Abstract
Lentiviral vectors (lentivectors) are effective for stimulation of cell-mediated and humoral immunity following subcutaneous and intramuscular immunization. However, lentivector genome integration carries a risk of perturbation of host gene expression. Here, we demonstrate that lentivectors with multiple mutations that prevent integration are also effective immunogens. First, systemic CD8+ T-cell responses to the model antigen ovalbumin were detected following subcutaneous injection of nonintegrating lentivectors. Transfer of transgenic OT1 T cells demonstrated that antigen presentation persisted for at least 30 days. Furthermore, an enhanced CD8+ T-cell response, peaking at 7 days, was stimulated by coexpression of p38 MAP kinase or an NF-κB activator from the same vector. Second, we demonstrated systemic CD8+ T-cell and antibody responses to the secreted hepatitis B virus (HBV) surface antigen expressed from a nonintegrating lentivector injected intramuscularly. The induction, specificity, and kinetics of antibody production closely mimicked those of natural HBV infection. In this case, both the vector genome and the immune response were maintained for at least 2 months. Together, our data indicate that nonintegrating lentivectors can be employed to generate effective vaccines.
Lentivectors are efficient tools for gene transfer because they can infect both dividing and nondividing cells. A number of recent studies have shown that lentivectors are promising vaccine candidates; they can induce protective and therapeutic immunity against tumors in mice (5, 7, 15, 25), generate CD8+ and CD4+ T-cell and antibody responses to the human tumor antigen NY-ESO-1 (9, 17, 22), induce protective humoral immunity to West Nile virus (13), and generate T-cell and antibody responses to human immunodeficiency virus (HIV) (14, 20). The clinically relevant subcutaneous injection route is thought to be particularly good at inducing T-cell responses, because lentivectors transduce skin-derived dendritic cells, which migrate to the draining lymph node and present antigen to T cells (11). Indeed, targeting of lentivectors to dendritic cells results in effective subcutaneous immunization (17, 31), and activating dendritic cells, by inclusion of signaling stimulators in the lentivector, increases the T-cell response following subcutaneous immunization (8, 24). Two studies have shown that intramuscular immunization of lentivectors stimulates anti-HIV envelope T-cell and antibody responses (4, 20).
The fact that lentivectors integrate into cellular DNA raises a possibility that they may be mutagenic. Clearly, they are less prone to inducing tumors than gammaretroviral vectors in a mouse insertional mutagenesis assay (19). However, we have detected lentiviral vector insertional gene activation in a cell line assay (2), and upregulation of adjacent genes by self-inactivating lentivectors has been reported (10). Recently, integration-deficient lentiviral vectors were described, with mutations in either integrase (23, 29, 30), the vector long terminal repeat (LTR) (1), or a combination (1). Following cell entry, reverse transcription, and nuclear transport, these vectors persist as circular episomes; if the target cell divides, these episomes are lost, but in nondividing cells, they persist and give rise to stable gene expression. These nonintegrating lentivectors have been shown to mediate long-term gene expression in nondividing tissues, such as retina (30), brain (23), and muscle (1).
Our aim was to compare integrating and nonintegrating lentivectors for their ability to work as vaccines. One previous report has compared anti-HIV gp120 T-cell and antibody responses in mice following intramuscular immunization with integrating or nonintegrating lentivectors encoding gp120 and GM-CSF (20). In their study, a single, relatively high dose of either vector generated prolonged CD8+ T-cell and antibody responses, with those to the nonintegrating lentivector being somewhat lower. We have now compared the two types of vector in subcutaneous immunization using the model antigen ovalbumin (OVA). This allowed us to measure the duration of antigen presentation by adoptive transfer of transgenic T cells and also vaccine efficacy in a tumor therapy model. In addition, to characterize the antibody responses generated by the two types of vector in comparison to DNA vaccination, we have used intramuscular immunization with vectors encoding the clinically relevant hepatitis B virus (HBV) surface antigen (HBsAg).
We report that nonintegrating lentivectors are effective in subcutaneous and intramuscular immunizations. Although a higher dose of nonintegrating vectors is required in the tumor therapy model, antigen presentation persists for up to 30 days with either vector, and the nonintegrating vector is effective for tumor therapy if dendritic cell-activating molecules are included. We also show that a single intramuscular injection of an integration-deficient lentivector expressing HBsAg gives strong and sustained humoral and cellular immune responses.
MATERIALS AND METHODS
Plasmids.
pDual-EGFP-IiOVA and pDual-MKK6-IiOVA are described in reference 8, and IiOVA is described in reference 25. For the pDual-vFLIP-IiOVA construct, the vFLIP fragment was subcloned into BamHI-NotI restriction sites to replace enhanced green fluorescent protein (EGFP). Mutations in the attachment sites and integrase (in the plasmid pCMVΔR8.74) are described in reference 1. To construct the HBsAg lentivector, pHR′SIN-SEW (6) was digested with BamHI, blunted by the DNA polymerase II Klenow fragment, and redigested with NotI to yield the vector backbone. Next, using suitable primers, the HBs(S) (ayw) fragment was amplified by PCR, using the pRc/CMV-HBs(S) reporter plasmid (kindly provided by Aldevron, ND) as a template.
Lentivector production and titration.
Lentivectors were produced by transfection of 293T cells by use of Fugene (for OVA vectors) or a PEI (for HBsAg vectors) protocol. As a control for pseudotransduction, viral vectors with an empty core (referred in the text as ghost vectors) were generated using the packaging constructs, and the pRc/CMV-HBs(S) reporter plasmid viral supernatant was harvested and concentrated using ultracentrifugation (25,000 × g for 2 h at 4°C). Aliquots of viruses were stored at −80°C. The titers of all lentivectors were determined using a colorimetric reverse transcriptase (RT) enzyme-linked immunosorbent assay (ELISA) kit (catalog no. 11468120910; Roche) according to the manufacturer's instructions; the titers/ml of the various vectors prepared in parallel showed little variation when quantitated by this method. We have previously reported that the vectors all show similar titers when quantitated by p24 ELISA or short-term transduction efficiency (1). Culture media were assayed using an HBsAg ELISA kit (Abazyme, MA) according to the supplied instructions.
Cells and mice.
