Abstract
Ornithine decarboxylase (ODC), the rate-limiting enzyme in polyamine biosynthesis, is a nonredundant and essential gene in all eukaryotes. During the mitotic cell cycle, ODC exhibits two activity peaks: one at the G1/S transition and one during the G2/M transition. The physiological role of this cell cycle-dependent ODC activity dynamic is not clear. Previous studies have reported a significant elevation of ODC activity during Xenopus oocyte maturation, which resembles mitotic G2/M transition. In order to study the roles of ODC activity in the oocytes, we utilized antisense morpholino (xODC mo) oligonucleotides to inhibit ODC translation. We report here that xODC mo abolished ODC activity increase during oocyte maturation. xODC mo-injected oocytes underwent germinal vesicle breakdown, emitted the first polar body, and reached metaphase II, thus completing nuclear maturation. However, the metaphase II oocytes exhibited high levels of reactive oxygen species and became apoptotic. When transferred to host frogs and subsequently ovulated, these eggs were fertilized but exhibited embryo fragmentation. Translation of ODC is therefore integral to cytoplasmic maturation, protecting metaphase II oocytes from reactive oxygen species-induced apoptosis.
Ornithine decarboxylase (ODC) is the rate-limiting enzyme in the cellular biosynthetic pathway to polyamines (putrescine, spermidine, and spermine). ODC is encoded by a nonredundant and essential gene in all eukaryotic organisms, from yeast (27) to mammals (21). ODC levels are high during embryogenesis and in cancers but low at the onset of cell senescence and during mammalian aging (11). Apart from this developmental regulation, ODC is also highly regulated through the cell cycle, exhibiting two activity peaks: one at the G1/S transition and one during the G2/M transition (22). The cell cycle-dependent ODC activity dynamic is brought about by translation and proteasome-mediated degradation. ODC has a very short half-life, estimated to be 10 min in mammalian cells (30). ODC degradation is catalyzed by the 26S proteasome without prior polyubiquitination (17). Instead, ODC antizyme, a polyamine-induced protein, binds ODC, serving to inhibit ODC activity and to promote proteasome-mediated ODC degradation (33). The cell cycle-dependent ODC activity dynamic underscores the multiple cellular functions of ODC and polyamines, and yet, what these functions are remains unclear.
Immature vertebrate oocytes are arrested at the diplotene stage of meiotic prophase; this process is often referred to as G2 arrest. Oocyte maturation encompasses germinal vesicle breakdown (GVBD) and, subsequently, the dramatic chromosomal dynamics culminating with the extrusion of the first polar body and the successful arrest of the mature egg in metaphase II. Mechanistically, therefore, oocyte maturation resembles mitotic G2/M transition. Indeed, an earlier study from the laboratory of E. E. Baulieu reported a significant elevation of ODC activity during Xenopus oocyte maturation (32). However, complete inhibition of ODC activity by difluoromethylornithine, a highly specific suicide inhibitor of ODC (16), did not block GVBD. But as GVBD marks only the beginning of chromosome changes (condensation), the results of that study do not exclude the involvement of ODC in post-GVBD events in oocyte maturation. In order to determine the physiological roles of ODC activity increase during oocyte maturation, we utilized morpholino oligonucleotides antisense to Xenopus ODC to inhibit ODC synthesis.
MATERIALS AND METHODS
All morpholino oligonucleotides were purchased from Gene Tools (Philomath, OR). Antibodies against human cytochrome c (Cyt c) were purchased from BD Pharmingen. Antibodies against Xenopus MAP kinase have been described previously (20). Antibodies against β-tubulin were from Developmental Studies Hybridoma Bank at the University of Iowa. Antibodies against phospho-Tyr15 Cdc2 were purchased from Cell Signaling Technology. These antibodies recognize only the inactive form of Cdc2 (18). The reactive oxygen species (ROS) detection kit was purchased from Molecular Probes. The other reagents were from Sigma unless otherwise stated.
The coding sequence of Xenopus ODC (1) was PCR amplified from a Xenopus cDNA library (23) and inserted into the pCS2-HA vector (3), creating the hemagglutinin (HA)-ODC expression vector. A human Bcl-2 expression plasmid in pBluescript was a gift from Gordon Shore (McGill University). cDNA constructs were linearized prior to in vitro transcription reactions by using an Ambion mMESSAGE mMACHINE kit (5′ capped). The 5′-capped mRNA was dissolved in water to 1 mg/ml, and 10 nl was microinjected per oocyte.
Oocytes were isolated by treating ovarian tissues with collagenase and incubated in OR2 medium (82.50 mM NaCl, 2.5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 1 mM Na2HPO4, 5 mM HEPES, pH 7.8) (14), except for oocytes used in the host transfer experiments (described later). When oocytes were examined for chromosome morphology, we monitored progesterone-treated oocytes every 10 min to withdraw individual GVBD oocytes (when the GVBD spot first appeared) for further incubation before fixation for chromosome analyses (15, 34). In these experiments, 20 oocytes were examined per group. In all other time course experiments, oocytes were randomly withdrawn at the indicated time following the addition of progesterone (1 μM), at the time when 50% (or 100%) of the treated oocytes exhibited the GVBD spot, or at the indicated time after 100% of the oocytes reached GVBD.
Analyzing poly(A) tail length.
We followed the ligation coupled reverse transcription-PCR (RT-PCR) approach as described by Charlesworth et al. (5). In this technique, a DNA oligonucleotide (P1; 5′-P-GGTCACCTTGATCTGAAGC-NH2-3′) with the 3′ end blocked by an amide (so only the 5′ end can be ligated) is ligated to the 3′ end of the RNA by using T4 RNA ligase. Another DNA oligonucleotide (P2; 5′-GCTTCAGATCAAGGTGACCTTTTT) that is complementary to P1 is used as a primer for cDNA synthesis using reverse transcriptase. PCR is then carried out using P2 and a gene-specific forward primer (xODC [5′-GAA GAT GCT AAT TAT TTA CTC AAG CAT]; the gene-specific primers for Mos and Cyclin B1 were 5′-GTT GCA TTG CTG TTT AAG TGG TAA and 5′-GTG GCA TTC CAA TTG TGT ATT GTT, respectively [5]).
ODC activity assay.
