Abstract
Cancer cells frequently exhibit overduplicated centrosomes that lead to formation of multipolar spindles, chromosome missegregation, and aneuploidy. However, the molecular events involved in centrosome overduplication remain largely unknown. Experimentally, centrosome overduplication is observed in p53-deficient cells arrested in S phase with hydroxyurea. Using this assay, we have identified distinct roles for Cdk2, microtubules, dynein, and Hsp90 in the overduplication of functional centrosomes in mammalian cells and show that Cdk2 is also required for the generation of centriolar satellites. Moreover, we demonstrate that nuclear export is required for centriolar satellite formation and centrosome overduplication, with export inhibitors causing a Cdk-dependent accumulation of nuclear centrin granules. Hence, we propose that centrosome precursors may arise in the nucleus, providing a novel mechanistic explanation for how nuclear Cdk2 can promote centrosome overduplication in the cytoplasm. Furthermore, this study defines a molecular pathway that may be targeted to prevent centrosome overduplication in S-phase-arrested cancer cells.
Equal segregation of genetic material between daughter cells requires the mitotic spindle to be bipolar, with monopolar or multipolar spindles leading to abnormal segregation. Because spindle poles are organized by centrosomes, maintaining the right number of centrosomes is critical to the genetic integrity of dividing cells. Centrosomes normally duplicate once per cell cycle in a process that is tightly coordinated with the DNA replication cycle (9, 26, 42, 48, 62). However, the vast majority of human cancer cells accumulate extra or “supernumerary” centrosomes and, likely as a consequence, exhibit aneuploidy and chromosome instability (12, 15, 54). Supernumerary centrosomes may arise through several different mechanisms. Prominent among these are (i) a failure of diploid cells to complete mitosis, leading to tetraploidization and doubling of the centrosome number; and (ii) a loss of coordination of the centrosome duplication and DNA replication cycles such that cells undergo multiple rounds of centrosome duplication within a single cell cycle. There is good evidence to suggest that both of these pathways contribute to supernumerary centrosomes in cancer cells (49).
Centrosome duplication in dividing cells occurs in a semiconservative templated process. In G1 phase of the cell cycle, a centrosome consists of two centrioles surrounded by pericentriolar material (PCM). Centrioles are stable cylinders of 200 nm by 500 nm composed of nine triplets of posttranslationally modified microtubules (MTs) arranged in a pinwheel configuration (11, 40). After progression into S phase, two new procentrioles appear, elongating perpendicularly from the proximal ends of the older centrioles. In late G2, the two centrosomes, each now containing a pair of centrioles, separate to the two poles of the mitotic spindle. However, despite description of this process at the morphological level many years ago (34), the molecular events involved in biogenesis of new centrioles are only now beginning to be resolved (9, 16, 23, 38, 51).
In addition to templated duplication, it has been demonstrated experimentally that centrioles can form using a de novo assembly pathway (30, 37, 41). Following laser ablation of centrioles, a focus of γ-tubulin appears in Chinese hamster ovary (CHO) cells, within which a random number of centrioles assemble (30), while in HeLa cells expressing a centrin1-green fluorescent protein (centrin1-GFP) construct, multiple small centrin-containing foci appear (37). These centrin-containing structures were termed “precentrioles,” and it has been suggested, although not shown, that these may also play a role during templated centrosome duplication. In the latter case, only those precentrioles that adhere to the docking site on the existing centrioles would subsequently elongate into full centrioles, with the others eventually disappearing. Importantly for genome integrity, de novo centriole formation is suppressed in the presence of preexisting centrioles (63); however, this control may be lost in cancer cells. Multiple centrioles also assemble in differentiating cells undergoing ciliogenesis (24). In this case, the multiple centrioles, referred to as basal bodies, subtend the MTs that form the ciliary axoneme. Basal bodies assemble via both a centriolar pathway, in which multiple basal bodies assemble around individual centrioles, and an acentriolar pathway, in which basal bodies form around electron-dense structures known as deuterosomes.
Cells lacking the tumor suppressor p53 overduplicate centrosomes when they are arrested in S phase with drugs such as hydroxyurea (HU) or aphidicolin (4, 60). This has clinical relevance, as many commonly used anticancer agents (e.g., 5-fluorouracil and arabinoside C) function by interfering with DNA replication. Application of these drugs could therefore inadvertently accelerate chromosome instability in those cells that survive the treatment as a result of promoting centrosome amplification (8). Furthermore, the E7 oncoprotein from high-risk human papillomaviruses induces centrosome overduplication within a single cell cycle (19). Due to the prevalence of amplified centrosomes in cancer cells, we set out to examine the molecular events required for centrosome overduplication during S-phase arrest. Treatment of cells with various inhibitors demonstrated a requirement for MTs, dynein, Hsp90, Cdk2, and nuclear export for functional centrosome overduplication. Unexpectedly, though, staining of these cells for centriole markers revealed the presence of intermediate structures reminiscent of those seen in the de novo duplication pathway and the centriolar and acentriolar pathways of ciliogenesis. Characterization of these structures in the presence of different inhibitors has enabled us to delineate a pathway for centrosome overduplication in S-phase-arrested cells.
MATERIALS AND METHODS
Cell culture and transfection.
U2OS osteosarcoma and mouse embryo fibroblast (MEF) cells were grown in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% heat-inactivated fetal calf serum, 100 IU/ml penicillin, and 100 μg/ml streptomycin at 37°C in a 5% CO2 atmosphere. CHO cells were grown in Ham's F12 medium supplemented with 10% fetal calf serum, 100 IU/ml penicillin, and 100 μg/ml streptomycin at 37°C in a 5% CO2 atmosphere. The CHO:centrin1-GFP cell line was generated by transfection of centrin1-GFP and subsequent clonal selection. Transient transfections were performed with either Lipofectamine 2000 (Invitrogen) or Fugene 6 (Roche) according to the manufacturer's instructions.
Fixed- and live-cell microscopy.
Immunofluorescence microscopy was performed as previously described (21). Primary antibodies used were against centrin (1 μg/ml) (Abcam), centrin2 (2 μg/ml) (N-17; Santa Cruz), myc (0.5 μg/ml) (Molecular Probes), C-Nap1 (1 μg/ml) (22), rootletin (0.52 μg/ml) (H. L. Tsai and A. M. Fry), pericentrin (2 μg/ml) (Abcam), PCM-1 (1:500) (14), Cep135 (1 μg/ml) (31), acetylated tubulin (0.5 μg/ml) (Sigma), GFP (0.5 μg/ml [Sigma] or 1 μg/ml [Abcam]), γ-tubulin (0.15 μg/ml) (Sigma), polyglutamylated tubulin (1 μg/ml) (Abcam), α-tubulin (0.3 μg/ml) (Sigma), RPA32/RPA2 (1:200) (Abcam), γH2AX (1 μg/ml) (Abcam), and XPC (5 μg/ml) (Abcam). Secondary antibodies used were Alexa Fluor 488- and 594-conjugated goat anti-rabbit and goat anti-mouse immunoglobulins G (IgGs) (1 μg/ml; Invitrogen). Time-lapse imaging was performed on a Leica TCS SP5 confocal microscope equipped with a Leica DMI 6000B inverted microscope using a 63× oil objective (numerical aperture, 1.4). Cells were cultured in glass-bottomed culture dishes (with no. 1.5 or no. 2 gridded cover glasses; MatTek Corporation) and maintained on the stage at 37°C using a microscope temperature control system (Life Imaging Services). To locate cells on gridded cover glasses, cells were viewed with a 20× dry objective (numerical aperture, 0.7).
Photobleaching studies.
Photobleaching was performed with a Leica TCS SP5 confocal microscope and analyzed using Leica LAS AF software as previously described (55). For fluorescence recovery after photobleaching (FRAP), regions of interest (ROIs) of 3 μm by 3 μm were bleached with five iterations and 100% laser power (488-nm argon laser). Two images were captured prior to bleaching and 30 images were obtained after bleaching at intervals of 10 s (centrin1-GFP foci), or 15 images were obtained at intervals of 2 s (cytoplasmic centrin1-GFP or centrosomal GPF-Nek2A-WT), using a 488-nm argon laser at 10% power. For fluorescence loss in photobleaching analysis, five images were acquired every 5 s prior to bleaching and between each bleach cycle. A bleach area encompassing the whole cell but excluding centrin1-GFP foci of interest was bleached with 10 iterations at 100% laser power. Ten bleach cycles were performed per cell.
