Abstract
Patterning and differentiation signals are often believed to drive the developmental program, including cell cycle exit of proliferating progenitors. Taking advantage of the spatial and temporal separation of proliferating and differentiated cells within the developing anterior pituitary gland, we investigated the control of cell proliferation during organogenesis. Thus, we identified a population of noncycling precursors that are uniquely marked by expression of the cell cycle inhibitor p57Kip2 and by cyclin E. In p57Kip2−/− mice, the developing pituitary is hyperplastic due to accumulation of proliferating progenitors, whereas overexpression of p57Kip2 leads to hypoplasia. p57Kip2-dependent cell cycle exit is not required for differentiation, and conversely, blockade of cell differentiation, as achieved in Tpit−/− pituitaries, does not prevent cell cycle exit but rather leads to accumulation of p57Kip2-positive precursors. Upon differentiation, p57Kip2 is replaced by p27Kip1. Accordingly, proliferating differentiated cells are readily detected in p27Kip1−/− pituitaries but not in wild-type or p57Kip2−/− pituitaries. Strikingly, all cells of p57Kip2−/−;p27Kip1−/− pituitaries are proliferative. Thus, during normal development, progenitor cell cycle exit is controlled by p57Kip2 followed by p27Kip1 in differentiated cells; these sequential actions, taken together with different pituitary outcomes of their loss of function, suggest hierarchical controls of the cell cycle that are independent of differentiation.
The cell cycle is a highly controlled process that integrates numerous signals involved in normal cell proliferation and tumorigenesis. Every step of the cell cycle is subject to regulation, and critical regulators of this process include the cyclins and cyclin-dependent kinases (cdk's). Most of our understanding of mechanisms controlling cell cycle progression comes from studies with cultured cells, but the precise role of cell cycle regulators in normal development is not fully understood. This is best exemplified by the relatively subtle phenotypes of many mouse mutants deficient for regulators thought to be critical for cell cycle progression based on cell culture studies (reviewed in reference 5).
Progression through the cell cycle is controlled by pairs of cyclins and cdk's. In cycling cells, progression through the G1 phase is dependent on the cyclin D-cdk4/6 complexes, whereas cyclin E-cdk2 is required for the G1/S transition and cyclins A and B together with cdk1 (cdc2) are required for G2/M progression (37). This process is also under the control of negative regulators, the cyclin-dependent kinase inhibitors (cdki's). These inhibitors are divided into two groups, the INK4 and Cip/Kip families (36). The INK4 family includes inhibitors of cyclin D-cdk4/6 complexes, such as p16INK4a, p15INK4b, p18INK4c, and p19INK4d. The Cip/Kip family consists of p21Cip1, p27Kip1, and p57Kip2; the primary target of these cdki's is the cyclin E-cdk2 complex.
Organogenesis can be viewed simplistically as a combination of patterning cues acting on differentially proliferating tissues, and thus the shape of organs/tissues is intimately dependent on cell cycle control and, in particular, on cell cycle exit of proliferating progenitors. The cell cycle inhibitors appear to have critical roles in timing of cell cycle exit prior to differentiation. For example, ablation of p57Kip2, a less-studied cdki, caused severe developmental abnormalities characterized by global organomegaly (42, 43). Furthermore, double knockout mice for p27Kip1 and p57Kip2 have defective cell cycle exit and differentiation of lens fiber cells (9, 44). Muscle differentiation and inner ear development are also regulated by cdki's (12, 28, 45). Such studies highlight the importance of cell cycle exit for proper development and suggest a close link with differentiation. However, we currently have limited insight into the developmental program that drives proliferating progenitors out of the cell cycle and into the differentiation process. The present study investigates the relationship between these two processes in the developing pituitary gland.
The pituitary has been a useful model for understanding the role of cell cycle regulators in tumorigenesis. Indeed, the pituitary is particularly sensitive to the loss of function of many cell cycle regulators, including p18INK4c, p27Kip1, and Rb (11, 13, 20, 23, 29). In contrast to the wealth of information on the implications of cdki's in pituitary tumor formation, less is known about their contributions to pituitary organogenesis and cell differentiation. Pituitary cells are very homogenous in their developmental origins, since they were shown by fate mapping studies using chick-quail chimeras (6, 7) to all be derived from the midline region of the anterior neural ridge, i.e., the stomodeal/oral ectoderm. At the molecular level, all these cells are marked by expression of Pitx1 (26, 27), which is first expressed in the stomodeal ectoderm and is maintained in all its derivatives, including pituitary cells. The pituitary thus develops as an invagination of the oral ectoderm known as Rathke's pouch. The epithelial fold that constitutes Rathke's pouch at embryonic day 9.5 (e9.5)/e10.5 separates from the oral ectoderm, and organogenesis proceeds as cells leave this epithelium to form a nascent anterior pituitary lobe. This early phase of pituitary organogenesis occurs between e11.5 and e13.5 and is associated with extensive cell proliferation (40). A number of transcription factors have been shown to be critical for this early phase of organogenesis and cell proliferation. They include the transcription factors Pitx1, Pitx2 (4, 39), Lhx3, and Lhx4 (35).
The proliferation of early pituitary progenitors (10) is followed closely by sequential differentiation of various postmitotic hormone-producing cells. The adult rodent pituitary is composed of three different lobes: the posterior lobe is made of axonal projections coming from the hypothalamus, whereas the intermediate lobe (IL) contains only proopiomelanocortin (POMC)-expressing melanotroph cells. The anterior lobe (AL) contains the following five lineages that appear sequentially: POMC-expressing corticotrophs (adrenocorticotropin [ACTH] secreting), thyrotrophs (thyroid stimulating hormone secreting), somatotrophs (growth hormone [GH] secreting), lactotrophs (prolactin secreting), and finally, gonadotrophs producing the gonadotropins luteinizing hormone and follicle-stimulating hormone. Cell-restricted transcription factors are essential for hormone gene transcription and cell differentiation. These include the T-box factor Tpit for corticotrophs and melanotrophs (25, 31, 32), SF1 and GATA-2 for gonadotrophs (3, 8, 19), and Prop-1 and Pit1 for somatotrophs and lactotrophs (2, 38).
In the present work, expression pattern analyses of cell cycle regulators and differentiation markers during early pituitary development led to the identification of spatially and temporally restricted noncycling precursor cells characterized by expression of p57Kip2 and cyclin E. In differentiated cells, p57Kip2 is replaced by p27Kip1. In order to define mechanisms of cell cycle control during pituitary development, we investigated pituitaries from p57Kip2−/− and p27Kip1−/− mice, as well as p57Kip2 gain-of-function mice. These studies suggest that the role of p57Kip2 in normal pituitary development is to allow exit of proliferating progenitors from the cell cycle without affecting cell differentiation, while p27Kip1 appears to prevent reentry of differentiated cells into the cycle. p57Kip2-positive noncycling precursors are maintained throughout adulthood in mice defective in differentiation, such as Tpit−/− mice. These studies indicate that cell cycle exit and differentiation are largely uncoupled and sequential during normal development. Although the pituitary offered the opportunity to identify the transient population of noncycling precursors, this work suggests a more widely relevant paradigm for development in many tissues.
MATERIALS AND METHODS
Mice and BrdU injection.
p57Kip2 (B6.129S7-Cdkn1ctm1Sje/J) and p27Kip1 (B6.129S4-Cdkn1btm1Mlf/J) mice were obtained from Jackson Laboratory and maintained and genotyped as described previously. Tpit knockout mice were previously described (32). For bromodeoxyuridine (BrdU) injection, pregnant females were injected intraperitoneally with 100 mg of BrdU/kg of body weight in phosphate-buffered saline 1 h before sacrifice.
Generation of transgenic mice.
The p57Kip2 cDNA was inserted downstream of the 4.2-kb Pitx1 promoter (18). Transgenic mice were generated as described previously (30). Pregnant females were injected with BrdU, and embryos were taken by cesarian at day e14.5. For genotyping, the primers 5′-TGCGCGCACTGTCGTTT-3′ (Pitx1) and 5′-TGGAAGTTGAAGTCCCAGCGGTT-3′ (p57Kip2) were used.
Tissue preparation and immunofluorescence.