Oocytes were rinsed twice in ice-cold phosphate-buffered saline, suspended in ice-cold lysis buffer (0.25 M Tris-HCl, pH 7.4, 1 mM EDTA, 1 mM dithiothreitol; 10 μl per oocyte), and lysed by forcing them through a pipette tip. Lysates were centrifuged at 13,000 × g for 10 min. Supernatants were incubated for 1 h at 37°C with 0.25 μCi of 14C-labeled ornithine (Amersham) and 50 mM pyridoxal 5-phosphate in either 96-well circles or 384-well squares, which were covered with 3MM paper prewet with a solution of barium hydroxide [Ba(OH)2] saturated with H2O. 14CO2 released from the decarboxylation reaction then reacted with Ba(OH)2 to form Ba14CO3 precipitates on the filter. The 3MM paper was rinsed with acetone, dried, and exposed to storage phosphor screens (Kodak) overnight, followed by scanning in Typhoon. Signals in the gray map were quantified using ImageJ, followed by analyses using Sigma Plot 8.0.
Caspase 3 activity assay (Sigma kit).
Oocytes were suspended in 1× lysis buffer (10 μl per oocyte) and lysed by forcing them through a pipette tip. The lysates were placed on ice for 10 min before centrifugation at 13,000 × g for 10 min at 4°C. The supernatants were analyzed immediately or stored at −80°C. To initiate caspase 3 activity assays with microtiter plate wells, 50 μl of lysate was mixed with 40 μl 1× assay buffer and 10 μl of caspase 3 substrate. The reaction was carried out at 37°C for 3 h in the dark. Absorbance at 405 nm was determined with Spectra Max (Molecular Devices) for each sample at the beginning of incubation (time zero) and at the end of the 3-h incubation. Shown are net optical density (OD) values (OD at 3 h − OD at time zero).
Cyt c release assay.
The following procedure was modified from the method of Nutt et al. (19). Oocytes were suspended in lysis buffer (20 mM HEPES, pH 7.5, 20 mM β-glycerophosphate, 15 mM MgCl2, 20 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 10 μM each of aprotenin and leupeptin; 10 μl per oocyte) and lysed by forcing them through a pipette tip. The lysates were centrifuged at 13,000 × g for 5 min at 4°C. The supernatants were filtered through a 0.1-μm ultrafree-MC filter (Millipore), and the eluates, which contained released Cyt c, were mixed with cold acetone (1:4 ratio). The mixture was centrifuged (13,000 × g for 5 min at 4°C) to precipitate proteins. The dried pellet was dissolved in sodium dodecyl sulfate sample buffer before immunoblot analysis with antibodies against human Cyt c. Each lane represented a sample derived from 5 to 10 oocytes.
ROS detection.
Oocytes were suspended in ROS assay buffer (50 mM NaCl, 2.0 mM MgCl2, 10 mM Tris-HCl, pH 7.8; 10 μl per oocyte). The lysates were then centrifuged at 13,000 × g for 10 min at 4°C. The supernatants were analyzed immediately or stored at −80°C. To assay for ROS, 100 μl of lysates was placed in dark 96-well plates, followed by the addition of 100 μl 50 μM carboxy-H2DCFDA (Molecular Probes) solution, which was prepared in ROS assay buffer. The reactions were read immediately (time zero) and at the indicated time intervals (up to 250 min), using Fusion (Parkard), with 495-nm excitation and 529-nm emission filter set. Data were expressed as numbers of relative fluorescence units.
Host transfer experiments.
We essentially followed the protocol described by Heasman et al. (12). Briefly, oocytes were isolated by manual defolliculation (14) in OCM (prepared daily by mixing 480 ml of Lebbovitz L-15 medium, 320 ml sterile water, 0.32 g of bovine serum albumin, 4 ml 200 mM glutamine, and gentamicin to give 0.5 mg/ml, pH 7.6 to 7.8). Oocytes were injected with xODC mo or mODC mo (100 ng per oocyte). The injected oocytes were cultured in OCM (instead of OR2, used elsewhere in this study) with 1 μM progesterone for 10 to 12 h (under these conditions, all treated oocytes had undergone GVBD, but few xODC mo-injected oocytes would have exhibited the apoptotic phenotype). Eggs were stained for 15 min with neutral red (0.025%) or Bismarck brown (0.02%) and rinsed in OCM before being surgically transferred into the abdominal cavity of a recipient female. The recipient females were primed with human chorionic gonadotropins (50 U per frog) 5 to 15 days prior to the day of egg transfer. Twelve hours prior to egg transfer, two or three frogs were injected each with 800 U of human chorionic gonadotropins. The female laying the best-quality eggs (12) was chosen for egg transfer. Two and a half to four hours after surgery, colored eggs were collected alongside host eggs for in vitro fertilization. We removed the jelly coat by incubation with 2% cysteine (pH 8) 90 min after fertilization, when host eggs started to cleave. We typically transferred 200 to 300 colored eggs (roughly 60% injected with xODC mo and 40% with mODC mo, stained with red or brown dye) into the same female. Twenty to fifty percent of the transferred colored eggs were collected during the specified period (2.5 to 4 h after transfer) and fertilized by rubbing them with freshly removed and minced testes. Because significant percentages (different from experiment to experiment) of the colored eggs did not cleave (irrespective of whether they were injected with mODC mo or xODC mo or uninjected), as noted by Heasman et al. (12), only cleaving colored embryos were counted in the final tally.
Statistics.
Student t tests were performed for pairwise comparisons. Student-Newman-Keuls tests were performed for comparison of multiple groups. Graphs and images are representative of at least three, most of the time more, independent experiments.
RESULTS
Inhibition of ODC synthesis caused apoptosis.