Drug treatments.
To monitor centrosome duplication during S-phase arrest, CHO and MEF cells were treated with 2 mM HU (Sigma) and U2OS cells were treated with 16 mM HU. Mimosine (0.2 mM; Sigma) was used to arrest cells in G1. To investigate the role of MTs, cells were additionally treated with 5 μM nocodazole (Sigma) or 5 μM paclitaxel (Taxol; Sigma). Cytochalasin D (10 μg/ml; Sigma) was used to disrupt the actin network. To inhibit dynein, cells were treated with either 300 μM erythro-9-[3-(2-hydroxynonyl)]adenine (EHNA; Sigma) or 1 to 20 μM sodium orthovanadate (Sigma). Geldanamycin (1 μM; Sigma) and 17-AAG [17-(allylamino)-17-demethoxygeldanamycin] (1 μM; Calbiochem) were used to inhibit Hsp90, while heat shock protein inhibitor 1 (HSPI-1) (100 μM; Calbiochem) was used to inhibit Hsp70. Roscovitine (180 μM; Calbiochem) or olomoucine (360 μM; Calbiochem) was used to inhibit Cdk activity. Leptomycin B (LMB) (20 nM; Calbiochem) was used to block nuclear export. Lysates of drug-treated cells were prepared and analyzed by Western blotting as previously described (22). Primary antibodies used were raised against Cdk2 phospho-T14 (1:10,000; Abcam), Cdk2 phospho-T160 (1:1,000; Cell Signaling Technologies), total Cdk2 (1:500; Santa Cruz), α-tubulin (0.3 μg/ml; Sigma), PCM-1 (1:750) (14), γ-tubulin (0.15 μg/ml; Sigma), and centrin (1:200; Abcam).
Microinjection experiments.
Anti-dynein intermediate chain (IC) (clone 70.1; Sigma) was injected into CHO cells cultured on Iwaki glass-bottomed culture dishes (with no. 1.5 cover glass; Barloworld Scientific) at 2 mg/ml in 0.75× phosphate-buffered saline (PBS), using an Eppendorf micromanipulator. Control injections were performed using mouse IgG (Sigma) at 2 mg/ml in 0.75× PBS. At 4 h postinjection, HU was added to the medium. Cells were fixed at 48 h postinjection with methanol at −20°C and processed for immunofluorescence microscopy.
Electron microscopy (EM) analysis.
Cultured cells were collected as pellets, washed twice with PBS, fixed with 3% glutaraldehyde in PBS, and postfixed in 1% OsO4. After being washed, samples were stained en bloc with 2% uranyl acetate in 30% ethanol and completely dehydrated through an ethanol series. Samples were infiltrated in propylene oxide-Spurr resin before final embedding and polymerization in Spurr resin. Thin sections of ∼80 nm in thickness were cut from each sample by use of a Reichert Ultracut E ultramicrotome, collected on copper mesh grids, counterstained with Reynold's lead citrate, and observed using a JEOL 1220 electron microscope at an accelerating voltage of 80 kV. Digital images were recorded using a SIS Megaview III digital camera with analysis software.
RESULTS
MTs are required for overduplication of functional centrosomes but not centrin granule formation.
To examine the molecular events required for centrosome overduplication, immunofluorescence microscopy was performed on CHO cells arrested in S phase with HU for 0, 24, or 48 h. As visualized with markers for the PCM (γ-tubulin or pericentrin) or centrioles (centrin, C-Nap1, Nek2, or ninein), centrosomes increased in number over the treatment period (Fig. 1a; see Fig. S1a in the supplemental material; data not shown). Moreover, centrosome duplication, as determined by γ-tubulin staining, was blocked in cells treated with nocodazole to depolymerize the MT network (Fig. 1b) (5). However, when these cells were visualized with centrin antibodies, many small granules as well as larger aggregates of these granules were seen concentrated at the nuclear periphery, indicating that early steps in the overduplication pathway may proceed in the absence of MTs (Fig. 1b).
FIG. 1.
MTs are required for overduplication of functional MT organizing centers but not for accumulation of perinuclear centrin granules. (a) CHO cells were treated with HU and fixed after 0, 24, and 48 h. To identify centrosomes, cells were stained with γ-tubulin (red) and centrin (green) antibodies. Insets show magnified views of centrosomes. (b) CHO cells were treated concurrently with HU and nocodazole for 48 h and then stained with antibodies against γ-tubulin (green or red), α-tubulin (red), and centrin (green), as indicated. (c) CHO:centrin1-GFP cells were treated concurrently with HU and nocodazole for 48 h and then stained with antibodies against α-tubulin (red), γ-tubulin (red or green), and GFP (green), as indicated. (d) CHO:centrin1-GFP cells were treated for 48 h with HU and nocodazole, the nocodazole was then washed out, and after 5 min, cells were fixed and stained with the antibodies indicated. (e) CHO:centrin1-GFP cells were treated for 48 h with HU and paclitaxel and then stained with antibodies against GFP (green) and α-tubulin, γ-tubulin, or C-Nap1 (red), as indicated. Merge panels also include a DNA stain (blue). Bars, 10 μm.
To study these events in more detail, we established a CHO cell line stably expressing centrin1-GFP. Although centrin1 is expressed mainly in neuronal, ciliated, and male germ cells (36), it acts as an excellent marker of normal and overduplicated centrioles when expressed ectopically in cultured cells (37, 39, 53). Visualizing centrosomes using GFP fluorescence and γ-tubulin staining revealed that centrosomes also undergo overduplication in this cell line upon HU treatment (see Fig. S1b in the supplemental material). Indeed, overexpression of centrin1-GFP (∼2.5-fold in excess of endogenous centrin) accelerated the rate of centrosome overduplication compared to that in wild-type CHO cells (see Fig. S1c to e in the supplemental material). Again, MTs were required for duplication of γ-tubulin-stained centrosomes but not for formation of multiple nucleus-associated centrin1-GFP granules (Fig. 1c). Time-lapse imaging revealed that individual centrin granules appeared in the cytoplasm following 2 h of HU and nocodazole treatment, whereas the aggregates became apparent after 4 to 6 h (see Fig. S2a in the supplemental material). These aggregates were generally static and distributed in a perinuclear manner, suggestive of an association with the nuclear envelope. Treatment of cells with cytochalasin D led to a loss of this perinuclear distribution, implying a role for actin in this association (see Fig. S2b in the supplemental material).
We next examined the ability of centrin granules to nucleate MTs following transfer of cells back to nocodazole-free medium. In line with the absence of γ-tubulin on these granules, only one or two functional MT organizing centers were observed colocalized with the γ-tubulin-positive centrosomes (Fig. 1d). Thus, in the absence of MTs, overduplication of γ-tubulin-containing centrosomes capable of MT nucleation is blocked, but centrin-containing granules and aggregates accumulate in the cytoplasm. Interestingly, treatment of cells with HU and paclitaxel did not prevent centrosome overduplication measured with centrin1-GFP, γ-tubulin, or C-Nap1, implying that it is the generation of polymerized MTs rather than MT dynamics per se that is important (Fig. 1e).
Centrin granules exhibit properties of centriolar satellites and basal body precursors.
A random number of centrin1-GFP foci appear during de novo centriole assembly following laser ablation of centrosomes in S-phase-arrested HeLa cells (37). However, HeLa cells do not overduplicate centrosomes under S-phase arrest in the presence of preexisting centrosomes. In our CHO cell experiments, preexisting centrosomes, which are thought to suppress de novo duplication, were still present. To examine the properties of the centrin granules in more detail, CHO cells treated for 48 h with HU and nocodazole were stained with antibodies against a range of centriolar and PCM proteins. This revealed that they also contained α-tubulin, polyglutamylated tubulin, and acetylated tubulin, demonstrating the presence of nocodazole-resistant modified tubulin (Fig. 2a). They also stained strongly for pericentrin, PCM-1, and Cep135, structural components of the PCM and centriolar satellites, but not for C-Nap1 and rootletin, components of the intercentriolar linker (3, 22) (Fig. 2b). Photobleaching studies indicated that the bulk of centrin1-GFP at centrosomes in untreated cells or following overduplication in the presence of HU alone was highly stable (29 to 31% recovery, with a half-life of >5 min), whereas in perinuclear aggregates generated in the presence of HU and nocodazole, a larger fraction of the centrin1-GFP exhibited mobility (46% recovery, with a half-life of >5 min). However, the centrin1-GFP in these aggregates was still much more restricted than the highly dynamic centrosomal protein Nek2 (25) or free cytoplasmic centrin1-GFP (Fig. 2c to e).
