coupled RNA binding by 500-fold, it was important to determine whether these mutations also reduced RNA unwinding activity. Indeed, a sharp drop in reaction amplitude (A) was observed for unwinding of a 34-bp duplex RNA (RNA2) by the acidic-domain mutants relative to wild-type NS3-4A (Fig. 5) (for wild-type NS3-4A, A = 0.41 ± 0.02; for Y1702A, A = 0.13 ± 0.01; for M1708A, A = 0.13 ± 0.01). Importantly, the suppressor mutation S1369R significantly increased RNA unwinding activity by the M1708A mutant (Fig. 5) (for S1369R/M1708A, A = 0.70 ± 0.02).
We wondered whether S1369R acts as a general suppressor for replication-deficient NS4A acidic-domain mutations. Thus, we sought to determine whether the S1369R mutation can restore the deficiencies in coupled RNA binding and unwinding by the Y1702A mutant. In previous studies, no spontaneous suppressors were isolated for the Y1702A mutant (18). We observed that the S1369R mutation slightly rescues the growth of the defective Y1702A replicon (data not shown). Interestingly, the S1369R mutation enhanced direct RNA binding by NS3-4A (Y1702A) roughly sixfold (from a Kd of 13 ± 1 nM to 2.0 ± 0.1 nM) (Fig. 2B and Table 1). In addition, S1369R rescued RNA unwinding by Y1702A to the levels observed for wild-type NS3-4A, and it increased the RNA unwinding rate constant by twofold (for wild-type NS3-4A, A = 0.41 ± 0.02 and kobs = 0.02 ± 0.01; for S1369R/Y1702A, A = 0.35 ± 0.01 and kobs = 0.04 ± 0.01) (Fig. 5). However, in RNA-stimulated ATPase assays, NS3-4A (S1369R/Y1702A) functionally bound RNA only slightly better than NS3-4A (Y1702A) (for Y1702A, Km = 0.5 ± 0.1 μM; for S1369R/Y1702A, Km = 0.3 ± 0.1 μM) (Fig. 4 and Table 2). Thus, the S1369R mutation in the NS3 helicase domain rescued the RNA unwinding of mutants containing either M1708A or Y1702A in the NS4A acidic domain, but it enhanced it to a greater extent in the case of M1708A. Furthermore, the S1369R mutation only fully rescued the functional RNA-binding defect of NS3-4A (M1708A) and not that of NS3-4A (Y1702A).
DISCUSSION
NS4A promotes strong, ATP-coupled RNA binding to NS3.
Here we show that the activated, ATP-bound state of NS3 binds RNA tightly only in the presence of NS4A, thereby establishing a central role for the NS4A cofactor in the helicase mechanism. This result is strikingly reminiscent of a recent study on the ATPase cycle mechanism of the DEAD-box RNA helicase DbpA, which showed strong thermodynamic coupling between RNA binding and specific stages in the cycle of ATP hydrolysis (12). In that case, RNA is bound tightly only during a fleeting stage of the cycle that immediately precedes and activates the helicase for productive ATP hydrolysis. The affinity of this state is 10-fold stronger than that of the ground state (12). Remarkably, the magnitude of this effect appears to be identical for NS3-4A, which displays a 10-fold increase in RNA affinity relative to the ground state [e.g., Km(ATPase) = 1.0 ± 0.1 nM and Kd(RNA) = 10 ± 1 nM]. Interestingly, this enhancement is not observed at all for NS3 in isolation, suggesting that NS4A is required for the proper power stroke and ATP utilization by the enzyme.
A comparison of RNA affinities for NS3-4A and NS3 is informative because it may suggest other potential roles for the cofactor. Although NS3-4A binds RNA most tightly during ATP hydrolysis, high affinity for RNA is maintained throughout the cycle (Kd or Km never go above 10 nM). Thus, NS3-4A remains tightly bound to its RNA substrate under all circumstances. This contrasts with NS3, which actually loosens its grip on RNA in the presence of ATP (for NS3, Kd = 9 ± 3 nM in the absence of ATP and Km = 100 ± 3 nM in the presence of ATP) (4).
NS4A-stimulated RNA binding and unwinding by NS3 are essential for replicon replication.