293T, HeLa, HT1080, and C2C12 cells were grown in Dulbecco's modified Eagle's medium (Gibco) supplemented with 10% fetal calf serum (FCS) in 10% CO2. EG7.OVA cells (an EL4 cell line stably transfected with OVA) were grown in RPMI-1640 medium (Gibco) supplemented with 10% FCS and 0.4 mg/ml of G418. C57BL/6 mice at least 6 weeks old were used for OVA experiments (Harlan, United Kingdom). OT1 transgenic mice were a kind gift from Alistair Noble, King's College, London, United Kingdom. All vaccinations were carried out subcutaneously with lentivector preparations in Hanks balanced salt solution or 100 μg of OVA protein in complete Freund's adjuvant. For adoptive transfer of OT1 cells, purified splenocytes were injected intravenously. For HBsAg experiments, 6- to 8-week-old BALB/c mice were injected once intramuscularly (right thigh) with 20 μl of each vector in phosphate-buffered saline. Four mice were injected per group for analyses at different time points after the immunization. Mice were bled from the tail vain at the indicated time points, and the sera were collected and kept at −80°C. Splenocytes isolated from mouse spleen and bone marrow-derived cells were cultured in RPMI complete medium supplemented with 10% FCS and penicillin-streptomycin under standard conditions. The muscle injection site was removed for DNA analysis.
Cell staining and FACS analysis.
Before the adoptive transfer experiments, OT1 cells were isolated from OT1 transgenic mice and stained using a carboxyfluorescein diacetate succinimidyl ester (CFSE) staining kit (Invitrogen) according to the manufacturer's instructions. For analysis of cell expansion, splenocytes were isolated and stained with the following antibodies: anti-mouse CD8-fluorescein isothiocyanate (Proimmune), Vα2-phycoerythrin (PE; Caltag), and Vβ5.1,5.2-allophycocyanin (APC; BD Biosciences). Samples were collected on a FACSCalibur (BD), using appropriate channels for APC and PE. The analysis was performed by gating first on forward scatter-side scatter and CD8-positive population and next on Vα2-PE-positive population and finally visualizing the samples as a function of FL1 fluorescence (for CFSE) and FL4 fluorescence (for APC).
ELISPOT analyses.
To assess gamma interferon (IFN-γ) production by splenocytes in OVA-vaccinated mice, spleens were extracted 11 days after vaccination, treated with red blood cell lysis buffer (Sigma), and stimulated overnight with the OVA major histocompatibility class I peptide SIINFEKL, residues 257 to 264 (Proimmune, Oxford, United Kingdom), in enzyme-linked immunospot (ELISPOT) plates (Millipore), which were coated with anti-IFN-γ antibody (BD Pharmingen) overnight at 4°C. The plates were developed the following day according to the manufacturer's instructions (Bio-Rad). For HBsAg-vaccinated mice, an Immobilon P (Millipore) plate was coated with purified rat anti-mouse IFN-γ antibody (capture antibody; eBioscience) at 37°C for 2 h. A single-cell suspension of splenocytes isolated from sacrificed mice was incubated with purified HBsAg (final concentration, 10 μg/ml) at 37°C for 48 h. Following incubation and subsequent washing, the plates were incubated with ExtrAvidin-alkaline phosphatase conjugate (Sigma).
Tumor experiments.
Groups of 10 mice (9 in the case of the vFLIP/DNW group) were injected subcutaneously with 2 × 106 EG7.OVA cells. The first vaccination was performed 3 days after tumor inoculation with 150 ng RT of lentivectors. The second vaccination was carried out 1 week later. Tumors were measured, and scores were calculated by multiplying the width and height of the tumoral mass. Mice were killed if the tumors exceeded 150 mm2 or if the mice showed signs of suffering.
Transgene detection.
Twenty-four and 72 h after subcutaneous injection, total RNA from local draining lymph nodes was extracted using TRI reagent (Ambion) according to the manufacturer's instructions. First-strand cDNA synthesis was carried out using a first-strand cDNA synthesis kit (NEB), using poly(A)-specific primers. PCR amplification was carried out using OVA-specific primers (forward, CCTATCTTCTGGCCTGGGAGTG; reverse, TCACAGGGTGGCAGCATCCAC), using HotStart Taq polymerase (Qiagen). DNA from the site of muscle injection (the anterior tibialis) was extracted using a DNEasy blood and tissue isolation kit (Qiagen). All samples in which amplification of the mouse β-actin gene was observed were included in subsequent PCR analyses for detection of all forms of the vector (the woodchuck hepatitis virus posttranscriptional regulatory element [WPRE; forward primer, 5′-TGGATTCTGCGCGGGA-3′; reverse primer, 5′-GAAGGAAGGTCCGCTGGATT-3′]) and that with 2-LTR U3 deleted (2-dLTR). The latter was detected by nested PCR. The first round of PCR was performed with 500 ng of DNA, with 20 picomoles each of the outer primers OPF (5′-GCTTAAGCCTCAATAAAGCTTGCCT-3′) and OPR (5′-AGAGAGCTCCCAGGCTCAG-3′). The second round of PCR was performed using the amplification product from the first round as the template and 50 picomoles each of the inner primers IPF (5′-GTGACTCTGGTAACTAGAGA-3′) and IPR (5′-CTGGTCTAACCAGAGAGAC-3′), spanning the 2-dLTR junction (U5-ΔU3).
RESULTS
Immunization with nonintegrating lentivectors stimulates a CD8+ T-cell response.
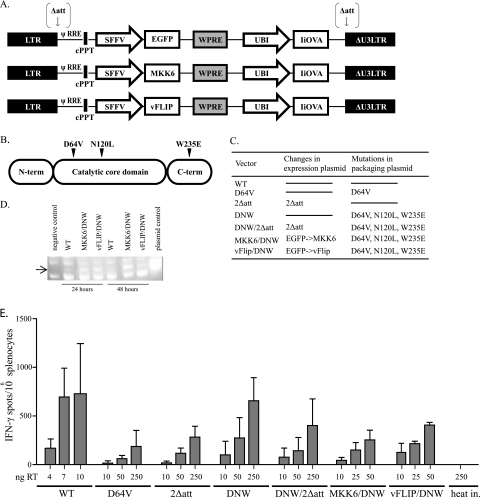
In order to examine immunization with nonintegrating lentivectors, we used vectors that were attenuated in integration, either by mutation of the att integrase recognition site in the LTR or by catalytic and noncatalytic mutations in integrase (1). Initially, we expressed the model antigen OVA and in some cases also expressed a constitutively active MKK6 protein, which stimulates the p38 pathway, or the vFLIP protein from Kaposi's sarcoma-associated herpesvirus, which stimulates the NF-κB pathway (Fig. 1A to C). We have previously reported that these activators enhance CD8+ T-cell responses in integrating lentivectors (8, 24). To confirm that the nonintegrating lentivectors express transgenes in vivo, we injected mice subcutaneously with equivalent doses (quantitated by an RT ELISA) of an integrating and each nonintegrating lentivector and then purified total RNA from the local draining lymph nodes. To detect transgene expression, we amplified RNA by using OVA-specific primers. Figure 1D shows that expression of OVA from each lentivector was detectable at 24 and 72 h postinjection in the lymph node. We have previously reported that subcutaneous injection of an integrating lentivector results in transduction of both skin-derived dendritic cells that migrate to the draining lymph node and resident dendritic cells and B cells in the lymph node (17).
