Abstract
Actin polymerization provides the driving force for the formation of cell-cell junctions and is mediated by two types of actin nucleators, Arp2/3 and formins. Proteins required for coordinately linking cadherin-mediated adhesion to Arp2/3-dependent versus formin-dependent nucleation have yet to be defined. Here we show a role for Abi, the Abi-binding partner Nap1, and the Nap1-binding protein Sra1 in the regulation of cadherin-dependent adhesion. We found that Abi, which is known to interact with Wave, leading to activation of the Arp2/3 complex, is also capable of interacting with the Diaphanous (Dia)-related formins in the absence of Wave. Knockdown of Abi, Nap1, Sra1, or Dia markedly inhibited cell-cell junctions, whereas knockdown of Wave or Arp2/3 produced mild and transient phenotypes. Dia and Abi colocalized with β-catenin at cell-cell junctions. Further, Dia and Wave bound to overlapping sites on Abi1, and Wave competed with Dia for Abi1 binding. Notably, an active Dia1 C-terminal fragment that localizes to cell-cell junctions rescued the abnormal junctions induced by depletion of Abi or Nap1 in epithelial cells. These findings uncover a novel link between cadherin-mediated adhesion and the regulation of actin dynamics through the requirement for an Abi/Dia complex for the formation and stability of cell-cell junctions.
The formation of adhesive contacts between cells is essential for tissue morphogenesis and homeostasis, processes that are critically dependent on adherens junctions (20). Adherens junctions link the actin cytoskeleton of neighboring cells by the binding of cadherins on contacting cells (20). Actin polymerization provides the driving force for the formation of adherens junctions (29). Assembly of intercellular adhesions is driven by lamellipodial or filopodial membrane protrusions in adjacent cells (8, 17, 29) dependent on the activity of Rho family GTPases, Rac and Cdc42 (9). De novo actin polymerization at various stages of junction formation has been linked to two distinct types of actin nucleator proteins, the Arp2/3 complex and the formins (16, 17). Actin nucleation through Arp2/3 produces the branched actin networks observed in lamellipodia (31). Formins nucleate unbranched actin filaments (11, 30), and formin-1 has been implicated in the formation of linear actin cables that radiate from cadherin-containing puncta (16). Cell-cell junction formation may involve coordinated regulation of actin polymerization by both Arp2/3 and formins. However, the proteins that coordinate actin polymerization by Arp2/3 and formins at sites of cell-cell adhesion have yet to be identified.
Arp2/3-dependent actin polymerization in response to Cdc42 or Rac activation is mediated by the Wiskott-Aldrich syndrome protein (WASP) family, which includes WASP, neural WASP (N-WASP), and the Wave proteins (27). WASP and N-WASP are activated by direct binding of Cdc42, while Wave proteins interact indirectly with Rac. Wave proteins form a complex with Nap1 (Hem2), Sra1 (PIR121), Abi1/Abi2, and HSPC300 (28). Active Rac binds to this complex by interaction with Sra1, which interacts with Nap1, and in turn Nap1 is linked to Wave by Abi proteins.
Abi1 and Abi2, which constitute the core of the Wave complex (15), were originally cloned as binding partners and substrates for the Abl tyrosine kinases (5, 23). Abi proteins contain multiple domains including SNARE, Wave-binding (WAB), homeodomain homologous region (HHR), and SH3 (5, 26). Abi1 and Abi2 localize to sites of dynamic actin polymerization (7, 12, 26, 36), and Abi1 is required for platelet-derived growth factor-induced membrane ruffling and lamellipodial protrusion (15, 21). We showed that both Abi1 and Abi2 are required for actin polymerization at the T-cell-B-cell contact site (36). A role for Abi in actin-dependent processes is also supported by our finding that small interfering RNA (siRNA)-mediated knockdown of Abi1 and Abi2 impairs cell-cell junctions (12). Subsequently it was reported that knockdown of Wave2, Abi1, or HSPC300 reduced E-cadherin signal intensity at sites of cell-cell contact in MDCK cells (33).
Here we show that the Abi proteins regulate the formation and stability of cell-cell junctions by interacting with the Diaphanous (Dia)-related formins. Our data reveal a role for Abi proteins in the regulation of distinct types of actin nucleation machines at intercellular junctions.
MATERIALS AND METHODS
Cell culture.
A431, 293T, and MDCK cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS).
Plasmids and siRNAs.
The pCMV-SCRIPT mDia1 and glutathione S-transferase (GST)-Dia1 N-terminal (residues 1 to 570) constructs (22) were the generous gifts of Michael K. Rosen (UT Southwestern Medical Center, Dallas, TX). Flag-Nap1, enhanced yellow fluorescent protein (EYFP)-Abi1 wild type (WT), and EYFP-Abi1 mutants were previously described (7). pLEGFP N-WASP was previously described (1). Green fluorescent protein (GFP)-WAVE1 (1) was subcloned into the pLEGFP-C1 vector (Clontech, Palo Alto, CA). The Dia1 N-terminal construct, FH1-FH2 (residues 571 to 1163) (22), and the Dia1 N-terminal mutants were amplified by PCR and cloned into pLEGFP-C1 vector. pLEGFP Dia1 ΔDD was generated using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). pLEGFP Dia1 and pLEGFP Dia1 ΔDD, which were derived from the mouse Dia1 sequence, were used as siRNA-resistant Dia1 mutants (Dia1•R and Dia1ΔDD•R) because the Dia1 siRNAs used were specifically targeted to human Dia1. The Dia1 dimerization domain (DD) was amplified by PCR and cloned into pGEX-3X vector. Human Dia2 (hDia2) cDNA was cloned from RNA extracted from 293T cells. hDia2 WT, N-terminal (residues 1 to 547), C-terminal (residues 548 to 1101), and hDia2 ΔDAD (residues 1 to 1002) mutants were amplified by PCR and cloned into the pLEGFP-C1 vector. RacV12 and RacN17 were subcloned into the EcoRI site of pGEX-5X-2. The siRNA sequences for human Nap1 (25) and Arp3 (24) were previously described (Dharmacon). Sequences of SMARTpool siRNAs for human Abi1, Abi2, Wave1, Wave2, N-WASP, Dia1, and Sra1 are available from Dharmacon.
Antibodies and reagents.
Polyclonal antibodies against Abi1 (6988) and Abi2 (5421) have been previously described (4). Rabbit polyclonal anti-Nap1B antibody was raised against the internal Nap1 peptide CVNKKSKKQTGKKGEPEREKPGVESMRKNR. Mouse monoclonal antibodies used were Abi1 (MBL, Woburn, MA; Biodesign, Saco, ME), β-catenin (BD Transduction Laboratories, Lexington, KY), E-cadherin (BD Transduction Laboratories), Wave1 (BD Transduction Laboratories), α-tubulin (Sigma, St. Louis, MO), Flag (Sigma), and GFP (Roche Applied Sciences). Rabbit polyclonal antibodies used were N-WASP (Abcam, Cambridge, MA), β-catenin (Sigma), and Dia1 (Bethyl Laboratories, Montgomery, TX). Goat antibodies used were Wave2 (Santa Cruz Biotechnology, Santa Cruz, CA), Abi2 (Santa Cruz Biotechnology), and Dia2 (Santa Cruz Biotechnology). Sra1 antibody was the kind gift of Theresia Stradal (German Research Centre for Biotechnology, Germany) (25). Secondary antibodies were obtained from Santa Cruz Biotechnology. Alexa Fluor 488 or 568 anti-mouse and anti-rabbit immunoglobulin G (IgG) and phalloidin were purchased from Invitrogen.