Embryos were fixed, embedded in paraffin, and treated as previously described (26, 27). Antibodies to the following proteins were used: phospho-histone H3 (pHH3) (Upstate), BrdU (DSHB), Tpit (25), cyclin E (M-20; Santa Cruz), cyclin D1 (BD Biosciences), cyclin D2 (M-20; Santa Cruz), p57Kip2 (H-91; Santa Cruz), p57Kip2 (Ab-3; Lab Vision), POMC (Cortex Biochem), α glycoprotein hormone subunit (αGSU) (AFP5191792; NIH), p27Kip1 (BD Biosciences), Ki67 (Ab-5; Lab Vision), cleaved caspase 3 (Cell Signaling), Pit1 (Simon Rhodes, University of Indiana), Prop-1 (Aimé Ryan, McGill University), and Pitx1 (26). Secondary antibody detection was achieved by immunofluorescence with Alexa-conjugated antibodies or using biotin-conjugated antibodies followed by Alexa-conjugated streptavidin (Invitrogen, Molecular Probes).
The Animal Ethics Review Committee of the Institut de Recherches Cliniques de Montréal (IRCM) approved all animal experimentation in conformity with the regulations of the Canadian Council on Animal Care.
RESULTS
Delay between cell cycle exit and pituitary differentiation.
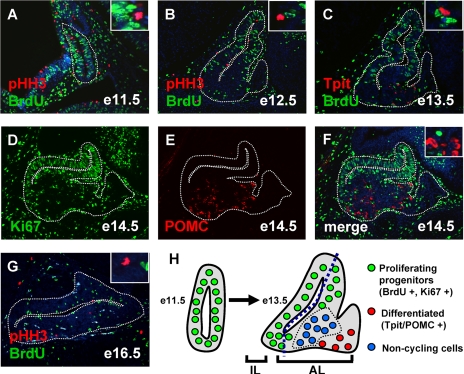
In order to study cell cycle control during pituitary organogenesis, we followed expression of cell cycle regulators as pituitary cells progressed from proliferative progenitors to differentiated cells. Proliferative cells were marked using Ki67 labeling and by BrdU incorporation, and mitotic cells were identified by pHH3 immunofluorescence; differentiated cells were revealed by hormone- or cell-specific transcription factor expression. At e11.5 and e12.5, the developing pituitary does not contain differentiated cells (25) and has a large number of proliferative cells, as revealed by BrdU and pHH3 labeling (Fig. 1A and B). At e13.5, BrdU-positive proliferating cells became concentrated on the dorsal side of the developing AL and the first differentiated cells appeared on the ventral side (Fig. 1C) as Tpit-positive corticotrophs (25). Proliferating cells identified by Ki67 expression were similarly found mostly around the lumen of the developing gland at e14.5 (Fig. 1D), and differentiated hormone-producing cells did not overlap with Ki67-expressing cells (Fig. 1E and F). Few proliferating cells remained at e16.5 (Fig. 1G), and the adult gland had just a few such cells per section (data not shown). The positions of proliferating (dorsal) and differentiated (ventral) cells in the developing AL are represented schematically in Fig. 1H; at these early stages, they were separated by cells that appeared to be neither proliferating nor differentiated.
FIG. 1.
Subpopulation of noncycling undifferentiated pituitary cells. (A, B, and G) Colabeling with the S phase marker BrdU (green) and the M phase marker pHH3 (red) identifies proliferating cells at different stages of pituitary development. Nuclei were stained blue with Hoechst 33258 dye. (C) The first corticotroph cells present at e13.5 are revealed by Tpit staining (red) in the AL. Colocalization with BrdU (green) reveals mutually exclusive expression patterns. (D to F) Proliferative cells identified by Ki67 expression (D) are clearly distinct from differentiated POMC-positive cells (E and F). (H) Schematic representation of the spatial organization of the different pituitary cell populations at e11.5 and e13.5. The proliferating progenitors (green) are found mostly around the lumen, while the differentiated cells (red) appear on the ventral side of the developing AL of the gland. A group of noncycling undifferentiated cells (blue) are present between proliferating and differentiated cells.
p57Kip2 and cyclin E expression marks a noncycling undifferentiated cell population.
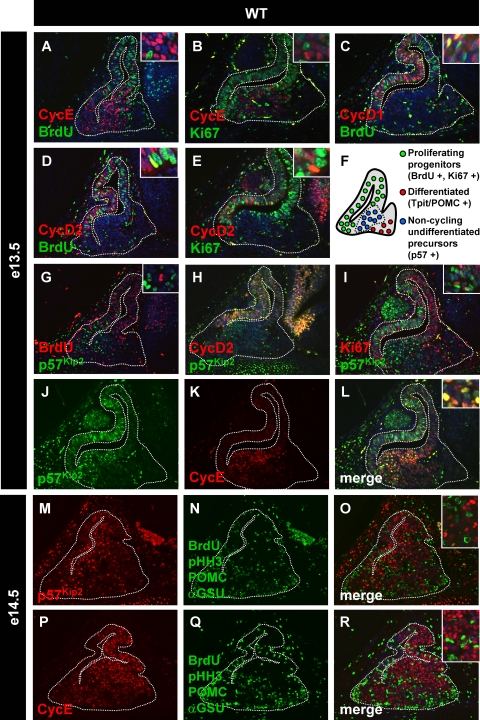
The e13.5 AL cell population that is neither proliferating nor differentiated was investigated for expression of various cell cycle regulators. It was found that cyclin E was particularly expressed in these cells (Fig. 2A and B) but that cyclin D1 or D2 was not (Fig. 2C to E). Expression of cyclins D1 and D2 in proliferating cells was expected. Cyclin E is reported to be expressed at the G1-to-S transition (37), but surprisingly, cyclin E-positive cells of the developing pituitary did not include any BrdU-positive cells (Fig. 2A) or Ki67-positive cells (Fig. 2B). The Cip/Kip family of cdki's has been associated with cell cycle exit (37). Since p57Kip2 (CDKN1C) was shown to be expressed particularly strongly during early pituitary development (21) and its knockout results in organomegaly (43), we investigated its expression in developing pituitaries. We performed colabeling of p57Kip2 with BrdU (Fig. 2G) and Ki67 (Fig. 2I). p57Kip2-positive cells were never found to be BrdU positive (Fig. 3G) and only rarely were Ki67 positive (Fig. 2I, inset). In order to ascertain that p57Kip2-positive AL cells are indeed noncycling, we also did colabeling of p57Kip2 with cyclin D2. Mostly singly positive cells for p57Kip2 were observed in the AL, although there were a few cyclin D2/p57Kip2 doubly positive cells (Fig. 2H). The similar distributions of p57Kip2- and cyclin E-positive cells suggest that they may overlap: indeed, p57Kip2 was coexpressed with cyclin E in most AL noncycling cells (Fig. 2J to L and 3J and K). The central location of p57Kip2- and cyclin E-positive cells in the e13.5 developing AL suggests that these cells may represent an intermediate population between proliferating and differentiated cells. All of these cells (Ki67-positive, p57Kip2/cyclin E-positive, and also differentiated cells) are of surface ectoderm origin and, as such, express Pitx1 (26, 27; data not shown); they are thus all of similar lineages.
FIG. 2.
p57Kip2 and cyclin E mark noncycling undifferentiated cells in the anterior pituitary. Colocalization experiments were performed with different proliferation and differentiation markers at e13.5 and e14.5, a developmental period when noncycling undifferentiated cells are most abundant. (A) BrdU-positive cells are not colabeled with cyclin E, a cyclin thought to be involved in G1-S transition. (B) Similarly, cyclin E does not colabel any cells with Ki67, a marker of proliferative cells. However, cyclin D1 (C) and cyclin D2 (D) colocalize partially with BrdU or Ki67 (E), suggesting that cyclin D expression is maintained until the beginning of S phase. p57Kip2-positive cells are BrdU negative (G) and mostly cyclin D2 (H) and Ki67 (I) negative. (J to L) Colocalization of p57Kip2 and cyclin E expression in cells of the developing anterior pituitary. (M to R) In order to clearly assess the differentiation status of cyclin E- and p57Kip2-positive cells, we performed colocalization of p57Kip2 (M) and cyclin E (P) with a mix of proliferation and differentiation markers (N and Q). Proliferating cells are identified by nuclear staining of BrdU and pHH3, while differentiated cells are marked by cytoplasmic labeling of POMC and αGSU (O and R). p57Kip2- and cyclin E-positive cells do not colabel with any of these markers, indicating that these noncycling cells are undifferentiated. (F) Schematic representation of the different cell populations at e13.5/e14.5 showing noncycling undifferentiated precursors physically located between proliferating progenitors and differentiated cells. WT, wild type.