FIG. 1.
Immunization with nonintegrating lentivectors. (A) All vectors have an invariant chain-OVA fusion (IiOVA) expressed under the control of the ubiquitin promoter and EGFP, activated MKK6, or vFLIP under the control of the SFFV promoter. Mutations in the attachment sites within the LTRs are indicated with arrows. LTR, HIV-1 LTR; ψ, HIV packaging signal; RRE, Rev response element; cPPT, central polypurine tract; UBI, human ubiquitin promoter; ΔU3 LTR, LTR with a deletion in the U3 region. (B) HIV-1 integrase consists of three domains: the N-terminal domain, the C-terminal domain, and a catalytic core domain. Mutations were introduced in two residues within the catalytic core (D64 and N120) and in one residue within the N-terminal domain (W235). (C) Summary of mutations within the expression and packaging plasmid for each of the vectors. WT, wild type. (D) OVA mRNA expression in lymph nodes. Samples were prepared from draining lymph nodes at 24 and 72 h after subcutaneous injection of 500 ng RT of the WT, DNW/2Δatt, MKK6/DNW, and vFLIP/DNW vectors as shown. (E) CD8+ T-cell responses to immunization. The x axis shows the dose of vector used for immunization measure by RT ELISA. The y axis depicts the number of IFN-γ spots per 106 splenocytes. The heat-inactivated control is 250 ng RT of WT vector inactivated at 95°C for 15 min.
We then immunized mice with various quantities of integrating and nonintegrating lentivectors and analyzed the systemic immune response by performing an ELISPOT assay for IFN-γ release by splenocytes in response to the OVA257-264 major histocompatibility class I peptide 11 days after vaccination. During production of vectors, there is a possibility of coprecipitation of proteins; we wanted to ensure that the induced immune responses were due to viral transduction, so we included a heat-inactivated control. We observed similar immunizations with each of the nonintegrating vectors that were dose dependent. We found that the minimum dose for inducing CD8+ T cells after immunization with nonintegrating vectors was 10 ng RT, and a dose of 250 ng RT gave a response comparable to that induced by vaccination with 10 ng RT of the integrating lentivector. Coexpression of a dendritic cell activator in a nonintegrating lentivector did not significantly enhance the number of IFN-γ-secreting cells in these experiments.
Antigens expressed from nonintegrating lentivectors are presented for up to 30 days.
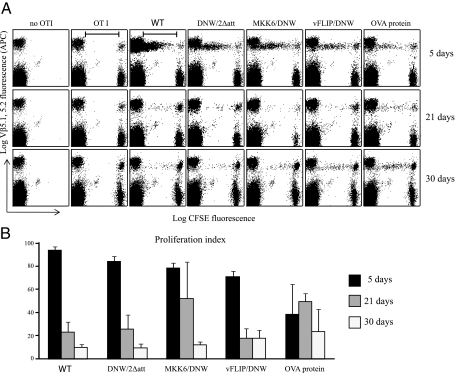
As the duration of antigen presentation might have a crucial impact on the outcome of vaccination, we analyzed this by adoptive transfer of CFSE-labeled OT1 transgenic cells into mice previously immunized with 150 ng RT of the lentivectors, and immunization with 100 μg of OVA protein in CFA was used as a control. We injected OT1 cells at three time points, 5, 21, and 30 days after immunization, and analyzed their proliferation after a further 5 days. The results are shown on a dot plot (Fig. 2A), and the proliferation index was calculated as the percentage of the starting population of CFSE-positive cells that underwent proliferation {[proliferated cells/(proliferated cells + remaining CFSE-positive cells)]} (Fig. 2B). Five days after immunization, at least 80% of the transferred cells proliferated in all the lentiviral groups. At 3 weeks postvaccination, there were still 20 to 30% of cells that proliferated, and this number decreased to about 10% at 30 days. These results indicated that antigen expressed from nonintegrating lentivectors was presented for as long as that expressed by integrating lentivectors. We have reported that 5 days after subcutaneous injection of lentivectors, transduced cells were found in the draining lymph node; however, after more than 10 days, they were no longer detected (17). We therefore conclude that the antigen-presenting cells migrate elsewhere after 10 days; the similar levels of persistence of presentation for nonintegrating and integrating vectors show that the antigen-presenting cells do not divide.
FIG. 2.
Persistence of antigen presentation. (A) OT1 cell proliferation induced by immunization with lentiviral vectors expressing IiOVA. Dot plot graphs show events gated on live cells and Vα 2.1 staining. Vβ5.1,5.2 fluorescence levels (y axis) are represented as a function of APC fluorescence. The top panel represents cell proliferation 5 days after vaccination, the middle panel 21 days, and the lower panel 30 days. The left column represents a group of mice that had no OT1 cells transferred and the next column a group of mice that had 2 × 106 CFSE-stained OT1 cells transferred but were not vaccinated. Five more groups follow, all of which underwent adoptive transfer after subcutaneous immunization with vectors/controls (the wild type [WT], DNW/2Δatt, MKK6/DNW, vFLIP/DNW, and 100 μg of OVA protein in complete Freund's adjuvant). (B) The proliferation index was calculated by dividing the number of cells that proliferated from the initial population of CFSE/Vβ5.1,5.2-positive cells (marked with a bar) divided by the total number of CFSE/Vβ5.1,5.2-positive cells. Different immunization groups are depicted on the x axis, and the proliferation index is marked on the y axis.