This in vitro biochemical analysis of NS4A function was complemented by functional genetic studies with the replicon system (18). Together, these data implicate the acidic domain of NS4A in stimulating the function of NS3 and strongly suggest that NS3 helicase activity and ATP-coupled RNA binding to NS3 are essential for the function of HCV. Although much has been written about the importance of the NS3 helicase activity during viral replication (1, 8, 19), no studies have directly linked RNA binding and helicase activity to a specific role in the viral life cycle. That NS3 serves as a helicase is inferred from the presence of conserved “helicase” motifs in the sequence and from in vitro biochemical studies of RNA unwinding by NS3. However, a diversity of new studies on the phylogenetically related group of “superfamily 2 helicases” has revealed that these proteins do not necessarily function solely in RNA and DNA unwinding and that other mechanistic roles in RNA binding, conformational switching, protein displacement, and other activities are equally likely (14, 21, 27, 34, 36). Nevertheless, a previous mutational study on the HCV replicon system is consistent with NS3 function as a helicase. It was observed that mutations that abrogate RNA binding and unwinding (R393A and F438A in NS3 helicase subdomain 2, as well as W501A in subdomain 3) abrogate RNA replication in the replicon (16). Our mutational analysis extends and complements this work.
Here we show that both the NS4A acidic domain and NS3 helicase domain 2 are important for strong, ATP-coupled RNA binding and for RNA unwinding in vitro and in vivo. Although the Y1702A and M1708A mutations do not significantly alter ground-state RNA binding by NS3-4A in the absence of ATP (Kd = 13 ± 1 nM and 5 ± 1 nM, respectively), these mutations significantly decreased RNA affinity in the presence of ATP, displaying markedly reduced RNA-stimulated ATPase activities (Km = 500 ± 100 nM and 500 ± 200 nM, respectively). In addition, these mutations severely inhibited efficient RNA unwinding. Importantly, the addition of the NS3 helicase domain 2 mutation S1369R to the M1708A mutant was restorative, compensating for both the reduced RNA binding affinity in the presence of ATP (Fig. 4 and Table 2) and the loss of robust RNA unwinding activity (Fig. 5). The compensatory S1369R mutation enhanced Y1702A RNA binding in the presence of ATP as well, but to a lesser extent than in the M1708A case (for S1369R/Y1702A, Km = 300 ± 100 nM). This probably accounts for the smaller improvement in RNA unwinding for Y1702A compared to S1369R/Y1702A (Fig. 5). Interestingly, we observed that the S1369R mutation by itself enhanced NS3-4A unwinding of a 34-bp RNA substrate (Fig. 5). The S1369R mutation may enhance activity by providing additional positive charge to an extended, basic surface on NS3 helicase subdomain 2. It has been suggested that this region of NS3 holds the RNA product strands apart after unwinding, thereby increasing the driving force of the helicase (22), and the S1369R mutation appears to enhance this effect. In addition, the S1369R mutation has recently been found to suppress replication defects caused by mutations in the HCV integral membrane protein NS4B (24), suggesting that the S1369R mutation may serve as a general replication enhancer through its enhancement of RNA unwinding activity. The fact that the Y1702A and M1708A mutations prevent or severely impair HCV replicon replication, respectively (18), indicates that tight RNA binding in the presence of ATP and robust RNA unwinding by NS3-4A are important for replicon replication, thereby providing an important link between the helicase behaviors that are monitored in vitro with the functions of the replicon in vivo.
Although the NS4A acidic domain mediates NS3-4A functional RNA binding with respect to ATPase activity and RNA unwinding, this domain may be important for HCV RNA replication for other reasons as well. Besides measuring the biochemical activities of NS3-4A (Y1702A) and NS3-4A (M1708A), we also examined other acidic-domain mutations that are known to reduce replicon growth (17). For example, mutation D1697A severely inhibited replicon growth (17) and, similar to the Y1702A and M1708A mutations, D1697A reduced functional RNA binding with respect to ATPase activity, as well as reduced RNA unwinding (data not shown). However, two other acidic-domain mutations that reduce replicon growth, R1698A and R1703A (17), did not significantly alter NS3-4A biochemical activities (data not shown). Thus, in addition to its role in the enzymatic activities of NS3-4A, the NS4A acidic domain is likely to have other crucial functions, such as mediating protein-protein interactions. Indeed, a recent study predicted that NS4A likely plays a central role in mediating HCV protein-protein interactions (5).