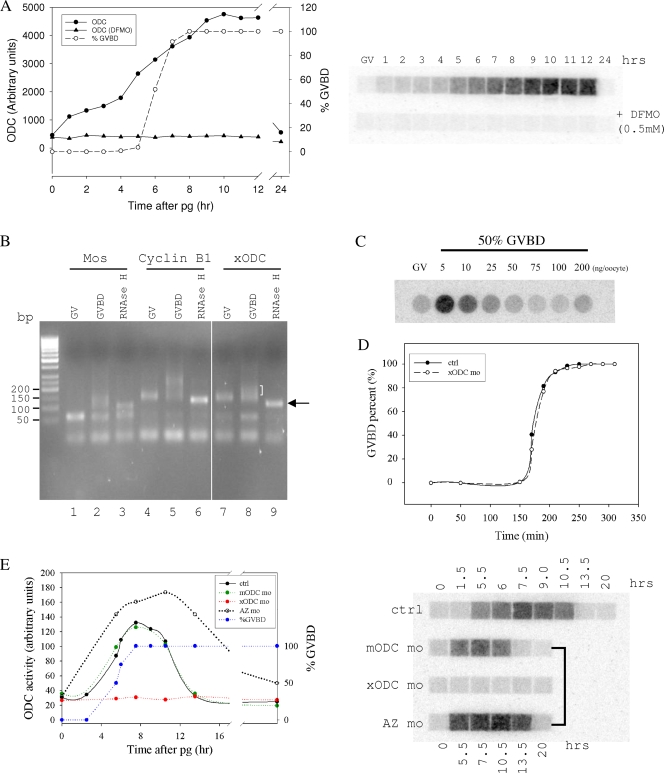
We carried out time course experiments to determine levels of ODC activity during oocyte maturation. As shown in Fig. 1A, ODC activity was undetectable in immature oocytes (also known as germinal vesicle [GV] oocytes), since the basal signal in these oocytes was not inhibited by high concentrations of the ODC inhibitor difluoromethylornithine. ODC activity gradually increased in the presence of progesterone, peaking after 100% of the oocytes reached GVBD. ODC activity remained high for several hours before returning to basal levels (also shown in Fig. 1E). Therefore, our results confirmed the conclusion made by Younglai et al. that ODC activity transiently increased during oocyte maturation (32).
FIG. 1.
ODC exhibited translation-mediated transient activity increase during oocyte maturation. (A) Oocytes were treated with progesterone. At the indicated times, oocytes were examined for GVBD (expressed as percentages) and 10 oocytes were withdrawn and lysed for ODC activity assays. Each sample was split and assayed in the absence (upper row) or the presence (lower row) of 0.5 mM difluoromethylornithine (DFMO). (B) Total RNA isolated from GV oocytes (lanes 1, 4, and 7) or GVBD oocytes (2 h after 100% of the oocytes reached GVBD; lanes 2, 3, 5, 6, 8, and 9) were analyzed by ligation-coupled RT-PCR. The RNA samples in lanes 3, 6, and 9 were first treated with oligo(dT) and RNase H to digest the poly(A) tail before being subjected to the ligation-coupled RT-PCR analyses. This experiment showed that ODC mRNA in GV oocytes had a short poly(A) tail (compared to the “tailless” mRNA in lane 9, indicated by an arrow), which was extended (polyadenylated) in GVBD oocytes (indicated by a half bracket). (C) Oocytes were injected with the indicated amounts of xODC mo and treated with progesterone. When 50% of the oocytes in each group reached GVBD, 10 oocytes were withdrawn, lysed, and assayed for ODC activity. GV represents an extract from uninjected and non-progesterone-treated oocytes. It can be concluded from this experiment that 50 to 100 ng xODC mo per oocyte can completely eliminate ODC translation during oocyte maturation. (D) Control oocytes (ctrl) or oocytes injected with ODC mo (100 ng per oocyte) were treated with progesterone and examined for GVBD at the indicated times after the addition of progesterone. (E) Uninjected oocytes (ctrl), oocytes injected with mODC mo, oocytes injected with xODC mo, and oocytes injected with AZ mo were treated with progesterone. At the indicated times, oocytes were examined for GVBD. Although only GVBD data for control oocytes are shown, none of the oligonucleotides affected the rate of GVBD, the percentage, or the time course (data not shown). At the indicated times, five oocytes were withdrawn from each group for ODC activity assays. On the right is an autoradiograph of a typical ODC assay. The graph shows the quantification of the same data.
Cytoplasmic polyadenylation of maternal mRNA is a major mechanism for mRNA activation and recruitment to polyribosomes during oocyte maturation (24). To determine whether ODC mRNA also undergoes polyadenylation during oocyte maturation, we employed the ligation-coupled RT-PCR approach (5) to analyze the lengths of poly(A) tails. As shown in Fig. 1B, ODC mRNA exhibited poly(A) tail lengthening during oocyte maturation, similar to MOS and cyclin B1, two other maternal mRNAs that are known to undergo cytoplasmic polyadenylation.
To determine whether the increase in ODC activity was the result of ODC translation, we injected morpholino oligonucleotides antisense to Xenopus ODC (xODC mo [5′-AGTCGTCATTGCTGAAGCTGTTCAT-3′]; 100 ng per oocyte unless otherwise indicated) into GV oocytes prior to the addition of progesterone. At 50 to 100 ng per oocyte, xODC mo completely inhibited the ODC activity increase (Fig. 1C). Inhibition of ODC activity increase by xODC mo did not affect progesterone-induced GVBD (Fig. 1D), consistent with the earlier conclusion that inhibition of ODC activity by difluormethylornithine did not inhibit progesterone-induced GVBD (32). To control for the specificity of xODC mo, we employed morpholino oligonucleotides antisense to mouse ODC (mODC mo [5′-CTCGTCCTTAGTAAAGCTGCTCATG-3′]; 100 ng per oocyte). The seven mismatches between mODC mo and the corresponding Xenopus ODC nucleotide sequence should be sufficient to render mODC mo ineffective (28) as an inhibitor of Xenopus ODC translation. Indeed, injection of mODC mo did not interfere with progesterone-induced ODC activity increase (Fig. 1E), nor did it affect progesterone-induced GVBD (not shown). Finally, we wished to determine whether ODC antizyme had any role in ODC turnover in the oocytes. We injected GV oocytes with morpholino oligonucleotides antisense to Xenopus antizyme (13) (AZ mo [5′-GGAGGATTTCACCATCCGGCCTCTC-3′]; 100 ng per oocyte). AZ mo significantly increased the overall level of ODC activity and delayed, but did not eliminate, ODC activity decrease (Fig. 1E).