FIG. 2.
Cytoplasmic centrin granules are highly reminiscent of centriolar satellites. (a) CHO:centrin1-GFP cells treated with HU and nocodazole for 48 h were stained with antibodies against GFP (green) and α-tubulin, polyglutamylated tubulin, or acetylated tubulin (red). (b) CHO:centrin1-GFP cells treated with HU and nocodazole for 48 h were stained for GFP (green) and the centrosomal proteins rootletin, C-Nap1, pericentrin, PCM-1, and Cep135 (red). In panels a and b, merge panels include DNA stained with Hoechst (blue). Bars, 10 μm. (c) FRAP was performed on untreated CHO:centrin1-GFP cells and cells treated with either HU or HU and nocodazole for 48 h. Three ROIs were bleached per cell. For untreated and HU-treated cells, one ROI included the centrosomes, while the other two were cytoplasmic. For cells treated with HU and nocodazole, three distinct centrin foci were bleached per cell to ensure that foci other than the original centrosomes were bleached. The relative GFP intensity within the bleached area was recorded as a measure of time. Each data point represents the average intensity ± standard deviation for at least 12 cells. (d) FRAP data for areas of cytoplasm without centrin foci and the centrosomes of CHO cells transiently transfected with GFP-Nek2A-WT. Each data point represents the average intensity ± standard deviation for at least 10 cells. (e) Fluorescence loss in photobleaching analysis of untreated CHO:centrin1-GFP cells and cells treated for 48 h with HU alone or HU and nocodazole. Each data point represents the average intensity ± standard deviation for at least 12 cells. (f) TEM analysis of centrioles in CHO:centrin1-GFP cells that were either untreated (−) or treated for 48 h with HU or HU plus nocodazole. Examples of centrioles (arrows) and centriolar satellites (arrowheads) are indicated. Bars, 500 nm. (g) Histogram showing the mean numbers of centriolar satellites within the EM sections that also contained centrioles from cells treated as indicated.
Using transmission EM (TEM), we found that in the presence of HU alone, multiple centrioles were detected, whereas in untreated cells or cells treated with HU and nocodazole, no more than two centrioles were seen per section (Fig. 2f). Under each condition, small (∼50 nm), densely staining granules were detected around the centrioles and were reminiscent of structures previously referred to as fibrous granules or centriolar satellites (1, 32, 59). Strikingly, in the HU-plus-nocodazole-treated cells, the number of these granules was significantly increased (Fig. 2g). Since fibrous granules/centriolar satellites contain centrin, polyglutamylated tubulin, and PCM-1 (36, 45), have been localized around the nuclear envelope as well as at the centrosome (1), and contribute to basal body replication in the centriolar and acentriolar pathways of ciliogenesis (1, 59), we suspect that the centrin-containing granules that we see by fluorescence microscopy are most likely the same structures.
The fact that the centrin-containing granules formed upon HU and nocodazole treatment also contained a subset of other centrosomal proteins was highly suggestive that these structures act as precursors for overduplicated centrosomes. To provide further evidence for this hypothesis, we first performed time-lapse imaging of centrin1-GFP cells, previously treated with HU plus nocodazole for 48 h, following transfer into nocodazole-free medium. Within approximately 1 to 2 h, the centrin aggregates had moved away from the nuclear periphery and coalesced into one or two large masses at the center of the cell (Fig. 3a). After 20 h, these cells were then fixed and stained for markers of functional centrosomes, namely, γ-tubulin and C-Nap1 (Fig. 3a and b). Bright-field and GFP staining was used to confirm relocalization of the same cells that were imaged during time-lapse microscopy. In all cells observed (n = 33), the majority of centrin aggregates were now positive for γ-tubulin and C-Nap1, confirming that these structures were indeed precursors of overduplicated centrosomes. To determine how quickly centrin aggregates assembled into functional centrosomes, cells were fixed at different times after transfer to nocodazole-free medium and stained for γ-tubulin (Fig. 3c). Quantification of the number of distinct γ-tubulin-stained structures revealed that this began to increase only after 5 to 10 h (Fig. 3d). Hence, upon nocodazole washout, the centrin-containing precursors rapidly coalesce into a mass at the center of the cell, presumably around the preexisting centrosome. However, the acquisition of markers of functional centrosomes, e.g., γ-tubulin, is somewhat delayed, consistent with an intervening period being required for additional processes such as the elongation of new centrioles.
FIG. 3.
Centrin aggregates represent precursors of overduplicated centrosomes. (a) CHO:centrin1-GFP cells grown in gridded glass-bottomed culture dishes were treated with HU and nocodazole for 48 h. Nocodazole was then washed out, and the cells were imaged with a Leica TCS SP5 microscope. At each time point, a z stack was acquired, and the images shown are the maximum intensity projections of the z stacks. Times are shown as hours:minutes. Following completion of the time series, the grid location of the cells was noted, and the cells were fixed and processed for immunofluorescence microscopy. Cells were stained with antibodies against GFP (green) and C-Nap1 (red), as indicated. Bright-field images show the field of view at the end of live-cell imaging and the fixed cells (BF). The cell shown is indicated (*). (b) The same as panel a, except that cells were stained with antibodies against GFP (green) and γ-tubulin (red). (c) CHO:centrin1-GFP cells were treated with HU and nocodazole for 48 h. Nocodazole was then washed out, and the cells were fixed at the indicated times. Cells were stained with antibodies against GFP (green) and γ-tubulin (red). In panels a to c, merge panels include DNA stained with Hoechst (blue). Bars, 10 μm. (d) Histogram representing mean numbers of γ-tubulin-staining centrosomes in cells treated with HU and nocodazole for 48 h before nocodazole was washed out for the indicated times (hours). Standard deviations are indicated.
Dynein is required for recruitment of γ-tubulin to duplicating centrosomes.
Previous reports indicated that dynein is not required for centrosome duplication during normal cell cycle progression (56). To examine the role of dynein in centrosome overduplication, we first used two chemical inhibitors, EHNA and vanadate, that target the dynein ATPase activity (52). Both inhibitors caused redistribution of the Golgi network consistent with dynein inhibition (data not shown). Staining for γ-tubulin and C-Nap1 revealed that both drugs prevented centrosome overduplication during S-phase arrest (Fig. 4a) and that this was dose dependent with vanadate (Fig. 4b). However, neither inhibitor prevented the appearance of centrin granules (Fig. 4c). As for the inhibition of MTs, TEM revealed no sections in which more than two centrioles were seen following dynein inhibition (Fig. 4d). Confirmation that dynein is required for centrosome overduplication was obtained by expressing a myc-dynamitin construct that disrupts dynein-dynactin function and injecting neutralizing anti-dynein antibodies. In both cases, the number of centrin foci increased, whereas the number of γ-tubulin-positive structures did not (Fig. 4e to h). However, unlike the perinuclear distribution of centrin aggregates seen in the absence of MTs, inhibition of dynein led to centrin aggregates that clustered around the preexisting centrosomes. Taken together, these data indicate that both MTs and dynein are required for the overduplication of centrioles during S-phase arrest but not for the formation of granules that contain multiple centriole precursors. However, MTs, but not dynein-dependent transport, are required to traffic these structures to the centrosome.
FIG. 4.