NS3 is a DExH/D-box protein that requires at least two cofactors.
Cofactor proteins are often required by DExH/D-box proteins for enzymatic activation and localization to specific substrates. For example, the DExH/D-box proteins RhlB and Dbp8 bind to the cofactor proteins RNase E and Esf2, respectively(11, 31). RhlB requires the RNase E cofactor to stimulate its ATPase activity and localize it to an unwinding substrate (15, 31). Dbp8 requires the Esf2 cofactor for ATPase stimulation and localization to rRNA (11). It appears that NS3hel requires not only the NS3 protease domain but also a protease domain that is modified by the NS4A cofactor to enhance RNA binding in the presence of ATP. Thus, the NS3 helicase requires two cofactors for function: one in cis (the protease domain) and one in trans (NS4A). Furthermore, it utilizes the NS4A cofactor in a manner similar to that of other DExH/D proteins, which typically use cofactors to stimulate some aspect of ATPase activity and its coupling with RNA (11, 31, 33). By using cofactors in this way, DExH/D proteins such as NS3 have acquired tightly regulated helicase functions that can be controlled and attenuated in response to the needs of the organism or pathogen.
Role of NS4A in ground-state binding of NS3 to RNA.
Although the present study demonstrates that NS4A facilitates productive binding of NS3 to RNA, this enhancement in RNA binding does not occur in the manner that we previously proposed (23). We show here that the NS4A cofactor does not enhance the rate constant for assembly of functional NS3-substrate complexes as reported previously. The reason for this discrepancy is that our previous studies utilized NS3 and NS3-4A protein expression construct designs that influenced their relative affinity for RNA. We have now shown that adding a positive charge to NS3 in the form of a histidine tag greatly enhances RNA binding (4). Extra positive charges can enhance direct binding, RNA-NS3 complex assembly, or RNA-stimulated ATPase activity (4). Our previous study concluded that NS4A enhances the rate constant for NS3 functional helicase complex formation on RNA substrates, but it did not account for differing N-terminal charges on the NS3 and NS3-4A proteins utilized in the study (23). The N terminus of the NS3 construct was neutral (23), while the N terminus of the NS3-4A construct was appended with positively charged residues (26).
We show here, using untagged proteins and identically tagged proteins, that the NS4A cofactor does not alter the apparent Kd of NS3 for RNA in the absence of ATP. Instead, it stimulates the coupling of RNA binding with ATPase activity, and it stimulates high-affinity binding during the process of ATP hydrolysis. Moreover, we show that this effect is important for the RNA unwinding activity of NS3.
Acknowledgments
We thank Victor Serebrov and Steven Ding for helpful discussions and comments on the manuscript.
R.K.F.B was supported by a Ruth Kirschstein postdoctoral fellowship from the National Institutes of Health (F32 GM071120-01A1). B.D.L. was supported by a National Institutes of Health award (K01CA107092). A.M.P. is an investigator with the Howard Hughes Medical Institute. This study was also supported by a generous grant from the National Institutes of Health (GM60620).
Footnotes
Published ahead of print on 19 January 2009.