Cell transfection and infection.
293T cells were transfected with the indicated plasmids using Fugene 6 transfection reagent (Roche Applied Science, Indianapolis, IN) according to the manufacturer's protocols. Retroviral transduction of A431 cells was performed as described previously (35). GFP-positive cells were sorted by fluorescence-activated cell sorting (FACS). The siRNAs were transfected using Oligofectamine (Invitrogen, Carlsbad, CA).
Immunofluorescence.
Cells plated on glass coverslips were fixed with 4% paraformaldehyde for 10 min, permeabilized by the addition of 0.1% Triton X-100 for 30 min, and incubated in 3% bovine serum albumin in phosphate-buffered saline (PBS), followed by incubation with primary antibodies for 1 h and then with Alexa Fluor 488 or 568 secondary antibodies for 1 h at room temperature. Alexa 488 or 568 phalloidin (Molecular Probes, Eugene, OR) was used for F-actin staining. Stained cells were visualized with an epifluorescence microscope or confocal microscope (Carl Zeiss, Thornwood, NY). For quantification of cell-cell junctions, 10 fields were selected by random scanning of the slides without knowledge of sample identity, and cells that stained positive for β-catenin at cell-cell contacts were counted to determine the percentages of cells with junctions. Where indicated, β-catenin-positive cell-cell contacts were classified into mature or abnormal junctions. Abnormal junctions lacked β-catenin staining at sites of cell-cell contact or displayed a discontinuous and diffuse pattern of β-catenin staining at cell-cell junctions. The intensity of β-catenin staining at sites of cell-cell contact was measured with MetaMorph software (Downingtown, PA). Results represent the means ± standard errors of the means (SEMs) of three independent experiments. All statistical analyses were performed by using one-way analysis of variance followed by Bonferroni's multiple comparison test (Graphpad software).
Cell lysis, immunoblotting, and immunoprecipitation.
Total lysates were prepared by lysis in ice-cold RIPA buffer (1% Triton X-100, 50 mM HEPES, pH 7.0, 150 mM NaCl, 2 mM EGTA, 0.25% sodium deoxycholate, 10 mM NaF, 1 mM Na3VO4, 0.2 mM phenylmethylsulfonyl fluoride, 10 μg/ml pepstatin, 10 μg/ml aprotinin, 10 μg/ml leupeptin). For immunoprecipitation, cells were lysed in ice-cold Triton lysis buffer (50 mM HEPES, pH 7.0, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA) or ice-cold Brij lysis buffer (1% Brij, 1% n-octyl-β-d-glucoside, 50 mM Tris-HCl, pH 7.6, 150 mM NaCl, 5 mM EDTA) containing inhibitors. Immunoprecipitation and immunoblotting were performed as previously described (7).
GST pull-down assays.
GST-Dia1 mutant, GST-RacV12, and GST-RacN17 constructs were introduced into Escherichia coli BL21-Codonplus (DE3)-RIL (Stratagene, La Jolla, CA) (7). GST fusion proteins were purified with glutathione-Sepharose 4B beads (Amersham, United Kingdom). GST-Dia1 mutant, GST-RacV12, and GST-RacN17 proteins were loaded onto glutathione-Sepharose 4B beads and incubated with cell lysates. After being washed four times with lysis buffer, samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting.
Functional adhesion assay.
Cadherin-dependent adhesion assays were performed as described previously (6) with some modifications. Wells (48-well plate) were coated with human E-cadherin/Fc chimeric protein overnight at 4°C, followed by blocking with 1% heat-inactivated bovine serum albumin in PBS for 1 h at 37°C. A431 cells were trypsinized and allowed to recover surface proteins for 1 h in suspension in Dulbecco's modified Eagle's medium containing 0.5% FBS at 37°C with constant, gentle shaking; 100,000 cells were plated per well, and adhesion was allowed to proceed for 1 h at 37°C. Unbound cells were discarded by being washed with PBS. Cells were fixed with cold methanol and stained with 4′,6-diamidino-2-phenylindole (DAPI) and counted.
RESULTS
Requirement for Abi, Nap1, and Diaphanous formins at cell-cell junctions.
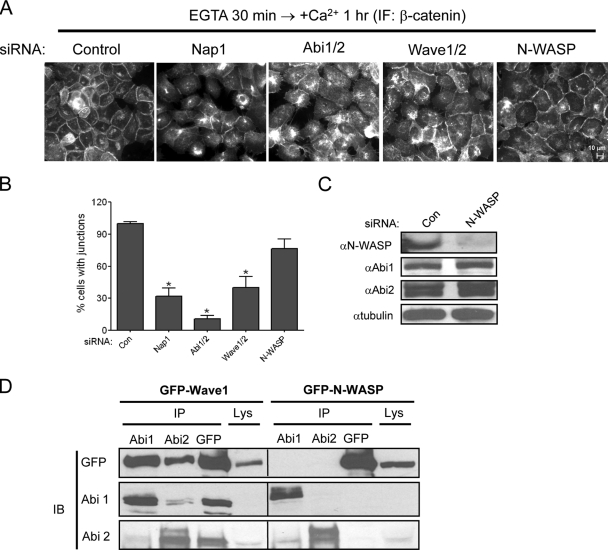
Rac localizes to cell-cell junctions, is activated during cell-cell adhesion, and regulates junction formation and stability (8, 33). Rac-dependent regulation of actin polymerization is thought to be mediated by a protein complex that contains Abi and the Abi-binding protein Nap1, as well as Sra1, HSPC300, and Wave (28). We previously reported that knockdown of Abi1 and Abi2 resulted in decreased cell-cell adhesion in HeLa cells (12). Current models predict that depletion of each component of the Wave complex would produce similar cellular phenotypes. Thus, we examined whether downregulation of Wave1 plus Wave2 or Nap1 by RNA interference in A431 epithelial cells resulted in cell-cell junction phenotypes similar to those produced following depletion of Abi. Unexpectedly, we observed that whereas knockdown of Nap1 or Abi1/Abi2 severely impaired the accumulation of β-catenin, a junctional marker, at sites of cell-cell contact in the presence of complete (calcium-containing) medium, knockdown of Wave1 plus Wave2 did not affect β-catenin localization at intercellular contacts (Fig. 1A and B). Depletion of Nap1 or Abi elicited the appearance of β-catenin-positive fingerlike extensions between adjacent cells and/or decreased β-catenin from sites of cell-cell contact (Fig. 1A and C). We next examined whether localization of β-catenin at cell-cell contacts required the presence of the Arp2/3 actin nucleation complex. Depletion of the Arp3 subunit of the Arp2/3 complex did not affect β-catenin localization (Fig. 1A and B). These findings suggest that in contrast to Abi or Nap1 knockdown, depletion of Wave and Arp2/3 failed to disrupt cell-cell junctions.
FIG. 1.