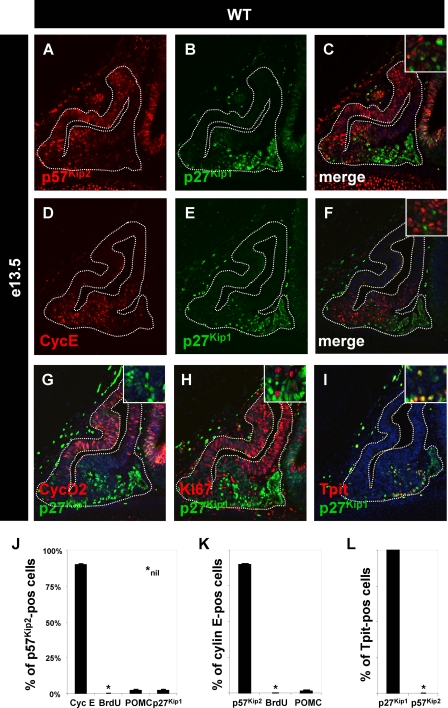
FIG. 3.
p27Kip1 replaces p57Kip2 during pituitary differentiation. The expression of another member of the Cip/Kip family, p27Kip1 (B and E), is complementary to the expression of p57Kip2 (A) and cyclin E (D) at e13.5. There is a small overlap between the two populations (C and F). The expression of p27Kip1 is not correlated with the proliferation marker cyclin D2 (G) or Ki67 (H). The ventral/rostral expression pattern of p27Kip1 suggests that it is expressed in nonproliferating differentiated cells. (I) Indeed, all Tpit-positive corticotroph cells coexpress p27Kip1. (J to L) Quantification of colabeling for different markers in the population of noncycling cells. These data are presented relative to p57Kip2 (J)-, cyclin E (K)-, and Tpit (L)-positive cells. WT, wild type.
In order to show that p57Kip2 and cyclin E doubly positive cells are neither proliferating nor differentiated, we used a mixture of antibodies against BrdU, pHH3, ACTH, and αGSU to label proliferating and differentiated cells. Labeling with various pairwise combinations of these antibodies (data not shown) yielded results that are entirely consistent with their combined use. These experiments showed that e14.5 p57Kip2-positive cells are not proliferating and almost never differentiated (Fig. 2M to O and 3J), and similar results were found for cyclin E-positive cells (Fig. 2P to R and 3K). Taken collectively, these data indicate that the centrally located (Fig. 2F) p57Kip2 and cyclin E doubly positive cells are noncycling and undifferentiated; their localization suggests that these cells may be noncycling precursors that have exited the cell cycle and are waiting for differentiation cues.
Throughout the above description, we have not discussed the developing IL that sits across the lumen opposite the AL. Although the IL appears to contain cells similar to those described in the AL, their intermingling in space and time makes it harder to identify separate cell populations. It is noteworthy, and consistent with AL data, that IL cells were not found to coexpress proliferation markers (BrdU or Ki67, as in Fig. 2G and I) and p57Kip2.
Since differentiated cells do not express p57Kip2 (Fig. 2M to O) and since p27Kip1 (CDKN1B) knockout mice exhibit differentiated pituitary tumors (11, 23, 29), we assessed the expression of p27Kip1 during pituitary development. In e13.5 pituitaries, p27Kip1 expression appeared complementary to that of p57Kip2 (Fig. 3A to C) and cyclin E (Fig. 3D to F), and p27Kip1-positive cells appeared to be distinct from proliferating progenitors, as assessed by cyclin D2 and Ki67 labeling (Fig. 3G and H). The ventral position of p27Kip1-positive cells (Fig. 3B) is consistent with the position of early differentiated cells, and indeed, all Tpit-positive (corticotroph) cells were found to be colabeled with p27Kip1 (Fig. 3I and L). Other p27Kip1-positive cells coexpressed αGSU (data not shown). This reciprocal pattern of p57Kip2 and p27Kip1 expression (Fig. 3J to L) clearly delineates the noncycling precursor and differentiated cell populations. In addition, the level of p57Kip2 expression in the e13.5 AL followed a dorsoventral gradient (Fig. 3A), and it should be noted that most cells of the developing gland expressed p57Kip2 at a low level, with the exclusion of BrdU-positive cells but including some p57Kip2/p27Kip1 doubly positive cells (Fig. 3J). Indeed, p57Kip2/p27Kip1 colabeling did reveal a subset of p57Kip2-positive and weakly p27Kip1-positive cells (Fig. 3J) that likely represent the transition between precursors and differentiated cells. Thus, p27Kip1 appears to replace p57Kip2 prior to or in parallel with differentiation.
p57Kip2 promotes cell cycle exit of proliferating progenitors.
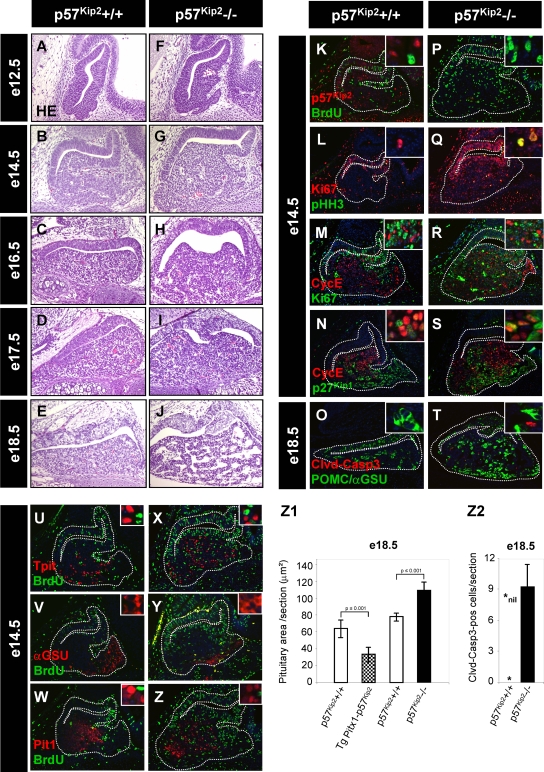
Since the cell cycle inhibitor p57Kip2 appears in a cell population that seems to be intermediate between proliferating progenitors and differentiated cells, we investigated p57Kip2-deficient mice to gain insight into the function of p57Kip2 in pituitary development. If p57Kip2 plays a role in cell cycle exit of pituitary progenitors, we would expect an effect of p57Kip2 deficiency between e10.5 and e14.5, when cell expansion takes place. We indeed observed signs of increased proliferation at e12.5 (compare Fig. 4F to Fig. 4A), and clear hyperplasia was observed at e14.5 and later (compare Fig. 4G, H, and I to Fig. 4B, C, and D). Quantification of the gland surface area at e14.5 confirmed this interpretation (Fig. 4Z1). BrdU incorporation was used to show increased proliferation in e14.5 p57Kip2−/− pituitaries (compare Fig. 4P to Fig. 4K). The AL hyperplasia of p57Kip2−/− pituitaries was also evident using colabeling for pHH3 and Ki67 (compare Fig. 4Q to Fig. 4L). Since normal p57Kip2-positive pituitary cells also express cyclin E, we tested whether the loss of p57Kip2 may affect cyclin E expression, and this was not the case (Fig. 4M and R). The Ki67-positive proliferating cells that appeared in the developing AL of p57Kip2−/− mice did not express cyclin E. This suggests that the switch-on of cyclin E is independent of p57Kip2 (Fig. 4M and R). We then tested whether the loss of p57Kip2 affects the appearance of p27Kip1. Since p27Kip1 expression was unaffected in p57Kip2−/− pituitaries (Fig. 4N and S), the control of p27Kip1 also appears independent of p57Kip2. In agreement with the data shown in Fig. 3J, it is noteworthy that a small number of cells coexpressed cyclin E and p27Kip1 in both normal and p57Kip2−/− pituitaries (Fig. 4N and S). Thus, cell cycle exit may be exerted by p27Kip1 in p57Kip2−/− pituitaries.