Effective tumor therapy by nonintegrating lentivectors.
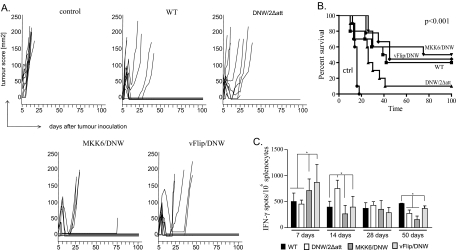
To evaluate the effectiveness of the OVA vectors in tumor therapy, we used EG7.OVA cells that rapidly develop in vivo into lymphomas. Three days after tumor inoculation, mice were vaccinated with the first 150-ng RT dose of lentivectors; then, immunization was repeated 1 week later with the same dose. The results are shown in Fig. 3. Tumors were visible about 5 days after inoculation and continued to grow in the control group until all mice had to be sacrificed by day 18 due to the sizes of the tumors. In the group vaccinated with integrating lentivector, eight mice showed initial regression. However, in four out of these eight mice, the tumors regrew; we have previously reported that this is due to the loss of OVA expression by the EG7 cell line (8). Mice vaccinated with nonintegrating lentivector showed survival that was prolonged in comparison to that for the control group, but after 40 days, only one mouse was tumor free. However, for mice vaccinated with nonintegrating lentivectors coexpressing dendritic cell activators, there was a highly significant improvement in the survival curve (Fig. 3B). In the group vaccinated with MKK6/DNW, all mice showed initial tumor regression and 50% of mice remained tumor free. In the group vaccinated with vFLIP/DNW, the patterns of tumor growth and regression were similar and four out of nine mice remained tumor free. All the mice that remained tumor free in these experiments were rechallenged either 4 or 9 months after the first tumor inoculation with the same dose of EG7.OVA cells. We observed that all the mice remained tumor free, which demonstrates that immunization with nonintegrating lentiviral vectors not only prolongs survival of mice with inoculated tumors but also confers complete protection from tumor rechallenge. The relevance of these results to human tumor vaccine development is obviously limited, and we are now pursuing the use of lentiviral vector immunization to break tolerance to self antigens.
FIG. 3.
Effective tumor therapy by nonintegrating lentivectors. (A) Groups of 10 mice were injected with the EG7 lymphoma cell line expressing OVA. Three and 10 days after tumor inoculation, mice were vaccinated with 150 ng RT of wild-type (WT), DNW/2Δatt, MKK6/DNW, or vFLIP/DNW vector or an integrating lentivector expressing EGFP as a control. Tumor growth was plotted as a function of tumor score (height by width) versus time in days. Mice were sacrificed when the tumor score exceeded 150 mm2. (B) Kaplan-Meier survival plot of mice vaccinated with a control vector, the WT, DNW/2Δatt, MKK6/DNW, and vFLIP/DNW. A log-rank test showed highly significant differences in survival between the groups (P < 0.001), a significant difference between survival in groups DNW/2Δatt and vFLIP/DNW (P = 0.03), and a very significant difference between DNW/2Δatt and MKK6/DNW (P = 0.01). (C) Time dependence of CD8+ T-cell responses. Mice were immunized with 150 ng RT of the WT, DNW/2Δatt, MKK6/DNW, and vFLIP/DNW. Animals were sacrificed 7, 14, 28, or 50 days after immunization, and an ELISPOT assay was performed. The outcome is represented as the number of IFN-γ spots per 1 × 106 splenocytes. At day 7, there was a very significant difference between groups vaccinated with MKK6/DNW or vFLIP/DNW and groups vaccinated with the WT or DNW/2Δatt. At day 14, DNW/2Δatt gave significantly higher responses than the three remaining groups. There were no differences between the groups at day 28. However, at day 50, the WT and vFLIP/DNW were significantly different from DNW/2Δatt and MKK6/DNW.
We were intrigued by the fact that tumor therapy with MKK6/DNW and vFLIP/DNW was more effective than that with DNW/2Δatt, as the CD8+ T-cell responses measured after 11 days were very similar (Fig. 1). We therefore immunized mice with 150 ng RT, the dose that was used in the tumor therapy experiment, and measured the CD8+ T-cell response over time. The assay carried out at 7 days postimmunization revealed significant differences in response between groups expressing dendritic cell activators and integrating or nonintegrating controls. By 28 days, there were no significant differences between the vectors, and at 50 days, the integrating vector and vFLIP/DNW had the highest levels (Fig. 3C). This could explain why nonintegrating lentivectors expressing dendritic cell activators perform better in tumor therapy. Dendritic cell activation apparently leads to earlier CD8+ T-cell expansion.
Sustainable expression of HBsAg from a nonintegrating lentivector.
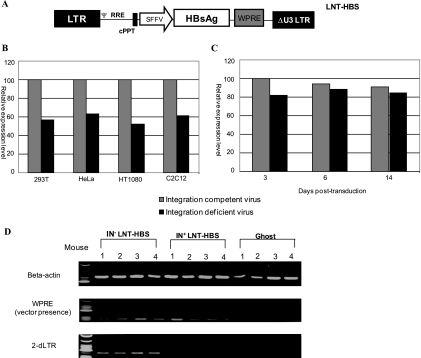
After establishing the efficacy of the nonintegrating lentivectors in tumor therapy, we chose to measure the immune response generated by a clinically relevant vaccine antigen. Toward this end, a self-inactivating vector expressing HBsAg from an internal spleen focus-forming virus (SFFV) promoter/enhancer was constructed, and both integrating and nonintegrating (D64V mutation) lentivectors were generated (Fig. 4A). To examine the expression level of the secreted HBsAg, we transduced various cell lines with 108 viral particles of the two vectors and measured HBsAg in the supernatant 72 h later. In these dividing cells, the level of HBsAg that accumulated with the use of the nonintegrating vector was approximately 50% of that seen with the integrating vector (Fig. 4B). As we have previously reported (1), the muscle cell line C2C12 can be differentiated into nondividing myotubes and then infected with lentivectors; in this case, nonintegrating lentivector genomes persist. In this system, we found that HBsAg expression levels for integrating and nonintegrating lentivectors were very similar for up to 2 weeks after transduction (Fig. 4C).
FIG. 4.