Dynein is required for overduplication of centrioles. (a) CHO cells were treated with HU and either EHNA or vanadate for 48 h before being stained with antibodies against γ-tubulin (red) and C-Nap1 (green). (b) Histogram indicating the mean numbers of γ-tubulin-stained centrosomes at 48 h in CHO cells treated with HU and the indicated concentrations of vanadate. Data are shown as the averages for three experiments ± standard deviations. Approximately 200 cells were counted per experiment. (c) CHO:centrin1-GFP cells were treated with HU and either EHNA or vanadate for 48 h before being stained with GFP (green) and γ-tubulin (red) antibodies. (d) TEM analysis of centrioles in CHO cells treated for 48 h with HU and EHNA. Bar, 500 nm. (e) CHO or CHO:centrin1-GFP cells, as indicated, were transiently transfected with myc-lamin A or myc-dynamitin for 24 h prior to being treated with HU for 48 h. myc-lamin A-transfected cells were stained with anti-γ-tubulin (red) and anti-myc (green) antibodies, while myc-dynamitin-transfected cells were stained with anti-myc (red) and anti-γ-tubulin or anti-centrin (green) antibodies, and in the case of CHO:centrin1-GFP cells, the GFP fluorescence alone (green) was monitored. (f) Histogram indicating the percentages of cells transfected with myc-lamin A or myc-dynamitin that accumulate extra (>2) γ-tubulin- and centrin-stained foci following 48 h of treatment with HU. Standard deviations are indicated. (g) CHO cells were microinjected with either mouse IgGs or anti-dynein IC antibodies and treated at 4 h postinjection with HU for 48 h. Cells were stained with anti-γ-tubulin or anticentrin antibodies (red). Injected cells were detected with anti-mouse secondary antibodies (green). Merge panels include a DNA stain (blue). Bars in panels a, c, e, and g, 10 μm. (h) Histogram indicating the percentages of cells injected with mouse IgGs or dynein IC antibodies that accumulate extra (>2) γ-tubulin- and centrin-expressing foci following 48 h of treatment with HU. Standard deviations are indicated.
Recruitment of γ-tubulin to duplicated centrioles requires Hsp90 but not Hsp70.
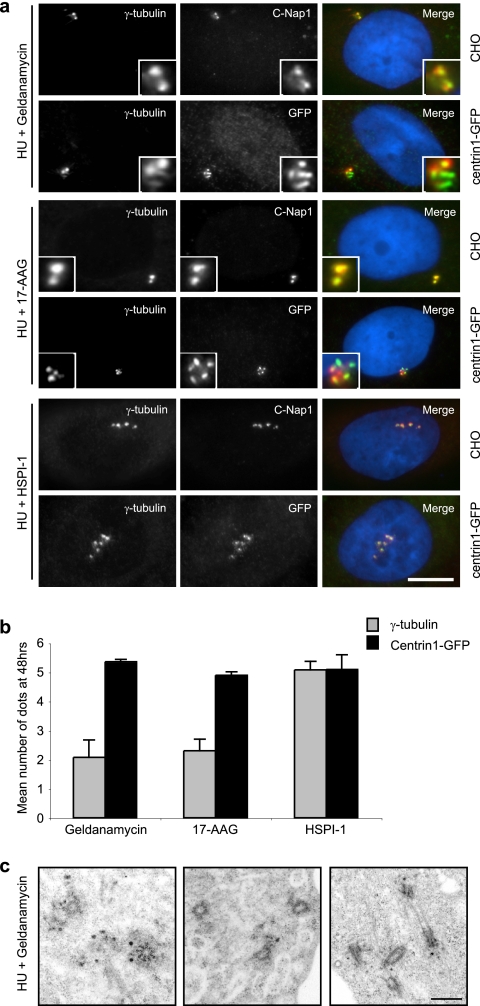
Duplication of the yeast equivalent of the centrosome, the spindle pole body, is disrupted in mutants that lead to a loss of the Hsp90 family of chaperones (64). We therefore examined whether heat shock proteins have a role in mammalian centrosome overduplication. Cells were treated for 48 h with HU in the presence of geldanamycin or 17-AAG, inhibitors of Hsp90, or HSPI-1, an inhibitor of Hsp70. Hsp90 inhibitors blocked duplication of γ-tubulin- and C-Nap1-positive centrosomes, but they did not prevent the accumulation of clustered centrin aggregates (Fig. 5a and b). In contrast, the Hsp70 inhibitor had no effect on centrosome overduplication, as judged by γ-tubulin, C-Nap1, or centrin staining (Fig. 5a and b). These results were not a consequence of α- or γ-tubulin instability in the absence of Hsp90 function but rather reflect a defect in centrosome recruitment, as the intensity of γ-tubulin at unduplicated centrosomes was also reduced in the presence of the Hsp90 inhibitor (see Fig. S3 in the supplemental material). Indeed, unlike MT or dynein inhibition, inhibition of Hsp90 did not prevent the generation of cells with multiple centrioles, as judged by TEM analysis (Fig. 5c). Therefore, we concluded that Hsp90 is not required for centriole overduplication but is required for recruitment of certain centrosomal proteins, including γ-tubulin, to overduplicated centrioles. Furthermore, we found that MTs, dynein, and Hsp90 are also all required for centrosome overduplication in S-phase-arrested human U2OS osteosarcoma cells and that MT inhibition similarly leads to perinuclear accumulation of centrin and pericentrin in these cells (see Fig. S4a to c in the supplemental material).
FIG. 5.
Hsp90 is required for recruitment of γ-tubulin to overduplicated centrioles. (a) CHO and CHO:centrin1-GFP cells were incubated with HU and geldanamycin or 17-AAG to inhibit Hsp90 or with HSPI-1 to inhibit Hsp70. After 48 h, cells were stained with anti-γ-tubulin antibodies (red). Cells were additionally costained with either C-Nap1 or GFP antibodies (green). Merge panels include a DNA stain (blue). Bar, 10 μm. (b) Histogram representing mean numbers of foci, as detected by staining for γ-tubulin (gray) or centrin1-GFP (black), after 48 h of treatment with HU and the Hsp inhibitor indicated. Standard deviations are indicated. (c) TEM images of centrioles in CHO cells treated for 48 h with HU and geldanamycin. Bar, 500 nm.
Cdk2 activity is required for formation of centrin foci.
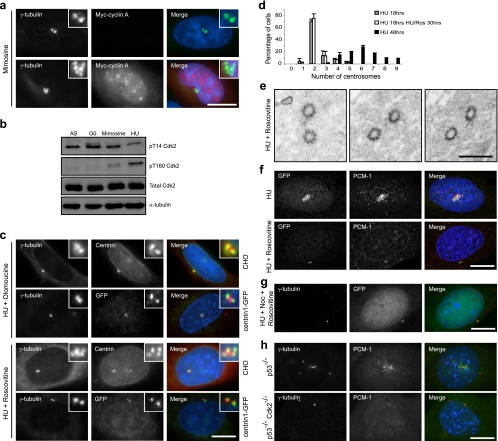
Inhibition of Cdk2 prevents overduplication of γ-tubulin-stained centrosomes in S-phase-arrested CHO cells (43). We therefore first examined whether centrosome overduplication in our system was dependent upon cell cycle status by arresting cells in G1 with mimosine. Under these conditions, centrosomes did not overduplicate. However, upon ectopic expression of cyclin A to prematurely activate Cdk2 during mimosine arrest, centrosome overduplication was observed (Fig. 6a). Indeed, Western blotting with phospho-specific antibodies confirmed that in mimosine-treated cells, endogenous Cdk2 is predominantly inactive, whereas in HU-treated cells, it is active (Fig. 6b).
FIG. 6.