FIG. 4.
p57Kip2 controls progenitor cell cycle exit but not differentiation. (A to J) Knockout of p57Kip2 affects pituitary development, as revealed by hematoxylin-eosin staining between e12.5 and e18.5. The absence of p57Kip2 leads to early pituitary hyperplasia between e12.5 and e17.5 (compare panels F to I to panels A to D) and later (e18.5) to tissue loss (compare panel J to panel E). (Z1) The pituitary hyperplasia was quantitated at e14.5 by surface area measurement for two to four wild-type and p57Kip2−/− pituitaries. Data represent the means ± standard errors of the means for measurements performed on six to eight sections for each of two to four pituitaries. Similar quantitations were performed for the control and transgenic pituitaries described in the legend to Fig. 5. (K to T) Proliferation and apoptosis in p57Kip2−/− pituitaries. p57Kip2 gene disruption leads to an increased number of proliferating cells in the AL, as revealed by BrdU incorporation (compare panel P to panel K) and by the proliferation markers Ki67 and pHH3 (compare panel Q to panel L). An increased number of cyclin E-positive cells is also observed in p57Kip2−/− pituitaries, but these cells appear to have exited the cell cycle, as they do not label for Ki67 (compare panel R to panel M). Expression of p27Kip1 is not altered in p57Kip2−/− pituitaries (compare panel S to panel N), but apoptotic cells are detected by cleaved caspase 3 immunofluorescence in knockout pituitaries (quantification is shown in panel Z2) and never in normal pituitaries (compare panel T to panel O), thus explaining the tissue loss observed in e18.5 pituitaries (J). (U to Z) Unaltered differentiation in p57Kip2−/− pituitaries. Expression of differentiation markers in wild-type (U to W) and p57Kip2 knockout (X to Z) pituitaries is similar for Tpit (corticotrophs) (U and X), αGSU (gonadotrophs) (V and Y), and Pit1 (somatotrophs, lactotrophs, and thyrotrophs) (W and Z). It is noteworthy that p57Kip2-dependent hyperplasia is accounted for by progenitor proliferation but does not involve proliferation of differentiated cells, as indicated by the absence of overlap between BrdU and differentiation markers.
The p57Kip2-dependent pituitary hyperplasia could have led to tumor formation. However, it was found that hyperplastic p57Kip2−/− pituitaries regressed starting at about e17.5 (compare Fig. 4H to J to Fig. 4 C to E). This regression appeared to be due to apoptosis, as supported by the appearance of cleaved caspase 3-positive cells. Whereas the normal pituitary did not have any caspase 3-positive cells (Fig. 4O), p57Kip2−/− pituitaries exhibited caspase 3-positive nuclei (Fig. 4T and Z2), in agreement with data for the pancreas (16). Similar results were obtained using a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (ApopTag) assay (see Fig. S1E and F in the supplemental material).
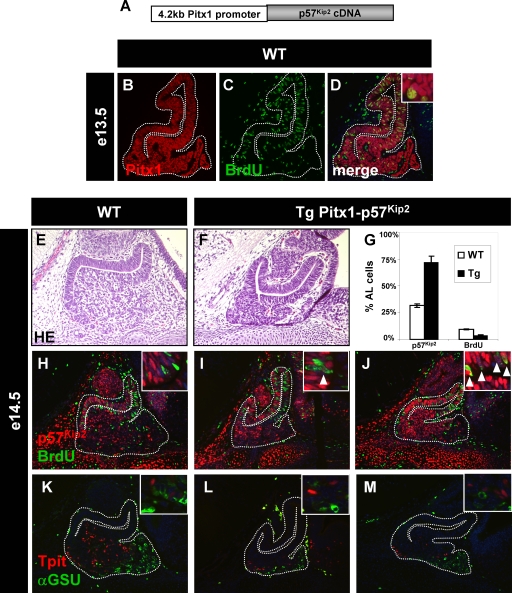
The p57Kip2 knockout suggests that this cell cycle inhibitor may control cell cycle exit of pituitary progenitors. In order to directly test this hypothesis, we constructed transgenic mice (Fig. 5A) to drive p57Kip2 expression in all cells (including proliferating BrdU-positive cells) of the developing pituitary, using the Pitx1 promoter (18, 26) (Fig. 5B to D). Transgenic embryos (e14.5) expressing this p57Kip2 transgene (Fig. 5G, I, and J) exhibited a hypoplastic anterior pituitary (compare Fig. 5F to Fig. 5E and 4Z1) and fewer BrdU-positive cells (Fig. 5G). Taken together, both p57Kip2 loss and gain of function support the model that this cell cycle inhibitor plays a key role.
FIG. 5.
Early p57Kip2 expression leads to pituitary hypoplasia but does not affect differentiation. A transgene driving p57Kip2 expression under the control of the Pitx1 promoter (A) will lead to early expression throughout the developing pituitary (B), including BrdU-positive cells (C and D). The ectopic expression of p57Kip2 leads to pituitary hypoplasia (compare panel F to panel E; see quantification in Fig. 4Z1). Two independent transgenic pituitaries are shown for p57Kip2 expression and BrdU incorporation (compare panels I and J to panel H) as well as for Tpit- and αGSU-positive cells (compare panels L and M to panel K). Differentiation is not impaired, as revealed by Tpit- and αGSU-positive cells (L and M). (G) The proportions of p57Kip2-positive and BrdU-positive cells in wild-type (WT) and transgenic pituitaries were quantitated for six to eight sections from three different embryos, and the differences are statistically significant (P ≤ 0.02).
p27Kip1 prevents cell cycle reentry of differentiated cells.
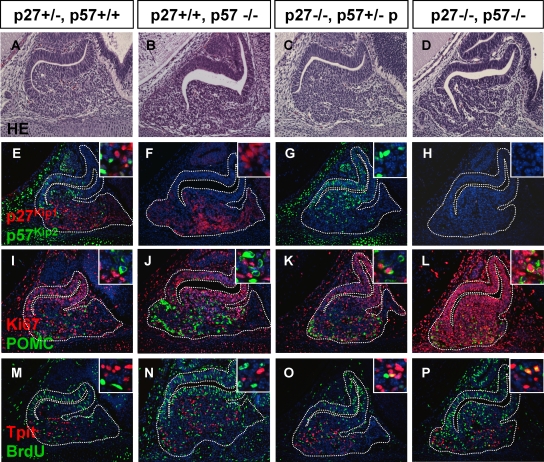
The replacement of p57Kip2 expression by p27Kip1 expression in differentiated cells suggests that p27Kip1 may be needed and/or sufficient to maintain differentiated cells (at least in early development) in a quiescent/nonproliferative state. In order to test this hypothesis, we investigated the p27Kip1 knockout on its own and in combination with p57Kip2 knockout (Fig. 6). Despite a predisposition to pituitary tumorigenesis later in adult mice (11, 13, 23, 29), the e14.5 p27Kip1−/− pituitaries did not display overt phenotypes (compare Fig. 6C and G to Fig. 6A and E); however, colabeling for Ki67 and POMC revealed doubly positive cells (Fig. 6K, inset), something that was almost never seen in wild-type (Fig. 6I) or p57Kip2−/− (Fig. 6J) mice. The proliferation of differentiated POMC-expressing cells was confirmed by BrdU and Tpit colabeling in p27Kip1−/− pituitaries but not in wild-type or p57Kip2−/− mice (compare Fig. 6O to Fig. 6M and N, respectively).
FIG. 6.
Double p27Kip1 and p57Kip2 knockout prevents pituitary cell cycle exit but not differentiation. In order to test whether p27Kip1 and p57Kip2 are sufficient for cell cycle exit during pituitary organogenesis, we crossed mice carrying null alleles for both genes. Data are shown for e14.5 pituitaries of p27Kip1+/−; p57Kip2+/+ (A, E, I, and M) mice, which are exactly like their wild-type sibs (not shown); for p27Kip1+/+; p57Kip2−/− mice (B, F, J, and N); for mice that are p27Kip1−/−; p57Kip2+/−, with the mutant allele being on the nonexpressed paternal chromosome (C, G, K, and O); and for double mutant mice (D, H, L, and P). For each genotype, hematoxylin-eosin stains are shown (A to D) together with immunofluorescence for p27Kip1 and p57Kip2 (E to H), Ki67 and POMC (I to L), and Tpit and BrdU (M to P). For double labeling, insets show colabeling or the absence thereof. Detailed analysis of Ki67-positive cells in double knockout pituitaries (L) revealed that the vast majority of the cells are Ki67 positive, including those that are POMC positive (P). Thus, the absence of cell cycle arrest in double knockout mice does not prevent differentiation into the corticotroph lineage.
The most striking result was obtained with p57Kip2−/−;p27Kip1−/− pituitaries: a deficiency of both cell cycle inhibitors prevented cell cycle exit of all pituitary cells expressing Ki67 (Fig. 6L), including POMC-expressing differentiated cells (inset). Consistent with the p27Kip1−/− phenotype, the double knockout pituitaries exhibited Tpit and BrdU doubly positive proliferating differentiated cells (Fig. 6P). Whereas p57Kip2 appears to direct cell cycle exit of pituitary progenitors in early development, these results suggest that p27Kip1 takes over from p57Kip2 in differentiated cells to prevent cell cycle reentry and to maintain quiescence.
Cell differentiation is independent of p57Kip2.