Dia1, Abi, and Nap1, but not Wave or Arp2/3, are required for cell-cell junctions. (A) A431 cells were transfected with the indicated siRNAs, and after 48 h in the presence of 2 mM calcium-containing media, cells were fixed and stained with anti-β-catenin antibody (red) and counterstained with DAPI (blue). The percentages of cells with β-catenin-positive junctions in randomly selected regions were analyzed. Results represent the means ± SEMs of at least three independent experiments (bar, 10 μm). *, P < 0.01. (B) A431 cells transfected with the indicated siRNAs were lysed, and the cell lysates were analyzed by blotting with the indicated antibodies. (C) A431 cells transfected with Nap1 or Abi1/2 siRNAs showed discontinuous junctions with β-catenin-positive fingerlike extensions between adjacent cells (bar, 10 μm). (D) Vertical sections of A431 cells transfected with Nap1 or Abi1/2 siRNAs. Cells were fixed and stained with anti-β-catenin antibody (bar, 10 μm).
It has been reported elsewhere that the protein stability of individual members of the Wave complex is dependent on their continued expression and assembly in several cell types (28). Consistent with these reports, knockdown of Nap1 or Abi in A431 cells produced marked downregulation of each other as well as of Wave1 and Wave2 (Fig. 1B). Notably, whereas knockdown of Wave1 and Wave2 with specific siRNAs effectively depleted the Wave proteins, it resulted in a partial depletion of Nap1 and Abi (Fig. 1B). Thus, the remaining pool of Nap1 and Abi proteins in the Wave-depleted cells might regulate cellular processes independently of Wave.
The Diaphanous-related formin-1 (Dia1/DRF1) actin nucleator has been reported to regulate intercellular adhesion in epithelial cells (2, 16, 19). Therefore, we examined the phenotypes induced by siRNA-mediated knockdown of Dia1 in A431 cells. Dia1 was effectively downregulated without altering Abi protein expression (Fig. 1B). Notably, depletion of Dia1 phenocopied the abnormal junctions elicited by Abi or Nap1 downregulation leading to decreased β-catenin accumulation at sites of cell-cell contact and disruption of intercellular contacts (Fig. 1A). These findings suggest that Abi and Nap1 might regulate cell-cell junctions independently of Wave and Arp2/3, possibly through pathways involving the Dia actin nucleator.
Nap1 and Abi are required for junction formation and stability: role of the Abi HHR domain.
Both Abi1 and Nap1 accumulated at epithelial cell-cell contacts, where they colocalized with β-catenin (Fig. 2A), and knockdown of Nap1 or Abi resulted in the appearance of abnormal cell-cell junctions (Fig. 1A and C). Vertical sections of z-series images of control A431 showed lateral localization of β-catenin at sites of cell contact (Fig. 1D) similar to that observed in MDCK cells (33). In contrast, β-catenin staining extended parallel to the plane of the coverslips in Nap1 or Abi knockdown cells (Fig. 1D), a pattern indicative of overlapping cells lacking intercellular junctions. Quantification of the height of the lateral domains stained with β-catenin in vertical sections showed a marked decrease in Nap1 or Abi1/2 siRNA-treated cells (3.61 ± 0.13 μm in control cells versus 1.85 ± 0.12 μm in Nap1 siRNA and 1.83 ± 0.086 μm in Abi1/2 siRNA; P < 0.001) (Fig. 1D).
FIG. 2.
The Abi1 HHR domain is required for binding to Nap1 and formation of cell-cell junctions. (A) Untransfected A431 cells or A431 cells expressing GFP-Nap1 were stained with the indicated antibodies, and confocal images were merged in the right panels. Abi1, Nap1, and β-catenin show complete colocalization at cell-cell junctions. (B) EYFP-Abi1 or the indicated EYFP-Abi1 mutants were cotransfected into 293T cells with Flag-Nap1. Nap1 protein was immunoprecipitated (IP) with anti-Flag antibodies. Immunoprecipitates were analyzed by immunoblotting (IB) with anti-GFP antibodies to detect coprecipitating Abi1 proteins (top panel) and for Flag-Nap1 with anti-Flag antibodies (middle panel). Total lysates were analyzed for expression of EGFP-Abi1 proteins by blotting with anti-GFP antibodies (bottom panel). The locations of the IgG heavy chain and the Abi1 Δ277-446 protein (*) are indicated. (C) Schematic diagram of Abi1. The core of the Nap1-binding domain is underlined and corresponds to the HHR domain. (D) A431 cells were transduced with retroviruses coding for GFP, GFP-Abi1 WT, or GFP-Abi1 ΔHHR as indicated. Cells were sorted for GFP by FACS, and cells expressing high GFP levels were fixed and stained with anti-β-catenin antibody (bar, 10 μm). (E) The percentages of cells with cell-cell junctions of randomly selected regions were analyzed. Results represent the means ± SEMs of at least three independent experiments, and at least 500 cells were counted in each experiment. *, P < 0.01.
Nap1 and Abi proteins constitute the core of the complex and interact with each other. Deletion of the Abi1 HHR domain (amino acids 111 to 163) or Abi1 amino acids 145 to 210, which include the HHR domain, prevented Abi1 from interacting with Nap1 (Fig. 2B and C). However, deletion of the Abi1 WAB or SNARE domains or sequences downstream of the HHR did not affect Abi1 binding to Nap1. These results show that Nap1 binding requires the conserved HHR domain of Abi1. Overexpression of an Abi1 mutant lacking Nap1 binding (Abi1ΔHHR) disrupts cell-cell junctions (Fig. 2D and E), which suggests that Nap1 binding is required for the integrity of intercellular adhesion.
Distinct temporal requirements for Wave versus Dia and Abi during formation of nascent junctions.
To further dissect the role of Abi and other actin regulatory factors during the formation of nascent junctions, we examined the re-formation of cadherin-dependent intercellular contacts in cells transfected with the indicated specific siRNAs (Fig. 3). Cadherin-dependent junctions were disrupted by chelating the calcium in the medium with EGTA and re-formed following a switch to high calcium in control cells, which displayed continuous cell-cell junctions with strong β-catenin staining that persisted at all times analyzed following the switch to high calcium (Fig. 3A and B). In contrast, depletion of Abi1/2 or Dia1 caused sustained inhibition of junction formation at all time points examined after the calcium switch (Fig. 3A and B). Notably, depletion of Wave resulted in transient inhibition of nascent junctions 1 h after the calcium switch (Fig. 3A and B), but junctions re-formed 4 and 10 h after the calcium switch in the Wave-depleted cells and were indistinguishable from control cells (Fig. 3A and B). The transient cell-cell junction defect elicited by Wave depletion might be explained by compensation by N-WASP because Abi1 was reported to interact with N-WASP (14). However, in contrast to the marked inhibition of nascent junction formation induced by depletion of Abi1/2, Nap1, or Wave1/2 1 hour after the switch to high calcium, knockdown of N-WASP did not affect the formation of nascent junctions in these cells at any time examined (Fig. 4A to C). Consistent with this result, GFP-Wave1, but not GFP-N-WASP, interacted strongly with endogenous Abi proteins in A431 cells expressing equivalent levels of the GFP-tagged proteins (Fig. 4D). Thus, Wave, but not N-WASP, is required during formation of nascent cell-cell junctions following the calcium switch.
FIG. 3.
Depletion of Wave transiently inhibits the formation of nascent junctions, but knockdown of Dia1 or Abi persistently impairs junction formation. (A) A431 cells transfected with the indicated siRNAs were incubated with 4 mM EGTA for 30 min to dissolve cell-cell junctions. After removal of EGTA, the cells were incubated for 1, 4, or 10 h in medium containing 2 mM calcium to re-form cadherin-dependent junctions. Cells were fixed and stained with anti-β-catenin antibody. Bar, 10 μm. (B) The percentages of cells with junctions in randomly selected regions were analyzed. Results represent the means ± SEMs of at least three independent experiments. *, P < 0.01.