Cdk2 activity is required for assembly of centriole precursors. (a) CHO cells transiently transfected with myc-cyclin A were treated with mimosine for 48 h. Cells were stained for γ-tubulin (green) and myc (red). (b) Cell extracts were prepared from asynchronous (AS) and G0 (serum starved for 72 h) CHO cells or cells treated with mimosine or HU for 48 h. Western blots were probed with antibodies against pT14 Cdk2, pT160 Cdk2, total Cdk2, and anti-α-tubulin antibodies. (c) CHO and CHO:centrin1-GFP cells were treated with HU and the Cdk inhibitor olomoucine or roscovitine for 48 h. Cells were stained with γ-tubulin (red) and centrin or GFP (green) antibodies. (d) Histogram indicating the numbers of centrosomes detected by γ-tubulin staining in CHO cells treated for 18 h with HU (gray bars), for 18 h with HU followed by 30 h with HU and roscovitine (white bars), or for 48 h with HU (black bars). Standard deviations are indicated. (e) TEM analysis of CHO:centrin1-GFP cells treated with HU and roscovitine for 48 h reveals no duplication of centrioles and the absence of centriolar satellites. Bar, 500 nm. (f) CHO:centrin1-GFP cells were treated with HU or HU plus roscovitine for 48 h. Cells were stained with GFP (green) and PCM-1 (red) antibodies. (g) CHO:centrin1-GFP cells were treated for 48 h with HU, nocodazole, and roscovitine before being stained with γ-tubulin (red) and GFP (green) antibodies. (h) p53−/− and p53−/− Cdk2−/− MEFs were treated with HU for 48 h before being stained with γ-tubulin (red) and PCM-1 (green) antibodies. In panels a, c, f, g, and h, merge images include a DNA stain (blue). Bars, 10 μm.
To directly test the requirement for Cdk activity, S-phase-arrested cells were treated with the Cdk-specific inhibitors olomoucine and roscovitine. Unexpectedly, this not only blocked duplication of γ-tubulin-positive structures but also prevented the formation of centrin granules (Fig. 6c). This was not the result of cells being arrested before S-phase entry, as similar results were seen with cells first arrested in S phase with HU prior to addition of the Cdk inhibitor (Fig. 6d). TEM analysis confirmed the lack of centriole duplication and the absence of centriolar satellites under these conditions (Fig. 6e), and staining for PCM-1 also showed a dramatic loss of centriolar satellites by fluorescence microscopy (92.4% ± 1.3%) (Fig. 6f). Similarly, perinuclear centrin granules did not assemble in cells (89.6% ± 2.7%) treated for 48 h with HU, nocodazole, and roscovitine, indicating that it is not the combination of HU and MT depolymerization that triggers centrin granule formation (Fig. 6g). Centrin granules also failed to form in cells treated with mimosine and nocodazole for 48 h (data not shown), supporting the hypothesis that high Cdk2 activity is required for these to assemble.
The specific requirement for Cdk2, as opposed to any other Cdk, in centrosome overduplication and centriolar satellite formation was confirmed using genetically deleted cells. HU-arrested p53−/− MEFs (76.8% ± 4.3%), unlike wild-type MEFs, overduplicated centrosomes when they were arrested in S phase with HU. However, p53−/− Cdk2−/− MEFs did not overduplicate centrosomes (11.2% ± 1.4%) and lost all pericentriolar satellite staining under these conditions (Fig. 6h). These results not only support the hypotheses that centrin granules are similar structures to centriolar satellites and are precursors of overduplicating centrioles but also reveal that Cdk2 regulates an early step of the overduplication process.
Centrosome overduplication requires nuclear export.
Cdk2 is a nuclear enzyme, yet centrosome duplication occurs in the cytoplasm. It has been postulated that nucleocytoplasmic shuttling of Cdk2, together with its cyclin partner, may be sufficient to allow Cdk2 to phosphorylate cytoplasmic proteins involved in centrosome duplication (28). However, we were intrigued by the concentration of centrin1-GFP in the nucleus in cells treated with HU, roscovitine, and nocodazole (Fig. 6g) and the accumulation of centrin aggregates around the nuclear periphery in the presence of MT inhibitors (Fig. 1b and c). To examine the possibility that nuclear export is required for centrosome overduplication, cells were treated for 48 h with HU and a specific inhibitor of the CRM1/exportin 1 export factor, LMB (33). This treatment completely blocked centrosome overduplication, as measured by γ-tubulin staining, and concomitantly led to the accumulation of centrin in the nucleus in CHO:centrin1-GFP, wild-type CHO, and U2OS cells and p53−/− MEFs (Fig. 7a and b; see Fig. S4d and e in the supplemental material). This result is consistent with a previous study showing that centrin, but not γ-tubulin, accumulates in the nucleus in LMB-treated cells (29). Importantly, nuclear centrin could be detected in each of these cell types in distinct foci reminiscent of the cytoplasmic granules (Fig. 7a; see Fig. S4d in the supplemental material). However, these foci did not stain for modified tubulins, pericentrin, PCM-1, or Cep135, suggesting that these are recruited only upon export to the cytoplasm (Fig. 7c and data not shown). Nevertheless, cytoplasmic centriolar satellites stained for PCM-1 disappeared upon LMB treatment, indicating that their formation also requires nuclear export (Fig. 7c; see Fig. S4f in the supplemental material), despite LMB having no effect on protein abundance of PCM-1, γ-tubulin, or centrin (Fig. 7d). Blocking nuclear export prevented the perinuclear appearance of centrin granules when cells were cotreated with nocodazole (87.8% ± 3.4%) (Fig. 7e). Moreover, combined treatment of CHO cells with LMB and roscovitine prevented formation of the granules within the nucleus (91.4% ± 2.4%), and nuclear centrin granules were not detected in p53−/− Cdk2−/− cells treated with LMB (94.5% ± 1.2%) (Fig. 7f). However, in line with our earlier observations, quantitative analysis revealed that total centrin staining was increased in the nucleus in cells treated with HU and either roscovitine or LMB, implying that in the absence of Cdk activity centrin accumulates in the nucleus in a diffuse form (Fig. 7g). Consistent with this, we found that blocking nuclear export led to the appearance of nuclear centrin granules only when cells were arrested in S phase with HU, not in cells arrested in G1 with mimosine, unless they ectopically expressed cyclin A (Fig. 7h and i). These results support a model in which nuclear export is required for centrosome overduplication because it allows export of centrin-containing granules generated in response to Cdk2 activity in the nuclei of S-phase cells.
FIG. 7.
Nuclear export is required for centrosome overduplication. (a) CHO or CHO:centrin1-GFP cells were treated with HU and LMB for 48 h. Cells were stained with γ-tubulin (red) and centrin or GFP (green) antibodies. (b) Histogram representing the numbers of centrosomes per cell, as observed by γ-tubulin staining, in cells treated with HU alone or HU plus LMB. (c) CHO:centrin1-GFP cells were treated with HU or HU plus LMB for 48 h. Cells were stained with GFP (green) and PCM-1 (red) antibodies. (d) Cell extracts were prepared from CHO cells treated with HU alone or HU plus roscovitine or LMB. Western blots were probed with antibodies against PCM-1, γ-tubulin, α-tubulin, and centrin. (e) CHO:centrin1-GFP cells were treated with HU, LMB, and nocodazole for 48 h before being stained with γ-tubulin (red) and GFP (green) antibodies. (f) CHO or CHO:centrin1-GFP cells were treated with HU, LMB, and roscovitine for 48 h, or p53−/− Cdk2−/− MEFs were treated with HU and LMB for 48 h. Cells were stained with γ-tubulin (red) and centrin or GFP (green) antibodies. (g) Histogram representing the relative fluorescence intensities of centrin and γ-tubulin in the nuclei of CHO cells treated with HU plus roscovitine or HU plus LMB relative to those in cells treated with HU alone (n, >40). (h) CHO:centrin1-GFP cells were treated for 48 h with LMB and either HU to arrest cells in S phase or mimosine to arrest cells in G1 before monitoring GFP fluorescence. Three examples of each are shown. (i) CHO:centrin1-GFP cells were transiently transfected with myc-cyclin A and treated with mimosine and LMB for 48 h. Cells were stained for GFP (green) and myc (red). In panels a, c, e, f, h, and i, merge panels include a DNA stain (blue). Bars, 10 μm.
Finally, we found that washout of Hsp90, dynein, MT, or nuclear export inhibitors led to a rapid increase in overduplicated centrosomes, as determined by γ-tubulin staining, whereas washout of the Cdk inhibitors roscovitine and olomoucine did not lead to overduplication of centrosomes within the same time frame (see Fig. S5 in the supplemental material). Hence, Cdk activation is an early, and possibly rate-limiting, step in the pathway that leads to centrosome overduplication, whereas the other factors are required to rapidly convert centrosome intermediates into functional centrosomes.