Vector expression and persistence. (A) Schematic representation of HIV-1-based self-inactivating vector expressing HBsAg from an internal SFFV-derived promoter/enhancer. HBsAg cDNA sequence is followed by the WPRE (W). The 3′ LTR contains a deletion of viral U3 region. RRE, Rev response element; cPPT, central polypurine tract. (B) Comparative expression levels of HBsAg from supernatant of transduced target cell lines infected separately at identical multiplicities of infection with integrating and nonintegrating LNT-HBS vector viruses. ELISA absorbance data for integrating vector were extrapolated to represent 100 on the scale for each cell type. (C) Comparison of surface antigen expression levels at different time points for differentiated C2C12 cells infected with integrating and nonintegrating LNT-HBS vector viruses. The ELISA absorbance at 3 days postinfection for the integrating vector was extrapolated to represent 100. Data represent means for two independent experiments performed in triplicates. (D) Detection of lentiviral vector sequences in immunized mice by PCR. DNA from immunized mice was extracted 60 days after the injection and amplified using different sets of primers as indicated (beta-actin, WPRE, and 2-dLTR). The amounts of template DNA varied from 100 to 500 ng, depending on the sample analyzed. The amplified products were separated on a 2% agarose gel and visualized by ethidium bromide staining. IN+, integration-competent virus; IN−, integration-deficient virus; LNT-NIGW, lentiviral vector expressing neomycin resistance gene from an internal SFFV promoter. Ψ, packaging signal.
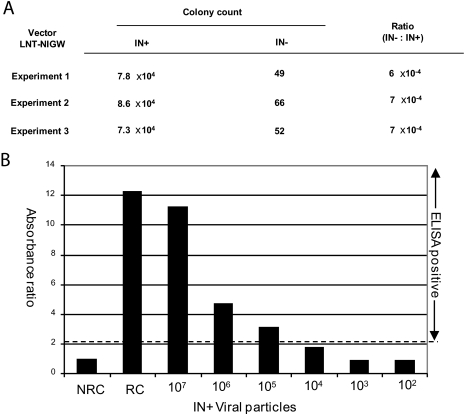
A low level of residual background integration has been reported to occur in spite of an integrase-deficient gag-pol packaging construct being employed (1). In this study, it is crucial to determine the contribution of the unintegrated vector over that of the integrated background in the expression of the surface antigen. For this purpose, HT1080 cells were infected with serial dilutions of integrating and nonintegrating LNT-NIGW viral particles and the transduced target cells were subjected to G418 selection. After selection, numbers of resistant colonies were counted and it was found that the neomycin resistance titers of integration-competent vector viruses ranged between 7.3 × 104 and 8.6 × 104 colonies per 107 viral particles, in comparison to 49 to 66 colonies for the integration-deficient vector virus. Therefore, the integration-deficient vector integrated between 6 × 104 and 7 × 104 times less frequently than the integration-competent vector (Fig. 5A). We further estimated the level of surface antigen expression from C2C12 target cells transduced with different amounts of the integration-competent LNT-HBS viral particles (range, 107 to 102 viral particles) and found that expression of surface antigen could not be detected when 104 viral particles or fewer were used for infection (Fig. 5B). We therefore concluded that by injecting 107 integration-deficient LNT-HBS viral particles, we could eliminate the possibility of any surface antigen expression from residually integrated vector viruses.
FIG. 5.
Residual integration activity of the integration-deficient vector. IN+, integration-competent virus; IN−, integration-deficient virus (D64V); LNT-NIGW, lentiviral vector expressing neomycin resistance gene from an internal SFFV promoter. (A) Colonies of G418-resistant HT1080 cells were counted after infection to determine the integration frequency per 107 viral particles. (B) Expression from integration-competent LNT-HBS. Differentiated C2C12 target cells were infected with serially diluted (10-fold) LNT-HBS vector viruses and assayed for HBsAg expression at 72 h postinfection. The optical density for each sample was divided by the optical density for the nonreactive control (NRC). A ratio of ≥2.1 denotes that the sample is positive for surface antigen expression. RC, reactive control.
We next examined the immune response to HBsAg following intramuscular delivery of integrating or nonintegrating lentivector, as this is an effective route for the induction of immune responses against secreted proteins. We therefore injected mice intramuscularly with 107 viral particles (equivalent to 75 ng of RT) of the two vectors and examined vector persistence using primers designed to amplify the WPRE sequence or the junction region between the end-joined, U3-deleted LTRs (2-dLTR). As shown in Fig. 4D, both vectors persisted for 60 days after injection and mice injected with the nonintegrating lentivector had PCR amplification products characteristic of the 2-dLTR circles that are a by-product of inhibition of integration.
Prolonged humoral and cellular immune responses to HBsAg.
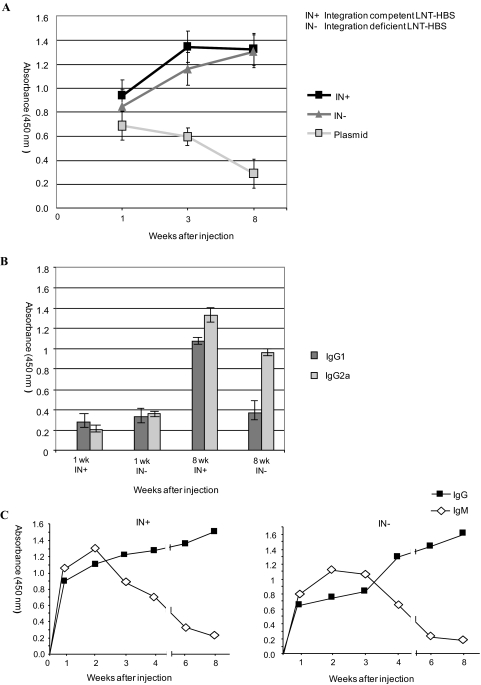
From previous human and primate vaccination studies, it is well established that circulating antibodies to HBsAg are sufficient to confer protection against HBV infection. We therefore investigated antibody responses elicited by a single intramuscular injection of 107 viral particles (equivalent to 75 ng of RT) of integrating and nonintegrating lentivectors. As a control, we injected plasmid DNA expressing HBsAg. HBsAg-specific antibodies (total immunoglobulin [Ig]) were detected within 1 week in mice injected with the integrating lentivector, which peaked by the third week and remained stable until the eighth week, when the experiment was terminated (Fig. 6A). A similar profile was observed for mice injected with the nonintegrating lentivector, except that antibody levels were slightly lower at the first two time points but rose to comparable levels at the eighth week. In mice immunized with plasmid, the antibody response declined steadily over the time period of our study.