REFERENCES
- 1.Appel, N., T. Schaller, F. Penin, and R. Bartenschlager. 2006. From structure to function: new insights into hepatitis C virus RNA replication. J. Biol. Chem. 2819833-9836. [DOI] [PubMed] [Google Scholar]
- 2.Beran, R. K., M. M. Bruno, H. A. Bowers, E. Jankowsky, and A. M. Pyle. 2006. Robust translocation along a molecular monorail: the NS3 helicase from hepatitis C virus traverses unusually large disruptions in its track. J. Mol. Biol. 358974-982. [DOI] [PubMed] [Google Scholar]
- 3.Beran, R. K., and A. M. Pyle. 2008. Hepatitis C viral NS3-4A protease activity is enhanced by the NS3 helicase. J. Biol. Chem. 28329929-29937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beran, R. K., V. Serebrov, and A. M. Pyle. 2007. The serine protease domain of hepatitis C viral NS3 activates RNA helicase activity by promoting the binding of RNA substrate. J. Biol. Chem. 28234913-34920. [DOI] [PubMed] [Google Scholar]
- 5.Campo, D. S., Z. Dimitrova, R. J. Mitchell, J. Lara, and Y. Khudyakov. 2008. Coordinated evolution of the hepatitis C virus. Proc. Natl. Acad. Sci. USA 1059685-9690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de la Cruz, J., D. Kressler, and P. Linder. 1999. Unwinding RNA in Saccharomyces cerevisiae: DEAD-box proteins and related families. Trends Biochem. Sci. 24192-198. [DOI] [PubMed] [Google Scholar]
- 7.Failla, C., L. Tomei, and R. De Francesco. 1994. Both NS3 and NS4A are required for proteolytic processing of hepatitis C virus nonstructural proteins. J. Virol. 683753-3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frick, D. N. 2007. The hepatitis C virus NS3 protein: a model RNA helicase and potential drug target. Curr. Issues Mol. Biol. 91-20. [PMC free article] [PubMed] [Google Scholar]
- 9.Frick, D. N., R. S. Rypma, A. M. Lam, and B. Gu. 2004. The nonstructural protein 3 protease/helicase requires an intact protease domain to unwind duplex RNA efficiently. J. Biol. Chem. 2791269-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallinari, P., C. Paolini, D. Brennan, C. Nardi, C. Steinkuhler, and R. De Francesco. 1999. Modulation of hepatitis C virus NS3 protease and helicase activities through the interaction with NS4A. Biochemistry 385620-5632. [DOI] [PubMed] [Google Scholar]
- 11.Granneman, S., C. Lin, E. A. Champion, M. R. Nandineni, C. Zorca, and S. J. Baserga. 2006. The nucleolar protein Esf2 interacts directly with the DExD/H box RNA helicase, Dbp8, to stimulate ATP hydrolysis. Nucleic Acids Res. 343189-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henn, A., W. Cao, D. D. Hackney, and E. M. De La Cruz. 2008. The ATPase cycle mechanism of the DEAD-box rRNA helicase, DbpA. J. Mol. Biol. 377193-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikeda, M., M. Yi, K. Li, and S. M. Lemon. 2002. Selectable subgenomic and genome-length dicistronic RNAs derived from an infectious molecular clone of the HCV-N strain of hepatitis C virus replicate efficiently in cultured Huh7 cells. J. Virol. 762997-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jankowsky, E., C. H. Gross, S. Shuman, and A. M. Pyle. 2001. Active disruption of an RNA-protein interaction by a DExH/D RNA helicase. Science 291121-125. [DOI] [PubMed] [Google Scholar]
- 15.Khemici, V., I. Toesca, L. Poljak, N. F. Vanzo, and A. J. Carpousis. 2004. The RNase E of Escherichia coli has at least two binding sites for DEAD-box RNA helicases: functional replacement of RhlB by RhlE. Mol. Microbiol. 541422-1430. [DOI] [PubMed] [Google Scholar]