DISCUSSION
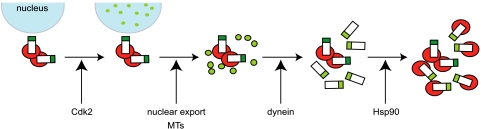
Cancer cells frequently contain supernumerary centrosomes, and at least for some of these cells, this is likely to have arisen by overduplication of centrosomes within a single cell cycle (12, 15, 47, 49). Yet the mechanism by which centrosomes overduplicate is poorly understood. Here we define a series of novel molecular events that are required for the overduplication of functional centrosomes, including the generation of centrin-containing precursors in the nucleus, their export to the cytoplasm, the recruitment of additional centrosome components, MT-dependent trafficking to the existing centrosome, formation of new centrioles, and recruitment of MT nucleating activity (Fig. 8).
FIG. 8.
Pathway for centrosome overduplication in S-phase-arrested cells. In this model of centrosome overduplication, the earliest precursors, which include the centrin protein, assemble in the nucleus dependent upon Cdk2 activity (green dots). Nuclear export is required for translocation of these precursors to the cytoplasm, where they recruit multiple centrosomal proteins, including posttranslationally modified tubulin, PCM-1, and pericentrin, and thereby resemble the fibrous granules/centriolar satellites that also act as precursors for basal body formation in the ciliogenesis pathway (green dots with black borders). MTs and dynein contribute to coalescence of these structures around the existing centrosome and to the formation of new centrioles (cylinders). Finally, Hsp90 is required for recruitment of γ-tubulin (red discs) to the overduplicated centrioles and for the formation of centrosomes capable of nucleating MTs.
The key observation in this study was that particular treatments block centrosome overduplication, as measured with some centrosome markers but not others. Hence, staining with single antibodies can be misleading. Inhibition of MTs, dynein, Hsp90, Cdk activity, and nuclear export all interferes with overduplication of γ-tubulin-containing centrosomes capable of nucleating MT asters. In contrast, only Cdk inhibition prevents the appearance of centrin-containing foci. TEM analysis was used to define whether or not morphologically distinct centrioles were assembled, revealing that inhibition of MTs, dynein, and Cdk activity blocked centriole overduplication, whereas inhibition of Hsp90 did not. These events were not restricted to one particular cell line, as they were observed in three independent centrin1-GFP-expressing CHO cell lines as well as in wild-type CHO cells, U2OS cells, and MEFs.
In placing these events on a pathway that leads to centrosome overduplication, we have made an assumption that centrin granules are genuine precursors of centrosomes. Crucially, these granules contain a large number of other well-defined centrosomal proteins, which together may constitute structures previously described as centriolar satellites and fibrous granules. Centrin, which is known to assemble into calcium-sensitive fibers in complex with the SfiI protein, has been localized to centriolar satellites, the PCM, and the distal lumen of centrioles (57). Moreover, centriolar satellites contain stabilized tubulin, pericentrin, PCM-1, and Cep135 and cluster around the centrosome in the presence of MTs, while fibrous granules which act as precursors for basal body assembly during the centriolar and acentriolar pathways of ciliogenesis (1, 59) also contain stabilized tubulin, centrin, and PCM-1 (32, 36, 45). Remarkably, during early studies of oviduct ciliogenesis, fibrous granules were seen in pockets of the nuclear envelope (1, 46), an observation highly reminiscent of the concentration of centrin aggregates at the nuclear envelope in the absence of MTs. Indeed, in the experiments that we carried out, treatments that blocked centrosome overduplication invariably led to reorganization of centriolar satellites, strongly supporting the notion that centriolar satellites play important roles in centrosome duplication. However, the precise relationship between centriolar satellites and centrosomes remains an area of debate, with some suggestion that satellites may coalesce to form the fibrous PCM lattice (6, 7). In other words, centriolar satellites and the PCM may be interconvertible structures with the larger foci that we see contributing to formation of the PCM. However, the centrin that appears within the centriolar lumen may also be delivered via centriolar satellites. On this basis, we propose that the small centrin foci initially detected in the nucleus and the larger, more complex cytoplasmic foci can both be considered genuine centrosome precursors.
Importantly, by combining time-lapse imaging and immunofluorescence microscopy of the same cells, we found that centrin1-GFP aggregates that formed during treatment of cells with HU and nocodazole acquired markers of functional overduplicated centrosomes upon washout of the nocodazole. This lends considerable weight to our hypothesis that centrin aggregates are genuine precursors of overduplicated centrosomes. Live-cell imaging revealed that upon nocodazole washout, the centrin1-GFP aggregates quickly clustered in the center of the cell. These clusters stained positively for γ-tubulin and C-Nap1, although there was a clear delay in the clustering and the acquisition of these markers. We speculate that this may represent a period during which additional events are proceeding, including, perhaps, the elongation of new centrioles. However, our studies do not address whether overduplicated centrioles formed via a templated or de novo pathway. It was previously reported that cells in which centrioles were laser ablated assembled centrin-containing granules, which were termed precentrioles because of their apparent contribution to the de novo assembly of centrioles (37). However, it may not be as simple as one or another pathway, as under certain experimental circumstances individual centrioles can act as templates for the growth of multiple centrioles (17, 31, 39). Furthermore, a recent study on the dynamics of centrin2-GFP suggested that in HU-arrested CHO cells, centrosomes initially duplicate in a standard templated manner and then overduplicate in a more random manner, perhaps involving both de novo formation and templating of multiple centrioles, with centrin contributing to both centriole and PCM formation (35). How exactly centrin participates in centriole formation remains obscure, though. One possibility is that centrin/SfiI fibers contribute to the cartwheel structure that transiently assembles before the elongation of new centrioles, and in this respect, it is interesting that Cep135, the mammalian homologue of the Chlamydomonas Bld10 protein that is thought to be part of the cartwheel, was also detected in the cytoplasmic centrin granules (20, 57).
Previous work using HU-arrested CHO cells demonstrated an essential role for Cdk2 in centrosome overduplication (43). This, together with other studies, suggested a mechanism by which cells might coordinate their centriole duplication and DNA replication cycles (26). More recent work, though, has questioned the role of Cdk2 in normal centrosome duplication and suggested that Cdk1 can compensate in the absence of Cdk2 (18, 27). Here we demonstrated, using both pharmacological inhibitors and genetically modified cells, that Cdk2 activity is essential not only for overduplication of functional centrosomes but also for the assembly of centrin-containing granules in the nucleus. Cdk2 is found predominantly in the nucleus, where it regulates the initiation of and passage through S phase by phosphorylating chromatin-associated replication factors (10). This raises the intriguing question of how it can also control a cytoplasmic process such as centrosome duplication. Cdk2, with its cyclin partners, does undergo nucleocytoplasmic shuttling, but this is not blocked by LMB (28). However, blocking nuclear export with LMB completely arrested centrosome overduplication and led to the accumulation of centrin granules in the nuclei of S-phase-arrested cells. Importantly, such granules were not present in G1-arrested cells unless cyclin A was overexpressed. This argues against these nuclear centrin granules representing foci of DNA damage that may be induced by HU treatment. Indeed, although centrin is implicated in nucleotide excision repair as part of the XPC complex (2), nuclear centrin granules did not colocalize with sites of DNA damage, as stained with various markers, including XPC itself (see Fig. S6 in the supplemental material).
Taken together, our data support a model in which, upon activation of Cdk2, centrin is assembled into centrosome precursors in the nucleus. These are then exported to the cytoplasm in a Crm1/exportin 1-dependent manner, where they recruit additional centrosomal components and form centriolar satellites. Indeed, the nucleocytoplasmic shuttling of centrin has been reported previously (29). This raises the obvious question of what Cdk2 phosphorylates to trigger formation of nuclear centrin granules. It is intriguing that centrin binds to CP110, a protein whose phosphorylation by Cdk2 promotes centrosome duplication (13, 61), and thus, phosphorylation of proteins such as CP110 might promote centrin recruitment and assembly of early centrosome intermediates.