FIG. 6.
Kinetics of humoral response in mice immunized separately with integrating (IN+), nonintegrating (IN−), and ghost LNT-HBS vector viruses. Sera were taken at different times after a single intramuscular injection, and pools were made from all sera (n = 4) taken at a single time point. (A) Total Ig response was measured by detecting bound antibodies with biotinylated anti-mouse Ig. (B) IgG response was detected by using rat anti-mouse IgG1 and IgG2a antibodies separately conjugated to horseradish peroxidase. (C) IgM-to-IgG class shift response was measured by detecting antibodies in mouse sera with biotin-conjugated anti-mouse IgM and previously described anti-mouse IgG1 antibody. IN+, integration-competent virus; IN−, integration-deficient virus.
We further investigated the Ig response by using anti-mouse monoclonal antibodies directed against both IgG1 and IgG2a subclasses. As shown in Fig. 6B and C, we observed identical trends of increasing IgG antibody levels for both the integrating and the nonintegrating lentivectors. Further, the IgM-to-IgG class shift, which is typical of natural infection and mediated by specific helper T cells, was also seen in both groups of immunized mice (Fig. 6C). For the mice injected with nonintegrating lentivector, IgG2a levels were significantly higher at week 8, which is characteristic of a Th1 response. Together, these data demonstrate an effective immunization against HBsAg with a single intramuscular injection of nonintegrating lentivector. The vector persistence that we detected in muscle (Fig. 4C) may explain the more-prolonged antibody response due to sustained expression of the antigen.
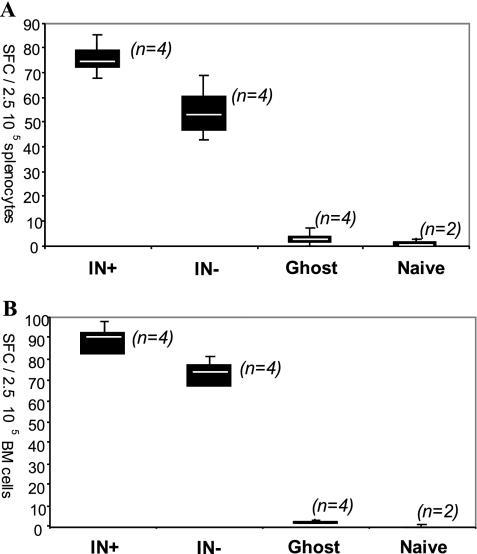
We then compared the systemic cellular immune responses after 60 days in both groups of immunized mice by using an IFN-γ ELISPOT assay on splenocytes and bone marrow cells stimulated with HBsAg. The responses to the integrating and nonintegrating lentivectors were very similar, while control mice immunized with empty particles (ghost) did not respond (Fig. 7A and B). To determine the phenotype of the IFN-γ producing cells, an intracellular staining for IFN-γ was performed on the splenocytes stimulated with HBsAg. The percentages of antigen-specific CD8+ T cells expressing IFN-γ were found to be 2.6% and 1.8% in the mice injected with integrating and nonintegrating lentivectors, respectively (data not shown).
FIG. 7.
T-cell responses measured for cells isolated 2 months after immunization with lentiviral vectors expressing HBsAg. An IFN-γ ELISPOT assay was performed with splenocytes (A) and bone marrow cells (B) stimulated for 48 h with HBsAg. IFN-γ-producing T cells are expressed as numbers of spot-forming cells (SFCs) per 250,000 cells after background subtraction. The median for each data set is indicated by the white center line of the box plots. n denotes the number of mice used in each set. IN+, integration-competent virus; IN−, integration-deficient virus.
DISCUSSION
In this study, we showed that integration-deficient lentiviral vectors were effective in subcutaneous immunization. A higher dose of these nonintegrating vectors was required to achieve the same CD8+ T-cell response that was induced by integrating lentivectors. However, the nonintegrating vectors were engineered to be as effective for tumor therapy by encoding activators of the p38 or NF-κB pathways in the vector. One striking observation was that antigen presentation following subcutaneous immunization with either the integrating or the nonintegrating lentivectors persisted for up to 30 days. This long-term antigen presentation has previously been reported following subcutaneous immunization with integrating lentivectors (11). In steady state, dendritic cells in the skin have a half-life of >21 days (Langerhans cells) or up to 12 days (dermal dendritic cells) (reviewed in reference 18). Skin-derived dendritic cells are critical for immune responses following lentiviral vector immunization (11). These cells are transduced by lentiviral vectors and migrate to draining lymph nodes within days of immunization (11). However, we could no longer detect transduced cells in the draining lymph node at more than 10 days after integrating lentivector immunization (17). The results that we present here suggest that the transduced cells migrate elsewhere, continue to present antigen, and do not divide. Toll-like receptor agonists and T-cell contact are known to increase Bcl2 family member expression in dendritic cells and promote cell survival (12), we must conclude that lentiviral vectors also do this efficiently.
One school of thought suggests that prolonged antigen presentation may lead to tolerance rather than immunization (32). However a recent study that induced antigen expression in dendritic cells in situ found that prolonged expression promoted immunization even in the absence of a dendritic cell activation signal (21). Indeed, vaccine strategies that prolong antigen presentation, for example, by expressing Bcl2 to promote dendritic cell survival, have been shown to be more effective (16). In some ways, lentiviral vector immunization mimics persistent viral infection, which can result in lifelong, high-level CD8+ T-cell responses, generated both from memory cells and from naive CD8+ T-cell recruitment (28).
We also examined T-cell and antibody responses to the secreted HBsAg following intramuscular injection of nonintegrating lentivectors. In agreement with a previous study of HIV gp120 responses (20), we found that the nonintegrating lentivectors persisted for at least 60 days at the injection site in muscle following this route of administration. Antibody titers were similar after immunization with integrating and nonintegrating lentivectors, and IgG titers rose over the 8 weeks of the experiment. In contrast, DNA vaccination with a plasmid encoding the same antigen generated a peak antibody response after 1 week, which then declined. This suggests that episome delivery by nonintegrating lentivectors is more effective than plasmid, perhaps because of more-efficient cell entry or because the structure of the episome promotes persistence or prolonged gene expression.