The expression of p57Kip2 in a cell population that appears intermediate between progenitors and differentiated cells and the apparent role of p57Kip2 in cell cycle exit of pituitary progenitors may suggest that p57Kip2 is also a signal for differentiation. This hypothesis was assessed at a stage of development (e14.5) when different lineages appeared, as revealed with Tpit as a marker for corticotrophs, αGSU as a marker for thyrotrophs and gonadotrophs, and Pit1 as a marker of GH and prolactin lineages. Immunofluorescence analysis of p57Kip2−/− pituitaries for these markers clearly showed that differentiation was not prevented in the absence of p57Kip2 (compare Fig. 4X to Z to Fig. 4U to W). Similarly, expression of Prop-1, Pit1, SF1, GH, and β-luteinizing hormone was unaltered in p57Kip2−/− e18.5 pituitaries (see Fig. S1 in the supplemental material). It is also noteworthy that the absence of p57Kip2 did not lead to proliferation of differentiated cells, as no BrdU-positive cells coexpressed a differentiation marker in p57Kip2−/− pituitaries (Fig. 4X to Z and 6J and N). It thus appears that only undifferentiated pituitary progenitors proliferate in the absence of p57Kip2. These conclusions were corroborated in p57Kip2 gain-of-function mice, since the hypoplastic transgenic pituitaries had a few Tpit- and αGSU-positive differentiated cells (compare Fig. 5L and M to Fig. 5K). As indicated above, the loss of p57Kip2 did not impair p27Kip1 expression (Fig. 4N and S and 6F), and as in normal pituitaries (Fig. 3), p27Kip1 was expressed in differentiated cells of p57Kip2−/− pituitaries (Fig. 6 and data not shown). It was similarly shown that the loss of p27Kip1 does not prevent POMC-expressing cell lineage differentiation (11) (Fig. 6K and O). It is striking that POMC-expressing cell differentiation was also not affected by the double loss of p57Kip2 and p27Kip1 (Fig. 6L and P), despite maintenance of the proliferative state in all pituitary cells.
While these data collectively suggest that p57Kip2 is critical for exit from the cell cycle during pituitary development, they also suggest that cell differentiation is controlled independently of p57Kip2 or p27Kip1 and not coupled to cell cycle exit.
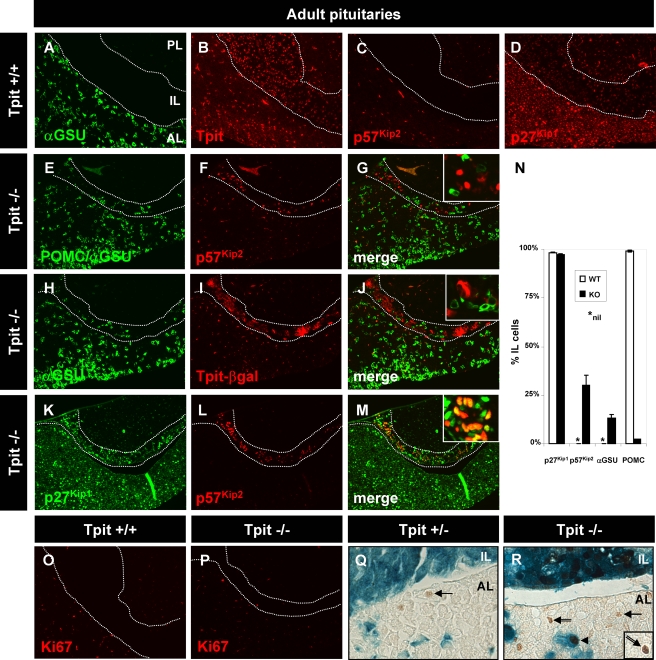
Undifferentiated Tpit−/− cells are blocked in a p57Kip2-positive noncycling state.
Pituitary differentiation thus appears independent of p57Kip2 (Fig. 4U to Z) and p27Kip1 (Fig. 6) (11). In order to assess whether differentiation has a regulatory function in control of cell cycle exit and/or p57Kip2 or p27Kip1 expression, we analyzed Tpit−/− pituitaries that are deficient for POMC-expressing cell lineage differentiation. The most striking phenotype of Tpit−/− pituitaries was a change in cell fate in the IL (32). Whereas the normal IL is constituted entirely of melanotrophs that are positive for Tpit (Fig. 7B), the mutant Tpit−/− IL contained mostly undifferentiated cells, together with a minority of cells that had changed fate to gonadotrophs (αGSU positive) (compare Fig. 7H to Fig. 7A) and, more rarely, POMC-expressing cells (Fig. 7N). About 30% of cells present in the Tpit−/− IL (Fig. 7N) remained p57Kip2 positive (Fig. 7F), in contrast to the absence of p57Kip2 in the normal IL (Fig. 7C), and they did not proliferate, as assessed by Ki67 labeling (Fig. 7P). None of the p57Kip2-positive cells colabeled with POMC or αGSU (Fig. 7E to G). The mutant Tpit−/− mice did not produce intact Tpit (32) but a chimeric gene product constituted of the Tpit N terminus fused to β-galactosidase (β-Gal) (Fig. 7I). This subset of cells did not colabel with the differentiation marker αGSU (Fig. 7H to J), indicating that the chimeric gene product is expressed in undifferentiated cells. Thus, gonadotroph (αGSU-expressing) differentiation appears to lead to extinction of the Tpit-βGal locus, and many cells of the Tpit−/− IL are blocked in the p57Kip2-positive noncycling state. This suggests that differentiation, whether into the POMC-expressing or gonadotroph (αGSU) lineage, switches off p57Kip2 expression. We have shown above that p27Kip1 is switched on in differentiated pituitary cells (Fig. 3). In order to test whether the failure of Tpit−/− IL cells to differentiate affects the expression of p27Kip1, we assessed its expression by immunofluorescence and found that most cells of the mutant IL expressed p27Kip1 (Fig. 7M), including the subset of p57Kip2-expressing cells (Fig. 7M and N). These data indicate that the control of p27Kip1 expression is independent of differentiation and of p57Kip2 expression.
FIG. 7.
In the absence of Tpit, undifferentiated cells are blocked in a p57Kip2-positive noncycling precursor state. Tpit loss of function (Tpit−/−) prevents melanotroph and corticotroph differentiation. The normal adult pituitary IL (between dotted lines) does not express cytoplasmic αGSU (A), but all of its melanotroph cells express nuclear Tpit (B). This tissue does not have p57Kip2-positive cells (C), but most cells are p27Kip1 positive (D). As previously described (32), a small number of gonadotroph cells appear in the IL in the absence of Tpit, as revealed by αGSU staining together with a few POMC-expressing cells (E). The Tpit−/− IL has a large number of p57Kip2-positive cells (F), in contrast to the normal IL (C). There is no overlap between differentiated and p57Kip2-positive cells (G). The Tpit targeting vector included a β-Gal gene fused in frame with the Tpit gene's first exon, leading to production of a Tpit-β-Gal fusion protein; expression of Tpit-β-Gal (I) is not codetected with αGSU (H) in gonadotrophs with a changed fate (J). The p57Kip2-positive cells of the mutant IL (L) are not identical to their counterparts in normal development, since they are also p27Kip1 positive (K to M). (N) Quantification of marker expression in the IL of normal and Tpit−/− pituitaries. (O and P) Tpit knockout does not affect proliferation of cells in either the adult IL or AL, as revealed by Ki67 labeling. (Q and R) Colabeling of Tpit−/− (R) and Tpit+/− (Q) pituitaries for Tpit-β-Gal (blue) and p57Kip2 (brown) reveals a significant persistence of p57Kip2 expression in both the AL and IL of the mutant pituitary. Both normal and mutant AL contain weak p57Kip2-positive nuclei close to the AL-IL border (arrows), but the Tpit−/− AL also has stronger-staining p57Kip2-positive cells that either coexpress Tpit-β-Gal (arrowhead) or do not (double arrow).
The Tpit−/− AL contained a small number of Tpit-βGal-positive cells (Fig. 7R), and some of these were shown to be POMC positive (32). p57Kip2 was rarely detected in the normal adult pituitary (Fig. 7C), but using a more sensitive immunoperoxidase assay rather than immunofluorescence, we could detect a few weak p57Kip2-positive cells, particularly in the AL close to the IL (Fig. 7Q, arrow); this region of the AL was recently shown to contain a pool/niche of adult pituitary stem cells (17). In the Tpit−/− AL, p57Kip2-positive cells were more frequent and included cells with a stronger signal (Fig. 7R, double arrow); in addition, some p57Kip2-positive cells were also Tpit-βGal positive (Fig. 7R, arrowhead), as observed in the IL. These data suggest that the block of differentiation produced by Tpit deficiency results in maintenance of p57Kip2 expression in the AL, as it does in the IL.