FIG. 4.
Formation of nascent junctions is independent of N-WASP. (A) A431 cells transfected with the indicated siRNAs were incubated with 4 mM EGTA for 30 min to dissolve junctions, washed, and then incubated for 1 h in medium containing 2 mM calcium to allow junctions to re-form. Cells were fixed and stained with anti-β-catenin antibody (bar, 10 μm). (B) The percentages of cells with cell-cell junctions of randomly selected regions were analyzed. Results represent the means ± SEMs of at least three independent experiments, and at least 500 cells were counted in each experiment. *, P < 0.01. (C) A431 cells were transfected with N-WASP siRNA or with a control siRNA. After 48 h, cells were lysed and cell lysates were analyzed by Western blotting with the indicated antibodies. (D) Abi preferentially interacts with Wave1 over N-WASP in A431 epithelial cells. A431 cells expressing EGFP-Wave1 or EGFP-N-WASP were lysed, and the endogenous Abi proteins were immunoprecipitated (IP) with the indicated anti-Abi1 or anti-Abi2 antibodies. The coimmunoprecipitated EGFP-Wave1 or EGFP-N-WASP was detected by Western blotting (IB) with anti-GFP antibodies. The levels of endogenous Abi1 and Abi2 are shown in the middle and bottom panels, respectively. Total lysates (Lys) were analyzed for expression of EGFP-Wave1 and EGFP-N-WASP by blotting with anti-GFP antibodies.
Abi1 interacts with Dia: roles for the Abi1 SNARE domain and Dia1 DD.
To examine the possibility that Abi and Dia formins might function together to regulate cell-cell junctions, we tested whether these proteins interacted. We found that Abi1 interacted with Dia1 and that this interaction is mediated primarily by the Dia1 amino-terminal (N-T) sequences with the Abi1 SNARE domain (Fig. 5). Formins are defined by highly conserved formin homology domains (FH1-FH2) in the carboxy terminus (C-T) (11). The FH1-FH2 domains stimulate actin polymerization, whereas the N-T sequences of the Dia formins contain regulatory domains that include the GTPase-binding domain that interacts with Rho-family GTPases, the Diaphanous inhibitory domain, the DD, and a coiled-coil domain. The Dia1 N-T encodes both Rho-dependent and -independent membrane targeting domains, which may involve interactions of the DD with unknown factors (3, 22). The Diaphanous inhibitory domain interacts with the diaphanous autoregulatory domain in the C-T, thereby maintaining Dia formins in an autoinhibited state that negatively regulates actin nucleation and inhibits plasma membrane localization (22). We found that the N-T sequences of Dia1 interacted with Abi1 (Fig. 5A and B). This interaction was observed with both endogenous Abi1 and ectopically expressed Myc-Abi1. In contrast, the FH1-FH2 domains of Dia1 did not show detectable binding to Abi1 (Fig. 5A). A complex containing endogenous Dia1 and Abi1 was also detected in A431 cells (Fig. 5A, far right panel). To identify the specific domains within the Dia1 N-T sequences that mediate Abi1 binding, we employed GFP-Dia1 N-T deletion mutants. We found that the Dia1 DD was primarily required for Abi1 binding (Fig. 5B). Moreover, the Dia1 DD alone was sufficient for binding to Abi1 (Fig. 5C). Thus, the Dia1 DD is necessary and sufficient for Abi1 binding. To identify the Abi1 domains involved in Dia1 binding, we employed GFP-Abi1 WT and mutant proteins. Endogenous Dia1 interacted with GFP-Abi1 WT, Abi1Δ3-32, Abi1Δ65-79, and Abi1ΔHHR but failed to interact with Abi1 lacking the SNARE domain (Fig. 5D). These results identify Dia1 as a binding partner of Abi1 and show that this interaction is mediated by the Dia1 DD and the Abi1 SNARE domain. Abi1 and Dia1 may interact directly or indirectly through additional proteins that might be recruited to Abi/Dia protein complexes in cells.
FIG. 5.
Abi1 interacts with the N-terminal domain of Dia1. (A) 293T cells coexpressing Myc-Abi1 with EGFP-Dia1 N-T or EGFP-Dia1 FH1-FH2 (left panels) and A431 cells expressing EGFP-Dia1 N-T or EGFP-Dia1 FH1-FH2 (middle panels) were lysed, and Abi1 proteins were immunoprecipitated (IP) and immunoblotted (IB) with anti-Abi1 antibodies (bottom panels). Cell lysates were incubated with anti-rabbit IgG where indicated. The coimmunoprecipitated EGFP-Dia1 fragments and expression of EGFP constructs in total cell lysates (Lys) were detected by immunoblotting with anti-GFP antibodies (upper panels). A431 cells were lysed, endogenous Abi1 was immunoprecipitated with anti-Abi1 antibodies (bottom right panel), and coimmunoprecipitated endogenous Dia1 was detected by blotting with anti-Dia1 antibodies (top right panel). (B) 293T cells expressing EGFP-Dia1 N-T or the indicated Dia1 mutants were lysed, and endogenous Abi1 was immunoprecipitated with anti-Abi1 antibodies. Coimmunoprecipitated EGFP-Dia1 proteins were detected with anti-GFP antibodies (upper panel), and Abi1 levels are shown in the lower panel. Total cell lysates were analyzed by blotting with anti-GFP antibodies. A schematic diagram of Dia1 full length and the Dia1 mutant proteins is shown at right. GBD, GTPase-binding domain; DID, Diaphanous inhibitory domain; CC, coiled-coil; DAD, Diaphanous autoregulatory domain. (C) The indicated GST proteins were incubated with lysates from 293T cells expressing EGFP-Abi1. Bound EGFP-Abi1 protein was detected with anti-GFP antibodies. The levels of immobilized GST-Dia1 proteins are shown (bottom panel). (D) A431 cells expressing EGFP-Abi1 WT or the indicated EGFP-Abi1 mutants were lysed, and endogenous Dia1 was immunoprecipitated with anti-Dia1 antibodies. Coimmunoprecipitated EGFP-Abi1 proteins were detected with anti-GFP antibodies, and the levels of Dia1 are shown (middle panel). Total cell lysates were analyzed for expression of EGFP-Abi1 constructs by blotting with anti-GFP antibodies.
To examine whether the DD is required for cell-cell junction formation, we carried out suppression-reconstitution experiments with siRNA-resistant forms of Dia1 WT and Dia1ΔDD lacking the Abi-binding site. We found that, whereas expression of the siRNA-resistant Dia1 WT restored cell-cell junctions in cells depleted of endogenous Dia1, expression of the siRNA-resistant Dia1ΔDD protein failed to restore cell-cell junctions in these cells (Fig. 6A and B). Moreover, expression of the Dia1ΔDD protein alone in control cells inhibited the integrity of cell-cell junctions (Fig. 6A), which might be explained by the inability of the Dia1ΔDD protein to localize to cell-cell junctions compared to WT Dia1 (Fig. 6C). The punctate and diffuse cytoplasmic distribution of the Dia1ΔDD protein is in agreement with a report showing that the DD is required for Rho-independent membrane localization of the Dia1 N terminus (3). In this regard, we found that the localization of endogenous Dia1 to cell-cell junctions is disrupted in cells depleted of Abi proteins (data not shown). Thus, the localization of Abi and Dia proteins to cell-cell junctions might be interdependent and involve both GTPase-dependent and -independent protein interactions.