- 16.Lam, A. M., and D. N. Frick. 2006. Hepatitis C virus subgenomic replicon requires an active NS3 RNA helicase. J. Virol. 80404-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindenbach, B. D., B. M. Pragai, R. Montserret, R. K. Beran, A. M. Pyle, F. Penin, and C. M. Rice. 2007. The C terminus of hepatitis C virus NS4A encodes an electrostatic switch that regulates NS5A hyperphosphorylation and viral replication. J. Virol. 818905-8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindenbach, B. D., B. M. Pragai, R. Montserret, R. K. Beran, A. M. Pyle, F. Penin, and C. M. Rice. 2007. The C terminus of hepatitis C virus NS4A encodes an electrostatic switch that regulates NS5A hyperphosphorylation and viral replication. J. Virol. 818905-8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindenbach, B. D., and C. M. Rice. 2005. Unravelling hepatitis C virus replication from genome to function. Nature 436933-938. [DOI] [PubMed] [Google Scholar]
- 20.Lohmann, V., F. Korner, J. Koch, U. Herian, L. Theilmann, and R. Bartenschlager. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285110-113. [DOI] [PubMed] [Google Scholar]
- 21.Lund, M. K., and C. Guthrie. 2005. The DEAD-box protein Dbp5p is required to dissociate Mex67p from exported mRNPs at the nuclear rim. Mol. Cell 20645-651. [DOI] [PubMed] [Google Scholar]
- 22.Myong, S., M. M. Bruno, A. M. Pyle, and T. Ha. 2007. Spring-loaded mechanism of DNA unwinding by hepatitis C virus NS3 helicase. Science 317513-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pang, P. S., E. Jankowsky, P. J. Planet, and A. M. Pyle. 2002. The hepatitis C viral NS3 protein is a processive DNA helicase with cofactor enhanced RNA unwinding. EMBO J. 211168-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paredes, A. M., and K. J. Blight. 2008. Genetic interaction between hepatitis C virus NS4B and NS3 important for RNA replication. J. Virol. [DOI] [PMC free article] [PubMed]
- 25.Py, B., C. F. Higgins, H. M. Krisch, and A. J. Carpousis. 1996. A DEAD-box RNA helicase in the Escherichia coli RNA degradosome. Nature 381169-172. [DOI] [PubMed] [Google Scholar]
- 26.Sali, D. L., R. Ingram, M. Wendel, D. Gupta, C. McNemar, A. Tsarbopoulos, J. W. Chen, Z. Hong, R. Chase, C. Risano, R. Zhang, N. Yao, A. D. Kwong, L. Ramanathan, H. V. Le, and P. C. Weber. 1998. Serine protease of hepatitis C virus expressed in insect cells as the NS3/4A complex. Biochemistry 373392-3401. [DOI] [PubMed] [Google Scholar]
- 27.Solem, A., N. Zingler, and A. M. Pyle. 2006. A DEAD protein that activates intron self-splicing without unwinding RNA. Mol. Cell 24611-617. [DOI] [PubMed] [Google Scholar]
- 28.Suzich, J. A., J. K. Tamura, F. Palmer-Hill, P. Warrener, A. Grakoui, C. M. Rice, S. M. Feinstone, and M. S. Collett. 1993. Hepatitis C virus NS3 protein polynucleotide-stimulated nucleoside triphosphatase and comparison with the related pestivirus and flavivirus enzymes. J. Virol. 676152-6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taliani, M., E. Bianchi, F. Narjes, M. Fossatelli, A. Urbani, C. Steinkuhler, R. De Francesco, and A. Pessi. 1996. A continuous assay of hepatitis C virus protease based on resonance energy transfer depsipeptide substrates. Anal. Biochem. 24060-67. [DOI] [PubMed] [Google Scholar]
- 30.Reference deleted.
- 31.Vanzo, N. F., Y. S. Li, B. Py, E. Blum, C. F. Higgins, L. C. Raynal, H. M. Krisch, and A. J. Carpousis. 1998. Ribonuclease E organizes the protein interactions in the Escherichia coli RNA degradosome. Genes Dev. 122770-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voet, D., and J. G. Voet. 1995. Biochemistry, 2nd ed. J. Wiley & Sons, Inc., New York, NY.
- 33.Weirich, C. S., J. P. Erzberger, J. S. Flick, J. M. Berger, J. Thorner, and K. Weis. 2006. Activation of the DExD/H-box protein Dbp5 by the nuclear-pore protein Gle1 and its coactivator InsP6 is required for mRNA export. Nat. Cell Biol. 8668-676. [DOI] [PubMed] [Google Scholar]
- 34.Yang, Q., and E. Jankowsky. 2005. ATP- and ADP-dependent modulation of RNA unwinding and strand annealing activities by the DEAD-box protein DED1. Biochemistry 4413591-13601. [DOI] [PubMed] [Google Scholar]
- 35.Yi, M., Y. Ma, J. Yates, and S. M. Lemon. 2007. Compensatory mutations in E1, p7, NS2, and NS3 enhance yields of cell culture-infectious intergenotypic chimeric hepatitis C virus. J. Virol. 81629-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5730-737. [DOI] [PubMed] [Google Scholar]