In conclusion, the analysis of Tpit−/− pituitaries indicates that p57Kip2-mediated cell cycle exit yields a transient population of noncycling precursors independently of the differentiation process but that this process feeds back to repress p57Kip2 expression.
DISCUSSION
The present work demonstrates the presence of noncycling precursor cells at a critical time in pituitary development, when proliferating progenitors exit the cell cycle and prepare to undergo differentiation. These noncycling precursors are restricted both spatially and temporally during normal development and are marked by expression of p57Kip2 and cyclin E. p57Kip2 appears to be a critical regulator of progenitor cell cycle exit, and its induction is independent of differentiation. Conversely, p27Kip1 is expressed in differentiated cells, and its presence protects cells from reentering the cell cycle.
The role of cyclin E expression in noncycling precursors is intriguing. Cyclin E could be involved in early differentiation processes similar to its purported role in Drosophila neuronal asymmetric cell division and lineage specification (1); however, its restricted expression in noncycling precursors is not suggestive of involvement in a particular differentiated lineage. Alternatively, cyclin E has been associated with endoreplication in trophoblast giant cells (15); our analyses clearly do not support such a possibility, since no Ki67 expression was observed in cyclin E-positive cells (Fig. 2B). Finally, this expression may be related to a kinase-independent function of cyclin E (14).
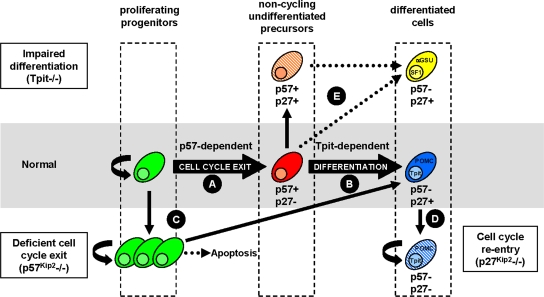
Thus, during normal pituitary development, p57Kip2 controls cell cycle exit of proliferating progenitors to yield noncycling undifferentiated precursors (Fig. 8A). These “noncycling precursors” may represent a mixture of cells, including uncommitted progenitors and cells that are engaged in a particular fate: we presently have markers of terminal differentiation, but none that could mark early commitment. The identification of weakly p57Kip2-positive cells close to the AL-IL border in the adult pituitary would be consistent with a sustained role of p57Kip2 in cell cycle exit of putative progenitors that were recently shown to have similar localization (17) and to be Sox2 positive (10). The p57Kip2- and cyclin E-positive cells may undergo differentiation into different lineages; for corticotrophs and melanotrophs, terminal differentiation depends on Tpit (Fig. 8B). Differentiation is accompanied by extinction of p57Kip2 and cyclin E expression and the appearance of p27Kip1. The expansion of proliferating progenitors in the absence of p57Kip2 (Fig. 8C), taken together with the small number of these cells in p57Kip2-overexpressing pituitaries, supports the conclusion that p57Kip2 is a critical regulator of cell cycle exit for pituitary progenitors. The organomegaly that has been reported for p57Kip2−/− mice suggests that similar situations might exist in other tissues (43); the nature of excess cells in other p57Kip2−/− tissues has not been defined. The importance of p57Kip2-dependent cell cycle exit for normal development is highlighted by the extensive cell loss and apoptosis observed in p57Kip2−/− pituitaries beyond e18.5. The role of p57Kip2 at this critical stage of pituitary organogenesis might be to control the size of the progenitor niche and ultimately organ size by limiting the expansion of progenitors. It should be noted that organ size is not definitive at birth, when most pituitary cells are differentiated, and that further tissue growth occurs in the postnatal period. The mechanisms of this postnatal growth remain to be explored. In contrast, p27Kip1 appears to play a predominant role in preventing differentiated cells (such as POMC- and Tpit-positive cells) (Fig. 6K and O) from reentering the cell cycle (Fig. 8D). These observations are consistent with the development of POMC-positive tumors in p27Kip1−/− pituitaries (11).
FIG. 8.
Uncoupling of proliferation and differentiation during pituitary development. The present work defined a transient population of noncycling undifferentiated precursors (blue) during normal pituitary development that are marked by the expression of p57Kip2 and cyclin E. (A) Following their expansion, proliferative pituitary progenitors (green) exit the cell cycle under the control of p57Kip2 to yield noncycling precursors (blue). (B) These transient cells then switch off p57Kip2 and switch on p27Kip1 in parallel with differentiation into various hormone-producing lineages. This sequence was characterized in detail for the Tpit-dependent corticotroph (POMC) lineage (red), which is the earliest to reach terminal differentiation in the anterior pituitary. (C) In the absence of p57Kip2, the cdki that appears to drive normal cell cycle exit, pituitary progenitors initially overgrow and then undergo extensive apoptosis late in fetal life. However, the absence of p57Kip2 does not prevent the later expression of p27Kip1 or differentiation into various lineages. The p57Kip2-dependent cell cycle exit of progenitors therefore appears to be controlled independently of cell differentiation. (D) Expression of p27Kip1 occurs with differentiation and protects differentiated cells from reentering the cell cycle. (E) In agreement with this, the blockade of cell differentiation observed in Tpit−/− mice leads to the accumulation of noncycling undifferentiated precursors (orange) that are p57Kip2 and p27Kip1 positive. However, a small fraction of cells in the pituitary IL of Tpit−/− mutant mice do differentiate, either through cell fate change into gonadotrophs (yellow) (αGSU and SF1 positive) or, more rarely, into POMC-positive cells. The cells that do differentiate switch off p57Kip2 expression, suggesting that the differentiation process itself is responsible for p57Kip2 extinction.
Temporal and hierarchical control of cell cycle by p57Kip2 and p27Kip1.
p57Kip2 and p27Kip1 are both members of the Cip/Kip family of cdki's, and within the context of cell cycle regulation, they are considered to have largely redundant activities as inhibitors of the cdk2-cyclin E complex (5). Their sequential expression during pituitary organogenesis and cell differentiation could be considered trivial and in keeping with their redundant activities. Indeed, the loss of function of either p57Kip2 or p27Kip1 did not result in unbridled cell proliferation, suggesting a compensation mechanism. This interpretation is likely simplistic, since the loss of p57Kip2 and p27Kip1 did not result in the same outcome. Indeed, p57Kip2 knockout results in maintenance of the proliferative status of pituitary progenitors, but it does not affect p27Kip1-dependent cell cycle exit of differentiated cells. In addition, p57Kip2 deficiency leads to extensive apoptosis in the developing anterior pituitary lobe (Fig. 4T and Z2). In contrast, p27Kip1 deficiency allows reentry of differentiated pituitary cells into the cell cycle but is not associated with any apoptotic phenotype (data not shown); in addition, p27Kip1 knockout eventually leads to differentiated pituitary tumor development (11). The activities of p57Kip2 and p27Kip1 are thus clearly distinct during the pituitary developmental sequence, although both contribute significantly to inhibition of cell cycling.
Thus, the sequential expression of these two Cip/Kip cdki's may be consistent with temporally different but redundant functions in inhibition of the cell cycle, but the different outcomes of their loss of function suggest that they may be involved in a hierarchical mechanism of cell cycle control that operates differently in progenitors/stem cells in comparison to differentiated cells. At present, there are few clues about the differential activities on p57Kip2 and p27Kip1 that may relate to their respective activities in progenitors versus differentiated cells, but clearly this is an important question to address. Unfortunately, the present work does not offer clues in this respect, as the data mostly indicated independent regulation of p57Kip2 and p27Kip1 as well as independent regulation of these cdki's during the differentiation program.
Independent programs for cell cycle exit and differentiation.
Strikingly, p57Kip2−/−, p27Kip1−/−, and p57Kip2−/−; p27Kip1−/− pituitaries have normal distributions of differentiated cells, indicating that accumulation of progenitors and the absence of these cdki's do not impair the differentiation program. Differentiation is thus controlled independently. Furthermore, the differentiated cells of p57Kip2−/− pituitaries express p27Kip1, as do normally differentiated pituitary cells, clearly showing that p27Kip1 expression is not dependent on the transient expression of p57Kip2. Conversely, expression of p57Kip2 itself, as well as p27Kip1 expression, is independent of differentiation, as shown in Tpit−/− mice, which accumulate noncycling precursors that coexpress p57Kip2 and p27Kip1 (Fig. 8E).