FIG. 6.
Ectopic expression of siRNA-resistant Dia1 wild type, but not Dia1 ΔDD, rescues the disruption of cell-cell junctions in Dia1-depleted cells. (A) A431 cells expressing siRNA-resistant EGFP-Dia1 (GFP-Dia1•R), siRNA-resistant EGFP-Dia1 ΔDD mutant (GFP-Dia1 ΔDD•R), or GFP were transfected with siRNAs to hDia1 or control siRNA. After 48 h, cells were fixed and stained with anti-β-catenin or anti-Abi1 antibody (red) and counterstained with DAPI (blue) (bars, 10 μm). (B) Total cell lysates were analyzed by blotting with the indicated antibodies. (C) A431 cells expressing EGFP-Dia1 full length, EGFP-Dia1 ΔDD, or EGFP-Dia1 FH1FH2 were incubated for 30 min in 20% FBS and then extracted with 0.3% Triton X-100 and fixed. Cells were counterstained with DAPI (blue), and GFP-Dia1 proteins were detected by confocal microscopy.
Dia and Wave bind to overlapping domains in the Abi1 N terminus.
We reported that depletion of Abi resulted in decreased cell-cell adhesion in HeLa cells (12). In contrast to A431 cells, which express high levels of Dia1 but have undetectable levels of hDia2 (hDia2/DRF2), HeLa cells express hDia2. Thus, we examined whether Abi1 could also interact with hDia2. Abi1 interacted with endogenous hDia2 (Fig. 7A), and deletion of the Abi1 SNARE domain inhibited this interaction (data not shown). The Abi1 SNARE partially overlaps the WAB domain (7) (Fig. 7B). Thus, we hypothesized that Wave may compete with Dia for binding to Abi1. To examine this possibility, we analyzed the binding of GFP-Abi1 to endogenous hDia2 in the presence of increasing amounts of Flag-Wave2. Increased Wave2 expression decreased the interaction between Abi1 and hDia2 (Fig. 7A), supporting the notion that binding to Abi1 by hDia2 and Wave2 may be mutually exclusive. In this regard, we found that Abi/Dia complexes can be detected in cells depleted of Wave proteins (data not shown). Thus, Abi/Dia complexes can exist independently of Abi/Wave complexes.
FIG. 7.
hDia2 and Wave2 bind to partially overlapping domains within the Abi1 N terminus, and hDia2 colocalizes with Abi1 at cell-cell junctions in response to activated Rac. (A) 293T cells were cotransfected with EGFP-Abi1 WT and increasing amounts of Flag-Wave2 as indicated. Endogenous hDia2 was immunoprecipitated with anti-Dia2 antibodies, and the coimmunoprecipitated EGFP-Abi1 was detected by immunoblotting with anti-GFP antibodies. hDia2 levels are shown (second panel from top). Total cell lysates were blotted with anti-GFP or anti-Flag antibodies as indicated. (B) Schematic diagram of Abi1 domains: WAB, SNARE, SH3, SR (serine-rich), PR (proline-rich), and Nap1-binding domain, which includes the HHR. Dia proteins bind to the Abi1 SNARE domain, and Wave proteins bind to the Abi1 WAB domain. The WAB (amino acids 18 to 79) and SNARE (amino acids 54 to 108) domains partially overlap. (C) Abi1 and hDia2 colocalize at cell-cell junctions in HeLa cells. HeLa cells expressing EGFP-hDia2 were transfected with activated Rac (RacV12), fixed, and stained with anti-β-catenin or anti-Abi1 antibody as indicated. Confocal images were merged in the right panels (bar, 10 μm). (D) Active hDia2 and hDia2 N-T interact with activated Rac. Purified GST, GST-RacV12, or GST-RacN17 proteins were immobilized on glutathione-Sepharose beads and incubated with 293T cell lysates expressing the indicated GFP-hDia2 constructs. hDia2 constructs were detected by Western blotting with anti-GFP antibody (upper panels). The levels of immobilized GST-Rac proteins are shown by Ponceau S staining (lower panels).
We and others showed that Dia1 localizes to cell-cell junctions (2, 16) (Fig. 6C); however, hDia2 has not been reported to localize to these structures. We found that GFP-hDia2 WT accumulated at β-catenin-positive cell-cell junctions in HeLa cells expressing activated RacV12 (Fig. 7C, left), which promotes strengthening of intercellular adhesions in these cells (35). Further, GFP-hDia2 colocalized with endogenous Abi1 at cell-cell junctions in cells expressing RacV12 (Fig. 7C, right). A role for hDia2 in Rac-dependent formation of cell-cell junctions is supported by our finding that the hDia2 N-T fragment and active hDia2 lacking the Diaphanous autoregulatory domain interacted with purified, activated GST-RacV12 (Fig. 7D). As expected, little or no interaction was detected between GST-RacV12 and hDia2 full-length or hDia2 C-T sequences (Fig. 7D). Thus, recruitment of hDia2 to cell-cell junctions might be regulated by activated Rac through direct binding to hDia2 or indirectly through interactions involving Abi protein complexes.
Active domains of Dia1 rescue abnormal junctions in Abi-depleted cells.
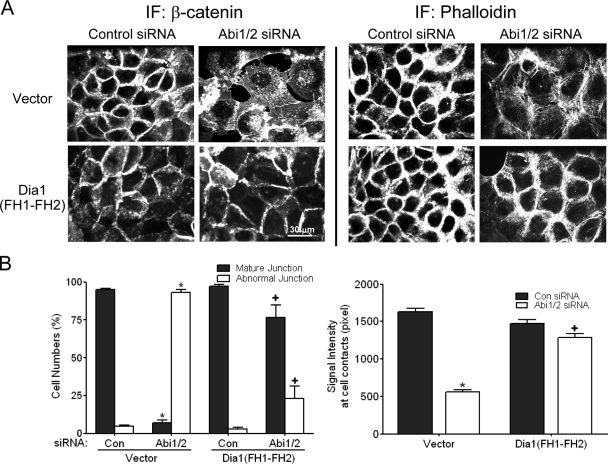
If Abi proteins regulate cell-cell junctions via Dia-dependent actin nucleation, then the abnormal adherens junctions induced by Abi depletion might be rescued by enhanced actin nucleation by the FH1-FH2 domains. To test this prediction, we employed the Dia1 FH1-FH2 domains, as the full-length Dia1 protein is autoinhibited. In this regard, a Dia1 protein lacking the N-T inhibitory sequences was shown to promote maintenance of cell-cell junctions (19), and a Dia1 C-T fragment containing the FH1-FH2 domains was reported to localize to cell-cell junctions (2). Consistent with these reports, we show that indeed the Dia1 FH1-FH2 fragment accumulates at cell-cell junctions in A431 epithelial cells (Fig. 6C). Knockdown of Abi1/2 disrupted β-catenin localization and the organization of actin filaments at sites of cell-cell contact (Fig. 8), and overexpression of the active FH1-FH2 domains of Dia1 restored β-catenin localization to junctions (Fig. 8A, left) and enhanced the accumulation of actin filaments at sites of cell-cell contact in the Abi-depleted cells (Fig. 8A, right). Mature junctions are characterized by strong and continuous accumulation of β-catenin at cell-cell contacts, whereas abnormal junctions display a diffuse and disorganized pattern of β-catenin staining. The percentage of cells with mature β-catenin-positive junctions decreased by greater than 90% in Abi-depleted cells, and this was accompanied by a concomitant increase in the percentage of cells with abnormal junctions (Fig. 8B, left). Notably, FH1-FH2 expression restored the appearance of mature junctions in Abi-depleted cells to levels that were comparable to those of control cells (Fig. 8B, left). Measurement of β-catenin signal intensity between cells that retained junctions showed that Abi depletion caused greater than a 75% decrease in β-catenin staining (Fig. 8B, right) and that expression of the FH1-FH2 domains of Dia1 rescued this deficit and enhanced β-catenin accumulation at sites of cell-cell contact to levels similar to those of control cells (Fig. 8B, right). Together, our data support a role for Abi proteins in the regulation of cell-cell junctions through pathways that require Dia formins.