There is an urgent need for novel vaccine vectors to tackle problems such as cancer and chronic infectious disease. In these settings, an immune system that has already been downregulated by mechanisms such as exhaustion or regulatory cell induction must be restimulated. In the case of cancer vaccines, the antigens are often self proteins expressed during development or in a particular cell lineage. In such situations, responses to the vaccine vector may dominate those to the cancer antigen, as has been observed for the modified vaccinia virus Ankara poxvirus (27). Also, preexisting immune responses to vaccine vectors derived from human pathogens, such as Ad5, may limit their efficacy in the clinic (3). Lentivectors are very promising vaccine candidates because they do not express viral proteins from the vector which may themselves be immunogenic. The envelope used on the vector can also be interchanged, which allows repeat immunization without the problem of induction of neutralizing antibodies. Several envelopes have been used for effective immunization in mice (17, 31). Lentivectors can also be used as part of a prime/boost immunization protocol, for example, with poxviruses (17, 22). The demonstration that nonintegrating lentivectors can be used as vaccines to generate T-cell and antibody responses is extremely important. Their genotoxicity is theoretically no different from plasmid immunization, which has a good clinical safety record, for example (26). The use of an attenuated vector, such as the Δatt mutation in a self-inactivating vector, is an important additional safety measure for preventing rescue of the episome by wild-type virus infection.
Acknowledgments
This project was supported by an MRC studentship to K.K. and a Fundação para a Ciência e a Tecnologia, Portugal, studentship to L.A. S.M. was supported by a BBSRC (United Kingdom) postdoctoral fellowship. A.J.T. was supported by the Wellcome Trust, M.P.B. by an EU grant, D.E. by an Arthritis and Rheumatism Campaign Career Development Award, and G.B. by a Marie Curie training fellowship (040855).
Footnotes
Published ahead of print on 28 January 2009.
REFERENCES
- 1.Apolonia, L., S. N. Waddington, C. Fernandes, N. J. Ward, G. Bouma, M. P. Blundell, A. J. Thrasher, M. K. Collins, and N. J. Philpott. 2007. Stable gene transfer to muscle using non-integrating lentiviral vectors. Mol. Ther. 151947-1954. [DOI] [PubMed] [Google Scholar]
- 2.Bokhoven, M., S. L. Stephen, S. Knight, E. F. Gevers, I. C. Robinson, Y. Takeuchi, and M. K. Collins. 2009. Insertional gene activation by lentiviral and gammaretroviral vectors. J. Virol. 83283-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchbinder, S. P., D. V. Mehrotra, A. Duerr, D. W. Fitzgerald, R. Mogg, D. Li, P. B. Gilbert, J. R. Lama, M. Marmor, C. Del Rio, M. J. McElrath, D. R. Casimiro, K. M. Gottesdiener, J. A. Chodakewitz, L. Corey, M. N. Robertson, et al. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372(9653)1881-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buffa, V., D. R. Negri, P. Leone, R. Bona, M. Borghi, I. Bacigalupo, D. Carlei, C. Sgadari, B. Ensoli, and A. Cara. 2006. A single administration of lentiviral vectors expressing either full-length human immunodeficiency virus 1 (HIV-1)HXB2 Rev/Env or codon-optimized HIV-1JR-FL gp120 generates durable immune responses in mice. J. Gen. Virol. 871625-1634. [DOI] [PubMed] [Google Scholar]
- 5.Chapatte, L., S. Colombetti, J. C. Cerottini, and F. Levy. 2006. Efficient induction of tumor antigen-specific CD8+ memory T cells by recombinant lentivectors. Cancer Res. 661155-1160. [DOI] [PubMed] [Google Scholar]
- 6.Demaison, C., K. Parsley, G. Brouns, M. Scherr, K. Battmer, C. Kinnon, M. Grez, and A. J. Thrasher. 2002. High-level transduction and gene expression in hematopoietic repopulating cells using a human imunodeficiency [sic] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum. Gene Ther. 13803-813. [DOI] [PubMed] [Google Scholar]
- 7.Dullaers, M., S. Van Meirvenne, C. Heirman, L. Straetman, A. Bonehill, J. L. Aerts, K. Thielemans, and K. Breckpot. 2006. Induction of effective therapeutic antitumor immunity by direct in vivo administration of lentiviral vectors. Gene Ther. 13630-640. [DOI] [PubMed] [Google Scholar]
- 8.Escors, D., L. Lopes, R. Lin, J. Hiscott, S. Akira, R. J. Davis, and M. K. Collins. 2008. Targeting dendritic cell signaling to regulate the response to immunization. Blood 1113050-3061. [DOI] [PubMed] [Google Scholar]
- 9.Garcia Casado, J., J. Janda, J. Wei, L. Chapatte, S. Colombetti, P. Alves, G. Ritter, M. Ayyoub, D. Valmori, W. Chen, and F. Levy. 2008. Lentivector immunization induces tumor antigen-specific B and T cell responses in vivo. Eur. J. Immunol. 381867-1876. [DOI] [PubMed] [Google Scholar]
- 10.Hargrove, P. W., S. Kepes, H. Hanawa, J. C. Obenauer, D. Pei, C. Cheng, J. T. Gray, G. Neale, and D. A. Persons. 2008. Globin lentiviral vector insertions can perturb the expression of endogenous genes in beta-thalassemic hematopoietic cells. Mol. Ther. 16525-533. [DOI] [PubMed] [Google Scholar]
- 11.He, Y., J. Zhang, C. Donahue, and L. D. Falo, Jr. 2006. Skin-derived dendritic cells induce potent CD8(+) T cell immunity in recombinant lentivector-mediated genetic immunization. Immunity 24643-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou, W. S., and L. Van Parijs. 2004. A Bcl-2-dependent molecular timer regulates the lifespan and immunogenicity of dendritic cells. Nat. Immunol. 5583-589. [DOI] [PubMed] [Google Scholar]