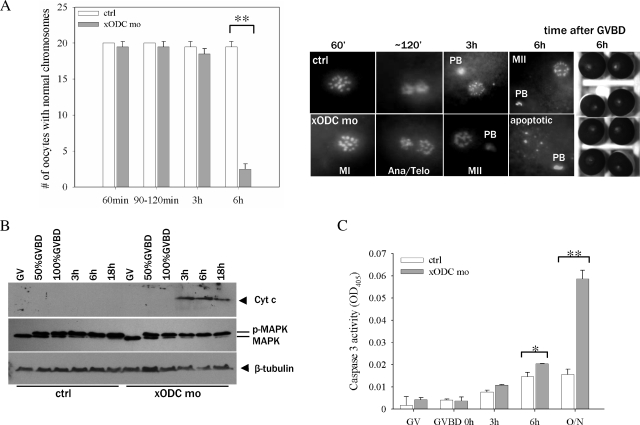
To determine whether xODC mo affected chromosomal dynamics following GVBD, we carried out time course experiments in which we examined the chromosome morphologies of oocytes fixed at different times following GVBD. We found that oocytes injected with xODC mo proceeded through the normal chromosome changes found in control oocytes (15, 34), emitting the first polar body and reaching metaphase II (Fig. 2A) (3 h after GVBD). However, 3 h later (6 h after GVBD), xODC mo-injected oocytes exhibited chromosome disarray, indicating disruption of the metaphase II spindle. In contrast, control oocytes maintained the typical metaphase II chromosome array. This difference was very pronounced, as uninjected oocytes, or oocytes injected with mODC mo (used as control morpholino oligonucleotides), routinely remained in stable metaphase II arrest after more than 20 h (overnight) treatment with progesterone (see Fig. 4A and 5C; also data not shown).
FIG. 2.
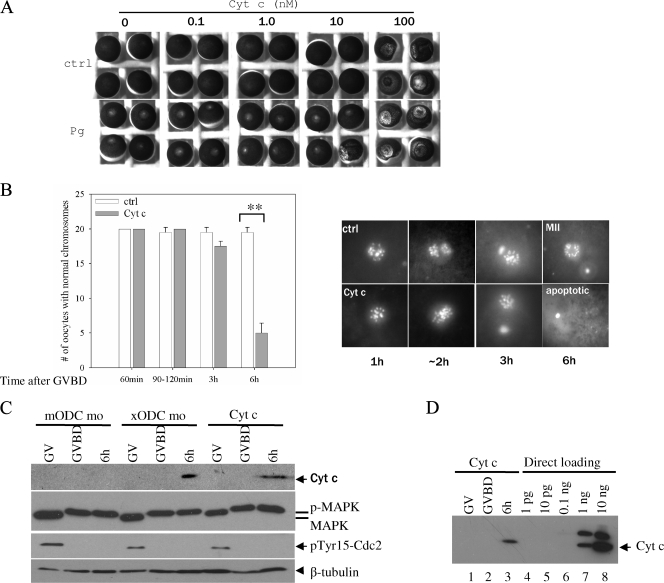
Inhibition of ODC translation caused apoptosis. (A) Control oocytes (ctrl) and xODC mo-injected oocytes were fixed for chromosome analyses at the indicated times following GVBD. The graph summarizes three independent experiments, showing numbers of oocytes (means with standard errors of the means [SEM]) with normal chromosomes (defined as indistinguishable from those observed in the control group) at the indicated times. Representative images are shown below, except for the 90- to 120-min group, which contained a mixture of metaphase I (similar to those in the 60-min group) and anaphase/telophase oocytes, but only the images of anaphase/telophase oocytes are shown. The bright-field images depict the exteriors of the two groups of oocytes (6 h following GVBD), indistinguishable even though the ODC mo oocytes were clearly apoptotic by chromosome morphology. MI, metaphase I; Ana/Telo, anaphase/telophase; MII, metaphase II; PB, polar body; **, P < 0.01 (Student's t test). (B) Control oocytes and xODC mo-injected oocytes were withdrawn at the indicated times for Cyt c release assays (upper panel). The same extracts were also blotted with anti-MAP kinase (middle panel) and anti-β tubulin (lower panel, loading control). p-MAPK, phosphorylated (active) MAP kinase; MAPK, inactive MAP kinase (seen in GV oocyte extracts). (C) Control oocytes and xODC mo-injected oocytes were withdrawn at the indicated times for caspase 3 activity assays. Shown are means (with SEM) for three independent experiments. O/N, overnight (15 to 20 h). *, P < 0.05; **, P < 0.01 (Student's t test).
FIG. 4.
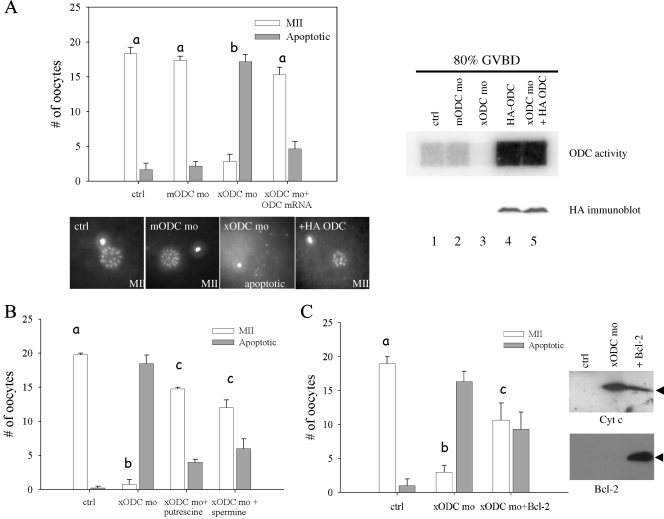
xODC mo-induced apoptosis can be rescued by HA-ODC, polyamines, and Bcl-2. (A) Control oocytes (ctrl) and oocytes injected with the indicated agents were treated overnight (15 to 20 h) with progesterone. Chromosome morphology was determined as indicative of normal metaphase II (MII) eggs or apoptosis. Shown are means (with SEM) for six experiments, with representative chromosome images below. Shown on the right are the results for an ODC activity assay (top) and an HA immunoblot analysis (bottom) of extracts, made when 80% of the oocytes of each group exhibited GVBD. Oocytes injected with HA-ODC mRNA alone were indistinguishable from control oocytes in terms of chromosome morphology (not shown). (B) Assays similar to those for panel A, except for inclusion, where indicated, of 5 mM putrescine or 5 mM spermine in OR2 medium at the same time as progesterone addition. Shown are means (with SEM) for three independent experiments. (C) Assays similar to those for panels A and B, with one group of oocytes injected with xODC mo and Bcl-2 mRNA. Shown are means (with SEM) for three independent experiments. On the right are representative immunoblots showing release of mitochondrial Cyt c (top) and expression of exogenous Bcl-2 (bottom). Differences in letters (a to c) denote significant differences in values (P < 0.01; Student-Newman-Keuls test).