FIG. 8.
The C-terminal FH1-FH2 domains of Dia1 rescue the loss of cell-cell junctions in Abi-depleted cells. (A) A431 cells expressing GFP alone or GFP-Dia1 (FH1-FH2) were transfected with siRNAs to Abi1/2 or control siRNA. After 48 h, cells were fixed and stained with anti-β-catenin antibody or phalloidin as indicated (bar, 30 μm). (B) Quantification of cells with mature or abnormal adherens junctions (left panel). The β-catenin signal intensity at sites of cell-cell contact in randomly selected regions was quantified using MetaMorph software (right panel). Results represent the means ± SEMs of at least three independent experiments. *, significant decrease or increase for control siRNA versus Abi1/2 siRNA (P < 0.01); +, significant increase or decrease for Abi1/2 siRNA versus Abi1/2 siRNA + Dia1 (FH1-FH2) (P < 0.01).
Impaired cell-cell junctions in Nap1-depleted cells are restored to normal by overexpression of the Dia1 actin polymerization domains.
The inhibitory phenotypes of Nap1 depletion on cell-cell junctions are similar to those elicited by Abi or Dia downregulation. Thus, Nap1 might function together with Abi and Dia proteins in the regulation of intercellular junctions, and Nap1 may be found in a complex with Dia proteins. We show that both ectopically expressed Dia1 (Fig. 9A) and endogenous Dia1 (Fig. 9B) coimmunoprecipitated with endogenous Nap1. Endogenous Abi1 was also present in these complexes (Fig. 9A and B). Similar to Abi1, Nap1 interacted with the N-T of Dia1 (Fig. 9A) and hDia2 (data not shown). The interaction between Nap1 and hDia2 appears to be mediated by Abi1, as Flag-Nap1 interacted with endogenous hDia2 only in the presence of GFP-Abi1 (data not shown). If Nap1 were to regulate cell-cell junctions through Dia-dependent pathways, then the abnormal junctions induced by Nap1 depletion might be rescued by the active Dia1 FH1-FH2 domains. Indeed, expression of the active Dia1 C-T domains promoted the appearance of mature junctions in the Nap1-depleted cells (Fig. 9C and D).
FIG. 9.
Abnormal junctions in Nap1-depleted cells are rescued by overexpression of the Dia1 actin polymerization domains. (A) 293T cells or A431 cells expressing the indicated EGFP-Dia1 proteins were lysed, and endogenous Nap1 was immunoprecipitated with anti-Nap1 antibodies (middle row of panels). The coimmunoprecipitated EGFP-Dia1 proteins were detected with anti-GFP antibodies (top panels), and endogenous Abi1 was detected with anti-Abi1 antibodies (bottom panels). (B) A431 cells were lysed, and endogenous Nap1 was immunoprecipitated with anti-Nap1 antibodies. Coimmunoprecipitated endogenous Dia1 and Abi1 proteins are shown. (C) A431 cells expressing GFP alone or GFP-Dia1 (FH1-FH2) were transfected with Nap1 or control siRNAs. After 48 h, cells were fixed and stained with anti-β-catenin antibody (bar, 30 μm). (D) Quantification of cells with mature or abnormal junctions. Results represent the means ± SEMs of at least three independent experiments. *, significant decrease or increase for control siRNA versus Nap1 siRNA (P < 0.01); +, significant increase or decrease for Nap1 siRNA versus Nap1 siRNA + Dia1 (FH1-FH2) (P < 0.01).
Sra1, Nap1, and Abi proteins are required for E-cadherin-mediated adhesion.
In addition to Nap1 and Abi, the Nap1-binding protein Sra1 is required to link Rac activation by diverse stimuli to downstream actin polymerization events (28). Thus, we examined whether loss of Sra1 affected cell-cell junctions. We found that, similarly to depletion of Nap1 or Abi1/2, knockdown of Sra1 in A431 epithelial cells promoted the downregulation of other components of the Wave protein complex without affecting the levels of Dia1 protein (Fig. 10A and B). Moreover, depletion of Sra1 impaired cell-cell junction formation and inhibited the accumulation of β-catenin (Fig. 10A) and E-cadherin (data not shown) to sites of cell-cell contact similar to that observed in cells depleted of Nap1, Abi1/2, or Dia1.
FIG. 10.
Sra1, Nap1, and Abi are required for cadherin-dependent adhesion. (A) A431 cells were transfected with control or Sra1-specific siRNAs, and after 48 h, cells were fixed and stained with anti-β-catenin antibody (left panels) (bar, 10 μm) or subjected to cell lysis (right panels). Total cell lysates were analyzed by Western blotting with the indicated antibodies. (B) A431 cells transfected with the indicated siRNAs were allowed to adhere for 1 h on E-cad-Fc-coated wells, and adherent cells were counted (upper panel). Results represent the means ± SEMs of at least three independent experiments. *, significant decrease for control siRNA versus Sra1 siRNA, Nap1 siRNA, or Abi1/2 siRNA (P < 0.01). A431 cells transfected with the indicated siRNAs were lysed, and cell lysates were analyzed by blotting with the indicated antibodies (lower panel). (C) Proposed model for the role of Abi in the regulation of cell-cell junctions through distinct types of actin nucleation complexes. Active Rac1 and RhoA may promote the recruitment of distinct Abi/Dia and Abi/Wave protein complexes. The Abi/Dia complex is required for junction formation and stability, whereas the Abi/Wave/Arp2/3 complex is involved in lamellipodial protrusions during the formation of nascent junctions.
In order to assess whether Abi, Nap1, and Sra1 are required for E-cadherin-dependent adhesion without interference from other adhesion receptors, we performed functional assays to quantify cell adhesion to surfaces coated with a chimeric protein comprised of the extracellular domain of human E-cadherin fused to Fc (E-cad-Fc). E-cadherin-mediated adhesion was dramatically decreased in A431 cells transfected with siRNAs specific for Abi1/2, Nap1, or Sra1 (Fig. 10A and B). Together our data support a role for Abi, Nap1, and Sra1 in the regulation of junction formation and the continuous maintenance of mature junctions downstream of cadherin engagement (Fig. 10C).
DISCUSSION
The assembly and maintenance of cadherin-mediated intercellular adhesions are critically dependent on at least two types of actin nucleation machines: the Arp2/3 complex and the formins. Initial cell-cell contact formation is driven by Arp2/3-dependent polymerization, which may also be required at later stages of intercellular adhesion for contact area extension and junction stabilization (33). Formin-mediated nucleation of linear actin filaments is observed at nascent junctions in keratinocytes (16), and linear actin bundles are present in close association with the cadherin/catenin complex at the edges of epithelial cell-cell contacts (32). One or more signaling molecules might function to coordinate Wave/Arp2/3- versus formin-mediated actin nucleation during cell-cell junction formation and maintenance. Here we show that Abi proteins form complexes with both Wave and the Dia formins and are required for E-cadherin-dependent adhesion.