Cell differentiation is accompanied by extinction of p57Kip2 expression: this was also observed in Tpit−/− mice, in which a cell fate change occurred. Indeed, cells that should have become POMC positive changed fate to become gonadotrophs (αGSU and SF1 positive); such cells also switch off p57Kip2 expression, as do normal cells. These data suggest that the differentiation program represses the progenitor program, as marked by p57Kip2 expression, and that in POMC-expressing cells, Tpit is not the sole repressor of p57Kip2. We do not know how this feedback is exerted, and this may differ between lineages. Collectively, this work demonstrates the independence of the programs for cell cycle exit from those for differentiation.
The control of p57Kip2 expression in progenitors may be cell autonomous, but it may also be triggered by extracellular signals; the Notch pathway may represent a candidate for this, as Rbp-J and Hes1 knockout mice exhibit premature differentiation and progenitor cell cycle exit (22, 33, 46). Accordingly, we found an overlap of Hes1- and BrdU-positive cells (see Fig. S1C and D in the supplemental material), suggesting active Notch signaling in proliferating progenitors; the number of Hes1 and BrdU doubly positive cells appeared greater in p57Kip2−/− e14.5 pituitaries, in agreement with an expansion of the progenitor pool of cells. Also, p57Kip2 expression was shown to be a direct target of the repressor Hes1 (16). The loss of Hes1 expression in normal development or in Hes1−/− mice would thus lead to p57Kip2 expression and progenitor cell cycle exit. The present demonstration of separate control for cell cycle exit and differentiation may warrant a reassessment of the relative roles of signals such as Notch, fibroblast growth factors, BMPs, and Wnt in early pituitary organogenesis.
Stem cells, cell cycle exit, and differentiation.
The proliferating pituitary cells identified in the present work likely represent progenitors rather than committed cells, as supported by the recent characterization of fetal and adult pituitary stem cells (10, 17). The uncoupling of cell cycle exit and differentiation shown here may be consistent with cancer stem cell models which propose that a small number of stem cells underlie the growth of differentiated tumors (41). It is noteworthy that progenitor cells, but not differentiated cells, are expanded in p57Kip2−/− pituitaries. The loss of p57Kip2 expression may thus predispose cells to tumorigenesis. This would be consistent with the identification of p57Kip2 mutations or imprinting defects that prevent p57Kip2 expression in Beckwith-Wiedemann syndrome, an overgrowth syndrome characterized by tumor susceptibility (24, 34).
Conversely, the program of cell cycle exit exerted by p57Kip2 may be an important feature for normal differentiation or for loss of “stemness.” Considerable effort is currently being applied toward the targeted differentiation of stem cells into specific differentiation pathways, such as for production of insulin-secreting pancreatic β cells or dopaminergic neurons. This differentiation is targeted by expression of restricted transcription factors that trigger the differentiation program. The uncoupling between cell cycle exit and differentiation may be an important and poorly recognized parameter for such targeted differentiation. Thus, programmed cell cycle exit of stem cells may be required for the efficient production of appropriately differentiated cells. The implications of the present finding for the therapeutic use of stem cells remain to be explored.
Supplementary Material
Acknowledgments
We are grateful to many colleagues in the lab for their comments and in particular to Claude Sardet and Jean-Marie Blanchard, Montpellier, for comments on the manuscript. We thank Simon Rhodes, University of Indiana, for his generous gift of Pit1 antibody and the Developmental Studies Hybridoma Bank, developed under the auspices of the NICHD and maintained by the Department of Biological Sciences, University of Iowa. The excellent work of Annie Vallée and Julie D'Amours in tissue preparations and animal maintenance, respectively, is acknowledged. We are grateful to Anne-Marie Pulichino for Tpit knockout mice. The expert secretarial assistance of Lise Laroche is greatly appreciated.
This work was supported by a fellowship from the Fonds des Chercheurs et Aide à la Recherche-Fonds de la Recherche en Santé du Québec (FCAR-FRSQ) and the Canadian Institutes of Health Research (CIHR) to S.B. and by research grants to J.D. from the CIHR and the National Cancer Institute of Canada.
We have no conflicts of interest to declare.
Footnotes
Published ahead of print on 12 January 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Berger, C., S. K. Pallavi, M. Prasad, L. S. Shashidhara, and G. M. Technau. 2005. Cyclin E acts under the control of Hox-genes as a cell fate determinant in the developing central nervous system. Cell Cycle 4422-425. [DOI] [PubMed] [Google Scholar]
- 2.Bodner, M., J. L. Castrillo, L. E. Theill, T. Deerinck, M. Ellisman, and M. Karin. 1988. The pituitary-specific transcription factor GHF-1 is a homeobox-containing protein. Cell 55505-518. [DOI] [PubMed] [Google Scholar]
- 3.Charles, M. A., T. L. Saunders, W. M. Wood, K. Owens, A. F. Parlow, S. A. Camper, E. C. Ridgway, and D. F. Gordon. 2006. Pituitary-specific Gata2 knockout: effects on gonadotrope and thyrotrope function. Mol. Endocrinol. 201366-1377. [DOI] [PubMed] [Google Scholar]
- 4.Charles, M. A., H. Suh, J. Drouin, S. A. Camper, and P. J. Gage. 2005. PITX genes are required for cell survival and Lhx3 activation. Mol. Endocrinol. 191893-1903. [DOI] [PubMed] [Google Scholar]
- 5.Ciemerych, M. A., and P. Sicinski. 2005. Cell cycle in mouse development. Oncogene 242877-2898. [DOI] [PubMed] [Google Scholar]
- 6.Couly, G. F., and N. M. Le Douarin. 1985. Mapping of the early neural primordium in quail-chick chimeras. I. Developmental relationships between placodes, facial ectoderm, and prosencephalon. Dev. Biol. 110422-439. [DOI] [PubMed] [Google Scholar]
- 7.Couly, G. F., and N. M. Le Douarin. 1987. Mapping of the early neural primordium in quail-chick chimeras. II. The prosencephalic neural plate and neural folds: implications for the genesis of cephalic human congenital abnormalities. Dev. Biol. 120198-214. [DOI] [PubMed] [Google Scholar]
- 8.Dasen, J. S., S. M. O'Connell, S. E. Flynn, M. Treier, A. S. Gleiberman, D. P. Szeto, F. Hooshmand, A. K. Aggarwal, and M. G. Rosenfeld. 1999. Reciprocal interactions of Pit1 and GATA2 mediate signaling gradient-induced determination of pituitary cell types. Cell 97587-598. [DOI] [PubMed] [Google Scholar]
- 9.Dyer, M. A., and C. L. Cepko. 2001. p27Kip1 and p57Kip2 regulate proliferation in distinct retinal progenitor cell populations. J. Neurosci. 214259-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fauquier, T., K. Rizzoti, M. Dattani, R. Lovell-Badge, and I. C. Robinson. 2008. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc. Natl. Acad. Sci. USA 1052907-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fero, M. L., M. Rivkin, M. Tasch, P. Porter, C. E. Carow, E. Firpo, K. Polyak, L. H. Tsai, V. Broudy, R. M. Perlmutter, K. Kaushansky, and J. M. Roberts. 1996. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell 85733-744. [DOI] [PubMed] [Google Scholar]
- 12.Figliola, R., and R. Maione. 2004. MyoD induces the expression of p57Kip2 in cells lacking p21Cip1/Waf1: overlapping and distinct functions of the two cdk inhibitors. J. Cell Physiol. 200468-475. [DOI] [PubMed] [Google Scholar]
- 13.Franklin, D. S., V. L. Godfrey, H. Lee, G. I. Kovalev, R. Schoonhoven, S. Chen-Kiang, L. Su, and Y. Xiong. 1998. CDK inhibitors p18(INK4c) and p27(Kip1) mediate two separate pathways to collaboratively suppress pituitary tumorigenesis. Genes Dev. 122899-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geng, Y., Y. M. Lee, M. Welcker, J. Swanger, A. Zagozdzon, J. D. Winer, J. M. Roberts, P. Kaldis, B. E. Clurman, and P. Sicinski. 2007. Kinase-independent function of cyclin E. Mol. Cell 25127-139. [DOI] [PubMed] [Google Scholar]