FIG. 5.
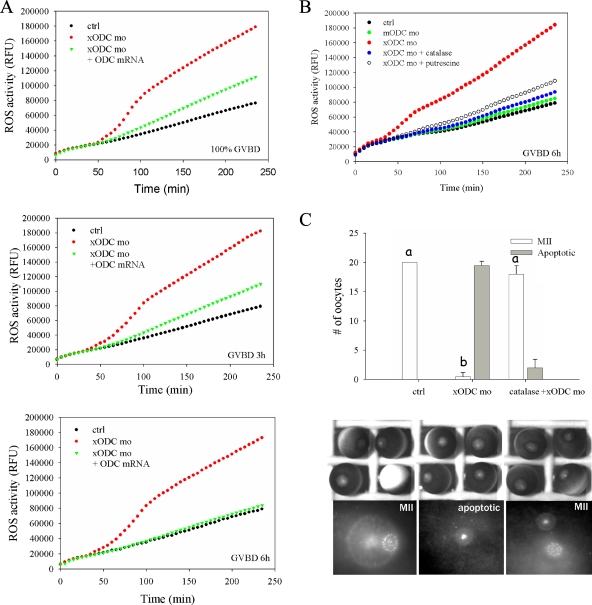
ODC deficiency resulted in elevated levels of ROS in oocytes. (A) Control oocytes (ctrl), oocytes injected with xODC mo, and oocytes injected with xODC mo plus HA-ODC mRNA were incubated in OR2 for 2 h before the addition of progesterone. Groups of 20 oocytes were withdrawn at the indicated times (at 100% GVDB or 3 h or 6 h after GVBD), lysed immediately, and assayed for ROS. The time points (in min) denote reaction times following the addition of carboxy-H2DCFDA. RFU, relative fluorescence units. (B) Uninjected oocytes (ctrl), oocytes injected with mODC mo, or oocytes injected with xODC mo alone or coinjected with catalase (350 ng per oocyte) were treated with progesterone. One group were injected with xODC mo and treated with progesterone in the presence of 5 mM putrescine. Oocytes in all groups were withdrawn 6 h after GVBD for ROS assays. (C) Control oocytes, oocytes injected with xODC mo, or oocytes coinjected with xODC mo and catalase (350 ng per oocyte) were incubated with progesterone overnight (15 to 20 h). Oocytes were fixed for chromosomal analyses. Shown are means (with SEM) for three independent experiments. Also shown are typical light images and chromosomal images of each group. One of the control oocytes was positioned upside-down, showing the vegetal hemisphere. Differences in letters (a and b) denote significant differences in values (P < 0.01; Student-Newman-Keuls test).
The chromosomal disarray in xODC mo-injected oocytes suggested that these oocytes might have undergone apoptosis. To confirm this notion, we analyzed mitochondrial Cyt c release (Fig. 2B) and caspase 3 activity (Fig. 2C); the results of these experiments clearly supported this notion. Furthermore, Cyt c release (3 h after GVBD) was detected prior to the chromosome disarray seen in xODC mo-injected oocytes. On the other hand, caspase 3 activity increase was more evident at the time of chromosome disarray. The delayed caspase 3 activation may reflect the requirement of other cytosolic factors in addition to mitochondrial Cyt c release (4). Control and xODC mo-injected oocytes both maintained phosphorylated (active) MAP kinase (Fig. 2B) and active maturation-promoting factor (MPF) (as measured by Cdc2 Tyr-15 dephosphorylation) (Fig. 3C), despite the clear apoptotic phenotype in xODC mo oocytes. These results indicated that mitochondrial Cyt c release and apoptosis are independent of the activity statuses of MAP kinase and MPF, two of the most important protein kinases involved in nuclear maturation. We did not observe significant terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining in xODC mo-injected oocytes at any stage (not shown). The lack of TUNEL signals was consistent with the notion that meiotic chromosomes in the eggs are too condensed for the nick end labeling enzyme to access in TUNEL assays (31).
FIG. 3.
Cyt c injection caused apoptosis in metaphase II oocytes. (A) GV oocytes were injected with the indicated (final intracellular) concentrations of horse heart Cyt c. Half of the injected oocytes were incubated in OR2 (ctrl) and the other half in OR2 containing progesterone. Following overnight incubation, representative oocytes were photographed. Note the mottled phenotype in oocytes injected with 100 nM Cyt c, with or without progesterone treatment. (B) Control oocytes and oocytes injected with 0.1 nM Cyt c were treated with progesterone. GVBD oocytes were further incubated for the indicated periods of time before fixation for chromosome examination. **, P < 0.01 (Student's t test). (C) Oocytes injected with mODC mo (as a control), oocytes injected with xODC mo, and oocytes injected with 0.1 nM Cyt c were either untreated (GV) or treated with progesterone, withdrawn at GVBD or 6 h after GVBD. The oocytes were subjected to Cyt c release assays (top panel) or analyzed for MAP kinase phosphorylation (activation, as described for Fig. 2B) and for Cdc2 Tyr15 dephosphorylation (activation). The bottom panel depicts β-tubulin as a loading control. p-MAPK, phosphorylated (active) MAP kinase; MAPK, inactive MAP kinase. (D) The same three samples from the Cyt c-injected oocytes shown in panel C were reblotted (lanes 1 to 3, each representing five oocytes) together with the indicated amounts of horse heart Cyt c directly loaded on the gel (lanes 4 to 8). We estimate that about 500 pg of Cyt c (about one-half of the amount loaded in lane 7) was released from five oocytes (lane 3), assuming that the antibodies recognized Cyt c from the two species (94% identical in amino acid sequence) with equal levels of efficiency.