Recent findings on the spatial and temporal localization of active Rac and Rho GTPases at epithelial cell-cell contacts (32) raise the possibility that distinct types of actin nucleation complexes function downstream of Rac and Rho to regulate various phases of cell-cell adhesion in a spatiotemporally coordinated manner. Active Rac and Arp2/3 localize transiently at nascent cell-cell junctions, dissipate from these sites as cadherin accumulates, and spread outward to the periphery of the expanding contacts (32). This initial phase is then followed by the accumulation of active Rho at the outside edges of the cell-cell contacts (32). Active Rho is likely to engage downstream effectors including Dia proteins, which play critical roles in the expansion and stabilization of cell-cell adhesions. However, some Dia proteins bind to both RhoA and Rac1 in a GTP-dependent manner (10). In this regard, we have shown that the active (open conformation) of hDia2 and the isolated hDia2 N-terminal fragment interact with active RacV12. These findings suggest that active Rac may promote the recruitment of specific Dia proteins to sites of cell-cell adhesion directly or, alternatively, may employ the Sra1/Nap1/Abi complex to recruit Dia family proteins to cell-cell junctions. Another possibility is that active RhoA might recruit Dia1 to cell-cell junctions, which in turn would promote the localization of Abi proteins to these sites. Thus, Abi/Wave and Abi/Dia complexes may function downstream of one or more Rho family GTPases at sites of cell-cell adhesion.
Unexpectedly, we found that depletion of Abi1/Abi2 resulted in a more severe deficit in junction formation than that induced by Wave1/Wave2 depletion. Whereas knockdown of Abi, Nap1, and Sra1 markedly impaired cell-cell junctions, knockdown of Wave or the Arp2/3 complex produced mild and transient effects. This finding raised the possibility that Abi/Nap1/Sra1 might function independently of Wave/Arp2/3 at sites of cell-cell contact. This possibility is consistent with reports that Dictyostelium discoideum Nap1 regulates cell motility and cell-substrate adhesion through Wave/SCAR-dependent and -independent pathways (13). Support for unique Abi roles that might not be shared by Wave is provided by the recent finding that loss of Dictyostelium Abi causes cytokinesis defects which are not induced by loss of Dictyostelium Wave/SCAR (18). Also, mammalian Abi1 has been found in protein complexes that do not contain Wave2 (14). Here we show that both Dia1 and hDia2 proteins are binding partners of Abi1. In this regard, it was recently reported that Abi1 also binds to the related mDia2/Drf3 through interactions involving the Abi1 SH3 domain as determined by pull-down assays with the purified Abi1-SH3 protein fragment fused to GST (34). In our studies we used deletion mutagenesis of specific Abi1 domains to identify the sites required for the Abi-Dia binding and found that the N-T sequences of Abi1 encoded within the SNARE domain mediate the interaction with both Dia1/DRF1 and hDia2/DRF2. The involvement of different Abi1 sequences in binding to various Dia family proteins might be due to the presence of distinct Abi-binding sites in Drf3/mDia2 which are absent in Dia1 and hDia2 or alternatively to the various cell types employed or to the different methods used to identify the domains mediating the interactions (GST-Abi1-SH3 pull-down assays versus coimmunoprecipitation of a series of Abi deletion mutants to detect interactions with Dia family proteins). Notably, we found that the Dia-binding site overlaps with the Wave-binding site within the Abi1 N terminus and that Wave competes with Dia for binding to Abi1. Thus, Abi1 is uniquely positioned to function as a molecular switch to coordinate the recruitment of Wave/Arp2/3 complexes or Dia formins at sites of cell-cell adhesion. This prediction is supported by our findings that depletion of Dia1 phenocopied the abnormal cell-cell junctions elicited by depletion of either Abi and that the active FH1-FH2 domains of Dia1 rescued the abnormal junctions observed in Abi-depleted cells.
Our data support a model where initial formation of cell-cell contacts is driven by dynamic lamellipodia which are dependent on the recruitment of the Sra1/Nap1/Abi/Wave/Arp2/3 complex to the leading edge in response to active Rac (15, 26). As cell-cell junctions mature, lamellipodial motility decreases, and Abi proteins at cadherin-containing complexes might promote the recruitment of Dia formins to nucleate linear actin filaments. The replacement of Wave with Dia from Abi protein complexes might be induced by phosphorylation regulated by yet-to-be-identified protein kinases localized to cell-cell junctions and/or the activation of distinct Rho family GTPases which are activated at various stages of junction formation. After the displacement of Wave from Abi, the Dia-binding site within the Abi N terminus would be available to engage Dia-dependent nucleation of unbranched actin filaments at sites of cell-cell adhesion. Alternatively, it is possible that Abi/Dia complexes might be recruited independently of Abi/Wave complexes to sites of cell-cell adhesion in response to activation of different Rho family GTPases. In sum, our findings have uncovered a novel role for distinct Abi protein complexes in the regulation of cadherin-mediated adhesion.
Acknowledgments
We thank Michael K. Rosen for the generous gift of reagents. We also thank Tadaomi Takenawa for Wave1 and Wave2 antibodies and Theresia E. Stradal for Sra1 antibody. We gratefully acknowledge Mike Cook for FACS-based sorting and Matthew Robinson for technical assistance.
This work was supported by NIH grants CA 70940, HL 084102, and AI 56266 to A.M.P.
Footnotes
Published ahead of print on 21 January 2009.