- 15.Geng, Y., Q. Yu, E. Sicinska, M. Das, J. E. Schneider, S. Bhattacharya, W. M. Rideout, R. T. Bronson, H. Gardner, and P. Sicinski. 2003. Cyclin E ablation in the mouse. Cell 114431-443. [DOI] [PubMed] [Google Scholar]
- 16.Georgia, S., R. Soliz, M. Li, P. Zhang, and A. Bhushan. 2006. p57 and Hes1 coordinate cell cycle exit with self-renewal of pancreatic progenitors. Dev. Biol. 29822-31. [DOI] [PubMed] [Google Scholar]
- 17.Gleiberman, A. S., T. Michurina, J. M. Encinas, J. L. Roig, P. Krasnov, F. Balordi, G. Fishell, M. G. Rosenfeld, and G. Enikolopov. 2008. Genetic approaches identify adult pituitary stem cells. Proc. Natl. Acad. Sci. USA 1056332-6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodyer, C. G., J. J. Tremblay, F. W. Paradis, A. Marcil, C. Lanctôt, Y. Gauthier, and J. Drouin. 2003. Pitx1 in vivo promoter activity and mechanisms of positive autoregulation. Neuroendocrinology 78129-137. [DOI] [PubMed] [Google Scholar]
- 19.Ingraham, H. A., D. S. Lala, Y. Ikeda, X. Luo, W. H. Shen, M. W. Nachtigal, R. Abbud, J. H. Nilson, and K. L. Parker. 1994. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev. 82302-2312. [DOI] [PubMed] [Google Scholar]
- 20.Jacks, T., A. Fazeli, E. M. Schmitt, R. T. Bronson, M. A. Goodell, and R. A. Weinberg. 1992. Effects of an Rb mutation in the mouse. Nature 359295-300. [DOI] [PubMed] [Google Scholar]
- 21.John, R. M., J. F. Ainscough, S. C. Barton, and M. A. Surani. 2001. Distant cis-elements regulate imprinted expression of the mouse p57(Kip2) (Cdkn1c) gene: implications for the human disorder, Beckwith-Wiedemann syndrome. Hum. Mol. Genet. 101601-1609. [DOI] [PubMed] [Google Scholar]
- 22.Kita, A., I. Imayoshi, M. Hojo, M. Kitagawa, H. Kokubu, R. Ohsawa, T. Ohtsuka, R. Kageyama, and N. Hashimoto. 2007. Hes1 and Hes5 control the progenitor pool, intermediate lobe specification, and posterior lobe formation in the pituitary development. Mol. Endocrinol. 211458-1466. [DOI] [PubMed] [Google Scholar]
- 23.Kiyokawa, H., R. D. Kineman, K. O. Manova-Todorova, V. C. Soares, E. S. Hoffman, M. Ono, D. Khanam, A. C. Hayday, L. A. Frohman, and A. Koff. 1996. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell 85721-732. [DOI] [PubMed] [Google Scholar]
- 24.Lam, W. W., I. Hatada, S. Ohishi, T. Mukai, J. A. Joyce, T. R. Cole, D. Donnai, W. Reik, P. N. Schofield, and E. R. Maher. 1999. Analysis of germline CDKN1C (p57KIP2) mutations in familial and sporadic Beckwith-Wiedemann syndrome (BWS) provides a novel genotype-phenotype correlation. J. Med. Genet. 36518-523. [PMC free article] [PubMed] [Google Scholar]
- 25.Lamolet, B., A. M. Pulichino, T. Lamonerie, Y. Gauthier, T. Brue, A. Enjalbert, and J. Drouin. 2001. A pituitary cell-restricted T-box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell 104849-859. [DOI] [PubMed] [Google Scholar]
- 26.Lanctôt, C., Y. Gauthier, and J. Drouin. 1999. Pituitary homeobox 1 (Ptx1) is differentially expressed during pituitary development. Endocrinology 1401416-1422. [DOI] [PubMed] [Google Scholar]
- 27.Lanctôt, C., B. Lamolet, and J. Drouin. 1997. The bicoid-related homeoprotein Ptx1 defines the most anterior domain of the embryo and differentiates posterior from anterior lateral mesoderm. Development 1242807-2817. [DOI] [PubMed] [Google Scholar]
- 28.Lee, Y. S., F. Liu, and N. Segil. 2006. A morphogenetic wave of p27Kip1 transcription directs cell cycle exit during organ of Corti development. Development 1332817-2826. [DOI] [PubMed] [Google Scholar]
- 29.Nakayama, K., N. Ishida, M. Shirane, A. Inomata, T. Inoue, N. Shishido, I. Horii, D. Y. Loh, and K. Nakayama. 1996. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 85707-720. [DOI] [PubMed] [Google Scholar]
- 30.Poulin, G., M. Lebel, M. Chamberland, F. W. Paradis, and J. Drouin. 2000. Specific protein-protein interaction between basic helix-loop-helix transcription factors and homeoproteins of the Pitx family. Mol. Cell. Biol. 204826-4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pulichino, A. M., S. Vallette-Kasic, C. Couture, Y. Gauthier, T. Brue, M. David, G. Malpuech, C. Deal, G. Van Vliet, M. De Vroede, F. G. Riepe, C. J. Partsch, W. G. Sippell, M. Berberoglu, B. Atasay, and J. Drouin. 2003. Human and mouse Tpit gene mutations cause early onset pituitary ACTH deficiency. Genes Dev. 17711-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pulichino, A. M., S. Vallette-Kasic, J. P. Y. Tsai, C. Couture, Y. Gauthier, and J. Drouin. 2003. Tpit determines alternate fates during pituitary cell differentiation. Genes Dev. 17738-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raetzman, L. T., J. X. Cai, and S. A. Camper. 2007. Hes1 is required for pituitary growth and melanotrope specification. Dev. Biol. 304455-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahman, N. 2005. Mechanisms predisposing to childhood overgrowth and cancer. Curr. Opin. Genet. Dev. 15227-233. [DOI] [PubMed] [Google Scholar]
- 35.Sheng, H. Z., K. Moriyama, T. Yamashita, H. Li, S. S. Potter, K. A. Mahon, and H. Westphal. 1997. Multistep control of pituitary organogenesis. Science 2781809-1812. [DOI] [PubMed] [Google Scholar]
- 36.Sherr, C. J., and J. M. Roberts. 1999. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 131501-1512. [DOI] [PubMed] [Google Scholar]
- 37.Sherr, C. J., and J. M. Roberts. 2004. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 182699-2711. [DOI] [PubMed] [Google Scholar]
- 38.Sornson, M. W., W. Wu, J. S. Dasen, S. E. Flynn, D. J. Norman, S. M. O'Connell, I. Gukovsky, C. Carriere, A. K. Ryan, A. P. Miller, L. Zuo, A. S. Gleiberman, B. Andersen, W. G. Beamer, and M. G. Rosenfeld. 1996. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature 384327-333. [DOI] [PubMed] [Google Scholar]
- 39.Suh, H., P. J. Gage, J. Drouin, and S. A. Camper. 2002. Pitx2 is required at multiple stages of pituitary organogenesis: pituitary primordium formation and cell specification. Development 129329-337. [DOI] [PubMed] [Google Scholar]
- 40.Ward, R. D., L. T. Raetzman, H. Suh, B. M. Stone, I. O. Nasonkin, and S. A. Camper. 2005. Role of PROP1 in pituitary gland growth. Mol. Endocrinol. 19698-710. [DOI] [PubMed] [Google Scholar]
- 41.Ward, R. J., and P. B. Dirks. 2007. Cancer stem cells: at the headwaters of tumor development. Annu. Rev. Pathol. Mech. Dis. 2175-189. [DOI] [PubMed] [Google Scholar]
- 42.Yan, Y., J. Frisen, M. H. Lee, J. Massague, and M. Barbacid. 1997. Ablation of the CDK inhibitor p57Kip2 results in increased apoptosis and delayed differentiation during mouse development. Genes Dev. 11973-983. [DOI] [PubMed] [Google Scholar]
- 43.Zhang, P., N. J. Liegeois, C. Wong, M. Finegold, H. Hou, J. C. Thompson, A. Silverman, J. W. Harper, R. A. DePinho, and S. J. Elledge. 1997. Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith-Wiedemann syndrome. Nature 387151-158. [DOI] [PubMed] [Google Scholar]
- 44.Zhang, P., C. Wong, R. A. DePinho, J. W. Harper, and S. J. Elledge. 1998. Cooperation between the Cdk inhibitors p27(KIP1) and p57(KIP2) in the control of tissue growth and development. Genes Dev. 123162-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang, P., C. Wong, D. Liu, M. Finegold, J. W. Harper, and S. J. Elledge. 1999. p21(CIP1) and p57(KIP2) control muscle differentiation at the myogenin step. Genes Dev. 13213-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu, X., J. Zhang, J. Tollkuhn, R. Ohsawa, E. H. Bresnick, F. Guillemot, R. Kageyama, and M. G. Rosenfeld. 2006. Sustained Notch signaling in progenitors is required for sequential emergence of distinct cell lineages during organogenesis. Genes Dev. 202739-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.