To further establish the apoptotic phenotype in xODC mo-injected oocytes, we sought to induce apoptosis in oocytes by direct injection of Cyt c. Injection of as little as 0.1 nM (final concentration in the oocyte cytoplasm, or ∼1.2 pg per oocyte) horse heart Cyt c (Sigma) into GV oocytes did not visibly affect the health of the oocytes or their response to progesterone, and they underwent GVBD (Fig. 3A). Remarkably, 3 h after reaching metaphase II, these oocytes exhibited the same chromosome disarray (Fig. 3B) and mitochondrial Cyt c release (Fig. 3C) as xODC mo-injected oocytes. On the basis of the results shown in Fig. 3D, we estimated that as much as 100 pg of Cyt c is released from each oocyte, suggesting that the injected Cyt c (1.2 pg per oocyte) acted via a positive-feedback mechanism (6) to induce mitochondrial Cyt c release and apoptosis. Apoptotic oocytes, whether resulting from xODC mo injection or direct Cyt c injection, still exhibited active MAP kinase and active Cdc2 (MPF) (Fig. 3C). The effective concentration of Cyt c (0.1 nM) is several orders lower than that (100 nM) required to cause the mottled phenotype in GV oocytes (Fig. 3A, top row), previously described as apoptotic by others (2, 19). The striking difference in Cyt c sensitivity between GV and metaphase II oocytes further supports the role for increased ODC activity in protecting metaphase II oocytes from apoptotic insult.
To eliminate any unforeseen, nonspecific effects of xODC mo, we carried out several rescue experiments. First, we designed an xODC mo-insensitive ODC expression construct by inserting the coding sequence of Xenopus ODC (1) downstream of an HA epitope (3). The presence of more than 30 nucleotides (HA-coding sequence) 5′ to the xODC mo target sequence should render HA-ODC mRNA insensitive to xODC mo, as morpholino oligonucleotides are effective only when targeted to the 5′ untranslated region or the vicinity of the ATG codon (28). Indeed, injection of HA-xODC mRNA resulted in a significant increase of ODC activity (Fig. 4A, right panel, lane 4), even in the presence of xODC mo (lane 5). The fusion protein was readily detectable by anti-HA immunoblotting (Fig. 4A, right panel, HA immunoblot). Most importantly, injection of HA-ODC mRNA rescued chromosomal abnormality caused by xODC mo (Fig. 4A, upper left panel, xODC mo+ODC mRNA). Second, we included various concentrations of individual polyamines in the incubation medium. Both putrescine and spermine exhibited significant ability to rescue the xODC mo-induced apoptotic phenotype, with maximum efficiency at 5 mM of the polyamines (Fig. 4B; also data not shown). Third, we tested the ability of the antiapoptotic mitochondrial protein Bcl-2 to reverse the phenotype. As shown in Fig. 4C, Bcl-2 mRNA injection significantly inhibited apoptosis in xODC mo-injected oocytes, as judged by chromosome morphology and by Cyt c release (Fig. 4C). The exogenous Bcl-2 protein was readily detectable in the mRNA-injected oocytes (Fig. 4C).
ODC synthesis is required for suppression of ROS.
ROS, particularly hydrogen peroxide, which is generated by multiple pathways under physiological conditions (7), have frequently been implicated in triggering apoptosis. We analyzed levels of ROS in control oocytes versus oocytes injected with xODC mo. At all time points tested, extracts derived from xODC mo-injected oocytes contained significantly higher levels of ROS than control oocytes (Fig. 5A). Remarkably, overexpression of HA-xODC by mRNA injection (Fig. 5A) or addition of 5 mM putrescine (Fig. 5B) virtually eliminated the increased levels of ROS in xODC mo-injected oocytes. Injection of ODC mRNA (Fig. 4A) or inclusion of putrescine in the medium (Fig. 4B) efficiently rescued the xODC mo-caused apoptotic phenotype. These data suggest that increased levels of putrescine in metaphase II oocytes, as reported previously (29), may act as ROS scavengers.
We reasoned that if the elevated levels of ODC and putrescine are indeed needed to protect oocytes from ROS insult, introducing exogenous antioxidant detoxification enzymes, superoxide dismutase (SOD), and catalase (O2− → H2O2 → O2 plus H2O, in which the first reaction is catalyzed by SOD and the second by catalase) may abrogate this need. Indeed, while oocytes injected with xODC mo exhibited elevated levels of ROS and underwent apoptosis subsequent to polar-body extrusion, oocytes injected with the catalase-xODC mo combination exhibited ROS levels similar to those in control metaphase II oocytes (Fig. 5B) and were healthy and arrested in metaphase II (Fig. 5C). On the other hand, we found that injection of SOD was not effective in preventing oocyte apoptosis (not shown), suggesting that H2O2 is the major ROS in the oocytes.
ODC translation is integral to healthy oocyte maturation.
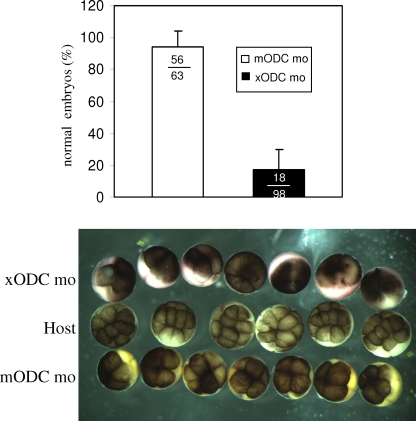
To examine the developmental potential of ODC-deficient eggs, we employed the host transfer strategy developed by Heasman et al. (12). Oocytes injected with mODC mo (used as control morpholino oligonucleotides) or xODC mo were treated with progesterone, stained with vital dyes, and transferred together to an ovulating host female. The ovulated, colored eggs were fertilized alongside host eggs. As shown in Fig. 6, embryos derived from mODC mo-injected oocytes underwent normal cleavage. These embryos often developed into swimming tadpoles (not shown). In contrast, the majority of embryos derived from xODC mo-injected oocytes exhibited clear fragmentation (Fig. 6) and died shortly after (not shown). We observed that embryos derived from transferred eggs (whether injected with morpholino oligonucleotides or not) often, but not always, developed more slowly than those derived from host eggs (Fig. 6, photograph). These results confirmed that translation of ODC during oocyte maturation is required for production of healthy eggs and that possible uptake of polyamines from the oviduct (following egg transfer) is not sufficient for rescue of the apoptotic phenotype.
FIG. 6.
ODC deficiency caused embryo fragmentation. This graph shows percentages of normal embryos derived from xODC mo-injected or mODC mo-injected oocytes. Only cleaving embryos were included in the tally. The total numbers of embryos in the three experiments were also included in the graph. Shown below are representative images of embryos derived from host eggs (uncolored, middle row), embryos derived from xODC mo-injected oocytes (top row, exhibiting fragmentation), and those derived from mODC mo-injected oocytes (bottom row, exhibiting normal, albeit slightly slower, cleavage). All three groups of embryos were from the same fertilization. In other experiments, the two vital dyes were switched between the two groups of transferred eggs, with no significant difference in experimental outcome.