REFERENCES
- 1.Burton, E. A., T. N. Oliver, and A. M. Pendergast. 2005. Abl kinases regulate actin comet tail elongation via an N-WASP-dependent pathway. Mol. Cell. Biol. 258834-8843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carramusa, L., C. Ballestrem, Y. Zilberman, and A. D. Bershadsky. 2007. Mammalian diaphanous-related formin Dia1 controls the organization of E-cadherin-mediated cell-cell junctions. J. Cell Sci. 1203870-3882. [DOI] [PubMed] [Google Scholar]
- 3.Copeland, S. J., B. J. Green, S. Burchat, G. A. Papalia, D. Banner, and J. W. Copeland. 2007. The diaphanous inhibitory domain/diaphanous autoregulatory domain interaction is able to mediate heterodimerization between mDia1 and mDia2. J. Biol. Chem. 28230120-30130. [DOI] [PubMed] [Google Scholar]
- 4.Courtney, K. D., M. Grove, H. Vandongen, A. Vandongen, A. S. LaMantia, and A. M. Pendergast. 2000. Localization and phosphorylation of Abl-interactor proteins, Abi-1 and Abi-2, in the developing nervous system. Mol. Cell. Neurosci. 16244-257. [DOI] [PubMed] [Google Scholar]
- 5.Dai, Z., and A. M. Pendergast. 1995. Abi-2, a novel SH3-containing protein interacts with the c-Abl tyrosine kinase and modulates c-Abl transforming activity. Genes Dev. 92569-2582. [DOI] [PubMed] [Google Scholar]
- 6.de Rooij, J., A. Kerstens, G. Danuser, M. A. Schwartz, and C. M. Waterman-Storer. 2005. Integrin-dependent actomyosin contraction regulates epithelial cell scattering. J. Cell Biol. 171153-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Echarri, A., M. J. Lai, M. R. Robinson, and A. M. Pendergast. 2004. Abl interactor 1 (Abi-1) Wave-binding and SNARE domains regulate its nucleocytoplasmic shuttling, lamellipodium localization, and Wave-1 levels. Mol. Cell. Biol. 244979-4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehrlich, J. S., M. D. Hansen, and W. J. Nelson. 2002. Spatio-temporal regulation of Rac1 localization and lamellipodia dynamics during epithelial cell-cell adhesion. Dev. Cell 3259-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Etienne-Manneville, S., and A. Hall. 2002. Rho GTPases in cell biology. Nature 420629-635. [DOI] [PubMed] [Google Scholar]
- 10.Faix, J., and R. Grosse. 2006. Staying in shape with formins. Dev. Cell 10693-706. [DOI] [PubMed] [Google Scholar]
- 11.Goode, B. L., and M. J. Eck. 2007. Mechanism and function of formins in the control of actin assembly. Annu. Rev. Biochem. 76593-627. [DOI] [PubMed] [Google Scholar]
- 12.Grove, M., G. Demyanenko, A. Echarri, P. A. Zipfel, M. E. Quiroz, R. M. Rodriguiz, M. Playford, S. A. Martensen, M. R. Robinson, W. C. Wetsel, P. F. Maness, and A. M. Pendergast. 2004. Abi2-deficient mice exhibit defective cell migration, aberrant dendritic spine morphogenesis, and deficits in learning and memory. Mol. Cell. Biol. 2410905-10922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibarra, N., S. L. Blagg, F. Vazquez, and R. H. Insall. 2006. Nap1 regulates Dictyostelium cell motility and adhesion through SCAR-dependent and -independent pathways. Curr. Biol. 16717-722. [DOI] [PubMed] [Google Scholar]
- 14.Innocenti, M., S. Gerboth, K. Rottner, F. P. Lai, M. Hertzog, T. E. Stradal, E. Frittoli, D. Didry, S. Polo, A. Disanza, S. Benesch, P. P. Di Fiore, M. F. Carlier, and G. Scita. 2005. Abi1 regulates the activity of N-WASP and WAVE in distinct actin-based processes. Nat. Cell Biol. 7969-976. [DOI] [PubMed] [Google Scholar]
- 15.Innocenti, M., A. Zucconi, A. Disanza, E. Frittoli, L. B. Areces, A. Steffen, T. E. Stradal, P. P. Di Fiore, M. F. Carlier, and G. Scita. 2004. Abi1 is essential for the formation and activation of a WAVE2 signalling complex. Nat. Cell Biol. 6319-327. [DOI] [PubMed] [Google Scholar]
- 16.Kobielak, A., H. A. Pasolli, and E. Fuchs. 2004. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat. Cell Biol. 621-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovacs, E. M., M. Goodwin, R. G. Ali, A. D. Paterson, and A. S. Yap. 2002. Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts. Curr. Biol. 12379-382. [DOI] [PubMed] [Google Scholar]
- 18.Pollitt, A. Y., and R. H. Insall. 2008. Abi mutants in Dictyostelium reveal specific roles for the SCAR/WAVE complex in cytokinesis. Curr. Biol. 18203-210. [DOI] [PubMed] [Google Scholar]
- 19.Sahai, E., and C. J. Marshall. 2002. ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nat. Cell Biol. 4408-415. [DOI] [PubMed] [Google Scholar]
- 20.Schöck, F., and N. Perrimon. 2002. Molecular mechanisms of epithelial morphogenesis. Annu. Rev. Cell Dev. Biol. 18463-493. [DOI] [PubMed] [Google Scholar]
- 21.Scita, G., J. Nordstrom, R. Carbone, P. Tenca, G. Giardina, S. Gutkind, M. Bjarnegard, C. Betsholtz, and P. P. Di Fiore. 1999. EPS8 and E3B1 transduce signals from Ras to Rac. Nature 401290-293. [DOI] [PubMed] [Google Scholar]
- 22.Seth, A., C. Otomo, and M. K. Rosen. 2006. Autoinhibition regulates cellular localization and actin assembly activity of the diaphanous-related formins FRLalpha and mDia1. J. Cell Biol. 174701-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi, Y., K. Alin, and S. P. Goff. 1995. Abl-interactor-1, a novel SH3 protein binding to the carboxy-terminal portion of the Abl protein, suppresses v-abl transforming activity. Genes Dev. 92583-2597. [DOI] [PubMed] [Google Scholar]
- 24.Steffen, A., J. Faix, G. P. Resch, J. Linkner, J. Wehland, J. V. Small, K. Rottner, and T. E. Stradal. 2006. Filopodia formation in the absence of functional WAVE- and Arp2/3-complexes. Mol. Biol. Cell 172581-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steffen, A., K. Rottner, J. Ehinger, M. Innocenti, G. Scita, J. Wehland, and T. E. Stradal. 2004. Sra-1 and Nap1 link Rac to actin assembly driving lamellipodia formation. EMBO J. 23749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stradal, T., K. D. Courtney, K. Rottner, P. Hahne, J. V. Small, and A. M. Pendergast. 2001. The Abl interactor proteins localize to sites of actin polymerization at the tips of lamellipodia and filopodia. Curr. Biol. 11891-895. [DOI] [PubMed] [Google Scholar]
- 27.Stradal, T. E., K. Rottner, A. Disanza, S. Confalonieri, M. Innocenti, and G. Scita. 2004. Regulation of actin dynamics by WASP and WAVE family proteins. Trends Cell Biol. 14303-311. [DOI] [PubMed] [Google Scholar]
- 28.Stradal, T. E., and G. Scita. 2006. Protein complexes regulating Arp2/3-mediated actin assembly. Curr. Opin. Cell Biol. 184-10. [DOI] [PubMed] [Google Scholar]
- 29.Vasioukhin, V., C. Bauer, M. Yin, and E. Fuchs. 2000. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell 100209-219. [DOI] [PubMed] [Google Scholar]
- 30.Wallar, B. J., and A. S. Alberts. 2003. The formins: active scaffolds that remodel the cytoskeleton. Trends Cell Biol. 13435-446. [DOI] [PubMed] [Google Scholar]
- 31.Welch, M. D., and R. D. Mullins. 2002. Cellular control of actin nucleation. Annu. Rev. Cell Dev. Biol. 18247-288. [DOI] [PubMed] [Google Scholar]
- 32.Yamada, S., and W. J. Nelson. 2007. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J. Cell Biol. 178517-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamazaki, D., T. Oikawa, and T. Takenawa. 2007. Rac-WAVE-mediated actin reorganization is required for organization and maintenance of cell-cell adhesion. J. Cell Sci. 12086-100. [DOI] [PubMed] [Google Scholar]
- 34.Yang, C., L. Czech, S. Gerboth, S. Kojima, G. Scita, and T. Svitkina. 2007. Novel roles of formin mDia2 in lamellipodia and filopodia formation in motile cells. PLoS Biol. 5e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zandy, N. L., M. Playford, and A. M. Pendergast. 2007. Abl tyrosine kinases regulate cell-cell adhesion through Rho GTPases. Proc. Natl. Acad. Sci. USA 10417686-17691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zipfel, P. A., S. C. Bunnell, D. S. Witherow, J. J. Gu, E. M. Chislock, C. Ring, and A. M. Pendergast. 2006. Role for the Abi/wave protein complex in T cell receptor-mediated proliferation and cytoskeletal remodeling. Curr. Biol. 1635-46. [DOI] [PubMed] [Google Scholar]