Finally, it is worth noting that while yeast centrin, Cdc31p, is essential for spindle pole body duplication, there is some debate over whether mammalian centrins are necessary for centrosome duplication (31, 44, 50, 58). However, in our study, centrin acts as a marker for centrosome precursors and we do not address whether it is an essential component of such structures. In summary, while there may be key differences in the control of normal centrosome duplication and that of overduplication, the clinical importance of this study is that it reveals novel mechanisms that might be targeted to prevent centrosome overduplication during chemotherapeutic strategies that arrest cancer cells in S phase.
Supplementary Material
Acknowledgments
We are very grateful to M. Bornens (Paris), R. Vallee (New York), J. Pines (Cambridge), and S. Shackleton (Leicester) for centrin1-GFP, myc-dynamitin, myc-cyclin A, and myc-lamin A plasmids, respectively; to S. Monkley, S. Macip (Leicester), and P. Kaldis (Singapore) for wild-type, p53−/−, and p53−/− Cdk2−/− MEFs, respectively; to A. Merdes (Montpellier) and E. Nigg (Martinsried) for PCM-1 and Cep135 antibodies, respectively; and to M. Mogensen (Norwich), S. Hyman, and N. Allcock (Leicester) for support with microinjection and EM studies. We thank all members of the lab for useful discussions.
This work was supported by grants to A.M.F. from the BBSRC, The Wellcome Trust, and Cancer Research UK.
Footnotes
Published ahead of print on 12 January 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Anderson, R. G., and R. M. Brenner. 1971. The formation of basal bodies (centrioles) in the rhesus monkey oviduct. J. Cell Biol. 5010-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araki, M., C. Masutani, M. Takemura, A. Uchida, K. Sugasawa, J. Kondoh, Y. Ohkuma, and F. Hanaoka. 2001. Centrosome protein centrin 2/caltractin 1 is part of the xeroderma pigmentosum group C complex that initiates global genome nucleotide excision repair. J. Biol. Chem. 27618665-18672. [DOI] [PubMed] [Google Scholar]
- 3.Bahe, S., Y. D. Stierhof, C. J. Wilkinson, F. Leiss, and E. A. Nigg. 2005. Rootletin forms centriole-associated filaments and functions in centrosome cohesion. J. Cell Biol. 17127-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balczon, R., L. Bao, W. E. Zimmer, K. Brown, R. P. Zinkowski, and B. R. Brinkley. 1995. Dissociation of centrosome replication events from cycles of DNA synthesis and mitotic division in hydroxyurea-arrested Chinese hamster ovary cells. J. Cell Biol. 130105-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balczon, R., C. E. Varden, and T. A. Schroer. 1999. Role for microtubules in centrosome doubling in Chinese hamster ovary cells. Cell. Motil. Cytoskeleton 4260-72. [DOI] [PubMed] [Google Scholar]
- 6.Baron, A. T., and J. L. Salisbury. 1988. Identification and localization of a novel, cytoskeletal, centrosome-associated protein in PtK2 cells. J. Cell Biol. 1072669-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baron, A. T., V. J. Suman, E. Nemeth, and J. L. Salisbury. 1994. The pericentriolar lattice of PtK2 cells exhibits temperature and calcium-modulated behavior. J. Cell Sci. 1072993-3003. [DOI] [PubMed] [Google Scholar]
- 8.Bennett, R. A., H. Izumi, and K. Fukasawa. 2004. Induction of centrosome amplification and chromosome instability in p53-null cells by transient exposure to subtoxic levels of S-phase-targeting anticancer drugs. Oncogene 236823-6829. [DOI] [PubMed] [Google Scholar]
- 9.Bettencourt-Dias, M., and D. M. Glover. 2007. Centrosome biogenesis and function: centrosomics brings new understanding. Nat. Rev. Mol. Cell. Biol. 8451-463. [DOI] [PubMed] [Google Scholar]
- 10.Blow, J. J., and A. Dutta. 2005. Preventing re-replication of chromosomal DNA. Nat. Rev. Mol. Cell. Biol. 6476-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bornens, M. 2002. Centrosome composition and microtubule anchoring mechanisms. Curr. Opin. Cell Biol. 1425-34. [DOI] [PubMed] [Google Scholar]
- 12.Brinkley, B. R. 2001. Managing the centrosome numbers game: from chaos to stability in cancer cell division. Trends Cell Biol. 1118-21. [DOI] [PubMed] [Google Scholar]
- 13.Chen, Z., V. B. Indjeian, M. McManus, L. Wang, and B. D. Dynlacht. 2002. CP110, a cell cycle-dependent CDK substrate, regulates centrosome duplication in human cells. Dev. Cell 3339-350. [DOI] [PubMed] [Google Scholar]
- 14.Dammermann, A., and A. Merdes. 2002. Assembly of centrosomal proteins and microtubule organization depends on PCM-1. J. Cell Biol. 159255-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Assoro, A. B., W. L. Lingle, and J. L. Salisbury. 2002. Centrosome amplification and the development of cancer. Oncogene 216146-6153. [DOI] [PubMed] [Google Scholar]
- 16.Delattre, M., C. Canard, and P. Gonczy. 2006. Sequential protein recruitment in C. elegans centriole formation. Curr. Biol. 161844-1849. [DOI] [PubMed] [Google Scholar]
- 17.Duensing, A., Y. Liu, S. A. Perdreau, J. Kleylein-Sohn, E. A. Nigg, and S. Duensing. 2007. Centriole overduplication through the concurrent formation of multiple daughter centrioles at single maternal templates. Oncogene 266280-6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duensing, A., Y. Liu, M. Tseng, M. Malumbres, M. Barbacid, and S. Duensing. 2006. Cyclin-dependent kinase 2 is dispensable for normal centrosome duplication but required for oncogene-induced centrosome overduplication. Oncogene 252943-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duensing, S., L. Y. Lee, A. Duensing, J. Basile, S. Piboonniyom, S. Gonzalez, C. P. Crum, and K. Munger. 2000. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc. Natl. Acad. Sci. USA 9710002-10007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dutcher, S. K. 2007. Finding treasures in frozen cells: new centriole intermediates. Bioessays 29630-634. [DOI] [PubMed] [Google Scholar]
- 21.Faragher, A. J., and A. M. Fry. 2003. Nek2A kinase stimulates centrosome disjunction and is required for formation of bipolar mitotic spindles. Mol. Biol. Cell 142876-2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fry, A. M., T. Mayor, P. Meraldi, Y.-D. Stierhof, K. Tanaka, and E. A. Nigg. 1998. C-Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle-regulated protein kinase Nek2. J. Cell Biol. 1411563-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goshima, G., R. Wollman, S. S. Goodwin, N. Zhang, J. M. Scholey, R. D. Vale, and N. Stuurman. 2007. Genes required for mitotic spindle assembly in Drosophila S2 cells. Science 316417-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hagiwara, H., N. Ohwada, and K. Takata. 2004. Cell biology of normal and abnormal ciliogenesis in the ciliated epithelium. Int. Rev. Cytol. 234101-141. [DOI] [PubMed] [Google Scholar]
- 25.Hames, R. S., R. E. Crookes, K. R. Straatman, A. Merdes, M. J. Hayes, A. J. Faragher, and A. M. Fry. 2005. Dynamic recruitment of Nek2 kinase to the centrosome involves microtubules, PCM-1, and localized proteasomal degradation. Mol. Biol. Cell 161711-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinchcliffe, E. H., and G. Sluder. 2001. “It takes two to tango”: understanding how centrosome duplication is regulated throughout the cell cycle. Genes Dev. 151167-1181. [DOI] [PubMed] [Google Scholar]