DISCUSSION
It is thought that in parallel to, but independently from, the dramatic chromosome changes (nuclear maturation), vertebrate oocytes also undergo “cytoplasmic maturation” in preparation for healthy fertilization and embryonic development (8). Although the molecular basis for cytoplasmic maturation is poorly understood, it is thought to entail synthesis of proteins and other metabolites (9, 26). ODC synthesis during oocyte maturation, as reported earlier by others (29, 32) and demonstrated here, is not required for polar body emission or transition to metaphase II (nuclear maturation) but is clearly integral for producing healthy eggs. Therefore, we conclude that ODC synthesis during oocyte maturation is an integral component of oocyte cytoplasmic maturation. It seems likely that the elevation of metabolic activity during oocyte maturation results in significant oxidative stress and that ODC synthesis, and the ensuing putrescine production, is a protective measure against ROS-induced apoptosis for production of healthy eggs. In contrast, translation of a number of other maternal mRNAs, including MOS and cyclin B, is required for proper execution of meiotic chromosome segregation and transition to metaphase II (nuclear maturation).
Deficiency of ODC in the mouse causes embryonic lethality at the blastocyst stage, with apoptosis in the inner cell mass (21). The ability of these embryos to undergo normal cleavage, compaction, and blastocoel formation is likely attributable to maternal ODC mRNA/protein present in the eggs (21). Our results confirm this prediction and, furthermore, demonstrate an elevation of ROS in ODC-deficient oocytes, thus suggesting ROS suppression as a likely mechanism for ODC in promoting cell survival. It is interesting to note that the increase of ODC activity during oocyte maturation was transient and that ODC activity always returned to the basal level in stable metaphase II oocytes. This is consistent with an early study indicating that ODC activity is undetectable in ovulated eggs but rises rapidly following fertilization (25). Although the precise mechanism for, and the physiological significance of, the diminishing of this ODC activity in metaphase II-arrested eggs remains unknown, it is worth noting a similar transient elevation of ODC activity during G2/M transition in the mitotic cell cycle (22). It is tempting to speculate that elevation of ODC during G2/M transition in mitotic cells may play a similar antiapoptotic role, as G2/M transition exhibits the highest levels of ROS and the most susceptibility to arsenic trioxide-induced apoptosis, at least in NB4 promyelocytic leukemia cells (10).
We demonstrated here that ODC activity was undetectable in prophase oocytes and that the increase of ODC activity during oocyte maturation can be eliminated by ODC mo. These results suggest that ODC translation is responsible for the increase of ODC activity during oocyte maturation. This notion is further supported by the apparent polyadenylation of ODC mRNA during oocyte maturation. On the other hand, given the very short half-life of ODC protein demonstrated in other cells (30), we cannot rule out the possibilities that ODC translation serves to maintain a steady-state level of ODC protein and that a posttranslational mechanism is responsible for the elevation of ODC activity seen during oocyte maturation. We have so far not been able to obtain antibodies capable of recognizing xODC (endogenous or overexpressed HA-xODC), despite having contracted a commercial source to produce antisera against two xODC peptides (Ac-FSARDIVEQKINEVSLSC-amide and Ac-LDRIVERFELPELQVGC-amide). Nonetheless, the robust and transient nature of the increase of ODC activity during oocyte maturation reaffirms the physiological importance of the cell cycle-dependent ODC activity dynamic observed in mitotic cells (22). Oocyte maturation, for the lack of DNA replication and gene transcription, represents a unique opportunity to further study the regulation of the ODC dynamic and its antiapoptotic role during G2/M transition.
Acknowledgments
This work was supported by research grants from NSERC and CIHR.
Footnotes
Published ahead of print on 21 January 2009.
REFERENCES
- 1.Bassez, T., J. Paris, F. Omilli, C. Dorel, and H. B. Osborne. 1990. Post-transcriptional regulation of ornithine decarboxylase in Xenopus laevis oocytes. Development 110955-962. [DOI] [PubMed] [Google Scholar]
- 2.Bhuyan, A. K., A. Varshney, and M. K. Mathew. 2001. Resting membrane potential as a marker of apoptosis: studies on Xenopus oocytes microinjected with cytochrome c. Cell Death Differ. 863-69. [DOI] [PubMed] [Google Scholar]
- 3.Booth, R. A., C. Cummings, M. Tiberi, and X. J. Liu. 2002. GIPC participates in G protein signaling downstream of IGF-1 receptor. J. Biol. Chem. 2776719-6725. [DOI] [PubMed] [Google Scholar]
- 4.Budihardjo, I., H. Oliver, M. Lutter, X. Luo, and X. Wang. 1999. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 15269-290. [DOI] [PubMed] [Google Scholar]
- 5.Charlesworth, A., L. L. Cox, and A. M. MacNicol. 2004. Cytoplasmic polyadenylation element (CPE)- and CPE-binding protein (CPEB)-independent mechanisms regulate early class maternal mRNA translational activation in Xenopus oocytes. J. Biol. Chem. 27917650-17659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, Q., B. Gong, and A. Almasan. 2000. Distinct stages of cytochrome c release from mitochondria: evidence for a feedback amplification loop linking caspase activation to mitochondrial dysfunction in genotoxic stress induced apoptosis. Cell Death Differ. 7227-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumollard, R., M. Duchen, and J. Carroll. 2007. The role of mitochondrial function in the oocyte and embryo. Curr. Top. Dev. Biol. 7721-49. [DOI] [PubMed] [Google Scholar]