- 13.Iglesias, M. C., M. P. Frenkiel, K. Mollier, P. Souque, P. Despres, and P. Charneau. 2006. A single immunization with a minute dose of a lentiviral vector-based vaccine is highly effective at eliciting protective humoral immunity against West Nile virus. J. Gene Med. 8265-274. [DOI] [PubMed] [Google Scholar]
- 14.Iglesias, M. C., K. Mollier, A. S. Beignon, P. Souque, O. Adotevi, F. Lemonnier, and P. Charneau. 2007. Lentiviral vectors encoding HIV-1 polyepitopes induce broad CTL responses in vivo. Mol. Ther. 151203-1210. [DOI] [PubMed] [Google Scholar]
- 15.Kim, J. H., N. Majumder, H. Lin, S. Watkins, L. D. Falo, Jr., and Z. You. 2005. Induction of therapeutic antitumor immunity by in vivo administration of a lentiviral vaccine. Hum. Gene Ther. 161255-1266. [DOI] [PubMed] [Google Scholar]
- 16.Kim, T. W., C. F. Hung, D. Boyd, J. Juang, L. He, J. W. Kim, J. M. Hardwick, and T. C. Wu. 2003. Enhancing DNA vaccine potency by combining a strategy to prolong dendritic cell life with intracellular targeting strategies. J. Immunol. 1712970-2976. [DOI] [PubMed] [Google Scholar]
- 17.Lopes, L., M. Dewannieux, U. Gileadi, R. Bailey, Y. Ikeda, C. Whittaker, M. P. Collin, V. Cerundolo, M. Tomihari, K. Ariizumi, and M. K. Collins. 2008. Immunization with a lentivector that targets tumor antigen expression to dendritic cells induces potent CD8+ and CD4+ T-cell responses. J. Virol. 8286-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez-Bravo, M., and C. Ardavin. 2008. In vivo induction of immune responses to pathogens by conventional dendritic cells. Immunity 29343-351. [DOI] [PubMed] [Google Scholar]
- 19.Montini, E., D. Cesana, M. Schmidt, F. Sanvito, M. Ponzoni, C. Bartholomae, L. Sergi Sergi, F. Benedicenti, A. Ambrosi, C. Di Serio, C. Doglioni, C. von Kalle, and L. Naldini. 2006. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat. Biotechnol. 24687-696. [DOI] [PubMed] [Google Scholar]
- 20.Negri, D. R., Z. Michelini, S. Baroncelli, M. Spada, S. Vendetti, V. Buffa, R. Bona, P. Leone, M. E. Klotman, and A. Cara. 2007. Successful immunization with a single injection of non-integrating lentiviral vector. Mol. Ther. 151716-1723. [DOI] [PubMed] [Google Scholar]
- 21.Obst, R., H. M. van Santen, R. Melamed, A. O. Kamphorst, C. Benoist, and D. Mathis. 2007. Sustained antigen presentation can promote an immunogenic T cell response, like dendritic cell activation. Proc. Natl. Acad. Sci. USA 10415460-15465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmowski, M. J., L. Lopes, Y. Ikeda, M. Salio, V. Cerundolo, and M. K. Collins. 2004. Intravenous injection of a lentiviral vector encoding N.Y.-ESO-1 induces an effective CTL response. J. Immunol. 1721582-1587. [DOI] [PubMed] [Google Scholar]
- 23.Philippe, S., C. Sarkis, M. Barkats, H. Mammeri, C. Ladroue, C. Petit, J. Mallet, and C. Serguera. 2006. Lentiviral vectors with a defective integrase allow efficient and sustained transgene expression in vitro and in vivo. Proc. Natl. Acad. Sci. USA 10317684-17689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rowe, H. M., L. Lopes, N. Brown, S. Efklidou, T. Smallie, S. Karrar, P. M. Kaye, and M. K. Collins. 2009. Expression of vFLIP in a lentiviral vaccine vector activates NF-κB, matures dendritic cells, and increases CD8+ T-cell responses. J. Virol. 831555-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rowe, H. M., L. Lopes, Y. Ikeda, R. Bailey, I. Barde, M. Zenke, B. M. Chain, and M. K. Collins. 2006. Immunization with a lentiviral vector stimulates both CD4 and CD8 T cell responses to an ovalbumin transgene. Mol. Ther. 13310-319. [DOI] [PubMed] [Google Scholar]
- 26.Sandstrom, E., C. Nilsson, B. Hejdeman, A. Brave, G. Bratt, M. Robb, J. Cox, T. Vancott, M. Marovich, R. Stout, S. Aboud, M. Bakari, K. Pallangyo, K. Ljungberg, B. Moss, P. Earl, N. Michael, D. Birx, F. Mhalu, B. Wahren, and G. Biberfeld. 2008. Broad immunogenicity of a multigene, multiclade HIV-1 DNA vaccine boosted with heterologous HIV-1 recombinant modified vaccinia virus Ankara. J. Infect. Dis. 1981482-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith, C. L., F. Mirza, V. Pasquetto, D. C. Tscharke, M. J. Palmowski, P. R. Dunbar, A. Sette, A. L. Harris, and V. Cerundolo. 2005. Immunodominance of poxviral-specific CTL in a human trial of recombinant-modified vaccinia Ankara. J. Immunol. 1758431-8437. [DOI] [PubMed] [Google Scholar]
- 28.Snyder, C. M., K. S. Cho, E. L. Bonnett, S. van Dommelen, G. R. Shellam, and A. B. Hill. 2008. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity 29650-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vargas, J., Jr., G. L. Gusella, V. Najfeld, M. E. Klotman, and A. Cara. 2004. Novel integrase-defective lentiviral episomal vectors for gene transfer. Hum. Gene Ther. 15361-372. [DOI] [PubMed] [Google Scholar]
- 30.Yanez-Munoz, R. J., K. S. Balaggan, A. MacNeil, S. J. Howe, M. Schmidt, A. J. Smith, P. Buch, R. E. MacLaren, P. N. Anderson, S. E. Barker, Y. Duran, C. Bartholomae, C. von Kalle, J. R. Heckenlively, C. Kinnon, R. R. Ali, and A. J. Thrasher. 2006. Effective gene therapy with nonintegrating lentiviral vectors. Nat. Med. 12348-353. [DOI] [PubMed] [Google Scholar]
- 31.Yang, L., H. Yang, K. Rideout, T. Cho, K. I. Joo, L. Ziegler, A. Elliot, A. Walls, D. Yu, D. Baltimore, and P. Wang. 2008. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat. Biotechnol. 26326-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zinkernagel, R. M., and H. Hengartner. 2004. On immunity against infections and vaccines: credo 2004. Scand. J. Immunol. 609-13. [DOI] [PubMed] [Google Scholar]