- 27.Hochegger, H., D. Dejsuphong, E. Sonoda, A. Saberi, E. Rajendra, J. Kirk, T. Hunt, and S. Takeda. 2007. An essential role for Cdk1 in S phase control is revealed via chemical genetics in vertebrate cells. J. Cell Biol. 178257-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackman, M., Y. Kubota, N. den Elzen, A. Hagting, and J. Pines. 2002. Cyclin A- and cyclin E-Cdk complexes shuttle between the nucleus and the cytoplasm. Mol. Biol. Cell 131030-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keryer, G., B. Di Fiore, C. Celati, K. F. Lechtreck, M. Mogensen, A. Delouvee, P. Lavia, M. Bornens, and A. M. Tassin. 2003. Part of Ran is associated with AKAP450 at the centrosome: involvement in microtubule-organizing activity. Mol. Biol. Cell 144260-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khodjakov, A., C. L. Rieder, G. Sluder, G. Cassels, O. Sibon, and C. L. Wang. 2002. De novo formation of centrosomes in vertebrate cells arrested during S phase. J. Cell Biol. 1581171-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kleylein-Sohn, J., J. Westendorf, M. Le Clech, R. Habedanck, Y. D. Stierhof, and E. A. Nigg. 2007. Plk4-induced centriole biogenesis in human cells. Dev. Cell 13190-202. [DOI] [PubMed] [Google Scholar]
- 32.Kubo, A., H. Sasaki, A. Yuba-Kubo, S. Tsukita, and N. Shiina. 1999. Centriolar satellites: molecular characterization, ATP-dependent movement towards centrioles and possible involvement in ciliogenesis. J. Cell Biol. 147969-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kudo, N., N. Matsumori, H. Taoka, D. Fujiwara, E. P. Schreiner, B. Wolff, M. Yoshida, and S. Horinouchi. 1999. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl. Acad. Sci. USA 969112-9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuriyama, R., and G. G. Borisy. 1981. Centriole cycle in Chinese hamster ovary cells as determined by whole-mount electron microscopy. J. Cell Biol. 91814-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuriyama, R., Y. Terada, K. S. Lee, and C. L. Wang. 2007. Centrosome replication in hydroxyurea-arrested CHO cells expressing GFP-tagged centrin2. J. Cell Sci. 1202444-2453. [DOI] [PubMed] [Google Scholar]
- 36.Laoukili, J., E. Perret, S. Middendorp, O. Houcine, C. Guennou, F. Marano, M. Bornens, and F. Tournier. 2000. Differential expression and cellular distribution of centrin isoforms during human ciliated cell differentiation in vitro. J. Cell Sci. 1131355-1364. [DOI] [PubMed] [Google Scholar]
- 37.La Terra, S., C. N. English, P. Hergert, B. F. McEwen, G. Sluder, and A. Khodjakov. 2005. The de novo centriole assembly pathway in HeLa cells: cell cycle progression and centriole assembly/maturation. J. Cell Biol. 168713-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leidel, S., and P. Gonczy. 2005. Centrosome duplication and nematodes: recent insights from an old relationship. Dev. Cell 9317-325. [DOI] [PubMed] [Google Scholar]
- 39.Loncarek, J., P. Hergert, V. Magidson, and A. Khodjakov. 2008. Control of daughter centriole formation by the pericentriolar material. Nat. Cell Biol. 10322-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marshall, W. F. 2001. Centrioles take center stage. Curr. Biol. 11R487-R496. [DOI] [PubMed] [Google Scholar]
- 41.Marshall, W. F., Y. Vucica, and J. L. Rosenbaum. 2001. Kinetics and regulation of de novo centriole assembly. Implications for the mechanism of centriole duplication. Curr. Biol. 11308-317. [DOI] [PubMed] [Google Scholar]
- 42.Mazia, D. 1987. The chromosome cycle and the centrosome cycle in the mitotic cycle. Int. Rev. Cytol. 10049-92. [DOI] [PubMed] [Google Scholar]
- 43.Meraldi, P., J. Lukas, A. M. Fry, J. Bartek, and E. A. Nigg. 1999. Centrosome duplication in mammalian somatic cells requires E2F and Cdk2-cyclin A. Nat. Cell Biol. 188-93. [DOI] [PubMed] [Google Scholar]
- 44.Middendorp, S., T. Kuntziger, Y. Abraham, S. Holmes, N. Bordes, M. Paintrand, A. Paoletti, and M. Bornens. 2000. A role for centrin 3 in centrosome reproduction. J. Cell Biol. 148405-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Million, K., J. Larcher, J. Laoukili, D. Bourguignon, F. Marano, and F. Tournier. 1999. Polyglutamylation and polyglycylation of alpha- and beta-tubulins during in vitro ciliated cell differentiation of human respiratory epithelial cells. J. Cell Sci. 1124357-4366. [DOI] [PubMed] [Google Scholar]
- 46.Nayak, R. K., D. R. Zimmerman, and E. N. Albert. 1976. Electron microscopic studies of estrogen-induced ciliogenesis and secretion in uterine tube of the gilt. Am. J. Vet. Res. 37189-197. [PubMed] [Google Scholar]
- 47.Nigg, E. A. 2002. Centrosome aberrations: cause or consequence of cancer progression? Nat. Rev. Cancer 2815-825. [DOI] [PubMed] [Google Scholar]
- 48.Nigg, E. A. 2007. Centrosome duplication: of rules and licenses. Trends Cell Biol. 17215-221. [DOI] [PubMed] [Google Scholar]
- 49.Nigg, E. A. 2006. Origins and consequences of centrosome aberrations in human cancers. Int. J. Cancer 1192717-2723. [DOI] [PubMed] [Google Scholar]
- 50.Paoletti, A., N. Bordes, R. Haddad, C. L. Schwartz, F. Chang, and M. Bornens. 2003. Fission yeast cdc31p is a component of the half-bridge and controls SPB duplication. Mol. Biol. Cell 142793-2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pelletier, L., E. O'Toole, A. Schwager, A. A. Hyman, and T. Muller-Reichert. 2006. Centriole assembly in Caenorhabditis elegans. Nature 444619-623. [DOI] [PubMed] [Google Scholar]
- 52.Penningroth, S. M. 1986. Erythro-9-[3-(2-hydroxynonyl)]adenine and vanadate as probes for microtubule-based cytoskeletal mechanochemistry. Methods Enzymol. 134477-487. [DOI] [PubMed] [Google Scholar]
- 53.Piel, M., P. Meyer, A. Khodjakov, C. L. Rieder, and M. Bornens. 2000. The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J. Cell Biol. 149317-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pihan, G. A., J. Wallace, H. Zhou, and S. J. Doxsey. 2003. Centrosome abnormalities and chromosome instability occur together in pre-invasive carcinomas. Cancer Res. 631398-1404. [PubMed] [Google Scholar]
- 55.Prosser, S. L., and A. M. Fry. Fluorescence imaging of the centrosome cycle in mammalian cells. Methods Mol. Biol., in press. [DOI] [PubMed]
- 56.Quintyne, N. J., and T. A. Schroer. 2002. Distinct cell cycle-dependent roles for dynactin and dynein at centrosomes. J. Cell Biol. 159245-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salisbury, J. L. 2007. A mechanistic view on the evolutionary origin for centrin-based control of centriole duplication. J. Cell Physiol. 213420-428. [DOI] [PubMed] [Google Scholar]
- 58.Salisbury, J. L., K. M. Suino, R. Busby, and M. Springett. 2002. Centrin-2 is required for centriole duplication in mammalian cells. Curr. Biol. 121287-1292. [DOI] [PubMed] [Google Scholar]
- 59.Sorokin, S. P. 1968. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J. Cell Sci. 3207-230. [DOI] [PubMed] [Google Scholar]
- 60.Tarapore, P., and K. Fukasawa. 2002. Loss of p53 and centrosome hyperamplification. Oncogene 216234-6240. [DOI] [PubMed] [Google Scholar]
- 61.Tsang, W. Y., A. Spektor, D. J. Luciano, V. B. Indjeian, Z. Chen, J. L. Salisbury, I. Sanchez, and B. D. Dynlacht. 2006. CP110 cooperates with two calcium-binding proteins to regulate cytokinesis and genome stability. Mol. Biol. Cell 173423-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsou, M. F., and T. Stearns. 2006. Controlling centrosome number: licenses and blocks. Curr. Opin. Cell Biol. 1874-78. [DOI] [PubMed] [Google Scholar]
- 63.Wong, C., and T. Stearns. 2003. Centrosome number is controlled by a centrosome-intrinsic block to reduplication. Nat. Cell Biol. 5539-544. [DOI] [PubMed] [Google Scholar]
- 64.Zarzov, P., H. Boucherie, and C. Mann. 1997. A yeast heat shock transcription factor (Hsf1) mutant is defective in both Hsc82/Hsp82 synthesis and spindle pole body duplication. J. Cell Sci. 1101879-1891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.