- 8.Eppig, J. J. 1996. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod. Fertil. Dev. 8485-489. [DOI] [PubMed] [Google Scholar]
- 9.Eppig, J. J., R. M. Schultz, M. O'Brien, and F. Chesnel. 1994. Relationship between the developmental programs controlling nuclear and cytoplasmic maturation of mouse oocytes. Dev. Biol. 1641-9. [DOI] [PubMed] [Google Scholar]
- 10.Gao, F., J. Yi, J. Q. Yuan, G. Y. Shi, and X. M. Tang. 2004. The cell cycle related apoptotic susceptibility to arsenic trioxide is associated with the level of reactive oxygen species. Cell Res. 1481-85. [DOI] [PubMed] [Google Scholar]
- 11.Gerner, E. W., and F. L. Meyskens, Jr. 2004. Polyamines and cancer: old molecules, new understanding. Nat. Rev. Cancer 4781-792. [DOI] [PubMed] [Google Scholar]
- 12.Heasman, J., S. Holwill, and C. C. Wylie. 1991. Fertilization of cultured Xenopus oocytes and use in studies of maternally inherited molecules. Methods Cell Biol. 36213-230. [DOI] [PubMed] [Google Scholar]
- 13.Ichiba, T., S. Matsufuji, Y. Miyazaki, and S. Hayashi. 1995. Nucleotide sequence of ornithine decarboxylase antizyme cDNA from Xenopus laevis. Biochim. Biophys. Acta 126283-86. [DOI] [PubMed] [Google Scholar]
- 14.Liu, X. S., and X. J. Liu. 2006. Oocyte isolation and enucleation. Methods Mol. Biol. 32231-41. [DOI] [PubMed] [Google Scholar]
- 15.Ma, C., H. A. Benink, D. Cheng, V. Montplaisir, L. Wang, Y. Xi, P. P. Zheng, W. M. Bement, and X. J. Liu. 2006. Cdc42 activation couples spindle positioning to first polar body formation in oocyte maturation. Curr. Biol. 16214-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metcalf, B. W., P. Bey, C. Danzin, M. J. Jung, P. Casara, and J. P. Vevert. 1978. Catalytic irreversible inhibition of mammalian ornithine decarboxylase (E.C. 4.1.1.17) by substrate and product analogues. J. Am. Chem. Soc. 1002551-2553. [Google Scholar]
- 17.Murakami, Y., S. Matsufuji, T. Kameji, S. Hayashi, K. Igarashi, T. Tamura, K. Tanaka, and A. Ichihara. 1992. Ornithine decarboxylase is degraded by the 26S proteasome without ubiquitination. Nature 360597-599. [DOI] [PubMed] [Google Scholar]
- 18.Nakajo, N., S. Yoshitome, J. Iwashita, M. Iida, K. Uto, S. Ueno, K. Okamoto, and N. Sagata. 2000. Absence of Wee1 ensures the meiotic cell cycle in Xenopus oocytes. Genes Dev. 14328-338. [PMC free article] [PubMed] [Google Scholar]
- 19.Nutt, L. K., S. S. Margolis, M. Jensen, C. E. Herman, W. G. Dunphy, J. C. Rathmell, and S. Kornbluth. 2005. Metabolic regulation of oocyte cell death through the CaMKII-mediated phosphorylation of caspase-2. Cell 12389-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohan, N., Y. Agazie, C. Cummings, R. Booth, M. Bayaa, and X. J. Liu. 1999. Rho-associated protein kinase α potentiates insulin-induced MAP kinase activation in Xenopus oocytes. J. Cell Sci. 1122177-2184. [DOI] [PubMed] [Google Scholar]
- 21.Pendeville, H., N. Carpino, J. C. Marine, Y. Takahashi, M. Muller, J. A. Martial, and J. L. Cleveland. 2001. The ornithine decarboxylase gene is essential for cell survival during early murine development. Mol. Cell. Biol. 216549-6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pyronnet, S., L. Pradayrol, and N. Sonenberg. 2000. A cell cycle-dependent internal ribosome entry site. Mol. Cell 5607-616. [DOI] [PubMed] [Google Scholar]
- 23.Rebagliati, M. R., D. L. Weeks, R. P. Harvey, and D. A. Melton. 1985. Identification and cloning of localized maternal RNAs from Xenopus eggs. Cell 42769-777. [DOI] [PubMed] [Google Scholar]
- 24.Richter, J. D., and W. E. Theurkauf. 2001. Development. The message is in the translation. Science 29360-62. [DOI] [PubMed] [Google Scholar]
- 25.Russell, D. H. 1971. Putrescine and spermidine biosynthesis in the development of normal and anucleolate mutants of Xenopus laevis. Proc. Natl. Acad. Sci. USA 68523-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schultz, R. M., and P. M. Wassarman. 1977. Specific changes in the pattern of protein synthesis during meiotic maturation of mammalian oocytes in vitro. Proc. Natl. Acad. Sci. USA 74538-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz, B., A. Hittelman, L. Daneshvar, H. S. Basu, L. J. Marton, and B. G. Feuerstein. 1995. A new model for disruption of the ornithine decarboxylase gene, SPE1, in Saccharomyces cerevisiae exhibits growth arrest and genetic instability at the MAT locus. Biochem. J. 31283-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Summerton, J. 1999. Morpholino antisense oligomers: the case for an RNase H-independent structural type. Biochim. Biophys. Acta 1489141-158. [DOI] [PubMed] [Google Scholar]
- 29.Sunkara, P. S., D. A. Wright, and K. Nishioka. 1981. An essential role for putrescine biosynthesis during meiotic maturation of amphibian oocytes. Dev. Biol. 87351-355. [DOI] [PubMed] [Google Scholar]
- 30.Tabor, C. W., and H. Tabor. 1984. Polyamines. Annu. Rev. Biochem. 53749-790. [DOI] [PubMed] [Google Scholar]
- 31.Van Blerkom, J., and P. W. Davis. 1998. DNA strand breaks and phosphatidylserine redistribution in newly ovulated and cultured mouse and human oocytes: occurrence and relationship to apoptosis. Hum. Reprod. 131317-1324. [DOI] [PubMed] [Google Scholar]
- 32.Younglai, E. V., F. Godeau, J. Mester, and E. E. Baulieu. 1980. Increased ornithine decarboxylase activity during meiotic maturation in Xenopus laevis oocytes. Biochem. Biophys. Res. Commun. 961274-1281. [DOI] [PubMed] [Google Scholar]
- 33.Zhang, M., C. M. Pickart, and P. Coffino. 2003. Determinants of proteasome recognition of ornithine decarboxylase, a ubiquitin-independent substrate. EMBO J. 221488-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang, X., C. Ma, A. L. Miller, H. A. Katbi, W. M. Bement, and X. J. Liu. 2008. Polar body emission requires a RhoA contractile ring and Cdc42-mediated membrane protrusion. Dev. Cell 15386-400. [DOI] [PMC free article] [PubMed] [Google Scholar]